Abstract
Humans and other primates demonstrate an exquisite ability to precisely shape their hand when reaching out to grasp an object. Here we used a recently developed transcranial magnetic stimulation paradigm to examine how information about an object's geometric properties is transformed into specific motor programs. Pairs of transcranial magnetic stimulation pulses were delivered at precise intervals to detect changes in the excitability of cortico-cortical inputs to motor cortex when subjects prepared to grasp different objects. We show that at least 600 ms before movement, there is an enhancement in the excitability of these inputs to the corticospinal neurons projecting from motor cortex to the specific muscles that will be used for the grasp. These changes were object- and muscle-specific, and the degree of modulation in the inputs was correlated with the pattern of muscular activity used later by individual subjects to grasp the objects. In a number of control experiments, we demonstrated that no change in excitability was observed during object presentation alone, under conditions in which subjects imagined grasping the object, or before movements involving the same muscles but without an object. This finding demonstrates a cortico-cortical mechanism subserving the transformation from the geometrical properties of an object to the outputs from motor cortex before grasp that is specific for object-driven movements.
Keywords: cortex, transcranial magnetic stimulation, I-wave, corticospinal
The vast majority of our physical interactions with the world take place through the hand. When grasping objects, humans effortlessly preshape their hands to appropriately match the three-dimensional structure of the object. Such behavior requires a complex transformation from the object's geometrical properties to the motor commands acting on the muscles of the hand. Several studies have supported the hypothesis that this transformation relies on a parieto-frontal circuit, involving projections from inferior intraparietal areas (area AIP in the macaque) to the ventral premotor cortex (area F5 in the macaque) (1-4). However, it is unclear how this representation is then transmitted to the motor areas that control hand-shaping. It has been proposed that in visually guided grasp, the information passes from the premotor cortex (F5) through cortico-cortical projections (1, 5, 6) to primary motor cortex (M1), which is known to be crucial for skilled hand function (7). To examine these cortico-cortical influences on M1 we have used a sensitive paired-pulse transcranial magnetic stimulation (TMS) protocol that allows the excitability of these inputs to be measured independently from the intrinsic changes in excitability of corticospinal output neurons in M1.
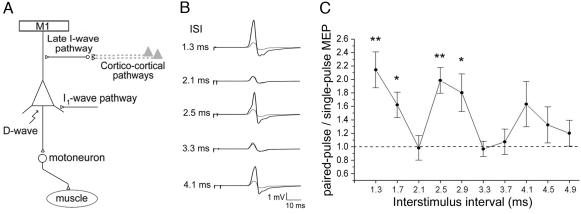
A single pulse of TMS produces repetitive excitation of corticospinal neurons in primary motor cortex (8-11). The first wave of excitation results from direct activation of corticospinal neurons and is called the direct or D wave (Fig. 1A). The succeeding indirect waves (I waves, termed I1, I2, I3, etc.) may arise either by intrinsic neural generators or by transsynaptic excitation of intracortical interneurons or axons that synapse on the corticospinal neurons (12, 13). There is evidence that the first I wave (I1) arises from different presynaptic structures than the later I waves (I2 onwards, Fig. 1A) (6, 12-16). Importantly, later I waves can reflect activity in cortico-cortical pathways transmitting information from other cortical areas (refs. 6-12 and Fig. 1A).
Fig. 1.
Effect of paired-pulse TMS on corticospinal outputs. (A) Diagram illustrating the possible elements giving rise to direct (D) and indirect (I) waves from corticospinal neurons in the motor cortex (12, 16). (B) Representative MEPs evoked by paired-pulse TMS for a range of ISI and single-pulse TMS (gray trace repeated at each interval). Each trace is the average of eight trials performed at rest without any behavioral task. (C) Paired-pulse/single-pulse MEP amplitude obtained from pilot data in eight subjects (see Methods). The MEP amplitude increased significantly (*, P = 0.05, **, P = 0.01) compared with baseline (dashed line) for the ISIs at 1.3, 1.7, 2.5, and 2.9 ms.
The presence of I waves can be revealed by using a paired-pulse TMS paradigm (17). When a suprathreshold TMS pulse is followed by a second subthreshold pulse over M1, the size of the response as measured by the motor-evoked potential (MEP) in a given muscle depends on the interstimulus interval (ISI) (Fig. 1B). If activity generated by the second stimulus coincides with one of the I waves arising from the first stimulus, then a larger descending corticospinal volley results, and facilitation of the MEP is seen. There is a marked modulation in the size of MEPs elicited at different ISIs (Fig. 1C), with interaction occurring at the inherent periodicity of the I wave generator: 1.2-1.5 ms between I waves. There is marked facilitation at ISIs of 1.3, 2.5, and 4.1 ms (Fig. 1C) with troughs at intermediate ISIs (2.1, 3.3, and 4.9 ms). The paired-pulse paradigm can, therefore, be used to assess the excitability of the elements within M1 that generate the I waves and, ultimately, any cortico-cortical projections to them. Importantly, paired-pulse facilitation is known to arise exclusively at the cortical level (17-19).
