Abstract
Chiral pesticides currently constitute about 25% of all pesticides used, and this ratio is increasing as more complex structures are introduced. Chirality occurs widely in synthetic pyrethroids and organophosphates, which are the mainstay of modern insecticides. Despite the great public concerns associated with the use of insecticides, the environmental significance of chirality in currently used insecticides is poorly understood. In this study, we resolved enantiomers of a number of synthetic pyrethroid and organophosphate insecticides on chiral selective columns and evaluated the occurrence of enantioselectivity in aquatic toxicity and biodegradation. Dramatic differences between enantiomers were observed in their acute toxicity to the freshwater invertebrates Ceriodaphnia dubia and Daphnia magna, suggesting that the aquatic toxicity is primarily attributable to a specific enantiomer in the racemate. In field sediments, the (-) enantiomer of cis-bifenthrin or cis-permethrin was preferentially degraded, resulting in relative enrichment of the (+) enantiomer. Enantioselective degradation was also observed during incubation of sediments under laboratory conditions. Enantioselectivity in these processes is expected to result in ecotoxicological effects that cannot be predicted from our existing knowledge and must be considered in future risk assessment and regulatory decisions.
Keywords: chiral contaminants, chirality, enantiomers, chiral pesticides, chiral selectivity
The significance of molecular chirality is widely recognized in life sciences (1, 2). A lesser-known fact is that many modern pesticides also contain chiral structures and thus consist of enantiomers (3, 4). About 25% of currently used pesticides are chiral, and this ratio is increasing as compounds with more complex structures are introduced into use (3). Enantiomers of the same compound have identical physical-chemical properties and thus appear as a single compound in standard analysis. For economic reasons, chiral pesticides are primarily used as mixtures of enantiomers, or racemates. However, enantiomers are known to selectively interact with biological systems that are usually enantioselective and may behave as drastically different compounds. The role of enantioselectivity in environmental safety is poorly understood for pesticides, and the knowledge gap is reflected in that the great majority of chiral pesticides are used and regulated as if they were achiral, that is, single compounds.
Studies on chiral pesticides started to appear in the early 1990s (4, 5–12). Studies so far show that microbial degradation of chiral pesticides is commonly enantioselective. As one enantiomer is preferentially degraded, the enantiomer ratio (ER), defined as the ratio of (+) enantiomers to (-) enantiomers, increasingly deviates from the original value (typically 1.0) (8, 9). Enantioselectivity was found to result in changes of ER in α-HCH along the polar bear food chain, causing ER to increase from ≈1.0 in cod to 2.3 in liver samples of polar bear (10). In the brain tissue of seals around Iceland, often only (+) α-HCH was found; (-) α-HCH was absent, giving indefinitely large ER values (5, 13). Enantioselectivity also has been observed under laboratory conditions in the soil degradation of some chiral herbicides and fungicides, including metolachlor, metalaxyl, and dichlorprop (4, 8, 14).
Most environmental research on chiral pesticides has thus far been limited to a few legacy chlorinated insecticides whose use was discontinued decades ago and some herbicides or fungicides. Little is known about currently used chiral insecticides. Many current insecticides have high activity against nontarget organisms and are also chiral. In particular, two classes of insecticides, synthetic pyrethroids (SPs) and organophosphates (OPs), are acutely toxic to a wide range of aquatic organisms at trace levels (15, 16). Contamination of surface aquatic ecosystems by these compounds is a great environmental concern (17, 18). All known SPs have chiral structures and contain four or eight enantiomers (19); many OPs are also chiral, typically consisting of two enantiomers (20). Although these compounds are in wide use, enantioselectivity in their environmental behavior is almost unknown (21).
In this study, we developed chiral-selective chromatographic methods to separate and isolate enantiomers from a number of SPs and OPs. Individual enantiomers were used for characterizing their differences in acute toxicity to two indicator aquatic invertebrates, Ceriodaphnia dubia and Daphnia magna. Enantioselectivity was further evaluated for SPs during their biodegradation in sediments under field and laboratory conditions.
