Abstract
Purpose
Double-hit lymphomas (DHLs) and double-expressor lymphomas (DELs) are subtypes of diffuse large B-cell lymphoma (DLBCL) associated with poor outcomes after standard chemoimmunotherapy. Data are limited regarding outcomes of patients with relapsed or refractory (rel/ref) DEL or DHL who undergo autologous stem-cell transplantation (ASCT). We retrospectively studied the prognostic impact of DEL and DHL status on ASCT outcomes in patients with rel/ref DLBCL.
Methods
Patients with chemotherapy-sensitive rel/ref DLBCL who underwent ASCT at two institutions and in whom archival tumor material was available were enrolled. Immunohistochemistry for MYC, BCL2, and BCL6 and fluorescence in situ hybridization (FISH) for MYC were performed. In cases with MYC rearrangement or copy gain, FISH for BCL2 and BCL6 was also performed.
Results
A total of 117 patients were included; 44% had DEL and 10% had DHL. DEL and DHL were associated with inferior progression-free survival (PFS), and DHL was associated with poorer overall survival (OS). The 4-year PFS in patients with DEL compared with those with non-DEL was 48% versus 59% (P = .049), and the 4-year OS was 56% versus 67% (P = .10); 4-year PFS in patients with DHL compared with those with non-DHL was 28% versus 57% (P = .013), and 4-year OS was 25% versus 61% (P = .002). The few patients with concurrent DEL and DHL had a poor outcome (4-year PFS, 0%). In multivariable models, DEL and DHL were independently associated with inferior PFS, whereas DHL and partial response (v complete response) at transplant were associated with inferior OS.
Conclusion
DEL and DHL are both associated with inferior outcomes after ASCT in patients with rel/ref DLBCL. Although ASCT remains a potentially curative approach, these patients, particularly those with DHL, are a high-risk subset who should be targeted for investigational strategies other than standard ASCT.
INTRODUCTION
High-dose chemotherapy followed by autologous stem-cell transplantation (ASCT) is a potentially curative treatment for patients with chemotherapy-sensitive relapsed or refractory (rel/ref) diffuse large B-cell lymphoma (DLBCL), but more than half of patients will ultimately relapse.1,2 Many prognostic factors are associated with post-ASCT outcome in patients with rel/ref DLBCL, including pre-ASCT remission status as assessed by positron emission tomography (PET), time to relapse, and prognostic indices (eg, second-line age-adjusted International Prognostic Index [IPI]).1,3-10 Although these clinical prognostic factors are useful, new tumor-specific biomarkers may allow for improved prognostication.
Double-hit lymphomas (DHLs) are a subset of DLBCL with concurrent chromosomal rearrangements involving the MYC and BCL2 and/or BCL6 genes, which comprise approximately 2% to 10% of newly diagnosed DLBCL. Patients with DHL have dismal outcomes with standard induction chemoimmunotherapy with rituximab added to cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).11-29 Double-expressor lymphomas (DELs) are DLBCLs with co-expression of the MYC and BCL2 proteins by immunohistochemistry (IHC) and comprise 21% to 34% of patients newly diagnosed with DLBCL. A subset of patients will have both DEL and DHL. Similar to DHL, patients with DEL also have poor outcomes after R-CHOP induction therapy, independent of DHL status.12,15,18,19,30,31 The prognostic impact of MYC and BCL2 coexpression in DLBCL also appears to be independent of other prognostic factors like cell-of-origin (COO) and IPI scores.15,18,19 Although MYC rearrangement (MYC-R) appears to be associated with inferior outcome in rel/ref DLBCL, there are limited data regarding the outcome of patients with rel/ref DHL or DEL who proceed to ASCT.32,33 In this retrospective study, we evaluated the prognostic impact of DEL and DHL status in patients with rel/ref aggressive B-cell non-Hodgkin lymphoma (NHL) undergoing ASCT.
METHODS
Patients
We performed a retrospective, multicenter study of adult patients with rel/ref aggressive B-cell NHL who underwent ASCT at the Dana-Farber Cancer Institute/Brigham and Women’s Hospital (DFCI/BWH; Boston, MA) and City of Hope National Medical Center (COH; Duarte, CA). Patients enrolled from DFCI/BWH were transplanted between January 2003 and June 2013, and COH patients were transplanted between January 2000 and December 2012. Patients with DLBCL and unclassifiable B-cell lymphoma, with features intermediate between Burkitt lymphoma and DLBCL, were eligible. Eligible patients had to have chemosensitive disease, defined as at least a partial response (PR) or complete response (CR) to salvage therapy. Patients with transformed indolent B-cell NHL, primary mediastinal B-cell lymphoma, primary CNS lymphoma, or Richter transformation of chronic lymphocytic leukemia were excluded. Patients who had not received rituximab before ASCT, patients who received a tandem procedure with ASCT followed by allogeneic hematopoietic stem-cell transplantation, and patients who received consolidative ASCT in their first response after induction therapy were also excluded. Eligible patients had to have an available formalin-fixed, paraffin-embedded tumor specimen. The most recent available biopsy before ASCT (ie, relapse biopsy) was used for testing. Patients underwent salvage therapy and ASCT according to institutional practices. The study was approved by the institutional review boards at both centers.
Immunohistochemistry
Confirmation of the histologic diagnosis and review of all IHC was performed by two or more hematopathologists at each institution (S.J.R., G.K.G, Y.K., L.L., and J.Y.S.). Any discrepancies were resolved by consensus at a multiheaded microscope. IHC was performed for MYC, BCL2, and BCL6 according to standard protocols, detailed in the Appendix (online only). The percentage of positive tumor cells was scored in deciles and the hematopathologists were blinded to patient outcome and the results of fluorescence in situ hybridization (FISH) cytogenetic testing. DEL was defined as MYC expression in ≥ 40% of tumor cells and BCL2 expression in ≥ 50% of tumor cells.19 COO was determined according to the Hans algorithm.34
FISH
FISH analysis for MYC rearrangement was performed on all patients using the Vysis LSI MYC dual color break-apart rearrangement probe (Abbott Molecular, Des Plaines, IL; https://www.abbottmolecular.com) according to the manufacturer’s instructions. At least 100 nuclei were counted; rearrangement was defined as the presence of breakapart signals in ≥ 10% of nuclei, and copy gain (CG) was defined as three or more signals in ≥ 30% of nuclei. In patients with MYC-R or CG, FISH for BCL2 and BCL6 was performed using Vysis LSI BCL2 and BCL6 dual color breakapart rearrangement probes (Abbott Molecular). DHL was defined as concurrent rearrangements of MYC and BCL2 and/or BCL6. Atypical DHL was defined as MYC CG without MYC-R but one or more of the following: BCL2 CG, BCL6 CG, BCL2 or BCL6 rearrangement; or MYC-R without BCL2 or BCL6 rearrangement but with BCL2 CG and/or BCL6 CG.24,35
Statistical Analysis
Fisher’s exact test and χ2 tests were used for group comparison of categorical variables. Kruskal-Wallis test was used for group comparison of continuous variables. Overall survival (OS) and progression-free survival (PFS) were estimated using the Kaplan-Meier method. The log-rank test was used to compare OS and PFS between subgroups. Cox proportional hazards models with forward variable selection (entry criterion P < .05) were used to evaluate predictors of PFS and OS. Because the outcomes were comparable for both centers, the analyses were not stratified by center. P values were two-sided with a significance level of .05. All data were analyzed using SAS version 9.3 (SAS Institute, Cary, NC; http://www.sas.com) and R version 3.1.2 (R Foundation, Vienna Austria; https://www.r-project.org).
