Abstract
In a natural community of 49 species (12 species of aphids and 37 species of their parasitoids), body lengths of 2,151 parasitoid individuals were, to an excellent approximation, related to the body lengths of their individual aphid hosts by a power law with an exponent close to 3/4. Two alternative models predict this exponent. One is based on surface area to volume relationships. The other is based on recent developments in metabolic ecology. Both models require a changing ratio (in both host and parasitoid) of length to diameter with increasing body length. These changing ratios are manifested differently in the two models and result in testably different predictions for the scaling of body form with increasing size. The estimated exponent of 3/4 for the relationship between individual host body size and individual parasitoid body size degrades to an exponent of nearly 1/2, and the scatter in the relationship between aphid and parasitoid body length is substantially increased, if the average length of a parasitoid species is examined as a function of the average length of its aphid host species instead of using measurements of individuals.
Keywords: allometry, aphids, development, metabolism, weight–length relations
Explaining the size of organisms is an enduring challenge to ecologists and evolutionary biologists (1, 2) to cellular and developmental biologists (3). Ecological studies of the relationship between consumer and resource body sizes (4–8) usually assume that the average body size of a species is an adequate approximation to the size of the individuals taking part in a particular trophic interaction. However, individuals of different size within one resource species may be selectively consumed by different consumer species or individuals of different size within a given consumer species. Vice versa, individuals of different size within one consumer species may selectively consume resource species of different average size or individuals of different size within a given resource species. To understand the relationship between consumer and resource body sizes, it is important that the data correctly represent the body sizes of the consumers and resources involved in the trophic interactions. What are the consequences of focusing on body sizes of consumer and resource individuals vs. average sizes of taxonomic species for understanding feeding relations in natural communities? To answer this question, here we report quantitative field data on body sizes in individual events of parasitism.
Animal consumers are often considerably larger than their prey (4), whereas parasites and pathogens are generally much smaller than their resources (5). Solitary insect parasitoids that complete their larval development on or in the body of other living insects, and require just a single host to complete development, lie between these extremes: they are often similar in size to their insect hosts. Parasitoid and host body sizes are well suited to shed light on the role of individual differences in consumer-resource body size relations because the variations in both parasitoid and host body sizes are likely to be of comparable magnitude.
Parasitoids are important components of all terrestrial ecological communities. Probably 1–2 million species are parasitoids (9), and they are thus a significant fraction of all species on this planet. As potentially important regulators of their host populations, parasitoids are intensively used in biological control (10). Most prior studies of the body sizes of hosts and parasitoids consider only a single species of host. The few studies (11–14) that consider host–parasitoid size relationships of multiple species have only one data point per species.
We studied quantitatively the relationship between final individual aphid host and parasitoid body length in a natural aphid-parasitoid community with multiple species of hosts and parasitoids. The objectives of the study were to (i) describe the relationship between final aphid host and parasitoid body size, (ii) analyze the consequences of focusing on body sizes of consumer and resource individuals vs. average sizes of taxonomic species for the apparent relationship between final aphid host and parasitoid body size, and (iii) offer two alternative explanations for the relationship between final aphid host and parasitoid body size. We hope that future studies will discriminate between these alternative explanations.
Methods
Aphid mummies that contained developing parasitoid larvae were collected from May to September 1994, in an abandoned field at Silwood Park. Müller et al. (15) described the aphid-parasitoid community at this site. Mummies were kept at room temperature in the laboratory until the parasitoids emerged. The mummies with diapausing parasitoids were kept in an outside insectary from the beginning of November 1994 to the beginning of February 1995. The samples were then brought back to the laboratory.
The aphid mummy and newly emerged parasitoid were pinned and later measured (length, reported in millimeters) and identified to species (Table 1). The lengths of the aphid mummy and the parasitoid were measured from the front of the head to the end of abdomen. The cauda of aphids was excluded because it is pronounced only in adult stages and some of the mummies were in the fourth-instar stage. Specimens that had been pinned in a distorted position were excluded. The data (listed in full in Data Set 1, which is published as supporting information on the PNAS web site) consist of 2,151 individual observations of the length of individual parasitoids emerging from aphid hosts and the length of their aphid mummy hosts.
Table 1. The species and number of observations of each link.