Materials and Methods
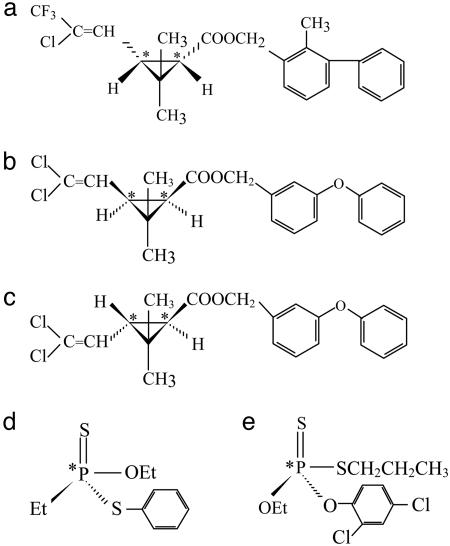
Chemicals. Analytical standards of racemic (Z)-cis-bifenthrin [>96%, 2-methylbiphenyl-3-ylmethyl (Z)-(1RS)-cis-3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-dimethylcyclopropanecarboxylate], fonofos [99.4%, O-ethyl S-phenyl (RS)-ethylphosphonodithioate], and profenofos [>93.7%, O-4-bromo-2-chlorophenyl O-ethyl S-(RS)-propyl phosphorothioate] were purchased from Chem Service (West Chester, PA). Racemic trans-permethrin [99.4%, phenoxybenzyl-(1SR)-(trans)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate], cis-permethrin (99.3%), and enantiopure (1R-cis)-bifenthrin (97.2%) were provided by FMC. The structures of these compounds are given in Fig. 1. Each of the selected insecticides (or isomers) contains one pair of enantiomers. Other solvents or chemicals used in this study were of pesticide-residue or HPLC grade.
Fig. 1.
Chemical structures of test compounds. (* indicates chiral position.) Shown are cis-bifenthrin (a), cis-permethrin (b), trans-permethrin (c), fonofos (d), and profenofos (e).
Chromatographic Separation and Analysis. Enantiomers were resolved and isolated on an 1100 Series HPLC (Agilent, Wilmington, DE) with chiral columns. After testing with a suite of commercially available columns, resolution of SP enantiomers was achieved on a Sumichiral OA-2500I column (Sumika Chemical Analysis Service, Osaka, Japan) by using 99.5% hexane and 0.5% 1,2-dichloroethane as the mobile phase. Resolution of OP enantiomers was achieved on a Chiralcel OJ column (Daicel Chemical Industries, Tokyo) by using 98% hexane and 2% ethanol (containing 5% methanol and 5% isopropanol) as the mobile phase. The injection volume was 20 μl, and the UV wavelength for detection was 230 ± 15 nm. The polarity (i.e., rotation sign) of the resolved enantiomers was determined by using an inline laser polarimeter detector (PDR-Chiral, Lake Park, FL). The light source for the chiral detector was a laser (675 nm), and the cell path was 50 mm. The resolved enantiomers were individually collected at the HPLC outlet, evaporated to dryness, and used in aquatic toxicity bioassays. Concentrations were determined by using peak area, assuming the same response factor for enantiomers originating from the same compound.
An Agilent 6890N GC–electron capture detector (ECD) system was used for quantitative analysis in experiments to evaluate enantioselectivity in degradation of SPs in sediments. An Agilent 5973 mass selective detector was used for structural confirmation. The temperature of the ECD was 310°C, and the detector makeup gas was N2 (60 ml·min-1). A suite of chiral capillary columns was tested, and optimal separation of enantiomers from cis-bifenthrin or cis-permethrin was achieved on a BGB-172 column (20% tert-butyldimethylsilyl-β-cyclodextrin dissolved in 15% diphenyl-polysiloxane and 85% dimethylpolysiloxane, GBG Analytik, Adliswil, Switzerland). The column was held at 180°C for 2 min, ramped at 1°C·min-1 to 225°C (first ramp), held at 225°C for 60 min, ramped at 5°C·min-1 to 230°C (second ramp), and then held at 230°C until complete elution. Preliminary experiments showed that no interconversion between enantiomers occurred under these conditions. Concentrations were determined by using peak area, assuming the same response factor for enantiomers originating from the same compound.