RESULTS
Patients
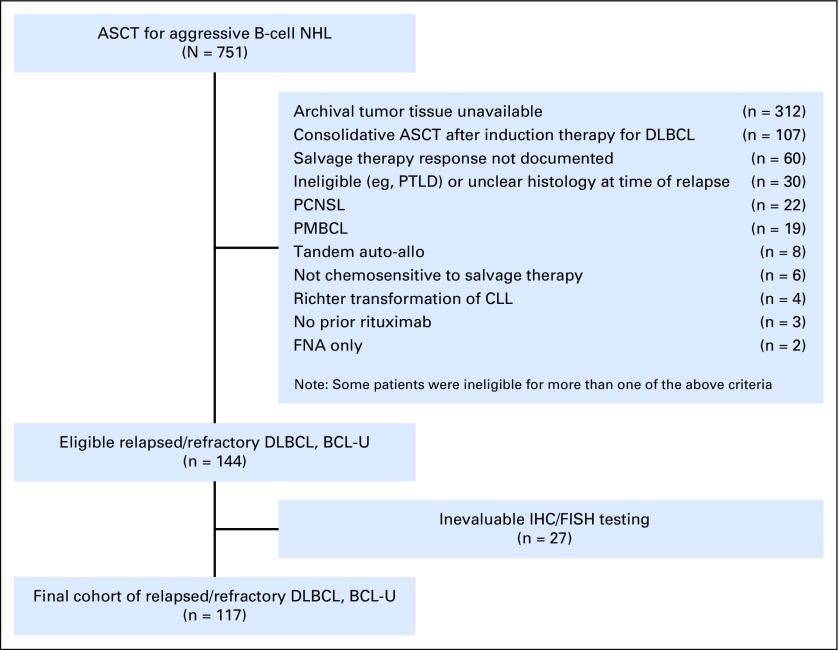
A total of 751 patients underwent ASCT for aggressive B-cell NHL at DFCI/BWH or COH during the study periods. Among those, 144 patients met the eligibility criteria and had available tumor tissue and clinical data. Complete data, with evaluable IHC and FISH testing, were available for 117 patients, who made up the study cohort (Appendix Fig A1, online only). Of the 144 patients, 114 had DLBCL, and three had unclassifiable B-cell lymphoma with features intermediate between Burkitt lymphoma and DLBCL. All patients had received prior anthracycline-based CHOP or CHOP-like chemotherapy except one patient who had received prior anthracycline-based chemotherapy for a distant history of localized breast cancer. The median follow-up time for survivors was 45 months (range, 7 to 115 months). For the entire cohort, the 4-year PFS and OS were 54% (95% CI, 44% to 63%) and 62% (95% CI, 52% to 71%), respectively. Overall, outcomes were similar in the study cohort compared with eligible patients in whom IHC or FISH testing was unevaluable (not shown). In addition, the outcome of patients transplanted at each center were similar: 4-year PFS of 50% in COH patients versus 56% in DFCI/BWH patients (P = .7), and a 4-year OS of 54% in COH versus 68% in DFCI/BWH patients (P = .2).
DEL, DHL, and Outcomes
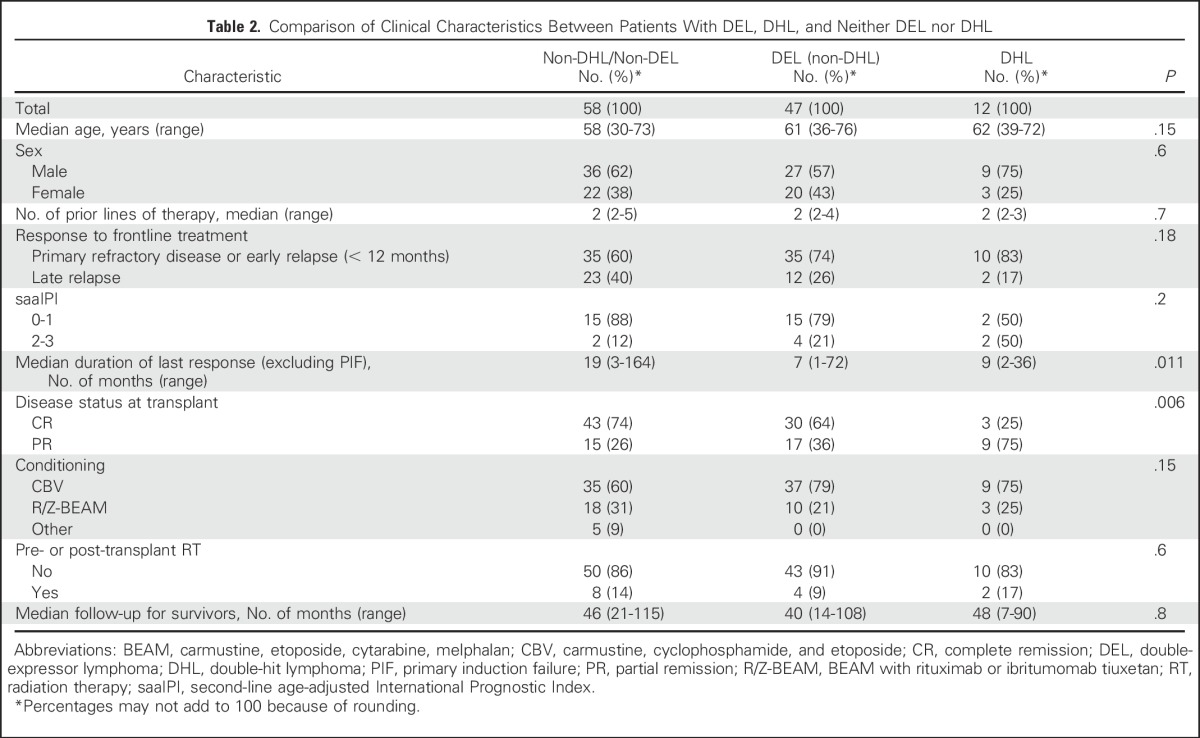
The IHC and FISH data are summarized in Table 1. DEL with MYC/BCL2 coexpression was observed in 52 patients (44%). MYC-R was present in 15 patients (13%), of whom 12 (10%) had DHL. Clinical characteristics in patients with DEL, DHL, or neither DEL nor DHL are listed in Table 2. Patients with DHL were more likely to have only a PR to salvage therapy (evaluated by PET in 105 patients and computed tomography [CT] in 12 patients; 75% of patients who underwent CT were in CR). Patients with DEL and those with DHL had a shorter time to relapse.
