Abstract
Purpose
Optimal assessment methods and criteria for reporting hearing outcomes in children who receive treatment with cisplatin are uncertain. The objectives of our study were to compare different ototoxicity classification systems, to evaluate the feasibility of including otoacoustic emissions and extended high frequency audiometry, and to evaluate a central review mechanism for audiologic results for cisplatin-treated children in the cooperative group setting.
Patients and Methods
Eligible participants were 1 to 30 years, with planned cisplatin-containing treatment. Hearing evaluations were conducted at baseline, before each cisplatin cycle, and at the end of therapy. Audiologic results were assessed and graded by the testing audiologist and by two central review audiologists using the American Speech-Language-Hearing Association Ototoxicity Criteria (ASHA), Common Terminology Criteria for Adverse Events, version 3.0 (CTCAE), and Brock Ototoxicity Grades (Brock). One central reviewer also used the International Society of Pediatric Oncology Ototoxicity Scale (SIOP).
Results
At the end of treatment, the prevalence of any degree of ototoxicity ranged from 40% to 56%, and severe ototoxicity ranged from 7% to 22%. Compared with CTCAE, SIOP detected significantly more ototoxicity (P = .004), whereas Brock criteria detected significantly fewer patients with any or severe ototoxicity (P < .001 for both). SIOP detected ototoxicity earlier than did the other scales. Agreement between the central reviewers and the institutional audiologist was almost perfect for ASHA and Brock, whereas the poorest agreement occurred with CTCAE.
Conclusion
The SIOP scale may be superior to ASHA, Brock, and CTCAE scales for classifying ototoxicity in pediatric patients who were treated with cisplatin. Future studies should evaluate inter-rater reliability of the SIOP scale.
INTRODUCTION
Cisplatin is a well-established chemotherapeutic agent that is used for several forms of childhood cancer, but its dose-limiting toxicity is hearing loss. Irreversible hearing loss occurs in approximately two thirds of children who are treated with cisplatin1 and is almost universal in specific subsets, such as young children with neuroblastoma who are treated with cisplatin and carboplatin.1-3 In children, cisplatin-induced ototoxicity has the potential to impact speech-language and social development, educatioxnal achievement, cognition, and quality of life.4,5
As survival has improved, strategies for mitigating or preventing the adverse effects of cancer therapy have assumed greater importance. As a result of differences in pediatric hearing assessment protocols,6,7 variability in hearing outcomes reporting,3,7,8 and differences in the mechanisms for collecting and reporting audiologic data in multicenter clinical trials,9,10 it is currently difficult to directly compare or pool ototoxicity data across studies. International standardization in the assessment and reporting of ototoxicity for pediatric patients with cancer would advance patient care and ototoxicity research.
Ototoxicity is typically monitored with serial audiometry, measured for frequencies 0.25 to 8 kHz. Extended high-frequency audiometry (EHF) and otoacoustic emissions (OAEs) are more sensitive measures of ototoxicity.11-14 EHF audiometry is the measurement of hearing thresholds at frequencies > 8 kHz. It detects ototoxicity earlier because ototoxic damage initially occurs at the base of the cochlea where high frequencies are encoded.15 OAEs provide an objective evaluation of cochlear outer hair cell function, and changes in OAEs may precede loss of hearing sensitivity.13,14
Children’s Oncology Group (COG) study ACCL05C1 was designed to inform future ototoxicity studies by identifying the optimal criteria for ototoxicity reporting, to evaluate the feasibility of more sensitive measures of ototoxicity, and to gain pilot experience with a central ototoxicity review mechanism prospectively among pediatric patients who were treated with cisplatin in a cooperative group setting. The specific objectives were to compare contemporaneous ototoxicity scales, evaluate the feasibility of including OAEs and EHF, and assess central review for audiologic results.
PATIENTS AND METHODS
This study was a multi-institutional, multinational COG prospective observational cohort study.
Study Participants
Participants were enrolled between May 2007 and February 2012. Eligibility criteria were 1 to 30 years of age at enrollment, planned treatment with any cisplatin-containing regimen, no prior history of cisplatin therapy, and, for patients enrolled after February 9, 2009, intent to offer enrollment into a companion clinical trial (ACCL0431) for which ACCL05C1 was the mechanism to collect hearing outcomes. ACCL0431 was a randomized trial that evaluated the efficacy of sodium thiosulfate for protection against cisplatin ototoxicity in pediatric patients.16 ACCL05C1 was approved by the National Cancer Institute’s Central Institutional Review Board and by each individual institutional review board at participating institutions. Informed consent or assent was obtained from participants and their guardians, as appropriate, before study entry.
Hearing Evaluation Procedures
A baseline audiologic evaluation was required before the first course of cisplatin, and monitoring evaluations were conducted within 1 week before each subsequent cisplatin course. An end-of-treatment evaluation was completed approximately 4 weeks after the final cisplatin treatment or 4 weeks after hematopoietic cell transplantation for patients who received the procedure.
