Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive disease characterized by fibrosis and abnormal vascularity. IL-13, a profibrotic cytokine that plays a role in IPF, functions through the Jak/STAT pathway after binding to the IL-13 receptor α1 (IL-13Rα1)/IL-4Rα complex. IL-13 also binds to IL-13Rα2, which has been thought to function as a nonsignaling decoy receptor, although possible signaling roles of this receptor have been proposed. CXCR3 and its IFN-inducible ligands—CXCL9, CXCL10, and CXCL11—have been implicated in vascular remodeling and fibroblast motility during the development of IPF. In this study, CXCR3 expression was demonstrated in cultured pulmonary fibroblasts from wild-type BALB/c mice and was found to be necessary for the IL-13–mediated gene and protein up-regulation of IL-13Rα2. In fibroblasts from CXCR3-deficient mice, STAT6 activation was prolonged. This study is the first to demonstrate the expression of CXCR3 in fibroblasts and its association with the expression of IL-13Rα2. Taken together, the results from this study point strongly to a requirement for CXCR3 for IL-13–mediated IL-13Rα2 gene expression. Understanding the function of CXCR3 in IL-13–mediated lung injury may lead to novel approaches to combat the development of pulmonary fibrosis, whether by limiting the effects of IL-13 or by manipulation of angiostatic pathways. The elucidation of the complex relationship between these antifibrotic receptors and manipulation of the CXCR3-mediated regulation of IL-13Rα2 may represent a novel therapeutic modality in cases of acute lung injury or chronic inflammation that may progress to fibrosis.
Keywords: CXCR3, fibroblasts, IL-13, IL-13Rα2, idiopathic pulmonary fibrosis
Clinical Relevance
The elucidation of the complex relationship between the two antifibrotic receptors, IL-13Rα2 and CXCR3, and manipulation of the CXCR3-mediated regulation of IL-13Rα2 may represent a novel therapeutic modality in cases of acute lung injury or chronic inflammation that may progress to fibrosis.
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive interstitial lung disease of unknown etiology. This condition is characterized by a histological pattern of usual interstitial pneumonia that includes alternating areas of normal lung, interstitial inflammation, fibrotic zones composed mainly of dense collagen and scattered fibroblastic foci, and “honeycomb” areas (i.e., cystic fibrotic air spaces that are frequently lined by bronchiolar epithelium and filled with mucin). Numerous treatment strategies have all but failed to modify the progressive nature of IPF, and the prognosis for this disease remains poor. Estimated median survival from time of diagnosis ranges from 2.5 to 3.5 years (1, 2).
Much attention has been paid recently to the origin and activation process of fibroblasts comprising the fibroblastic foci, which are the pathologic hallmarks of IPF (3), as well as the role of vascular remodeling and angiogenesis associated with these areas of injury (4–7).
The contribution of IL-13 and its signaling pathways to the development of pulmonary fibrosis has been well characterized (3, 8, 9). The effects of IL-13 are mediated through a complex receptor system that includes IL-13 receptor α (IL-13Rα1), IL-4Rα, and IL-13Rα2. IL-13 binds IL-13Rα1 with moderate affinity, which induces this receptor to heterodimerize with IL-4Rα, forming a transmembrane complex that binds IL-13 with high affinity (10, 11). This dimerization then induces phosphorylation of Jak proteins associated with the cytoplasmic tails of the receptor subunits and the subsequent activation of the STAT6 transcription factor (11, 12).
The exact mechanism by which IL-13Rα2 regulates IL-13 signaling remains unclear. Because the cytoplasmic tail of IL-13Rα2 is short and lacks an obvious signaling motif, IL-13Rα2 has previously been thought to function as a nonsignaling decoy receptor to attenuate responses to IL-13 by sequestering the molecule to limit its bioavailability (8, 13, 14). However, several recent studies highlight possible signaling roles of IL-13Rα2 (15–17) and have generated much interest in the function of this receptor.
The CXC subfamily of chemokines is characterized by four highly conserved cysteine residues in the protein sequence, with the first two cysteine residues separated by a nonconserved amino acid (C-X-C). These chemokines are involved in promoting the trafficking of various leukocytes and in recruiting mesenchymal progenitor cells such as fibrocytes (18).
CXCR3 and its IFN-inducible ligands, CXCL9, CXCL10, and CXCL11, have been implicated as having a role in the pathogenesis of pulmonary fibrosis. Studies with bronchoalveolar lavage fluid from patients with IPF have demonstrated marked angiogenic activity that may be attributed to the overexpression of the angiogenic ELR+ CXC chemokines CXCL5 and CXCL8 relative to the down-regulation of CXCL10 and CXCL11 (19–21).
Similarly, in the bleomycin model of lung injury, the levels of the angiogenic ELR+ CXC chemokines CXCL2 and CXCL3 and of CXCL10 and CXCL11 in the lung were found to be directly and inversely correlated, respectively, with measures of fibrosis. Attenuation of pulmonary fibrosis was achieved by depletion of endogenous CXCL2 and CXCL3 or by administration of exogenous CXCL10 or CXCL11, and these antifibrotic effects were attributed, at least in part, to reduced angiogenesis in the damaged lung tissue (19, 20).
To investigate the role of CXCR3 in the regulation of IL-13Rα2, wild-type and CXCR3−/− BALB/c animals were used as a source of fibroblasts. The expression of CXCR3 was demonstrated on these cells. Our results show that, in the absence of CXCR3, IL-13–mediated IL-13Rα2 gene and protein up-regulation is greatly attenuated. Also, we demonstrate that IL-13–mediated STAT6 signaling is heightened and prolonged in the absence of CXCR3. Finally, we postulate that CXCR3 may have constitutive activity, which influences the expression of IL-13Rα2. Understanding the influence of CXCR3 on the regulation of IL-13Rα2 and further investigations of the influence of IFN-γ signaling over these processes could lead to novel strategies for the treatment of pulmonary fibrosis.
Materials and Methods
Source of Wild-Type and Gene-Targeted Animal Colonies
Pulmonary fibrosis was induced as previously described (20, 22) in female mice (6–8 wk of age) by intratracheal instillation of FITC (Sigma, St. Louis, MO) in 40 μl sterile PBS (2.1 mg/ml) or control animals were instilled with 40 μl of sterile PBS. Mice were killed at 8 days after FITC.
Specific pathogen-free wild-type BALB/c mice were obtained from Charles River UK (Lothian, UK). CXCR3−/− mice (23) used were rederived by embryonic transfer at University College Dublin Biomedical Facility. Animal experimental protocols were approved by the University College Dublin Animal Research Ethics Committee and performed in accordance with the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and the 2010 UKCCCR guidelines on the use of animals in research (24).