Table 1.
Summary of IHC and FISH Data (N = 117)
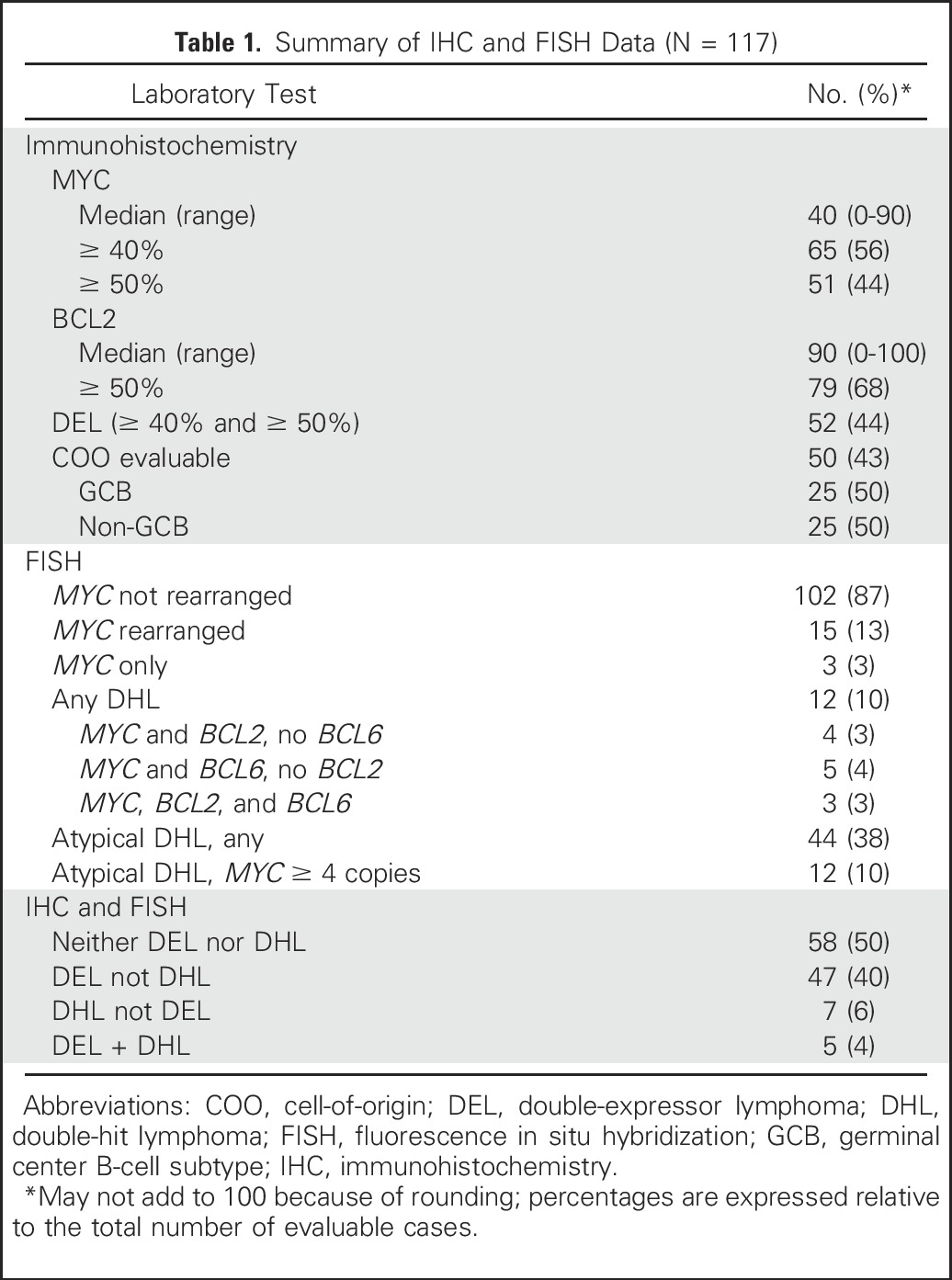
Table 2.
Comparison of Clinical Characteristics Between Patients With DEL, DHL, and Neither DEL nor DHL
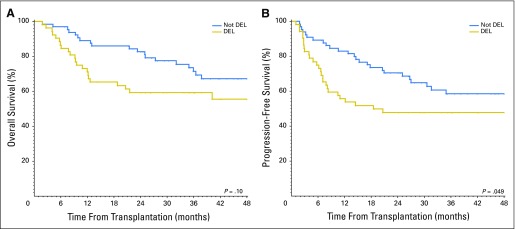
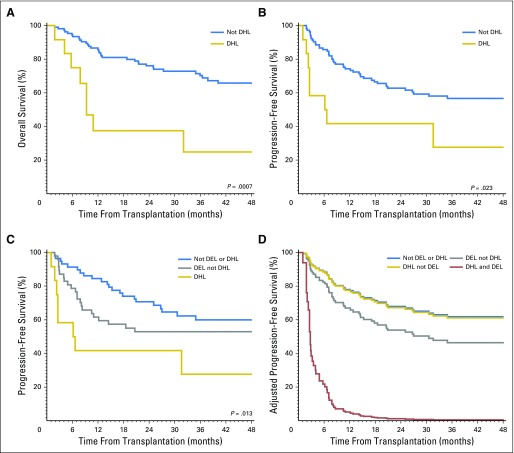
The 4-year PFS in patients with DEL was inferior to that of patients without MYC/BCL2 coexpression: 48% (95% CI, 34% to 61%) versus 59% (95% CI, 45% to 70%; P = .049). The 4-year OS in patients with DEL was 56% (95% CI, 40% to 69%) versus 67% (95% CI, 53% to 79%; P = .1; Figs1A and 1B). The 4-year PFS and OS in patients with DHL were inferior to that of patients without DHL: 28% (95% CI, 6% to 57%) versus 57% (95% CI, 46% to 66%; P = .013), and 25% (95% CI, 5% to 54%) versus 66% (95% CI, 55% to 75%; P < .001), respectively (Figs 2A and 2B). Patients with DHL had poorer PFS (28%; 95% CI, 6% to 57%) and OS (25%; 95% CI, 5% to 54%) compared with patients with DEL but not DHL (PFS 53%, 95% CI, 38% to 66%; OS 61%, 95% CI, 45% to 74%), and patients with neither DEL nor DHL (PFS 60%, 95% CI, 46% to 72%; OS 70%, 95% CI, 55% to 80%; three-way P value for PFS, P = .013; OS, P = .002; Fig 2C).
Fig 1.
Graphs of (A) overall survival and (B) progression-free survival after autologous stem-cell transplantation in patients with DEL compared with patients without DEL. DEL, double-expressor lymphoma.
Fig 2.
Graphs of overall survival and progression-free survival after autologous stem-cell transplantation in patients with DHL compared with patients without DHL. (A) Overall survival. (B) Progression-free survival. (C) Progression-free survival in patients with DHL compared with patients with DEL without DHL and patients with neither DEL nor DHL. (D). Progression-free survival curves adjusted for baseline covariates, stratifying patients by disease status into nonDEL/nonDHL (n = 58), DEL/nonDHL (n = 47), nonDEL/DHL (n = 7), and DEL/DHL (n = 5). DEL, double-expressor lymphoma; DHL, double-hit lymphoma.