Audiologic assessments included bilateral measurement of pure tone air conduction thresholds at frequencies of 0.5, 1, 2, 3, 4, 6, and 8 kHz; otoscopy; and middle ear immittance measurement with tympanometry. Audiometric methods included standard audiometry, conditioned play audiometry, or visual reinforcement audiometry, depending on the age and development of the child. Bone-conduction threshold measurement was indicated if air conduction thresholds at 0.5 to 4 kHz were > 20 dB hearing level (HL) or if otoscopy or tympanometry revealed conductive middle ear pathology. When audiometry was unreliable or unobtainable because of age or health status, the protocol recommended estimation of hearing thresholds with frequency-specific evoked auditory brainstem potentials, auditory brainstem response (ABR), or auditory steady state response (ASSR) at frequencies of 0.5 to 4 kHz and 8 kHz, if possible, when this testing was feasible and available.
To evaluate the feasibility of OAEs and EHF, institutions were asked to include these measures with each audiologic evaluation, if available. Distortion product evoked otoacoustic emissions (DPOAEs) or transient evoked otoacoustic emissions were obtained when middle ear function was normal, and EHF thresholds were requested for participants ≥ 5 years of age.17
Ototoxicity Classification Systems Evaluated
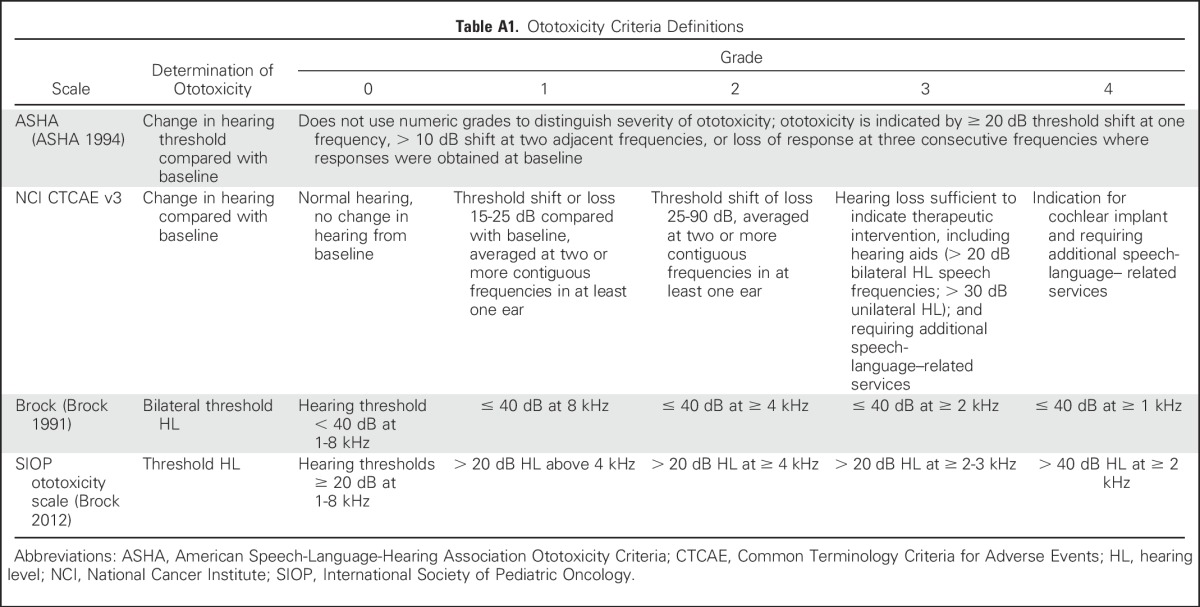
Four ototoxicity systems were evaluated, the American Speech-Language-Hearing Association Ototoxicity Criteria (ASHA),18 Common Terminology Criteria for Adverse Events (CTCAE) version 3.0,19 the Brock Criteria (Brock),20 and the International Society of Pediatric Oncology Ototoxicity Scale (SIOP).7 Specific definitions are listed in Appendix Table A1 (online only).
ASHA18 is a binary criterion (yes or no) that is designed for early ototoxicity detection. Ototoxicity is defined as a ≥ 20-dB decrease in pure tone threshold at one test frequency or a ≥ 10-dB decrease in pure tone threshold at two adjacent test frequencies. ASHA ototoxic change criteria exceeds test-retest variability and indicates a loss of hearing as a result of ototoxicity.
CTCAE19 grading is the standard approach for toxicity reporting in National Cancer Institute clinical trials. Ototoxicity is graded on an ordinal scale from 1 to 4, with 4 being the most severe. Grades 1 and 2 are based on change in hearing thresholds from baseline, and grades 3 and 4 relate to recommendations for hearing intervention. CTCAE version 3.0 was the current version at the time of study activation.
Brock20 was developed to compare hearing outcomes at the end of treatment in international clinical trials; a statistically significant relationship has been established between cumulative cisplatin dose and Brock grade.18 Ototoxicity is graded on an ordinal scale of 1 to 4, where 4 is the most severe. Grades are based on hearing threshold levels ≥ 40 dB HL, rather than a change in threshold compared with baseline.
SIOP7 was also developed to report hearing outcomes in international clinical trials for pediatric patients who were treated with platinum chemotherapy. Grading is based on hearing thresholds > 20 dB HL by using an ordinal scale of 1 to 4, where 4 is the most severe.
