Abstract
The lymphoma-inducing potential of Ig heavy-chain enhancer- and promoter-regulated Epstein-Barr virus (EBV) nuclear antigen 1 (EBNA1) was evaluated in three transgenic FVB mouse lineages. EBNA1 was expressed at a higher level in transgenic B220(+) splenocytes than in EBV-infected lymphoblastoid cell lines. EBNA1 was also expressed in B220(-) transgenic splenocytes and thymocytes. Before killing and assessments at 18-26 months, EBNA1-transgenic mice did not differ from control mice in mortality. At 18-26 months EBNA1-transgenic mice did not differ from littermate control in ultimate body weight, in spleen size or weight, in lymph node, kidney, liver, or spleen histology, in splenocyte fractions positive for cluster of differentiation (CD)3ε, CD4, CD8, CD62L, B220, CD5, IgM, IgD, MHC class II, CD11b, or CD25, or in serum IgM, IgG, or total Ig levels. Lymphomas were not found in spleens or other organs of 18- to 26-month-old EBNA1-transgenic (n = 86) or control (n = 45) FVB mice. EBNA1-transgenic lineages had a higher pulmonary adenoma prevalence than did littermate controls (39% versus 7%). However, the adenoma prevalence was not higher in EBNA1-transgenic mice than has been described for FVB mice, and EBNA1 was not expressed in normal pulmonary epithelia or adenomas.
Keywords: cancer, herpesvirus, latent, persistence
In initial lymphocyte infection, Epstein-Barr Virus (EBV) causes B lymphocyte proliferation through a latency III program of expression of six nuclear proteins (EBNAs), two different integral membrane proteins, two small RNAs, and Bam A-initiated transcripts (1, 2). Although, in vitro, latency III-infected lymphocytes are able to grow endlessly as lymphoblastoid cell lines (LCLs), EBV proteins expressed in latency III-infected cells, in vivo, engender T cell responses that limit infected cell survival. EBV mostly persists long term in nonproliferating, latency I- or II-infected B cells that express EBNA1 or EBNA1 and latent infection membrane proteins, respectively (2-4). Latency I or latency II are also characteristic of Burkitt's lymphoma, Hodgkin's disease, and nasopharyngeal carcinoma (2). EBNA1 is expressed in almost all latent EBV infections and associated malignant diseases.
EBNA1 enables efficient EBV episome replication, transcription, and maintenance in latently infected dividing cells (5-21). EBNA1 amino acids 379-641 include a dimerization and DNA-binding domain, which binds cognate 30-bp inverted repeat DNA sequences. EBV origin of plasmid replication (oriP) consists of 20 tandem cognate 30-bp repeats and four others 1 kbp away in a dyad symmetry (22). OriP has intrinsic origin activity. EBNA1 enhances oriP-dependent episome replication, transcription, and maintenance (7, 8). EBNA1 amino acids 1-378 are necessary for chromosome association, transcriptional enhancement, and episome persistence, although not for initial DNA replication (21, 23-25). Within amino acids 1-378 is an irregular glycine/alanine repeat, which inhibits EBNA1 processing through the proteasome/TAP pathway (26, 27).
Because EBNA1 is broadly expressed in latent EBV infection and associated malignancies, EBNA1 could affect cell growth or survival by binding to putative cognate sequences in cell DNA or through interactions with cell proteins. However, EBNA1 enhancement of transcription requires multiple tandem repeats of a dimeric EBNA1 cognate recognition site, and such sites are not in cell DNA (28). Furthermore, expression of a dominant negative EBNA1 in an LCL with integrated EBV genomes blocked EBNA1 effects through cognate DNA but did not alter cell growth or gene expression (20). Moreover, EBNA1 has little effect on transcription of an EBNA1-dependent reporter integrated at most sites in cell DNA (20, 29). Alternatively, EBNA1 might alter cell growth or survival through an interaction with a cell protein (30-39). However, once EBV DNA has integrated into cell DNA, EBNA1 is not required for LCL growth, and LCLs with EBNA1-null mutant EBV genomes are indistinguishable from wild-type EBV-transformed B lymphocytes in their growth (12).
The experiments reported here were undertaken to determine whether EBNA1-transgenic expression in mice can cause lymphoma. Previously, an IgH constant region enhancer (Eμ)- and polyoma virus early promoter (PyP)-regulated EBNA1 (EμPyP-EBNA1)-transgenic mouse lineage was described in which EBNA1 protein was barely detectable in lymphocytes, and 6- to 8-month-old mice uniformly died of lymphomas in which EBNA1 was barely detectable (40). This uniform lymphoma mortality was similar to that observed in Eμ-enhanced c-myc transgenic mice (41, 42) and was in contrast to a second EμPyP-EBNA1-transgenic mouse lineage (40), which had readily detectable EBNA1 protein in spleen and thymus cells without significant mortality from lymphoma. The “inverse relationship between the levels of detectable EBNA1 and the penetrance of disease” (40) with EμPyP-EBNA1 could have been due to a cis-acting EμPyP effect on expression of a putative cellular oncogene in the lineage with uniform lymphoma mortality. Indeed, EBNA1 expression in primary or continuous cells in culture has been associated with relatively small effects on cell growth, survival, or gene expression (29, 37-39, 43-45). We therefore initiated a series of EBNA1-transgenic mouse experiments to study the effect of EBNA1 protein in mouse B lymphocytes at levels similar to that of latent EBV infection in human B lymphocytes.