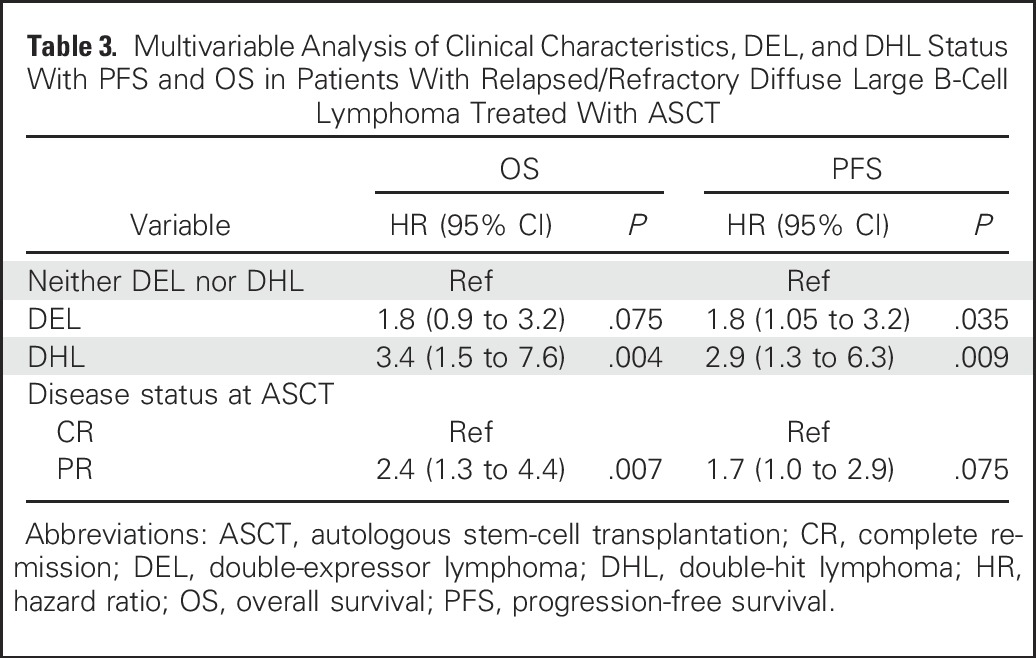
Cox models with forward variable selection were constructed for PFS and OS, including all clinical characteristics shown in Table 2 as well as DEL and DHL status as covariates. In these models, the only factors significantly associated with PFS were DEL (hazard ratio [HR], 1.8; 95% CI, 1.05 to 3.2; P = .035) and DHL (HR, 2.9; 95% CI, 1.3 to 6.3; P = .009). The factors significantly associated with OS were DHL (HR, 3.4; 95% CI, 1.5 to 7.6; P = .004) and remission status at ASCT (HR for PR, 2.4; 95% CI, 1.3 to 4.4; P = .007). Table 3 displays the final PFS and OS model output using DEL and DHL status as well as remission status as covariates. When transplant center was forced into the model, there was no significant impact on outcome (not shown).
Table 3.
Multivariable Analysis of Clinical Characteristics, DEL, and DHL Status With PFS and OS in Patients With Relapsed/Refractory Diffuse Large B-Cell Lymphoma Treated With ASCT
Because of the overlap between the DEL and DHL groups, we sought to disentangle the impact of DEL and DHL status in an exploratory analysis. Figure 2D shows PFS curves, adjusted for baseline covariates, of four groups of patients separated by both DEL and DHL status. Compared with patients in all other subgroups, patients with concurrent DEL and DHL had dismal outcomes (4-year PFS, 0%). In a bivariable model using remission status at ASCT and DEL/DHL status (as a four-group variable), the HRs for progression or death (using patients without DEL or DHL as the reference group) were 1.5 (95% CI, 0.8 to 2.6; P = .2) for DEL/nonDHL, 1.4 (95% CI, 0.4 to 4.5; P = .6) for nonDEL/DHL, and 11.7 (95% CI, 3.8 to 35.6; P < .001) for DEL+DHL. In similar models for OS, the corresponding HRs for death were 1.4 (95% CI, 0.7 to 2.7; P = .4), 1.7 (95% CI, 0.5 to 5.9; P = .4), and 10.5 (95% CI, 3.4 to 32.8; P < .001).
Additional IHC and FISH Analyses
We evaluated additional potential IHC and FISH biomarkers of outcome in our cohort. MYC overexpression (positive in ≥ 40% tumor cells) combined with low BCL6 expression (positive in ≤ 25% of tumor cells) was present in 29 patients (16%) but was not significantly associated with outcome in our cohort (Appendix).30 Atypical DHL, with concurrent chromosomal abnormalities of MYC and BCL2 and/or BCL6 other than concurrent rearrangements, was not associated with outcome in actuarial or multivariable analyses, even when four or more copies of MYC were present (Appendix).35 A subset of patients (n = 50) were evaluable for COO using the Hans algorithm: 25 patients (50%) had a germinal center B-cell (GCB) subtype, and 25 (50%) had a non-GCB subtype. Among patients with GCB subtype tumors, 24% had DEL, compared with 36% DEL among patients with non-GCB subtype tumors (P = .7). DHL was present in 20% of patients with the GCB subtype compared with 4% of patients with the non-GCB subtype (P = .3). In this subset of patients with COO data, 15 patients had DEL and six patients had DHL; 60% of DEL patients (nine of 15) had non-GCB subtype and 83% of DHL patients (five of six) had GCB subtype tumors. Overall, there was no apparent difference in outcome based on COO: 4-year PFS was 47% in the GCB subgroup compared with 43% for patients with the non-GCB tumors (P = .8); the corresponding 4-year OSs were 48% versus 66%, respectively (P = .67). This remained true in multivariable analyses for OS and PFS limited to patients with COO data available.
