Abstract
Upstream interventions – e.g. housing programs and community health worker interventions-address socioeconomic and behavioral factors that influence health outcomes across diseases. Studying these types of interventions in clinical trials raises a methodological challenge: how should researchers measure the effect of an upstream intervention in a sample of patients with different diseases? This paper addresses this question using an illustrative protocol of a randomized controlled trial of collaborative-goal setting versus goal-setting plus community health worker support among patients multiple chronic diseases: diabetes, obesity, hypertension and tobacco dependence.
At study enrollment, patients met with their primary care providers to select one of their chronic diseases to focus on during the study, and to collaboratively set a goal for that disease. Patients randomly assigned to a community health worker also received six months of support to address socioeconomic and behavioral barriers to chronic disease control. The primary hypothesis was that there would be differences in patients’ selected chronic disease control as measured by HbA1c, body mass index, systolic blood pressure and cigarettes per day, between the goal-setting alone and community health worker support arms. To test this hypothesis, we will conduct a stratum specific multivariate analysis of variance which allows all patients (regardless of their selected chronic disease) to be included in a single model for the primary outcome. Population health researchers can use this approach to measure clinical outcomes across diseases.
Keywords: Randomized controlled trial, Upstream medicine, Socioeconomic determinants
1. Introduction
Historically, most randomized controlled trials were designed to test biomedical interventions within disease-specific populations. Policymakers including the Patient-Centered Outcomes Research Institute (PCORI) have argued for a shift away from disease-specific biomedical research, towards ‘upstream’ research. Upstream interventions target underlying socioeconomic and behavioral determinants –e.g. access to care, health literacy, food security – that influence health outcomes across diseases. Examples of upstream interventions include housing programs [1,2], income supplementation [3] and community health worker interventions [4].
Studying these types of interventions in clinical trials raises an important methodologic question: how should researchers measure the effect of an upstream intervention in a sample of patients with different diseases? Historically, outcomes of most intervention trials were disease-specific, e.g., glycosylated hemoglobin (HbA1c) for diabetes. Population health researchers now must decide how to determine treatment effect in a trial that may include patients with diabetes, hypertension and obesity, each with distinct clinical outcomes. In order to conduct these trials, researchers must address “the fundamental question of how to define benefit or harm [of an intervention] when multiple conditions coexist.” [5]
Traditionally, public health researchers have tried to address this problem by using “universal outcome measures on which all diseases exert an effect” (such as self-rated health) [6], or else distal “hard” outcomes that are objective and easily measurable (such as mortality). These approaches have limitations: universal outcomes are often self-reported and do not reflect important clinical changes that may not directly be felt by the patient (such as blood pressure improvement). On the other hand, distal outcomes like mortality take a long time to measure and are often hard to detect without very large sample sizes. For these reasons, intermediary clinical outcomes like HbA1c remain important for researchers, yet are restrictive due to their disease-specific nature.
This paper describes an alternative study design that allows for measurement of clinical outcomes across patients with different diseases. We illustrate this approach using the protocol for a randomized controlled trial of a community health worker intervention conducted in a sample of patients with multiple chronic diseases: diabetes, obesity, hypertension and tobacco dependence.
2. Design and methods
2.1. Study sponsorship and IRB approval
This work was supported by a grant from Agency for Healthcare Research and Quality Patient Centered Outcomes Research Institutional Career Development Program (K12 HS 21706-1) and funding from the University of Pennsylvania Institute for Translational Medicine and Therapeutics. This trial is registered (ClinicalTrials.gov Identifier: NCT01900470) and approved by the Institutional Review Board of the University of Pennsylvania.
2.2. Background
A growing body of literature suggests that community health workers, trained laypeople who share socioeconomic background with patients, can effectively address socioeconomic and behavioral factors that influence a range of health outcomes [4]. Most prior community health worker interventions have been disease-specific [6–18] making them hard to scale across populations, and creating fragmentation for the growing number of patients with multiple co-morbidities. IMPaCT (Individualized Management for Patient-Centered Targets) [19–23] is a standardized community health worker intervention that focuses on upstream factors and can be applied across diseases. This intervention has been demonstrated to improve post-hospital outcomes, including access to primary care and hospital readmission, in a randomized controlled trial of general medical inpatients with a variety of diagnoses [21]. We adapted the intervention for use in the outpatient setting [20] and designed a randomized controlled trial to test its effectiveness in a population of patients with a variety of chronic diseases.
2.3. Study design overview
Patients in the study were uninsured or publicly-insured individuals who lived in high-poverty, urban neighborhoods, and were diagnosed with two or more of the following chronic diseases: diabetes, obesity, hypertension and tobacco dependence. At the time of enrollment, each patient met with his/her primary care provider to select one of their multiple chronic diseases to focus on during the study period, and to set a goal for that disease (Fig. 1). This type of collaborative goal-setting motivates patients and facilitates good patient-provider communication [24]. Collaborative goal-setting has been demonstrated to improve number of health-related behaviors [25–30] and outcomes [31] [32,33].
Fig. 1.
