Abstract
Purpose
To determine whether hospice use by patients with cancer is associated with their families’ perceptions of patients’ symptoms, goal attainment, and quality of end-of-life (EOL) care.
Methods
We interviewed 2,307 families of deceased patients with advanced lung or colorectal cancer who were enrolled in the Cancer Care Outcomes Research and Surveillance study (a multiregional, prospective, observational study) and died by 2011. We used propensity-score matching to compare family-reported outcomes for patients who did and did not receive hospice care, including the presence and relief of common symptoms (ie, pain, dyspnea), concordance with patients’ wishes for EOL care and place of death, and quality of EOL care. We also examined associations between hospice length of stay and these outcomes among hospice enrollees.
Results
In a propensity-score-matched sample of 1,970 individuals, families of patients enrolled in hospice reported more pain in their patient compared with those not enrolled in hospice. However, families of patients enrolled in hospice more often reported that patients received “just the right amount” of pain medicine (80% v 73%; adjusted difference, 7 percentage points; 95% confidence interval [CI], 1 to 12 percentage points) and help with dyspnea (78% v 70%; adjusted difference, 8 percentage points; 95% CI, 2 to 13 percentage points). Families of patients enrolled in hospice also more often reported that patients’ EOL wishes were followed (80% v 74%; adjusted difference, 6 percentage points; 95% CI, 2 to 11 percentage points) and “excellent” quality EOL care (57% v 42%; adjusted difference, 15 percentage points; 95% CI, 11 to 20). Families of patients who received > 30 days of hospice care reported the highest quality EOL outcomes.
Conclusion
Hospice care is associated with better symptom relief, patient-goal attainment, and quality of EOL care. Encouraging earlier and increased hospice enrollment may improve EOL experiences for patients with cancer and their families.
INTRODUCTION
Patients with advanced cancer often experience pain, dyspnea, and distress at the end of life (EOL), and use intensive, hospital-based services near death.1-3 Hospice offers an alternative, patient-centered model of care focused on relieving suffering, and often delivers services within the home environment. ASCO recently adopted timely hospice enrollment (defined as > 3 days before death) as a key quality measure for patients with incurable, poor-prognosis cancers4 because hospice has been associated with less hospital-based EOL medical care, better quality of life, and improved caregiver outcomes.2,5-9
Despite this, few studies have examined the associations between receipt of hospice and the symptoms experienced by patients with advanced cancer and receipt of medical care congruent with their preferences.7 Similarly, few studies have assessed whether these outcomes differ by the duration of hospice enrollment.2,10-14 This is important because although hospice use has increased since the 1990s,3,15 many patients with advanced cancer are enrolled ≤ 3 days of death.3
In this study, we examined whether hospice was associated with family members’ reports of the presence and relief of common symptoms, patient-goal attainment, and quality of EOL care, using data from the Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium. We also assessed whether longer hospice stays were associated with better family-reported outcomes among hospice enrollees.
METHODS
Dataset
Data came from the CanCORS I and II studies, which enrolled participants from 2003 through 2005 and followed patients or family through 2011. CanCORS enrolled patients with newly diagnosed lung or colorectal cancers from five regions (northern California; Los Angeles County, California; North Carolina; Iowa; and Alabama), five integrated health systems, and 15 Veterans Affairs hospitals.16 CanCORS participants were representative of patients diagnosed with lung or colorectal cancer in the US regions covered by the SEER program.17
Trained staff interviewed participants (or a family member or close friend, if the patient was too ill or had died) in English, Spanish, or Chinese, using computer-assisted telephone software at three time points after diagnosis: (1) approximately 4 to 6 months, (2) 1 year (for patients alive at the first interview), and (3) 5 to 7 years later (for patients alive 1 year after diagnosis). The study was approved by the institutional review boards at all sites.
Cohort
The study cohort included participants with advanced-stage lung or colorectal cancer at diagnosis or recurrence who died by 2011 and whose family or close friend participated in a postdeath interview. For patients deceased at the time of initial study contact, the next of kin was invited to participate. Patients who were alive for the first interview were asked to identify a primary family member or friend “familiar with your care since diagnosis” and a secondary respondent (in case the first could not be reached). Because 95% of interviews were completed by family (eg, spouse/partner, child) we refer to respondents as family. The final cohort included 2,307 decedents.
Outcome Variables
Outcomes of interest included family members’ perceptions of patients’ symptom prevalence and control, concordance of EOL care with patients’ preferences, and quality of EOL care. Specifically, we examined symptom burden (ie, pain or dyspnea), symptom control, overall quality of EOL care, and goal attainment (ie, whether patients’ EOL wishes were followed and whether they died in their preferred place).
To assess symptoms, family members were asked whether, in the last days to weeks of life, patients had “pain or took pain medication” (yes/no) or had “shortness of breath” (yes/no). Family members who affirmed the presence of pain or dyspnea were asked the following: “How much medicine did the patient receive for his/her pain?” and/or “How much help...with his/her breathing did the patient receive?” Response options included “less than he/she wanted,” “just the right amount,” and “more than he/she wanted.”
We examined concordance with patients’ previously stated wishes for EOL care, asking the family: “During the last month of life, did he/she prefer a course of treatment that focused on extending life as much as possible, even if it meant more pain and discomfort, or on a plan that focused on relieving pain and discomfort as much as possible, even if that meant not living as long?” Next, family members were asked: “To what extent were these wishes followed in the medical treatment he/she received during the last month of life?” Response options included: “a great deal,” “somewhat,” “not at all,” “don’t know,” or “refused.” We defined concordance as family who responded “a great deal” to this question. We also assessed the concordance between the patients’ previously stated preferred place of death (reported by family) and where patients actually died. We defined concordance as patients whose actual place of death matched their preferred place. Family members also rated the overall quality of care received by patients in the last place where they spent ≥ 48 hours before death (rating options were excellent, very good, good, fair, or poor). We defined high-quality EOL care as care that was rated excellent.