Aquatic Toxicity Assays. Enantioselectivity in aquatic toxicity was evaluated through 96-h acute toxicity assays by using C. dubia and D. magna. The overall procedure for the test was similar to the Environmental Protection Agency guidelines (22). Test animals were supplied by Aquatic Biosystems (Fort Collins, CO). Briefly, test solutions (15 ml) containing a given enantiomer (or a racemate) over a known concentration range were prepared in 20 ml borosilicate vials and used for exposure to five active C. dubia neonates aged <20 h. All vials were monitored at 24-h intervals until reaching 96 h. The concentration that caused 50% mortality of the test population (LC50) (μg·l-1) was determined by probit analysis with toxcalc (Tidepool, McKinleyville, CA). The test procedure for D. magna was roughly similar to that used for C. dubia, except that the volume of test solution was 50 ml, and five adult animals were used for exposure. The exposure was carried out under static conditions for 96 h, and LC50 was calculated by probit analysis with toxcalc.
Analysis of Field Sediments. Sediments containing residues of cis-bifenthrin and cis-permethrin were collected at a site next to a nursery in Southern California and were used for evaluation of changes in ER as a result of natural attenuation. The dried sediment was accumulated from surface runoff over a 4-year period. Samples were taken by using a hand auger to depths of 0–15, 15–30, and 30–45 cm. The sediment samples were airdried, mixed, and passed through a 0.5-mm sieve. Aliquots (5.0 g) of sediments were extracted by mixing twice with acetonehexane (1:1 vol/vol), and the extracts were concentrated to a small volume for analysis by GC. Preliminary experiments showed that the recovery of the above procedure was >90% for cis-bifenthrin or cis-permethrin in soil or sediment. Three replicates were used for each sediment sample.
Incubation Experiments. Enantioselectivity in SP degradation was further evaluated through incubation experiments. Sediment samples were collected from a sedimentation pond and a runoff channel at a nursery site in Southern California. The pond sediment contained 0.65% organic carbon and 5% clay; the channel sediment contained 6.4% organic carbon and 19% clay. Compared with sediments used in the above experiment, sediments from the sedimentation pond and channel were newly deposited. The sediments were sampled from the surface layer (0–5 cm) and were used without air-drying to preserve the original microbial activity. Five grams (dry weight equivalent) of the sediment was placed in a 20-ml glass vial and mixed with 5 ml of deionized water. The sample vials were covered with aluminum foil and incubated at room temperature (21 ± 1°C). Replicate samples were removed at different times and stored in a freezer (-21°C) before analysis. For analysis, the sediment was quantitatively transferred to a Teflon centrifuge tube, mixed with hexane-acetone (1:1 vol/vol), and then centrifuged. After the supernatant was decanted, the sediment phase was extracted two more times with fresh solvents. The extracts were combined, dried with 50 g of anhydrous sodium sulfate, and then concentrated to a small volume to be used for analysis by GC.
Results and Discussion
Enantiomer Separation and Analysis. Resolution of enantiomers was highly column-specific in both the HPLC and GC analyses. In the HPLC analysis, complete separation was achieved only on the Sumichiral OA-2500I column for SP enantiomers and on the Chiralcel OJ column for OP enantiomers (Table 1). In GC analysis, separation was achieved only on the BGB-172 column for cis-bifenthrin and cis-permethrin. Under the conditions described here, the (+) enantiomer of SPs consistently eluted before the (-) enantiomer on the Sumichiral OA-2500I column (Table 1). The use of the enantiopure standard (1R-cis) bifenthrin confirmed that the (+) enantiomer of cis-bifenthrin was (1R-cis) bifenthrin, whereas the (-) bifenthrin corresponded to (1S-cis) bifenthrin. For the selected OPs, the (+) enantiomer also eluted before the corresponding (-) enantiomer on the Chiralcel OJ column (Table 1). The adequate resolution allowed individual enantiomers to be recovered for use in bioassays.