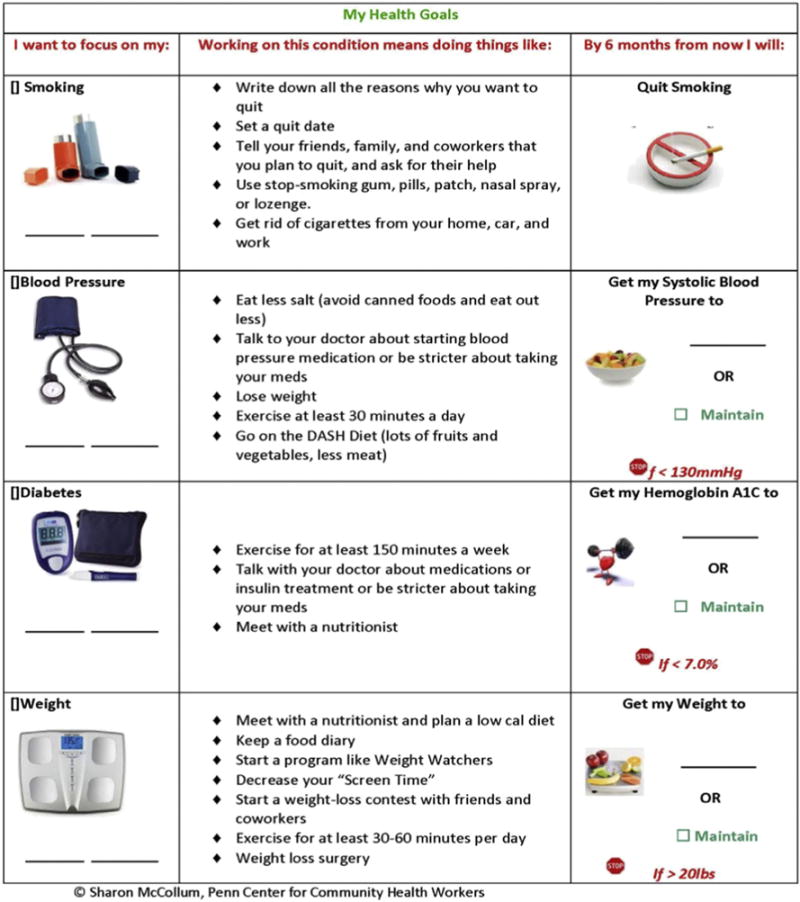
Collaborative goal-setting aid.
However, we reasoned that patients with low socioeconomic status and multiple chronic conditions would likely require intensive support beyond collaborative goal-setting in order to improve their chronic disease control. Therefore, in this study, patients were randomly assigned to collaborative goal-setting alone vs. goal-setting plus six months of support from a community health worker to help address socioeconomic and behavioral barriers to chronic disease control.
2.4. Study aims
The objective of this study was to compare the effect of collaborative goal-setting alone versus goal-setting plus community health worker support on outcomes among a population of patients with low socioeconomic status and multiple chronic conditions. The primary hypothesis was that there would be differences in patients’ selected chronic disease control as measured by HbA1c, body mass index (BMI), systolic blood pressure (SBP) and cigarettes per day (CPD), between the goal-setting alone and community health worker support arms. The secondary hypotheses were that compared with goal-setting alone, community health worker support would result in greater improvements in patient-reported quality of care, self-rated health, patient activation, and all-cause hospitalizations assessed by statewide claims data.
2.5. Setting and participants
Study enrollment was conducted between July 12th, 2013 and October 15th, 2014 at two urban academic adult internal medicine clinics. Analysis of study results is ongoing. Eligible patients: 1) had ≥1 visit in a study clinic during the prior year and an upcoming appointment; 2) lived in a high-poverty 5-ZIP code region in Philadelphia; 3) were uninsured or publicly insured; 4) were diagnosed with 2 or more of the following chronic diseases: hypertension, diabetes, obesity, asthma/emphysema with tobacco dependence. These diagnoses were defined using electronic medical record ICD-9CM codes from the year prior to study enrollment, or in the case of obesity, by a Body Mass Index (BMI) of 30 or greater at the last visit. Patients were excluded if they: 1) had previously worked with a community health worker or 2) lacked capacity to provide informed consent.
2.6. Enrollment
In order to increase real-world applicability of the intervention, the only data elements required to identify eligible patients–height, weight, ICD-9CM codes, insurance and ZIP code—were readily available within the electronic medical record of study clinics. Bioinformatics staff at the clinical sites developed a list of eligible patients; this list was automatically refreshed weekly and sent securely to trained research assistants. Research assistants called patients with upcoming primary care appointments to explain the study and ask patients if they would be willing to spend additional time during their scheduled clinic appointment for study enrollment. When interested patients arrived on the day of the clinic visit, the research assistant obtained written informed consent.
2.7. Study procedures and randomization
2.7.1. Collaborative goal-setting
Study procedures are shown in Fig. 2. After providing written consent, research assistants used a 2-min script (Appendix 1) to explain a low-literacy visual aid (Fig. 1) that patients used to select one of their multiple chronic conditions to focus on during the study period. The visual aid listed the chronic diseases that each patient had and their current level of control. It also described evidence-based behaviors proven to benefit each chronic disease. Research assistance prompted patients to think about what types of behaviors they might actually be willing to perform, and use this intention to make their disease selection.
Fig. 2.