Materials and Methods
EBNA1-Transgenic Construct, Plasmids, and Antibodies. An Eμ and Pμ, FLAG-tagged EBNA1-expression plasmid, pEμPμFE1 was made by ligating the Klenow-treated EBV B95 strain DNA encoding EBNA1 (46) into the blunt-ended BamHI site of the EμPμ vector (47). FLAG-epitope oligonucleotides 5′-CTAGG TACCA TGGAC TACAA GGACG ACGAT GACA and 5′-CTAGT GTCAT CGTCG TCCTT GTAGT CCATG GTAC were annealed and ligated into the SpeIsite5′ to the EBNA1 ORF resulting in pEμPμFE1. Orientation and coding frame sequence were verified. EBNA1 and oriP-dependent reporter plasmid pFL, pCDNAFE1, and pGKβ-galactosidase have been described (20).
Mouse monoclonal antibodies specific for the EBNA1 carboxyl terminus and FLAG epitope were purchased from Applied Bio-systems and Sigma, respectively. Anti-mouse antibodies or anti-biotin streptavidin conjugates for flow cytometric analyses were purchased from Pharmingen and included anti-CD16 (Fc block, clone 2.4G2), hamster biotin cluster of differentiation (CD) 3ε (145-2C11), nonlabeled or FITC-CD45R/B220 (RA3-6B2), FITC or R-PE-CD8a (Ly-2) (clone 53-6.7), R-PE-CD5 (Ly-1) (53-7.3), FITC-CD25 (IL-2 receptor alpha) (7D4), R-PE-IgM (R6-60.2), FITC-IgD (11-26c.2a), Biotin-CD62L (L selectin, LECAM-1, Ly-22) (clone MEL-14), R-PE-CD4 (L3T4) (clone GK1.5), Biotin-I-Ad/I-Ed, FITC-CD11b (Integrin a, M, Mac-1 a chain) (M1/70), FITC-Pan NK (DX5), streptavidin-horseradish peroxidase conjugate, streptavidin-FITC, and streptavidin-cy-chrome. Antibodies for ELISA, mouse IgM and IgG isotype control, unlabeled or alkaline phosphatase-conjugated goat anti-mouse IgM+IgG+IgA (H+L), goat F(ab′)2 anti-mouse IgM, and goat anti-mouse IgG (gamma chain-specific) were purchased from Southern Biotechnology Associates. CD45R(B220) or CD8+ (Ly2) antibodies conjugated to microbeads for magnetic cell sorting were from Miltenyi Biotec (Auburn, CA).
Transient Transfection and Reporter Assays. For BJAB cell reporter assays, 10 × 106 cells were cotransfected with 30 μg of pEμPμFE1, pCDNAFE1 or pcDNA-FLAG vector control, 2 μg of pFL, and 1 μg of pGKβ-galactosidase (20).
EBNA1-Transgenic Mice. Specific pathogen-free FVB and C57BL/6 mice were purchased from Taconic Farms. Mice were screened for endemic mouse viruses and mycoplasma and maintained under microisolator-barrier conditions in a BL1-N facility under an animal experimentation protocol approved by the Institutional Animal Care and Use committee at Harvard Medical School.
The SalI 5.6-kb pEμPμFEBNA1 DNA fragment was microinjected into fertilized FVB zygotes, which were implanted into oviducts of pseudopregnant Swiss-Webster mice (47). Progeny were screened for EBNA1 DNA by PCR with 50 ng of genomic tail DNA prepared with an Easy DNA kit (Invitrogen) and P1s (5′-GGGGA AGAGC GCTGT GCACA GAAAG) with P1as (FSPEas, 5′-CTAGT GTCAT CGTCG TCCTT GTAGT CCATG GTAC) for amplification of base pairs -319 to +23 relative to the ATG of FLAG or P2s (5′-GCAGG AGGTG GAGGC CGGGG TCG) and P2as (5′-TGATG ACTGA CTACT GGGAC TCC) for amplification of base pairs +987 to +1175 by 35 cycles at 94°C for 30 sec, 60°C for 30 sec, and 72°C for 30 sec. Three independent EBNA1-transgenic lineages, 58, 68, and 73, were identified by PCR in 25 newborn mice. The three EμPμFE1 DNA transgenic lineages were maintained by mating with FVB mice for nine generations and then crossed through six successive generations into C57BL/6.
At the 11th generation of the three lineages, the unique EμPμFE1 DNA from upstream of the FLAG encoding DNA through the beginning of the EBNA1 ORF into DNA encoding the glycine/alanine repeat and from DNA encoding the glycine/alanine repeat to the 3′ end of the EBNA1 ORF was PCR-amplified [by using pfu turbo polymerase (Stratagene)], cloned, and sequenced. These PCR amplifications used forward primer 5′-CGT CGA GGA TCC TTG GCA TCT GTG TTT TCT TTC TCA-3′ with reverse primer 5′-CGT CGA CTC GAG CCG CTC CTG CTC CTG CTC CTG-3′ and forward primer 5′-CGT CGA GGA TCC CAG GAG CAG GAG GTG GAG GCC-3′ with reverse primer 5′-CGT CGA CTC GAG TGA GGG CGT CTC CTA ACA AGT TAC-3′.
Cell Preparation and FACS Analyses. Spleen, thymus, bone marrow, lymph nodes, stomach, lung, kidney, brain, and liver were examined and collected in cold RPMI medium 1640 plus 0.5% FCS. Cells were extracted from tissues by mincing between frosted slide glasses on ice, filtered through a 30 μM mesh filter (Miltenyi Biotec), and centrifuged at 300 × g for 10 min at 4°C. After suspension in 50 μl of RPMI medium 1640, RBCs were instantly lysed by adding 0.5 ml of deionized water. RPMI medium 1640 with 0.5% FCS (5 ml) was added. Intact cells were filtered through a 30 μM filter, counted with a hemocytometer, and used for FACS staining or immunoblotting. Serum Ig levels were assayed by ELISA (47). For magnetic cell-sorting separation of B220(+) B lymphocytes or CD8+ T lymphocytes, 107 splenocytes were mixed with 90 μl of labeling buffer, PBS with 0.5% BSA, and 10 μl of B220 or CD8 microbeads and separated according to the manufacturer's protocol.