Procedures for Ototoxicity Determination
For each hearing evaluation, ototoxicity determination and grading was conducted by the testing audiologist and two central study audiologists (BB, KK). Raw audiologic data, including audiograms, tympanograms, OAE printouts, ABR waveforms, and the evaluation report, were faxed from the institutions to the COG Data Center and were then distributed to the study audiologists for independent central review. If the records indicated that specific tests were completed but the raw data were not submitted, missing data were requested from the institution. Central reviewers were blinded to the institutional audiologist’s assessment and to each other’s assessments. Any initial discrepancies between the two central reviews were discussed and resolved to achieve consensus. Because SIOP was added after completion of the study—as it was not available at the time of study development—it was only evaluated by one central reviewer (K.K.).
Audiograms were reviewed to determine if ototoxicity occurred according to ASHA and were graded for severity of hearing loss according to CTCAE, Brock, and SIOP. If middle ear pathology or conductive hearing loss was present, ototoxicity determination was based on bone conduction thresholds, and if bone conduction thresholds were not obtained, then the assessment was categorized as not evaluable. Ototoxicity for EHF was determined by using ASHA. When ABRs or ASSRs were measured, results were classified as normal or abnormal by the testing audiologist. If the ABR or ASSR was categorized as abnormal, ototoxic change in ABR and ASSR thresholds—relative to a previous ABR or ASSR—was determined according to ASHA. ABR and ASSR results and behavioral audiometric thresholds were not directly compared, and ototoxicity grading was not applied to ABR and ASSR results. OAEs were classified as abnormal if a loss of OAEs occurred at any frequency within the 2- to 8-kHz range when middle ear function was normal.
Comparison of Ototoxicity Systems
Two approaches were used to compare the four different ototoxicity systems. First, within each patient, the earliest date of detection of ototoxicity was determined for each of the four measures (ASHA, CTCAE, Brock, and SIOP). These dates were ranked, with rank 1 for the earliest and rank 4 the latest. If more than one ototoxicity measure was met at the same time point, both were given a score that represented the mean of the corresponding ranks. For example, if three scales were second in detecting ototoxicity, each received a score of 3, which was the average of ranks 2 to 4. If none of the measures met ototoxicity criteria at any time point on study, the date was arbitrarily set as September 30, 2015, later than any study evaluation date. Rank scores among all patients were summarized for each measure; thus, a lower rank score indicates earlier detection of ototoxicity by that measure. Because not every audiogram was evaluable by all four scales, the rank score reflected a combination of sensitivity and feasibility.
Second, we reviewed false-positive rates for each ototoxicity system. A false positive was defined as identification of ototoxicity at one time point and normal hearing or no ototoxicity on a subsequent evaluation. If more than one false positive occurred in the same patient, each instance was counted. The last hearing assessment could never be designated a false positive as there would be no later assessment to confirm or change the assignment of ototoxicity.
Statistical Analysis
Descriptive statistics summarized patient characteristics, the number of evaluable assessments, and the prevalence of ototoxicity and severe ototoxicity (grades 3 or 4) by the different ototoxicity measures at the end of therapy and among all time points. McNemar’s test was used to compare the frequency of ototoxicity or severe ototoxicity by two different measures at the end of therapy. Two-sided nonparametric binomial sign test was used to compare rank scores for initial detection of ototoxicity between two measures; the scale with the lowest average rank was used as reference and compared with each of the other three scales. Bonferroni adjustment for the six possible pairwise comparisons would require a comparison between two scales to have a P value of < .0083 to be considered statistically significant. Initial agreement between the two central reviewers and between the institutional review and consensus central review was examined by using the simple Kappa statistic for comparisons between two categories and the weighted Kappa when data included more than two categories. Any ototoxicity, ototoxicity grade (1 to 4), and ototoxicity severity (severe v none or mild) were compared.
Strength of agreement was defined as slight (0.00 to 0.20), fair (0.21 to 0.40), moderate (0.41 to 0.60), substantial (0.61 to 0.80), or almost perfect (0.81 to 1.00).21 All analyses were performed by using SAS (SAS/STAT User’s Guide, Version 9.3; SAS Institute, Cary, NC).
