Abstract
Purpose
Despite increasing awareness of accrual challenges, it is unknown if accrual of older patients to breast cancer treatment trials is improving.
Methods
We examined accrual of older patients to Alliance for Clinical Trials in Oncology systemic therapy breast cancer trials during 1985-2012 and compared disease characteristics and reasons for therapy cessation for older (age ≥ 65 years and ≥ 70 years) versus younger (age < 65 years and < 70 years) participants. To examine accrual trends, we modeled age as a function of time, using logistic regression.
Results
Overall, 17% of study participants were ≥ 65 years of age. Approximately 15%, 24%, and 24% of participants in adjuvant, neoadjuvant, and metastatic trials were age ≥ 65 years, and 7%, 15%, and 13% were age ≥ 70 years, respectively. The odds of a patient age ≥ 65 years enrolling significantly increased over time for adjuvant trials (odds ratio [OR] per year, 1.04; 95% CI, 1.04 to 1.05) but decreased significantly for neoadjuvant and metastatic trials (OR, 0.62; 95% CI, 0.58 to 0.67 and OR, 0.98, 95% CI, 0.97 to 1.00). Similar trends were seen for those age ≥ 70 years but these were statistically significant for adjuvant and neoadjuvant trials only (OR, 1.05, 95% CI, 1.04 to 1.07; and OR, 0.57, 95% CI, 0.52 to 0.62). In general, those age ≥ 65 years (v those < 65 years) in adjuvant studies had a higher mean number of lymph nodes involved and more hormone receptor-negative tumors, although tumor sizes were similar. Early protocol treatment cessation was also more frequent in those age ≥ 65 years (50%) versus < 65 years (35.9%) across trials.
Conclusion
Older patients with breast cancer remain largely underrepresented in cooperative group therapeutic trials. We observed some improvement in accrual to adjuvant trials but worsening of accrual for neoadjuvant/metastatic trials. Novel strategies to increase accrual of older patients are critical to meaningfully change the evidence base for this growing patient population.
INTRODUCTION
Breast cancer is a disease of aging and nearly 72,000 breast cancers occur annually in US women ≥ 70 years of age.1,2 Although most breast cancers in older women are lower-risk tumors and most older patients with breast cancer die of other causes,3-5 approximately 19,000 breast cancer deaths occur yearly in US women ≥ 70 years of age, accounting for 47% of breast cancer deaths.6
Although most cancers occur in older patients, accrual of older patients to cancer clinical trials has been a persistent challenge. Consequently, the availability of prospective data for older patients with breast cancer is limited. Although some evidence suggests that older patients are just as likely to enroll in clinical trials as younger patients if one is offered,7 multiple barriers to accrual have been identified, including comorbidity, physician/patient preferences, socioeconomic factors, insurance, concerns about loss of continuity with primary oncologists, lack of knowledge about clinical trials, and age itself.7-18 In addition, others have cited time and travel considerations and trial phase as barriers for all patients.16,19 Thus far, specific efforts to improve enrollment of older patients with cancer to clinical trials within the cooperative group setting have included educational interventions,20 focused committees, policy statements,21-23 and the development of a limited number of trials dedicated to older patients.24-26 Additional attempts to improve accrual across all ages have examined strategies to improve the consent process and the methods for opting in/out of enrollment.27-29
Because of keen awareness of accrual challenges for older patients, designing clinical trials specific to older patients with cancer has been a priority.22,23,30 We have seen some successes with Cancer and Leukemia Group B (CALGB) trials 9343 and 49907,24-26 though there is concern that we continue to struggle with enrollment of older patients.22 The Alliance for Clinical Trials in Oncology (“Alliance”; formed by the merger of legacy groups CALGB, American College of Surgeons Oncology Group [ACOSOG], and North Central Cancer Treatment Group [NCCTG]) has a national therapeutic protocol program in breast cancer31 and has completed many practice-changing, large-scale systemic trials to date. However, it is not known if accrual of older patients to these trials has improved. In this retrospective analysis (A151527), we examined whether enrollment of older patients with breast cancer to Alliance systemic therapy clinical trials has improved over time. We also examined disease characteristics and the reasons for study treatment cessation for those age ≥ 65 years and ≥ 70 years versus younger patients.