In Vitro Gel Shift. Nuclear extracts were prepared from 2 × 107 EBNA1-converted BJAB (BFE1) cells, and total cellular extracts were prepared from transgenic or control FVB splenocytes (48). The oriP consensus dsDNA, GATCC TGGAT AGCAT ATGCT ATCCAG, was labeled, and 5 μg of extract protein was incubated with an ≈30,000-cpm probe DNA at room temperature in buffer containing 25 mM KCl, 500 ng of poly dIdC, 10% glycerol, 20 mM Tris·HCl (pH 7.5), 0.1 mM DTT, 10 mM MgCl2, and 1 mM EDTA. For supershift or competition, 1 μg of EBNA1 monoclonal anti-body or 100 M excess of unlabeled double-stranded wild type or mutant, GATCC ACCTT AGCAT ATGCT AAGGTG, cold probe was preincubated with extract before the addition of labeled probe. The reaction was run on 3.5% polyacrylamide gels in 1× Tris·glycine buffer (pH 10.6) for 1 h at 300 V.
Autopsy, Immunohistochemical Staining, and Microscopic Analyses. Transgenic and control mice were usually killed at 18-26 months. At autopsy, body weight and spleen size and weight were measured. Abdominal and thoracic viscera and lymph nodes were examined. Abnormalities were recorded. Spleen, kidney, liver, lung, and lymph nodes were collected in 10% formalin, sectioned, stained with hematolylin/eosin, and examined by two mouse pathologists, a human pathologist, and two investigators. Transgene-positive or control mice that were sick or found dead were similarly autopsied when circumstances permitted.
Immunohistochemical staining of formalin-fixed and paraffin-embedded tissues used 1.3 μg/ml mouse EBNA1 monoclonal antibody or 0.4 μg/ml CD45R/B220 (RA3-6B2) (Pharmingen) and indirect peroxidase staining, according to the supplier's protocols (DAKO).
Results
Expression of FLAG-Tagged EBNA1 Under Control of Eμ and Pμ. The EμPμ (47) was used to achieve FLAG-tagged EBNA1 expression in B lymphocytes and, to a lesser extent, in T lymphocytes. An N-terminal FLAG tag has not affected EBNA1 activity in oriP-dependent DNA replication, transcription, or episome maintenance assays (20, 21). Before zygote microinjection, the DNA sequence of the EμPμ FLAG-tagged EBNA1 expression plasmid, pEμPμFE1, was confirmed from the promoter through DNA encoding the FLAG epitope into the EBNA1 ORF and from the 3′ end of the EBNA1 ORF into the vector DNA (Fig. 1A).
Fig. 1.
FLAG-epitope-tagged EBNA1 transgene expression construct, functional test, and PCR detection of transgenes. (A) Transgene expression plasmid pEμPμFE1 is composed of Eμ and Pμ upstream of the FLAG-tagged EBNA1 ORF. (B) EBV-negative BJAB B lymphoblasts were transiently cotransfected with pEμPμFE1, pCDNAFE1, or pcDNA FLAG vector control, reporter plasmid pFL, and control pGKβ-galactosidase DNA. Relative luciferase activity is fold over the luciferase activity of pFL transfected with vector control DNA and was also corrected for transfection efficiency as assayed by β-galactosidase. (C) PCR screening used primer sets 1 and 2 to identify pEμPμFE1 DNA in 58, 68, and 73 founder mice among 25 progeny from embryo microinjections.
The pEμPμFE1 plasmid was also tested in EBV-negative human B lymphoma (BJAB) cells for activation of transcription from pFL, a plasmid that has an oriP-dependent promoter upstream of a luciferase reporter (20). In BJAB cells, pEμPμFE1 activated the oriP enhancer ≈140-fold and was similar in activity and in EBNA1-expression levels to pcDNAFE1 (20), whereas pEμPμB7.2, which expresses B7.2 (47), was indistinguishable from vector-control DNA and did not activate oriP-dependent gene expression (Fig. 1B and data not shown).
Three Independent Transgenic EBNA1 Lineages. Twenty-five progeny were born from pseudopregnant Swiss-Webster recipients of pEμPμFE1-injected FVB zygotes. Two primer sets specific for pEμPμFE1 were used for PCR screening; three of 25 founder mice (58, 68, and 73) were positive for both PCR products, whereas 22 of 25 were negative for both PCR products (Fig. 1C).
Founder mice 58, 68, and 73 were mated with FVB mice. F1 progeny positive for pEμPμFE1 DNA were identified from each founder. Transgene-positive 58, 68, and 73 F1 mice were mated with FVB mice, and all three transgenes transmitted to F2 progeny. Transgene transmission to F2 progeny was sex-independent. Furthermore, ≈50% of F2 progeny were transgene-positive; 3 of 9 for lineage 58, 10 of 21 for lineae 68, and 13 of 28 for lineage 73. Sex-independent transgene transmission to ≈50% of progeny was also characteristic of the ensuing 12 generations.
To discern differences in phenotype among the three lineages, mice from each lineage were analyzed at each phase of the study and compared with negative control littermates. To maximize lymphoma detection in EBNA1 lineages, 86 EBNA1 mice in three lineages were observed through maturity to advanced age, killed at 18-26 months, and autopsied in comparison with 45 control mice. For most outcome measures, 57 of the EBNA1 mice in three lineages were matched with 45 littermate controls.