Independent Variables
Receipt of hospice was assessed by asking the family whether the patient ever received hospice care (yes/no). The family reported the duration of hospice services received, categorized as ≤ 3 days, 4 to 7 days, 8 to 30 days, or > 30 days before death. We compared ≤ 3 days of hospice services with longer stays because prior studies suggested that short hospice stays are associated with worse patient quality of life and EOL care, compared with longer durations.2,5
Additional Baseline Covariates
Sociodemographic characteristics and treatment preferences.
In the baseline survey, patients or their family reported sex, age at death, race/ethnicity, marital status, whether English was spoken at home, education, income, and insurance.
Clinical covariates.
Cancer type and stage at diagnosis data were obtained from the medical record or, if unavailable, from cancer registries. The family reported patients’ smoking status, comorbid medical conditions, and receipt of cancer-directed surgery, chemotherapy, or radiation. We also documented the region where patients resided and whether they received treatment within an integrated health-care system.
Statistical Analysis
We compared, with descriptive statistics, sociodemographic and clinical characteristics between patients who did or did not receive hospice care. We examined family-reported outcomes by hospice enrollment after using propensity score matching to balance measurable confounders between those who received hospice and those who did not.18 We used logistic regression to assess patient factors associated with hospice and then matched nonhospice enrollees with hospice enrollees based on their estimated propensity of hospice enrollment using 1:1 matching via a greedy algorithm with a caliper of 0.20. Standardized differences in observable characteristics of the matched cohort were ≤ 7% (Appendix Table A1, online only).
We compared relief of EOL symptoms, concordance with family-reported patient EOL preferences, and family-reported quality of EOL care, using separate logistic regression models among the propensity-matched cohort. We fit linear-binomial models with an identity link to estimate adjusted differences in the likelihood of each outcome.19
In a second set of analyses among patients enrolled in hospice, we examined associations between each of the dependent variables and hospice length of stay (categorized with indicator variables as ≤ 3 days, 4 to 7 days, 8 to 30 days, > 30 days), while adjusting for all the covariates described. We calculated adjusted rates for each outcome by hospice duration, using ≤ 3 days as the reference category.
Missing data on outcomes varied between 1% (quality of EOL care) and 19% (EOL wishes); missing data for covariates ranged between 1% (stage) and 15% (income). We used multiple imputation methods to create five complete data sets, imputing values for missing outcomes and covariates, and repeated all analyses on each imputed data set, combining results using standard methods for multiple imputed data for our primary analysis.20,21 Sensitivity analyses were conducted to examine associations between hospice and each outcome in a second sample without imputation of outcome data. Because patients with lung and colorectal cancer may differ, we repeated analyses stratified by disease site tested for the interaction of hospice and disease site. Two-sided P values < .05 were considered statistically significant; analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, NC).
RESULTS
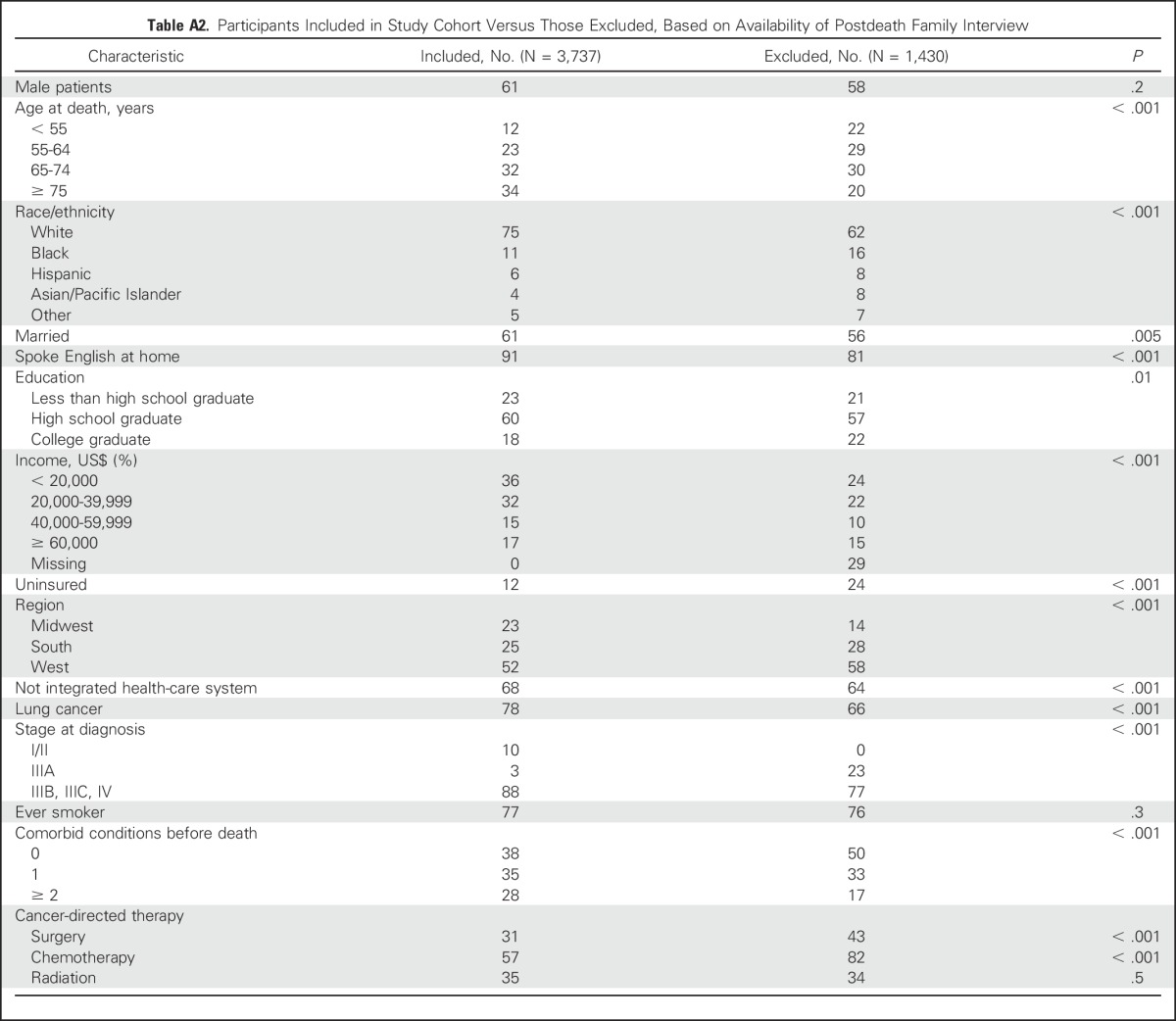
Among 3,737 participants with advanced-stage cancer at diagnosis or recurrence, 2,307 had an after-death family interview and were included in the cohort. These individuals were older, less educated, and had more advanced-stage disease and comorbid medical conditions than participants without an after-death interview; they also received fewer cancer-directed therapies (Appendix Table A2, online only).
