Abstract
Purpose
Previous studies suggest that adherence to adjuvant endocrine therapy (AET) for patients with breast cancer is suboptimal, especially among minorities, and is associated with out-of-pocket medication costs. This study aimed to determine whether there are racial/ethnic differences in 1-year adherence to AET and whether out-of-pocket costs explain the racial/ethnic disparities in adherence.
Methods
This retrospective cohort study used the SEER-Medicare linked database to identify patients ≥ 65 years of age with hormone receptor–positive breast cancer who were enrolled in Medicare Part D from 2007 to 2009. The cohort included non-Hispanic whites, blacks, Hispanics, and Asians. Out-of-pocket costs for AET medications were standardized for a 30-day supply. Adherence to tamoxifen, aromatase inhibitors (AIs), and overall AET (tamoxifen or AIs) was assessed using the medication possession ratio (≥ 80%) during the 12-month period.
Results
Of 8,688 patients, 3,197 (36.8%) were nonadherent to AET. Out-of-pocket costs for AET medication were associated with lower adjusted odds of adherence for all four cost categories compared with the lowest category of ≤ $2.65 (P < .01). In the univariable analysis, Hispanics had higher odds of adherence to any AET at initiation (OR, 1.30; 95% CI, 1.07 to 1.57), and blacks had higher odds of adherence to AIs at initiation (OR, 1.27; 95% CI, 1.04 to 1.54) compared with non-Hispanic whites. After adjusting for copayments, poverty status, and comorbidities, the association was no longer significant for Hispanics (OR, 0.95; 95% CI, 0.78 to 1.17) or blacks (OR, 0.96; 95% CI, 0.77 to 1.19). Blacks had significantly lower adjusted odds of adherence than non-Hispanic whites when they initiated AET therapy with tamoxifen (OR, 0.54; 95% CI, 0.31 to 0.93) after adjusting for socioeconomic, clinic, and prognostic factors.
Conclusion
Racial/ethnic disparities in AET adherence were largely explained by women's differences in socioeconomic status and out-of-pocket medication costs.
INTRODUCTION
Black and Hispanic women experience an increased risk of breast cancer death compared with non-Hispanic white women.1-4 These racial/ethnic disparities in mortality have been attributed to late stage at diagnosis,2,3 socioeconomic status,4 tumor subtypes,5,6 and the initiation and timing of effective, recommended treatment of breast cancer.2,4 One way to significantly reduce breast cancer mortality is to improve adherence to recommended treatment.7 Adherence to guidelines for systemic adjuvant endocrine therapy (AET) is associated with improved disease-free survival for women with early-stage breast cancer.8-12 Treatment with tamoxifen can reduce 5-year mortality by up to 26%.8,10,13
AET treatment includes tamoxifen and the aromatase inhibitors (AIs) exemestane, anastrozole, and letrozole. The National Comprehensive Cancer Network recommends that postmenopausal women diagnosed with early breast cancer receive either AI as initial adjuvant therapy for 5 years, or tamoxifen for 2 to 3 years followed by an AI to complete 5 years, or tamoxifen alone for 5 years for women who have contraindications to AIs.14 In general, the drugs are taken orally every day.14 There is no clear indication whether tamoxifen or AIs should be the first line of adjuvant endocrine treatment for older postmenopausal women. Understanding adherence during the first 12 months of treatment following breast cancer diagnosis may provide evidence for the effective use of one drug over the other.
Despite the effectiveness of AET in improving survival and decreasing cancer recurrence, adherence rates remain low. In studies of treatment adherence, in which adherence is defined as possessing ≥ 80% of the prescribed medication over a 1-year period, 55% to 75% of patients with breast cancer are adherent.16 Low adherence is associated with the number of other medications prescribed for comorbidities,17 demographic characteristics such as age,18,19 and AET adverse effects.16,20-24 Previous studies have examined lower adherence rates for nonwhite women, a finding that may contribute to the disparity in breast cancer mortality observed between minorities and white women.18,25,26 A study by Hershman et al25 found that household net worth partially explains the racial disparity in AET adherence. However, the study examined overall AET medication but not the AIs or tamoxifen separately. This is important because we recently found that Hispanic and black women were more likely to initiate AET with AIs than were non-Hispanic white women.27 It is important to examine differences in the type of AET medication because the out-of-pocket costs for AIs and tamoxifen vary, and higher copayments are inversely associated with AET adherence.28,29 A review by Ursem et al26 underscored the importance of drug costs on the impact of adherence for low-income women. However, little evidence exists about which AET medications at initiation are associated with 12-month adherence in a diverse cohort of postmenopausal women and whether out-of-pocket costs for AET explain the adherence disparities. Therefore, the objective of this study was to identify whether there are racial/ethnic and sociodemographic differences in 1-year adherence to AET overall and adherence to tamoxifen and the AIs separately and to determine to what extent out-of-pocket costs explain the racial/ethnic disparities in AI and tamoxifen adherence.
METHODS
Data Source
This study used the National Cancer Institute's SEER-Medicare linked database for patients from 2007 to 2009 with Medicare Part D claims up to December 2010.27,30,31 Briefly, information collected includes patient demographics, primary tumor site, tumor morphology and stage at diagnosis, first course of treatment, and follow-up vital statistics.