| Wasp species
|
Aphid species
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| 13 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 29 | 0 |
| 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 15 | 76 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 16 | 99 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 |
| 17 | 0 | 0 | 0 | 0 | 0 | 233 | 0 | 0 | 0 | 0 | 0 | 0 |
| 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 288 | 0 | 0 |
| 19 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 2 | 0 |
| 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 28 | 0 | 42 | 0 |
| 21 | 0 | 4 | 0 | 0 | 0 | 0 | 41 | 0 | 0 | 14 | 0 | 0 |
| 22 | 0 | 0 | 68 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 94 | 0 |
| 24 | 0 | 0 | 101 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 25 | 10 | 10 | 1 | 0 | 0 | 0 | 9 | 2 | 0 | 22 | 3 | 3 |
| 26 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 0 |
| 27 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 28 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 29 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 |
| 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 1 |
| 31 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 32 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 31 | 0 | 0 |
| 33 | 42 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 2 |
| 34 | 0 | 0 | 0 | 0 | 0 | 24 | 0 | 0 | 0 | 0 | 0 | 0 |
| 35 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
| 36 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 37 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 |
| 38 | 0 | 0 | 0 | 0 | 0 | 170 | 0 | 0 | 0 | 0 | 0 | 0 |
| 39 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 45 | 0 |
| 40 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 14 | 0 | 20 | 0 |
| 41 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 42 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| 43 | 8 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 29 | 0 | 9 | 0 |
| 44 | 18 | 0 | 0 | 1 | 0 | 7 | 1 | 0 | 41 | 55 | 33 | 1 |
| 45 | 78 | 1 | 3 | 0 | 0 | 0 | 2 | 0 | 1 | 10 | 23 | 5 |
| 46 | 2 | 0 | 0 | 0 | 0 | 26 | 1 | 0 | 1 | 1 | 3 | 0 |
| 47 | 16 | 0 | 0 | 0 | 3 | 18 | 0 | 0 | 0 | 18 | 78 | 0 |
| 48 | 5 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 1 | 8 | 0 | 1 |
| 49 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
Species: 1, Acyrthosiphon pisum; 2, Amphorophora rubi; 3, Aphis grossulariae/Aphis epilobii; 4, Aulocorthum solani; 5, Brachycaudus cardui; 6, Capitophorus carduinis; 7, Macrosiphum funestum; 8, Megoura viciae; 9, Metopolophium albidum; 10, Microlophium carnosum; 11, Sitobion fragariae/Sitobion avenae; 12, Sitobion ptericolens; 13, Aphelinus abdominalis; 14, Aphelinus varipes; 15, Aphidius eadyi; 16, Aphidius ervi; 17, Aphidius matricariae; 18, Aphidius microlophii; 19, Aphidius picipes; 20, Aphidius rhopalosiphi; 21, Aphidius urticae; 22, Binodoxys acalephe; 23, Ephedrus plagiator; 24, Praon abjectum; 25, Praon dorsale; 26, Praon volucre; 27, Alloxysta brachyptera; 28, Alloxysta circumscripta; 29, Alloxysta cursor; 30, Alloxysta fulviceps; 31, Alloxysta fuscicornis; 32, Alloxysta halterata; 33, Alloxysta macrophadna; 34, Alloxysta brevis; 35, Alloxysta ramulifera; 36, Alloxysta ruficollis; 37, Alloxysta semiaperta; 38, Alloxysta tscheki; 39, Alloxysta victrix; 40, Phenoglyphis villosa; 41, Phenoglyphis xanthochroa; 42, Syrphophagus mamitus; 43, Asaphes suspensus; 44, Asaphes vulgaris; 45, Coruna clavata; 46, Dendrocerus aphidum; 47, Dendrocerus carpenteri; 48, Dendrocerus dubiosus; 49, Dendrocerus laevis. Species: 1–12, aphids; 13–26, primary parasitoids; 27–41, hyperparasitoids; 43–49, mummy parasitoids; Species 42 is both a hyperparasitoid and a mummy parasitoid. Primary parasitoid genus Aphidius belongs to Aphidiinae (Ichneumonoidea, Braconidae), and genus Aphelinus belongs to Aphelinidae (Chalcidoidea). Secondary parasitoid genera split into (family number 1) Alloxystinae (Cynipoidea, Charipidae) for genera Alloxysta and Phenoglyphis, (family number 2) Pteromalidae (Chalcidoidae) for genera Asaphes and Coruna, (family number 3) Megaspilidae (Ceraphronoidea) for genus Dendrocerus, and (family number 4) Encyrtidae (Chalcidoidea) for genus Syrphophagus.
All of the parasitoids in this study are solitary Hymenoptera (parasitoid wasps): that is, only one parasitoid individual develops within one host individual. In primary parasitoids (Braconidae: Aphidiinae, Aphelinidae), the female usually attacks nymphal instars of a living unparasitized aphid and lays one egg within the aphid. The developing parasitoid larva feeds on the aphid. When the aphid contains more than one larva of the same (superparasitism) or different (multiparasitism) species, the larvae fight until one remains. Normally, the larva kills the aphid when the aphid is in the last-instar or adult stage. At this point, the aphid is transformed into a mummy in which the parasitoid larva pupates.
The primary parasitoids may be parasitized by secondary parasitoids that may attack the primary parasitoid larva inside the still-living aphid (true hyperparasitoids, Charpidae: Alloxystinae) or after the aphid has mummified (mummy parasitoids, Pteromalidae, Megaspilidae, and Encytridae). The development of the hyperparasitoid egg(s) and their consumption of the primary parasitoid host are delayed until the primary parasitoid has killed and mummified its aphid host (16). Primary parasitoids and hyperparasitoids can potentially influence the final size of the aphid host, whereas mummy parasitoids cannot.