Overall, EBNA1 transgene-positive mice were indistinguishable from negative control mice in appearance, weight, growth, and development. Incidental deaths between birth and killing at 18-26 months were 25 of 124 (20%) among EBNA1 mice versus 18 of 67 (27%) among control mice. The most common findings at death were skin infection or perineal hernia. One dead 14-month-old EBNA1-transgenic mouse with partially autolyzed organs had spleen and brain histology consistent with a lymphoma.
Body weights recorded at autopsy for EBNA1-transgenic mice were similar among mice of the three lineages and negative control mice: 31 ± 7 g for 86 EBNA1 mice of three lineages versus 30 ± 7 g for 45 control mice. Thus, EBNA1 had no adverse effect on premature mortality or postmature animal weight (Fig. 2A).
Fig. 2.
Comparison of body weight, spleen size, spleen cell types, and serum Ig levels in EBNA1-transgenic and littermate control mice. (A) Comparison of body weight, spleen weight, and spleen length at 18-26 months. (B) Comparison of total nucleated spleen cell numbers. (C) Flow cytometry analysis of splenocyte protein surface marker expression at 18-24 months. Six independent experiments were done, and the values shown are averages with standard deviation based on 26 EBNA1-transgenic and 26 control mice (CD3ε), 32 transgenic and 32 control mice (CD4), 29 transgenic and 29 control mice (CD8), 3 transgenic and 3 control mice (CD62L, MHCII, and CD11b), 19 transgenic and 19 control mice (B220), 15 transgenic and 15 control mice (CD5), 15 transgenic and 15 control mice (IgM), 6 transgenic and 6 control mice (IgD), and 4 transgenic and 4 control mice (CD25). (D) Duplicate ELISA analyses of serum Ig levels from 39 EBNA1 and 34 control 18- to 24-month-old mice. Error bars, 2 SD.
EBNA1 Expression in Transgenic Mice. Transgenic EBNA1 protein expression was initially evaluated by Western blot of cells from organs of 2- to 3-month-old mice. EBNA1 expression was similar in the three lineages and was highest in B220(+) splenocytes (Fig. 3A and data not shown). When equal numbers of cells were loaded, EBNA1 expression in transgenic B220(+) splenocytes was ≈50% of the level in EBV-transformed LCLs (Fig. 3A). However, mouse splenocytes have <10% of the protein of human LCLs or BJAB cells as assessed by protein assay or by tubulin blot (Fig. 3A Lower). When adjusted for equal protein loading, EBNA1 levels in transgenic B220(+) splenocytes were 3-fold higher than LCLs and similar to BJAB-converted FLAG-EBNA1-expressing cells (21) (Fig. 3B). Expression was lower in transgenic B220(-) splenocytes and CD8(+) thymocytes (Fig. 3 A-C and data not shown). EBNA1 was also detected in Western blots of bone marrow, lymph node, and peripheral blood extracts from mice of all three lineages and was not detected in liver, brain, stomach, kidney, or lung extracts (Fig. 3 A and C and data not shown). By Western blot, EBNA1 protein expression in transgenic splenocytes was constant at 2, 3, 19, or 21 months of age, across the three lineages, and through 11 generations (Fig. 3 and data not shown; variations in expression level in individual experiments were artifacts). Furthermore, EBNA1 from 10th-generation lineages 68 and 73 transgenic splenocyte extracts induced the same gel shift as EBNA1 stably expressed in BJAB cells (Fig. 3D). Moreover, PCR-amplified EBNA1 DNA sequences from the promoter through DNA encoding the FLAG epitope and the 5′ end of the EBNA1 ORF into the GC-rich DNA encoding the EBNA1 Gly-Ala repeat and from the 3′ end of the Gly-Ala repeat through the 3′ EBNA1 ORF into vector DNA in the 11th generation of the three EBNA1 transgenes were identical to the original sequences. Thus, across the three EBNA1 transgene lineages, over multiple generations, EBNA1 was stable and non-mutated through critical domains, functional by gel shift, expressed in B220(+) splenocytes at higher levels than in LCLs, and expressed in B220(-) splenocytes and CD8(+) thymocytes at levels almost comparable to LCLs (Fig. 3 and data not shown).
Fig. 3.
EBNA1-transgenic protein expression. (A) EBNA1 protein levels in third-generation lineages 58, 68, and 73 B220 (+) and (-) splenocytes, CD8(+) thymic cells, and total bone marrow cells were compared with levels in equal numbers of EBV-transformed human B lymphocytes, LCLs, or EBV-negative BJAB lymphoblasts (BJ). FV, FVB control cells. The protein loaded in each lane is indicated. Western blots of the same extracts with EBNA1 and tubulin antibody are shown. (B) Western blots of 40-μg protein extracts from BJ cells, BJ cells that had been converted 2-3× EBNA1 overexpression, BJFE1, or from spleens of 12th-generation lineage 73 and littermate control mice. (C) Western blot of protein extracts from kidney (Ki), liver (Li), stomach (St), brain (Br), thymus (Th), and spleen (Sp) of lineage 58 or littermate control with EBNA1-specific antibody. ns, nonspecific reactivity with hepatocyte extracts. (D) In vitro gel shift with nuclear extracts from BJFE1 cells or total cell extract from lineages 73 and 68 or littermate control splenocytes. Extracts were incubated with labeled EBNA1-cognate DNA probe in the presence or absence of EBNA1 (E1) or control (C) antibody, or wild-type (W) or mutant (M) competitor DNA.