METHODS
Data Source
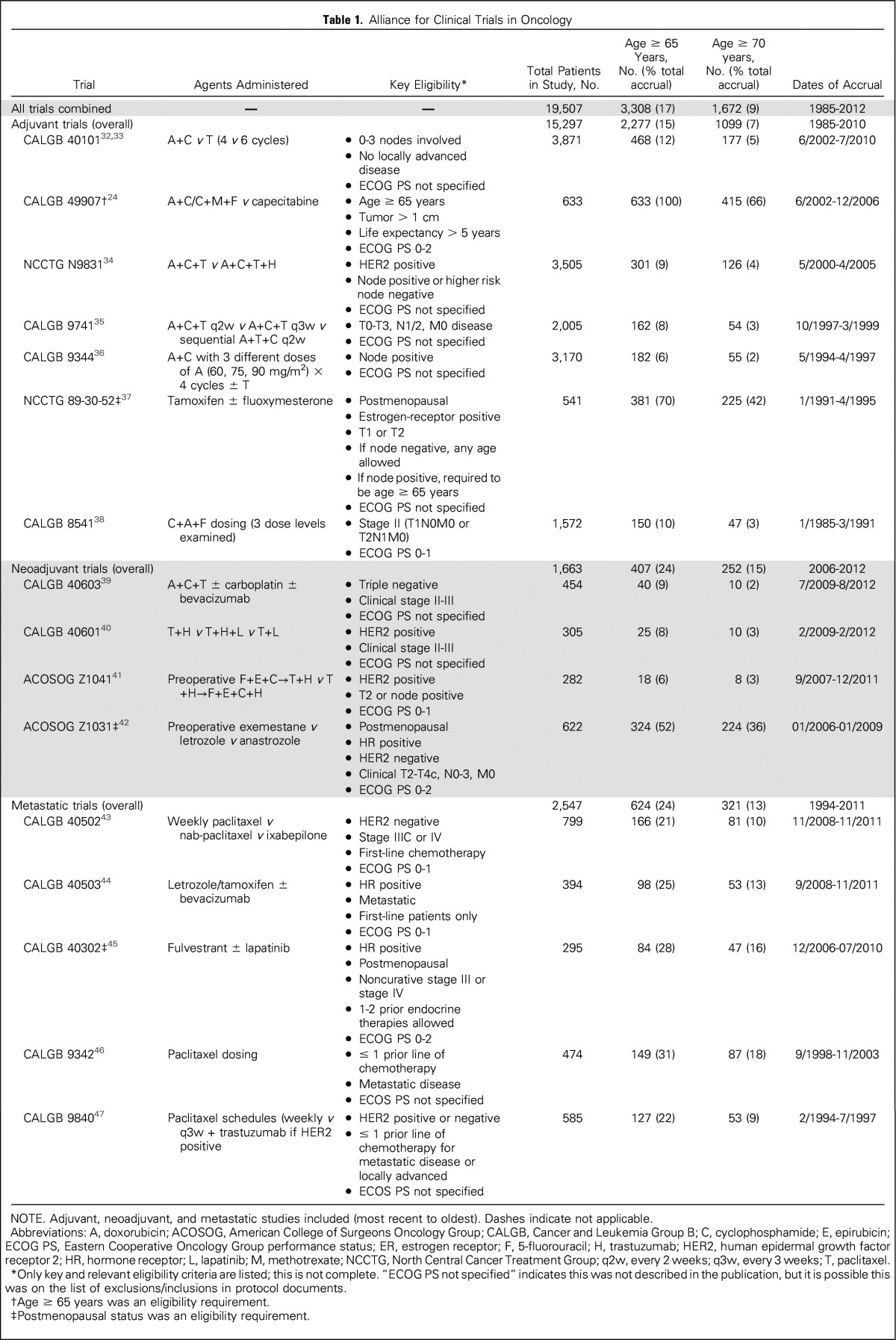
We reviewed the Alliance portfolio of accrued systemic therapy trials in neoadjuvant, adjuvant, and metastatic breast cancer therapeutic settings during 1985-2012. Because of specific concerns about the lack of enrollment of older patients in systemic treatment trials in particular, we focused our analysis on therapeutic systemic trials only and did not include local or supportive therapy protocols. Table 1 lists the trials included.
Table 1.
Alliance for Clinical Trials in Oncology
Variables of Interest
Our primary end point was the proportion of older patients enrolled in studies over time, using the date of protocol registration for each patient. We determined whether the probability of an older patient enrolling in a study changed significantly over time. We examined both the overall time trend for all studies and the time trends specific to trial type (separately) for adjuvant, neoadjuvant, and metastatic trials. Our secondary goal was to better understand whether disease characteristics for trial participants differed by age by examining tumor size, number of nodes involved, hormone receptor status, and human epidermal growth factor 2 (HER2) receptor status (when available) for older versus younger women in each adjuvant study. We limited comparisons of disease characteristics to adjuvant trials because neoadjuvant and metastatic trials primarily enrolled patients with homogeneous staging and tumor subtypes. When available, we also examined baseline Eastern Cooperative Oncology Group (ECOG) performance status (PS)48 and reasons for protocol therapy cessation by age.
Statistical Analysis
To determine whether the proportion of older patients enrolling in trials changed over time, we modeled age as a function of time, using logistic regression, both for age ≥ 65 years (v < 65 years) and age ≥ 70 years (v < 70 years). This was done separately for adjuvant, neoadjuvant, and metastatic trials, using data pooled across all available studies for each trial setting. Of note, CALGB 49907, NCCTG 89-30-52, and ACOSOG Z1031 primarily targeted postmenopausal and/or older women, enrolling patients age ≥ 65 years at rates of 100%, 70%, and 52%, respectively. Therefore, in a sensitivity analysis, we repeated analyses with these trials excluded.
To assess the degree to which disease characteristics differed with age for each adjuvant study, we compared subtype (estrogen receptor [ER], progesterone receptor [PR], and HER2 receptor status), tumor size, and the number of nodes involved for patients (when data were available) in each adjuvant study, using χ2 tests for comparisons of receptor status and tumor size and paired t tests for comparison of nodes. We also examined differences in ECOG PS and reasons for protocol therapy cessation (overall and for each trial type separately) by age, using χ2 tests.
Because this study used preexisting data, we received exemption from the Dana-Farber Cancer Institute Office for Human Research Studies for these analyses. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center.
RESULTS
Accrual Over Time
We included 16 Alliance protocols, which enrolled 19,507 patients. The proportion of patients age ≥ 65 years and ≥ 70 years enrolled in each study, agents administered, key eligibility, and dates of accrual are listed in Table 1. No trial had an upper limit for age as part of eligibility requirements. Overall, 3,308 (17%) of the 19,507 trial participants registered during 1985-2012 were age ≥ 65 years and 1,672 (9%) were age ≥ 70 years.
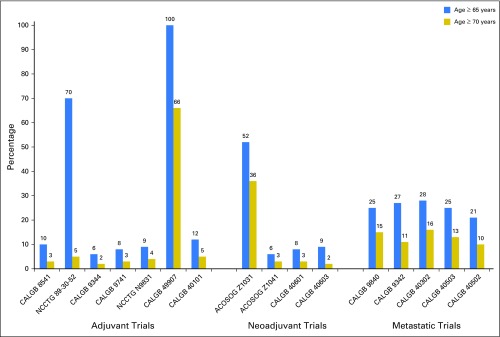
Among the 15,297 women who enrolled in adjuvant trials, 15% were age ≥ 65 years and 7% were ≥ 70 years. For the 1,663 women enrolled in neoadjuvant studies, 24% were age ≥ 65 years and 15% of participants were ≥ 70 years. Of the 2,547 participants in metastatic protocols, 24% were age ≥ 65 years and 13% were ≥ 70 years. Figure 1 displays the proportion of older women enrolled by trial. Distinct peaks in enrollment for older patients are visible for CALGB 49907 (adjuvant chemotherapy for patients age ≥ 65 years), NCCTG 89-30-52, and ACOSOG Z1031 (hormonal therapy-based trials for postmenopausal women).
Fig 1.
Proportions of patients age ≥ 65 years and ≥ 70 years enrolled in each clinical trial. ACOSOG, American College of Surgeons Oncology Group; CALGB, Cancer and Leukemia Group B; NCCTG, North Central Cancer Treatment Group.