Immunohistochemistry
Analysis of archived lung tissue from human subjects fulfilling current clinical diagnostic criteria for IPF (25) was approved by the St. Vincent’s University Hospital Ethics Committee. Formalin-fixed, paraffin-embedded tissues (control and FITC treated) were processed for immunohistochemical localization of CXCR3 using a DAKO autostainer with anti-mouse CXCR3 antibody (1:50 dilution) (C20; Santa Cruz Biotechnology, Santa Cruz, CA) and anti-human CXCR3 antibody (0.5 μg/ml) (MAB160; R&D Systems, Minneapolis, MN).
Culture of Primary Lung Fibroblasts
Lungs from male and female CXCR3−/− and wild-type BALB/c mice (8–10 wk of age) were used as a source of primary lung fibroblasts as previously described (26). For all experiments, primary lung fibroblasts were used between passages 3 and 8 and seeded at 5 × 104/ml per well.
RNA Extraction, cDNA Synthesis, and PCR
Total RNA was isolated from cells using an RNEasy Kit (Qiagen, Manchester, UK) and reverse-transcribed into cDNA using Superscript II reverse transcriptase (Invitrogen, Paisley, UK). Primers directed toward gene transcripts of interest (Sigma) included IL-13Rα2 forward, 5′-TGGAGCACACCTGGAGGACCC-3′; IL-13Rα2 reverse, 5′-ACAGAGGGTATCTTCATAAGC-3′; 18S ribosomal RNA (rRNA) forward, 5′-GTGGAGCGATTTGTCTGGTT-3′; and 18S rRNA reverse, 5′-CGCTGAGCCAGTCAGTGTAG-3′. Quantitative real-time RT-PCR was performed with Taqman Gene Assay probes (Applied Biosystems, Paisley, UK). The ΔΔCt method was used to relatively quantify the levels in gene expression, with 18S rRNA as an endogenous control.
Fluorescence Microscopy
Wild-type and CXCR3−/− murine primary lung fibroblast cultures were examined for the presence of specific markers using anti-mouse CD45-FITC (BD Biosciences, Oxford, UK), anti-mouse CXCR3-PE (Abcam, Cambridge, UK), and biotinylated anti-mouse collagen I (Rockland Immunochemicals, Gilbertsville, PA) antibodies or the appropriate isotype controls. Cells stained with the biotinylated collagen I antibody were incubated with streptavidin-allophycocyanin (APC; BioLegend, London, UK). Slides were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) nucleic acid stain (Invitrogen) and visualized.
Western Blotting Analysis
Western blotting was performed on normalized concentrations of protein samples using standard protocols. Primary antibodies used in this study included anti–IL-13Rα2 (R&D Systems), anti-STAT6 (Cell Signaling, Beverly, MA), and antiphospho STAT6 (Cell Signaling).
Sircol Collagen Assay
The Sircol collagen assay (Biocolor, Carrickfergus, UK) protocol was performed as per the manufacturer’s instructions.
Proliferation Assay
Wild-type and CXCR3−/− fibroblasts were assessed for proliferation using the BrdU assay (Roche, Basel, Switzerland) as per the manufacturer’s protocol.
Statistics
Statistical analysis for all data sets was performed using GraphPad Prism version 5.01 (GraphPad, San Diego, CA). The nonparametric Mann-Whitney U test was used to analyze data from each set of experiments, and data are representative of at least three independent experiments.
Results
CXCR3 Is Expressed in Lung Fibroblasts in Human IPF and in Mouse Lung in Response to Injury with FITC
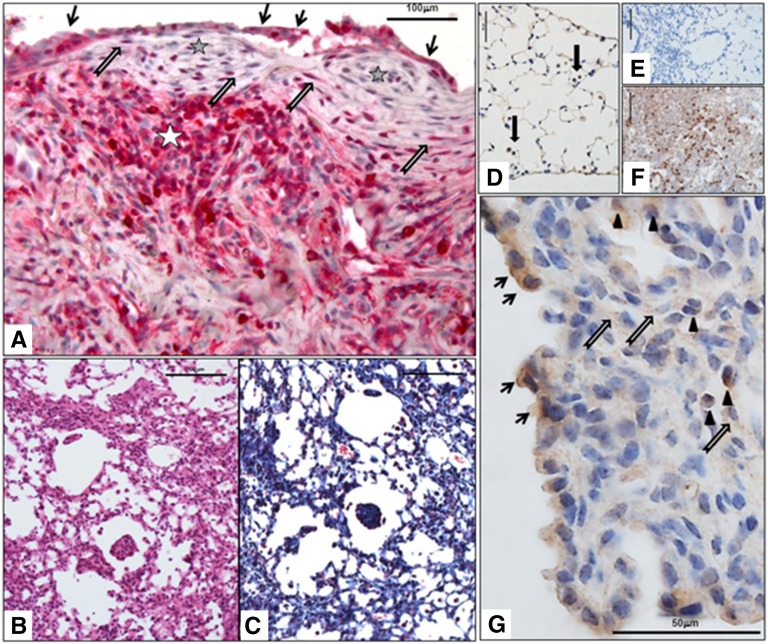
CXCR3 expression was assessed in lung surgical biopsy samples of patients with IPF. CXCR3 was expressed in various cell types, including inflammatory cells, type 2 pneumocytes, and fibroblasts in fibroblastic foci (Figure 1A).
Figure 1.
Chemokine (C-X-C) receptor 3 (CXCR3) is expressed in lung fibroblasts in idiopathic pulmonary fibrosis (IPF) and in mouse lung in response to injury with FITC. CXCR3 expression was evaluated in biopsy samples of patients with IPF and in mice treated with FITC. (A) CXCR3 expression in human IPF lung: expression in type 2 hyperplastic pneumocytes (black arrows), in lymphoid aggregate (white star), and in fibroblasts (gray arrows) in the fibroblastic foci (gray stars). Scale bar, 50 μm. (B) Hematoxylin and eosin stain of FITC mouse lung showing fibroinflammatory changes. Scale bar, 100 μm. (C) Masson’s trichome stain of the same area shown in B showing fibrosis (in blue). Scale bar, 100 μm. (D) CXCR3 expression in control mouse lung: positive staining in alveolar macrophages (black arrows). Scale bar, 100 μm. (E) Ig control for CXCR3 staining in control mouse lung. Scale bar, 100 μm. (F) Mouse spleen positive control for CXCR3 in lymphocytes. Scale bar, 100 μm. (G) CXCR3 expression in FITC mouse lung: expression in type 2 hyperplastic pneumocytes (black arrows), in inflammatory cells such as lymphocytes (arrowheads), and in fibroblasts (gray arrows). Scale bar, 100 μm.