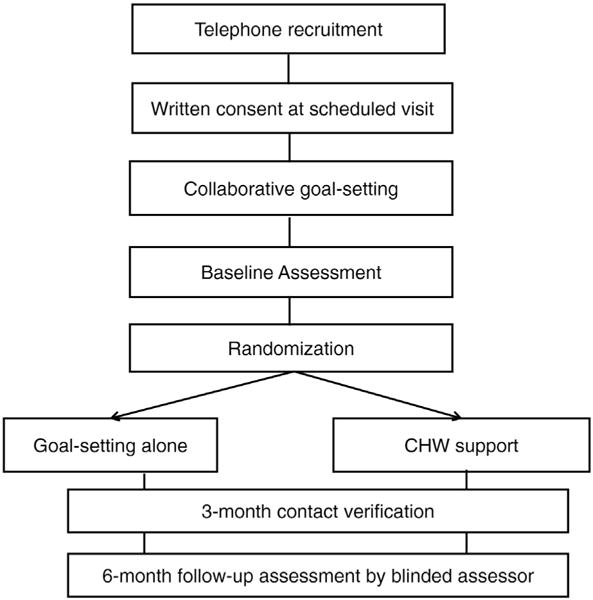
Study Procedures.
After selecting a disease to focus on, patients brought the aid with them into the exam room and reviewed their choice with their primary care provider. The provider then helped them to set a specific disease management goal for their selected disease: a systolic blood pressure goal for hypertension, HbA1c goal for diabetes, weight goal for obesity, or smoking cessation for asthma/COPD and tobacco dependence. Patients and providers were allowed to set a maintenance goal (same as their baseline value).
This goal-setting process was designed to take less than five minutes of providers’ time so that it could be folded in to the busy workflow of a real-world primary care visit. Primary care providers at study clinics were offered a 60-min training session on collaborative goal-setting, which focused on principles of goal-setting theory [34] including the importance of setting realistic goals.
Research assistants were observed during an initial training period by their supervisors (a clinical research coordinator) and the principal investigator in order to assess fidelity to the collaborative goal-setting script. Providers were prompted by research assistants to reconsider goals that were overly ambitious (Appendix 1).
2.7.2. Baseline assessment and randomization
After collaborative goal-setting, the research assistant completed a brief baseline survey, and recorded height, weight and blood pressure measured by clinic staff. The research assistant then walked patients to on-site blood testing facilities for measurement of HbA1c. We assessed HbA1c for all patients, not just those with a diagnosis of diabetes because of the higher rate of undiagnosed diabetes among low-income, minority populations. While the patient was undergoing blood testing, the research assistant notified a coordinator that enrollment was complete. The coordinator (who was not involved with outcomes assessment) randomly assigned each participant to an experimental arm using a computer-generated, randomization algorithm with permuted variable block sizes with a concealed sequence. In addition, randomization was stratified by selected chronic disease [35]. The coordinator notified community health workers of study assignment. The community health worker met each patient at the laboratory, notified him/her of the study assignment, and immediately initiated the IMPaCT intervention for patients randomized to receive community health worker support.
2.7.3. Outcomes assessment and incentives
All patients were contacted by research assistants at three months after enrollment in order to verify contact information. At five months post-enrollment, research assistants called patients in order to schedule a six-month follow-up assessment. The follow-up assessment was conducted at the study clinic and included a brief verbal survey, recording of height, weight and blood pressure measured by clinic staff and measurement of HbA1c. In order to minimize missing six-month outcomes data in study of highly vulnerable and often transient patients, research assistants made three attempts to contact each patient via telephone and then conducted a home visit if the patient was never reached by phone or was unable to come in for follow-up. Research assistants also extracted clinical data from the electronic medical record that occurred within 4 weeks of the study completion date for patients who were unable to be reached for a complete six-month follow-up assessment.
Participating patients received a $10 pre-paid gift card upon completion of the baseline survey, $20 upon completion of baseline laboratory testing, $30 upon completion of the 6-month follow-up assessment.
2.8. Interventions
2.8.1. Goal-setting alone
After collaborative goal-setting with primary care providers as described above, patients received usual care in accordance with guidelines at each site.
2.8.2. Goal-setting plus community health worker support
IMPaCT is an intervention in which community health workers provide patients of low socioeconomic status with coaching, social support, advocacy and navigation in order to help them reach health goals [22, 23, 36]. The intervention has been described in detail elsewhere [19, 21, 22], but briefly, consists of three stages: goal-setting, tailored support and connection with long-term support. On the day of enrollment, community health workers used a semi-structured interview guide to get to know their patients holistically, assess social and behavioral determinants of health (e.g. food insecurity, housing instability, drug and alcohol use, family stress, etc.). Community health workers use this conversation to help patients formulate action plans for addressing their social/behavioral determinants of health and achieving their chronic disease management goal. A key question in the semi-structured interview is: “What will you need to do in order to reach the health goal you set with your doctor?” This allows patients to have control over the action-planning process and to create tailored strategies. For instance, one patient might consider ‘stable housing’ and ‘access to fresh produce’ the most important steps towards reaching his health goal. Another patient might describe wanting ‘a reason to get out of bed in the morning since my son was murdered.’ These individualized goals become the basis for tailored action plans. Each action plan consists of several components: a measurable goal (e.g. moving into a new apartment), patient confidence in being able to achieve the goal, a list of resources that might help patients to achieve that goal, and concrete next steps [22].