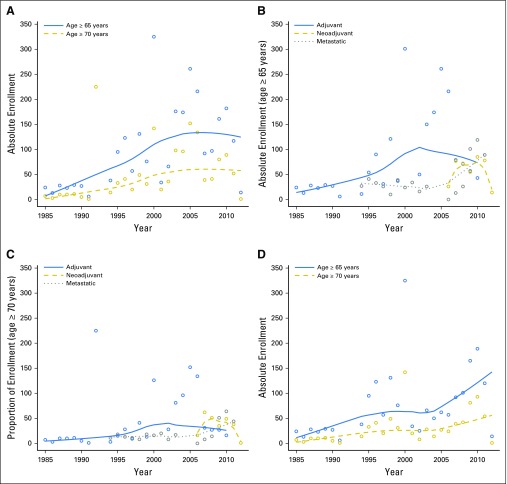
The absolute numbers of patients age ≥ 65 years and age ≥ 70 years increased somewhat over the study period (Appendix Fig A1, online only), although this was not consistent over time. For example, the years with the lowest absolute numbers of enrolled patients age ≥ 65 years were 1991 (n = 7), 2012 (n = 15), and 1985 (n = 31), whereas the highest numbers of enrolled older patients occurred during 2000 (n = 467), 2010 (n = 282), and 2009 (n = 246).
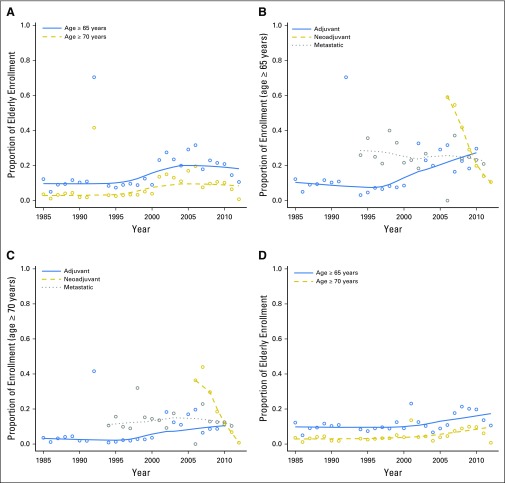
Figure 2 displays the trends in overall accrual over time by age (Fig 2A), trends in accrual by trial type for age ≥ 65 years (Fig 2B) and ≥ 70 years (Fig 2C), and trends by ages ≥ 65 years and ≥ 70 years after excluding CALGB 49907/NCCTG 89-30-52/ACOSOG Z1031 (Fig 2D). To further illustrate potential trends in each of these plots, we included locally weighted scatterplot smoothing lines (a type of nonparametric regression).
Fig 2.
Unadjusted proportions of patients age ≥ 65 years and ≥ 70 years enrolled in all trials (A), by trial type for age ≥ 65 years and ≥ 70 years (B, C), and after exclusion of Cancer and Leukemia Group B (CALGB) 49907, North Central Cancer Treatment Group (NCCTG) 89-30-52, and American College of Surgeons Oncology Group (ACOSOG) Z1031 (D).
Results from our logistic regression model indicate that the odds of a patient age ≥ 65 years enrolling slightly increased with each year in adjuvant trials, with an odds ratio (OR) for enrollment of 1.04 per year (95% CI, 1.04 to 1.05; P < .001). Thus, over 5 years, this translates into a 20% increase in the odds of enrolling patients age ≥ 65 years. In contrast, the odds of a patient age ≥ 65 years enrolling decreased significantly by year in neoadjuvant and metastatic trials (OR, 0.62; 95% CI, 0.58 to 0.67; P < .001; and OR, 0.98, 95% CI, 0.97 to 1.00, P = .03, respectively). Similar trends were seen for patients age ≥ 70 years, but these were statistically significant for adjuvant and neoadjuvant trials only (OR, 1.05; 95% CI, 1.04 to 1.07; and OR, 0.57; 95% CI, 0.52 to 0.62, respectively). After exclusion of patients enrolled in CALGB 49907/NCCTG 89-30-52/ACOSOG Z1031, results were unchanged for adjuvant and metastatic trials, but no significant time trend was found for neoadjuvant trials.
Disease Characteristics and ECOG PS by Age
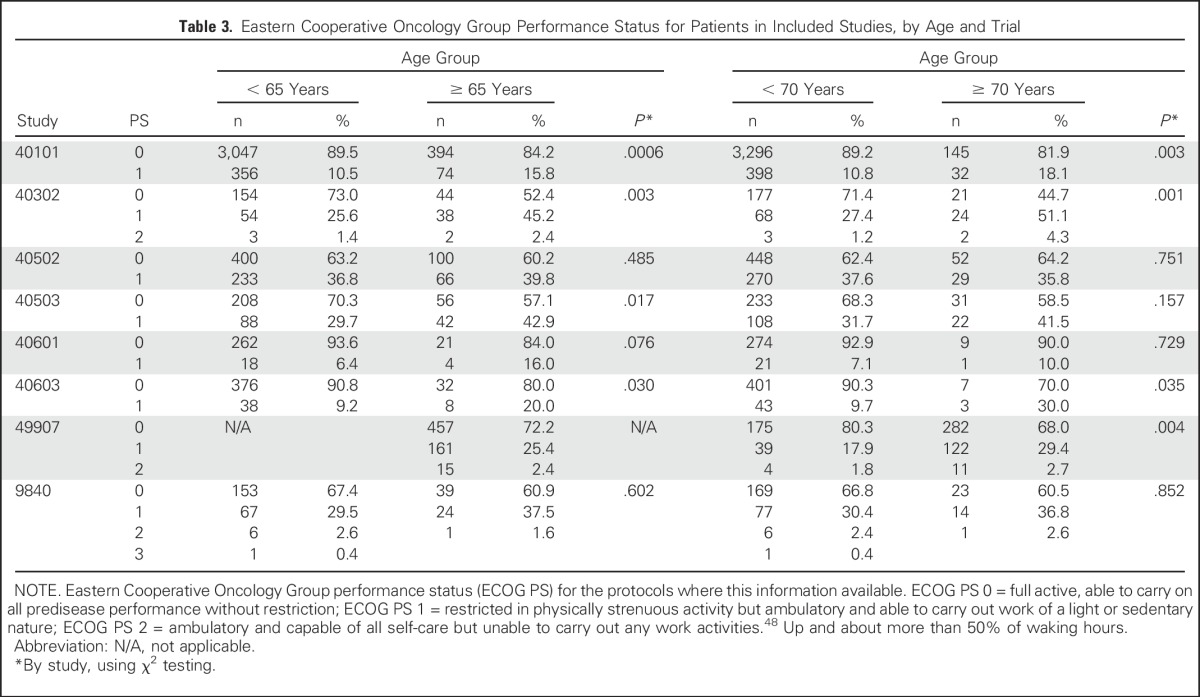
Disease characteristics by age for adjuvant trials CALGB 40101, 49907, 9344, 9741, 9344, 8541, and N9831 are listed in Table 2 (detailed characteristics for NCCTG 89-30-52 were not available). In general, across adjuvant trials, the proportion of women with ER-negative and PR-negative tumors was significantly higher for older versus younger women. Comparisons by HER2 status were limited because these data were not routinely collected in older studies and because all patients in N9831 had HER2-positive disease. For CALGB studies 40101, 8541, 9344, and 9741, the mean number of nodes in women age ≥ 65 years was higher than in younger patients. Mean tumor sizes were similar by age for all adjuvant studies (P > .05 for all five protocols), although when categorized as < 2 cm or ≥ 2 cm, CALGB 40101 had a higher proportion of patients age ≥ 65 years with tumors ≥ 2 cm compared with those age < 65 years (49% v 44%, respectively; P = .019). Results for age ≥ 70 years were similar to those with a cutoff age of 65 years (data not shown). Patients age ≥ 65 years and ≥ 70 years had significantly higher proportions of participants with ECOG PS 1 (v 0) in CALGB studies 40101, 40302, and 40603 compared with younger women. ECOG PS was similar by age in CALGB 40502 and 9840 (Table 3).
