Abstract
Simple organisms including yeast and flies with mutations in the IGF-1 and Tor-S6K pathways are dwarfs, are highly protected from toxins, and survive up to 3 times longer. Similarly, dwarf mice with deficiencies in the growth hormone-IGF-I axis are also long-lived and protected from diseases. We recently reported that humans with Growth Hormone Receptor Deficiency (GHRD) rarely develop cancer or diabetes. These findings are in agreement with the effect of defects in the Tor-S6K pathways in causing dwarfism and protection of DNA. Because protein restriction reduces both GHR-IGF-1 axis and Tor-S6K activity, we examined links between protein intake, disease, and mortality in over 6,000 US subjects in the NHANES CDC database. Respondents aged 50–65 reporting a high protein intake displayed an increase in IGF-I levels, a 75% increased risk of overall mortality and a 3–4 fold increased risk of cancer mortality and these findings were confirmed in mouse studies. These studies point to a conserved link between proteins and amino acids, GHR-IGF-1/insulin, Tor-S6k signaling, aging, and diseases.
Mutations in growth pathways: from yeast to humans
Nutrient sensing pathways that regulate metabolism and growth can also promote aging and mortality [1].
Yeast, C. elegans and Drosophila
In S. cerevisiae, multiple pathways represent the central pro-growth and pro-aging signaling network activated by nutrients. The TOR1–Sch9 pathway is primarily activated by amino acids whereas the Ras2–cAMP–PKA pathway is turned on predominantly by glucose [1]. The activation of these pathways by nutrients inhibits the activity of the serine/threonine kinase, Rim15 (regulator of IME2), and consequently the activity of stress resistance transcription factors Msn2/Msn4 (multicopy suppressor of SNF1 mutation) and Gis1 (glycogenin-like gene 1–2 suppressor), which play critical roles in lifespan regulation [2]. Genetic disruption of TOR1–Sch9, Ras2, or both, extends lifespan in this organism by up to fivefold [3, 4]. In agreement with an effect of nutrient signaling on both aging and mortality, yeast cells deficient in TOR1–Sch9 or Ras2–cAMP–PKA signaling display reduced genomic instability and greater resistance to oxidative stress [3, 5, 6]. Reduced activity of the IIS (insulin/Igf-like signaling) pathway has been shown to extend life span in C. elegans and other multicellular organisms [7, 8]. This life-span increase requires the forkhead FoxO transcription factor, daf-16, which regulates genes involved in a wide range of defensive activities including cellular stress response, antimicrobial activity, and detoxification of xenobiotics and free radicals. The TOR pathway interacts intimately with IIS, and inhibition of its activity, including reduction of TOR and S6 kinase, as in yeast, can increase life span in C. elegans [1]. In Drosophila, as in C. elegans, reduced IIS can extend life span, establishing its evolutionarily conserved role [9] For example, mutations in the insulin receptor (InR), which is homologous to the mammalian insulin and IGF-I receptors and to C. elegans daf-2, results in a smaller body size, infertility in females and significantly increases longevity [10] and mutation of the insulin receptor substrate (IRS) homolog chico, results in reduced body size and increases lifespan of female flies by 48% [11]. Moreover, down-regulation of TOR pathway activity genetically [12] or by rapamycin [13]extends life span as it does in yeast and C. elegans. Extension of life span by rapamycin requires autophagy, reduced S6K activity, and eukaryotic initiation factor 4E binding protein (4E-BP) and is associated with reduced protein turnover[13], similar to what is seen in C. elegans. The effects of dietary restriction on lifespan in Drosophila appear to be mediated by reduced consumption of amino acids, particularly essential amino acids rather than reduction of sugar intake [14].