The major part of the interaction (searching, decision to attack a host, and final oviposition by female wasps, and growth of the primary parasitoid larvae) took place in the field. The aphid host is mummified just before the primary parasitoid larva pupates so growth of the primary parasitoid ceases at host mummification. Thus, the data represent the relationship between host and primary parasitoid body size under field situations (not in the laboratory). Larvae of secondary parasitoids grow within the aphid mummies so that some growth of secondary parasitoids may have occurred under laboratory conditions.
A link is a pair consisting of one host species and one parasitoid species known to feed on the host species. Link averages refer to the geometric mean body size of the host and parasitoid individuals actually observed in each link. Species averages are the geometric mean body size of all individuals of a species. Analyses using arithmetic means instead of geometric means lead to entirely parallel conclusions.
Analysis of covariance in combination with Tukey's honestly significant difference criterion (17) was used to analyze the effect of species identity on the regression slope. All individual data points are treated as statistically independent in calculating P values. Further details are given in Supporting Text, which is published as supporting information on the PNAS web site.
Results
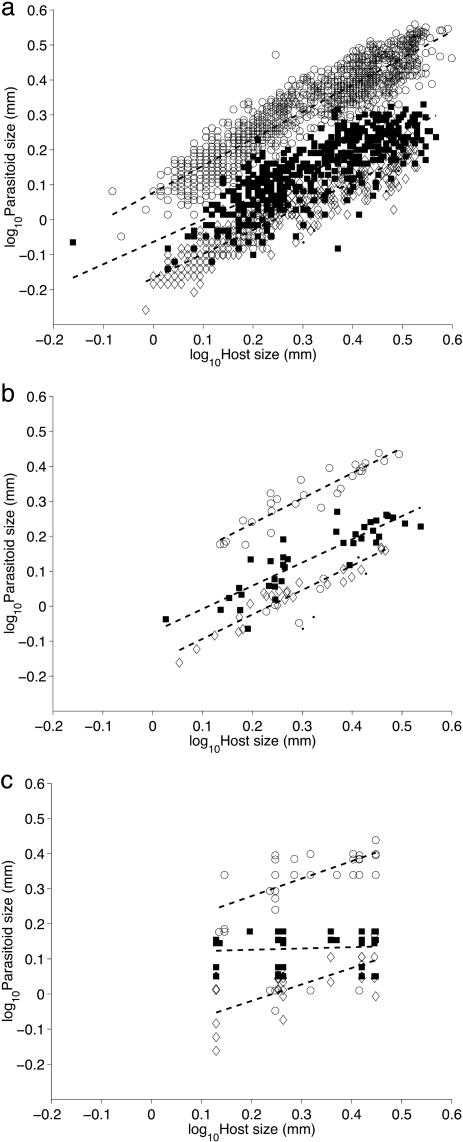
Across individuals of all species, the sizes of emerging parasitoids were strongly positively related to final aphid size for primary, hyper-, and mummy parasitoids (Fig. 1A and Table 2). On a log-log scale, there was no significant difference in regression slope between primary and secondary parasitoids (P = 0.16), but the intercepts and overall regressions were both significantly different (P < 0.001). The slope of the relationship was significantly less than 1 (P < 0.001) and was close to 3/4 for both primary (b = 0.77, r2 ≈ 0.85) and secondary (b = 0.76, r2 ≈ 0.72) parasitoids (Table 2). The regression slopes for individual species differed extensively among themselves and differed widely from the regression slopes of 0.76 or 0.77 over all individuals. The slopes 0.76 or 0.77 represented the interspecific trend over a wider range of body sizes than the species-specific clouds of points. The minimum and maximum lengths of all 2,151 aphids were 0.69 and 3.97 mm, respectively, whereas the aphid hosts of individual species of parasitoids covered a narrower range of body sizes (Table 3, which is published as supporting information on the PNAS web site).
Fig. 1.
The relationship between aphid host body size and parasitoid body size. (A) Individual data. Each marker represents the body size of a parasitoid individual and the body size of the individual aphid host from which the parasitoid emerged. (B) Link-average data. Each marker represents the geometric-average body size of the individuals of the host and parasitoid involved in one trophic link. (C) Species-average data. Each marker represents the geometric-average body size of the host and parasitoid species. ○, primary parasitoids; ⋄, hyperparasitoids; ▪, mummy parasitoids; ·, secondary parasitoid Syrphophagus mamitus (see Methods). Dashed lines in A–C are the ordinary least-squares regression lines of log10 (lengths) after excluding observations of Aphelinus abdominalis, Aphelinus varipes, and Syrphophagus mamitus (see Supporting Text).