Control Balb/c mice lung showed expression of CXCR3 in alveolar macrophages (Figure 1D). Balb/c mice treated with FITC displayed evidence of lung fibrosis as shown by hematoxylin-eosin stain and Masson’s trichrome stain (Figures 1B and 1C) as well as expression of CXCR3 in inflammatory cells, type 2 hyperplastic pneumocytes, and fibroblasts (Figure 1G).
Basal Gene Expression Profile of Isolated Pulmonary Fibroblasts
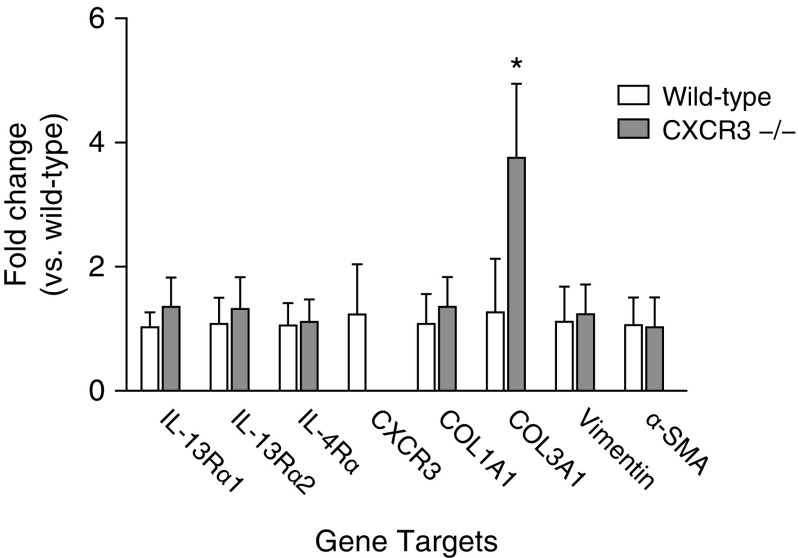
Primary pulmonary fibroblasts from wild-type and CXCR3−/− mice were cultured to 80% confluency and collected for total cellular RNA isolation with subsequent reverse transcription to cDNA. Quantitative real-time PCR was used to determine the relative expression of each gene transcript of interest for comparison between wild-type and CXCR3−/− genotypes. Figure 2 shows no significant differences between the two genotypic strains regarding gene expression of the cytokine receptors IL-13Rα1, IL-13Rα2, and IL-4Rα. CXCR3 mRNA was detected in wild-type fibroblasts but was not found in CXCR3−/− fibroblasts. No difference was found in gene expression of the cytoskeletal proteins vimentin and α–smooth muscle actin or in the expression of collagen I, type 1. Basal expression of the collagen III, type 1 (COL3A1) gene was significantly higher in fibroblasts from CXCR3−/− animals than from wild-type animals.
Figure 2.
Basal gene expression of wild-type and CXCR3−/− cultured primary fibroblasts. Quantitative real-time RT-PCR using the ΔΔCt method of analysis was used to quantify the copy number of each gene transcript of interest in primary pulmonary fibroblasts isolated from wild-type and CXCR3−/− animals. Data are shown as fold change as compared with wild type. Statistical analysis by the Mann-Whitney U test is indicated for four or five animals per strain. *P < 0.05 versus wild type. α-SMA, α-smooth muscle actin; COL1A1, collagen 1A1; COL3A1, collagen 3A1.
CXCR3 Is Expressed on Cultured Pulmonary Fibroblasts from Wild-Type Mice
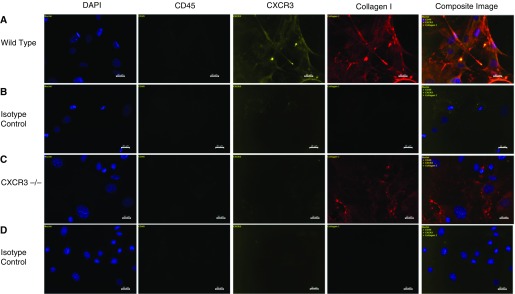
Cultured pulmonary fibroblasts were characterized by staining with fluorophore-conjugated antibodies directed against CD45, CXCR3, and collagen I or with the associated isotype control antibodies (Figure 3). DAPI staining (blue) identifies the nuclei of the cells, whereas FITC (green) staining is associated with CD45. PE (yellow) staining indicates CXCR3 on the cell surface, and APC (red) indicates collagen I. Spleen cells from wild-type mice were used as a positive control for CD45. Cultures were deemed negative for CD45 expression. PE staining and APC staining were shown to be colocalized in wild-type cell cultures, whereas PE staining in CXCR3−/− cultures was not above the background level. Because of the overlapping spindle-shape morphology of the cells, it was difficult to quantify a percentage of dual or triple-stained cells; however, in all instances viewed, a CXCR3-positive cell was also collagen I positive. The absence of CD45 and the presence of collagen I are indicative of a fibroblastic phenotype, and the positive PE signal demonstrates the presence of CXCR3 on the cell surface of wild-type cells.
Figure 3.
CXCR3 and collagen I are coexpressed in wild-type murine pulmonary fibroblasts. Fluorescence microscopy analysis of primary pulmonary cells isolated from wild-type and CXCR3−/− animals was performed after incubation with antibodies directed against CD45 (FITC-conjugated), CXCR3 (phycoerythrin-conjugated), or collagen I (biotinylated)/streptavidin (allophycocyanin-conjugated) or against the appropriate isotype controls. Cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) to visualize cell nuclei. Composite images from individual and overlaid fluorescent signals from cells stained with DAPI (blue), CD45-FITC (green [not seen because these cells are all negative for CD45]), CXCR3-PE (yellow), and biotinylated collagen I/streptavidin-APC (red) or with the corresponding isotype controls are shown. Figure is representative of results from three independent experiments.