In the second stage, community health workers provided hands-on support guided by patients’ action plans. For example, if a patient wanted to find affordable, fresh produce, the community health worker may have accompanied them to a food pantry. Community health workers conducted follow-up at least once per week for 6 months through telephone, text, home or community visits. Action plans created during the initial meeting were revisited during these follow-up encounters; additional action plans could be created throughout the 6- month intervention. Community health workers also reviewed their patients’ action plans with their managers, who provided feedback and trouble-shooting. During follow-up encounters, community health workers also encouraged patients to monitor progress towards their chronic disease management goal by measuring glucose, weight, blood pressure or number of cigarettes smoked. Community health workers also communicated with patients’ primary care team about patients’ progress towards chronic disease management goals through electronic medical record messages or if providers were willing via telephone calls and team huddles.
Finally, community health workers led a weekly support group that facilitated discussion of motivation for health behavior change and management of psychosocial stressors [19]. The group was intended to create social networks between patients who could support each other in maintaining healthy behaviors after the intensive 6-month community health worker support ended. Discussion topics were selected by participants on a weekly basis but include possible areas such as habit change, motivation, working through difficulties, and relationships with friends and family members. The support was offered once per week and was open to all patients. Sessions were located in the primary care clinic (as opposed to community sites) because this allowed patients to utilize transportation benefits linked to clinical care. These sessions were considered drop-in sessions and were available to participants even after the 6-month intensive intervention period ended in order to prevent the ‘voltage drop’ in support that often occurs after an intervention ends.
Detailed manuals describing recruiting, training, supervision and workflow of community health workers (including guidelines for facilitation of the peer support group) are available online (http://chw.upenn.edu/). Briefly, community health workers were recruited by circulating job descriptions through a network of community-based organizations (e.g. block captain associations, recreation centers, churches). This approach is more selective than public advertising because it is targeted to potential ‘natural helpers’ within the community. Applicants for the community health worker position undergo several rounds of screening during the hiring process including meet-and-greets, interviews with case-based scenarios and employer reference checks. These hiring strategies are designed to identify individuals who are good listeners, non-judgmental and reliable. Community health workers who are ultimately hired undergo a month-long college-accredited training that covers topics such as the mechanics of the IMPaCT intervention, action-planning, motivational interviewing and trauma-informed care. After the month-long classroom training, community health workers undergo an on the job training through apprenticeship with a senior community health worker. This continues until each new trainee demonstrates proficiency in core competences of the IMPaCT model. Community health workers are supervised by a manager, who is typically a master’s level social worker. The manager provides real-time support, caseload supervision, ongoing training, and also helps to integrate community health workers with clinical care teams. Managers also assess intervention fidelity and performance through a recurring series of weekly assessments: detailed chart reviews of a sample of community health worker patient documentation, quarterly day-long observation of community health workers, calls to patients to assess their experience with community health workers, and a performance dashboard that reports key metrics (chronic disease control, number of contacts, hospitalizations) for each patient on a community health worker’s caseload. A manager can supervise between 4 and 6 community health workers who may be located in different practices or hospitals. A given manager’s ‘team’ of community health workers forms a unit that comes together for ongoing training, burnout prevention and support.
2.9. Measures
2.9.1. Baseline measures
Research assistants collected the following baseline data: age, race [37], ethnicity [37], employment [37], severity of illness (ACG score) [38], household income and size [37], unmet or delayed need for medical care [39], number of emergency room visits and hospital admissions in the prior twelve months [39], and usual source of care [39]. The baseline questionnaire also included the SF-12 survey [40], the Patient Activation Measure (PAM) [41], the ENRICHD Social Support Instrument (ESSI) [42], the Single-Item Literacy Screen [43], the Trauma History Questionnaire (THQ) [44], the Single-Item Drug Screening [45], Single-Item Alcohol Screening [46], perceived stress [47], and questions assessing homelessness and food insecurity [48]. In addition, the baseline questionnaire included an assessment of commitment and self-efficacy for achieving the collaboratively chronic disease management goal [49]. This assessment was taken from Kanfer et al.’s assessment of motivation; specifically we used domains that pertained to commitment to an assigned goal, self-confidence for goal attainment and attention to the goal assignment [49]. For patients assigned to community health worker support, community health workers documented action plans and detailed encounter notes.
2.9.2. Outcome measures
Research assistants blinded to study arms and hypotheses conducted an in-clinic follow-up assessment at 6 months post-enrollment. The pre-specified primary outcome is change in patient’s selected chronic disease between enrollment and 6-month follow-up. Secondary outcome measures include change in self-rated health (SF-12), [40] quality of patient-centered care (CAHPS-PCMH) [50], change in patient activation (PAM) [41], and all-cause hospitalizations (at 6 and 12 months) [51].
2.9.3. Process measures
Community health workers documented the following process measures: initiation of the intervention, action plans, completion of action plans (including resolution of social/behavioral barriers) and detailed encounter notes. Research assistants un-blinded to study arms conducted brief exit interviews with patients who received community health worker support and obtained open-ended feedback about the experience of working with a community health worker.
2.10. Analysis
Our pre-specified primary and secondary hypotheses are being tested with two-sided p-values using an intent-to-treat approach. We are analyzing all available data and using multiple imputations for missing data. Statistical analyses are being performed using SAS for Windows (version 9.4: SAS Institute, Cary, NC) and STATA/MP for Unix (version 14.0: StataCorp, College Station, Texas).