Table 2. Ordinary least square regressions of log parasitoid length as a function of log aphid host length using body size data of individuals (A), the geometric mean body size of the individuals involved in each link (B), and the geometric mean body size of all individuals of each species (C).
| Relationship | Data type | a (± 95% c.l.) | b (± 95% c.l.) | r | n | P |
|---|---|---|---|---|---|---|
| log10(PP) = a + blog10(H) | A | 0.0780 ± 0.0061 | 0.7694 ± 0.0182 | 0.9239 | 1,177* | <0.001 |
| B | 0.09218 ± 0.0379 | 0.7208 ± 0.1149 | 0.9326 | 27* | <0.001 | |
| C | 0.1759 ± 0.0643 | 0.5056 ± 0.1922 | 0.7349 | 27* | <0.001 | |
| log10(SP) = a + blog10(H) | A | –0.1405 ± 0.0096 | 0.7644 ± 0.0305 | 0.8493 | 939 | <0.001 |
| B | –0.1291 ± 0.0400 | 0.7171 ± 0.1215 | 0.8274 | 66 | <0.001 | |
| C | 0.0157 ± 0.0535 | 0.2292 ± 0.1636 | 0.3302 | 66 | 0.0034 | |
| log10(HP) = a + blog10(H) | A | –0.1665 ± 0.0070 | 0.6862 ± 0.0255 | 0.9339 | 411 | <0.001 |
| B | –0.1632 ± 0.0271 | 0.6972 ± 0.0910 | 0.9571 | 27 | <0.001 | |
| C | –0.1135 ± 0.0628 | 0.4685 ± 0.1982 | 0.7140 | 27 | <0.001 | |
| log10(MP) = a + blog10(H) | A | –0.0632 ± 0.0141 | 0.6374 ± 0.0409 | 0.8026 | 521 | <0.001 |
| B | –0.0746 ± 0.0412 | 0.6644 ± 0.1197 | 0.8854 | 39 | <0.001 | |
| C | 0.1185 ± 0.0476 | 0.0373 ± 0.1428 | 0.0892 | 39 | 0.2999 |
H, aphid host size; PP, primary parasitoid size; SP, secondary parasitoid size; HP, hyperparasitoid size; MP, mummy parasitoid size (all in millimeters). The correlation coefficient is denoted by r, n is the number of observations, and P is the probability that the slope is not different from 0. For the intercept a and the slope coefficient b, 95% c.l. gives the 95% confidence limits. For example, in the first line, the 95% confidence interval of the estimate b = 0.7694 is from 0.7512 to 0.7876.
Observations involving primary parasitoids Aphelinus abdominalis and Aphelinus varipes excluded (see Supporting Text)
When link averages were used instead of body sizes of individuals, the intercept and slope of the relationship between aphid host and parasitoid body sizes were similar to those obtained when using individual data (Fig. 1B and Table 2) for both primary and secondary parasitoids. The slopes were significantly less than 1 (P < 0.001) but not significantly different from 3/4 (P > 0.077). On a log-log scale, a significant amount of the variation in average parasitoid size was explained by the average size of the aphid mummy (r2 ≈ 0.87 for primary parasitoids, r2 ≈ 0.68 for secondary parasitoids collectively, r2 ≈ 0.92 for hyperparasitoids, and r2 ≈ 0.78 for mummy parasitoids).
Using the geometric-average body size of all of the individuals of a species (i.e., species averages) instead of body sizes of individuals or link averages markedly affected the relationship between aphid host and parasitoid body size (Fig. 1C and Table 2). For primary parasitoids and hyperparasitoids, the intercepts increased, whereas the slopes decreased to the neighborhood of 1/2. The slopes were significantly less than both 1 (P < 0.001) and 3/4 (P < 0.008). Much less of the variation in parasitoid size was explained by the size of the aphid mummy (r2 ≈ 0.54 for primary parasitoids and r2 ≈ 0.51 for hyperparasitoids). For mummy parasitoids, the relationship between final aphid size and parasitoid size was no longer statistically significant (P > 0.29). The average size of a mummy parasitoid species was unrelated to the average size of the aphid species.
Further details of the results, such as a comparison of the relationship between hyper- and mummy parasitoids, as well as a description of species-specific relationships, are given in Supporting Text.
Discussion
This analysis of data on body sizes of individual aphids and parasitoids in a natural community of 49 species led to three conclusions. First, the relationship between emerging parasitoid body size and final aphid host body size depended on the type of parasitoid (primary, hyper-, or mummy parasitoid) as well as on the species identity of the parasitoid. Second, using the average size of a species to study the relation between consumer and resource body sizes generated a misleading picture of the real feeding interactions between an individual host and an individual parasitoid. Third, the body lengths of parasitoid individuals were related to the body lengths of aphid host individuals by a power law with an exponent close to 3/4. We discuss these conclusions and conclude by suggesting their implications for community dynamics.
Effects of Aggregation to Species Averages and of Species Identity. In the data analyzed here, going from individuals' body lengths to species-average body lengths dramatically lowered the estimated exponent (from nearly 3/4 to nearly 1/2 for primary parasitoids) of an allometric relationship, and lowered the proportion of variance that the relationship explains. According to Jensen's inequality, the average of differing individual body masses, each raised to a power b < 1, is less than the species-average body mass raised to the power b (18). Allometric functions may be corrected to allow for averaging, and the corrections matter when averaging over systems where the range in body size is large compared with the average body size (19). The statistical literature on the pitfalls of averaging nonlinear functions is extensive (19).