RESULTS
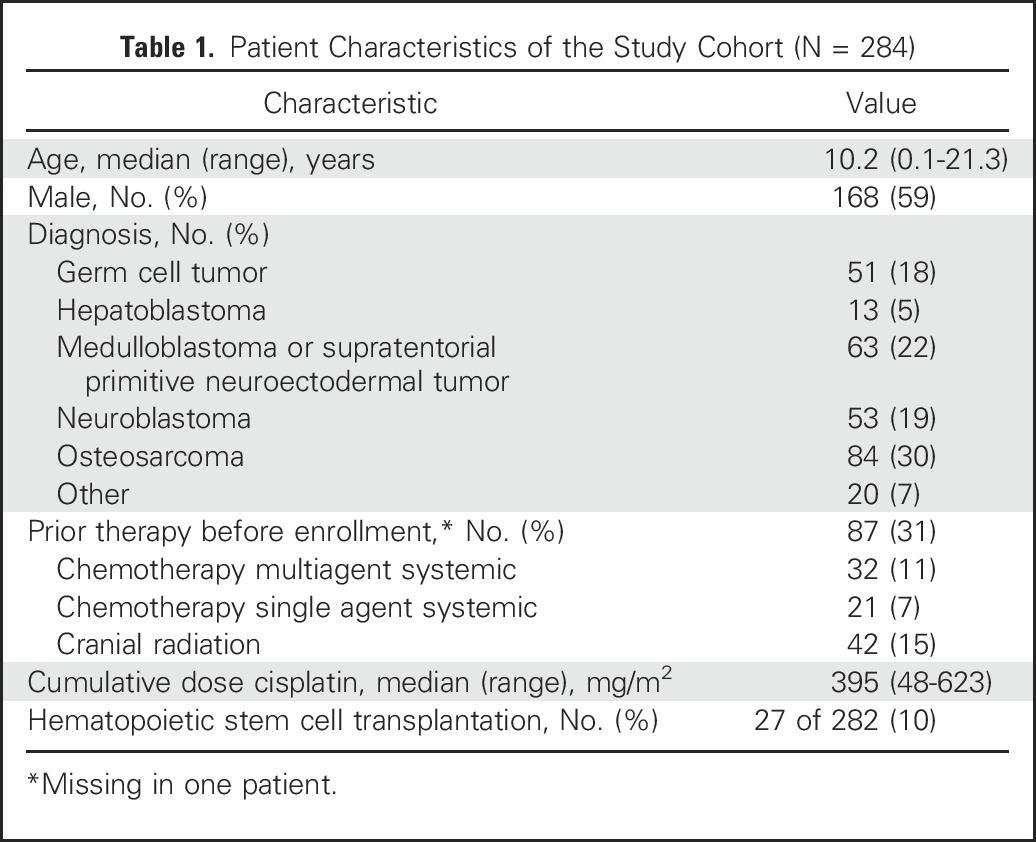
During the study period, 301 participants from 53 institutions enrolled, of whom 131 coenrolled in ACCL0431. There were 17 participants who were ineligible (prior receipt cisplatin [n = 1], enrolled after February 9, 2009, and not eligible for ACCL0431 [n = 16]), which left 284 eligible participants. Table 1 lists the demographic characteristics of the cohort. Median age was 10.2 years (range, 0.1 to 21.3 years) and the median cumulative dose of cisplatin was 395 mg/m2 (range, 48 to 623 mg/m2). There were 27 patients who underwent hematopoietic stem cell transplantation.
Table 1.
Patient Characteristics of the Study Cohort (N = 284)
A total of 1,436 audiologic evaluations were reviewed. Hearing assessment methods and the number of evaluations that included OAEs and EHF are listed in Table 2. Central review for ototoxicity was not possible for 54 evaluations (4%) as a result of missing test data.
Table 2.
Hearing Assessment Methods
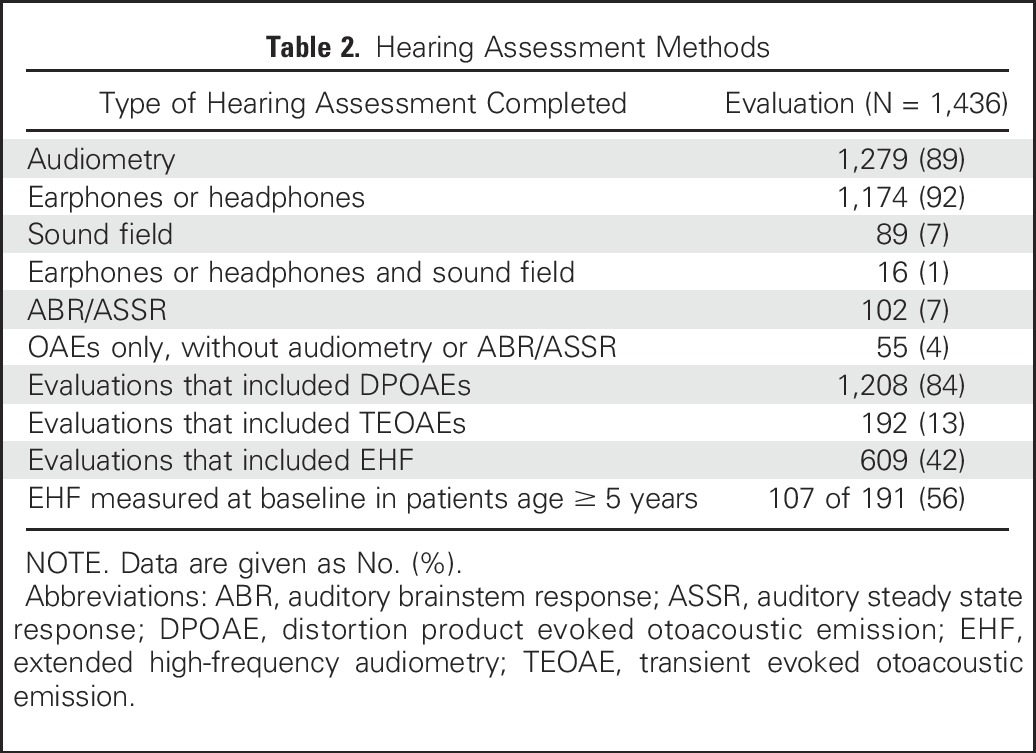
Table 3 lists the prevalence of ototoxicity and severe ototoxicity at the end of treatment by the four ototoxicity systems. A discordant pair occurred in the comparison of two systems when ototoxicity was classified by one system and not the other. Compared with CTCAE, SIOP detected significantly more ototoxicity (11 v 1 discordant pairs; P = .004), whereas Brock criteria detected significantly fewer patients with any ototoxicity (0 v 19 discordant pairs) or severe ototoxicity (0 v 22 discordant pairs; P < .001 for both). In 19 patients who had ABR or ASSR at the end of treatment, ototoxicity occurred in eight and could not be determined in two patients due to lack of a prior comparison. Ototoxicity in EHF thresholds occurred in 69 (68%) of 101 patients and 25 patients (25%) had ototoxicity in EHF range but not in the conventional frequencies. DPOAEs were categorized as abnormal in 120 (60%) of 201 patients at the end of therapy.