Here we investigate the interaction between the I wave components of MEPs in two hand muscles that occurs during preparation for grasp of a visible object. We used this approach to monitor any changes in the excitability of cortico-cortical inputs that might be important for visual cues to the grasp. In addition, we examined responses during object presentation alone, during motor imagery, and during simple and complex movements that use the same muscles as the grasp but lack a target object. We show that before a grasping movement, there is an object-specific facilitation at one particular ISI of the MEP in the muscles that will be used to shape the hand. This facilitation was not observed in any of the control conditions.
Methods
Subjects. A total of 39 healthy volunteers (20 males and 19 females aged 19-32) gave informed consent and participated in the study, which was approved by a local ethics committee.
TMS and Electromyography (EMG) Recording. Two Magstim 200 magnetic stimulators (Magstim, Whitland, U.K.) were used to deliver two pulses through the same figure-of-eight coil (7 cm in diameter). The coil handle was oriented at 45° to the midline, pointing laterally and backwards with posteriorly directed current, and stimuli were applied to the “hot spot” on the scalp where a low-threshold MEP could be evoked in EMG recordings from two intrinsic hand muscles abductor digiti minimi (ADM) (abducts the little finger) and first dorsal interosseous (1DI) (abducts and flexes the index finger). Surface EMG was recorded from these muscles with bipolar (belly-tendon) surface electrodes, and the EMG was sampled at 4 kHz and high-pass filtered (5-4,000 Hz). The resting motor threshold (as defined by Rossini et al. in ref. 20) was assessed for both muscles and found to be very similar (41.8% and 41.7% of maximum stimulator output for 1DI and ADM, respectively). The mean intraindividual difference in threshold between the two muscles was 0.06 ± 0.9% of stimulator output.
The intensity of the first (S1) and second TMS stimuli (S2) were set, respectively, at 130% and 90% of the resting motor threshold for the 1DI muscle. In each trial, subjects received either S1 alone or S1 followed by S2 with an interstimulus interval (ISI) of 1.3, 2.1, 2.5, 3.3, and 4.1 ms (16), making six stimulus conditions in total. The 1.3-, 2.5-, and 4.1-ms ISIs were timed to produce optimal facilitation between I wave components evoked by the first and second stimuli, leading to enhanced descending corticospinal activity and a larger MEP (16). The 2.1- and 3.3-ms ISIs, falling in troughs of the I wave excitability cycle (Fig. 1 B and C) are not considered to elicit any significant facilitation of the MEP. To confirm the effects of specific ISIs on the MEP, preliminary tests were carried out on eight subjects at rest (four males and four females, aged 23-35 years); the ISIs tested started at 1.3 ms and increased in 0.4-ms steps (results are shown in Fig. 1C).
Experimental Protocols. Experimental control was performed with signal software (Cambridge Electronic Design, Cambridge, U.K.).
Object Presentation Alone. Ten subjects (five males and five females aged 21-27) sat with their right hands resting pronated on a table; this resting position was the same for all other conditions. Subjects were presented with two different objects, a vertically oriented handle (9 cm high and 5 cm deep) or a large disk (12 cm in diameter and 2 cm deep) (Fig. 2A), and were instructed to watch them. Computer-controlled visual occlusion spectacles (PLATO, Translucent Technologies, Toronto) were used to prevent vision while the objects were changed. The total visual presentation time of each object was 2 s with an intertrial interval of 8 s. TMS was delivered 1,200 ms (±10% jitter) from the opening of the spectacles. A total of 96 trials were performed with combinations of two objects and six TMS conditions repeated eight times in a pseudorandom order.
Fig. 2.
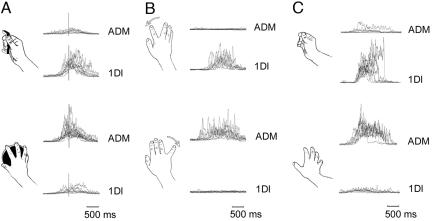
Superimposed averages (10 trials) of rectified EMG from 1DI and ADM for each subject and condition. (A) Object grasping for handle (Upper) and disk (Lower). (B) Simple movements of the index finger (Upper) and little finger (Lower). (C) Complex hand movements that activate the same muscles as handle grasp (Upper) and disk grasp (Lower). Individual traces are aligned to the time of contact with the object (indicated by the vertical line in A) in the grasp condition and to the onset of movement in both the simple and complex hand movement conditions.