The close adjustment between the body sizes of individual aphid hosts and their individual primary parasitoids suggested that parasitoids developed under the joint influence of the host and the primary parasitoids, and that secondary parasitoids were influenced by both their hosts and their primary parasitoids. These mutual influences may include indirect influences of the genes of the individual aphid, primary parasitoid, and secondary parasitoid (3). Both the type of parasitoid (primary, hyper-, or mummy parasitoid) and the species identity of the plant, aphid, or parasitoid affected the relationship between the emerging parasitoid body size and the final aphid host body size. To accurately predict the body size of an emerging parasitoid, it was not sufficient to know the body size of the attacked host individual and the type of parasitoid. The species identities of the aphid and parasitoid also mattered.
Within each type of parasitoid (primary, hyper-, and mummy parasitoid), the relationship between parasitoid and aphid body size was different for different plant and aphid species. Aphid species identity did not seem to matter much for the slope of the relationship within any given parasitoid species. The explanation of this apparent contradiction is that most parasitoid species in this community were very host-specific and mainly attacked one aphid species. The slope of the relationship differed for different aphid species mainly because they were attacked by different parasitoid species. For the few parasitoid species where the data allowed the effect of aphid species identity on the regression slope to be analyzed within a parasitoid species, the effects of aphid species identity were, with one exception, insignificant. The type and species identity of the parasitoid seemed to matter most for the relationship between parasitoid and aphid body size. Plant and aphid species identities were important through the association with different parasitoid species and because different aphid species had different body sizes.
Two Models of Allometric Length Scaling in Parasitoid and Host. The conclusion that the body lengths of emerging parasitoid individuals were related to the body lengths of aphid host individuals by a power law with an exponent close to 3/4 raises the question: what explains 3/4? We propose two testable explanations. In summary, the first model assumes that emerging parasitoid mass scales in proportion to the host's surface area. This model must be rejected if observations indicate that the parasitoid gets relatively fatter, whereas the host gets relatively thinner, with increasing length. The second model assumes that parasitoid metabolic rate scales in proportion to host metabolic rate. Contrary to the first model, this second model must be rejected if the parasitoid gets relatively thinner and the host gets relatively fatter as they get longer.
As a preliminary to the first model, an overly simple physical model may be constructed from the observation that the aphid host breathes through tracheae. For biological background and interesting observations on insect tracheal systems, see Kozlowski and Konarzewski (ref. 20, p. 287). Suppose that the rate of oxygen supply to the host's tissue is limited by its surface area, or by a quantity proportional to its surface area. Savage et al. (ref. 19, p. 258) traced this “surface-area rule” as well as the basic ideas in the rest of this paragraph back to a paper by Rubner in 1883 (40). Suppose the parasitoid body density (weight per unit of volume) is independent of size, so that parasitoid volume is proportional to parasitoid mass, and suppose that emerging parasitoid mass is limited by the rate of oxygen supply within the host, i.e., by the host's surface area. Let L be the length of the host and A be the surface area of the host. Let λ be the length of the parasitoid and let μ be the mass of the parasitoid (proportional to volume by assumption). If the parasitoid body form is the same at all lengths (an assumption of geometric similarity), so that its mass or volume is proportional to the cube of its length, then λ3 ≈ μ ≈ A (where ≈ means proportionality). If the host body form is the same at all lengths (geometric similarity), so that A ≈ L2, then λ3 ≈ L2 or λ ≈ L2/3. The exponent 2/3 is nearly 3/4, but not near enough to describe our data accurately.
Our first model refines this classic model by abandoning either or both of the assumptions that the parasitoid and the host body forms are the same at all body lengths. Suppose, for example, that the host body form may be approximated by a cylinder with basal diameter D and length L perpendicular to the base, so that A = πD(D/2 + L), and that the parasitoid body form may also be approximated by a cylinder of basal diameter Δ and length λ perpendicular to the base, so that μ ≈ Δ2λ. Suppose that Δ ≈ λ1-δ and that D ≈ L1+ε, for some δ and ε.If δ > 0, then parasitoid body form is predicted to become relatively thinner with increasing length. Here, “relatively thinner” means that Δ does not increase by as large a proportion as λ increases, whether or not Δ increases absolutely as λ increases absolutely. Likewise, if ε > 0, then host body form is predicted to become relatively fatter with increasing length. Here, “relatively fatter” means that D increases by a higher proportion than L increases. However, we do not assume that δ > 0 and ε > 0.
If parasitoid mass is proportional to the host surface area, μ ≈ A, then μ ≈ Δ2λ ≈ λ3-2δ ≈ A ≈ D(D/2 + L) ≈ L2+ε(Lε/2 + 1). If ε is small enough that, for the relevant range of variation in values of L, the proportional variation in L2+ε(Lε/2 + 1) is largely accounted for by the proportional variation in L2+ε (an assumption that will be checked below), then approximately λ3-2δ ≈ L2+ε or λ ≈ L(2+ε)/(3-2δ). Any combination of values of δ and ε such that 3/4 = (2+ε)/(3 - 2δ), or equivalently 4ε + 6δ = 1, will reproduce the estimated 3/4 slope of the linear relationship between log parasitoid body length and log host body length. The constraint 4ε + 6δ = 1 can be satisfied by δ > 0 and ε > 0, for example, δ = ε = 1/10. To satisfy 4ε + 6δ = 1 and δ > 0 and ε > 0 requires that ε ≤ 1/4 and δ ≤ 1/6.