Table 3.
Incidence of Any Grade Ototoxicity and Severe Ototoxicity at the End of Treatment by Grading Criteria by Central Review
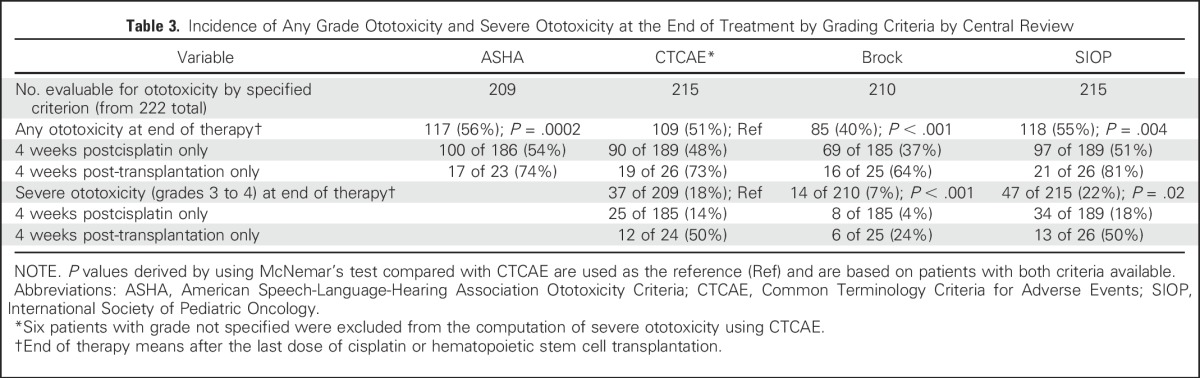
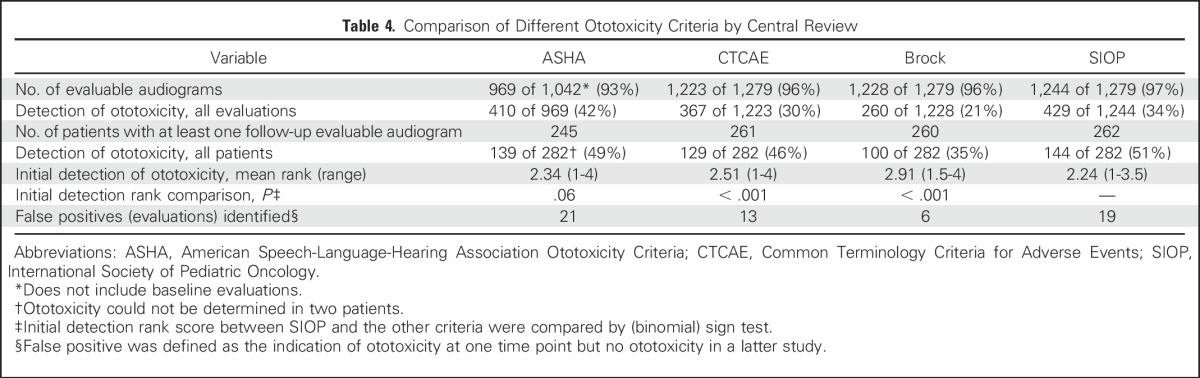
Table 4 lists the number of assessments that indicated ototoxicity for all audiometric evaluations combined. The number of evaluable audiograms was comparable between CTCAE, Brock, and SIOP, with slightly fewer by ASHA. Fewer patients had at least one audiogram that was evaluable by ASHA compared with the other scales. When evaluating time to detection of ototoxicity, on average, SIOP detected ototoxicity the earliest with the lowest mean rank score of 2.24, followed by ASHA, CTCAE, and Brock. Brock never detected ototoxicity before SIOP, ASHA, or CTCAE. Table 4 also illustrates that false positives were highest for ASHA and SIOP and lowest for Brock.
Table 4.