2.10.1. Primary outcome
The key methodologic challenge in this study is the fact that patients have different chronic diseases, each with its own disease-specific outcome (HbA1c, BMI, SBP and cigarettes per day). Similar studies have approached this challenge by creating dichotomous variables -such as improved control (yes/no) or achievement of guideline-based control (yes/no)—that can summarize each patient’s disease-specific outcome [17]. This approach is intuitive but loses a significant amount of information and fails to capture incremental improvements. These incremental gains are often very important and lead to greater harm reduction than achievement of guidelines. For example, reducing systolic blood pressure from 200 mmHg to 150 mmHg, leads to greater harm reduction than lowering blood pressure from 150 mmHg to 130 mmHg [52].
We chose to measure the primary outcome as a continuous variable: change at six months in the chronic disease patients selected as their focus for the study (measured by changes in HbA1c, BMI, SBP and CPD). The primary treatment effect is the between-arm difference in in these values. To assess the effect of the intervention on the chosen outcome, we are conducting a stratum specific multivariate analysis of variance (MANOVA) [53] MANOVA is an extension of ANOVA which allows for multiple outcome variables for each participant to be compared simultaneously. This also allows for all patients (regardless of their selected chronic disease) to be fitted into a single model for the primary outcome. ANOVA tests for the difference in means between two or more groups, while MANOVA tests for the difference in two or more vectors of means. Testing the multiple dependent variables is accomplished by creating new dependent variables that maximize group differences. These artificial dependent variables are linear combinations of the measured dependent variables. MANOVA has several advantages over ANOVA. First, by measuring several dependent variables in a single trial, there is a better chance of discovering which factor is truly important. Second, it can protect against Type I errors that might occur if multiple ANOVA’s were conducted independently. Additionally, it can reveal differences not discovered by ANOVA tests. We use this approach to first test whether there is any treatment effect on any of the outcomes. We then use a stratified approach to estimate and test the average treatment effect on a particular outcome among patients who chose to focus on that outcome.
We also use multivariate models based on generalized estimating equations to account for the correlation of outcomes measured on the same participant and conduct hypothesis tests using joint multivariate Wald test statistics. We use permutation-based p-values for these tests since they preserve correct type I error without making any distributional assumptions. Difference-in-difference analyses are then based on these multivariate models.
2.10.2. Sample size considerations
Prior to this RCT, we conducted a single-arm pilot (n = 30) of the intervention and compared change in control of selected chronic disease (HBa1C, BM1, systolic blood pressure and cigarettes per day) in the 3 month time period prior to enrollment with changes in the 3 months after enrollment. We used population data from the primary care practice to obtain a population mean and standard deviation for change in each chronic disease metric over similar time periods. We then calculated a standardized score (z-score) for change in selected disease for each patient in the study.
We calculated the mean standardized change and compared this number between the time period prior to enrollment and the time period after enrollment. In our population, a z-score of 1 was equivalent to 3.3 cigarettes = 20.5 mmHg = 1.5%HgbA1c = 16.7 lbs. We found a difference of 1.5 standardized units between comparators which was the basis for our sample size calculation.
Like the MANOVA, calculating a standardized score across disease outcomes allowed us to compare patients across different diseases. Standardized scores are easier to use for sample size calculations, but because MANOVA does not result in loss of information, we used this for our primary analysis. This approach is described in greater detail elsewhere [53].
Thus, determination of targeted sample size was based on detecting a difference in the primary outcome of 1.5 standardized units between treatment and control arms. To achieve at least 80% power with a type I error rate of 5% we required 212 total participants. To account for a 30% drop out rate, we aimed to accrue 302 participants.
2.10.3. Secondary outcomes
We are using appropriate functional forms of multivariable regression models, adjusting for any imbalance in baseline differences, to compare secondary outcomes. Specifically, we are testing difference in treatment arms using linear regression for change in self-rated health (SF-12) and change in patient activation (PAM) [41]; logistic regression for the proportion of patients reporting each quality rating (CAHPS-PCMH); and negative binomial regression for all-cause hospitalizations.
2.10.4. Missing data
We are performing an intention-to-treat analysis based on random assignment and using multiple imputation for missing data [54] simulating a multivariate normal distribution. The imputation model includes outcome variables, variables in the primary model that are imbalanced or stratified at baseline and variables associated with missing outcome information. Each of 5 imputed data sets will be analyzed and the results combined for inference. The resulting data set of complete primary outcomes will be used in the multivariate model. To assess the robustness of our results, we are performing additional analyses such as including only patients with complete data.
3. Discussion
This randomized controlled trial will test whether an upstream intervention -community health worker —leads to improvements in meaningful clinical outcomes compared to collaborative goal-setting alone. Importantly, by using multivariate analysis of variance (MANOVA), we are able to answer this question for a sample of patients with diverse diseases while conducting a single, population-based study, rather than conducting several separate disease-specific studies.