Preparation for Object-Specific Grasp. The same subjects then participated in a grasp experiment. At the same moment the spectacles opened, one of two 200-ms auditory tones of different frequencies was played instructing subjects to either prepare to grasp or not grasp the object. In half of the subjects, the instructions referring to the tones were reversed. TMS pulses were again delivered 1,200 ms (±10% jitter) after visual presentation. The TMS pulse was the subject's cue to start the movement on the “grasp” trials. The spectacles remained open for 4,800 ms after the pulse to allow visual guidance of the grasping movement. Intertrial intervals lasted 8 s. A total of 160 trials were performed with combinations of two objects, two conditions (grasp/no grasp) and five of the six TMS conditions (3.3 ms ISI was excluded to shorten the procedure) repeated eight times in a pseudorandom order. The moment of first contact with the object was recorded by using a touch-sensitive electrical circuit.
Preparation for a Simple Movement. Ten additional subjects participated (four males and six females, aged 19-28). On each trial, one of two 200-ms acoustic tones instructed a subject to make either a little finger or index finger abduction cued by the TMS pulse and maintain the abducted posture for ≈1 s. TMS was delivered 1,200 ms (±10% jitter) after the start of the tone, and trials were separated by 8 s. A total of 96 trials were performed with combinations of the two movements and six TMS conditions repeated eight times in a pseudorandom order.
Preparation for a Complex Movement. Ten additional subjects (six male and four female, aged 22-27) participated, and the paradigm was the same as the simple movement except that subjects had to generate complex hand movements that activated the muscles in a similar way to a natural grasp of the two objects. Subjects performed several training trials and were guided by the experimenter on how to modify the movement so that they could stereotypically reproduce, during the experiment, the EMG patterns seen in grasp. These subjects were naïve about the task and never saw or had information about the objects.
Motor Imagery. Nine additional subjects (five male and four female, aged 21-32) participated in this experiment; they were presented with one of the two objects in randomized order and instructed to imagine grasping either the handle or the disk once they had seen it. The spectacles were opened for 1 s at the beginning of each trial with an intertrial interval of 6 s (±10% jitter). TMS was delivered 800 ms after the spectacles closed. A total of 72 trials were performed with combinations of both objects and six TMS conditions repeated six times in a pseudo-random order.
Data Analysis. In all experiments, trials were discarded if EMG activity was present in the 800-ms period preceding the TMS pulses. For accepted trials, amplitudes of the raw unrectified MEPs were measured from negative peak to positive peak. As a measure of facilitation by paired-pulse TMS, we calculated the ratio between the average amplitude of MEP evoked by paired-pulse TMS and the average amplitude of the single-pulse MEP for the corresponding condition. A ratio value >1 indicates facilitation by paired-pulse TMS (Fig. 3 Upper). These data were split into three groups corresponding to the three different I wave facilitatory ISIs (1.3, 2.5, and 4.1 ms). Three repeated measures ANOVA were performed separately on data obtained with these three ISIs as a function of muscle (two factors) and the experimental factors. The factors were: object-presentation alone, object (two); object-guided grasp, grasp/no grasp (two) and object (two); simple movement, index/little abduction (two); complex movement, handle/disk shaping (two); and motor imagery, object (two).
Fig. 3.
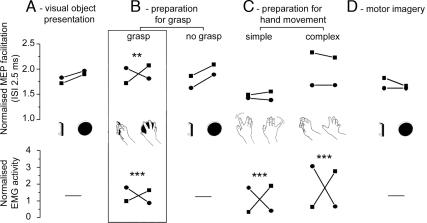
Facilitation of MEP obtained with an ISI of 2.5 ms (Upper) and EMG activation for the ADM (▪) and 1DI (•) (Lower) with the key result highlighted with a box outline. (A) Object presentation alone. (B) Preparation for grasp (Left) and no-grasp condition (Right). (C) Preparation for simple movement (Left) and complex movement (Right). (D) Motor imagery. The values given in Upper represent the ratio of the peak-to-peak amplitude of the MEPs obtained with paired pulses at 2.5 ms to that obtained with a single TMS pulse for the same condition. All values were obtained with the muscles at rest. Lower shows EMG activity during active grasp (B), a simple movement of the index or little finger, or a more complex hand movement (C). Values given represent the mean value of the normalized EMG recorded in the 300 ms preceding contact with the object (B) or onset of movement (C) (for details see the last paragraph of Methods). Data was obtained from three different groups of subjects (n = 10 for A and B, n = 20 for C, and n = 9 for D; see Methods). The significance of the interactions is indicated by asterisks (**, P < 0.001 and ***, P < 0.0001, see Methods).