Table 1. Separation of enantiomers of selected synthetic pyrethroids and organophosphates on chiral HPLC columns.
| Retention time, min
|
||
|---|---|---|
| Compound | (+) Enantiomers | (–) Enantiomers |
| cis-Bifenthrin* | 10.57 | 11.25 |
| cis-Permethrin* | 17.41 | 19.06 |
| trans-Permethrin* | 27.32 | 28.29 |
| Fonofos† | 12.73 | 14.86 |
| Profenofos† | 23.29 | 27.35 |
Results from the Sumichiral OA-2500I column
Results from the Chiralcel OJ column
On the β-cyclodextrin-coated BGB-172 GC column, cis-bifenthrin gave two separated peaks with retention times of 54.3 min (peak I) and 55.5 min (peak II) under the described conditions. Elution of (1R-cis) bifenthrin under the same conditions produced only peak I, confirming that peak I corresponded to (1R-cis) or (+) bifenthrin, leaving peak II as (1S-cis) or (-) bifenthrin. Analysis of cis-permethrin gave two separated peaks with retention times of 84.0 min (peak I) and 85.4 min (peak II) (Fig. 2). The two enantiomers in trans-permethrin were not separated under the given conditions. Isomers of cis-permethrin obtained from HPLC preparation were individually eluted on GC under the same conditions. The analysis showed that peak I in Fig. 2 was derived from the (+) enantiomer and that peak II was derived from the (-) enantiomer. The developed GC method was subsequently used for quantitative analysis of enantiomer concentrations for cis-bifenthrin and cis-permethrin in the degradation experiments.
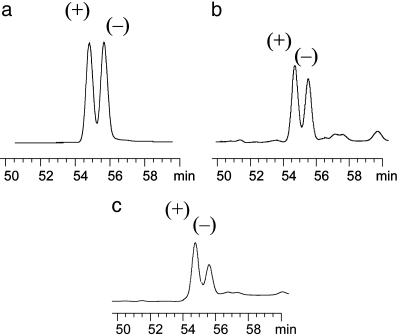
Fig. 2.
Chiral GC chromatograms of cis-bifenthrin in aged sediments from different depths. Results are shown for depths of 0–15 (a), 15–30 (b), and 30–45 (c) cm.
Enantioselectivity in Aquatic Toxicity. The acute aquatic toxicity was measured for individual enantiomers and racemates by using C. dubia and D. magna as the test animals (Table 2). From the LC50 values for the racemate, all insecticides possess outstanding toxicity against C. dubia or D. magna, with LC50 < 1 μg·l-1. However, there consistently was a significant difference in LC50 between the two enantiomers of the same compound. Invariably, when one enantiomer showed high activity, the other enantiomer exhibited activity that was drastically lower. For the selected SPs, the (+) enantiomer was more active than the (-) enantiomer by 17–38 times (Table 2). The (-) enantiomer in cis-permethrin and trans-permethrin was so inactive that only a threshold of 6 μg·l-1 was obtained for the LC50 against either C. dubia or D. magna. From the LC50 values measured for individual enantiomers, it was estimated that the (+) enantiomer contributed at least 95–97% of the activity against C. dubia and 94–96% of the activity against D. magna in the racemate. For the selected chiral OPs, it was observed that the (-) enantiomer was consistently more active than the (+) enantiomer. The activity of the racemate was attributable primarily to the (-) enantiomer for both C. dubia (92–94%) and D. magna (87–94%). In a previous study, a 22-fold difference was observed between the R and S forms of malaoxon, an oxidation product of malathion, in their inhibitory potency for acetylcholinesterase from bovine erythrocytes (21). Therefore, for these current chiral insecticides, although racemates are commercially used, only one of the two enantiomers may be expected to contribute significantly to the aquatic toxicity observed for the racemate.
Table 2. LC50 values in μg·l-1 of enantiomers (+ or –) and racemate (±) of selected synthetic pyrethroid and organophosphate insecticides.