This study has several limitations. First, it was conducted in a single, urban academic medical center and thus, may not be generalizable to broader populations. Second, this is a comparative effectiveness study that did not include a usual care arm. As a result, we will not be able to determine whether either collaborative goal-setting or community health worker support were more effective than usual care. Third, because most outcomes are assessed only at the end of the 6-month intervention period, we lack the ability to measure persistence of any treatment effect. One exception is all-cause hospitalizations as reported in a state-wide administrative claims database which will be assessed at 6 and 12 months post-enrollment. Finally, the community health worker intervention being tested in this study requires a significant amount of program-level infrastructure which may not be available in most outpatient practices. However, since the passage of the Affordable Care Act, community health worker have become a rapidly growing workforce: according to the United States Bureau of Labor there were 115,700 community health worker in the United States with a projected growth of 13% per year [55]. The IMPaCT community health worker intervention is well-described and is being replicated in diverse practice environments including the Veterans Affairs administration and federally qualified health centers. An open-source toolkit (http://chw.upenn.edu/tools) describing the intervention has been accessed by nearly 800 unique organizations across the country. These factors promote generalizability of the intervention studied in this trial.
4. Conclusion
In past decades, randomized controlled trials have been well-oiled engines to rigorously test disease-specific biomedical interventions. Yet, we now know that biomedical interventions have a relatively minor influence on health, and in fact contribute only 10%56 to the prevention of premature death in the United States. Far greater are the influences of socioeconomic and behavioral factors which together account for nearly 55% of premature death [56]. Recognition of these facts has spurred the resurgence of population health and an increased emphasis on upstream interventions that can address the fundamental and underlying determinants of health across diseases. Yet, much of the literature on upstream interventions has continued to focus on patients with single diseases, limiting scalability. The approach described in this paper can help researchers use the randomized controlled trial to measure clinical outcomes across adults with different conditions test interventions. This innovation may help randomized controlled trials remain relevant in the new era of population health research.
Appendix 1. Research assistant collaborative goal-setting script
Goal card
Here is your goal card. These are four of the most common chronic conditions we see in this doctor’s office. You came across our radar because of your_____,_____,_____ (poorly controlled conditions), so we want to encourage you to start to think about ways you can improve your control of that/those condition(s). What we are going to do now is to choose which one of these conditions that you can get under better control that you would like to focus on over the next 6 months.
How are you going to make that decision? Well, I will tell you about some of the things that are important for controlling each condition and as I go through this information, you should ask yourself: am I going to do that? I
For example, if you wanted to get your _____ under control, some of the things you might need to do include: __________. Do these seem like things that you might be able to do? Let’s go through your other conditions in the same way. To get your _____ under control, you might need to: __________. Do these seem like things that you might be able to do? Ok, let’s summarize and decide which condition you think you have the best chance of getting under control over the next 6 months.
Introduction to the primary care provider
Hi I’m_____, a Program Assistant with the IMPaCT Primary Care Program. Here is your patient’s goal card. I have boxed the conditions (s)he has in poor control. We just spent some time talking about which of these _____conditions he would like to focus on improving over the next 6 months. (S)he feels that he has the best chance of getting his _____under control, so has chosen to focus on that. Would you be able to take a few minutes to discuss with your patient whether you think that is the best choice of condition for them? Also, please help them to pick a specific target goal for their condition, for example a _____goal for their_____. The most important thing is to be realistic because we have found that not achieving goals can cause patients to lose confidence and do worse in the long term. We have found that there is sometimes a tendency to push past evidence-based guidelines so please do not choose goals that are beyond the noted values on the right. They are the lowest values we would recommend setting as a goal.
After the provider visit
[Check goal card to make sure it is filled out properly and that the goal is realistic. If goal seems unrealistic compared with others (Systolic BP 2 point HgbA1c drop, > 20 lb weight loss) tell the PCP: “The goal that you both have set is more difficult than most others I have seen. I want to remind you that it is important to set realistic goals because we have found that not achieving goals can cause patients to lose confidence and do worse in the long term.”]
References
- 1.Leventhal T, Brooks-Gunn J. Moving to opportunity: an experimental study of neighborhood effects on mental health. Am J Public Health. 2003;93:1576–1582. doi: 10.2105/ajph.93.9.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leventhal T, Dupere V. Moving to opportunity: does long-term exposure to ‘low-poverty’ neighborhoods make a difference for adolescents? Soc Sci Med. 2011;73:737–743. doi: 10.1016/j.socscimed.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 3.Costello EJ, Compton SN, Keeler G, Angold A. Relationships between poverty and psychopathology: a natural experiment. JAMA. 2003;290:2023–2029. doi: 10.1001/jama.290.15.2023. [DOI] [PubMed] [Google Scholar]