DISCUSSION
Patients with DEL or DHL have poor outcomes with standard R-CHOP chemoimmunotherapy.11-31 However, little is known regarding the outcomes in patients with rel/ref DEL or DHL who undergo ASCT, which remains the standard of care for patients with chemosensitive rel/ref DLBCL. We performed this retrospective multicenter study, therefore, to examine the prognostic impact of DEL and DHL status on ASCT outcomes in patients with rel/ref DLBCL. In this cohort of transplanted patients selected only based on the availability of tumor tissue and clinical data, the prevalence of DEL (44%) was slightly higher than what has typically been reported in newly diagnosed DLBCL cohorts, and the prevalence of DHL (10%) was on the high end of the reported range of DHL prevalence in upfront DLBCL.12,15,18,19,30,31 A higher prevalence of DEL and DHL might be expected in a rel/ref population; however, our study included only patients with chemosensitive rel/ref DLBCL who underwent ASCT. A higher prevalence of DEL and DHL may have been observed in a cohort of patients with rel/ref DLBCL identified at the time of relapse or refractory disease. Indeed, in the Collaborative Trial in Relapsed Aggressive Lymphoma (CORAL) study, fewer than half of patients with rel/ref DLBCL with MYC-R underwent ASCT per protocol.32
A comparison of clinical characteristics between patients with DEL, DHL, and neither DEL nor DHL revealed that even among patients with chemotherapy-sensitive rel/ref DLBCL, there were differences in the depth and duration of response to induction or salvage therapy. In our cohort, patients with DHL were less likely to be in CR after salvage therapy. Also, patients with DEL and patients with DHL had a shorter time to relapse after induction therapy. Second-line IPI data were available in only a small minority of patients; therefore, we were unable to draw meaningful conclusions about differences in IPI scores among groups. In other studies, patients with DEL were older15,18 and more likely to have a non-GCB subtype,15,18,19 whereas DHL patients were more likely to have a GCB subtype.21,24,25,27,28 As expected, in our cohort, DEL was more common in the patients with the non-GCB subtype, and DHL was more common in those with the GCB subtype, although these differences were not statistically significant, likely because of the limited number of patients with COO data. Likewise, patients with DEL and patients with DHL were older than those without DEL or DHL, although not significantly so.
Similar to what has been reported in studies of patients newly diagnosed with DLBCL, DEL and DHL were both associated with poorer outcomes after ASCT in patients with rel/ref DLBCL. As observed previously,15,19 the adverse impact of DHL on outcome appeared to be greater than that of DEL. The 4-year post-ASCT PFS and OS observed in patients with rel/ref DHL in our cohort (28% and 25%, respectively) are similar to the post-ASCT outcomes in patients with rel/ref DLBCL with MYC-R reported in the prospective CORAL study (14% and 23%, respectively).32 In our cohort, there were not enough patients with MYC-R alone to fully assess the impact of solitary MYC-R compared with DHL.36 Multivariable analyses confirmed that both DEL and DHL were associated with poorer outcome in our cohort. In fact, DEL and DHL were the only factors associated with PFS. This raises the possibility that the impact of some traditional prognostic factors may be confounded by DHL/DEL status,1,3-10,37 and argues that future studies of ASCT for DLBCL should include both IHC and FISH assessment for DEL and DHL in addition to other prognostic factors.
In an exploratory analysis of the impact of isolated DEL or DHL versus concurrent DEL+DHL, we observed that patients with concurrent DEL+DHL appeared to have the worst prognosis, with limited impact of isolated DEL or DHL. This finding is similar to prior observations that not all patients with DLBCL with MYC-R also have MYC overexpression by IHC,12,16,19,30 and that patients with MYC-R or DHL without concurrent MYC overexpression may exhibit less aggressive clinical behavior.12,19 Although our ability to draw conclusions about this finding is limited by the small size of the DHL groups in the cohort, this is a potentially important result that requires further study, because it may allow the identification of a small group of patients with dismal outcomes after ASCT.
Other potential tumor-specific biomarkers were not associated with outcome in our study. In contrast to the findings by Horn et al,30 in which low BCL6 expression combined with MYC expression or gene rearrangement was associated with poorer outcome after R-CHOP, BCL6 expression did not add prognostic value in our study. Likewise, in the subset of patients with available COO classification by the Hans algorithm, COO subtype was not significantly associated with outcome.37 Finally, unlike the reports by Oki et al24 and Li et al,35 which suggested that outcomes were similar in patients newly diagnosed with DLBCL with traditionally defined DHL and atypical DHL, atypical DHL was not associated with post-ASCT outcome in our cohort.
A strength of our study is the prospective IHC and FISH testing performed by blinded investigators. Testing was repeated in a blinded fashion in cases where it had been previously performed as part of routine clinical care. Whenever possible, biopsies performed at the time of rel/ref disease were used. In a prior study of rel/ref DLBCL, FISH testing was generally concordant between initial diagnostic and relapse biopsy specimens, but some patients with MYC-R at relapse did not have MYC-R at diagnosis.32 Despite the use of biopsy specimens obtained at the time of disease relapse (when possible), we may have underestimated the true prevalence of DHL in our cohort because we used only breakapart FISH probes. In one study, 10% of MYC rearrangements were identified only with a dual-fusion MYC probe.12 Because we did not use dual-fusion probes, we also do not have information about the MYC translocation partner (immunoglobulin v nonimmunoglobulin), which may provide additional prognostic information.11,14 Although we performed all testing prospectively, IHC was performed at different institutions using slightly different protocols, including different clones for certain antibodies. Although this could be considered a limitation, it is more reflective of real-world practice. Finally, because of its retrospective nature, our study is subject to inherent limitations and potential biases. However, patients were drawn from consecutive cohorts of patients with rel/ref DLBCL who underwent ASCT at both centers, and all eligible patients with available tissue were included. Moreover, there was no apparent difference in outcome between patients with or without evaluable FISH and IHC data.
In our retrospective, multicenter study of post-ASCT outcomes in patients with rel/ref DLBCL, we found that both DEL and DHL were associated with inferior outcomes, and that patients with concurrent DEL and DHL appeared to have a dismal outcome. This supports the concept that the double-hit/double-expressor biology appears to render DLBCL resistant to and less likely to be cured by chemotherapy, as has been demonstrated in the upfront setting. Nevertheless, a significant proportion of patients with rel/ref DEL experienced durable remissions after ASCT, especially patients with isolated DEL without DHL, suggesting that the presence of DEL alone should not be considered a contraindication to ASCT. Although some patients with rel/ref DHL had long-term remission after ASCT (isolated DHL without DEL), the low survival rate in this group argues that alternative transplantation strategies, including allogeneic hematopoietic stem-cell transplantation or peri-ASCT relapse prevention strategies should be studied.
Appendix
Fig A1.
Flowchart of patient enrollment in this study. ASCT, autologous stem-cell transplantation; auto-allo, autologous-allogeneic; BCL-U, unclassifiable B-cell lymphoma with features intermediate between Burkitt lymphoma and DLBCL; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; FISH, fluorescence in situ hybridization; FNA, fine-needle aspiration; IHC, immunohistochemistry; NHL, non-Hodgkin lymphoma; PCNSL, primary CNS lymphoma; PMBCL, primary mediastinal B-cell lymphoma; PTLD, post-transplant lymphoproliferative disease.
Immunohistochemistry Methods
Formalin-fixed, paraffin-embedded tissue was sectioned at 4- to 5-µm thick and fixed to unstained slides. At Dana-Farber Cancer Institute/Brigham and Women’s Hospital, MYC immunohistochemistry (IHC) was performed using the Ventana platform (Roche, Basel, Switzerland, http://www.roche.com) with the Y69 antibody (Abcam, Cambridge, UK, http://www.abcam.com), as previously described (Kluk MJ et al: PLoS One 7:e33813, 2012). All other IHC was performed using the BOND-III system (Leica Biosystems, Buffalo Grove, IL, http://www.leicabiosystems.com) according to standard protocols using the following antibodies: COH - MYC Y69 (Epitomics, Burlingame, CA, http://www.epitomics.com) at 1:100 dilution, BCL2 clone E17 (Abcam) at 1:200 dilution, and BCL6 clone PG-B6p (Dako, Carpinteria, CA, http://www.dako.com) at 1:25 dilution; Dana-Farber Cancer Institute/Brigham and Women’s Hospital - BCL2 clone 124 (Dako) at 1:200 dilution; and BCL6 GI191E/A8 (Cell Marque, Rocklin, CA, http://www.cellmarque.com) at 1:3000 dilution.