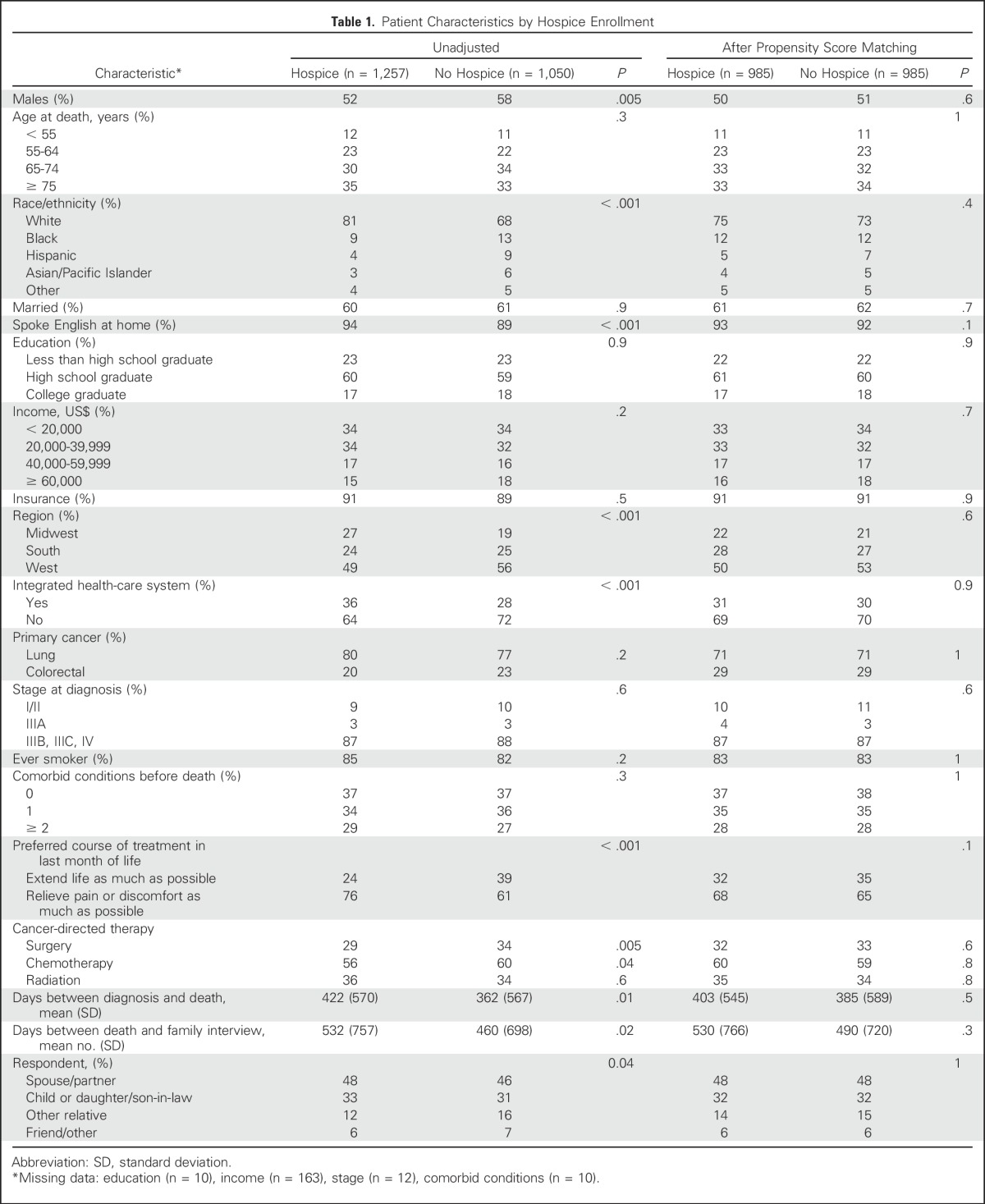
Among these 2,307 participants, 1,257 (55%) enrolled in hospice before death. Those enrolled in hospice were more likely to be non-Hispanic white (81% v 68%; P < .001), speak English at home (94% v 89%; P < .001), and receive care in an integrated health system (36% v 28%; P < .001) than those who did not receive hospice (Table 1). They were also more likely to have a family-reported preference for pain relief over life extension (76% v 61%; P < .001), and less likely to have received surgery (29% v 34%; P = .005) or chemotherapy (56% v 60%; P = .04).