Table 2.
Disease Characteristics by Adjuvant Trial for Older Versus Younger Women*
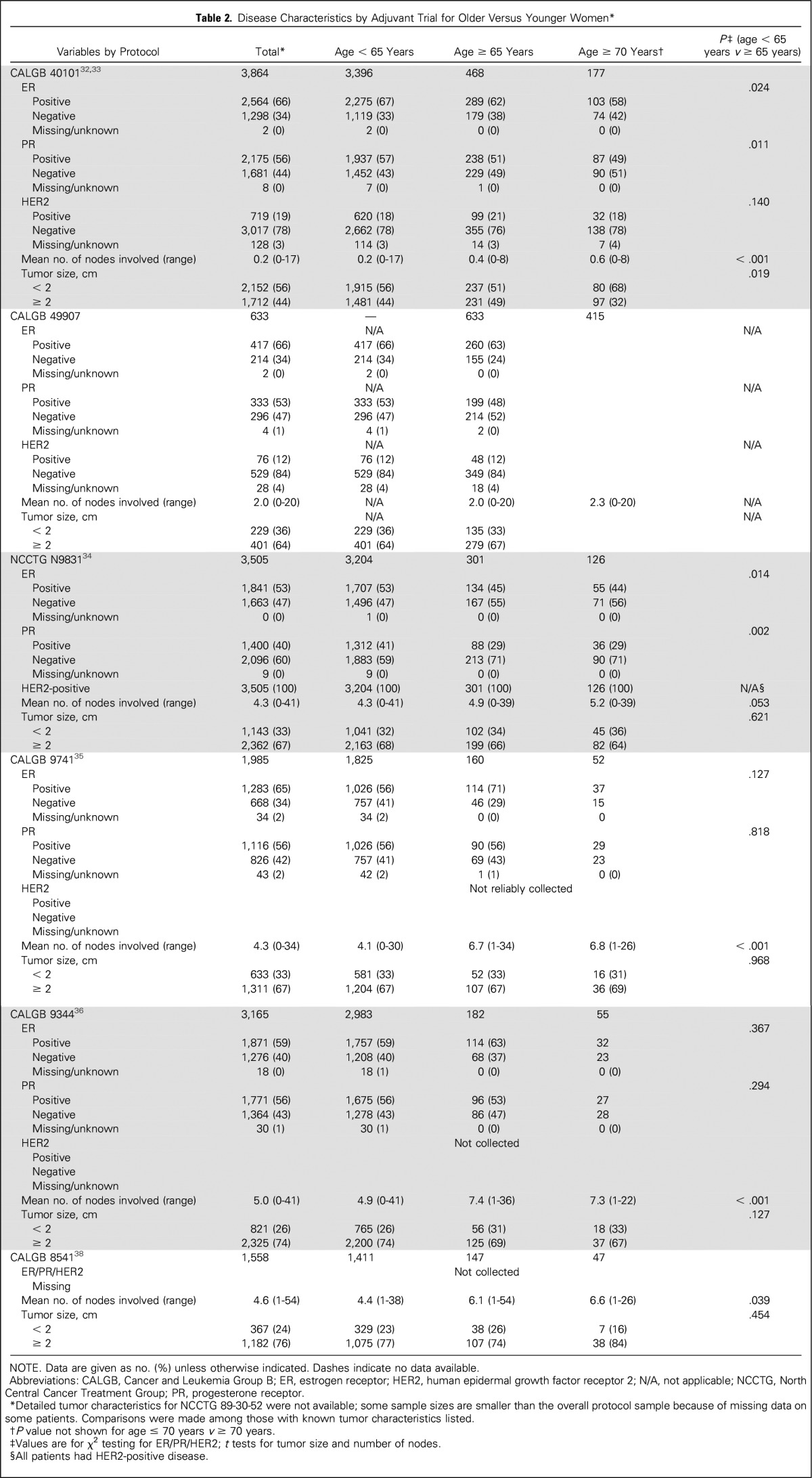
Table 3.