Study Design and Population
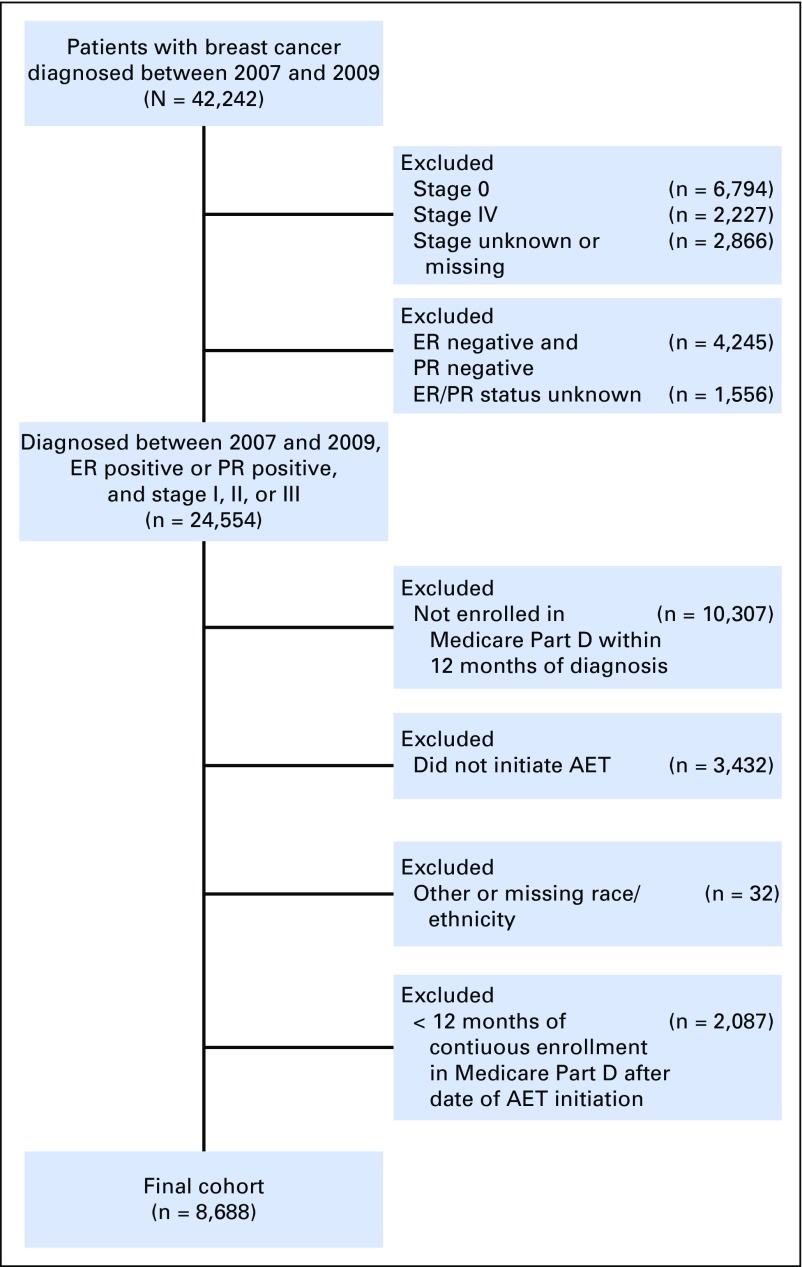
This was a retrospective cohort study restricted to women who were indicated for AET treatment.14 We included women ≥ 65 years of age with stage I to III hormone receptor–positive breast cancer enrolled in Medicare for at least 12 months before and after the date they filled their first AET prescription (either tamoxifen or AI; Fig 1). Women were excluded if they had unknown or estrogen receptor– and progesterone receptor–negative hormone receptor status, were not enrolled in Medicare Part D or both Parts A and B, or were enrolled in a health maintenance organization from the year of diagnosis to the last follow-up.
Fig 1.
Study diagram for identifying the cohort of women with hormone receptor–positive breast cancer who initiated adjuvant endocrine therapy (AET) from 2007 to 2009. ER, estrogen receptor; PR, progesterone receptor.
Dependent Variable
Medicare Part D drug claims contain information on person-specific drug use, such as date of service, product generic name identifier, quantity dispensed, and days of supply. Initiation of AET was defined as a single prescription for tamoxifen or an AI on the basis of the generic drug name up to 1 year after the date of breast cancer diagnosis. AIs were defined as anastrozole, exemestane, or letrozole. Adherence was defined by the medication possession ratio (≥ 80%) on the basis of the number of pills supplied over the 12 months following the initial AET prescription.
Independent Variables
We identified women who belonged to four racial/ethnic groups: non-Hispanic white, black/African American, Hispanic, and Asian. Race was identified using the SEER variable combined with the Hispanic origin variable.32 If race/ethnicity data were missing or unknown in the SEER registry, we used Medicare data to identify the patient’s race/ethnicity (Fig 1).
Demographic information included age (65 to 115 years) and marital status. Socioeconomic information obtained from the 2000 US Census included the percentage of residents living below federal poverty level (FPL) at the census tract level and whether they lived in a metropolitan region.4,31 Tumor characteristics included American Joint Committee on Cancer tumor stage, size, grade, and lymph node status. Chemotherapy use, radiation therapy, and surgery were identified through procedure codes on Medicare claims made within 6 months of diagnosis.4 The 18 comorbid conditions were ascertained from diagnoses or procedure codes in Medicare claims data that were made between 1 year before and 1 month after breast cancer diagnosis. Comorbidity scores were then generated using the Charlson comorbidity index, which assigns different weights according to the severity of different conditions,33 described in detail elsewhere.31,33-35
Out-of-pocket costs for AET medications were measured as the total out-of-pocket payments made by the patient for the 12-month study period at the time AET medications were filled. The dollar sum of the total payments made by the patient was divided by the number of days of medication supplied to obtain the cost per pill; this was multiplied by 30 to standardize the 30-day amounts because prescriptions could have been for a 30-, 60-, or 90-day supply at each fill. These costs included copayments, deductibles, and coinsurance associated with the prescription. The mean 30-day out-of-pocket cost for AET medication was categorized into quintiles: $0 to $2.65, $2.66 to $10.00, $10.01 to $41.25, $41.26 to $105.55, or > $105.55.