Table 1.
Patient Characteristics by Hospice Enrollment
Family-Reported Outcomes by Hospice Enrollment
Among the 1,050 patients who did not enroll in hospice, 985 were matched with a patient who was enrolled in hospice, resulting in a final cohort of 1,970 patients (985 matched pairs; Table 1). In adjusted analyses (Table 2), families of patients who received hospice care were more likely to report pain or use of pain medication, compared with those not receiving hospice (91% v 81%; adjusted difference, 10 percentage points; 95% CI, 7 to 13 percentage points). Among those who reported symptoms, families of patients enrolled in hospice were more likely to report that patients received “just the right amount of pain medicine” (80% v 73%; adjusted difference, 7 percentage points; 95% CI, 1 to 12 percentage points) and “just the right amount of help with breathing” (78% v 70%; adjusted difference, 8 percentage points; 95% CI, 2 to 13 percentage points), compared with those not enrolled in hospice. They were also less likely to report receiving “too little pain medicine” (8% v 11%; adjusted difference, 3 percentage points; 95% CI, 0 to 6 percentage points) or “less help with breathing than wanted” (12% v 18%; adjusted difference, 6 percentage points; 95% CI, 2 to 11 percentage points). Families of hospice enrollees were not more likely to report that patients received “too much pain medicine.”
Table 2.
Hospice and Family-Reported End-of-Life Symptoms, Care Quality, and Goal Attainment in Propensity-Matched Sample
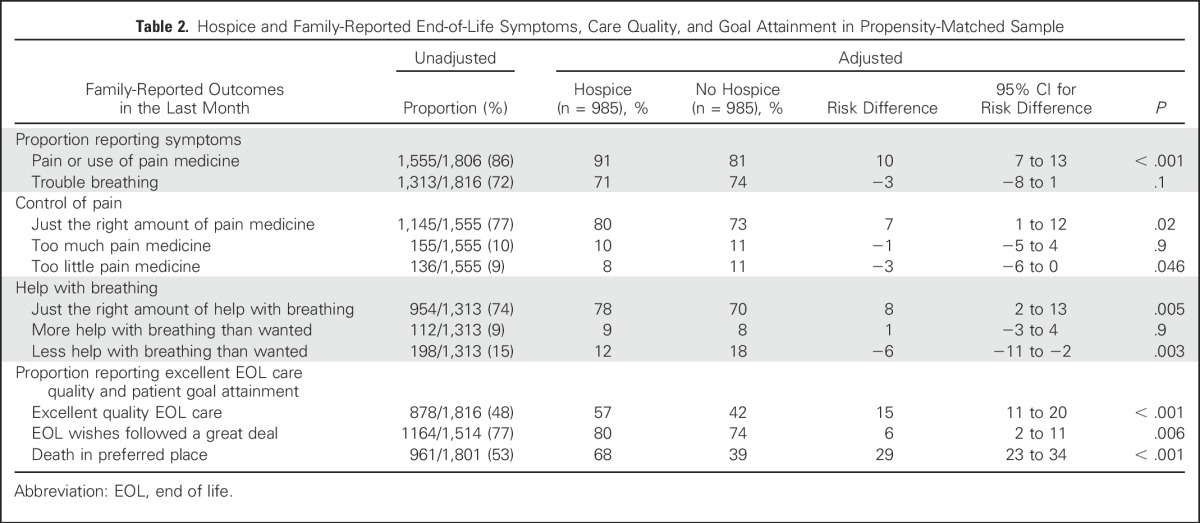
Families of patients enrolled in hospice were more likely to report that patients’ EOL wishes were followed “a great deal” (80% v 74%; adjusted difference, 6 percentage points; 95% CI, 2 to 11 percentage points). Decedents who received hospice care were also more likely to die in their preferred place (68% v 39%; adjusted difference, 29 percentage points; 95% CI, 23 to 34 percentage points). Families also reported excellent quality of EOL care more often for patients who received hospice (57% v 42%; adjusted difference, 15 percentage points; 95% CI, 11 to 20; Table 2), compared with those who did not.
Family-Reported Outcomes by Hospice Length of Stay
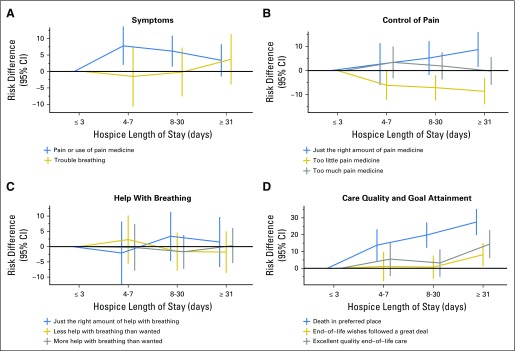
Among the 1,257 patients enrolled in hospice, the median length of enrollment was 21 days (interquartile range, 7 to 56 days). Overall, longer hospice stays were associated with family perceptions that patients received “just the right amount of pain medication,” greater patient-goal attainment, and higher rates of family-reported excellent quality of EOL care, compared with short stays (Table 3; Fig 1). Specifically, families of patients who received > 30 days of hospice care more often reported “just the right amount of pain medicine” than those enrolled ≤ 3 days (85% v 76%; adjusted difference, 9 percentage points; 95% CI, 2 to 16). Similarly, families of patients who received ≥ 8 days of hospice less often reported that patients received “too little pain medicine” compared with those enrolled ≤ 3 days; findings were similar when comparing > 30 days with ≤ 3 days of hospice. Family-reported help with dyspnea did not vary by hospice duration.