We now check the intermediate assumption that the proportional variation in L2+ε(Lε/2 + 1) is largely accounted for by the proportional variation in L2+ε. Because we are estimating slopes on log scales, what matters is the ratio of changes in L2+ε(Lε/2 + 1) over the range in our data (Table 3) from minimum Lmin = 0.69 mm to maximum Lmax = 3.97. Recall that 1/4 is the largest value of ε compatible with the measured exponent of 3/4 if δ > 0 and ε > 0. With ε = 1/4, Lmin2+ε(Lminε/2 + 1) = 0.63 and Lmax2+ε(Lmaxε/2 + 1) = 37.95. The ratio of the latter to the former is ≈60.1. By using the power-law approximation with ε = 1/4, Lmin2+ε = 0.43, and Lmax2+ε = 22.27. The ratio of the latter to the former is ≈51.3. The ratio of these ratios, 60.1/51.3 = 1.17 = (Lmaxε/2 + 1)/(Lminε/2 + 1), is not grossly different from 1. Even if ε = 1/2, (Lmaxε/2 + 1)/(Lminε/2 + 1) = 1.41. The intermediate assumption, then, is reasonable for the observed range of L.
In addition to the case where δ > 0 and ε > 0, the constraint 4ε + 6δ = 1 can be satisfied in other ways, for example, by ε = -1/2, δ = +1/2, so that both the parasitoid and the host are predicted to become relatively thinner (and, moreover, because ε = -δ in this instance, the host and parasitoid get relatively thinner at the same rate). Alternatively, the constraint 4ε + 6δ = 1 can be satisfied, for example, by ε = +3/4, δ = -1/3, so that now the parasitoid is predicted to get relatively fatter (unlike before), whereas host body form is predicted to become relatively fatter with increasing length (as when δ > 0 and ε > 0). Only the combination δ < 0 and ε < 0 is incapable of satisfying the constraint 4ε + 6δ = 1. The model must be rejected if observations indicate that the parasitoid gets relatively fatter, whereas the host gets relatively thinner with increasing length.
In a second model, instead of assuming that emerging parasitoid body mass scales in proportion to host surface area, an alternative approach assumes that emerging parasitoid metabolic rate β scales in proportion to host metabolic rate B. This assumption follows if the emerging metabolic rate (rate of oxygen consumption) for the parasitoid is limited by the rate of nutrient supply from the host, which in turn is determined by the metabolic rate of the host. The metabolic rate of the host may be proportional to its surface area, in which case B and A may be used interchangeably in these derivations. If metabolic rate scales with body mass according to β ≈ μη and B ≈ My for the parasitoid and host, and if body mass scales with length according to μ ≈ λσ and M ≈ Ls for the parasitoid and host, then β ≈ B implies that λ ≈ Lsy/(ση). If η = y, whether their common value be 2/3 or 3/4 or any other non-zero value, then λ ≈ Ls/σ. Geometric similarity of body form requires s = σ = 3, leading to the prediction that λ ≈ L, which is not consistent with the data unless the assumption that η = y is abandoned. However, the relation η = y can be maintained if, as in the previous model, body diameters scale with increasing body lengths according to Δ ≈ λ1-δ for parasitoids and D ≈ L1+ε for hosts. Then, instead of geometric similarity, one has σ = 3 - 2δ and s = 3 + 2ε. Imposing agreement with the observed 3/4 slope of log parasitoid body length as a function of log aphid length, i.e., 3/4 = s/σ = (3 + 2ε)/(3 - 2δ), leads to 6δ + 8ε = -3. Possible solutions of this constraint include δ = +1/2, ε = -3/4 (the parasitoid and host both get relatively thinner as they get longer), δ = -2/3, ε = +1/8 (the parasitoid and host both get relatively fatter as they get longer), and δ = -1/3, ε = -1/8 (the parasitoid gets relatively fatter, whereas the host gets relatively thinner as they get longer). If ε = -δ, then δ = 3/2 and Δ ≈ λ-1/2 for parasitoids and D ≈ L-1/2 for hosts. In this case, both parasitoids and hosts thin absolutely (their diameters decrease with increasing length) as well as thinning relatively. Only the combination δ > 0 and ε > 0 is excluded. Contrary to the previous approach, this model (on the assumption that η = y) must be rejected if the parasitoid gets relatively thinner and the host gets relatively fatter as they get longer.
The system of simultaneous constraints 6δ + 4ε = 1 and 6δ + 8ε = -3 has a unique solution δ = 5/6 and ε = -1. Only for these values of δ and ε would it be impossible to distinguish the two models on the basis of the body-form scaling exponents alone. Both models assumed that Δ ≈ λ1-δ and that D ≈ L1+ε. If δ = 5/6, ε = -1, then Δ ≈ λ1/6, and D ≈ L0, i.e., the diameter of the parasitoid would increase very slowly with increasing parasitoid length, whereas the diameter of the aphid would not increase at all with increasing aphid length. Otherwise, if power-law scaling of body form were a good approximation to the data for both parasitoid and host, the estimated values of δ and ε would reject one or both models. Thus, these models are readily testable with appropriate data for parasitoids and aphids on how body diameter scales with body length.