The amplitude of the MEPs from single-pulse trials was z-score normalized to the grand average of all single-pulse MEPs from the same muscle within the same subject and averaged across subjects. Multiple t tests for paired samples were performed within the same muscle between the different conditions.
For each grasp trial, the rectified EMG from each muscle was integrated for the 300-ms period preceding contact with the object and for the 800-ms period after contact. These two epochs separated the EMG activity involved in the preshaping of the hand from that associated with the actual grasp of the object (21). For the simple and complex movements, we integrated 800 ms of the rectified EMG starting from the onset of significant EMG activity. The data obtained from the rectified EMG traces were then normalized within each muscle to the grand average of the rectified EMG traces for that experiment.
Results
Muscle Activity During Grasp. The two objects used in this study, a handle and a disk, required activation of different muscle groups during preshaping of the hand to grasp (Fig. 2 A). Grasping the handle involved significantly greater EMG activity in 1DI, the muscle that abducts and flexes the index finger, compared with grasping of the disk. Conversely, the muscle that abducts the little finger (ADM) was more active for grasp of the disk than the handle (Fig. 3B Lower Left; interaction P = 0.0001).
Object Presentation Alone. The first experiment involved subjects being visually presented with either the handle or the disk and instructed simply to observe the object. Paired-pulse TMS stimulation was used to determine whether there was any facilitation of the MEP in either muscle during observation (Fig. 3A Upper). We found that object identity had no significant differential effect on the facilitation associated with any of the tested ISIs (1.3 ms, P = 0.10; 2.5 ms, P = 0.88; and 4.1 ms, P = 0.12) (Table 1). In addition, we found that object identity had no significant effect on the amplitude of single-pulse MEPs obtained from either muscle (ADM, P = 0.63; 1DI, P = 0.68), and no interaction was present between object and muscle for single-pulse MEPs (P = 0.29)
Table 1. Mean values of the normalized MEPs obtained at the three different facilitatory ISIs.
| ISI, 1.3 ms
|
ISI, 2.5 ms
|
ISI, 4.1 ms
|
||||||
|---|---|---|---|---|---|---|---|---|
| Condition | Movement | Task | ADM | 1DI | ADM | 1DI | ADM | 1DI |
| Visual object presentation | Handle | 1.73 (0.51) | 1.88 (0.61) | 1.70 (0.60) | 1.82 (0.58) | 1.69 (0.74) | 1.66 (0.79) | |
| Disc | 1.91 (0.71) | 1.73 (0.60) | 1.88 (0.53) | 1.96 (0.61) | 1.42 (0.39) | 1.35 (0.33) | ||
| Preparation for grasp | Grasp | Handle | 1.76 (0.86) | 1.95 (0.64) | 1.72 (0.73) | 2.02 (0.75) | 1.58 (0.79) | 1.64 (0.56) |
| Disc | 1.91 (0.80) | 2.00 (0.64) | 2.08 (1.02) | 1.81 (0.77) | 1.61 (0.73) | 1.42 (0.36) | ||
| No grasp | Handle | 1.89 (1.05) | 1.75 (0.73) | 1.86 (0.89) | 1.62 (0.74) | 1.71 (0.95) | 1.36 (0.86) | |
| Disc | 2.18 (0.98) | 1.96 (0.63) | 2.08 (0.87) | 1.89 (0.63) | 1.81 (0.70) | 1.49 (0.49) | ||
| Preparation for hand movement | Simple | Index finger | 1.79 (0.78) | 1.72 (0.76) | 1.46 (0.34) | 1.40 (0.32) | 1.26 (0.34) | 1.18 (0.22) |
| Little finger | 1.55 (0.49) | 1.82 (1.10) | 1.52 (0.49) | 1.36 (0.32) | 1.17 (0.37) | 1.18 (0.36) | ||
| Complex | Handle-like | 2.45 (1.00) | 1.71 (0.55) | 2.31 (1.16) | 1.66 (0.53) | 1.47 (0.39) | 1.27 (0.44) | |
| Disc-like | 2.55 (2.00) | 1.71 (0.55) | 2.21 (1.30) | 1.66 (0.66) | 1.61 (0.78) | 1.23 (0.28) | ||
| Motor imagery | Handle | 1.58 (0.78) | 1.55 (0.92) | 1.80 (1.36) | 1.59 (1.02) | 1.24 (0.25) | 1.31 (0.46) | |
| Disc | 1.62 (0.62) | 1.72 (0.92) | 1.63 (1.59) | 1.60 (0.85) | 1.49 (0.85) | 1.39 (0.61) | ||
SD values are in parentheses. Values represent the peak amplitude of the MEP obtained with paired-pulse TMS divided by single-pulse TMS for the same condition.