CXCR3 Deletion Attenuates the Up-Regulation of IL-13Rα2 mRNA in Response to IL-13
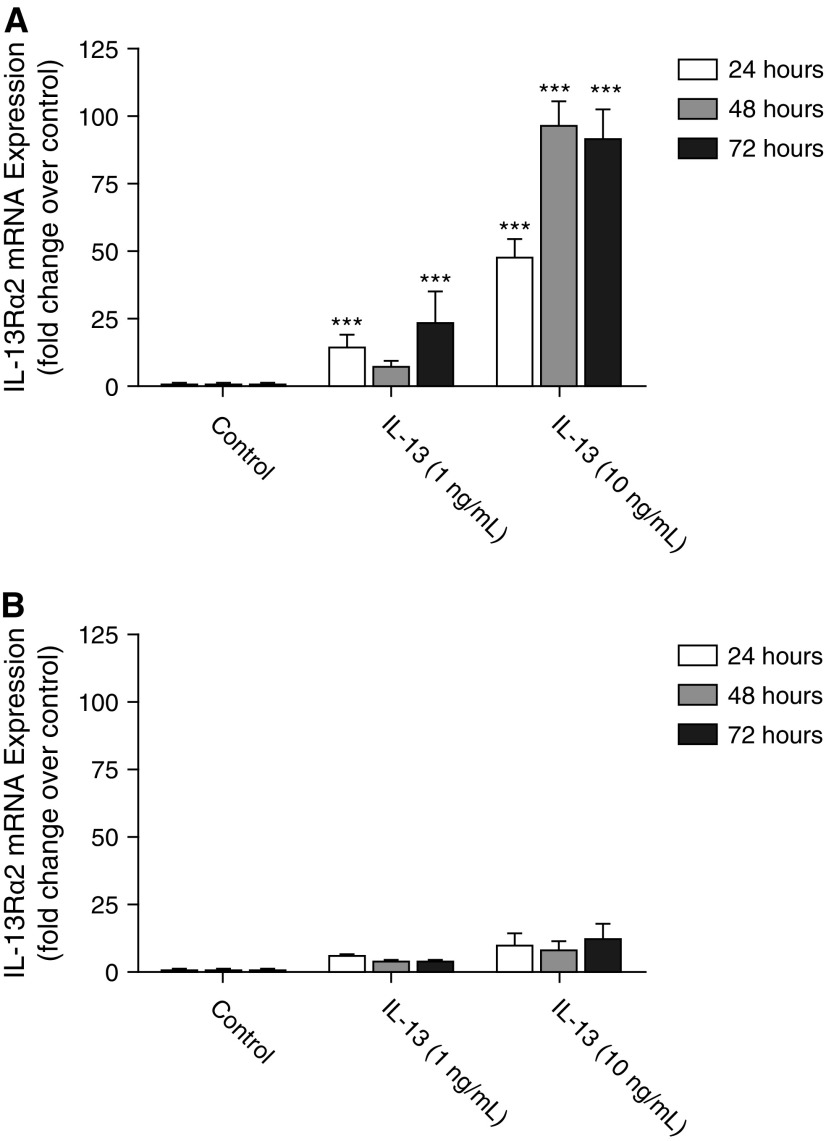
To investigate the role of CXCR3 in the regulation of IL-13Rα2 expression, cultured primary fibroblasts from wild-type and CXCR3−/− mice were serum starved overnight before stimulation with 1 or 10 ng/ml IL-13 for 24, 48, or 72 hours. Time-matched control wells were treated with PBS only. After the appropriate stimulation period, total cellular RNA was isolated and quantified, and equal amounts of RNA were reverse transcribed into cDNA using random primers. Quantitative real-time PCR was used to determine the relative expression of IL-13Rα2 gene transcript in each sample. Data are presented as fold change as compared with the time-matched control sample. Figure 4A shows that, in the wild-type fibroblasts, IL-13 was a potent inducer of IL-13Rα2 gene expression at the low (1 ng/ml) and at the higher (10 ng/ml) concentration at the 24-, 48-, and 72-hour time points. In CXCR3-deficient animals, however, this up-regulation of IL-13Rα2 was attenuated (Figure 4B).
Figure 4.
CXCR3 deletion attenuates the up-regulation of IL-13Rα2 gene expression in response to IL-13. Cultured primary pulmonary fibroblasts isolated from wild-type (A) and CXCR3−/− (B) mice were serum starved overnight before stimulation with increasing amounts of IL-13 (0–10 ng/ml) for 24 to 72 hours. Quantitative real-time RT-PCR was used to quantify the relative expression of the IL-13Rα2 gene transcript for each condition by the ΔΔCt method of analysis. Data are presented as fold change as compared with the time-matched control treatment (PBS only). Statistical analysis by the Mann-Whitney U test is indicated for seven or eight independent experiments: ***P < 0.001 versus time-matched control treatment. mRNA, messenger RNA.
CXCR3 Deletion Attenuates the Up-Regulation of IL-13Rα2 in Response to IL-13, and STAT6 Activation Is Altered in CXCR3−/− Fibroblasts
Cultured primary fibroblasts from wild-type and CXCR3−/− mice were serum starved overnight before the addition of 1, 10, or 50 ng/ml IL-13 to the media for 48 hours. Time-matched control wells were treated with PBS only. After the appropriate stimulation period, total cellular protein was isolated. Similar to the gene expression data obtained, IL-13 was a potent inducer of IL-13Rα2 protein expression in wild-type fibroblasts at the low (10 ng/ml) and at the higher (50 ng/ml) concentration at 48 hours (Figure 5A). In CXCR3-deficient animals, however, this up-regulation of IL-13Rα2 was attenuated. The higher-molecular-weight (55 kD) protein band that is recognized by the anti–IL-13Rα2 antibody corresponds to the membrane-bound, full-length IL-13Rα2 protein, whereas the lower-molecular-weight band (44 kD) corresponds to the ΔEx10 IL-13Rα2 protein, which is lacking the transmembrane region of the full-length protein and is therefore soluble (27).
Figure 5.
CXCR3 deletion attenuates the up-regulation of IL-13Rα2 in response to IL-13 and alters gene expression of the IL-13Rα2 splice variants in wild-type and CXCR3−/− fibroblasts. (A) Cultured primary pulmonary fibroblasts isolated from wild-type and CXCR3−/− mice were serum starved overnight before treatment with increasing concentrations of IL-13 for 48 hours. Total cellular protein was isolated and quantified by Bradford assay, and equal amounts of protein were separated on 10% SDS-PAGE gel. β-Actin was used as a loading control. Figure is representative of three independent experiments. (B) Cultured primary pulmonary fibroblasts isolated from wild-type and CXCR3−/− mice were serum starved overnight before treatment with increasing concentrations of IL-13 for 48 hours. RT-PCR analysis was performed using primers specific to the gene transcript of IL-13Rα2 and 18S ribosomal RNA (rRNA) (as an endogenous control). NTC, no-template control from cDNA synthesis. IL-13Rα2 cDNA previously cloned and sequenced by our group was run as a positive control. Figure is representative of three independent experiments. (C) Cultured primary pulmonary fibroblasts isolated from wild-type and CXCR3−/− mice were serum starved overnight before treatment with 10 ng/ml of IL-13 for varying times between 0 minutes and 6 hours. Total cellular protein was isolated and quantified by Bradford assay, and equal amounts of protein were separated on a 10% SDS-PAGE gel. Proteins were transferred to polyvinyl difluoride membranes, and membranes were probed for phosphorylated STAT6 (Y641) before exposure to film for 20 seconds. Stripped membranes were probed for total STAT6 as a loading control. Figure is representative of three independent experiments. STAT6, signal transducer and activator of transcription 6.