Rodents
In mice, mutations that cause either deficiency in the levels of plasma GH and IGF-1 or a reduction in IGF-1 signaling lead to as much as a 50% increase in life span [15–17]. Homozygous Ames dwarf mutations in the Prop-1 gene (df/df) prevent the generation of the anterior pituitary cells that produce growth hormone, thyroid stimulating hormone, and prolactin and young adult df/df mice are approximately one third of the size of control mice but survive > 50% longer [15]. This effect of dwarf mutations on life span appears to be caused by the absence of plasma GH, which stimulates the secretion of IGF-1 from liver cells but can act on many different cell types [18]. In fact, IGF-1 is reduced dramatically in the plasma of df/df mice. The plasma GH deficiency appears to mediate the effects of Prop-1 (Ames dwarf) and Pit-1 (Snell dwarf) mutations on longevity, since the mice that cannot release GH in response to growth hormone releasing hormone (GHRH) also live longer [18]. Furthermore, dwarf mice with high plasma GH, but a 90% lower circulating IGF-1 (growth hormone receptor/GH binding protein, GHR/BP, null mice) live longer than their wild type littermates [16]. Female mice lacking one copy of IGF-1 receptor (IGF-IR+/−) were shown to live 33% longer than their wild type controls [17], while a separate study using mice of a different background showed only a modest (5%) increase in mean lifespan of female Igf1r+/− compared to controls ([19]. Moreover, LID mice, with a 70% reduction in circulating IGF-1 do not live longer [20] raising the possibility that circulating IGF-1 levels are only responsible for part of the pro-aging effects of GH. Taken together these studies suggest that the reduction in GHR signaling in many different tissues, some of which promote IGF-1 and insulin generation, is responsible for a significant portion of the life span increase in dwarf GH deficient and GHR/BP null mice. Analogous to the activation of yeast Sch9 and Ras by glucose, the mammalian IGF-1 receptor activates both Akt/mTOR/S6K and Ras, and regulates glucose metabolism and cellular proliferation [21]. Several studies have shown that treatment of mice with rapamycin, a pharmacological inhibitor of mTOR, resulted in lifespan extension [22–26] by retarding multiple aspects of aging including neoplasias [27]. Accumulating evidence has implicated increased IGF-1 or IGF-1 signaling as risk factors in a variety of cancers [28], suggesting that this pro-mitotic pathway can promote aging and also the damage and mutations necessary for tumorigenesis. A reduction in adenylyl cyclase activity by deletion of the adenylyl cyclase 5 (AC5) gene was also shown to extend life span and increase resistance to oxidative stress in mice [29], suggesting that these pathways, including homologs of Akt, S6 kinase and cAMP/PKA may play a partially conserved role in the regulation of aging and stress resistance in organisms ranging from yeast to mice [30].
Humans
Recently, we showed that humans with growth hormone receptor deficiency (GHRD), exhibiting major deficiencies in serum IGF-1 and insulin levels, displayed no cancer mortality or diabetes. Despite having a higher prevalence of obesity, combined deaths from cardiac disease and stroke in this group were similar to those in their relatives [31]. Our findings were also supported by a study of 230 GHRD subjects, which reported the absence of cancer in these individuals [32]. Although, neither GHR nor GH releasing hormone receptor deficient subjects appear to be long lived, mutations that reduce the activity of the IGF-IR protein were overrepresented among centenarians, suggesting that lower activity but not severe deficiency in GH/IGF-I signaling may be more beneficial for longevity extension[33, 34]. Moreover, lower IGF-1 levels were also shown to significantly predict survival, specifically in females, and in individuals with a history of cancer [35].
CR and protein/amino acid restriction
Caloric restriction (CR) has been consistently shown to increase longevity in a number of animal models, including yeast, C. elegans, and mice [36]. In yeast, amino acid scarcity which increases lifespan, acts through the Tor/Sch9 pathway [37], and amino acid withdrawal and repletion studies point to the TORC1 complex as a major amino acid transducer in mammalian cells [38, 39] which include the essential amino acids, leucine and methionine and to a lesser extent the non-essential amino acid arginine [40]. In yeast, we recently showed that methionine restriction does not extend lifespan and instead threonine and valine can promote stress sensitization by activating the mTOR pathway. Treatment of WT DBY746 yeast cells with serine, threonine and valine, and to a lesser extent methionine, promoted sensitization to peroxide induced stress, and treatment with myriocin, a Pkh blocker, to prevent Sch9 phosphorylation, only rescued serine sensitization but had no effect on methionine/threonine/valine treatment [37]. Moreover, the TORC1 inhibitor, Rapamycin was capable of suppressing the sensitization caused by threonine and valine, but was completely ineffective in suppressing the serine-dependent effects [37]. In rodents, reduced dietary intake of protein or certain essential amino acids, namely methionine and tryptophan, can also extend longevity [41]. However, the effect of CR on the lifespan of non-human primates remains controversial, and may be heavily influenced by dietary composition [36].
As mentioned above, we recently reported that humans with GHRD and major deficiencies in serum IGF-1 and insulin levels, displayed no cancer mortality or diabetes [31]. Protein restriction or restriction of particular amino acids, such as methionine and tryptophan, may explain part of the effects of CR and GHRD mutations on longevity and disease risk, since protein restriction is sufficient to reduce IGF-1 levels and can reduce cancer incidence or increase longevity in model organisms, independently of calorie intake [42–51]. In a recent study of 6,381 US men and women from the NHANES III database we reported that high protein intake correlated with a 75% increase in overall mortality, and increased risk of death from cancer and diabetes in respondents aged 50–65 whereas the opposite was true in respondents older than 65 [36]. Mouse studies confirmed that high protein intake was associated with higher rate of cancer progression in young mice whereas it was beneficial in older mice [36]. A higher protein intake after age 65 may be necessary to reduce age-dependent weight loss and prevent decrease in IGF-1 levels, which may be associated with frailty and mortality [36]. Studies have also noted poor nutrient absorption in older mice [52] and possibly limited systemic availability of dietary amino acids that may contribute to decreased muscle protein synthesis in humans [36].