Statistical Analysis
Differences in the distribution of sociodemographic and tumor characteristics were first examined across racial/ethnic groups and then by adherence to AET (combined, tamoxifen at initiation, and AI at initiation).We used the χ2tests to assess significant differences between groups with respect to categorical variables, and analysis of variance tests were used to assess differences for continuous variable age. Three multivariable logistic regression models were performed to assess the association of race/ethnicity and AET adherence, tamoxifen only, and AIs only. Collinearity of all independent variables was tested using multiple collinearity tests, and no variable was removed because none had a value > 0.7, and the variance inflation factor was > 10. We considered a priori significance level at P < .05. To assess whether racial/ethnic differences in adherence were explained by covariates, each variable was screened individually in the model along with race/ethnicity. The association between race and adherence was assessed after demographic, prognostic, and clinic factors and out-of-pocket AET costs were added. As a sensitivity analysis, we ran these regression models excluding patients with continuous Medicare-Medicaid dual coverage during the 12-month study period to see whether the main effects changed. Analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC).
RESULTS
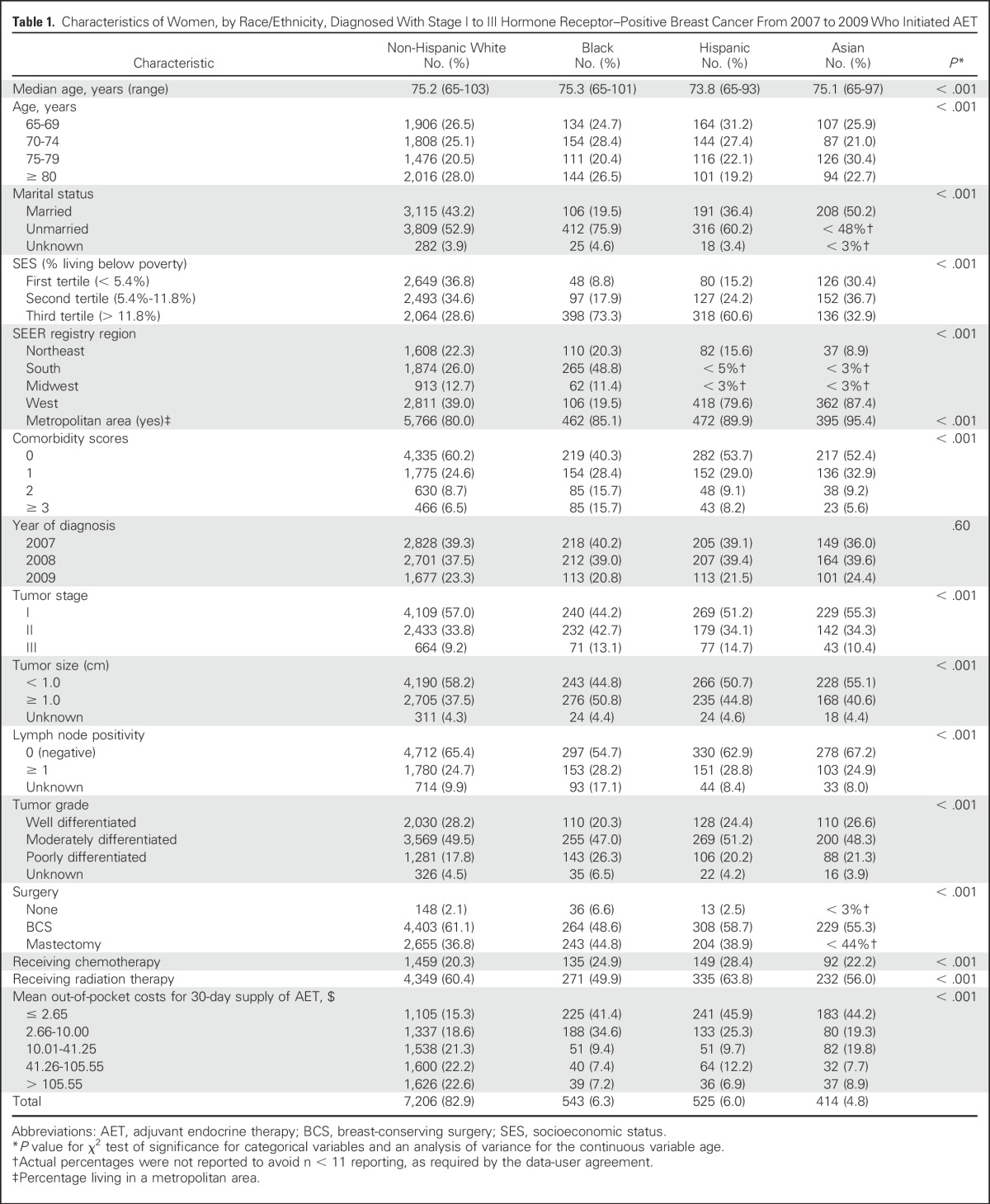
We identified 8,688 patients diagnosed with hormone receptor–positive breast cancer who initiated AET therapy (Fig 1). The mean age at diagnosis was 75.1 years (standard deviation, 7.0; range, 65 to 103 years) for all patients and was 75.2, 75.3, 73.8, and 75.1 years for non-Hispanic whites, blacks, Hispanics, and Asians, respectively. The mean ages and other demographic, treatment, and prognostic factors differed significantly among racial/ethnic groups except for year at diagnosis (P < .05; Table 1). The majority of women were non-Hispanic white (82.9%), followed by black (6.3%) and Hispanic (6.0%). The majority of black (73.3%) and Hispanic (60.6%) patients lived in areas where > 11.8% of the population were below the FPL compared with non-Hispanic white patients (29.6%). A greater proportion of black patients (31.4%) had comorbidity scores of ≥ 2 compared with non-Hispanic white patients (15.2%). A larger proportion of black, Hispanic, and Asian patients had out-of-pocket costs of $0 to $2.65 for a 30-day supply of AET medication compared with non-Hispanic white patients (41.4%, 45.9%, and 44.2% v 15.3%, respectively).
Table 1.