Preparation for Object-Specific Grasp. In the second experiment, subjects were then required to grasp the object that was visually presented to them. They were told not to initiate their movement until they received the TMS pulse and were instructed to grasp the object in a self-paced, natural manner. On average the first sign of EMG activity began 645 ± 278 (SD) ms after the TMS “start” signal (range across subjects was 278-1,261 ms). Thus, the TMS pulse occurred during object presentation and several hundred milliseconds before movement initiation. We found a clear and highly significant modulation of the MEP obtained with the 2.5 ms ISI that showed a significant interaction between object identity and the muscle (Fig. 3B Upper Left, P = 0.001; and Table 1): for 1DI the modulation was larger for the handle compared with the disk, and for ADM, modulation was larger for the disk compared with the handle. No significant interaction between the muscle and the object was found by using other ISIs (1.3 ms, P = 0.67; 4.1 ms, P = 0.23) (Table 1).
To examine how long before movement this modulation could be seen, the same analysis was performed dividing the subjects in two groups of five, according to their mean movement latency; in the first group the five subjects with shorter latency (278-516 ms) and in the second group the five subjects with longer latency (611-1,261 ms). An analogous significant interaction was found between MEP amplitude at ISI 2.5 ms and object identity in both the first (P < 0.05) and the second (P < 0.05) groups. This result shows that the modulation of the cortical output generated by TMS is seen at least 611 ms before movement.
In half of the trials, subjects were instructed, by an auditory cue, not to grasp the object (“no grasp” trials; see Methods). There was no significant object-based modulation of the MEP for any ISI during these trials (1.3 ms, P = 0.51; 2.5 ms, P = 0.87; 4.1 ms, P = 0.92) (Fig. 3B Upper Right and Table 1).
Normalized (z-score) single-pulse MEPs obtained from the same muscle did not differ significantly according to the different objects presented in either the grasp trials (ADM, P = 0.95; 1DI, P = 0.12) or in the no-grasp trials (ADM, P = 0.32; 1DI, P = 0.25). No interaction was found between object and recorded muscle for the single-pulse MEPs in either condition (grasp, P = 0.20; no grasp, P = 0.67)
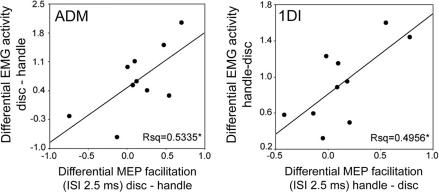
We also examined how, across subjects, the difference in the MEP obtained during preparation to grasp (MEP handle vs. MEP disk) at an ISI of 2.5 ms correlated with the difference in EMG activity for the two movements (EMG handle vs. EMG disk). We found that across subjects there was a significant correlation with the EMG activity during shaping of the grasp before object contact (Fig. 4; ADM, P < 0.05; 1DI, P < 0.05) but not with the EMG involved in grasp after contact with the object (ADM, P = 0.07; 1DI, P = 0.66). Thus, those subjects who exhibited a particularly strong difference in their use of the two muscles between the two objects when shaping their hands to grasp also showed a marked difference in the amount of MEP facilitation for the two objects during object presentation. Interestingly, unlike most subjects, two of the subjects activated ADM more for the handle than for disk (bottom left in Fig. 4 Left). Importantly, in these two subjects we observed a reversal of the MEP facilitation compared with the other subjects, consistent with the reversal of their EMG pattern.
Fig. 4.
Amount of paired-pulse facilitation related to pattern of grasp muscle activity across subjects. Correlation between the amount of MEP facilitation obtained with an ISI of 2.5 ms and muscle activity during the preshaping of the hand in ADM (Left) and 1DI (Right) (see Fig. 3B Left). The abscissa plots the difference between the peak amplitude of the MEP obtained while subjects (n = 10) prepared grasp of either the disk or the handle (muscle inactive). The ordinate represents the difference in EMG activity between the two objects, measured in the 200 ms preceding the contact with the object. *, P < 0.05.
Preparation for Simple or Complex Movements Without an Object. To confirm that the changes in excitability caused by paired TMS at 2.5 ms ISI were specific to object-driven grasp, we examined two further groups of subjects who made either simple or complex movements of the hand that did not involve grasp of an object. For the simple movements, subjects were required to prepare abduction movements of either their index or little finger in response to the TMS pulses (Fig. 2B). For the complex movements (Fig. 2C), the movements were chosen to match, in the absence of either an object or even the mention of an object, the EMG patterns seen in the preshaping of the hand during grasp. On average the first sign of EMG activity began 636 ± 166 (SD) ms after the TMS start signal (range across subjects was 426-998 ms) for the complex hand movements and 528 ± 176 (SD) ms after the TMS start signal (range across subjects was 280-735 ms) for the simple movements. In both the simple and complex movements the pattern of muscle use showed a differentiation with movement type similar to the one seen between grasping movements and object identity (Fig. 3C Lower, both P = 0.0001). However, as shown in Fig. 3C Upper, we found that these types of movement had no significant effect on the relative amount of facilitation in either muscle associated with any of the ISIs tested (simple: 1.3 ms, P = 0.14; 2.5 ms, P = 0.48; 4.1 ms, P = 0.64; complex: 1.3 ms, P = 0.78; 2.5 ms, P = 0.41; 4.1 ms, P = 0.46) (Table 1).