In several temperate and tropical habitats, for a variety of insect taxa at various levels of taxonomic resolution, linear regressions of log body dry mass as a function of log length had slopes notably <3 in the overwhelming majority of cases (21–23); the one reported exception with slope >3 (ref. 23, p. 107) occurred among Hemiptera (a taxon sometimes considered to include aphids) in a Massachusetts mixed forest. When all taxa were combined, slopes were <3 in all habitats, and lower in tropical than in temperate habitats. Some of these regressions used individuals as the units of observation (21, 22); others used “morphospecies” (23). If the general conclusion of these studies that longer insects are thinner than shorter insects applies to individual parasitoids and aphids of diverse species, then future observations are predicted to show that δ > 0 and ε < 0, a combination of constraints that is compatible with both models above.
Both of these phenomenological models are simpler than the developmental processes known to be at work, whether the aphid host remains alive and growing, or the parasitoid has killed its host. Future models may be expected to take account of these processes as more detailed data become available.
In the present data, the slopes of log λ as a function of log L differed among parasitoid species (Table 3). If a changing ratio of diameter to body length explained the slope, as both models suggest, then different parasitoid species should show different slopes if they differed in how their body shape changed with size, another testable prediction.
The supposition that B ≈ M3/4 is supported by a variety of data (19), although not by all data for all organisms under all methods of analysis (24). It is also supported by the influential modeling of West et al. (25, 26), although not by all commentators on their work (e.g., refs. 20 and 24). West et al. (27) and Savage et al. (19) responded to their critics. Because aphids and their parasitoids are poikilotherms, the 3/4 exponent cannot be explained by arguments that are specific to homeotherms, such as those derived in terms of mammalian respiratory and circulatory systems (ref. 28, chapter 4).
Implications of Individual Measurements for Community Dynamics. Direct measurements of the size of the individuals taking part in individual trophic interactions could significantly influence views of the role of body size in community dynamics. In the laboratory (29, 30), bigger individual parasitoids generally emerge from individual hosts that are bigger at parasitization. In many insect parasitoids, bigger female adults have higher fecundity or other indicators of fitness (31). Thus, bigger host size could be associated with higher female parasitoid fitness, other things being equal (32–34). Because female parasitoid body size is well correlated with longevity and egg load, the size of the host will affect host–parasitoid community dynamics.
Our current understanding of host–parasitoid population dynamics comes largely from models of single host–single parasitoid interactions (35), some of which consider the dynamical effects of host quality on parasitoid quality (36–38). So far, models of the dynamics of multiple species of hosts and parasitoids do not include explicit variation of host quality. However, female parasitoids are selective in attacking hosts as a function of host size. Understanding the dynamics of parasitoid communities (39) is likely to involve an understanding of how host size affects the parasitoids that emerge, and how parasitoids choose among the host sizes available. The average size of a species is probably limited as a means of achieving such understanding.
Supplementary Material
Acknowledgments
We thank Bo Ebenman, James Gillooly, Annie Jonsson, William Murdoch, and Roger Nisbet for comments on the manuscript; Irma Adriaanse for practical assistance with sampling and pinning; Robert Belshaw for assistance with identification of primary parasitoids; Frank van Veen for assistance with identification of hyperparasitoids; and Joshua Lederberg for helpful guidance to literature. J.E.C. thanks John Lawton for the invitation to visit the Centre for Population Biology, Imperial College, Silwood Park, in 1995, which led to collaboration between J.E.C. and C.B.M.; Kathe Rogerson for assistance; and Mr. and Mrs. William T. Golden for hospitality during this work. This work was supported in part by National Institutes of Health Grant 1 P50 GM68763-02 (to V.M.S.) and U.S. National Science Foundation Grants BSR92-07293 and DEB-9981552 (to J.E.C.).
Author contributions: J.E.C. and H.C.J.G. designed research; J.E.C. and C.B.M. performed research; J.E.C. and V.M.S. contributed new reagents/analytic tools; J.E.C. and T.J. analyzed data; and J.E.C., T.J., C.B.M., H.C.J.G., and V.M.S. wrote the paper.
References
- 1.Colinvaux, P. (1978) Why Big Fierce Animals Are Rare: An Ecologist's Perspective (Princeton Univ. Press, Princeton).
- 2.Witting, L. (1998) Trends Ecol. Evol. 13, 25. [DOI] [PubMed] [Google Scholar]
- 3.Gomer, R. H. (2001) Nat. Rev. Mol. Cell Biol. 2, 48-54. [DOI] [PubMed] [Google Scholar]
- 4.Cohen, J. E., Pimm, S. L., Yodzis, P. & Saldaña, J. (1993) J. Anim. Ecol. 62, 67-78. [Google Scholar]
- 5.Memmott, J., Martinez, N. D. & Cohen, J. E. (2000) J. Anim. Ecol. 69, 1-15. [Google Scholar]
- 6.Vézina, A. F. (1985) Oecologia 67, 555-565. [DOI] [PubMed] [Google Scholar]
- 7.Warren, P. H. & Lawton, J. H. (1987) Oecologia 74, 231-235. [DOI] [PubMed] [Google Scholar]
- 8.Dixon, A. F. G. & Hemptinne, J.-L. (2001) Ecology 82, 1847-1856. [Google Scholar]
- 9.Godfray, H. C. J. (1994) Parasitoids: Behavioral and Evolutionary Ecology (Princeton Univ. Press, Princeton).