|
C. dubia
|
D. magna
|
|||||
|---|---|---|---|---|---|---|
| Compound | (±) | (+) | (–) | (±) | (+) | (–) |
| cis-BF | 0.144 ± 0.017 | 0.076 ± 0.016 | 1.342 ± 0.165 | 0.175 ± 0.030 | 0.081 ± 0.014 | 1.803 ± 0.211 |
| cis-PM | 0.539 ± 0.027 | 0.156 ± 0.027 | >6.0 | 0.788 ± 0.112 | 0.388 ± 0.070 | >6.0 |
| trans-PM | 0.519 ± 0.055 | 0.197 ± 0.030 | >6.0 | 0.738 ± 0.046 | 0.307 ± 0.045 | >6.0 |
| Fonofos | 0.22 ± 0.03 | 2.24 ± 0.29 | 0.15 ± 0.04 | 0.58 ± 0.04 | 3.45 ± 0.33 | 0.23 ± 0.05 |
| Profenofos | 0.17 ± 0.02 | 1.68 ± 0.95 | 0.14 ± 0.07 | 0.69 ± 0.11 | ± 2.32 ± 0.21 | 0.35 ± 0.08 |
BF, bifenthrin; PM, permethrin.
Enantioselectivity in Degradation. Enantioselectivity in degradation was evaluated for the selected SPs by studying changes in ER during degradation under field and laboratory conditions. Analysis of aged field sediment samples showed that all samples contained relatively high levels of bifenthrin and permethrin. The ER in commercial formulations was close to 1.0 for cis-bifenthrin (1.02) and cis-permethrin (0.99). The averaged ER value for cis-bifenthrin (1.02) remained close to 1.0 in the surface sediment (0–15 cm) but increased to 1.11 in the 15–30-cm layer and further to 1.32 in the 30–45-cm layer (Table 3). The increase in ER was significant (P < 0.05) for both depths, suggesting that the (-) enantiomer was preferentially degraded compared with the (+) enantiomer in the subsurface sediment. A similar, but stronger, trend was observed for cis-permethrin (Table 3). Although ER for cis-permethrin was close to 1.0 in the surface sediment (1.05), it increased to 1.30 in the 15–30-cm layer and further to 1.54 in the 30–45-cm layer (Table 3). Preferential degradation of the (-) enantiomer resulted in relative enrichment of the aquatically active (+) enantiomer, as shown in Fig. 2 for cis-bifenthrin. The absence of enantioselectivity near the surface may be attributable to the fact that microbial degradation was limited because of the relatively low moisture content (14.5% for the 0–15-cm layer, with the top 5–8 cm near air-dry). The moisture increased to 29.3% for the 15–30-cm layer and further to 34.5% for the 30–45-cm layer. Therefore, it is likely that conditions in the subsurface layers were more favorable for microbial growth, resulting in more extensive biodegradation of the (+) enantiomer. It is also possible that the oxidation state in the sediment varied as a function of depth, with the surface layers being more aerobic than the deeper layers. The oxidation state may have affected the characteristics and enantioselectivity of the microbial transformations.
Table 3. Concentrations in μg·kg-1 of bifenthrin and permethrin enantiomers and ER in sediments from a nursery sediment disposal site (n = 3).
| (Z)-cis-bifenthrin
|
cis-Permethrin
|
|||||
|---|---|---|---|---|---|---|
| Sample* | (+) | (–) | ER | (+) | (–) | ER |
| A* | ||||||
| 0–15 cm | 69.7 ± 1.3 | 68.1 ± 1.2 | 1.02 ± 0.02 | 26.7 ± 1.9 | 25.3 ± 1.6 | 1.05 ± 0.02 |
| 15–30 cm | 93.3 ± 1.3 | 84.0 ± 1.1 | 1.11 ± 0.03 | 45.6 ± 1.6 | 35.0 ± 1.7 | 1.30 ± 0.02 |
| 30–45 cm | 50.3 ± 10.6 | 38.3 ± 1.5 | 1.32 ± 0.03 | 15.8 ± 1.5 | 10.3 ± 1.4 | 1.54 ± 0.03 |
| B* | ||||||
| 0–15 cm | 152.9 ± 2.3 | 149.6 ± 2.4 | 1.02 ± 0.03 | 43.3 ± 1.2 | 41.5 ± 1.4 | 1.02 ± 0.03 |
| 15–30 cm | 177.2 ± 1.5 | 159.9 ± 2.5 | 1.11 ± 0.03 | 65.5 ± 1.2 | 48.8 ± 1.1 | 1.33 ± 0.02 |
| C* | ||||||
| 0–15 cm | 143.2 ± 2.4 | 139.9 ± 2.4 | 1.32 ± 0.02 | 50.6 ± 1.3 | 48.2 ± 1.2 | 1.05 ± 0.03 |
| 15–30 cm | 189.9 ± 2.5 | 170.4 ± 3.1 | 1.11 ± 0.03 | 80.7 ± 1.5 | 60.3 ± 1.6 | 1.32 ± 0.03 |
| Formulation | — | — | 1.02 ± 0.03 | — | — | 0.99 ± 0.02 |
—, not applicable.