Eastern Cooperative Oncology Group Performance Status for Patients in Included Studies, by Age and Trial
Reasons for Protocol Treatment Cessation
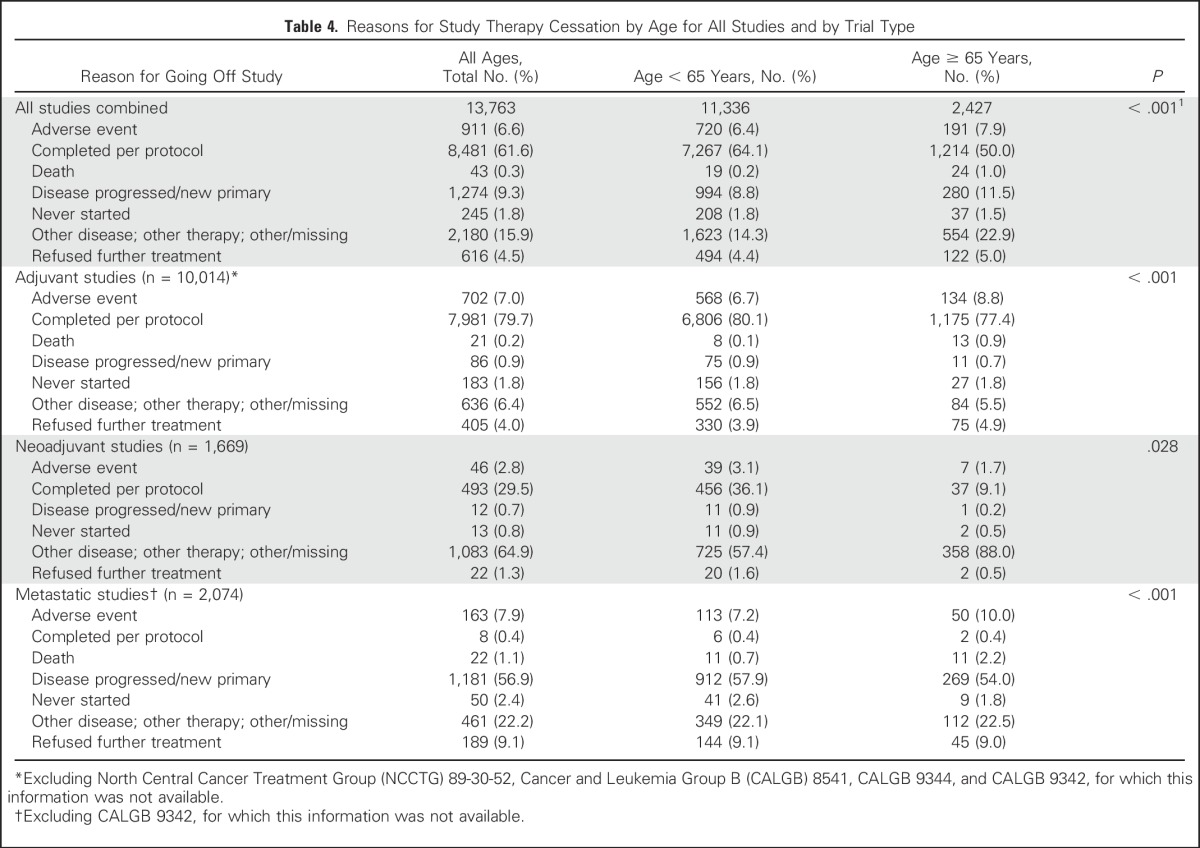
Table 4 lists the reasons for stopping protocol treatment (based on trials with this information available). Overall, reasons for going off treatment differed significantly for women age ≥ 65 years versus those age < 65 years (overall P < .0001), and early protocol treatment cessation was more frequent in those age ≥ 65 years (50%) versus < 65 years (35.9%) across trials. For those treated in adjuvant trials (Table 4), small differences by age were seen in the percentage of women who went off protocol treatment because they completed therapy, with 80.1% of women age < 65 years completing therapy as planned versus 77.4% of women age ≥ 65 years (overall P < .0001). For neoadjuvant trials, comparisons were limited because 65% of patients did not have clearly documented reasons for stopping study therapy. Overall, deaths during a study were uncommon (1% of women age ≥ 65 years v 0.2% for those age < 65 years). Cessation of therapy because of adverse events occurred more frequently for those age ≥ 65 years compared with those < 65 years (7.9% v 6.4% overall; P < .0001), though differences were small.
Table 4.
Reasons for Study Therapy Cessation by Age for All Studies and by Trial Type
DISCUSSION
The US population is aging and the number of older patients with breast cancer is expected to rise significantly in the coming decades.49-53 Currently, the median age at diagnosis is 62 years and approximately 42% of all new breast cancer cases annually are diagnosed in women age ≥ 65 years.1 On average, an 80-year-old woman currently living in the United States will live another 9 years54 and will benefit from optimized systemic therapy for her breast cancer. Furthermore, nearly 10% of older patients present with stage III-IV disease,55 and even more have node-positive/earlier-stage disease with a high probability of recurrence, where chemotherapy can provide benefit.56-58 Improving the availability of prospective evidence for this growing number of patients is crucial to inform decision-making and to optimally serve patients’ medical, emotional, and functional needs.30
In this analysis of Alliance systemic therapy studies during 1985-2012, we observed small increases in accrual of older patients to adjuvant trials but a significant decrease in accrual to trials in the neoadjuvant and metastatic disease settings. In general, with the exception of selected trials dedicated to older patients, the proportion of patients enrolling to clinical trials who were age ≥ 65 years and, in particular, ≥ 70 years remained low throughout the study period, with less than 20% of participants age ≥ 65 years and less than 10% of participants age ≥ 70 years. The absolute numbers of older patients enrolled over time also remained low, though some years saw higher accrual than others.
Of note, despite a trend for decreased accrual for metastatic and neoadjuvant trials for older patients, overall, numerically higher proportions of older patients enrolled in these trials compared with adjuvant studies, with approximately 24% of participants aged ≥ 65 years in both metastatic and neoadjuvant studies (v 15% on adjuvant trials). The reasons for this finding are not clear from the data available but may be related to a lower threshold for treatment in the neoadjuvant/metastatic settings because of symptomatic or higher burden of disease. Furthermore, all the patients with metastatic disease in the trials included in this analysis received single-agent chemotherapy, which may be more appealing for providers and older patients than doublet treatment. Although there were some differences noted by age in PS for trial participants, essentially all patients across studies had ECOG PS of 0-1 and met trial eligibility criteria. It is possible that some older patients who would have otherwise participated in protocols were disproportionately not eligible because of poor PS, but we do not have information on patients not enrolled in studies. However, given that most older patients with cancer have life expectancies of > 16 years at age 70,54 and that approximately half of older patients with breast cancer having three or fewer comorbid conditions,4 it is unlikely that the majority of older patients not enrolled in trials were truly ineligible.
Not surprisingly, trials administering endocrine therapies also enrolled higher proportions of older participants, though results for accrual of older patients were similar even after excluding these trials. In contrast, in the adjuvant setting, there may be a higher threshold to treat older patients with chemotherapy in general, thus leading to fewer older patients enrolled in adjuvant studies. Consistent with this, we observed more nodal involvement and more hormone receptor-negative cancers for older (v younger) women enrolled in adjuvant studies. Although it is often appropriate to withhold adjuvant systemic therapy in older patients with significant comorbidity and/or low-to-intermediate risk tumors, there are likely many cases in which older women are being undertreated,56,57,9-64 possibly reflected by their disproportionately low enrollment in adjuvant trials. For example, in CALGB 9344, an adjuvant trial for women with node-positive cancers, only 6% of trial participants were age ≥ 65 years and 2% were age ≥ 70 years. On average, older women on this trial had more than seven positive lymph nodes compared with an average of more than five nodes for patients age < 65 years, suggesting a higher threshold to treat despite similar mortality benefits from adjuvant chemotherapy for older and younger women.55,57,58 However, we do not have information on whether older women received treatment off-study.