BCL6 IHC Results
Patients with MYC overexpression (defined as positive in ≥ 40% tumor cells) by IHC combined with low BCL6 expression (defined as positive in ≤ 25% of tumor cells) had similar outcomes to patients who did not have MYC overexpression and low BCL6 expression, with a 4-year progression-free survival (PFS) of 55% (95% CI, 31% to 73%) in patients categorized as MYChigh/BCLlow compared with 54% (95% CI, 43% to 64%) in all others (P = .8).
Atypical DHL Results
In total, 44 patients (38%) had atypical DHL, including 12 (10%) with four or more copies of MYC. The 4-year PFS and OS among patients with IHC and fluorescence in situ hybridization data who had atypical DHL, but not classic DHL, were 58% (95% CI, 41% to 72%) and 77% (95% CI, 60% to 87%), respectively, compared with 28% (95% CI, 6% to 57%) and 25% (95% CI, 5% to 54%) in patients with classic DHL, and 56% (95% CI, 43% to 67%) and 59% (95% CI, 44% to 71%) in patients with neither classic nor atypical DHL (P = .028 and P = .002 for three-way PFS and OS comparisons, respectively; P = .7 for comparison of PFS; and P = .3 for OS comparison between atypical DHL and non-DHL). Similar findings were observed in the subset of patients with atypical DHL who had four or more copies of MYC, in whom 4-year PFS and OS were 56% (95% CI, 23% to 79%) and 83% (95% CI, 48% to 96%), respectively. The absence of prognostic relevance of atypical DHL in this cohort was confirmed in multivariable models including DEL, DHL, and remission status (hazard ratio, 1.0 for PFS in patients with any atypical DHL; P = .9; hazard ratio, 1.0 for PFS in patients with atypical DHL with four or more copies of MYC; P = 1.0).
Footnotes
Supported by a Conquer Cancer Foundation/ASCO Young Investigator Award (A.F.H.) and National Cancer Institute (NCI) Grants No. NIH 2K12CA001727-21 and P50 CA107399 (A.F.H.); the Dana-Farber Cancer Institute Award Fund for Collaborative Research Initiatives in Hematologic Oncology (A.F.H., D.M.W., S.J.R., P.A.); the Harold and Virginia Lash/David Lash Fund for Lymphoma Research; and NCI Grant No. P30CA033572 for work performed in the COH Pathology Core.
The content is solely the responsibility of the authors and does not necessarily the official views of the National Institutes of Health.
This study was presented in part at the Annual Meeting of the American Society of Hematology meeting, Orlando, FL, December 2015.
See accompanying Editorial on page 1
AUTHOR CONTRIBUTIONS
Conception and design: Alex F. Herrera, Matthew Mei, Lawrence Low, Haesook T. Kim, Auayporn P. Nademanee, Joycelynne M. Palmer, David M. Weinstock, Stephen J. Forman, Dennis D. Weisenburger, Young Kim, Scott J. Rodig, Amrita Krishnan, Philippe Armand
Provision of study materials or patients: Alex F. Herrera, Matthew Mei, Lawrence Low, Gabriel K. Griffin, Joo Y. Song, Reid W. Merryman, Victoria Bedell, Christine Pak, Heather Sun, Jennifer R. Brown, Lihua E. Budde, Wing C. Chan, Robert Chen, Matthew S. Davids, Arnold S. Freedman, David C. Fisher, Eric D. Jacobsen, Caron A. Jacobson, Ann S. LaCasce, Joyce Murata-Collins, Auayporn P. Nademanee, German A. Pihan, Leslie Popplewell, Tanya Siddiqi, Aliyah R. Sohani, Jasmine Zain, David M. Weinstock, Stephen J. Forman, Dennis D. Weisenburger, Young Kim, Scott J. Rodig, Amrita Krishnan, Philippe Armand
Collection and assembly of data: Alex F. Herrera, Matthew Mei, Lawrence Low, Haesook T. Kim, Gabriel K. Griffin, Joo Y. Song, Reid W. Merryman, Victoria Bedell, Christine Pak, Heather Sun, Tanya Paris, Tracey Stiller, Jennifer R. Brown, Lihua E. Budde, Robert Chen, Matthew S. Davids, Arnold S. Freedman, David C. Fisher, Eric D. Jacobsen, Caron A. Jacobson, Ann S. LaCasce, Joyce Murata-Collins, Auayporn P. Nademanee, Joycelynne M. Palmer, German A. Pihan, Leslie Popplewell, Tanya Siddiqi, Aliyah R. Sohani, Jasmine Zain, David M. Weinstock, Stephen J. Forman, Dennis D. Weisenburger, Young Kim, Scott J. Rodig, Amrita Krishnan, Philippe Armand
Data analysis and interpretation: Alex F. Herrera, Matthew Mei, Lawrence Low, Haesook T. Kim, Gabriel K. Griffin, Joo Y. Song, Reid W. Merryman, Victoria Bedell, Tracey Stiller, Jennifer R. Brown, Lihua E. Budde, Wing C. Chan, Robert Chen, Matthew S. Davids, Arnold S. Freedman, David C. Fisher, Eric D. Jacobsen, Caron A. Jacobson, Ann S. LaCasce, Joyce Murata-Collins, Auayporn P. Nademanee, Joycelynne M. Palmer, German A. Pihan, Raju Pillai, Leslie Popplewell, Tanya Siddiqi, Aliyah R. Sohani, Jasmine Zain, Steven T. Rosen, Larry W. Kwak, David M. Weinstock, Stephen J. Forman, Dennis D. Weisenburger, Young Kim, Scott J. Rodig, Amrita Krishnan, Philippe Armand
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Relapsed or Refractory Double-Expressor and Double-Hit Lymphomas Have Inferior Progression-Free Survival After Autologous Stem-Cell Transplantation
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Alex F. Herrera
Research Funding: Seattle Genetics, Pharmacyclics, Genentech, Immune Design, Sequenta
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Matthew Mei
No relationship to disclose
Lawrence Low
No relationship to disclose
Haesook T. Kim
No relationship to disclose
Gabriel K. Griffin
No relationship to disclose
Joo Y. Song
Consulting or Advisory Role: Seattle Genetics
Reid W. Merryman
No relationship to disclose
Victoria Bedell
No relationship to disclose
Christine Pak
No relationship to disclose
Heather Sun
No relationship to disclose
Tanya Paris
No relationship to disclose
Tracey Stiller
No relationship to disclose
Jennifer R. Brown
Consulting or Advisory Role: Gilead Sciences, Pharmacyclics, Janssen Pharmaceuticals, Genentech, Celgene, ProNAi, Sun Pharma, Boehringer Ingelheim, Infinity Pharmaceuticals
Lihua E. Budde
No relationship to disclose
Wing C. Chan
No relationship to disclose
Robert Chen
Consulting or Advisory Role: Seattle Genetics, Merck, Genentech
Speakers' Bureau: Seattle Genetics, Genentech, Millennium Pharmaceuticals
Research Funding: Seattle Genetics, Pharmacyclics, Merck, Millennium Pharmaceuticals