Immunohistochemical staining of EBNA1-transgenic and control spleens showed that EBNA1 protein was expressed at very high levels in the nuclei of a small number of transgenic splenocytes that were mostly in B220(+) splenocyte-rich areas and was at a lower level in the nuclei of many other B220(+) and B220(-) splenocytes (Fig. 4 Upper and data not shown), consistent with Western blot results (Fig. 3A).
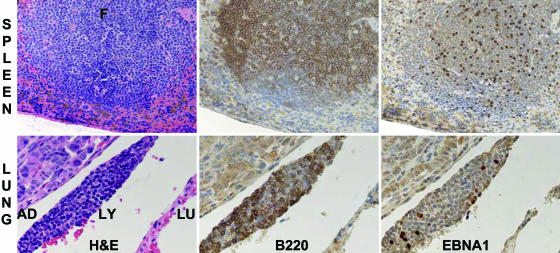
Fig. 4.
An EBNA1-transgenic mouse spleen lymphoid follicle (F) and a lung adenoma are shown (Upper and Lower, respectively). Tissues were stained with hematoxylin/eosin, B220 antibody, or EBNA1 antibody. The mouse lung (Lower) has a typical adenoma (AD) in the upper left corner, a peripulmonary lymphoid deposit (LY) in the center, and adjacent normal lung (LU) in the lower right corner. EBNA1 staining of the adenoma and normal lung resulted in light brown background cytoplasm staining and little or no nuclear staining, whereas ≈30% of the lymphoid nuclei stained light to dark brown. Photomicrographs were at ×100 (Upper) and ×400 (Lower), using a Zeiss photomicroscope and digital camera.
EBNA1 Did Not Alter Lymphocyte Differentiation, Proliferation, or Survival. To initially identify an EBNA1 effect on lymphocyte proliferation or survival, total splenocyte cell numbers in 17 EBNA1-transgenic mice of three lineages were compared to that of nine littermate controls at 2-6 months of age. EBNA1-transgenic mice had 61 × 106 ± 20 × 106 total splenocytes, whereas control mice had 59 × 106 ± 19 × 106 splenocytes (Fig. 2B). Furthermore, EBNA1-transgenic mice total splenocyte numbers were not different among the three lineages.
In studies of mature and aging mice, 42 EBNA1-transgenic mice of the three lineages and 36 littermate control mice were killed at 18-26 months of age, and splenocyte preparations were compared for the percentage of cells CD3ε+, CD4+, CD8+, CD62L+, B220+, IgM+, IgD+, CD5+, MHC class II+, CD25+, and CD11b+ (Fig. 2C). EBNA1-transgenic mouse splenocyte cell fractions were similar to those of negative littermate control mice for each cell surface marker. Furthermore, for 86 EBNA1-transgenic mice of the three lineages that were killed for studies at 18-26 months of age, spleen length and weight were not different from that of 45 control mice. EBNA1-transgenic mouse spleens were 17 ± 3.2 mm long and weighed 116 ± 80 mg, whereas EBNA1-negative littermate control mouse spleens were 17.1 ± 2.1 mm long and weighed 109 ± 46 mg (Fig. 2A). IgM, IgG, and total Ig levels in 39 EBNA1-transgenic mice and 34 littermate control mice at 18-26 months were also similar (Fig. 2D). These data indicate that EBNA1 does not affect B, T, or macrophage cell differentiation, proliferation, or survival, basal IgM, IgG, or total Ig levels, or spleen size, weight, or total cell number.
EBNA1 Did Not Cause Lymphoid Hyperplasia or Lymphoma. Female FVB mice have an expected lymphoma or histiocytic sarcoma prevalence at autopsy of 12% at 24 months, whereas male FVB mice have a lower incidence (49). When 86 EBNA1-transgenic and 45 control mice were killed at 18-26 months and autopsied with visualization of abdominal lymph nodes, spleen, liver, kidney, adrenal glands, and lung, abnormal lymph nodes were not observed. Abnormalities of other organs resulted in focused histological examination in search of lymphoma or other tumors. In addition, lymph node, spleen, liver, kidney, adrenal, and lung sections were routinely examined. Surveys failed to find any difference between EBNA1-transgenic and negative littermate control spleens in lymphoid follicle size, mantle or marginal zone size, cell density, red pulp size, or cell composition (Fig. 4 and data not shown). Aside from the one EBNA1-transgenic mouse that died of a probable lymphoma at 14 months, lymphoma was not detected at autopsy in 24 other EBNA1-transgenic mice that incidentally died or at autopsy in 86 EBNA1-transgenic mice that were specifically killed for autopsy and organ histology at 18-26 months. One EBNA1-transgenic 19-month-old mouse was found at autopsy and organ histology to have a histiocytic sarcoma of the uterus and pleura. Lymphomas or histiocytic sarcomas were not found at autopsy of 18 control mice that incidentally died or of 45 control mice that were specifically killed for autopsy and organ histology at 18-26 months. Thus, EBNA1 expression in B and T lymphocytes was not associated with an increase in lymphoid hyperplasia or lymphoma.
