Abstract
Purpose
Children with acute lymphoblastic leukemia (ALL) are generally instructed to take mercaptopurine (6-MP) in the evening and without food or dairy products. This study examines the association between 6-MP ingestion habits and 6-MP adherence, red cell thioguanine nucleotide (TGN) levels, and risk of relapse in children with TMPT wild-type genotype.
Methods
Participants included 441 children with ALL receiving oral 6-MP for maintenance. Adherence was monitored over 48,086 patient-days using the Medication Event Monitoring System; nonadherence was defined as adherence rate < 95%. 6-MP ingestion habits examined included: takes 6-MP with versus never with food, takes 6-MP with versus never with dairy, and takes 6-MP in the evening versus morning versus varying times.
Results
Median age at study was 6 years (range, 2 to 20 years); 43.8% were nonadherent. Certain 6-MP ingestion habits were associated with nonadherence (taking 6-MP with dairy [odds ratio (OR), 1.9; 95% CI, 1.3 to 2.9; P = .003] and at varying times [OR, 3.4; 95% CI, 1.8 to 6.3; P = .0001]). After adjusting for adherence and other prognosticators, there was no association between 6-MP ingestion habits and relapse risk (6-MP with food: hazard ratio [HR], 0.7; 95% CI, 0.3 to 1.9; P = .5; with dairy: HR, 0.3; 95% CI, 0.07 to 1.5; P = .2; taken in evening/night: HR, 1.1; 95% CI, 0.2 to 7.8; P = .9; at varying times: HR, 0.3; 95% CI, 0.04 to 2.7; P = .3). Among adherent patients, there was no association between red cell TGN levels and taking 6-MP with food versus without (206.1 ± 107.1 v 220.6 ± 121.6; P = .5), with dairy versus without (220.1 ± 87.8 v 216.3 ± 121.3; P =.7), or in the evening/night versus morning/midday versus varying times (218.8 ± 119.7 v 195.5 ± 82.3 v 174.8 ± 93.4; P = .6).
Conclusion
Commonly practiced restrictions surrounding 6-MP ingestion might not influence outcome but may hinder adherence. Future recommendations regarding 6-MP intake during maintenance therapy for childhood ALL should aim to simplify administration.
INTRODUCTION
Contemporary therapy for childhood acute lymphoblastic leukemia (ALL) remains dependent on a prolonged (approximately 2-year) maintenance phase of therapy during which daily oral mercaptopurine (6-MP) is critical for sustaining durable remissions.1-3 We previously showed that poor adherence to 6-MP is prevalent and is associated with an increased risk of relapse, reinforcing the need for adequate systemic exposure to 6-MP during maintenance.4-6 On the basis of international consensus recommendations, children with ALL receiving maintenance therapy across clinical trials are generally instructed to take 6-MP every evening without food or dairy products (ie, on an empty stomach, 1 to 2 hours before or after meals)7; however, these consensus recommendations are based on limited and conflicting evidence.8-17 In addition, 6-MP tablets are intended to be swallowed whole18; however, in pediatric patients these tablets are commonly chewed or crushed before ingestion.19 In this study, we examined the association between 6-MP ingestion habits and red cell 6-thioguanine nucleotide (TGN) levels, risk of relapse, and adherence to oral 6-MP in a multi-institutional cohort of racially and geographically diverse children with ALL receiving maintenance therapy.
METHODS
Study Participants
After obtaining approval from local institutional review boards, participants were enrolled in the Children’s Oncology Group prospective clinical trial AALL03N1 (NCT00268528) at one of 87 participating institutions (Appendix Table A1, online only). Written informed consent/assent was obtained from patients and/or their parents/legal guardians. Eligible patients were diagnosed with ALL at age 21 years or younger, entered the study in first remission during the maintenance phase of therapy, were receiving oral 6-MP at 75 mg/m2/dose (modified only for toxicity or illness), and belonged to one of four self-reported racial/ethnic groups (Asian, African American, Hispanic, or non-Hispanic white). Participation on a Children’s Oncology Group therapeutic trial for ALL treatment was not required.
Adherence Monitoring
Adherence to 6-MP intake was monitored electronically using the Medication Event Monitoring System (MEMS; WestRock Healthcare, Sion, Switzerland), which uses microelectronic technology to record the date and time of each bottle opening (Appendix Figure A1, online only). Patients/parents were informed of the purpose of MEMS and instructed to take all doses of 6-MP from the MEMS bottle throughout the 6-month duration of the study; MEMS data were downloaded at the end of the study period (Appendix Figure A2, online only).5 Patients taking liquid formulations of 6-MP were not included in this analysis.
Institutions completed monthly reports detailing the 6-MP dose prescribed each day and dates when 6-MP was held for illness or toxicity. In addition, institutions submitted reports of participant clinical status (ie, remission, relapse, second neoplasm, or noncancer death, and date of last contact) every 6 months for the first 5 years postenrollment and then annually.
The 6-MP dose intensity (DI) was defined as the ratio of 6-MP dose actually prescribed to the planned protocol dose of 75 mg/m2/d. MEMS-based adherence rate was defined as the ratio of the number of days with MEMS cap openings (X) to the number of days 6-MP was prescribed (N), and was reported as a percentage (X/N × 100); days when 6-MP was held by the prescriber were removed from the denominator (N). On the basis of our previous finding of high relapse rates associated with 6-MP adherence rates < 95%,5,6 patients with mean MEMS adherence rates < 95% over the study period were classified as nonadherent.