- 4.Viswanathan M, Kraschnewski JL, Nishikawa B, et al. Outcomes and costs of community health worker interventions: a systematic review. Med Care. 2010;48:792–808. doi: 10.1097/MLR.0b013e3181e35b51. [DOI] [PubMed] [Google Scholar]
- 5.Tinetti ME, Studenski SA. Comparative effectiveness research and patients with multiple chronic conditions. N Engl J Med. 2011;364:2478–2481. doi: 10.1056/NEJMp1100535. [DOI] [PubMed] [Google Scholar]
- 6.Tang TS, Funnell M, Sinco B, et al. Comparative effectiveness of peer leaders and community health workers in diabetes self-management support: results of a randomized controlled trial. Diabetes Care. 2014;37:1525–1534. doi: 10.2337/dc13-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heisler M, Choi H, Palmisano G, et al. Comparison of community health worker-led diabetes medication decision-making support for low-income Latino and African American adults with diabetes using e-health tools versus print materials: a randomized, controlled trial. Ann Intern Med. 2014;161:S13–S22. doi: 10.7326/M13-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heisler M, Vijan S, Makki F, Piette JD. Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Ann Intern Med. 2010;153:507–515. doi: 10.7326/0003-4819-153-8-201010190-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer MS, Rosland AM, Kieffer EC, et al. Effectiveness of a community health worker intervention among African American and Latino adults with type 2 diabetes: a randomized controlled trial. Am J Public Health. 2011;101:2253–2260. doi: 10.2105/AJPH.2010.300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang TS, Funnell MM, Sinco B, Spencer MS, Heisler M. Peer-led, empowerment-based approach to self-management efforts in diabetes (PLEASED): a randomized controlled trial in an African American community. Ann Fam Med. 2015;13(Suppl 1):S27–S35. doi: 10.1370/afm.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ephraim PL, Hill-Briggs F, Roter DL, et al. Improving urban African Americans’ blood pressure control through multi-level interventions in the achieving blood pressure control together (ACT) study: a randomized clinical trial. Contemp Clin Trials. 2014;38:370–382. doi: 10.1016/j.cct.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper LA, Roter DL, Carson KA, et al. A randomized trial to improve patient-centered care and hypertension control in underserved primary care patients. J Gen Intern Med. 2011;26:1297–1304. doi: 10.1007/s11606-011-1794-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long JA, Jahnle EC, Richardson DM, Loewenstein G, Volpp KG. Peer mentoring and financial incentives to improve glucose control in African American veterans: a randomized trial. Ann Intern Med. 2012;156:416–424. doi: 10.1059/0003-4819-156-6-201203200-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim K, Choi JS, Choi E, et al. Effects of community-based health worker interventions to improve chronic disease management and care among vulnerable populations: a systematic review. Am J Public Health. 2016;106:e3–e28. doi: 10.2105/AJPH.2015.302987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine DM, Bone LR, Hill MN, et al. The effectiveness of a community/academic health center partnership in decreasing the level of blood pressure in an urban African-American population. Ethn Dis. 2003;13:354–361. [PubMed] [Google Scholar]
- 16.Allen JK, Dennison-Himmelfarb CR, Szanton SL, et al. Community outreach and cardiovascular health (COACH) trial: a randomized, controlled trial of nurse practitioner/community health worker cardiovascular disease risk reduction in urban community health centers. Circ Cardiovasc Qual Outcomes. 2011;4:595–602. doi: 10.1161/CIRCOUTCOMES.111.961573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adair R, Christianson J, Wholey DR, et al. Care guides: employing nonclinical laypersons to help primary care teams manage chronic disease. J Ambul Care Manage. 2012;35:27–37. doi: 10.1097/JAC.0b013e31823b0fbe. [DOI] [PubMed] [Google Scholar]
- 18.Becker DM, Yanek LR, Johnson WR, Jr, et al. Impact of a community-based multiple risk factor intervention on cardiovascular risk in black families with a history of premature coronary disease. Circulation. 2005;111:1298–1304. doi: 10.1161/01.CIR.0000157734.97351.B2. [DOI] [PubMed] [Google Scholar]
- 19.Kangovi S, Leri D, Clayton C, Lang A, Grande D, Long JA, Rosin R. Penn Center for Community Health Workers. 2016 Dec; 2013 Access date. http://chw.upenn.edu/
- 20.Kangovi S, Carter T, Charles D, et al. Toward a scalable, patient-centered community health worker model: adapting the IMPaCT intervention for use in the outpatient setting. Popul Health Manag. 2016 doi: 10.1089/pop.2015.0157. [DOI] [PubMed] [Google Scholar]
- 21.Kangovi S, Mitra N, Grande D, et al. Patient-centered community health worker intervention to improve posthospital outcomes: A randomized clinical trial. JAMA Intern Med. 2014;174:535–543. doi: 10.1001/jamainternmed.2013.14327. [DOI] [PubMed] [Google Scholar]
- 22.Kangovi S, Grande D, Carter T, et al. The use of participatory action research to design a patient-centered community health worker care transitions intervention. Healthcare. 2014;2:136–144. doi: 10.1016/j.hjdsi.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Kangovi S, Grande D, Trinh-Shevrin C. From rhetoric to reality-community health workers in post-reform U.S. health care. N Engl J Med. 2015;372:2277–2279. doi: 10.1056/NEJMp1502569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bodenheimer T, Handley MA. Goal-setting for behavior change in primary care: an exploration and status report. Patient Educ Couns. 2009;76:174–180. doi: 10.1016/j.pec.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Schafer LC, Glasgow RE, McCaul KD. Increasing the adherence of diabetic adolescents. J Behav Med. 1982;5:353–362. doi: 10.1007/BF00846162. [DOI] [PubMed] [Google Scholar]