Characteristics of Women, by Race/Ethnicity, Diagnosed With Stage I to III Hormone Receptor–Positive Breast Cancer From 2007 to 2009 Who Initiated AET
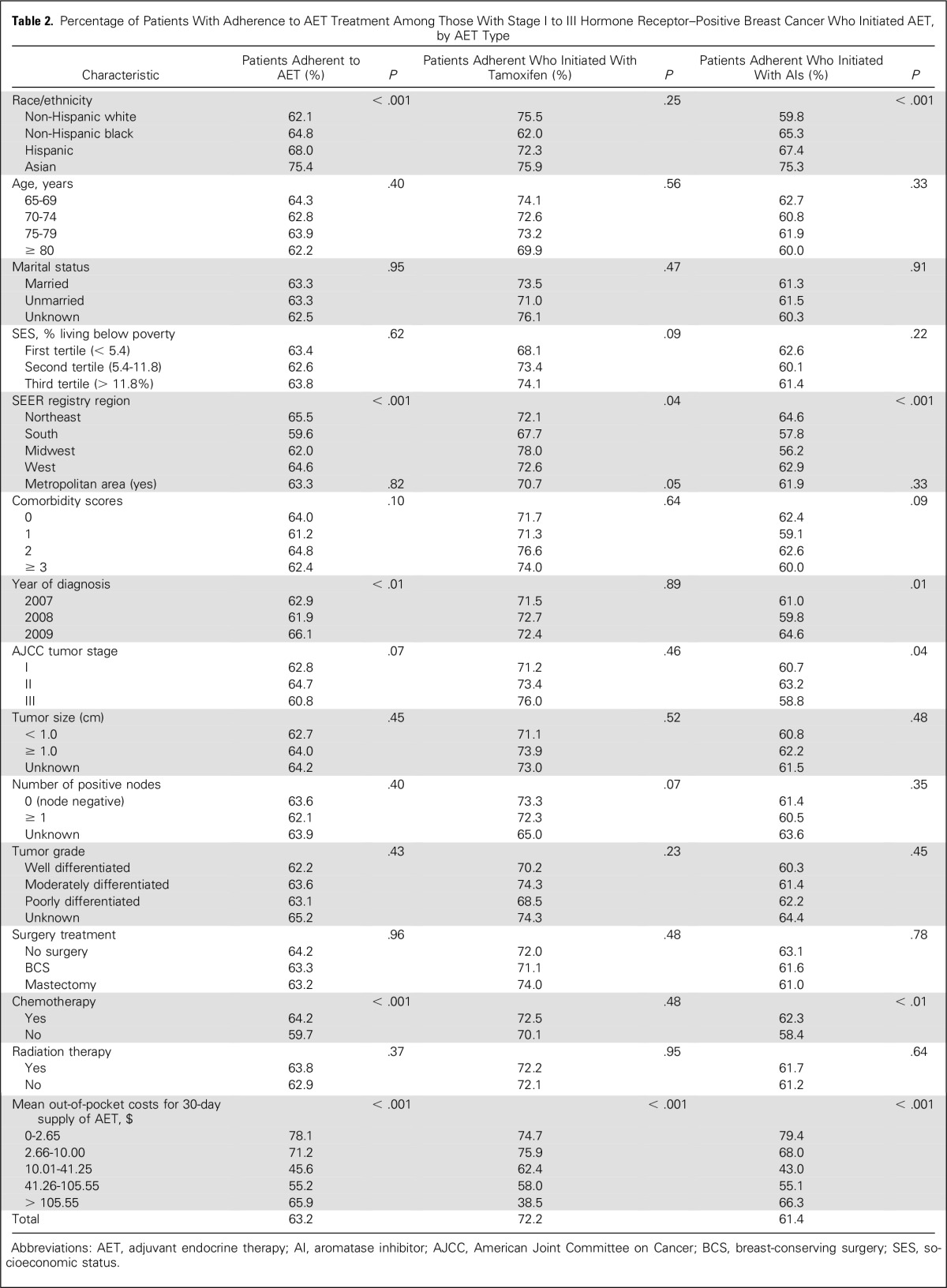
Of the 8,688 patients, 5,491 (63.2%) were adherent during the 12-month study period. Non-Hispanic whites were 3% to 13% less adherent than blacks, Hispanics, or Asians (62.1% v 64.8%, 68.0%, and 75.4%, respectively). Adherence to tamoxifen (72.2%) was higher than AIs (61.4%). Adherence to tamoxifen was lowest for patients who paid > $105.55 for mean out-of-pocket costs for a 30-day supply of AET (38.5%) and higher for those who paid ≤ $2.65 (74.7%; Table 2).
Table 2.