Sociodemographic Data
A sociodemographic questionnaire was completed by patients (if ≥ 18 years of age) or their parents (if <18 years of age) at study entry. The purpose of this questionnaire was to determine characteristics such as race/ethnicity, household income, and parental educational level.
Thiopurine Methyltransferase Genotype
Thiopurine methyltransferase (TPMT) genotype was determined in all patients using leukocyte DNA and polymerase chain reaction–based methods specific for the TPMT *2, *3A, *3B, and *3C variant alleles as previously described20; a TPMT wild-type genotype (normal activity) was assigned when none of these variants were detected. Only patients with wild-type genotype were included in this analysis.
Red Cell TGN Levels
Red cell TGN levels21 were measured monthly for each participant during the study period (1,395 measurements) to represent systemic exposure to 6-MP over the prior 1 to 4 weeks. TGN measurements obtained from patients who had received red blood cell transfusions within the previous 90 days (n = 53) were excluded from the TGN level analysis. 6-MP DI-adjusted TGN was calculated as the ratio of monthly TGN to 6-MP DI.
6-MP Ingestion Habits
The ingestion habits of 6-MP were elicited via parent- (for patients < 18) or self- (for patients ≥18) report questionnaire at four study time points (days 29, 57, 113, and 141; see study schema, Appendix Figure A3, online only). Responses were dichotomized as follows: takes 6-MP with food versus never with food; takes 6-MP with milk/dairy versus never with milk/dairy; swallows 6-MP tablet whole versus crushed/chewed; and follows an established 6-MP routine versus no routine. Timing of 6-MP intake was determined by MEMS records, and patients were categorized into one of two time frames (morning/midday: 5:00 AM to 3:59 PM; evening/night: 4:00 PM to 4:49 AM local time) if ≥ 75% of doses fell within one of the time frames; otherwise, the patients were classified as taking 6-MP at varying times of day.
Statistical Analysis
Predictors of 6-MP ingestion habits.
Multivariable logistic regression modeling (adjusted for age at study, sex, race/ethnicity, parental educational level, household income, National Cancer Institute [NCI] risk group,22 and cytogenetic blast characteristics) was used to determine the association of patient characteristics with 6-MP ingestion habits. Each patient was assigned a 6-MP ingestion phenotype, defined as ever answering yes at any of the four study time points to the 6-MP ingestion habit being examined (eg, a patient answering yes to “Takes 6-MP with food” at one or more time points was categorized as taking 6-MP with food in the logistic regression models).
6-MP ingestion habits and adherence.
Time-varying generalized estimating equation analysis (adjusted for age at study, study month, race/ethnicity, income, and parental education) was used to determine the association between 6-MP ingestion habits and MEMS-based adherence. 6-MP ingestion habits were measured at each of the 4 study months, and MEMS-based adherence was measured for the 28 days immediately preceding the questionnaire completion.
6-MP ingestion habits and red cell TGN levels.
This analysis was restricted to adherent patients. Multivariable linear modeling (adjusted for age at study, study month, race/ethnicity) was used to determine the association between 6-MP ingestion habits and DI-adjusted average TGN levels for the four time points associated with questionnaire completion.
6-MP ingestion habits and risk of relapse.
Cumulative incidence of first relapse at any site was calculated, treating subsequent neoplasms and noncancer deaths as competing risks. Proportional subdistribution hazards modeling (adjusted for age at study, race/ethnicity, time from start of maintenance, NCI risk group,22 cytogenetics, and adherence rate) was used to determine the association between 6-MP ingestion habits and relapse risk, treating subsequent neoplasms and noncancer deaths as competing risks.
PROCs FREQ, PHREG, GENMOD, and GLM of SAS software, version 9.4 (SAS Institute, Cary, NC) and the SAS macro PSHREG were used for analysis. Because of multiple comparisons made in the analyses, two-sided tests with P ≤ .01 were considered statistically significant.