Noting that IL-13 treatment results in differential increases in expression of membrane-bound and soluble IL-13Rα2 protein in wild-type fibroblasts as compared with CXCR3−/− fibroblasts, experiments were designed to examine the IL-13–induced splicing variation of IL-13Rα2 in the two strains. Wild-type and CXCR3−/− fibroblasts were serum starved overnight before stimulation with 1, 10, or 50 ng/ml IL-13 for 48 hours. Time-matched control wells were treated with PBS only. Total cellular RNA was isolated and quantified, and equal amounts of RNA were reverse transcribed to cDNA. RT-PCR was performed on cDNA samples using primers that span exon 10 in the IL-13Rα2 sequence so that the full-length IL-13Rα2 transcript would yield a product of 395 base pairs and the ΔEx10 splice variant would yield a shorter product of 295 base pairs. Figure 5B shows that, in the wild-type fibroblasts, stimulation with 10 or 50 ng/ml of IL-13 resulted in a robust up-regulation of the full length and the ΔEx10 transcripts of the IL-13Rα2 gene. In the absence of CXCR3, however, this up-regulation was attenuated.
To investigate the role of CXCR3 in downstream IL-13 signal transduction, wild-type and CXCR3−/− fibroblasts were serum starved overnight before stimulation with 10 ng/ml of IL-13 for various time points from 0 minutes (PBS only) to 6 hours. Total STAT6 and STAT6 phosphorylated at residue Tyrosine 641 (pSTAT6) were detected. The results from these experiments are shown in Figure 5C. In wild-type fibroblasts, STAT6 activation begins at 10 minutes and reaches a peak at 30 minutes, declining by 4 hours. In contrast, in CXCR3−/− fibroblasts, STAT6 activation begins at 5 minutes, reaches the same peak at 30 minutes, and declines at 6 hours.
CXCR3−/− Fibroblasts Demonstrate Increased Fibrotic Functional Capacities to Wild-Type Fibroblasts
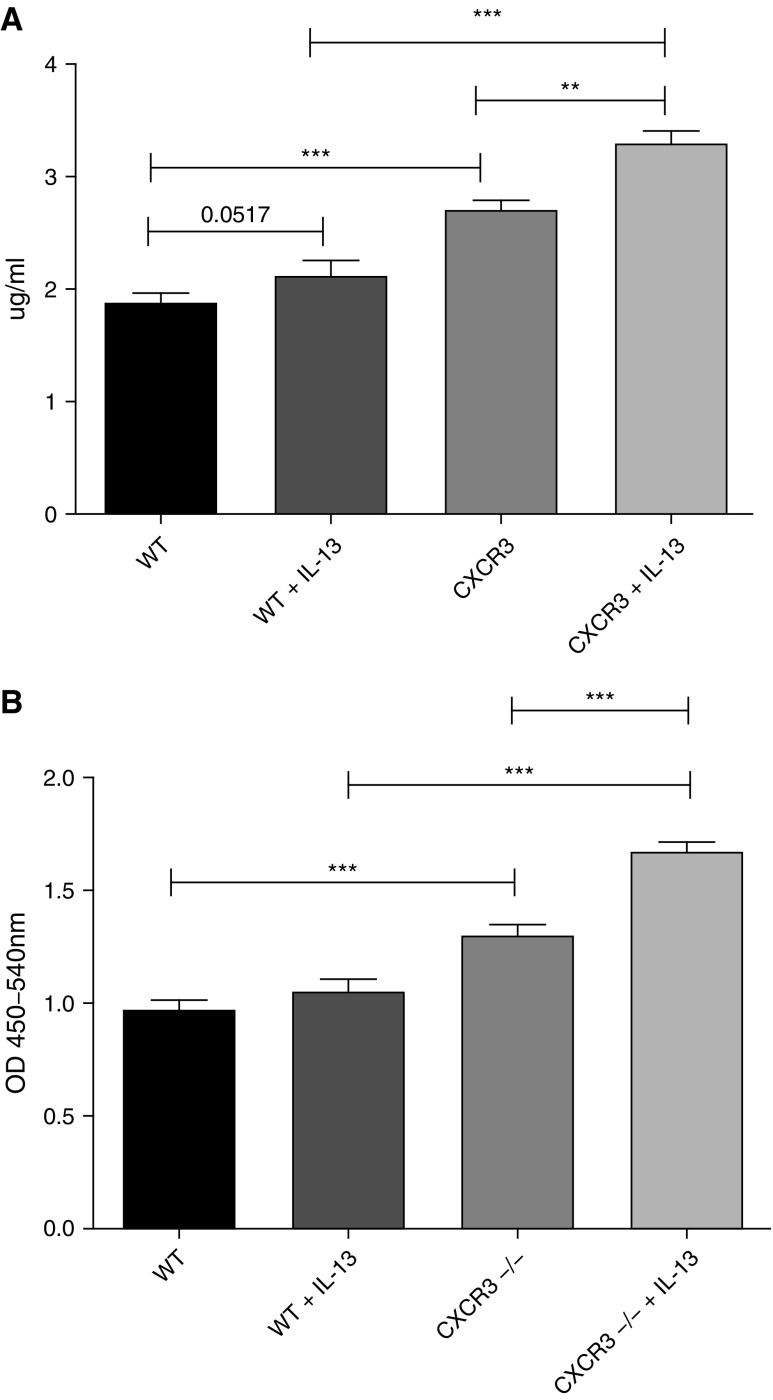
To investigate the role of CXCR3 as an antifibrotic receptor, we performed two functional assays on wild-type and CXCR3−/− fibroblasts. Wild-type and CXCR3−/− fibroblasts were serum starved overnight before stimulation with 10 ng/ml of IL-13 for 48 hours. Collagen production was measured and, after IL-13 stimulation, wild-type and CXCR3−/− fibroblasts demonstrated significant increases in collagen production (Figure 6A). However, this increase in collagen production was significantly higher in unstimulated and stimulated CXCR3−/− fibroblasts.
Figure 6.
CXCR3−/− fibroblasts demonstrate increased fibrotic functional capacities to wild-type fibroblasts. Wild-type and CXCR3−/− fibroblasts were treated with IL-13 (10 ng/ml) for 48 hours and assessed for soluble collagen production (A) and proliferation (B) as measured by the Sircol soluble collagen assay and BrdU proliferation assay, respectively. CXCR3−/− fibroblasts produce significantly more soluble collagen than wild-type fibroblasts at the basal level and in response to IL-13. CXCR3−/− fibroblasts proliferate significantly more than wild-type at a basal level and in response to IL-13. **P < 0.01, ***P < 0.001 (Mann-Whitney U test). OD, optical density.