The single-pulse MEPs within the same muscle showed no significant differences for either the simple movements (ADM, P = 0.65; 1DI, P = 0.15) or for the ADM muscle in the complex movements, (ADM, P = 0.28) but showed a tendency toward significance for the 1DI muscle in the complex movement (1DI, P = 0.05). No interaction was present between object and muscle for single-pulse MEPs in either condition (simple movement, P = 0.13; complex movement, P = 0.28). Furthermore, no significant correlation was found between the differential muscle activity for the simple or complex movements and the differential amplitude of the MEP obtained with an ISI of 2.5 ms.
Motor Imagery. To examine the role of motor imagery, an additional group of subjects were required to imagine grasping the object that they had just seen. We found that object identity had no significant differential effect on the facilitation associated with any of the tested ISIs (interaction: 1.3 ms, P = 0.46; 2.5 ms, P = 0.83; 4.1 ms, P = 0.36). MEPs obtained with single-pulse stimulation from either muscle were not significantly different according to the different objects presented (ADM, P = 0.39; 1DI, P = 0.12) and did not show any interaction of the object and muscle factors (P = 0.70, see Fig. 3D and Table 1).
Discussion
When subjects shape their hand to grasp a handle or a disk there is a characteristic pattern of muscle activity in the intrinsic muscles of the hand that move the index (1DI) and little finger (ADM); greater activity is seen in 1DI for the handle compared with the disk and vice versa for the ADM. Here we have shown that, before grasp, there is a modulation of the corticospinal output to the muscles that depends on which object subjects are preparing to grasp, and the modulation matches the crossed pattern seen in the EMG during grasp (compare Fig. 3B Upper Left and Lower Left). An examination of the level of muscle activity during shaping of the hand for grasp in each muscle showed that there were individual differences between the subjects. We found that these differences were significantly correlated with the individual subjects' differences in the modulation of the respective paired-pulse MEP for the handle and disk before grasp (Fig. 4).
These results show that, during preparation for grasp, the modulation of the MEP obtained at ISI 2.5 ms is a predictor of muscle activity that will be used during grasp. In other words, paired-pulse TMS reveals the changes in cortical excitability underlying the organized pattern of muscle activity needed to match the three-dimensional properties of an object, long before the movement has started. Indeed these changes were detected on average 645 ms before any EMG activity was present in the muscle. We have demonstrated robust and significant modulation of the MEP observed at one particular ISI, 2.5 ms, which we shall argue results from interaction between different I waves. I wave interactions at other ISIs showed no significant object-based modulation.
A number of additional conditions confirmed the specificity of the result to object-driven grasp. First, the muscle-specific facilitation at ISI 2.5 ms was only present if the subject was instructed to grasp; no such facilitation was found during object observation alone (see Fig. 3A Upper). Second, we tested nonobject driven movements involving either simple or complex hand movements. These tasks resulted in a pattern of EMG activity similar to the grasp condition, and although they could result in a facilitation of the paired-pulse MEP (Fig. 3C Upper Right), there was no significant task-specific effect. Moreover, we did not observe any facilitation when paired-pulse TMS was delivered while subjects imagined performing the grasp (Fig. 3D Upper). These results highlight the grasp-related nature of the modulation shown in Fig. 3B [cf. Järveläinen et al. (22)]. The control experiments rule out the possibility of a nonspecific premovement facilitation and indeed suggest that the paired-pulse paradigm may allow us to discriminate between preparation for movement and motor imagery.
Thus, the paired-pulse technique we have used is sensitive enough to detect aspects of the motor plan in the early pre-movement stage. Although separate I waves form a component of the single-pulse MEP, we saw no modulation of this MEP in our task. The interaction between different I waves produces a nonlinear increase in descending corticospinal activity, larger than that evoked by a single TMS pulse (6). With the coil orientation and stimulus intensity used here, the single-pulse MEP is predominately driven by the I1 component (14, 15, 23). Because we saw no effects on modulation at the shortest ISI, this component appears to be relatively insensitive to behavioral modulation (24).