- 10.Mills, N. J. (1994) in Individuals, Populations and Patterns in Ecology, eds. Leather, S. A., Watt, A. D., Mills, N. J. & Walters, K. F. A. (Intercept, Andover, U.K.), pp. 213-222.
- 11.Blackburn, T. M. (1991) J. Anim. Ecol. 60, 151-164. [Google Scholar]
- 12.Blackburn, T. M. (1991) Funct. Ecol. 5, 65-74. [Google Scholar]
- 13.Hails, R. S. (1989) Oecologia 81, 28-32. [DOI] [PubMed] [Google Scholar]
- 14.LeMasurier, A. D. (1987) Ecol. Entomol. 12, 383-393. [Google Scholar]
- 15.Müller, C. B., Adriaanse, I. C. T., Belshaw, R. & Godfray, H. C. J. (1999) J. Anim. Ecol. 68, 346-370. [Google Scholar]
- 16.Sullivan, D. J. (1987) Annu. Rev. Entomol. 32, 323-389. [Google Scholar]
- 17.Zar, J. H. (1999) Biostatistical Analysis (Prentice–Hall, Upper Saddle River, NJ).
- 18.Cohen, J. E., Jonsson, T. & Carpenter, S. R. (2003) Proc. Natl. Acad. Sci. USA 100, 1781-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savage, V. M., Gillooly, J. F., Woodruff, W. H., West, G. B., Allen, A. P., Enquist, B. J. & Brown, J. H. (2004) Funct. Ecol. 18, 257-282. [Google Scholar]
- 20.Kozlowski, J. & Konarzewski, M. (2004) Funct. Ecol. 18, 283-289. [Google Scholar]
- 21.Rogers, L. E., Hinds, W. T. & Buschbom, R. L. (1976) Ann. Entomol. Soc. Am. 69, 387-389. [Google Scholar]
- 22.Rogers, L. E., Buschbom, R. L. & Watson, C. R. (1977) Ann. Entomol. Soc. Am. 70, 51-53. [Google Scholar]
- 23.Schoener, T. W. (1980) Ann. Entomol. Soc. Am. 73, 106-109. [Google Scholar]
- 24.Dodds, P. S., Rothman, D. H. & Weitz, J. S. (2001) J. Theor. Biol. 209, 9-27. [DOI] [PubMed] [Google Scholar]
- 25.West, G. B., Brown, J. H. & Enquist, B. J. (1997) Science 276, 122-126. [DOI] [PubMed] [Google Scholar]
- 26.West, G. B., Brown, J. H. & Enquist, B. J. (1999) Science 284, 1677-1679. [DOI] [PubMed] [Google Scholar]
- 27.West, G. B., Brown, J. H. & Enquist, B. J. (2004) Funct. Ecol. 18, 188-196. [Google Scholar]
- 28.Peters, R. H. (1983) The Ecological Implications of Body Size (Cambridge Univ. Press, Cambridge, U.K.).
- 29.Harvey, J. A., Harvey, I. F. & Thompson, D. J. (1994) Ecology 75, 1420-1428. [Google Scholar]
- 30.Sequeira, R. & Mackauer, M. (1992) Ecology 73, 183-189. [Google Scholar]
- 31.Vinson, S. B. & Iwantsch, G. F. (1980) Annu. Rev. Entomol. 25, 397-419. [Google Scholar]
- 32.Kazmer, D. J. & Luck, R. F. (1995) Ecology 76, 412-425. [Google Scholar]
- 33.Visser, M. E. (1994) J. Anim. Ecol. 63, 963-978. [Google Scholar]
- 34.West, S. A., Flanagan, K. E. & Godfray, H. C. J. (1996) J. Anim. Ecol. 65, 631-639. [Google Scholar]
- 35.Hassell, M. P. (2000) The Spatial and Temporal Dynamics of Host-Parasitoid Interactions (Oxford Univ. Press, Oxford).
- 36.Murdoch, W. W., Nisbet, R. M., Luck, R. F., Godfray, H. C. J. & Gurney, W. S. C. (1992) J. Anim. Ecol. 61, 533-541. [Google Scholar]
- 37.Murdoch, W. W., Briggs, C. J. & Nisbet, R. M. (1997) J. Anim. Ecol. 66, 542-556. [Google Scholar]
- 38.Shea, K., Nisbet, R. M., Murdoch, W. W. & Yoo, H. J. S. (1996) J. Anim. Ecol. 65, 743-755. [Google Scholar]
- 39.Hochberg, M. E. & Ives, A. R., eds. (2000) Parasitoid Population Biology (Princeton Univ. Press, Princeton).
- 40.Rubner, M. (1883) Zeitschrift für Biologie 19, 536-562. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.