Reassuringly, we observed small differences in the proportion of patients who stopped therapy for adverse events by age, and ≤ 10% of all patients stopped therapy for this reason, regardless of age. Consistent with this, Muss et al24 reported that 92% of patients age ≥ 65 years receiving anthracycline-based therapy on CALGB 49907 completed treatment. Deaths while participating in a study in our analysis were more common for older patients overall (1% for age ≥ 65 years v 0.2% for age < 65 years), but nearly half of deaths in older patients occurred in the metastatic setting and were likely related to disease progression. Less than 1% of older patients on adjuvant trials had death as the documented reason for therapy cessation.
Our results suggest that current strategies to increase enrollment of older adults to Alliance trials have had limited effectiveness. Although chemotherapy-based clinical trials targeting older women, such as CALGB 49907 and the local therapy trial CALGB 9343 (tamoxifen with or without radiation therapy for women undergoing breast conservation) have demonstrated the ability to accrue patients to large-scale, practice-changing trials on a national level, protocols dedicated to older patients remain few. Consequently, there has been new momentum to reassess how we approach accrual challenges across the cancer spectrum. Recently proposed, overarching strategies to improve prospective evidence have outlined strong recommendations for the following: (1) leverage trial designs by including specific analyses of outcomes for older patients and (2) improve the research environment for enrollment (eg, nationally based incentives for enrolling older patients, improving the information collected for older patients).23,30
Additional ways to incorporate older patients22 could include strategies that either mandate enrollment of a prespecified proportion or number of older patients in National Institutes of Health-supported studies or a dedicated “expansion cohort” of older patients where toxicity, functional decline, and outcomes can be closely monitored. An alternative strategy could require that all new National Clinical Trials Network (NCTN) protocols undergo review to ensure specific considerations and outcomes are included for older adults. Third, ensuring that clinical trials are extended to community sites rather than academic institutions will likely improve the appeal of clinical trials for older patients. With any of these approaches, we may not need to rely on dedicated clinical trials for older patients to gain prospective data. This would also reduce the need to pool clinical trial data from heterogeneous groups of patients to answer questions about the experiences, toxicities, and disease outcomes for older patients.58,65
We recognize several important limitations. We did not have information on the numbers of women approached or not approached, the characteristics of providers and practices, trial burden or requirements, or why patients did not enroll. Second, because the focus of our analysis was limited to Alliance systemic therapy trials, our results cannot be extrapolated across all breast cancer studies. Furthermore, we do not have information on how our results compare with those from other cooperative group settings or clinical trials in general, because of the lack of previously published data. Third, we did not have complete information on PS, reasons for therapy cessation, and tumor characteristics for some trials. Also, some tumor characteristics may be misclassified in some cases because they relied on previously collected data. Finally, interpretation of results from neoadjuvant trials may be limited by the smaller number of patients enrolled overall and the fact that two of these trials (CALGB 40603 and 40601) targeted patients with tumor subtypes that occur less frequently in older (v younger) patients.66
Despite these limitations, we were able to examine breast cancer systemic treatment trials over time on a national scale, further highlighting previous findings across all cancers22,23 and identifying areas in urgent need of improvement within breast cancer NCTN trials. Our findings serve as an urgent call to action: Novel strategies for accrual of older patients to clinical trials are critical to meaningfully change the evidence-base for this growing group. The NCTN has the power to effect meaningful change on a national level with regard to the care of older patients with cancer and should seize this opportunity to forge a new path in clinical trial strategy.
ACKNOWLEDGMENT
We thank all patients who have been enrolled in Alliance trials and the individual Alliance study teams for their prior data collection, analyses, and collaboration. We also thank Vera Suman, PhD, Alliance statistician, for her assistance with data analysis.
Appendix
Fig A1.
Absolute numbers of patients enrolled onto clinical trials for all patients age ≥ 65 years and age ≥ 70 years (A), by trial type for age ≥ 65 years and ≥ 70 years (B, C), and after exclusion of Cancer and Leukemia Group B (CALGB) 49907, North Central Cancer Treatment Group (NCCTG) 89-30-52, and American College of Surgeons Oncology Group (ACOSOG) Z1031 (D).
Footnotes
Supported by the National Cancer Institute under Awards No. UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), U10CA032291, U10CA047559, U10CA047577, U10CA077597, U10CA077651, U10CA180790, U10CA180791, U10CA180838, U10CA180857, and U10CA180867. R.A.F. also receives funding from the Susan G. Komen Foundation and American Cancer Society. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Presented as a poster at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 2-6, 2016.