EBNA1-Transgenic Mice Had a Higher Prevalence of Pulmonary Adenomas. Male and female FVB mice have a described 40% prevalence of pulmonary adenomas on autopsy at 24 months, with many adenomas exhibiting progression toward carcinoma (49). EBNA1-transgenic mice had the described 38% (17 of 45) pulmonary adenoma prevalence at 18-26 months and only 2 of 17 showed degeneration toward carcinoma. However, matched littermate control mice had only 7% (3 of 41) pulmonary adenoma prevalence. The difference was apparent in lineages 58, 68, and 73, where the pulmonary adenoma prevalence was 5 of 14 (36%) versus 0 of 19 among littermate controls, 6 of 15 (40%) versus 1 of 11 (9%), and 6 of 16 (38%) versus 2 of 11 (18%). The higher prevalence of pulmonary adenomas in EBNA1 transgenes than in littermate controls could be due to an EBNA1 effect on pulmonary cells or to a more thorough search for palpable nodules in organs of EBNA1 transgene mice to avoid missing an EBNA1-associated lymphoma. The very low prevalence of pulmonary adenomas in littermate control mice versus previous FVB mice (49) was consistent with the latter possibility.
A direct EBNA1 effect on pulmonary adenoma prevalence would require EBNA1 expression in normal alveolar/bronchial epithelial cells or in adenoma cells. EBNA1-transgenic lung protein extracts were separated by SDS/PAGE and Western blotted for EBNA1. EBNA1 was readily detected in EBNA1-transgenic mouse splenocyte extracts and was not detectable in lung extracts (data not shown). However, the sensitivity level was not adequate to exclude EBNA1 expression in specific epithelial cells. Immune histochemistry with EBNA1 monoclonal antibody was sensitive and cell-type-specific in spleen histochemistry (Fig. 4 Upper); EBNA1 was readily detected in peri- or intrapulmonary lymphoid cell nuclei (Fig. 4 Lower and data not shown). However, EBNA1 was not detected in adjacent pulmonary epithelial cell nuclei or in adenomas; these nuclei were nearly uniformly without tint of EBNA1-specific brown stain, whereas lymphoid cell nuclei ranged from light to very dark brown (Fig. 4 Lower).
Discussion
These experiments evaluated the consequences of transgenic EBNA1 protein expression in lymphocytes of three independent lineages of FVB mice. EBNA1 was expressed in transgenic B220(+) B lymphocytes at a higher level relative to total cell protein than in a typical EBV-infected LCL. EBNA1 was also expressed at close to LCL levels in B220(-) B and T lymphocytes, including CD8(+) T cells, and was not detectable in liver, lung, kidney, or brain. EμPμ-driven FLAG-tagged EBNA1 was fully active in enhancing oriP-dependent gene expression in human B lymphoblasts. The EBNA1 gene in the three transgenic mouse lineages had the expected unique nucleotide and imputed amino acid sequence. Moreover, the EBNA1 proteins had the expected size and specificity for binding EBNA1-cognate DNA.
EBNA1 expression did not alter splenocyte number, spleen CD4(+), CD8(+), or total T cells, CD5(+), B220(+), IgM(+), IgD(+), MHC II, or CD11b(+) cells, IgM, IgG, or total Ig levels, incidental mortality, or total weight. Upon autopsy at 18-26 months of age, EBNA1 expression did not alter spleen length, weight, or architecture. Gross and microscopic examination of spleen and other organs did not reveal lymphoid hyperplasia or lymphoma in 45 control mice, 57 EBNA1-transgenic littermates, or 29 other EBNA1-transgenic mice without littermate controls. In all, lymphoma was not found in 86 fully mature EBNA1-transgenic mice or in 45 fully mature control mice.
At 24 months, FVB mice have a described lymphoma prevalence for female mice of 6% (49). For both EBNA1-transgenic and control mice, ≈2/3 of the mice that were maintained to 18-26 months before killing and autopsy were female. An EBNA1-related increase in lymphoma prevalence should have resulted in lymphoma detection among the 86 EBNA1 transgene-positive mice that were autopsied at 18-26 months. Thus, EBNA1 does not substantially affect lymphoma prevalence in the FVB genetic background.
Because inbred mouse strains differ in lymphoma predisposition (50-52), these FVB mouse data do not exclude the possibility that EBNA1 could cause lymphoma in C57BL/6 mice (40, 53). For example, Eμ-enhanced c-Myc expression induces pre-B and B lymphomas in C57BL/6 mice, mostly T cell lymphomas in C3H mice, and T and B cell lymphomas in C57BL/6 × C3H F1 mice (41, 42, 54). However, as noted in the Introduction, readily detectable EBNA1 protein expression in EμPyP EBNA1 C57BL/6 mice, albeit at levels less than latent EBV infection in LCLs, resulted in 0 of 46 mice with neoplastic lymphoma histology after killing at ≤18 months and 17 of 94 mice with neoplastic lymphoma histology after killing at 19-24 months (40). This prevalence was higher than in control mice (40) but less than the described C57BL/6 lymphoma prevalence of ≈30% at 24 months (53). Thus, EBNA1 protein may not have a substantial effect in C57BL/6 mice, as we report in three lineages of FVB mice with EBNA1 protein at levels at least as high as those in LCLs.
These three FVB EBNA1 transgene lineages have been crossed with C57BL/6 mice through six generations to determine whether EBNA1 has a phenotypic effect in C57BL/6 mice. At the third cross, incidental death did not occur in 18 EBNA1-transgenic mice that were kept for 1 year. Complete data for three lineages after five or six backcrosses with C57BL/6 will require another 2.5 years.
Most previous studies of EBNA1 effects on cells in culture are also consistent with EBNA1's having no effect (or a small effect) on cell growth or survival. EBNA1 expression in human lymphocytes did not increase thymidine incorporation (43). Conditional EBNA1 dominant negative expression in an LCL with integrated EBV DNA did not alter cell growth or gene expression (20). Furthermore, LCLs transformed by an EBNA1-negative EBV were not different in growth from wild-type virus-transformed cells (12). Moreover, the increased tumor induction that follows SCID mouse injection with EBV reinfected Akata Burkitt lymphoma cells is attributed to the EBV small RNAs and not to EBNA1 (55, 56). Nevertheless, EBNA1 can interact with USP7, the p53 ubiquitin protease, and EBNA1 overexpression can inhibit p53-overexpression-mediated apoptosis (32, 33, 37).