RESULTS
Patient Characteristics
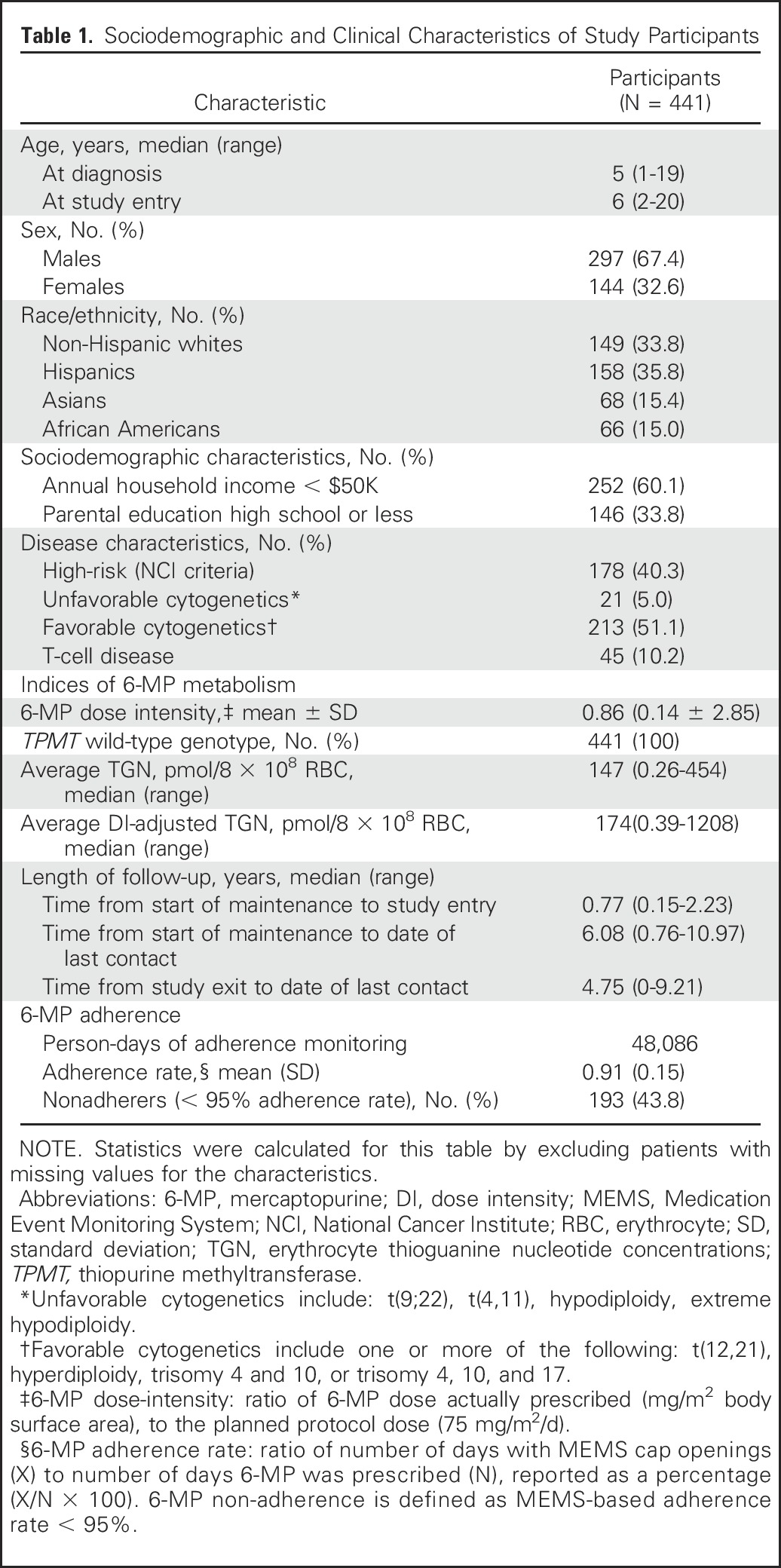
Clinical and sociodemographic characteristics are summarized in Table 1. The cohort consisted of 441 patients. The median age at study participation was 6 years (range, 2 to 20 years); 67% of the cohort were males; 33.8% of the cohort was non-Hispanic white, 35.8% Hispanic, 15.4% Asian, and 15.0% African American; and 40.3% of the cohort had high-risk disease as per NCI definition.
Table 1.
Sociodemographic and Clinical Characteristics of Study Participants
Predictors of 6-MP Ingestion Habits
Coadministration of 6-MP with food.
Twenty-eight percent of the cohort reported taking 6-MP with food at least some of the time. Age ≥ 12 years at study (odds ratio [OR], 1.7; 95% CI, 1.1 to 2.4; P = .01; referent group: < 12 years) was a significant predictor of taking 6-MP with food.
Coadministration of 6-MP with milk/dairy products.
Fifteen percent of the cohort reported taking 6-MP with milk or dairy products at least some of the time. There were no patient or clinical characteristics significantly associated with taking 6-MP with milk/dairy products.
Swallowing 6-MP tablets whole.
Twenty-three percent of the cohort reported chewing the tablets, and 10% ingested the tablet after it had been crushed. Age at study < 12 years (OR, 5.0; 95% CI, 1.8 to 13.7; P = .002; referent group: ≥ 12 years) was a significant predictor of not swallowing 6-MP tablets whole.
Timing of 6-MP doses.
Ninety-one percent of the cohort took 6-MP in the evening or at night, 3% in the morning or midday, and 6% at varying times. There were no patient or clinical characteristics significantly associated with timing of 6-MP doses.
Daily 6-MP routine.
Fourteen percent of the cohort reported having no set routine for taking 6-MP. There were no patient or clinical characteristics significantly associated with not having a daily routine for taking 6-MP.
Association Between 6-MP Ingestion Habits and Nonadherence
MEMS-based adherence was monitored over 48,086 patient-days; 43.8% of the cohort was nonadherent to 6-MP. Taking 6-MP with milk or dairy products (OR, 1.9; 95% CI, 1.3 to 3.0; P = .003; referent group: never taking 6-MP with milk/dairy) and taking 6-MP at varying times of day (OR, 3.4; 95% CI, 1.8 to 6.3; P < .001; referent group: taking 6-MP in evening/night) were associated with a higher risk of nonadherence. The risk for nonadherence was reduced in those who took 6-MP in the morning/midday (OR, 0.2; 95% CI, 0.1 to 0.5; P = .001; referent group: taking 6-MP in evening/night).