Matthew S. Davids
Consulting or Advisory Role: Infinity Pharmaceuticals, Genentech, Gilead Sciences, Janssen Pharmaceuticals, Pharmacyclics, TG Therapeutics, Celgene, Abbvie
Research Funding: Genentech, TG Therapeutics, Infinity Pharmaceuticals, Pharmacyclics
Arnold S. Freedman
No relationship to disclose
David C. Fisher
Consulting or Advisory Role: Seattle Genetics, Pharmacyclics
Eric D. Jacobsen
Consulting or Advisory Role: Spectrum Pharmaceuticals, Seattle Genetics
Research Funding: Celgene
Caron A. Jacobson
Consulting or Advisory Role: Kite Pharma, Pharmacyclics
Ann S. LaCasce
Consulting or Advisory Role: Forty Seven
Joyce Murata-Collins
No relationship to disclose
Auayporn P. Nademanee
Consulting or Advisory Role: Seattle Genetics, Gilead Sciences
Speakers' Bureau: Seattle Genetics
Research Funding: Seattle Genetics
Joycelynne M. Palmer
No relationship to disclose
German A. Pihan
No relationship to disclose
Raju Pillai
Research Funding: Trillium Therapeutics
Leslie Popplewell
Honoraria: Cardinal Health
Tanya Siddiqi
Speakers' Bureau: Pharmacyclics, Seattle Genetics
Research Funding: Pharmacyclics (Inst), Juno Therapeutics (Inst), Kite Pharma (Inst), Acerta Pharma (Inst), MedImmune (Inst), Genentech (Inst), TG Therapeutics (Inst), Merck (Inst), Boehringer Ingelheim (Inst), Karyopharm Therapeutics (Inst)
Travel, Accommodations, Expenses: Kite Pharma
Aliyah R. Sohani
Research Funding: Sysmex America
Jasmine Zain
Honoraria: Seattle Genetics, Celgene, Spectrum Pharmaceuticals
Consulting or Advisory Role: Seattle Genetics, Celgene, Spectrum Pharmaceuticals
Speakers' Bureau: Seattle Genetics, Celgene, Spectrum Pharmaceuticals
Steven T. Rosen
Honoraria: Celgene, Genentech, Seattle Genetics
Consulting or Advisory Role: Celgene, Genentech Health Practices Consulting, Seattle Genetics
Speakers' Bureau: Celgene, Seattle Genetics
Larry W. Kwak
Stock or Other Ownership: XEME BioPharma, Antigenics
Consulting or Advisory Role: XEME BioPharma, Celltrion, Sella Life Sciences
David M. Weinstock
Honoraria: Seattle Genetics, Infinity Pharmaceuticals, Genentech
Consulting or Advisory Role: Novartis, Pangaea Biotech
Research Funding: Novartis
Expert Testimony: Monsanto
Stephen J. Forman
No relationship to disclose
Dennis D. Weisenburger
Honoraria: Seattle Genetics
Consulting or Advisory Role: Seattle Genetics
Speakers' Bureau: Seattle Genetics
Travel, Accommodations, Expenses: Seattle Genetics
Young Kim
Research Funding: Seattle Genetics
Scott J. Rodig
Honoraria: Perkin Elmer, Bristol-Myers Squibb
Consulting or Advisory Role: AstraZeneca, Perkin Elmer
Research Funding: Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: Patent pending for use of anti-galectin 1 antibodies for diagnostic use
Travel, Accommodations, Expenses: Roche
Amrita Krishnan
Stock or Other Ownership: Celgene (I), Infinity Pharmaceuticals (I)
Consulting or Advisory Role: Celgene, Spectrum Pharmaceuticals, Onyx, Janssen Oncology, Takeda
Speakers' Bureau: Celgene, Millennium Pharmaceuticals, Onyx, Janssen Oncology
Research Funding: Celgene (Inst), Takeda (Inst)
Philippe Armand
Consulting or Advisory Role: Bristol-Myers Squibb, Merck Sharp & Dohme, Infinity Pharmaceuticals
Research Funding: Merck Sharp & Dohme (Inst), Bristol-Myers Squibb (Inst), Sequenta (Inst), Tensha Therapeutics (Inst), Sigma-Tau (Inst), Otsuka (Inst), Pfizer (Inst), Affimed Therapeutics (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck Sharp & Dohme, Sequenta
REFERENCES
- 1.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 3.Alousi AM, Saliba RM, Okoroji GJ, et al. Disease staging with positron emission tomography or gallium scanning and use of rituximab predict outcome for patients with diffuse large B-cell lymphoma treated with autologous stem cell transplantation. Br J Haematol. 2008;142:786–792. doi: 10.1111/j.1365-2141.2008.07277.x. [DOI] [PubMed] [Google Scholar]
- 4.Armand P, Welch S, Kim HT, et al. Prognostic factors for patients with diffuse large B cell lymphoma and transformed indolent lymphoma undergoing autologous stem cell transplantation in the positron emission tomography era. Br J Haematol. 2013;160:608–617. doi: 10.1111/bjh.12176. [DOI] [PubMed] [Google Scholar]
- 5.Derenzini E, Musuraca G, Fanti S, et al. Pretransplantation positron emission tomography scan is the main predictor of autologous stem cell transplantation outcome in aggressive B-cell non-Hodgkin lymphoma. Cancer. 2008;113:2496–2503. doi: 10.1002/cncr.23861. [DOI] [PubMed] [Google Scholar]
- 6.Dickinson M, Hoyt R, Roberts AW, et al. Improved survival for relapsed diffuse large B cell lymphoma is predicted by a negative pre-transplant FDG-PET scan following salvage chemotherapy. Br J Haematol. 2010;150:39–45. doi: 10.1111/j.1365-2141.2010.08162.x. [DOI] [PubMed] [Google Scholar]
- 7.Schot BW, Zijlstra JM, Sluiter WJ, et al. Early FDG-PET assessment in combination with clinical risk scores determines prognosis in recurring lymphoma. Blood. 2007;109:486–491. doi: 10.1182/blood-2005-11-006957. [DOI] [PubMed] [Google Scholar]
- 8.Roland V, Bodet-Milin C, Moreau A, et al. Impact of high-dose chemotherapy followed by auto-SCT for positive interim [18F] FDG-PET diffuse large B-cell lymphoma patients. Bone Marrow Transplant. 2011;46:393–399. doi: 10.1038/bmt.2010.130. [DOI] [PubMed] [Google Scholar]
- 9.Hamlin PA, Zelenetz AD, Kewalramani T, et al. Age-adjusted International Prognostic Index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2003;102:1989–1996. doi: 10.1182/blood-2002-12-3837. [DOI] [PubMed] [Google Scholar]
- 10.Guglielmi C, Gomez F, Philip T, et al. Time to relapse has prognostic value in patients with aggressive lymphoma enrolled onto the Parma trial. J Clin Oncol. 1998;16:3264–3269. doi: 10.1200/JCO.1998.16.10.3264. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen MO, Gang AO, Poulsen TS, et al. MYC translocation partner gene determines survival of patients with large B-cell lymphoma with MYC- or double-hit MYC/BCL2 translocations. Eur J Haematol. 2014;92:42–48. doi: 10.1111/ejh.12212. [DOI] [PubMed] [Google Scholar]