The proliferative capabilities of wild-type and CXCR3−/− fibroblasts were measured after IL-13 stimulation for 48 hours. Unstimulated CXCR3−/− fibroblasts demonstrated significant increases in proliferation compared with unstimulated wild-type fibroblasts (Figure 6B). When stimulated with IL-13, CXCR3−/− fibroblasts displayed significant increases in proliferation.
IL-13 Down-Regulates CXCR3 Gene Expression in Pulmonary Fibroblasts
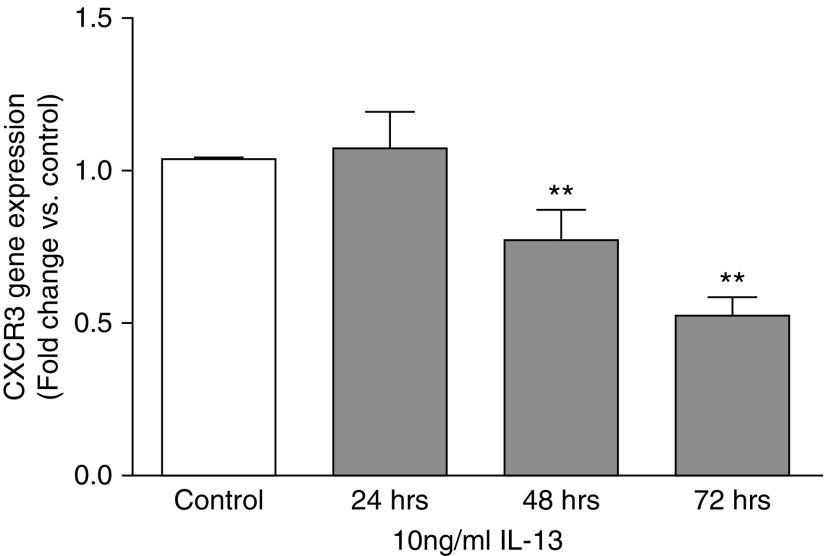
To assess the role of IL-13 on the regulation of CXCR3 gene expression, pulmonary fibroblasts isolated from wild-type BALB/c mice were cultured to 80% confluency before stimulation with 10 ng/ml of IL-13 for 24, 48, or 72 hours. Total RNA was isolated and reverse transcribed into cDNA using random primers. Quantitative real-time PCR was used to determine the relative expression of CXCR3 with time-matched controls. Figure 7 shows that, at 24 hours, no significant difference was observed in CXCR3 gene expression with IL-13 treatment, but, after exposure to IL-13 for 48 and 72 hours, gene expression of CXCR3 decreased significantly. These data demonstrate that IL-13 is a negative regulator of CXCR3 expression that it does so in a time-dependent manner. This down-regulation of CXCR3 expression by IL-13 also occurs in a dose-dependent fashion (data not shown).
Figure 7.
IL-13 down-regulates CXCR3 gene expression in pulmonary fibroblasts in a time-dependent manner. Cultured primary pulmonary fibroblasts isolated from wild-type mice were serum starved overnight before stimulation with 10 ng/ml IL-13 for 24 to 72 hours. Total RNA was isolated from cultured cells and reverse transcribed into cDNA. Quantitative real-time RT-PCR was used to quantify the relative expression of the CXCR3 gene transcript for each condition by the ΔΔCt method of analysis. Data are presented as fold change as compared with the time-matched control treatment (PBS only). Statistical analysis by the Mann-Whitney U test is indicated for five independent experiments. **P < 0.01 versus control treatment.
Discussion
Fibroblasts are the main effector cells in fibrogenesis, and, once activated by various stimuli arising from tissue injury, these cells proliferate and contribute to increased synthesis and deposition of extracellular matrix components (28). Ex vivo, we have demonstrated CXCR3 expression in pulmonary human and murine fibroblasts. In vitro, fibroblasts were cultured from lung explants of wild-type and CXCR3−/− mice to investigate the role of CXCR3 in the regulation of IL-13Rα2. The results of these studies demonstrate that CXCR3 contributes to the IL-13–mediated up-regulation of IL-13Rα2 gene and protein expression.
Targeted disruption of CXCR3 in mice resulted in a loss of CXCR3 gene and protein expression in cultured fibroblasts from lung explants. No other changes in gene expression were detected in these cells, except for a significant increase in COL3A1 in the absence of CXCR3. After tissue injury, the secretion of type III collagen is transient during the time of fibroblast proliferation and activation, and this isoform is ultimately replaced by type I collagen, which imparts greater tensile strength to the healing wound (29). The significantly higher unstimulated gene expression of type III collagen in fibroblasts lacking CXCR3, as compared with wild-type fibroblasts, suggests that these cells may be in a constitutively proliferative and/or activated state, without prior stimulation. Proliferation assay data, however, showed few significant differences in proliferation capacity between the two genotypes after cytokine or chemokine stimulation (data not shown).
CXCR3-deficient mice have previously been shown to display increased fibrosis in response to bleomycin (30), and IL-13 is known to play a role in this process. Treatment of CXCR3-deficient mice with IL-13 (details are provided in the online supplement) results in increased CCL17 expression in response to IL-13 compared with wild-type mice, thereby suggesting an altered role for the IL-13 receptors because CCL17 is regulated by IL-13.
CXCL10/IP-10 has been reported to negatively regulate fibroblast migration after bleomycin-induced lung injury (31), and, although the mechanism and receptor through which it functions on nonleukocytes has not been fully elucidated, some evidence for a CXCR3-independent pathway exists (31, 32). CXCL11 was not found to have an effect on pulmonary fibroblasts, and CXCR3 did not seem to be expressed on these cells (19). Because the expression of CXCR3 on pulmonary fibroblasts has been disputed, it was necessary to rule out the possibility of a heterogeneous cell population in lung explant cultures as the reason for the observed CXCR3 expression. The major possibility for contaminating the cell population in our cultures was the presence of leukocytes, which are immune effector cells that have been shown to express CXCR3. Cultured cells were characterized according to expression of CD45, CXCR3, and collagen I to demonstrate a fibroblastic phenotype. Wild-type cells gave a strong positive signal for CXCR3, whereas CXCR3−/− cells did not.