Acknowledgments
We thank Rebecca Greenwald, Kenneth Izumi, Mee Soo Chang, and Jeffery Kutok, who provided advice and help. This work was supported by National Cancer Institute Grant CA47006 and the Korea Research Foundation Program grant (during 1997).
Author contributions: M.-S.K., A.S., E.C.-M., S.C.H., and E.K. designed research; M.-S.K., H.L., T.Y., H.W., R.B., S.C.H., and E.K. performed research; M.-S.K., H.W., E.C.-M., R.B., and E.K. analyzed data; and M.-S.K. and E.K. wrote the paper.
Abbreviations: EBV, Epstein-Barr virus; EBNA1, EBV nuclear antigen 1; LCL, lymphoblastoid cell line; oriP, origin of plasmid replication; Eμ, IgH constant region enhancer; PyP, polyoma virus early promoter; EμPyP-EBNA1, EμPyP-regulated EBNA1; BJAB, human B lymphoma; F, FLAG tag; CD, cluster of differentiation.
References
- 1.Kieff, E. & Rickinson, A. B. (2001) in Fields Virology, ed. Howley, P. M. (Lippincott, Philadelphia), Vol. 2, pp. 2511-2574. [Google Scholar]
- 2.Rickinson, A. B. & Kieff, E. (2001) in Fields Virology, ed. Howely, P. M. (Lippincott, Philadelphia), Vol. 2, pp. 2575-2628. [Google Scholar]
- 3.Hochberg, D., Middeldorp, J. M., Catalina, M., Sullivan, J. L., Luzuriaga, K. & Thorley-Lawson, D. A. (2004) Proc. Natl. Acad. Sci. USA 101, 239-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuppers, R. (2003) Nat. Rev. Immunol. 3, 801-812. [DOI] [PubMed] [Google Scholar]
- 5.Yates, J., Warren, N., Reisman, D. & Sugden, B. (1984) Proc. Natl. Acad. Sci. USA 81, 3806-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yates, J. L., Warren, N. & Sugden, B. (1985) Nature 313, 812-815. [DOI] [PubMed] [Google Scholar]
- 7.Reisman, D. & Sugden, B. (1986) Mol. Cell. Biol. 6, 3838-3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawlins, D. R., Milman, G., Hayward, S. D. & Hayward, G. S. (1985) Cell 42, 859-868. [DOI] [PubMed] [Google Scholar]
- 9.Davenport, M. G. & Pagano, J. S. (1999) J. Virol. 73, 3154-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gahn, T. A. & Sugden, B. (1995) J. Virol. 69, 2633-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee, M. A., Diamond, M. E. & Yates, J. L. (1999) J. Virol. 73, 2974-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humme, S., Reisbach, G., Feederle, R., Delecluse, H. J., Bousset, K., Hammerschmidt, W. & Schepers, A. (2003) Proc. Natl. Acad. Sci. USA 100, 10989-10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambinder, R. F., Mullen, M. A., Chang, Y. N., Hayward, G. S. & Hayward, S. D. (1991) J. Virol. 65, 1466-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambinder, R. F., Shah, W. A., Rawlins, D. R., Hayward, G. S. & Hayward, S. D. (1990) J. Virol. 64, 2369-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wysokenski, D. A. & Yates, J. L. (1989) J. Virol. 63, 2657-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldsmith, K., Bendell, L. & Frappier, L. (1993) J. Virol. 67, 3418-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bochkarev, A., Barwell, J. A., Pfuetzner, R. A., Bochkareva, E., Frappier, L. & Edwards, A. M. (1996) Cell 84, 791-800. [DOI] [PubMed] [Google Scholar]
- 18.Cruickshank, J., Shire, K., Davidson, A. R., Edwards, A. M. & Frappier, L. (2000) J. Biol. Chem. 275, 22273-22277. [DOI] [PubMed] [Google Scholar]
- 19.Kapoor, P. & Frappier, L. (2003) J. Virol. 77, 6946-6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang, M. S., Hung, S. C. & Kieff, E. (2001) Proc. Natl. Acad. Sci. USA 98, 15233-15238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung, S. C., Kang, M. S. & Kieff, E. (2001) Proc. Natl. Acad. Sci. USA 98, 1865-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koons, M. D., Van Scoy, S. & Hearing, J. (2001) J. Virol. 75, 10582-10592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackey, D. & Sugden, B. (1999) Mol. Cell. Biol. 19, 3349-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackey, D. & Sugden, B. (1997) J. Biol. Chem. 272, 29873-29879. [DOI] [PubMed] [Google Scholar]
- 25.Marechal, V., Dehee, A., Chikhi-Brachet, R., Piolot, T., Coppey-Moisan, M. & Nicolas, J. C. (1999) J. Virol. 73, 4385-4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levitskaya, J., Coram, M., Levitsky, V., Imreh, S., Steigerwald-Mullen, P. M., Klein, G., Kurilla, M. G. & Masucci, M. G. (1995) Nature 375, 685-688. [DOI] [PubMed] [Google Scholar]