Percentage of Patients With Adherence to AET Treatment Among Those With Stage I to III Hormone Receptor–Positive Breast Cancer Who Initiated AET, by AET Type
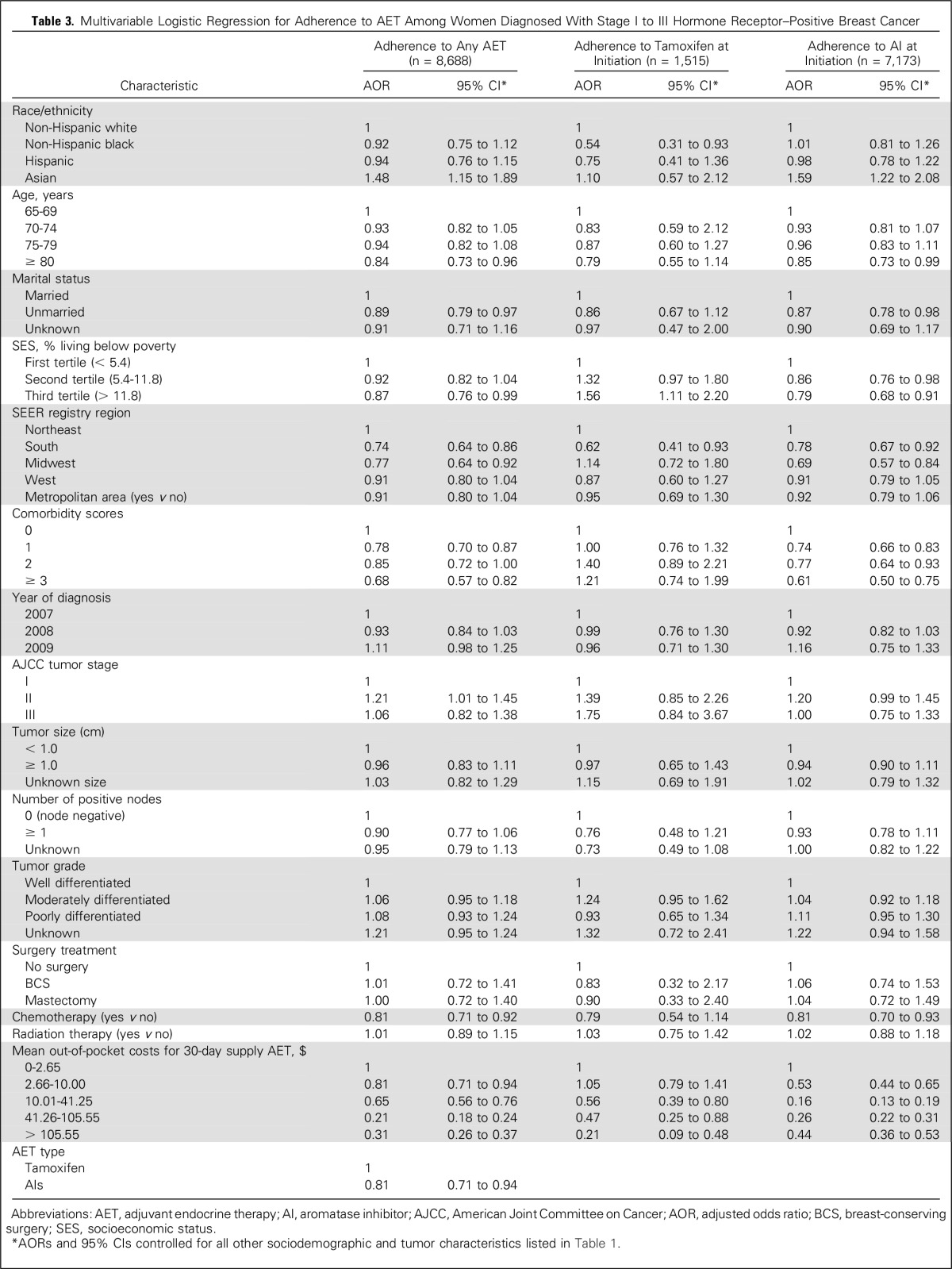
In the adjusted analyses, adherence to any AET was not significantly associated with being Hispanic or black compared with being non-Hispanic white (Table 3). However, being Asian, compared with being non-Hispanic white, was significantly associated with higher adherence (odds ratio [OR], 1.48; 95% CI, 1.15 to 1.89). Blacks compared with non-Hispanic whites were associated with lower odds of adherence to tamoxifen at initiation (OR, 0.54; 95% CI, 0.31 to 0.93), even after controlling for all other study variables. Patients who initiated AI therapy had significantly lower odds of adherence than those who initiated tamoxifen therapy (OR, 0.81; 95% CI, 0.71 to 0.94). Mean out-of-pocket costs for a 30-day supply of AET medication for all cost categories (> $10.01) compared with the lowest quintile (≤ $2.65; P < 0.01) was associated with lower odds of adherence to any AET, tamoxifen, or AI at initiation. For instance, women with mean 30-day, out-of-pocket costs of $10.01 to $41.25 had significantly lower odds of adherence than did those with out-of-pocket costs of ≤ $2.65 (OR, 0.65; 95% CI, 0.56 to 0.76). Unmarried women had significantly lower odds of adherence compared with married women (OR, 0.89; 95% CI, 0.79 to 0.97). Those who lived in areas where > 11.8% of the population was below the FPL had lower odds of adherence compared with those living in areas where < 5.4% was below the FPL (OR, 0.89; 95% CI, 0.76 to 0.99). Patients with comorbidity scores > 3 had lower odds of adherence compared with patients without comorbidities (OR, 0.68; 95% CI, 0.57 to 0.82). In this cohort, 15.4% of patients had Medicare-Medicaid dual coverage. After excluding these patients from the analysis, the main results remained significant, although the effect size moved away from the null. For example, patients who paid between $10.01 and $41.25 compared with those who paid 0$ to $2.65 had an adjusted odds of adherence of 0.65 (95% CI, 0.56 to 0.76), whereas in the sensitivity analysis, excluding dual coverage patients, the adjusted odds of adherence was 0.29 (95% CI, 0.23 to 0.35) for the same groups.
Table 3.