AUTHOR CONTRIBUTIONS
Conception and design: Rachel A. Freedman, Jacqueline M. Lafky, Hyman B. Muss, Harvey J. Cohen, Eric P. Winer, Clifford A. Hudis, Alvaro Moreno-Aspitia, Arti Hurria
Administrative support: Rachel A. Freedman, Jacqueline M. Lafky
Collection and assembly of data: Rachel A. Freedman, Jared C. Foster, Drew K. Seisler, Jacqueline M. Lafky, Constance Cirrincione
Data analysis and interpretation: Rachel A. Freedman, Jared C. Foster, Drew K. Seisler, Jacqueline M. Lafky, Hyman B. Muss, Harvey J. Cohen, Jeanne Mandelblatt, Ann H. Partridge, Lisa A. Carey, Constance Cirrincione, Alvaro Moreno-Aspitia, Gretchen Kimmick, Aminah Jatoi, Arti Hurria
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Accrual of Older Patients With Breast Cancer to Alliance Systemic Therapy Trials Over Time: Protocol A151527
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Rachel A. Freedman
Research Funding: Puma Biotechnology (Inst), Genentech (Inst), Eisai (Inst)
Jared C. Foster
No relationship to disclose
Drew K. Seisler
No relationship to disclose
Jacqueline M. Lafky
No relationship to disclose
Hyman B. Muss
Consulting or Advisory Role: Pfizer
Harvey J. Cohen
No relationship to disclose
Jeanne Mandelblatt
No relationship to disclose
Eric P. Winer
No relationship to disclose
Clifford A. Hudis
Consulting or Advisory Role: Pfizer, Roche, Novartis, Lilly
Ann H. Partridge
Consulting or Advisory Role: Pfizer
Lisa A. Carey
Research Funding: GlaxoSmithKline, Genentech
Constance Cirrincione
No relationship to disclose
Alvaro Moreno-Aspitia
No relationship to disclose
Gretchen Kimmick
Research Funding: Roche (Inst), Genentech (Inst), Abraxis BioScience (Inst), Bristol-Myers Squibb (Inst), Puma Biotechnology (Inst), Wyeth (Inst), Novartis (Inst), GlaxoSmithKline (Inst), Johnson & Johnson (Inst)
Travel, Accommodations, Expenses: Genomic Health
Aminah Jatoi
Research Funding: Entera Health, Boston Biologics
Arti Hurria
Consulting or Advisory Role: GTx, Seattle Genetics, Boehringer Ingelheim, On Q Health, Sanofi, OptumHealth Care Solutions
Research Funding: GlaxoSmithKline, Celgene, Novartis
REFERENCES
- 1.National Cancer Institute, Surveillance, Epidemiology and End Results Program: SEER Stat Fact Sheets: Female breast cancer. http://seer.cancer.gov/statfacts/html/breast.html
- 2.American Cancer Society: Breast cancer facts and figures 2015-2016. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-046381.pdf
- 3.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120:104–110. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 4.Yancik R, Wesley MN, Ries LA, et al. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 5.Schonberg MA, Marcantonio ER, Li D, et al. Breast cancer among the oldest old: Tumor characteristics, treatment choices, and survival. J Clin Oncol. 2010;28:2038–2045. doi: 10.1200/JCO.2009.25.9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Cancer Society: Homepage. http://www.cancer.org/
- 7.Kemeny MM, Peterson BL, Kornblith AB, et al. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol. 2003;21:2268–2275. doi: 10.1200/JCO.2003.09.124. [DOI] [PubMed] [Google Scholar]
- 8.Kornblith AB, Kemeny M, Peterson BL, et al. Survey of oncologists’ perceptions of barriers to accrual of older patients with breast carcinoma to clinical trials. Cancer. 2002;95:989–996. doi: 10.1002/cncr.10792. [DOI] [PubMed] [Google Scholar]
- 9.Javid SH, Unger JM, Gralow JR, et al. A prospective analysis of the influence of older age on physician and patient decision-making when considering enrollment in breast cancer clinical trials (SWOG S0316) Oncologist. 2012;17:1180–1190. doi: 10.1634/theoncologist.2011-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 11.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 13.Simon MS, Du W, Flaherty L, et al. Factors associated with breast cancer clinical trials participation and enrollment at a large academic medical center. J Clin Oncol. 2004;22:2046–2052. doi: 10.1200/JCO.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Gross CP, Filardo G, Mayne ST, et al. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103:483–491. doi: 10.1002/cncr.20792. [DOI] [PubMed] [Google Scholar]
- 15.Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31:536–542. doi: 10.1200/JCO.2012.45.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basche M, Barón AE, Eckhardt SG, et al. Barriers to enrollment of elderly adults in early-phase cancer clinical trials. J Oncol Pract. 2008;4:162–168. doi: 10.1200/JOP.0842001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimmick G. Clinical trial accrual in older cancer patients: The most important steps are the first ones. J Geriatr Oncol. 2016;7:158–161. doi: 10.1016/j.jgo.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Ayodele O, Akhtar M, Konenko A, et al. Comparing attitudes of younger and older patients towards cancer clinical trials. J Geriatr Oncol. 2016;7:162–168. doi: 10.1016/j.jgo.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Avis NE, Smith KW, Link CL, et al. Factors associated with participation in breast cancer treatment clinical trials. J Clin Oncol. 2006;24:1860–1867. doi: 10.1200/JCO.2005.03.8976. [DOI] [PubMed] [Google Scholar]
- 20.Kimmick GG, Peterson BL, Kornblith AB, et al. Improving accrual of older persons to cancer treatment trials: A randomized trial comparing an educational intervention with standard information: CALGB 360001. J Clin Oncol. 2005;23:2201–2207. doi: 10.1200/JCO.2005.01.222. [DOI] [PubMed] [Google Scholar]
- 21.Hurria A, Cohen HJ, Extermann M. Geriatric oncology research in the cooperative groups: A report of a SIOG special meeting. J Geriatr Oncol. 2010;1:40–44. doi: 10.1016/j.jgo.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurria A, Dale W, Mooney M, et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol. 2014;32:2587–2594. doi: 10.1200/JCO.2013.55.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurria A, Levit LA, Dale W, et al. Improving the evidence base for treating older adults with cancer: American Society of Clinical Oncology statement. J Clin Oncol. 2015;33:3826–3833. doi: 10.1200/JCO.2015.63.0319. [DOI] [PubMed] [Google Scholar]
- 24.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055–2065. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: Long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31:2382–2387. doi: 10.1200/JCO.2012.45.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 27.Coyne CA, Xu R, Raich P, et al. Randomized, controlled trial of an easy-to-read informed consent statement for clinical trial participation: A study of the Eastern Cooperative Oncology Group. J Clin Oncol. 2003;21:836–842. doi: 10.1200/JCO.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Treweek S, Lockhart P, Pitkethly M, et al: Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open 3:e002360, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treweek S, Pitkethly M, Cook J, et al: Strategies to improve recruitment to randomised controlled trials. Cochrane Database Syst Rev 4:MR000013, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Hurria A, Naylor M, Cohen HJ. Improving the quality of cancer care in an aging population: Recommendations from an IOM report. JAMA. 2013;310:1795–1796. doi: 10.1001/jama.2013.280416. [DOI] [PubMed] [Google Scholar]
- 31.Alliance for Clinical Trials in Oncology: Homepage. https://www.allianceforclinicaltrialsinoncology.org/main/public/index.xhtml