Our results can be interpreted in relation to the neurophysiology of premotor-motor cortex interactions in the macaque monkey. Although it has long been known that TMS excites cortico-cortical inputs to motor cortex, our study has pinpointed significant facilitation at an ISI of 2.5 ms, which is most likely to reflect an augmented I2 wave of corticospinal activity (16, 17, 25). Although TMS cannot define which cortico-cortical inputs are modulated during preparation to grasp, we know that the ventral premotor cortex is activated in both monkeys (5, 26) and humans (27, 28) before and during visually guided grasp and that projections from premotor to motor cortex can exert a particularly powerful modulation of the later I waves, including the I2 (6). Thus we speculate that visual presentation of a graspable object activates cortico-cortical inputs to M1, including those from premotor cortex, that represent the object to be grasped in terms of the actions required to grasp it. Increased activity in cortico-cortical pathways terminating on corticospinal pathways via the late I wave pathway, as shown speculatively in Fig. 1 A, could explain the specific interaction between I waves generated by the first and second TMS stimuli at an ISI of 2.5 ms. At this interval it is unlikely that the early I waves generated by the first stimulus could interact with the second stimulus, whereas it could interact with the later I waves (I2 and I3); these components are known to be influenced by cortico-cortical inputs (6, 12) and to be readily modifiable (6, 24).
It is noteworthy that in both hand muscles investigated we found a correlation between the degree of I wave facilitation in the inactive muscle and the pattern of muscle activity during preshaping, which is largely dependent on the geometric properties of the object (1) (Fig. 4). In contrast, there was no correlation with EMG activity recorded after contact with the object, which reflects mainly grip force instead of object geometry (29). Interestingly, inactivation of area F5 in the macaque induces selective impairment in hand preshaping but not in grip force adjustments (30).
In conclusion, these results show the presence of an early premovement modulation of the excitability of the motor cortex that is specifically related to the geometrical properties of the visually presented target object. This modulation depends on a specific interaction between I waves that is modulated by changes in cortico-cortical neural elements transmitting object-based coding for hand shape to the motor cortex. It should be stressed that this conclusion does not necessarily imply a physiological role of the I waves per se, which may only arise as a result of intense stimulation of the motor cortex. However, our results suggest that monitoring of interactions between I waves provides a sensitive tool for the investigation of object-based transformation for grasping. This approach has revealed a possible cortico-cortical mechanism subserving the transformation from the geometrical properties of an object to the outputs from motor cortex that control hand shape.
Acknowledgments
We thank Patrick Haggard, Peter Kirkwood, and John Rothwell for help with this project. L.C. has been supported by a European Union Marie Curie Training Grant, and M.V. has been supported by a fellowship from the German Academic Exchange Service (Deutscher Akademischer Austauschdienst). The work was supported by the Biotechnology and Biological Sciences Research Council, the Human Frontier Science Program, and the Wellcome Trust.
Author contributions: L.C., M.V., T.B., D.M.W., and R.N.L. designed research; L.C., M.V., and G.P. performed research; L.C. analyzed data; and L.C., M.V., T.B., D.M.W., and R.N.L. wrote the paper.
Abbreviations: ADM, abductor digiti minimi; EMG, electromyography; I wave, indirect wave; ISI, interstimulus interval; MEP, motor-evoked potential; 1DI, first dorsal interosseous; TMS, transcranial magnetic stimulation.
References
- 1.Jeannerod, M., Arbib, M. A., Rizzolatti, G. & Sakata, H. (1995) Trends Neurosci. 18, 314-320. [PubMed] [Google Scholar]
- 2.Sakata, H., Taira, M., Murata, A. & Mine, S. (1995) Cereb. Cortex 5, 429-438. [DOI] [PubMed] [Google Scholar]
- 3.Geyer, S., Matelli, M., Luppino, G. & Zilles, K. (2000) Anat. Embryol. 202, 443-474. [DOI] [PubMed] [Google Scholar]
- 4.Rizzolatti, G. & Luppino, G. (2001) Neuron 1, 889-901. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh, S., Brinkman, C. & Porter, R. (1987) J. Comp. Neurol. 259, 424-444. [DOI] [PubMed] [Google Scholar]
- 6.Shimazu, H., Maier, M. A., Cerri, G., Kirkwood, P. A. & Lemon, R. N. (2004) J. Neurosci. 24, 1200-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter, R. & Lemon, R. N. (1993) Corticospinal Function and Voluntary Movement (Clarendon, Oxford).