Multivariable Logistic Regression for Adherence to AET Among Women Diagnosed With Stage I to III Hormone Receptor–Positive Breast Cancer
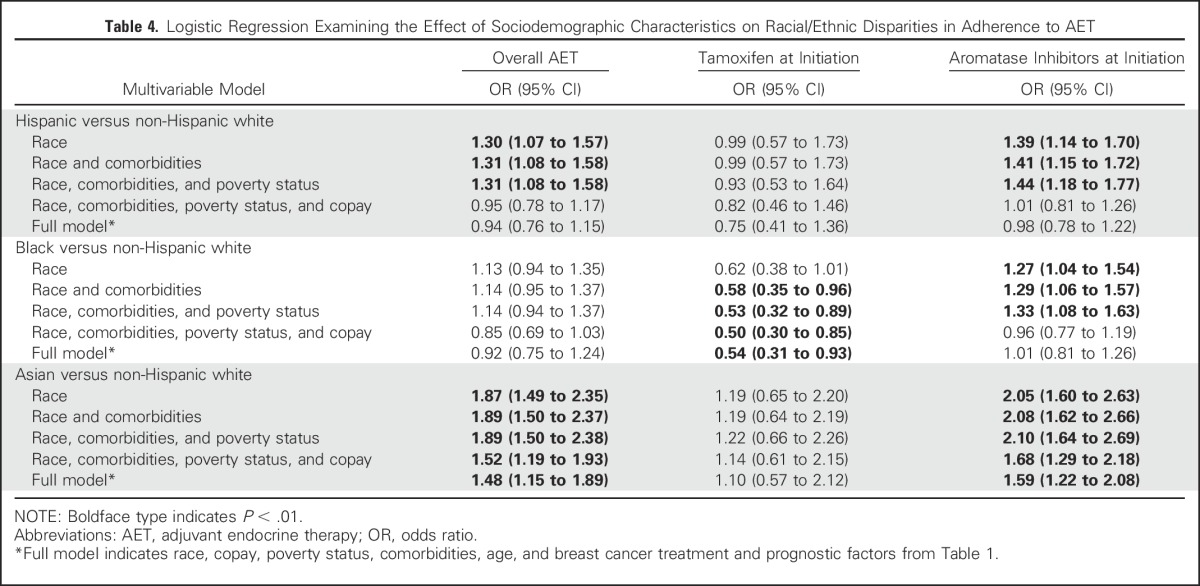
The relationships between race, out-of-pocket AET costs, and adherence are illustrated in multiple models (Table 4). In the unadjusted models, the odds of AET adherence for Hispanics were 30% greater than for non-Hispanic whites. After adjustment for comorbidities and FPL, the association persisted. Including FPL in the model, however, did affect the OR for the association between race/ethnicity and adherence for patients who initiated with tamoxifen or an AI, but the association persisted. However, after considering copay, the association was no longer significant (OR, 0.95; 95% CI, 0.78 to 1.17). The addition of further clinical and sociodemographic variables only had a slight additional effect on the relationship between Hispanics and adherence in the full model (OR, 0.94; 95% CI, 0.76 to 1.15). The same relationship between race, copayment, and adherence was observed among Hispanics and blacks who initiated treatment with AIs compared with non-Hispanic whites. Asians had significantly greater odds of adherence compared with non-Hispanic whites, even after controlling for comorbidities, FPL status, copayment, and all other study variables for AET (OR, 1.48; 95% CI, 1.15 to 1.89) and for the AIs (OR, 1.59; 95% CI, 1.22 to 2.08).
Table 4.
Logistic Regression Examining the Effect of Sociodemographic Characteristics on Racial/Ethnic Disparities in Adherence to AET
DISCUSSION
This study found that out-of-pocket cost for a 30-day supply of AET medication was a main driver of the observed association of AET adherence for Hispanics compared with non-Hispanic whites. Prior studies did not find significant differences in AET adherence by race/ethnicity in younger, privately insured populations.25,36 However, to our knowledge, this is the first study to examine racial/ethnic differences by type of AET in Medicare beneficiaries ≥ 65 years of age. Despite controlling for out-of-pocket AET costs, black women who initiated tamoxifen still had significantly lower odds of adherence than non-Hispanic white women. This finding is different from a recent study by Hershman et al,25 which found that after controlling for net worth and copayment, there was no significant association between race and adherence. That study, however, examined overall AET use and not specifically AIs or tamoxifen.25
We observed that a suboptimal proportion of women (63.2%) were adherent to AET medication. This is lower than previous reports using similar claims-based methodology among insured women, which reported 72% to 81% 12-month adherence to AET.28,37-39 Such studies, however, included a larger proportion of younger women taking tamoxifen.28,37-39 This is important because our study had more women taking AIs (82.6%), which was associated with lower odds of adherence. Furthermore, the mean age of our study cohort was between 74 and 75 years, and extreme ages have been found to be associated with lower adherence as well as a higher number of comorbidities.40,41
Adherence to tamoxifen was better than to the AIs, which may be driven largely by cost because tamoxifen is available to patients in generic form.29 However, even after adjustment, women who initiated treatment with an AI had significantly lower odds of adherence than did those who began with tamoxifen (OR, 0.81; 95% CI, 0.71 to 0.94), which may be affected by other factors, such as the AI adverse effects.10,11,42,43 It should be noted that Arimidex (anastrozole; AstraZeneca, Wilmington, DE), although available now in generic form, was only available in brand name until the tail end of our study (August 2010). This is significant, given that we previously found that Hispanic and black women were significantly more likely to have initiated therapy with AI than were non-Hispanic white women,27 which, together with the findings from this study, may partly explain the racial/ethnic disparities in adherence.
Tamoxifen adherence was higher in patients who lived in areas with > 11.8% of the population below the FPL compared with those in areas with fewer people below the FPL, even after controlling for all other characteristics. This can be explained, in part, by the fact that minority patients (black and Hispanic) also had lower out-of-pocket costs ($0 to $2.65) and may be more likely to have Medicare-Medicaid dual coverage, which would cover the cost of medications.44 The finding on the association between higher out-of-pocket AET costs and lower risk of adherence is similar to other retrospective cohort studies, which report that, on average, copayments decrease the odds of adherence to AIs or tamoxifen.28,29,37,39 Similarly, we found that older women and women with fewer comorbidities had higher odds of adherence.28,37-39
This study has several strengths and could add new information to the literature on AET adherence for elderly women with breast cancer. Because Medicare data were linked with SEER registry data, we were able to assess the date of cancer diagnosis, which allowed us to study AET adherence during the first year of initiating therapy, which may be the most critical to address suboptimal adherence because women who discontinue AET do so within the first few months.18,37 Next, we were able to include detailed baseline demographic and clinical characteristics, which may have confounded the observed associations with adherence and which were not available in other studies.25,28,29,39,45 Prior reports on AET adherence that use insurance claims data alone may have misclassified patients initiating adjuvant treatment because those data did not have information on cancer diagnosis date, stage at diagnosis, and estrogen-receptor status.25,28
Our study was limited first, by the population, which included only women ≥ 65 years of age enrolled in Medicare Part D. Therefore, results may not be generalizable to younger patients or those not enrolled in Part D. Second, there could be unmeasured confounding factors, such as psychosocial factors related to the quality of care that women receive, including physician-patient communication, for example, that may influence women’s AET adherence but could not be captured in this study.46 Third, there may have been misclassification of race/ethnicity in the database. However, misclassification bias may be minimal because the SEER cancer registry uses incidence data for Hispanics on the basis of the validated North American Association of Central Cancer Registries Hispanic/Latino Identification Algorithm.47 Also, we used race/ethnicity data from the Medicare data set, which was also well validated for accuracy of race/ethnicity to augment the information on missing or unknown race/ethnicity in SEER.32 Fourth, calculating adherence using prescription claims assumes that patients take the medications as often as they refill prescriptions. However, pharmacy records may be considered the most accurate and valid estimation of actual medication use in large populations over time.48,49
Under the Patient Protection and Affordable Care Act, Medicare will cover a larger proportion of generic and brand name drugs. In 2016, patients on Medicare Part D received a 55% discount when buying Part D–covered brand-name drugs and a 58% discount when buying generic-name prescription drugs. Because our results indicated that lower out-of-pocket costs explained most racial/ethnic differences in AET adherence, the influence of the Patient Protection and Affordable Care Act, by decreasing the amount of out-of-pocket costs for AET medication, will likely improve adherence for all racial/ethnic groups enrolled in Medicare Part D.