Table 3.
Adjusted Associations Between Hospice Length of Stay and Family-Reported End-of-Life Symptoms, Care Quality, and Goal Attainment Among Patients Enrolled in Hospice (n = 1,257)
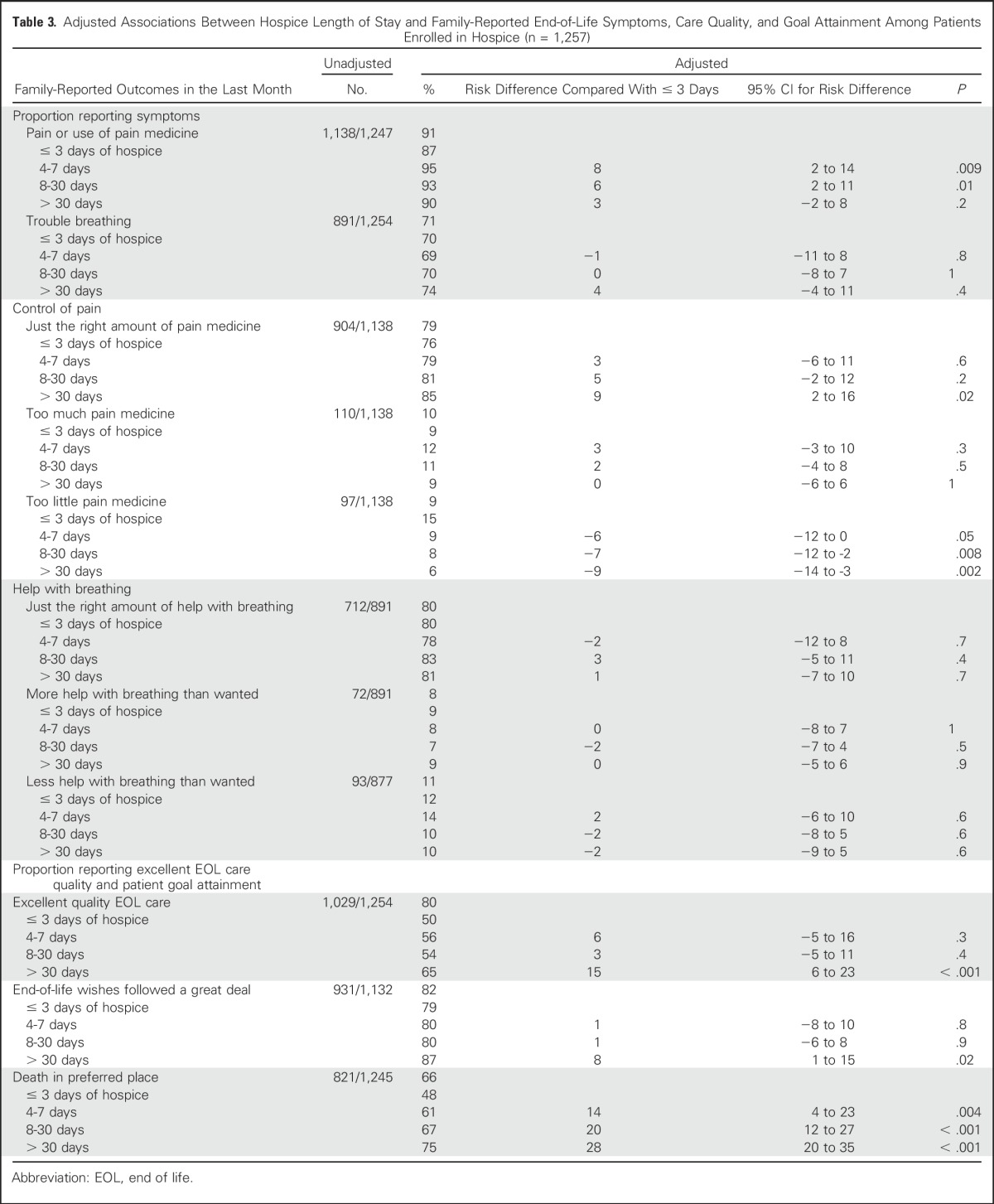
Fig 1.
Adjusted associations between hospice length of stay and family-reported end-of-life outcomes among patients enrolled in hospice (n = 1,257), expressed as risk differences and 95% CI (bars), compared with the reference (≤ 3 days): (A) symptoms, (B) control of pain, (C) help with breathing, and (D) care quality and goal attainment. Analyses adjusted for all patient characteristics listed in Table 1, independent of significance.
Families of patients who received > 30 days of hospice more often reported that patients’ EOL wishes were followed “a great deal” compared with patients enrolled ≤ 3 days (87% v 79%; adjusted difference, 8 percentage points; 95% CI, 1 to 15) and more often rated the quality of EOL care “excellent” (65% v 50%; adjusted difference, 15 percentage points; 95% CI, 6 to 23). The longer patients were enrolled in hospice, the more likely they were to die in their preferred place.
Sensitivity Analyses
In sensitivity analyses, we examined associations between hospice enrollment and each outcome after excluding individuals with missing values for dependent variables. The results were consistent with the main findings except that associations between hospice and “EOL wishes followed a great deal” no longer reached statistical significance (data not shown). Results were also similar when stratified by cancer type (P ≥ .09 for interaction between hospice and cancer type for all outcomes; data not shown).