Association Between 6-MP Ingestion Habits and TGN Levels
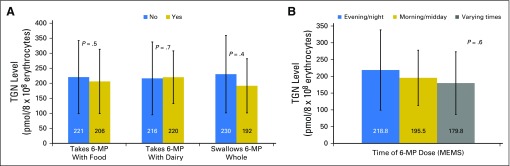
Among adherent patients, DI-adjusted TGN levels (measured in pmol/8 × 108 erythrocytes) were comparable between those who took 6-MP with food (206.1 ± 107.1) versus never took 6-MP with food (220.6 ± 121.6; P = .5); took 6-MP with dairy (220.1 ± 87.8) versus never took 6-MP with dairy (216.3 ± 121.3; P = .7); swallowed 6-MP tablet whole (191.5 ± 90.1) versus crushed/chewed 6-MP (230.4 ± 128.7; P = .4; Fig 1A); or took 6-MP in the evening/night (218.8 ± 119.7) versus morning/midday (195.5 ± 82.3), versus varying times (174.8 ± 93.4; P = .6; Fig 1B).
Fig 1.
Average dose intensity–adjusted thioguanine nucleotide (TGN) levels in adherent, TPMT wild-type patients with acute lymphoblastic leukemia according to (A) taking mercaptopurine (6-MP) with food versus never with food; with milk or dairy products versus never with milk/dairy; swallowing 6-MP tablet whole versus crushed/dissolved/chewed; (B) taking 6-MP in the evening/night versus morning/midday versus varying times of day. Adjusted mean values by time-varying generalized estimating equation analysis. MEMS, Medication Event Monitoring System; TPMT, thiopurine methyltransferase.
Association Between 6-MP Ingestion Habits and Risk of Relapse
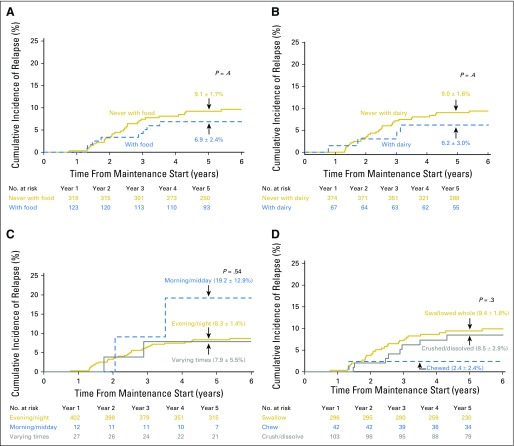
The cumulative incidence of first relapse (8.6% ± 1.4%) at 5 years from start of maintenance did not differ significantly among patients who: took 6-MP with food (6.9% ± 2.4%) versus never with food (9.1% ± 1.7%; P = .4; Fig 2A); took 6-MP with milk/dairy (6.2% ± 3.0%) versus never with milk/dairy (9.0% ± 1.6%; P = .4; Fig 2B); took 6-MP in the evening/night (8.3% ± 1.4%) versus morning/midday (19.2% ± 12.9%) versus varying times (7.9% ± 5.5%; P = .5; Fig 2C); or swallowed the 6-MP tablet whole (9.4% ± 1.8%) versus crushed (8.5% ± 2.9%) versus chewed (2.4% ± 2.4%; P = .3; Fig 2D).
Fig 2.
Cumulative incidence of first relapse of acute lymphoblastic leukemia according to (A) taking mercaptopurine (6-MP) with food versus never with food; (B) taking 6-MP with milk or dairy products versus never with milk/dairy; (C) taking 6-MP in the evening/night versus morning/midday versus varying times of day; (D) swallowing 6-MP tablet whole versus crushed/dissolved versus chewed.
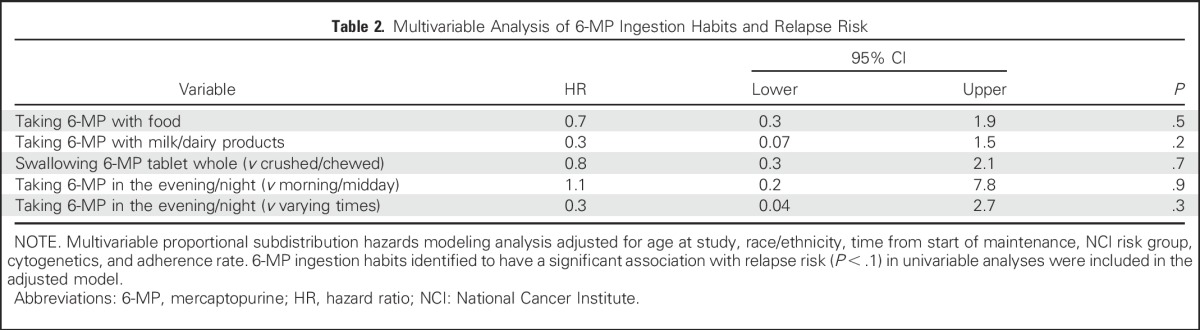
The multivariable analysis, which was adjusted for demographic characteristics, clinical prognosticators, and adherence rate, failed to identify any association between relapse risk and: taking 6-MP with food versus never with food (hazard ratio [HR], 0.7; 95% CI, 0.3 to 1.9; P = .5); taking 6-MP with milk/dairy products versus never with milk/dairy products (HR, 0.3; 95% CI, 0.07 to 1.5; P = .2); swallowing the 6-MP tablet whole versus crushed/chewed (HR, 0.8; 95% CI, 0.3 to 2.1; P = .7); taking 6-MP in the morning/midday versus evening/night (HR, 1.1; 95% CI, 0.2 to 7.8; P = .9); or taking 6-MP at varying times of day versus evening/night (HR, 0.3; 95% CI, 0.04 to 2.7; P = .3; Table 2).