- 32.Shulman LN, Berry DA, Cirrincione CT, et al. Comparison of doxorubicin and cyclophosphamide versus single-agent paclitaxel as adjuvant therapy for breast cancer in women with 0 to 3 positive axillary nodes: CALGB 40101 (Alliance) J Clin Oncol. 2014;32:2311–2317. doi: 10.1200/JCO.2013.53.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shulman LN, Cirrincione CT, Berry DA, et al. Six cycles of doxorubicin and cyclophosphamide or Paclitaxel are not superior to four cycles as adjuvant chemotherapy for breast cancer in women with zero to three positive axillary nodes: Cancer and Leukemia Group B 40101. J Clin Oncol. 2012;30:4071–4076. doi: 10.1200/JCO.2011.40.6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: Joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 36.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 37.Ingle JN, Suman VJ, Mailliard JA, et al. Randomized trial of tamoxifen alone or combined with fluoxymesterone as adjuvant therapy in postmenopausal women with resected estrogen receptor positive breast cancer. North Central Cancer Treatment Group Trial 89-30-52. Breast Cancer Res Treat. 2006;98:217–222. doi: 10.1007/s10549-005-9152-1. [DOI] [PubMed] [Google Scholar]
- 38.Wood WC, Budman DR, Korzun AH, et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med. 1994;330:1253–1259. doi: 10.1056/NEJM199405053301801. [DOI] [PubMed] [Google Scholar]
- 39.Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance) J Clin Oncol. 2015;33:13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carey LA, Berry DA, Cirrincione CT, et al. Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J Clin Oncol. 2016;34:542–549. doi: 10.1200/JCO.2015.62.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buzdar AU, Suman VJ, Meric-Bernstam F, et al. Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): A randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1317–1325. doi: 10.1016/S1470-2045(13)70502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: Clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype--ACOSOG Z1031. J Clin Oncol. 2011;29:2342–2349. doi: 10.1200/JCO.2010.31.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rugo HS, Campone M, Amadori D, et al. A randomized, phase II, three-arm study of two schedules of ixabepilone or paclitaxel plus bevacizumab as first-line therapy for metastatic breast cancer. Breast Cancer Res Treat. 2013;139:411–419. doi: 10.1007/s10549-013-2552-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dickler MN, Barry WT, Cirrincione CT, et al. Phase III trial evaluating letrozole as first-line endocrine therapy with or without bevacizumab for the treatment of postmenopausal women with hormone receptor-positive advanced-stage breast cancer: CALGB 40503 (Alliance) J Clin Oncol. 2016;34:2602–2609. doi: 10.1200/JCO.2015.66.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burstein HJ, Cirrincione CT, Barry WT, et al. Endocrine therapy with or without inhibition of epidermal growth factor receptor and human epidermal growth factor receptor 2: A randomized, double-blind, placebo-controlled phase III trial of fulvestrant with or without lapatinib for postmenopausal women with hormone receptor-positive advanced breast cancer-CALGB 40302 (Alliance) J Clin Oncol. 2014;32:3959–3966. doi: 10.1200/JCO.2014.56.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winer EP, Berry DA, Woolf S, et al. Failure of higher-dose paclitaxel to improve outcome in patients with metastatic breast cancer: Cancer and leukemia group B trial 9342. J Clin Oncol. 2004;22:2061–2068. doi: 10.1200/JCO.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 47.Seidman AD, Berry D, Cirrincione C, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: Final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008;26:1642–1649. doi: 10.1200/JCO.2007.11.6699. [DOI] [PubMed] [Google Scholar]
- 48.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 49.Edwards BK, Howe HL, Ries LA, et al. Annual report to the nation on the status of cancer, 1973-1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94:2766–2792. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 50.Yancik R, Ries LA. Cancer in older persons: An international issue in an aging world. Semin Oncol. 2004;31:128–136. doi: 10.1053/j.seminoncol.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 51.Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 52.Yancik R. Population aging and cancer: A cross-national concern. Cancer J. 2005;11:437–441. doi: 10.1097/00130404-200511000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25:1029–1036. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walter LC, Schonberg MA. Screening mammography in older women: A review. JAMA. 2014;311:1336–1347. doi: 10.1001/jama.2014.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schonberg MA, Marcantonio ER, Ngo L, et al. Causes of death and relative survival of older women after a breast cancer diagnosis. J Clin Oncol. 2011;29:1570–1577. doi: 10.1200/JCO.2010.33.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giordano SH, Duan Z, Kuo YF, et al. Use and outcomes of adjuvant chemotherapy in older women with breast cancer. J Clin Oncol. 2006;24:2750–2756. doi: 10.1200/JCO.2005.02.3028. [DOI] [PubMed] [Google Scholar]
- 57.Elkin EB, Hurria A, Mitra N, et al. Adjuvant chemotherapy and survival in older women with hormone receptor-negative breast cancer: Assessing outcome in a population-based, observational cohort. J Clin Oncol. 2006;24:2757–2764. doi: 10.1200/JCO.2005.03.6053. [DOI] [PubMed] [Google Scholar]
- 58.Muss HB, Woolf S, Berry D, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293:1073–1081. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 59.Bouchardy C, Rapiti E, Fioretta G, et al. Undertreatment strongly decreases prognosis of breast cancer in elderly women. J Clin Oncol. 2003;21:3580–3587. doi: 10.1200/JCO.2003.02.046. [DOI] [PubMed] [Google Scholar]
- 60.Freedman RA, Hughes ME, Ottesen RA, et al. Use of adjuvant trastuzumab in women with human epidermal growth factor receptor 2 (HER2)-positive breast cancer by race/ethnicity and education within the National Comprehensive Cancer Network. Cancer. 2013;119:839–846. doi: 10.1002/cncr.27831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freedman RA, He Y, Winer EP, et al. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713–719. doi: 10.1200/JCO.2008.17.9234. [DOI] [PubMed] [Google Scholar]
- 62.Freedman RA, Vaz-Luis I, Barry WT, et al. Patterns of chemotherapy, toxicity, and short-term outcomes for older women receiving adjuvant trastuzumab-based therapy. Breast Cancer Res Treat. 2014;145:491–501. doi: 10.1007/s10549-014-2968-9. [DOI] [PubMed] [Google Scholar]
- 63.Griggs JJ, Hawley ST, Graff JJ, et al. Factors associated with receipt of breast cancer adjuvant chemotherapy in a diverse population-based sample. J Clin Oncol. 2012;30:3058–3064. doi: 10.1200/JCO.2012.41.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Griggs JJ, Culakova E, Sorbero ME, et al. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol. 2007;25:2522–2527. doi: 10.1200/JCO.2006.10.2749. [DOI] [PubMed] [Google Scholar]
- 65.Muss HB, Berry DA, Cirrincione C, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: The Cancer and Leukemia Group B Experience. J Clin Oncol. 2007;25:3699–3704. doi: 10.1200/JCO.2007.10.9710. [DOI] [PubMed] [Google Scholar]
- 66.Howlader N, Altekruse SF, Li CI, et al: US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 106(5), 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]