- 12.Tzankov A, Xu-Monette ZY, Gerhard M, et al. Rearrangements of MYC gene facilitate risk stratification in diffuse large B-cell lymphoma patients treated with rituximab-CHOP. Mod Pathol. 2014;27:958–971. doi: 10.1038/modpathol.2013.214. [DOI] [PubMed] [Google Scholar]
- 13.Pillai RK, Sathanoori M, Van Oss SB, et al. Double-hit B-cell lymphomas with BCL6 and MYC translocations are aggressive, frequently extranodal lymphomas distinct from BCL2 double-hit B-cell lymphomas. Am J Surg Pathol. 2013;37:323–332. doi: 10.1097/PAS.0b013e31826cebad. [DOI] [PubMed] [Google Scholar]
- 14.Copie-Bergman C, Cuillière-Dartigues P, Baia M, et al. MYC-IG rearrangements are negative predictors of survival in DLBCL patients treated with immunochemotherapy: A GELA/LYSA study. Blood. 2015;126:2466–2474. doi: 10.1182/blood-2015-05-647602. [DOI] [PubMed] [Google Scholar]
- 15.Hu S, Xu-Monette ZY, Tzankov A, et al: MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: A report from The International DLBCL Rituximab-CHOP Consortium Program. Blood 121:4021-4031, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valera A, López-Guillermo A, Cardesa-Salzmann T, et al. MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica. 2013;98:1554–1562. doi: 10.3324/haematol.2013.086173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visco C, Tzankov A, Xu-Monette ZY, et al. Patients with diffuse large B-cell lymphoma of germinal center origin with BCL2 translocations have poor outcome, irrespective of MYC status: A report from an International DLBCL rituximab-CHOP Consortium Program Study. Haematologica. 2013;98:255–263. doi: 10.3324/haematol.2012.066209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3460–3467. doi: 10.1200/JCO.2011.41.4342. [DOI] [PubMed] [Google Scholar]
- 19.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117:2319–2331. doi: 10.1182/blood-2010-09-297879. [DOI] [PubMed] [Google Scholar]
- 21.Snuderl M, Kolman OK, Chen YB, et al. B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are aggressive neoplasms with clinical and pathologic features distinct from Burkitt lymphoma and diffuse large B-cell lymphoma. Am J Surg Pathol. 2010;34:327–340. doi: 10.1097/PAS.0b013e3181cd3aeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akyurek N, Uner A, Benekli M, et al. Prognostic significance of MYC, BCL2, and BCL6 rearrangements in patients with diffuse large B-cell lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Cancer. 2012;118:4173–4183. doi: 10.1002/cncr.27396. [DOI] [PubMed] [Google Scholar]
- 23.Kojima M, Nishikii H, Takizawa J, et al. MYC rearrangements are useful for predicting outcomes following rituximab and chemotherapy: multicenter analysis of Japanese patients with diffuse large B-cell lymphoma. Leuk Lymphoma. 2013;54:2149–2154. doi: 10.3109/10428194.2013.771398. [DOI] [PubMed] [Google Scholar]
- 24.Oki Y, Noorani M, Lin P, et al. Double hit lymphoma: The MD Anderson Cancer Center clinical experience. Br J Haematol. 2014;166:891–901. doi: 10.1111/bjh.12982. [DOI] [PubMed] [Google Scholar]
- 25.Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: A multicenter retrospective analysis. Blood. 2014;124:2354–2361. doi: 10.1182/blood-2014-05-578963. [DOI] [PubMed] [Google Scholar]
- 26.Savage KJ, Johnson NA, Ben-Neriah S, et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009;114:3533–3537. doi: 10.1182/blood-2009-05-220095. [DOI] [PubMed] [Google Scholar]
- 27.Johnson NA, Savage KJ, Ludkovski O, et al. Lymphomas with concurrent BCL2 and MYC translocations: The critical factors associated with survival. Blood. 2009;114:2273–2279. doi: 10.1182/blood-2009-03-212191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrans S, Crouch S, Smith A, et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol. 2010;28:3360–3365. doi: 10.1200/JCO.2009.26.3947. [DOI] [PubMed] [Google Scholar]
- 29.Cohen JB, Geyer SM, Lozanski G, et al. Complete response to induction therapy in patients with Myc-positive and double-hit non-Hodgkin lymphoma is associated with prolonged progression-free survival. Cancer. 2014;120:1677–1685. doi: 10.1002/cncr.28642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horn H, Ziepert M, Becher C, et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121:2253–2263. doi: 10.1182/blood-2012-06-435842. [DOI] [PubMed] [Google Scholar]
- 31.Perry AM, Alvarado-Bernal Y, Laurini JA, et al. MYC and BCL2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with rituximab. Br J Haematol. 2014;165:382–391. doi: 10.1111/bjh.12763. [DOI] [PubMed] [Google Scholar]
- 32.Cuccuini W, Briere J, Mounier N, et al. MYC+ diffuse large B-cell lymphoma is not salvaged by classical R-ICE or R-DHAP followed by BEAM plus autologous stem cell transplantation. Blood. 2012;119:4619–4624. doi: 10.1182/blood-2012-01-406033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Den Neste E, Schmitz N, Mounier N, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant. 2016;51:51–57. doi: 10.1038/bmt.2015.213. [DOI] [PubMed] [Google Scholar]
- 34.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 35.Li S, Seegmiller AC, Lin P, et al. B-cell lymphomas with concurrent MYC and BCL2 abnormalities other than translocations behave similarly to MYC/BCL2 double-hit lymphomas. Mod Pathol. 2015;28:208–217. doi: 10.1038/modpathol.2014.95. [DOI] [PubMed] [Google Scholar]
- 36.Caponetti GC, Dave BJ, Perry AM, et al. Isolated MYC cytogenetic abnormalities in diffuse large B-cell lymphoma do not predict an adverse clinical outcome. Leuk Lymphoma. 2015;56:3082–3089. doi: 10.3109/10428194.2015.1034699. [DOI] [PubMed] [Google Scholar]
- 37.Gu K, Weisenburger DD, Fu K, et al. Cell of origin fails to predict survival in patients with diffuse large B-cell lymphoma treated with autologous hematopoietic stem cell transplantation. Hematol Oncol. 2012;30:143–149. doi: 10.1002/hon.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]