In conclusion, most women (63.2%) in our study were adherent during the first year of treatment. We did not find a significant difference in AET adherence among Hispanic and black patients compared with non-Hispanic whites after adjusting for out-of-pocket costs of the medication. We did, however, find significantly lower odds of tamoxifen adherence among blacks compared with non-Hispanic whites. Out-of-pocket costs for AET medication is associated with adherence, and racial/ethnic disparities in AET adherence were largely explained by women's differences in socioeconomic status and out-of-pocket AET costs. These results suggest that economic factors may significantly contribute to disparities in the quality of breast cancer care. Future studies should account for economic and treatment factors. Long-term outcomes associated with poor adherence to tamoxifen and AI treatment by race/ethnicity need further investigation. Because out-of-pocket costs for AET medication accounted for the majority of racial/ethnic differences in adherence to AET, health plans, drug companies, and providers should work together to ensure patients pay little-to-no out-of-pocket costs for this treatment, which will likely lead to the reduced racial/ethnic disparities in breast cancer mortality.
ACKNOWLEDGMENT
We acknowledge the efforts of the National Cancer Institute, Centers for Medicare and Medicaid Services, Information Management Services, and the SEER program tumor registries in the creation of this database. The interpretation and reporting of these data are the sole responsibilities of the authors.
Footnotes
A.J.F. is a postdoctoral fellow supported by a University of Texas Health Science Center at Houston School of Public Health Cancer Education and Career Development Program grant from the National Cancer Institute (Grant No. R25-CA57712). This study was also supported, in part, by the Agency for Healthcare Research and Quality (Grant No. R01-HS018956) and the Cancer Prevention and Research Institute of Texas (Grant No. RP130051).
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Association Between Out-Of-Pocket Costs, Race/Ethnicity, and Adjuvant Endocrine Therapy Adherence Among Medicare Patients With Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Albert J. Farias
No relationship to disclose
Xianglin L. Du
No relationship to disclose
REFERENCES
- 1.Ooi SL, Martinez ME, Li CI. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat. 2011;127:729–738. doi: 10.1007/s10549-010-1191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silber JH, Rosenbaum PR, Clark AS, et al. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. 2013;310:389–397. doi: 10.1001/jama.2013.8272. [DOI] [PubMed] [Google Scholar]
- 3.Banegas MP, Li CI. Breast cancer characteristics and outcomes among Hispanic black and Hispanic white women. Breast Cancer Res Treat. 2012;134:1297–1304. doi: 10.1007/s10549-012-2142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du XL, Fang S, Meyer TE. Impact of treatment and socioeconomic status on racial disparities in survival among older women with breast cancer. Am J Clin Oncol. 2008;31:125–132. doi: 10.1097/COC.0b013e3181587890. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien KM, Cole SR, Tse CK, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16:6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 7.De Geest S, Sabaté E. Adherence to long-term therapies: Evidence for action. Eur J Cardiovasc Nurs. 2003;2:323. doi: 10.1016/S1474-5151(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 8.Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haque R, Ahmed SA, Fisher A, et al. Effectiveness of aromatase inhibitors and tamoxifen in reducing subsequent breast cancer. Cancer Med. 2012;1:318–327. doi: 10.1002/cam4.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 11.Coombes RC, Hall E, Gibson LJ, et al: Intergroup Exemestane Study: A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350:1081-1092, 2004 [Erratum: N Engl J Med 351:2461, 2004; N Engl J Med 355:1746, 2006] [DOI] [PubMed] [Google Scholar]
- 12.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines), Breast Cancer (ed Version 2.2015). https://www.nccn.org/professionals/physician_gls/f_guidelines_nojava.asp [Google Scholar]
- 15.American Cancer Society: Breast Cancer Facts & Figures 2013-2014. Atlanta, GA, American Cancer Society, Atlanta, GA, 2013 [Google Scholar]
- 16.Banning M. Adherence to adjuvant therapy in post-menopausal breast cancer patients: A review. Eur J Cancer Care (Engl) 2012;21:10–19. doi: 10.1111/j.1365-2354.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- 17.Kimmick G, Anderson R, Camacho F, et al. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol. 2009;27:3445–3451. doi: 10.1200/JCO.2008.19.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 19.Fink AK, Gurwitz J, Rakowski W, et al. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor--positive breast cancer. J Clin Oncol. 2004;22:3309–3315. doi: 10.1200/JCO.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 20.Adisa AO, Lawal OO, Adesunkanmi AR. Paradox of wellness and nonadherence among Nigerian women on breast cancer chemotherapy. J Cancer Res Ther. 2008;4:107–110. doi: 10.4103/0973-1482.42640. [DOI] [PubMed] [Google Scholar]
- 21.Winterhalder R, Hoesli P, Delmore G, et al. Self-reported compliance with capecitabine: Findings from a prospective cohort analysis. Oncology. 2011;80:29–33. doi: 10.1159/000328317. [DOI] [PubMed] [Google Scholar]
- 22.Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat. 2008;107:167–180. doi: 10.1007/s10549-007-9548-1. [DOI] [PubMed] [Google Scholar]