Our results demonstrate that, in the presence of CXCR3, IL-13 is a potent inducer of IL-13Rα2 gene expression in pulmonary fibroblasts, and this induction is attenuated in the absence of CXCR3 in these cells. Western blot analysis shows that IL-13 stimulation of wild-type fibroblasts primarily induces the expression of the 55-kD isoform of IL-13Rα2, which corresponds to the membrane-bound form of the receptor (mIL-13Rα2) (27, 33, 34), whereas this induction is greatly attenuated in fibroblasts lacking CXCR3. The 44-kD isoform, which corresponds to the soluble IL-13Rα2 receptor (sIL-13Rα2) (27, 33, 34), tends to increase as well in the wild-type cells after IL-13 stimulation, although not as dramatically as the membrane-bound form. There is a slight increase of IL-13Rα2 expression after stimulation with increasing concentrations of IL-13 in the absence of CXCR3, suggesting that a lesser mechanism is also used for the induction of this receptor. The lesser mechanism is unidentified. Similarly, the up-regulation of full-length and ΔEx10 gene transcripts was induced with a lower concentration of IL-13 stimulation in wild-type (10 ng/ml) as compared with CXCR3−/− fibroblasts (50 ng/ml), and the up-regulation seen in the wild-type cells is attenuated in the absence of CXCR3.
A marked difference was observed between the IL-13–mediated phosphorylation of STAT6 in wild-type as compared CXCR3−/− cells. STAT6 activation occurred earlier and was prolonged in the absence of CXCR3, and IL-13–activated STAT6 appeared to be more abundant in CXCR3−/− than in wild-type cells. Functionally, CXCR3−/− cells are more fibrotic than wild-type cells. They produce more collagen at a basal level and when stimulated with IL-13. The CXCR3−/− cells also display increased proliferation compared with wild-type cells when stimulated with IL-13. Given that IL-13Rα2 up-regulation in response to IL-13 is attenuated in the absence of CXCR3 and that IL-13Rα2 is known to act as a decoy receptor, the bioavailablility of IL-13 may be increased at the IL-13Rα1/IL-4α receptor complex, which may thus potentiate the profibrotic actions of IL-13. These results suggest that CXCR3 is also important for limiting the profibrotic actions of IL-13 indirectly through the regulation of the antifibrotic receptor IL-13Rα2.
CXCR3 is a member of the large family of GPCRs, many of which are known for their constitutive activities in the absence of ligand in normal physiology and in pathological conditions. These constitutively active GPCRs include the β2-adrenergic receptor (35), the δ opioid receptor (36), the growth hormone secretagogue receptor (37), and the melanocortin-4 receptor (38), and loss-of-function mutations that selectively eliminate the normal basal signaling of these receptors are thought to influence the development of human disease (38) or to alter the processes of physiological development (39). The potential exists that, in instances of IPF, CXCR3 may have decreased basal signaling capacity, although this has not been investigated. If this is true, then therapeutic modalities to increase signaling function of CXCR3 may be of benefit in the treatment of the disease.
Taken together, the results from this study point strongly to a requirement for CXCR3 to attain IL-13–mediated IL-13Rα2 gene expression. Further studies investigating the downstream signaling mechanisms of CXCR3 that may play a role in this regulation need to be performed. Understanding the function of CXCR3 in IL-13–mediated lung injury may lead to novel approaches to combat the development of pulmonary fibrosis, whether by limiting the effects of IL-13 or by manipulation of angiostatic pathways. Further investigations into the possible constitutive activity of CXCR3 and its role in IL-13Rα2 expression may have implications for novel therapies to treat this disease.
Acknowledgments
Acknowledgments
The authors thank Dr. Dimitri Scholz and Andrew Gaffney for technical assistance with fluorescence microscopy experimental protocols and data analysis and Ms. Catherine Moss for technical assistance with real-time PCR.
Footnotes
This work was supported by Science Foundation Ireland.
Author Contributions: Conception and experimental design: J.C.B., R.V.L., J.W., R.K., J.A.B., and M.P.K. Providing materials: J.A.B. Performing experiments: J.C.B., R.V.L., J.W., A.F., D.B., and R.K. Analysis and interpretation: J.C.B., R.V.L., J.W., A.F., S.C.D., I.P.C., S.L.O’B., D.B., R.K., and M.P.K. Drafting the manuscript for important intellectual content: J.C.B., R.V.L., J.W., R.K., and M.P.K.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0433OC on December 16, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest. 2012;122:2756–2762. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 3.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner-Warwick M. Precapillary systemic-pulmonary anastomoses. Thorax. 1963;18:225–237. doi: 10.1136/thx.18.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renzoni EA, Walsh DA, Salmon M, Wells AU, Sestini P, Nicholson AG, Veeraraghavan S, Bishop AE, Romanska HM, Pantelidis P, et al. Interstitial vascularity in fibrosing alveolitis. Am J Respir Crit Care Med. 2003;167:438–443. doi: 10.1164/rccm.200202-135OC. [DOI] [PubMed] [Google Scholar]
- 6.Cosgrove GP, Brown KK, Schiemann WP, Serls AE, Parr JE, Geraci MW, Schwarz MI, Cool CD, Worthen GS. Pigment epithelium-derived factor in idiopathic pulmonary fibrosis: a role in aberrant angiogenesis. Am J Respir Crit Care Med. 2004;170:242–251. doi: 10.1164/rccm.200308-1151OC. [DOI] [PubMed] [Google Scholar]
- 7.Peao MN, Aguas AP, de Sa CM, Grande NR. Neoformation of blood vessels in association with rat lung fibrosis induced by bleomycin. Anat Rec. 1994;238:57–67. doi: 10.1002/ar.1092380108. [DOI] [PubMed] [Google Scholar]
- 8.Mentink-Kane MM, Wynn TA. Opposing roles for IL-13 and IL-13 receptor alpha 2 in health and disease. Immunol Rev. 2004;202:191–202. doi: 10.1111/j.0105-2896.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- 9.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2:103–121. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang JG, Hilton DJ, Willson TA, McFarlane C, Roberts BA, Moritz RL, Simpson RJ, Alexander WS, Metcalf D, Nicola NA. Identification, purification, and characterization of a soluble interleukin (IL)-13-binding protein: evidence that it is distinct from the cloned Il-13 receptor and Il-4 receptor alpha-chains. J Biol Chem. 1997;272:9474–9480. doi: 10.1074/jbc.272.14.9474. [DOI] [PubMed] [Google Scholar]
- 11.Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111:677–690, quiz 691. doi: 10.1067/mai.2003.1333. [DOI] [PubMed] [Google Scholar]