DISCUSSION
In this large, population-based cohort study of patients with advanced-stage cancer and high symptom burden, family members of patients enrolled in hospice were more likely to report that patients received “just the right” level of help with pain and dyspnea—not “too much,” or “too little.” Hospice use was also associated with higher rates of family-reported patient-goal attainment and quality of EOL care. In addition, patients who received > 30 days of hospice were more likely to receive optimal pain management, care that was congruent with their wishes, and care that family described as of “excellent” quality, compared with patients enrolled for ≤ 3 days. Together, our findings suggest that encouraging hospice enrollment, particularly enrollment weeks before death, may improve EOL experiences of patients with cancer.
To date, relatively few studies have examined bereaved family members’ perspectives on hospice care in population-based cohorts. We found that fewer than half of family members reported “excellent” quality of EOL care and nearly one-quarter felt that patients’ EOL wishes were not followed a “great deal.” These results demonstrate that a substantial proportion of patients with cancer have unmet needs close to death. Our examination of the prevalence and relief of symptoms by receipt of hospice services extends findings from earlier studies,7,22 demonstrating that although patients enrolled in hospice have more symptoms, their symptoms are better controlled overall. Of note, the families of patients enrolled in hospice were not more likely to report that patients received “too much” pain medicine; rather, they were more likely to report that patients received “just the right amount.”
A unique feature of this study was our ability to examine the relationship between the length of hospice enrollment and several important patient- and family-centered EOL outcomes, while adjusting for patients’ treatment preferences. Existing quality measures, endorsed by the National Quality Forum and ASCO, identify ≤ 3 days and ≤ 7 days of hospice services as poor-quality EOL care.23,24 Consistent with this, one prior study demonstrated that patients who received < 1 week of hospice care reported similar quality EOL care to those not receiving hospice.2 In our study, the families of patients who received > 8 days of hospice care were more likely to report that patients died in their preferred place and less likely to report inadequate pain control, compared with ≤ 3 days. However, > 70% of patients with lung or colorectal cancer experienced difficulty breathing, and relief of dyspnea did not vary by hospice length of stay; future studies should examine strategies to manage dyspnea more effectively. Additionally, we observed few differences between patients with very short stays (≤ 3 days) and moderately short stays (4 to 7 or 8 to 30 days); the best outcomes were generally seen in patients who received > 30 days of hospice.
Although early hospice enrollment may not be possible for all patients,10 our data suggest that more attention should be focused on efforts to enroll patients with cancer into hospice earlier because the median length of stay for patients enrolled in hospice care in the United States is only 17.4 days.25 The requirement to forego cancer-directed therapy remains a major barrier to early hospice referral26,27; however, early palliative care is associated with earlier discontinuation of chemotherapy and hospice referrals, without impacting the number of chemotherapy regimens received.28
Our study had some limitations. Although CanCORS participants were representative of patients diagnosed with lung and colorectal cancers in SEER regions,17 our findings may not be generalizable to patients with other cancers or those without involved family caregivers. In addition, although we measured and adjusted for many of the patient and caregiver characteristics that influence hospice enrollment (eg, treatment preferences), other confounding influences may not have been measured (eg, we lacked information about patients’ rate of functional decline and referring providers, both of which influence the intensity of EOL care provided).29,30 Moreover, patients with long hospice length of stays may differ from patients with shorter stays in ways that we could not measure. The data are older, particularly for patients who died soon after diagnosis. However, recent evidence demonstrates that EOL care remains intensive3,22,31; thus, patients’ and family members’ experiences are likely to be similar today. Finally, we relied on family members’ reports of patients’ preferred and actual place of death to determine goal attainment.
Despite these limitations, our study has many strengths. The study population included patients of all ages (36% were < 65 years of age) with a wide range of insurance types, which has previously been associated with hospice lengths of stay.32 It also included patients who recurred with advanced cancer and may have different experiences near death than patients with advanced cancer at diagnosis.
In conclusion, despite a higher symptom burden among patients enrolled in hospice, hospice care was associated with better symptom management, patient-goal attainment, and quality of EOL care. In addition, patients who received > 30 days of hospice care had the best family-reported EOL outcomes, compared with those who received ≤ 3 days. Together, these results suggest that current EOL care quality measures may be too narrowly focused on increasing hospice enrollment to > 3 or > 7 days of services. Future studies should examine whether multifaceted approaches (eg, early palliative care referrals,33 sensitive provision of information about hospice care early in the disease course, and an audit and feedback system to monitor physicians’ rates and timing of hospice referrals) might result in the provision of more preference-sensitive, high-quality, and value-based EOL care for patients with cancer. Future studies should also examine the impact of cancer immunotherapy on the timing of hospice referral; although these therapies are often less toxic than chemotherapy, they may be increasingly used in the last weeks of life.34
ACKNOWLEDGMENT
We thank Lin Ding for conducting statistical analyses. Dr Ding’s work on this project was supported by research grants to Harvard Medical School from the National Cancer Institute.
Appendix
Table A1.