Table 2.
Multivariable Analysis of 6-MP Ingestion Habits and Relapse Risk
DISCUSSION
In this prospective study of 441 children with ALL during the maintenance phase of therapy, we identified variability in 6-MP ingestion habits: 9% of the cohort took their 6-MP dose at times other than the evening, 14% had no established routine for taking 6-MP, 15% reported taking their 6-MP with milk or dairy products and 28% with food, and 33% crushed or chewed the tablet before swallowing. Yet, despite this variability, none of these 6-MP ingestion habits were significantly associated with red cell TGN levels or with the risk of leukemia relapse. Nonetheless, we did find an association between 6-MP ingestion habits and adherence to 6-MP.
We found that taking 6-MP with milk/dairy products was associated with a 1.9-fold increased risk, and taking 6-MP at varying times of day was associated with a 3.4-fold increased risk for nonadherence; our study was not designed to discern the underlying reasons for these 6-MP ingestion habits. In a previous qualitative study, we found that the recommendation to administer 6-MP in the evening, combined with restrictions regarding food and milk intake in the hours surrounding 6-MP ingestion, presented significant barriers to administration.19
We found a lack of association between relapse risk and ingesting 6-MP with food, which is consistent with findings from a previous study.12 However, in contrast to our finding of a lack of association between relapse risk and 6-MP administration time, the previous study reported a significantly inferior outcome when 6-MP was taken in the morning as opposed to the evening. This previous study was limited in that information regarding 6-MP dosing schedule was collected at only one point in time, and adherence was not taken into account.12 A recent follow-up by the same group, with prospective collection of 6-MP dosing schedule (similar to our study design), concurs with our finding of no significant association between the time of day that 6-MP was administered and relapse risk.13
Restrictions regarding timing of administration and ingestion of food and dairy products in relation to 6-MP dosing date back to the 1980s.9 The physiologic basis for the postulated reduced 6-MP absorption in the presence of milk or dairy products stems from the high concentration of xanthine oxidase in milk products and the known metabolic pathway in which 6-MP is converted to the inactive metabolite, thiouric acid, by xanthine oxidase during normal first-pass metabolism in the liver.17 In the 1980s, small studies of children with ALL (n = 10 to n = 17) reported significantly reduced bioavailability when 6-MP was administered after a milk-containing breakfast, as compared with administration following an overnight fast,15,23 whereas similar small studies of 6-MP bioavailability after coadministration with food or milk were inconclusive.16,24 Nevertheless, given the known association between xanthine oxidase and 6-MP inactivation, and on the basis of the available (albeit limited) evidence, restrictive recommendations regarding ingestion of food and milk products in relation to 6-MP dosing were adopted in the mid-1980s, to guide maintenance therapy for children with ALL.9 Concerns over diurnal variation in 6-MP absorption also arose during this time frame.9,11 Suggestions that circadian rhythm might influence 6-MP absorption, and reports of improved outcomes in children who took their oral chemotherapy in the evening,11 led to recommendations for preferential administration of 6-MP dosing in the evening rather than at other times of the day.9,11 Subsequent larger studies reinforced these findings,12,25 leading to international consensus regarding the recommendations for evening administration of 6-MP in a fasting state, which largely remain in effect today.7
Given the suggestion of improved 6-MP bioavailability when administered in the fasting state, as well as the potential for optimized drug metabolism with evening dosing, these restrictive recommendations may have seemed reasonable when implemented. In the current study, we demonstrate quite conclusively that in adherent patients, the red cell TGN levels do not differ by coadministration of food or dairy or whether the 6-MP tablet is swallowed whole or crushed or chewed; furthermore, they do not differ by the time of 6-MP ingestion.
These results, taken in context with results from the previous studies discussed here, suggest that recommendations to take 6-MP in the evening in a fasting state—aimed at enhancing 6-MP absorption—may present barriers to adherence and are probably unnecessary. We have previously shown that poor 6-MP adherence is associated with an increased risk of relapse in childhood ALL,4,5 and we have also previously shown the importance of maintaining steady thiopurine exposure (through maximizing 6-MP adherence and minimizing dosing changes and drug interruptions) to minimize relapse in these children.26 Because it is clear that 6-MP will not be absorbed at all if it is not taken, our findings point to the importance of prioritizing adherence over restrictions regarding 6-MP ingestion.
This study must be considered in the context of certain limitations. The study was observational in design, and therefore we cannot draw conclusions regarding cause-and-effect relationships. In addition, although the magnitude of association between taking 6-MP in the morning or at varying times was large and statistically significant, this result was based on a small sample size. Furthermore, NUDT15 status and minimal residual disease status, two variables that may also influence thiopurine DI or relapse rates, respectively, were not available for inclusion in this analysis. Nevertheless, the study had several strengths, including its longitudinal, prospective design; the large and diverse study population; the collection of 6-MP-related ingestion behaviors, MEMS-based adherence, 6-MP DI, and TGN levels > 48,000 patient-days; as well as leukemia outcome (relapse) over a median of 6 years of follow-up, which allowed for determination of the association between 6-MP ingestion habits, TGN levels, adherence, and leukemia relapse in children with ALL.