Comparison of Different Ototoxicity Criteria by Central Review
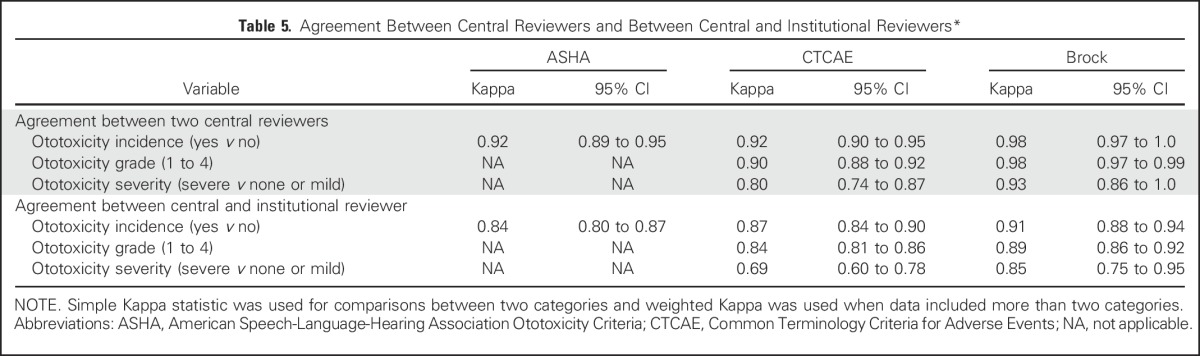
Agreement in the designation of ototoxicity and ototoxicity grade for all evaluations is shown in Table 5. Agreement between the two central reviewers was almost perfect. Agreement between the consensus central review and institutional audiologist was almost perfect for ASHA and Brock but was worst for CTCAE.
Table 5.
Agreement Between Central Reviewers and Between Central and Institutional Reviewers*
DISCUSSION
In this prospective, multi-institutional, multinational clinical trial among a large cohort of cisplatin-treated children and adolescents, variability of ototoxicity (40% to 56%) and severe ototoxicity (7% to 22%) reported by the different approaches was substantial. The current lack of an international standard for ototoxicity reporting prevents comparison of results within and across diseases and studies.
We found that SIOP might be the optimal criteria on the basis of the high number of evaluable assessments, sensitivity, and earliest time to detection of ototoxicity. ASHA had the lowest number of evaluable assessments, as it requires comparison with baseline and does not use a severity grading scale. Brock had the lowest false-positive rate and the highest inter-rater agreement; however, the scale identified ototoxicity in fewer patients, at a later time in treatment, and reported significantly fewer patients as having any ototoxicity and severe ototoxicity. Because Brock does not capture ototoxicity until hearing thresholds are ≤ 40 dB HL, it does not detect mild hearing loss that is communicatively and educationally important for developing children and adolescents.22,23 CTCAE was not the optimal measure by any evaluation and had the worst agreement between local and central audiologists.
In previous pediatric multicenter clinical studies, approximately 30% of hearing assessments were not evaluable for ototoxicity as a result of incomplete or missing test results or lack of a frequency-specific measurement.3,9,10 In contrast, only 4% of audiologic evaluations were missing data for central review and 3% of end-of-treatment audiograms were not evaluable for ototoxicity in this study. Our favorable results may have occurred because central review was completed soon after audiology results were submitted by the institution to COG and any missing test data were requested in real time. In addition, as the testing audiologist was asked to grade the results, he or she was aware of the information needed for ototoxicity grading.
There have been concerns about the reliability of institutional ototoxicity reporting. In a study of 120 children who were treated for hepatoblastoma, prevalence of CTCAE grade 3 and 4 ototoxicity was 4% by institutional reporting compared with 38% by central auditory specialist review.9 Having institutional audiologists review and report ototoxicity may have overcome these challenges as there was substantial to almost perfect agreement between institutional review and central review in our study. However, in light of its feasibility in the cooperative group setting demonstrated here, we believe central audiology review should be used in future clinical trials in which ototoxicity is a primary end point because it ensures consistency in the analysis of outcomes. This is important in the pediatric setting when test results may be incomplete or confounded by conductive middle ear pathology. In addition, collection of raw audiology data allows rescoring of ototoxicity by alternate approaches that might be developed in the future, as occurred in our study with SIOP.
OAEs were included in 84% of evaluations and are likely feasible for ototoxicity monitoring in future COG clinical trials; however, OAEs cannot estimate hearing thresholds, and, at this time, there are no accepted criteria for ototoxic change or grading of OAEs. Consistent with other studies, EHF was more sensitive to ototoxicity than was conventional audiometry12,24; however, it may not be feasible to implement in COG group-wide trials as it was only obtained in 56% of participants who were ≥ 5 years of age. The most common reason cited by institutional audiologists for not including EHF was lack of EHF instrumentation.
ASHA and SIOP had the highest rates of false positives, as defined by this study. Although we considered reversals in ototoxicity designation as false positives, it is possible that these changes could reflect a process of fluctuation in hearing levels with recovery during ototoxic treatment. Truong et al25 reported fluctuating tinnitus and hearing loss with accompanying changes in DPOAEs in a patient age 16 years during cisplatin chemotherapy. They hypothesized that in some dosing regimens, early acute ototoxicity may damage strial cells and supporting cells within the cochlea that have the potential to recover, whereas damage to outer hair cells results in permanent hearing loss.