- 27.Levitskaya, J., Sharipo, A., Leonchiks, A., Ciechanover, A. & Masucci, M. G. (1997) Proc. Natl. Acad. Sci. USA 94, 12616-12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horner, D., Lewis, M. & Farrell, P. J. (1995) Intervirology 38, 195-205. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy, G. & Sugden, B. (2003) Mol. Cell. Biol. 23, 6901-6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang, D., Frappier, L., Gibbs, E., Hurwitz, J. & O'Donnell, M. (1998) Nucleic Acids Res. 26, 631-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huber, M. D., Dworet, J. H., Shire, K., Frappier, L. & McAlear, M. A. (2000) J. Biol. Chem. 275, 28764-28773. [DOI] [PubMed] [Google Scholar]
- 32.Holowaty, M. N., Sheng, Y., Nguyen, T., Arrowsmith, C. & Frappier, L. (2003) J. Biol. Chem. 278, 47753-47761. [DOI] [PubMed] [Google Scholar]
- 33.Holowaty, M. N., Zeghouf, M., Wu, H., Tellam, J., Athanasopoulos, V., Greenblatt, J. & Frappier, L. (2003) J. Biol. Chem. 278, 29987-29994. [DOI] [PubMed] [Google Scholar]
- 34.Van Scoy, S., Watakabe, I., Krainer, A. R. & Hearing, J. (2000) Virology 275, 145-157. [DOI] [PubMed] [Google Scholar]
- 35.Wang, Y., Finan, J. E., Middeldorp, J. M. & Hayward, S. D. (1997) Virology 236, 18-29. [DOI] [PubMed] [Google Scholar]
- 36.Huang, S., Stupack, D., Mathias, P., Wang, Y. & Nemerow, G. (1997) Proc. Natl. Acad. Sci. USA 94, 8156-8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennedy, G., Komano, J. & Sugden, B. (2003) Proc. Natl. Acad. Sci. USA 100, 14269-14274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kube, D., Vockerodt, M., Weber, O., Hell, K., Wolf, J., Haier, B., Grasser, F. A., Muller-Lantzsch, N., Kieff, E., Diehl, V. & Tesch, H. (1999) J. Virol. 73, 1630-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruf, I. K., Rhyne, P. W., Yang, H., Borza, C. M., Hutt-Fletcher, L. M., Cleveland, J. L. & Sample, J. T. (1999) Mol. Cell. Biol. 19, 1651-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson, J. B., Bell, J. L. & Levine, A. J. (1996) EMBO J. 15, 3117-3126. [PMC free article] [PubMed] [Google Scholar]
- 41.Adams, J. M., Harris, A. W., Pinkert, C. A., Corcoran, L. M., Alexander, W. S., Cory, S., Palmiter, R. D. & Brinster, R. L. (1985) Nature 318, 533-538. [DOI] [PubMed] [Google Scholar]
- 42.Adams, J. M., Harris, A. W., Langdon, W. Y., Pinkert, C. A., Brinster, R. L., Palmiter, R. D., Corcoran, L., Alexander, W. S., Graham, M. W. & Cory, S. (1986) Curr. Top. Microbiol. Immunol. 132, 1-8. [DOI] [PubMed] [Google Scholar]
- 43.Gross, T. G., Sakai, K. & Volsky, D. J. (1986) Biochem. Biophys. Res. Commun. 134, 1260-1268. [DOI] [PubMed] [Google Scholar]
- 44.Chuang, T. C., Way, T. D., Lin, Y. S., Lee, Y. C., Law, S. L. & Kao, M. C. (2002) FEBS Lett. 532, 135-142. [DOI] [PubMed] [Google Scholar]
- 45.Daibata, M., Kubonishi, I. & Miyoshi, I. (1996) J. Virol. 70, 9003-9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baer, R., Bankier, A. T., Biggin, M. D., Deininger, P. L., Farrell, P. J., Gibson, T. J., Hatfull, G., Hudson, G. S., Satchwell, S. C., Seguin, C., et al. (1984) Nature 310, 207-211. [DOI] [PubMed] [Google Scholar]
- 47.Sethna, M. P., van Parijs, L., Sharpe, A. H., Abbas, A. K. & Freeman, G. J. (1994) Immunity 1, 415-421. [DOI] [PubMed] [Google Scholar]
- 48.Luftig, M., Yasui, T., Soni, V., Kang, M. S., Jacobson, N., Cahir-McFarland, E., Seed, B. & Kieff, E. (2004) Proc. Natl. Acad. Sci. USA 101, 141-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahler, J. F., Stokes, W., Mann, P. C., Takaoka, M. & Maronpot, R. R. (1996) Toxicol. Pathol. 24, 710-716. [DOI] [PubMed] [Google Scholar]
- 50.Akagi, K. & Yamamura, K. (2001) Jpn. J. Cancer Res. 92, 499-505. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Akagi, K., Miyazaki, J. & Yamamura, K. (1992) Jpn. J. Cancer Res. 83, 269-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yukawa, K., Kikutani, H., Inomoto, T., Uehira, M., Bin, S. H., Akagi, K., Yamamura, K. & Kishimoto, T. (1989) J. Exp. Med. 170, 711-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Babbitt, J. T., Kharazi, A. I., Taylor, J. M., Bonds, C. B., Mirell, S. G., Frumkin, E., Zhuang, D. & Hahn, T. J. (2000) Carcinogenesis 21, 1379-1389. [PubMed] [Google Scholar]
- 54.Lombardi, L., Newcomb, E. W. & Dalla-Favera, R. (1987) Cell 49, 161-170. [DOI] [PubMed] [Google Scholar]
- 55.Komano, J., Sugiura, M. & Takada, K. (1998) J. Virol. 72, 9150-9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Komano, J., Maruo, S., Kurozumi, K., Oda, T. & Takada, K. (1999) J. Virol. 73, 9827-9831. [DOI] [PMC free article] [PubMed] [Google Scholar]