In conclusion, results from this study suggest that commonly practiced restrictions surrounding 6-MP ingestion (ie, taking 6-MP only in the evening, without food/dairy), might not influence outcome but may hinder adherence. On the basis of these findings, we believe that future recommendations regarding 6-MP intake during maintenance therapy for childhood ALL should aim to simplify and reduce variability in administration and enhance adherence—by removing restrictions regarding coadministration of food and milk products and emphasizing the importance of establishing a consistent daily 6-MP routine that integrates well into the family lifestyle—to maximize the likelihood of success.
Appendix
Fig A1.

Medication Event Monitoring System (MEMS) pill bottle and TrackCap.
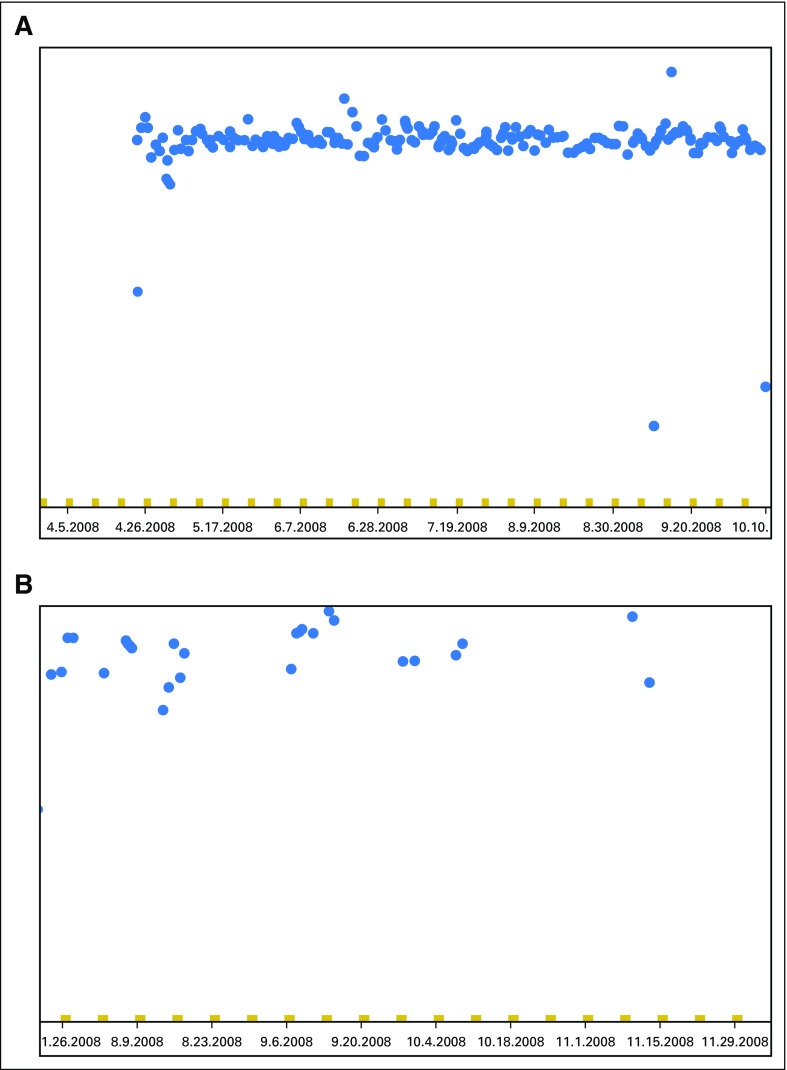
Fig A2.
Examples of Medication Event Monitoring System data download. (A) Patient with consistent mercaptopurine intake across the study period (each blue dot represents one bottle opening). (B) Patient with frequently missed mercaptopurine doses taken at irregular intervals.
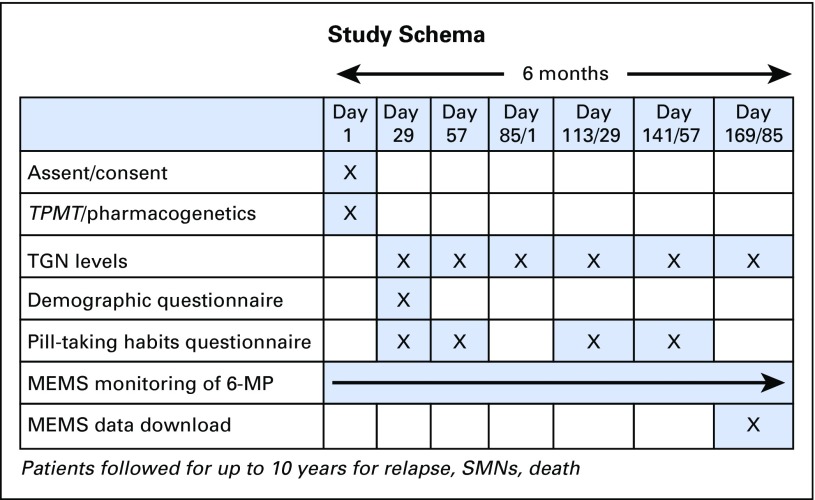
Fig A3.
Study schema. 6-MP, mercaptopurine; MEMS, Medication Event Monitoring System; SMN, subsequent malignant neoplasm; TGN, thioguanine nucleotide; TPMT, thiopurine methyltransferase.
Table A1.