The strengths of our report are the large number and diversity of children and adolescents included and the number of participating institutions, which improve the generalizability of our findings. Other strengths are the use of two independent central audiology reviewers as well as novel approaches to compare ototoxicity systems; however, our results must be interpreted in light of the limitations of the study. First, because SIOP was developed after initiation of this trial, inter-rater reliability of this approach could not be evaluated, although given the excellent agreement in ototoxicity designations between the two central reviewers, we do not anticipate that this absence would have affected our conclusions. Second, as there is not a gold standard measure of ototoxicity, one cannot calculate specificity or sensitivity. Third, another recently developed ototoxicity criteria, the Chang Criteria,6 was not evaluated in this study.
In conclusion, SIOP may be superior to ASHA, Brock, and CTCAE scales for classifying ototoxicity in pediatric patients who are treated with cisplatin. Future studies should evaluate inter-rater reliability of the SIOP scale.
ACKNOWLEDGMENT
We thank Beth Hausenauer for technical assistance and expertise. Presented at the 2015 Cisplatin Chemoprotection Conference, Stevenson, WA, and the 2015 Children’s Oncology Group Fall Meeting, Dallas, TX, October 6-9, 2015.
Appendix
Table A1.
Ototoxicity Criteria Definitions
Footnotes
Supported by the Community Clinical Oncology Program Research Base Grant No. U10-CA095861, NCI Community Oncology Research Program Research Base Grant No. UG1-CA189955, and the National Clinical Trials Network Statistics and Data Center Award Grant No. U10-CA180899.
The views expressed in this paper are the authors’ own and not an official position of the institutions or funder. Oregon Health and Science University (OHSU), Portland Veterans Affairs Medical Center (PVAMC), and the Department of Veterans Affairs have a significant financial interest in Fennec, a company that may have a commercial interest in the results of this research and technology. E.A.N., inventor of technology licensed to Fennec, has divested himself of all potential earnings. These potential conflicts of interest were reviewed and managed by the OHSU Integrity Program Oversight Council and the OHSU and PVAMC Conflict of Interest in Research Committees.
AUTHOR CONTRIBUTIONS
Conception and design: Kristin R. Knight, David Freyer, Richard Aplenc, Bonnie Bliss, Eleanor Hendershot, Dale F. Kraemer, Jane Meza, Edward A. Neuwelt, Brad H. Pollock, Lillian Sung
Financial support: Edward A. Neuwelt, Lillian Sung
Administrative support: Lanie Lindenfeld, Edward A. Neuwelt, Lillian Sung
Provision of study materials or patients: Edward A. Neuwelt, Lillian Sung
Collection and assembly of data: Kristin R. Knight, Mary Bancroft, Bonnie Bliss, Biljana Gillmeister, Lanie Lindenfeld, Jane Meza, Edward A. Neuwelt
Data analysis and interpretation: Kristin R. Knight, Lu Chen, David Freyer, Bonnie Bliss, Ha Dang, Dale F. Kraemer, Edward A. Neuwelt, Brad H. Pollock, Lillian Sung
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Group-Wide, Prospective Study of Ototoxicity Assessment in Children Receiving Cisplatin Chemotherapy (ACCL05C1): A Report From the Children’s Oncology Group
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Kristin R. Knight
No relationship to disclose
Lu Chen
No relationship to disclose
David Freyer
No relationship to disclose
Richard Aplenc
Honoraria: Sigma-Tau
Expert Testimony: Dana Wiggins
Travel, Accommodations, Expenses: Sigma-Tau
Mary Bancroft
No relationship to disclose
Bonnie Bliss
No relationship to disclose
Ha Dang
No relationship to disclose
Biljana Gillmeister
No relationship to disclose
Eleanor Hendershot
No relationship to disclose
Dale F. Kraemer
Employment: 21st Century Oncology (I)
Lanie Lindenfeld
No relationship to disclose
Jane Meza
No relationship to disclose
Edward A. Neuwelt
Other Relationship: Fennec Pharmaceuticals
Brad H. Pollock
Consulting or Advisory Role: Astellas Pharma
Lillian Sung
No relationship to disclose
REFERENCES
- 1.Punnett A, Bliss B, Dupuis LL, et al. Ototoxicity following pediatric hematopoietic stem cell transplantation: A prospective cohort study. Pediatr Blood Cancer. 2004;42:598–603. doi: 10.1002/pbc.20036. [DOI] [PubMed] [Google Scholar]
- 2.Parsons SK, Neault MW, Lehmann LE, et al. Severe ototoxicity following carboplatin-containing conditioning regimen for autologous marrow transplantation for neuroblastoma. Bone Marrow Transplant. 1998;22:669–674. doi: 10.1038/sj.bmt.1701391. [DOI] [PubMed] [Google Scholar]