- 12.Wills-Karp M, Finkelman FD. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci Signal. 2008;1:pe55. doi: 10.1126/scisignal.1.51.pe55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donaldson DD, Whitters MJ, Fitz LJ, Neben TY, Finnerty H, Henderson SL, O'Hara RM, Jr, Beier DR, Turner KJ, Wood CR, et al. The murine IL-13 receptor alpha 2: molecular cloning, characterization, and comparison with murine IL-13 receptor alpha 1. J Immunol. 1998;161:2317–2324. [PubMed] [Google Scholar]
- 14.Wood N, Whitters MJ, Jacobson BA, Witek J, Sypek JP, Kasaian M, Eppihimer MJ, Unger M, Tanaka T, Goldman SJ, et al. Enhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor alpha 2. J Exp Med. 2003;197:703–709. doi: 10.1084/jem.20020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 16.Fichtner-Feigl S, Young CA, Kitani A, Geissler EK, Schlitt HJ, Strober W.IL-13 signaling via IL-13R alpha2 induces major downstream fibrogenic factors mediating fibrosis in chronic TNBS colitis Gastroenterology 20081352003–2013, e1–7 [DOI] [PubMed] [Google Scholar]
- 17.Mandal D, Levine AD. Elevated IL-13Ralpha2 in intestinal epithelial cells from ulcerative colitis or colorectal cancer initiates MAPK pathway. Inflamm Bowel Dis. 2010;16:753–764. doi: 10.1002/ibd.21133. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Strieter RM, Gomperts BN, Keane MP. The role of CXC chemokines in pulmonary fibrosis. J Clin Invest. 2007;117:549–556. doi: 10.1172/JCI30562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burdick MD, et al. CXCL11 attenuates bleomycin-induced pulmonary fibrosis via inhibition of vascular remodeling. Am J Respir Crit Care Med. 2005;171:261–268. doi: 10.1164/rccm.200409-1164OC. [DOI] [PubMed] [Google Scholar]
- 20.Keane MP, Belperio JA, Arenberg DA, Burdick MD, Xu ZJ, Xue YY, Strieter RM. IFN-gamma-inducible protein-10 attenuates bleomycin-induced pulmonary fibrosis via inhibition of angiogenesis. J Immunol. 1999;163:5686–5692. [PubMed] [Google Scholar]
- 21.Belperio JA, Keane MP, Burdick MD, Lynch JP, 3rd, Zisman DA, Xue YY, Li K, Ardehali A, Ross DJ, Strieter RM. Role of CXCL9/CXCR3 chemokine biology during pathogenesis of acute lung allograft rejection. J Immunol. 2003;171:4844–4852. doi: 10.4049/jimmunol.171.9.4844. [DOI] [PubMed] [Google Scholar]
- 22.Belperio JA, Dy M, Burdick MD, Xue YY, Li K, Elias JA, Keane MP. Interaction of IL-13 and C10 in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2002;27:419–427. doi: 10.1165/rcmb.2002-0009OC. [DOI] [PubMed] [Google Scholar]
- 23.Hancock WW, Lu B, Gao W, Csizmadia V, Faia K, King JA, Smiley ST, Ling M, Gerard NP, Gerard C. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–1520. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DA, Glennie MJ, et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010;102:1555–1577. doi: 10.1038/sj.bjc.6605642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JFO, Flaherty KR, Lasky JA, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baglole CJ, Reddy SY, Pollock SJ, Feldon SE, Sime PJ, Smith TJ, Phipps RP. Isolation and phenotypic characterization of lung fibroblasts. Methods Mol Med. 2005;117:115–127. doi: 10.1385/1-59259-940-0:115. [DOI] [PubMed] [Google Scholar]
- 27.Tabata Y, Chen W, Warrier MR, Gibson AM, Daines MO, Hershey GKK. Allergy-driven alternative splicing of IL-13 receptor α2 yields distinct membrane and soluble forms. J Immunol. 2006;177:7905–7912. doi: 10.4049/jimmunol.177.11.7905. [DOI] [PubMed] [Google Scholar]
- 28.Zeisberg M, Strutz F, Muller GA. Role of fibroblast activation in inducing interstitial fibrosis. J Nephrol. 2000;13:S111–S120. [PubMed] [Google Scholar]
- 29.Sephel GC, Davidson JM.Repair, regeneration and fibrosis. In: Rubin R, Strayer D, Rubin E, editors. Rubin's pathology: clinicopathologic foundations of medicine. Baltimore, MD: Lippincott Williams and Wilkins; 2012. pp. 83–114 [Google Scholar]
- 30.Jiang D, Liang J, Hodge J, Lu B, Zhu Z, Yu S, Fan J, Gao Y, Yin Z, Homer R, et al. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest. 2004;114:291–299. doi: 10.1172/JCI16861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campanella GS, Colvin RA, Luster AD. CXCL10 can inhibit endothelial cell proliferation independently of CXCR3. PLoS One. 2010;5:e12700. doi: 10.1371/journal.pone.0012700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang D, Liang J, Campanella GS, Guo R, Yu S, Xie T, Liu N, Jung Y, Homer R, Meltzer EB, et al. Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest. 2010;120:2049–2057. doi: 10.1172/JCI38644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen W, Tabata Y, Gibson AM, Daines MO, Warrier MR, Wills-Karp M, Hershey GK. Matrix metalloproteinase 8 contributes to solubilization of IL-13 receptor alpha2 in vivo. J Allergy Clin Immunol. 2008;122:625–632. doi: 10.1016/j.jaci.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen W, Sivaprasad U, Tabata Y, Gibson AM, Stier MT, Finkelman FD, Hershey GK. IL-13R alpha 2 membrane and soluble isoforms differ in humans and mice. J Immunol. 2009;183:7870–7876. doi: 10.4049/jimmunol.0901028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerione RA, Codina J, Benovic JL, Lefkowitz RJ, Birnbaumer L, Caron MG. The mammalian beta 2-adrenergic receptor: reconstitution of functional interactions between pure receptor and pure stimulatory nucleotide binding protein of the adenylate cyclase system. Biochemistry. 1984;23:4519–4525. doi: 10.1021/bi00315a003. [DOI] [PubMed] [Google Scholar]
- 36.Costa T, Cotecchia S. Historical review: Negative efficacy and the constitutive activity of G-protein-coupled receptors. Trends Pharmacol Sci. 2005;26:618–624. doi: 10.1016/j.tips.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW. High constitutive signaling of the ghrelin receptor–identification of a potent inverse agonist. Mol Endocrinol. 2003;17:2201–2210. doi: 10.1210/me.2003-0069. [DOI] [PubMed] [Google Scholar]
- 38.Srinivasan S, Lubrano-Berthelier C, Govaerts C, Picard F, Santiago P, Conklin BR, Vaisse C. Constitutive activity of the melanocortin-4 receptor is maintained by its N-terminal domain and plays a role in energy homeostasis in humans. J Clin Invest. 2004;114:1158–1164. doi: 10.1172/JCI21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoue H, Kangawa N, Kinouchi A, Sakamoto Y, Kimura C, Horikawa R, Shigematsu Y, Itakura M, Ogata T, Fujieda K. Identification and functional analysis of novel human growth hormone secretagogue receptor (GHSR) gene mutations in Japanese subjects with short stature. J Clin Endocrinol Metab. 2011;96:E373–E378. doi: 10.1210/jc.2010-1570. [DOI] [PubMed] [Google Scholar]