Standardized Differences Before and After Propensity Score Matching
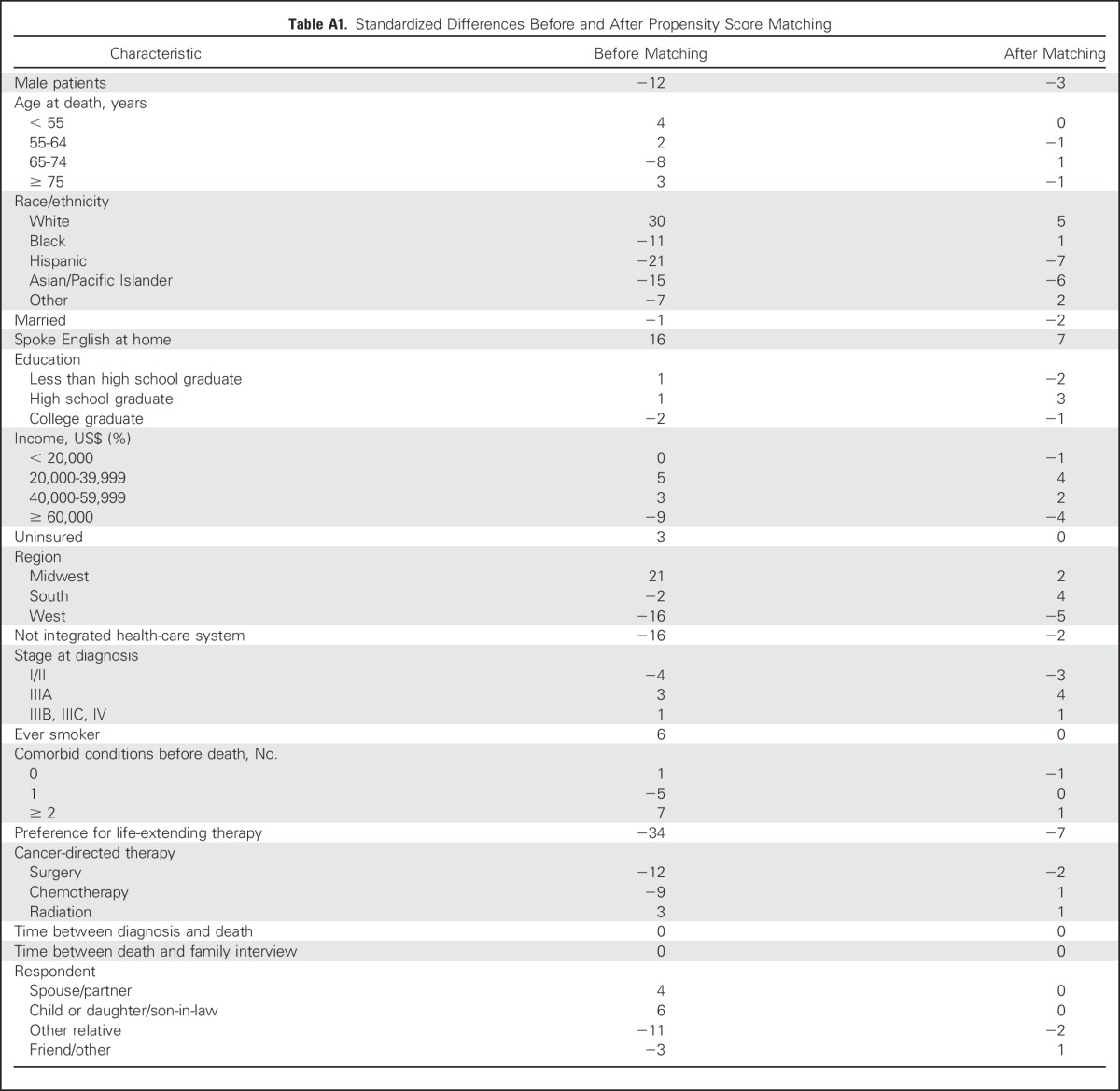
Table A2.
Participants Included in Study Cohort Versus Those Excluded, Based on Availability of Postdeath Family Interview
Footnotes
This work was supported by the National Institutes of Health, National Cancer Institute (NCI) Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium (Grants no. U01 CA093344 to Dana-Farber Cancer Institute; U01 CA093324 to Harvard Medical School and Northern California Cancer Center; U01 CA093332 to Dana-Farber Cancer Institute and Cancer Research Network; U01 CA093348 to RAND Corporation and University of California, Los Angeles; U01 CA093329 to University of Alabama at Birmingham; U01 CA093339 to University of Iowa; U01 CA093326 to University of North Carolina; and R01 CA1604021 to N.L.K.). The CanCORS II work was funded by Grant no. 5U01CA093344-08 from the NCI to the CanCORS Consortium Statistical Coordinating CenterA.A.W. is supported by Grant no. K07 CA166210 from the NCI. N.L.K. is also supported by Grant no. K24 CA181510 from the NCI.
Presented, in part, at the ASCO Palliative Care in Oncology Symposium, San Francisco, CA, September 9-10, 2015.
AUTHOR CONTRIBUTIONS
Conception and design: Pallavi Kumar, Alexi A.Wright, Jennifer S. Temel, Nancy L. Keating
Financial support: Nancy L. Keating
Administrative support: Nancy L. Keating
Provision of study materials or patients: Nancy L. Keating
Collection and assembly of data: Nancy L. Keating
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Family Perspectives on Hospice Care Experiences of Patients With Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Pallavi Kumar
No relationship to disclose
Alexi A. Wright
No relationship to disclose
Laura A. Hatfield
No relationship to disclose
Jennifer S. Temel
Research Funding: Pfizer (Inst)
Nancy L. Keating
No relationship to disclose
REFERENCES
- 1.Teunissen SC, Wesker W, Kruitwagen C, et al. Symptom prevalence in patients with incurable cancer: A systematic review. J Pain Symptom Manage. 2007;34:94–104. doi: 10.1016/j.jpainsymman.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for Medicare beneficiaries: Site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309:470–477. doi: 10.1001/jama.2012.207624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Society of Clinical Oncology The Quality Oncology Practice Initiative. 2013http://qopi.asco.org/program.html.