Conclusions
In conclusion, studies in model organisms and humans suggest a conserved link between proteins/amino acids, GHR-IGF-1/insulin, Tor-S6k signaling, aging, and diseases. Protein or amino acid restriction may explain part of the beneficial effects of GHRD in mice and humans, as both result in IGF-1 reduction and protection from cancer, stress resistance and DNA damage. Our observations in humans and mice are also consistent with studies in yeast, worms and flies, which implicate the amino acid responsive TOR/S6K pathway in lifespan regulation and point toward simple interventions focused on amino acids to extend lifespan and improve healthspan.
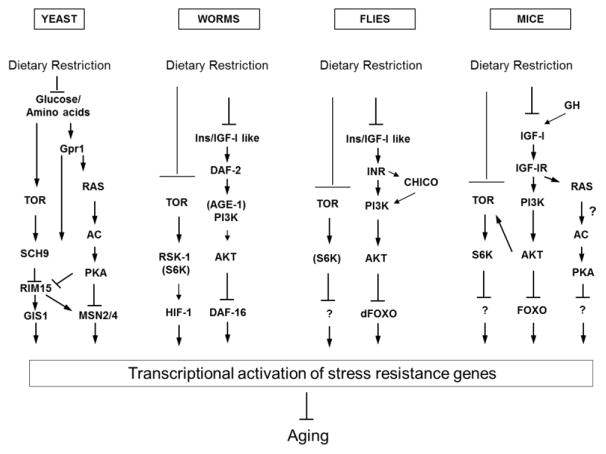
Figure 1.
A model for conserved nutrient signaling pathways that regulate aging
Dietary restriction reduces the activity of conserved pro-aging genes and pathways directly by downregulating TOR and RAS-PKA (yeast), and indirectly, through reduced levels of IGF-1 and Ins/IGF-1 like growth factors (C. elegans, Drosophila and mice). This results in increased expression and/or activity of stress resistance genes that promote lifespan extension.
Highlights.
Mutations in GH-IGF-1 and TOR-S6K pathways extend lifespan and protect from toxins.
These pathways and their components are highly conserved across species.
Protein restriction reduces both GHR-IGF-1 axis and TOR-S6K activity.
These findings point to a conserved link between proteins, GHR-IGF-1 and TOR-S6K.
Acknowledgments
This work was funded by NIA/NIH grants AG034906 and AG02642 to VDL.
Footnotes
Conflict of interest
VDL has equity interests in DSR Pharma Inc. VDL and PB have filed provisional patents related to the development of GHR blockers.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328(5976):321–6. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mirzaei H, Suarez JA, Longo VD. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol Metab. 2014;25(11):558–66. doi: 10.1016/j.tem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabrizio P, et al. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292(5515):288–90. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 4.Wei M, et al. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008;4(1):e13. doi: 10.1371/journal.pgen.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madia F, et al. Longevity mutation in SCH9 prevents recombination errors and premature genomic instability in a Werner/Bloom model system. J Cell Biol. 2008;180(1):67–81. doi: 10.1083/jcb.200707154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabrizio P, et al. Chronological aging-independent replicative life span regulation by Msn2/Msn4 and Sod2 in Saccharomyces cerevisiae. FEBS Lett. 2004;557(1–3):136–42. doi: 10.1016/s0014-5793(03)01462-5. [DOI] [PubMed] [Google Scholar]
- 7.Kenyon C. A conserved regulatory system for aging. Cell. 2001;105(2):165–8. doi: 10.1016/s0092-8674(01)00306-3. [DOI] [PubMed] [Google Scholar]
- 8.Dorman JB, et al. The age-1 and daf-2 genes function in a common pathway to control the lifespan of Caenorhabditis elegans. Genetics. 1995;141(4):1399–406. doi: 10.1093/genetics/141.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piper MD, et al. Separating cause from effect: how does insulin/IGF signalling control lifespan in worms, flies and mice? J Intern Med. 2008;263(2):179–91. doi: 10.1111/j.1365-2796.2007.01906.x. [DOI] [PubMed] [Google Scholar]
- 10.Tatar M, et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292(5514):107–10. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 11.Clancy DJ, et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292(5514):104–6. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- 12.Kapahi P, et al. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14(10):885–90. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjedov I, et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11(1):35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grandison RC, et al. Effect of a standardised dietary restriction protocol on multiple laboratory strains of Drosophila melanogaster. PLoS One. 2009;4(1):e4067. doi: 10.1371/journal.pone.0004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown-Borg HM, et al. Dwarf mice and the ageing process. Nature. 1996;384(6604):33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 16.Coschigano KT, et al. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141(7):2608–13. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 17.Holzenberger M, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421(6919):182–7. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 18.Flurkey K, et al. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98(12):6736–41. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bokov AF, et al. Does reduced IGF-1R signaling in Igf1r+/− mice alter aging? PLoS One. 2011;6(11):e26891. doi: 10.1371/journal.pone.0026891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tikoo S, Sengupta S. Time to bloom. Genome Integr. 2010;1(1):14. doi: 10.1186/2041-9414-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kandel ES, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res. 1999;253(1):210–29. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- 22.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–5. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2(98):ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anisimov VN, et al. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol. 2010;176(5):2092–7. doi: 10.2353/ajpath.2010.091050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anisimov VN, et al. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10(24):4230–6. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- 26.Miller RA, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66(2):191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkinson JE, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11(4):675–82. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4(7):505–18. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 29.Yan L, et al. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130(2):247–58. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 30.Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299(5611):1342–6. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- 31.Guevara-Aguirre J, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3(70):70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol. 2011;164(4):485–9. doi: 10.1530/EJE-10-0859. [DOI] [PubMed] [Google Scholar]
- 33.Suh Y, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008;105(9):3438–42. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aguiar-Oliveira MH, et al. Longevity in untreated congenital growth hormone deficiency due to a homozygous mutation in the GHRH receptor gene. J Clin Endocrinol Metab. 2010;95(2):714–21. doi: 10.1210/jc.2009-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milman S, et al. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell. 2014;13(4):769–71. doi: 10.1111/acel.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levine ME, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19(3):407–17. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirisola MG, et al. Serine- and threonine/valine-dependent activation of PDK and Tor orthologs converge on Sch9 to promote aging. PLoS Genet. 2014;10(2):e1004113. doi: 10.1371/journal.pgen.1004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avruch J, et al. Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab. 2009;296(4):E592–602. doi: 10.1152/ajpendo.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40(2):310–22. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinha Roy R, et al. Coupling growth-factor engineering with nanotechnology for therapeutic angiogenesis. Proc Natl Acad Sci U S A. 2010;107(31):13608–13. doi: 10.1073/pnas.1006007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orentreich N, et al. Low methionine ingestion by rats extends life span. J Nutr. 1993;123(2):269–74. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- 42.Cruzen C, Colman RJ. Effects of caloric restriction on cardiovascular aging in non-human primates and humans. Clin Geriatr Med. 2009;25(4):733–43. ix–x. doi: 10.1016/j.cger.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raffaghello L, et al. Fasting and differential chemotherapy protection in patients. Cell Cycle. 2010;9(22):4474–6. doi: 10.4161/cc.9.22.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panici JA, et al. Is altered expression of hepatic insulin-related genes in growth hormone receptor knockout mice due to GH resistance or a difference in biological life spans? J Gerontol A Biol Sci Med Sci. 2009;64(11):1126–33. doi: 10.1093/gerona/glp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang CC, et al. Different forms of HSV-1 VP22a within purified virion and infected cells. J Microbiol Immunol Infect. 2000;33(3):141–8. [PubMed] [Google Scholar]
- 46.Hsieh M, et al. Identification of the spinocerebellar ataxia type 7 mutation in Taiwan: application of PCR-based Southern blot. J Neurol. 2000;247(8):623–9. doi: 10.1007/s004150070131. [DOI] [PubMed] [Google Scholar]
- 47.Liu YC, et al. Diffuse multiple coronary arteries to left ventricular fistulas. Zhonghua Yi Xue Za Zhi (Taipei) 2000;63(7):573–6. [PubMed] [Google Scholar]
- 48.Kaeberlein M, Kennedy BK. Large-scale identification in yeast of conserved ageing genes. Mech Ageing Dev. 2005;126(1):17–21. doi: 10.1016/j.mad.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi RF, Shima H, de Souza M. Women and AIDS: profile of an infected population and its social implications. Rev Lat Am Enfermagem. 1998;6(5):59–65. [PubMed] [Google Scholar]
- 50.Ocampo A, et al. Mitochondrial respiratory thresholds regulate yeast chronological life span and its extension by caloric restriction. Cell Metab. 2012;16(1):55–67. doi: 10.1016/j.cmet.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189(4):1177–201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sengupta S, Sharma CG, Jordan VC. Estrogen regulation of X-box binding protein-1 and its role in estrogen induced growth of breast and endometrial cancer cells. Horm Mol Biol Clin Investig. 2010;2(2):235–243. doi: 10.1515/HMBCI.2010.025. [DOI] [PMC free article] [PubMed] [Google Scholar]