- 23.Browall M, Ahlberg K, Karlsson P, et al. Health-related quality of life during adjuvant treatment for breast cancer among postmenopausal women. Eur J Oncol Nurs. 2008;12:180–189. doi: 10.1016/j.ejon.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Lorizio W, Wu AH, Beattie MS, et al. Clinical and biomarker predictors of side effects from tamoxifen. Breast Cancer Res Treat. 2012;132:1107–1118. doi: 10.1007/s10549-011-1893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hershman DL, Tsui J, Wright JD, et al. Household net worth, racial disparities, and hormonal therapy adherence among women with early-stage breast cancer. J Clin Oncol. 2015;33:1053–1059. doi: 10.1200/JCO.2014.58.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ursem CJ, Bosworth HB, Shelby RA, et al. Adherence to adjuvant endocrine therapy for breast cancer: importance in women with low income. J Womens Health (Larchmt) 2015;24:403–408. doi: 10.1089/jwh.2014.4982. [DOI] [PubMed] [Google Scholar]
- 27.Farias AJ, Du XL. Ethnic differences in initiation and timing of adjuvant endocrine therapy among older women with hormone receptor-positive breast cancer enrolled in Medicare Part D. Med Oncol. 2016;33:19. doi: 10.1007/s12032-016-0732-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29:2534–2542. doi: 10.1200/JCO.2010.33.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hershman DL, Tsui J, Meyer J, et al. The change from brand-name to generic aromatase inhibitors and hormone therapy adherence for early-stage breast cancer. J Natl Cancer Inst. 2014;106:dju319. doi: 10.1093/jnci/dju319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nattinger AB, McAuliffe TL, Schapira MM. Generalizability of the surveillance, epidemiology, and end results registry population: Factors relevant to epidemiologic and health care research. J Clin Epidemiol. 1997;50:939–945. doi: 10.1016/s0895-4356(97)00099-1. [DOI] [PubMed] [Google Scholar]
- 31.Du XL, Fang S, Vernon SW, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110:660–669. doi: 10.1002/cncr.22826. [DOI] [PubMed] [Google Scholar]
- 32.NAACCR Race and Ethnicity Work Group: NAACCR Guideline for Enhancing Hispanic/Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2.1]. Springfield IL, North American Association of Central Cancer Registries. http://www.naaccr.org/LinkClick.aspx?fileticket=6E20OT41TcA%3D
- 33.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 34.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: Differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. discussion 1081-1090. [DOI] [PubMed] [Google Scholar]
- 35.Hu CY, Delclos GL, Chan W, et al. Assessing the initiation and completion of adjuvant chemotherapy in a large nationwide and population-based cohort of elderly patients with stage-III colon cancer. Med Oncol. 2011;28:1062–1074. doi: 10.1007/s12032-010-9644-7. [DOI] [PubMed] [Google Scholar]
- 36.Livaudais JC, Lacroix A, Chlebowski RT, et al. Racial/ethnic differences in use and duration of adjuvant hormonal therapy for breast cancer in the women’s health initiative. Cancer Epidemiol Biomarkers Prev. 2013;22:365–373. doi: 10.1158/1055-9965.EPI-12-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Partridge AH, LaFountain A, Mayer E, et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–562. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 38.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sedjo RL, Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Res Treat. 2011;125:191–200. doi: 10.1007/s10549-010-0952-6. [DOI] [PubMed] [Google Scholar]
- 40.Roberts MC, Wheeler SB, Reeder-Hayes K: Racial/ethnic and socioeconomic disparities in endocrine therapy adherence in breast cancer: A systematic review. Am J Public Health 105:e4-e15, 2015 (suppl 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owusu C, Buist DS, Field TS, et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2008;26:549–555. doi: 10.1200/JCO.2006.10.1022. [DOI] [PubMed] [Google Scholar]
- 42.Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncology. 2006;71:1–9. doi: 10.1159/000100444. [DOI] [PubMed] [Google Scholar]
- 43.Chlebowski RT, Kim J, Haque R. Adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Cancer Prev Res (Phila) 2014;7:378–387. doi: 10.1158/1940-6207.CAPR-13-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riley GF, Warren JL, Harlan LC, et al. Endocrine therapy use among elderly hormone receptor-positive breast cancer patients enrolled in Medicare Part D. Medicare Medicaid Res Rev. 2011;1:E1. doi: 10.5600/mmrr.001.04.a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Livaudais JC, Hershman DL, Habel L, et al. Racial/ethnic differences in initiation of adjuvant hormonal therapy among women with hormone receptor-positive breast cancer. Breast Cancer Res Treat. 2012;131:607–617. doi: 10.1007/s10549-011-1762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maly RC, Umezawa Y, Ratliff CT, et al. Racial/ethnic group differences in treatment decision-making and treatment received among older breast carcinoma patients. Cancer. 2006;106:957–965. doi: 10.1002/cncr.21680. [DOI] [PubMed] [Google Scholar]
- 47.Bonito A, Bann C, Eicheldinger C, et al: Creation of New Race/Ethnicity Codes and Socioeconomic Status (SES) Indicators for Medicare Beneficiaries. Final Report. Sub-Task 2. Rockville, MD, RTI International, 2008 [Google Scholar]
- 48.Choo PW, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37:846–857. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Steiner JF, Koepsell TD, Fihn SD, et al. A general method of compliance assessment using centralized pharmacy records. Description and validation. Med Care. 1988;26:814–823. doi: 10.1097/00005650-198808000-00007. [DOI] [PubMed] [Google Scholar]