Participating Children’s Oncology Group Institutions
Footnotes
Supported in part by the National Cancer Institute Grants No. R01CA096670, U10CA098543, U10CA098413, U10CA095861, U10CA180886, U10CA180899, UG1CA189955, P30CA21765, and R37CA36401; and by the National Institute of General Medical Sciences Grant No. P50GM115279; the Children's Oncology Group; the St. Baldrick's Foundation; and the American Lebanese Syrian Associated Charities.
Presented in part at the American Society of Hematology Annual Meeting, San Francisco, CA, December 8, 2014.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Clinical trial information: NCT00268528.
Listen to the podcast by Dr Winick at ascopubs.org/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: Wendy Landier, Lindsey Hageman, Mary V. Relling, Smita Bhatia
Financial support: Smita Bhatia
Administrative support: Wendy Landier, Lindsey Hageman, Mary V. Relling, Smita Bhatia
Provision of study materials or patients: Wendy Landier, Lindsey Hageman, Nancy Kornegay, Bruce C. Bostrom, Jacqueline Casillas, David S. Dickens, Anne L. Angiolillo, Glen Lew, Kelly W. Maloney, Leo Mascarenhas, A. Kim Ritchey, Amanda M. Termuhlen, William L. Carroll, Smita Bhatia
Data analysis and interpretation: Wendy Landier, Lindsey Hageman, Yanjun Chen, William E. Evans, Mary V. Relling, F. Lennie Wong, Smita Bhatia
Collection and assembly of data: Wendy Landier, Lindsey Hageman, Nancy Kornegay, Bruce C. Bostrom, Jacqueline Casillas, David S. Dickens, Anne L. Angiolillo, Glen Lew, Kelly W. Maloney, Leo Mascarenhas, A. Kim Ritchey, Amanda M. Termuhlen, William L. Carroll, Smita Bhatia
Data analysis and interpretation: Wendy Landier, Lindsey Hageman, Yanjun Chen, William E. Evans, Mary V. Relling, F. Lennie Wong, Smita Bhatia
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Mercaptopurine Ingestion Habits, Red Cell Thioguanine Nucleotide Levels, and Relapse Risk in Children With Acute Lymphoblastic Leukemia: A Report From the Children’s Oncology Group Study AALL03N1
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Wendy Landier
Research Funding: Merck Sharp & Dohme (Inst)
Lindsey Hageman
No relationship to disclose
Yanjun Chen
No relationship to disclose
Nancy Kornegay
No relationship to disclose
William E. Evans
No relationship to disclose
Bruce C. Bostrom
No relationship to disclose
Jacqueline Casillas
No relationship to disclose
David S. Dickens
No relationship to disclose
Anne L. Angiolillo
No relationship to disclose
Glen Lew
No relationship to disclose
Kelly W. Maloney
No relationship to disclose
Leo Mascarenhas
Honoraria: Bayer HealthCare Pharmaceuticals
Consulting or Advisory Role: Bayer HealthCare Pharmaceuticals, Eli Lilly (Inst)
Research Funding: AstraZeneca/MedImmune (Inst)
Travel, Accommodations, Expenses: Bayer HealthCare Pharmaceuticals, AstraZeneca/MedImmune, Eli Lilly
A. Kim Ritchey
No relationship to disclose
Amanda M. Termuhlen
No relationship to disclose
William L. Carroll
No relationship to disclose
Mary V. Relling
No relationship to disclose
F. Lennie Wong
No relationship to disclose
Smita Bhatia
No relationship to disclose
REFERENCES
- 1.Koren G, Ferrazini G, Sulh H, et al. Systemic exposure to mercaptopurine as a prognostic factor in acute lymphocytic leukemia in children. N Engl J Med. 1990;323:17–21. doi: 10.1056/NEJM199007053230104. [DOI] [PubMed] [Google Scholar]
- 2.Riehm H, Gadner H, Henze G, et al. Results and significance of six randomized trials in four consecutive ALL-BFM studies. Haematol Blood Transfus. 1990;33:439–450. doi: 10.1007/978-3-642-74643-7_81. [DOI] [PubMed] [Google Scholar]
- 3.Toyoda Y, Manabe A, Tsuchida M, et al. Six months of maintenance chemotherapy after intensified treatment for acute lymphoblastic leukemia of childhood. J Clin Oncol. 2000;18:1508–1516. doi: 10.1200/JCO.2000.18.7.1508. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia S, Landier W, Hageman L, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: A Children’s Oncology Group study. Blood. 2014;124:2345–2353. doi: 10.1182/blood-2014-01-552166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia S, Landier W, Shangguan M, et al. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: A report from the children’s oncology group. J Clin Oncol. 2012;30:2094–2101. doi: 10.1200/JCO.2011.38.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia S, Landier W, Shangguan M, et al: Nonadherence to oral mercaptopurine in a multi-racial cohort of children with acute lymphoblastic leukemia and its impact on relapse: A Children’s Oncology Group study (AALL03N1). Presented at the Am Soc Hematol Annual Meeting, Atlanta, GA, December 8-11, 2012 [Google Scholar]