- 8.Day, B. L., Dressler, D., Maertens de Noordhout, A., Marsden, C. D., Nakashima, K., Rothwell, J. C. Thompson, P. D. (1989) J. Physiol. (London) 412, 449-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothwell, J. C. (1991) Electroencephalogr. Clin. Neurophysiol. Suppl. 43, 29-35. [PubMed] [Google Scholar]
- 10.Edgley, S. A., Eyre, J. A., Lemon, R. N. & Miller, S. (1997) Brain 120, 839-853. [DOI] [PubMed] [Google Scholar]
- 11.Pascual-Leone, A., Davey, N. J., Rothwell, J. C., Wassermann, E. M. & Puri, B. K. (2002) Handbook of Transcranial Magnetic Stimulation (Arnold Publishers, London).
- 12.Amassian, V. E., Stewart, M., Quirk, G. J. & Rosenthal, J. L. (1987) Neurosurgery 20, 74-93. [PubMed] [Google Scholar]
- 13.Ziemann, U. & Rothwell, J. C. (2000) J. Clin. Neurophysiol. 17, 397-405. [DOI] [PubMed] [Google Scholar]
- 14.Werhahn, K. J., Fong, J. K., Meyer, B. U., Priori, A., Rothwell, J. C., Day, B. L. & Thompson, P. D. (1994) Electroencephalogr. Clin. Neurophysiol. 93, 138-146. [DOI] [PubMed] [Google Scholar]
- 15.Sakai, K., Ugawa, Y., Terao, Y., Hanajima, R., Furubayashi, T. & Kanazawa, I. (1997) Exp. Brain. Res. 113, 24-32. [DOI] [PubMed] [Google Scholar]
- 16.Ilic, T. V., Jung, P. & Ziemann, U. (2002) J. Physiol. (London) 545, 153-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziemann, U., Tergau, F., Wassermann, E. M., Wischer, S., Hildebrandt, J. & Paulus, W. (1998) J. Physiol. (London) 511, 181-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Lazzaro, V., Rothwell, J. C., Oliviero, A., Profice, P., Insola, A., Mazzone, P. & Tonali, P. (1999) Exp. Brain Res. 129, 494-499. [DOI] [PubMed] [Google Scholar]
- 19.Hanajima, R., Ugawa, Y., Terao, Y., Enomoto, H., Shiio, Y., Mochizuki, H., Furubayashi, T., Uesugi, H., Iwata, N. K. & Kanazawa, I. (2002) J. Physiol. (London) 538, 253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossini, P. M., Barker, A. T., Berardelli, A., Caramia, M. D., Caruso, G., Cracco, R. Q., Dimitrijevic, M. R., Hallett, M., Katayama, Y. & Lucking, C. H. (1994) Electroencephalogr. Clin. Neurophysiol. 91, 79-92. [DOI] [PubMed] [Google Scholar]
- 21.Brochier, T., Spinks, R. L., Umilta, M. A. & Lemon, R. N. (2004) J. Neurophysiol. 92, 1770-1782. [DOI] [PubMed] [Google Scholar]
- 22.Järveläinen, J., Schürmann, M. & Hari, R. (2004) Neuroimage 23, 187-192. [DOI] [PubMed] [Google Scholar]
- 23.Kaneko, K., Kawai, S., Fuchigami, Y., Morita, H. & Ofuji, A. (1996) Electroencephalogr. Clin. Neurophysiol. 101, 478-482. [DOI] [PubMed] [Google Scholar]
- 24.Cerri, G., Shimazu, H., Maier, M. A. & Lemon, R. N. (2003) J. Neurophysiol. 90, 832-842. [DOI] [PubMed] [Google Scholar]
- 25.Di Lazzaro, V., Restuccia, D., Oliviero, A., Profice, P., Ferrara, L., Insola, A., Mazzone, P., Tonali, P. & Rothwell, J. C. (1998) J. Physiol. (London) 508, 625-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murata, A., Fadiga, L., Fogassi, L., Gallese, V., Raos, V. & Rizzolatti, G. (1997) J. Neurophysiol. 78, 2226-2230. [DOI] [PubMed] [Google Scholar]
- 27.Grezes, J., Armony, J. L., Rowe, J. & Passingham, R. E. (2003) Neuroimage 18, 928-937. [DOI] [PubMed] [Google Scholar]
- 28.Handy, T. C., Grafton, S. T., Shroff, N. M., Ketay, S. & Gazzaniga, M. S. (2003) Nat. Neurosci. 6, 421-427. [DOI] [PubMed] [Google Scholar]
- 29.Maier, M. A. & Hepp-Reymond, M.C. (1995) Exp. Brain. Res. 103, 108-122. [DOI] [PubMed] [Google Scholar]
- 30.Fogassi, L., Gallese, V., Buccino, G., Craighero, L., Fadiga, L. & Rizzolatti, G. (2001) Brain 124, 571-586. [DOI] [PubMed] [Google Scholar]