- 5.Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315:284–292. doi: 10.1001/jama.2015.18604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright AA, Keating NL, Balboni TA, et al. Place of death: Correlations with quality of life of patients with cancer and predictors of bereaved caregivers’ mental health. J Clin Oncol. 2010;28:4457–4464. doi: 10.1200/JCO.2009.26.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291:88–93. doi: 10.1001/jama.291.1.88. [DOI] [PubMed] [Google Scholar]
- 8.Obermeyer Z, Makar M, Abujaber S, et al. Association between the Medicare hospice benefit and health care utilization and costs for patients with poor-prognosis cancer. JAMA. 2014;312:1888–1896. doi: 10.1001/jama.2014.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ornstein KA, Aldridge MD, Garrido MM, et al. Association between hospice use and depressive symptoms in surviving spouses. JAMA Intern Med. 2015;175:1138–1146. doi: 10.1001/jamainternmed.2015.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teno JM, Casarett D, Spence C, et al. It is “too late” or is it? Bereaved family member perceptions of hospice referral when their family member was on hospice for seven days or less. J Pain Symptom Manage. 2012;43:732–738. doi: 10.1016/j.jpainsymman.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Rickerson E, Harrold J, Kapo J, et al. Timing of hospice referral and families’ perceptions of services: Are earlier hospice referrals better? J Am Geriatr Soc. 2005;53:819–823. doi: 10.1111/j.1532-5415.2005.53259.x. [DOI] [PubMed] [Google Scholar]
- 12.Schockett ER, Teno JM, Miller SC, et al. Late referral to hospice and bereaved family member perception of quality of end-of-life care. J Pain Symptom Manage. 2005;30:400–407. doi: 10.1016/j.jpainsymman.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Yamagishi A, Morita T, Kawagoe S, et al. Length of home hospice care, family-perceived timing of referrals, perceived quality of care, and quality of death and dying in terminally ill cancer patients who died at home. Support Care Cancer. 2015;23:491–499. doi: 10.1007/s00520-014-2397-7. [DOI] [PubMed] [Google Scholar]
- 14.Miller SC, Kinzbrunner B, Pettit P, et al. How does the timing of hospice referral influence hospice care in the last days of life? J Am Geriatr Soc. 2003;51:798–806. doi: 10.1046/j.1365-2389.2003.51253.x. [DOI] [PubMed] [Google Scholar]
- 15.Wright AA, Hatfield LA, Earle CC, et al. End-of-life care for older patients with ovarian cancer is intensive despite high rates of hospice use. J Clin Oncol. 2014;32:3534–3539. doi: 10.1200/JCO.2014.55.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: The Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22:2992–2996. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Catalano PJ, Ayanian JZ, Weeks JC, et al. Representativeness of participants in the cancer care outcomes research and surveillance consortium relative to the Surveillance, Epidemiology, and End Results program. Med Care. 2013;51:e9–e15. doi: 10.1097/MLR.0b013e318222a711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbaum PR RD. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–524. [Google Scholar]
- 19.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 20.Resche-Rigon M, White IR, Bartlett JW, et al. Multiple imputation for handling systematically missing confounders in meta-analysis of individual participant data. Stat Med. 2013;32:4890–4905. doi: 10.1002/sim.5894. [DOI] [PubMed] [Google Scholar]
- 21.He Y, Zaslavsky AM, Landrum MB, et al. Multiple imputation in a large-scale complex survey: A practical guide. Stat Methods Med Res. 2010;19:653–670. doi: 10.1177/0962280208101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teno JM, Freedman VA, Kasper JD, et al. Is care for the dying improving in the United States? J Palliat Med. 2015;18:662–666. doi: 10.1089/jpm.2015.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Quality Forum Cancer Endorsement Maintenance. 2011 http://www.qualityforum.org/Projects/Cancer_Endorsement_Maintenance_2011.aspx
- 24.Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer care near the end of life: Is it a quality-of-care issue? J Clin Oncol. 2008;26:3860–3866. doi: 10.1200/JCO.2007.15.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Hospice and Palliative Care Organization: NHPCO Facts and Figures: Hospice care in America. 2015 Edition. Alexandria, VA, National Hospice and Palliative Care Organization, 2015, p. 5 [Google Scholar]
- 26.Wright AA, Katz IT. Letting go of the rope--aggressive treatment, hospice care, and open access. N Engl J Med. 2007;357:324–327. doi: 10.1056/NEJMp078074. [DOI] [PubMed] [Google Scholar]
- 27.Casarett DJ, Fishman JM, Lu HL, et al. The terrible choice: Re-evaluating hospice eligibility criteria for cancer. J Clin Oncol. 2009;27:953–959. doi: 10.1200/JCO.2008.17.8079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greer JA, Pirl WF, Jackson VA, et al. Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J Clin Oncol. 2012;30:394–400. doi: 10.1200/JCO.2011.35.7996. [DOI] [PubMed] [Google Scholar]
- 29.Kelley AS, Ettner SL, Morrison RS, et al. Determinants of medical expenditures in the last 6 months of life. Ann Intern Med. 2011;154:235–242. doi: 10.7326/0003-4819-154-4-201102150-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obermeyer Z, Powers BW, Makar M, et al. Physician characteristics strongly predict patient enrollment in hospice. Health Aff (Millwood) 2015;34:993–1000. doi: 10.1377/hlthaff.2014.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen RC FA, Tian F, Basak R, et al: Aggressive care at the end-of-life for younger patients with cancer: Impact of ASCO’s Choosing Wisely campaign. J Clin Oncol 34, 2016 (suppl; abstr LBA10033) [Google Scholar]
- 32.O’Connor NR, Hu R, Harris PS, et al. Hospice admissions for cancer in the final days of life: Independent predictors and implications for quality measures. J Clin Oncol. 2014;32:3184–3189. doi: 10.1200/JCO.2014.55.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 34.Nipp RD BA, Rubin KM, Blackmon SM, et al: Palliative care and hospice use among melanoma patients treated with immunotherapy. J Clin Oncol 33, 2015 (suppl 29S; abstr 116) [Google Scholar]