- 7.Aricó M, Baruchel A, Bertrand Y, et al. The seventh international childhood acute lymphoblastic leukemia workshop report: Palermo, Italy, January 29--30, 2005. Leukemia. 2005;19:1145–1152. doi: 10.1038/sj.leu.2403783. [DOI] [PubMed] [Google Scholar]
- 8.Balis FM, Jeffries SL, Lange B, et al. Chronopharmacokinetics of oral methotrexate and 6-mercaptopurine: Is there diurnal variation in the disposition of antileukemic therapy? Am J Pediatr Hematol Oncol. 1989;11:324–326. [PubMed] [Google Scholar]
- 9.Poplack DG, Balis FM, Zimm S.The pharmacology of orally administered chemotherapy. A reappraisal Cancer 58473–480.1986. (suppl 2) [DOI] [PubMed] [Google Scholar]
- 10.Hrushesky WJ, Bjarnason GA. Circadian cancer therapy. J Clin Oncol. 1993;11:1403–1417. doi: 10.1200/JCO.1993.11.7.1403. [DOI] [PubMed] [Google Scholar]
- 11.Rivard GE, Infante-Rivard C, Hoyoux C, et al. Maintenance chemotherapy for childhood acute lymphoblastic leukaemia: Better in the evening. Lancet. 1985;2:1264–1266. doi: 10.1016/s0140-6736(85)91551-x. [DOI] [PubMed] [Google Scholar]
- 12.Schmiegelow K, Glomstein A, Kristinsson J, et al. Impact of morning versus evening schedule for oral methotrexate and 6-mercaptopurine on relapse risk for children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 1997;19:102–109. doi: 10.1097/00043426-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Clemmensen KK, Christensen RH, Shabaneh DN, et al. The circadian schedule for childhood acute lymphoblastic leukemia maintenance therapy does not influence event-free survival in the NOPHO ALL92 protocol. Pediatr Blood Cancer. 2014;61:653–658. doi: 10.1002/pbc.24867. [DOI] [PubMed] [Google Scholar]
- 14.Pinkerton CR, Welshman SG, Glasgow JF, et al. Can food influence the absorption of methotrexate in children with acute lymphoblastic leukaemia? Lancet. 1980;2:944–946. doi: 10.1016/s0140-6736(80)92105-4. [DOI] [PubMed] [Google Scholar]
- 15.Riccardi R, Balis FM, Ferrara P, et al. Influence of food intake on bioavailability of oral 6-mercaptopurine in children with acute lymphoblastic leukemia. Pediatr Hematol Oncol. 1986;3:319–324. doi: 10.3109/08880018609031233. [DOI] [PubMed] [Google Scholar]
- 16.Lönnerholm G, Kreuger A, Lindström B, et al. Oral mercaptopurine in childhood leukemia: Influence of food intake on bioavailability. Pediatr Hematol Oncol. 1989;6:105–112. doi: 10.3109/08880018909034276. [DOI] [PubMed] [Google Scholar]
- 17.de Lemos ML, Hamata L, Jennings S, et al. Interaction between mercaptopurine and milk. J Oncol Pharm Pract. 2007;13:237–240. doi: 10.1177/1078155207080802. [DOI] [PubMed] [Google Scholar]
- 18.Teva Pharmaceuticals USA: FDA-approved package insert for Purinethol (mercaptopurine) 50-mg scored tablets. Sellersville, PA, Teva Pharmaceuticals USA, 2011 [Google Scholar]
- 19.Landier W, Hughes CB, Calvillo ER, et al. A grounded theory of the process of adherence to oral chemotherapy in Hispanic and Caucasian children and adolescents with acute lymphoblastic leukemia. J Pediatr Oncol Nurs. 2011;28:203–223. doi: 10.1177/1043454211409582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yates CR, Krynetski EY, Loennechen T, et al. Molecular diagnosis of thiopurine S-methyltransferase deficiency: Genetic basis for azathioprine and mercaptopurine intolerance. Ann Intern Med. 1997;126:608–614. doi: 10.7326/0003-4819-126-8-199704150-00003. [DOI] [PubMed] [Google Scholar]
- 21.Su Y, Hon YY, Chu Y, et al. Assay of 6-mercaptopurine and its metabolites in patient plasma by high-performance liquid chromatography with diode-array detection. J Chromatogr B Biomed Sci Appl. 1999;732:459–468. doi: 10.1016/s0378-4347(99)00311-4. [DOI] [PubMed] [Google Scholar]
- 22.Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 23.Burton NK, Barnett MJ, Aherne GW, et al. The effect of food on the oral administration of 6-mercaptopurine. Cancer Chemother Pharmacol. 1986;18:90–91. doi: 10.1007/BF00253074. [DOI] [PubMed] [Google Scholar]
- 24.Lafolie P, Björk O, Hayder S, et al. Variability of 6-mercaptopurine pharmacokinetics during oral maintenance therapy of children with acute leukemia. Med Oncol Tumor Pharmacother. 1989;6:259–265. doi: 10.1007/BF02985158. [DOI] [PubMed] [Google Scholar]
- 25.Lau RC, Matsui D, Greenberg M, et al. Electronic measurement of compliance with mercaptopurine in pediatric patients with acute lymphoblastic leukemia. Med Pediatr Oncol. 1998;30:85–90. doi: 10.1002/(sici)1096-911x(199802)30:2<85::aid-mpo3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 26.Bhatia S, Landier W, Hageman L, et al. Systemic exposure to thiopurines and risk of relapse in children with acute lymphoblastic leukemia: A Children’s Oncology Group study. JAMA Oncol. 2015;1:287–295. doi: 10.1001/jamaoncol.2015.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]