- 3.Landier W, Knight K, Wong FL, et al. Ototoxicity in children with high-risk neuroblastoma: Prevalence, risk factors, and concordance of grading scales—A report from the Children’s Oncology Group. J Clin Oncol. 2014;32:527–534. doi: 10.1200/JCO.2013.51.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurney JG, Tersak JM, Ness KK, et al. Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: A report from the Children’s Oncology Group. Pediatrics. 2007;120:e1229–e1236. doi: 10.1542/peds.2007-0178. [DOI] [PubMed] [Google Scholar]
- 5.Schreiber JE, Gurney JG, Palmer SL, et al. Examination of risk factors for intellectual and academic outcomes following treatment for pediatric medulloblastoma. Neuro-oncol. 2014;16:1129–1136. doi: 10.1093/neuonc/nou006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang KW, Chinosornvatana N. Practical grading system for evaluating cisplatin ototoxicity in children. J Clin Oncol. 2010;28:1788–1795. doi: 10.1200/JCO.2009.24.4228. [DOI] [PubMed] [Google Scholar]
- 7.Brock PR, Knight KR, Freyer DR, et al. Platinum-induced ototoxicity in children: A consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol. 2012;30:2408–2417. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: Underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23:8588–8596. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- 9.Katzenstein HM, Chang KW, Krailo M, et al. Amifostine does not prevent platinum-induced hearing loss associated with the treatment of children with hepatoblastoma: A report of the Intergroup Hepatoblastoma Study P9645 as a part of the Children’s Oncology Group. Cancer. 2009;115:5828–5835. doi: 10.1002/cncr.24667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang KW. Clinically accurate assessment and grading of ototoxicity. Laryngoscope. 2011;121:2649–2657. doi: 10.1002/lary.22376. [DOI] [PubMed] [Google Scholar]
- 11.Coradini PP, Cigana L, Selistre SG, et al. Ototoxicity from cisplatin therapy in childhood cancer. J Pediatr Hematol Oncol. 2007;29:355–360. doi: 10.1097/MPH.0b013e318059c220. [DOI] [PubMed] [Google Scholar]
- 12.Knight KR, Kraemer DF, Winter C, et al. Early changes in auditory function as a result of platinum chemotherapy: Use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. J Clin Oncol. 2007;25:1190–1195. doi: 10.1200/JCO.2006.07.9723. [DOI] [PubMed] [Google Scholar]
- 13.Littman TA, Magruder A, Strother DR. Monitoring and predicting ototoxic damage using distortion-product otoacoustic emissions: Pediatric case study. J Am Acad Audiol. 1998;9:257–262. [PubMed] [Google Scholar]
- 14.Ress BD, Sridhar KS, Balkany TJ, et al. Effects of cis-platinum chemotherapy on otoacoustic emissions: The development of an objective screening protocol. Third place--Resident Clinical Science Award 1998. Otolaryngol Head Neck Surg. 1999;121:693–701. doi: 10.1053/hn.1999.v121.a101567. [DOI] [PubMed] [Google Scholar]
- 15.Blakley BW, Myers SF. Patterns of hearing loss resulting from cis-platinum therapy. Otolaryngol Head Neck Surg. 1993;109:385–391. doi: 10.1177/019459989310900302. [DOI] [PubMed] [Google Scholar]
- 16.Freyer DR. The effects of sodium thiosulfate (STS) on cisplatin-induced hearing loss: A report from the Children’s Oncology Group. J Clin Oncol. 2014;32:5s. (abstr 10017) [Google Scholar]
- 17.Beahan N, Kei J, Driscoll C, et al. High-frequency pure-tone audiometry in children: A test-retest reliability study relative to ototoxic criteria. Ear Hear. 2012;33:104–111. doi: 10.1097/AUD.0b013e318228a77d. [DOI] [PubMed] [Google Scholar]
- 18.American Speech-Language-Hearing Association Guidelines for the audiologic management of individuals receiving cochleotoxic drug therapy. Rockville, MD, ASHA, 1994, pp 11-19 (suppl 12)
- 19.National Cancer Institute . Common terminology criteria for adverse events v3.0 (CTCAE) https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brock PR, Bellman SC, Yeomans EC, et al. Cisplatin ototoxicity in children: A practical grading system. Med Pediatr Oncol. 1991;19:295–300. doi: 10.1002/mpo.2950190415. [DOI] [PubMed] [Google Scholar]
- 21.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 22.Wake M, Tobin S, Cone-Wesson B, et al. Slight/mild sensorineural hearing loss in children. Pediatrics. 2006;118:1842–1851. doi: 10.1542/peds.2005-3168. [DOI] [PubMed] [Google Scholar]
- 23.Lewis DE, Valente DL, Spalding JL. Effect of minimal/mild hearing loss on children’s speech understanding in a simulated classroom. Ear Hear. 2015;36:136–144. doi: 10.1097/AUD.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abujamra AL, Escosteguy JR, Dall’Igna C, et al. The use of high-frequency audiometry increases the diagnosis of asymptomatic hearing loss in pediatric patients treated with cisplatin-based chemotherapy. Pediatr Blood Cancer. 2013;60:474–478. doi: 10.1002/pbc.24236. [DOI] [PubMed] [Google Scholar]
- 25.Truong MT, Winzelberg J, Chang KW. Recovery from cisplatin-induced ototoxicity: A case report and review. Int J Pediatr Otorhinolaryngol. 2007;71:1631–1638. doi: 10.1016/j.ijporl.2007.06.021. [DOI] [PubMed] [Google Scholar]