- 26.Alexy B. Goal setting and health risk reduction. Nurs Res. 1985;34:283–288. [PubMed] [Google Scholar]
- 27.Epstein LH, Wing RR, Koeske R, Ossip D, Beck SA. Comparison of lifestyle change and programmed aerobic exercise on weight and fitness changes in obese children. Behav Ther. 1982;13:651–665. [Google Scholar]
- 28.Martin JE, Dubbert PM, Katell AD, et al. Behavioral-control of exercise in sedentary adults - studies 1 through 6. J Consult Clin Psychol. 1984;52:795–811. doi: 10.1037//0022-006x.52.5.795. [DOI] [PubMed] [Google Scholar]
- 29.Estabrooks PA, Nelson CC, Xu S, et al. The frequency and behavioral outcomes of goal choices in the self-management of diabetes. Diabetes Educator. 2005;31:391–400. doi: 10.1177/0145721705276578. [DOI] [PubMed] [Google Scholar]
- 30.Patrick K, Caifas KJ, Norman GJ, et al. Randomized controlled trial of a primary care and home-based intervention for physical activity and nutrition behaviors -PACE + for adolescents. Arch Pediatr Adolesc Med. 2006;160:128–136. doi: 10.1001/archpedi.160.2.128. [DOI] [PubMed] [Google Scholar]
- 31.Glasgow RE, La Chance PA, Toobert DJ, Brown J, Hampson SE, Riddle MC. Long-term effects and costs of brief behavioural dietary intervention for patients with diabetes delivered from the medical office. Patient Educ Couns. 1997;32:175–184. doi: 10.1016/s0738-3991(97)00039-6. [DOI] [PubMed] [Google Scholar]
- 32.Glasgow RE, Toobert DJ. Brief, computer-assisted diabetes dietary self-management counseling: effects on behavior, physiologic outcomes, and quality of life. Med Care. 2000;38:1062–1073. doi: 10.1097/00005650-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Baron P, Warters RG. Effects of goal-setting and of goal levels on weight-loss induced by self-monitoring. Int Rev Appl Psychol. 1982;31:369–382. [Google Scholar]
- 34.Locke EA, Latham GP. New Developments in Goal Setting and Task Performance. 2013;xxiv:664. [Google Scholar]
- 35.Piantadosi S. Block Stratified Randomization Windows Version 6.0. 2010 [Google Scholar]
- 36.Kangovi S, Long JA, Emanuel E. Community health workers combat readmission. Arch Intern Med. 2012;172:1756–1757. doi: 10.1001/2013.jamainternmed.82. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control. Behavioral Risk Factor Surveillance System Questionnaire. Atlanta, Georgia: National Center for Chronic Disease Prevention and Health Promotion; Dec, 2016. 2012 Access date. https://www.cdc.gov/brfss/questionnaires/index.htm. [Google Scholar]
- 38.Weiner JP, Starfield BH, Steinwachs DM, Mumford LM. Development and application of a population-oriented measure of ambulatory care case-mix. Med Care. 1991;29:452–472. doi: 10.1097/00005650-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Health Tracking Household Survey. Inter-University Consortium for Political and Social Research. 2007 [Google Scholar]
- 40.Maruish ME, Kosinski M. A Guide To The Development Of Certified Short Form Interpretation And Reporting Capabilities. Lincoln, RI: Quality Metric Inc.; 2009. [Google Scholar]
- 41.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39:1005–1026. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell PH, Powell L, Blumenthal J, et al. A short social support measure for patients recovering from myocardial infarction: the ENRICHD social support inventory. J Cardpulm Rehabil. 2003;23:398–403. doi: 10.1097/00008483-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Morris NS, MacLean CD, Chew LD, Littenberg B. The single item literacy screener: evaluation of a brief instrument to identify limited reading ability. BMC Fam Pract. 2006;7(21) doi: 10.1186/1471-2296-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hooper LM, Stockton P, Krupnick JL, Green BL. Development, use, and psychometric properties of the trauma history questionnaire. J Loss Trauma. 2011;16:258–283. [Google Scholar]
- 45.Smith PC, Schmidt SM, Allensworth-Davies D, Saitz RA. Single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155–1160. doi: 10.1001/archinternmed.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;24:783–788. doi: 10.1007/s11606-009-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen S, T Kamarck RA. Mermelstein, Global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 48.Gelberg L, Gallagher TC, Andersen RM, Koegel P. Competing priorities as a barrier to medical care among homeless adults in Los Angeles. Am J Public Health. 1997;87:217–220. doi: 10.2105/ajph.87.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanfer R, Ackerman PL. Motivation and cognitive-abilities - an integrative aptitude treatment interaction approach to skill acquisition. J Appl Psychol. 1989;74:657–690. Table 1. [Google Scholar]
- 50.Scholle SH, Vuong O, Ding L, et al. Development of and field test results for the CAHPS PCMH survey. Med Care. 2012;50(Suppl:S2–S10) doi: 10.1097/MLR.0b013e3182610aba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin J. Pennsylvania Health Care Cost Containment Council. 2016 Accessed December 18, 2016 http://www.phc4.org/
- 52.Cruickshank JM, Thorp JM, Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet. 1987;1:581–584. doi: 10.1016/s0140-6736(87)90231-5. [DOI] [PubMed] [Google Scholar]
- 53.EHK K, Mitra S. Estimating Scaled Treatment Effects With Multiple Outcomes. Cornell University Library; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.RJA L, Rubin DB. Statistical Analysis With Missing Data. second. Wiley; Hoboken, N.J.: 2002. [Google Scholar]
- 55.U.S. Department of Labor BoLS, editor. Occupational Outlook Handbook, 2016–17 Edition. Health Educators and Community Health Workers; 2016. [Google Scholar]
- 56.Schroeder SA. Shattuck Lecture. We can do better-improving the health of the American people. N Engl J Med. 2007;357:1221–1228. doi: 10.1056/NEJMsa073350. [DOI] [PubMed] [Google Scholar]