A, B, and C indicate sampling location
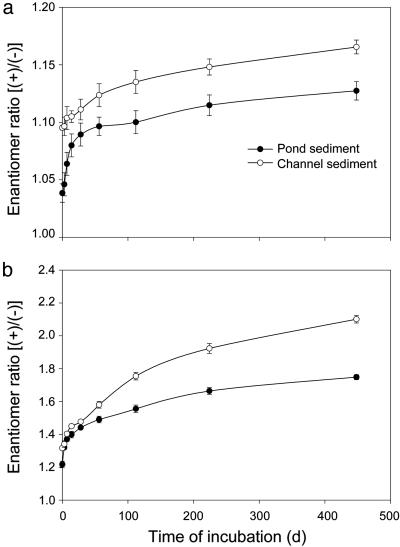
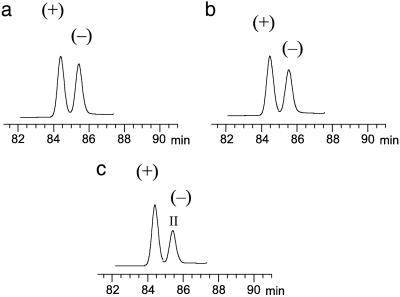
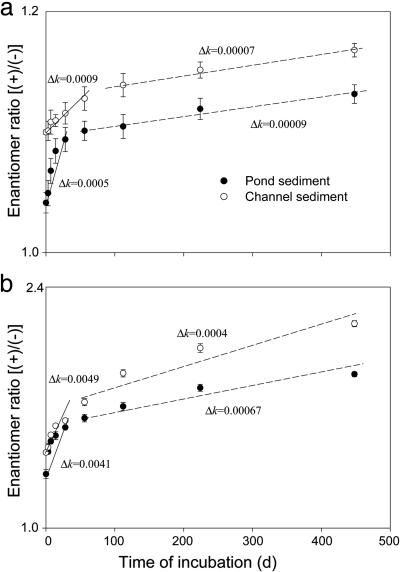
Changes in ER were further determined for cis-bifenthrin and cis-permethrin during 448-day incubation of pesticide-contaminated sediments under laboratory conditions. For both cis-bifenthrin and cis-permethrin, ER consistently increased with time (Fig. 3). The initial ER for cis-bifenthrin in the pond sediment was 1.04, but it gradually increased to 1.12 after 448 days of incubation. For the channel sediment, the ER increased from 1.10 at the beginning to 1.16 at the end of incubation. More pronounced changes were observed for degradation of cis-permethrin. In the pond sediment, the initial ER for cis-permethrin was 1.22, but it increased to 1.75 after the incubation. In the channel sediment, the initial ER was 1.32; the ER increased to 2.08 at the end of incubation (Fig. 3). Therefore, the (-) enantiomer was preferentially degraded over the (+) enantiomer for both cis-bifenthrin and cis-permethrin in both pond and channel sediments. The selective degradation resulted in relative enrichment of the aquatically active (+) enantiomer, as shown in Fig. 4 for cis-permethrin. This selectivity was in agreement with that observed for the aged field sediments.
Fig. 3.
Changes in ER during incubation of pyrethroid insecticide-contaminated sediments under laboratory conditions. Results are shown for cis-bifenthrin (a) and cis-permethrin (b). Vertical bars are standard errors of three replicates.
Fig. 4.
Chiral GC chromatograms of cis-permethrin in sediment after different periods of incubation at room temperature. Results are shown for incubation periods of 40 (a), 220 (b), and 446 (c) days.
Assuming that degradation of enantiomers followed firstorder kinetics with a rate constant of k(-) for the (-) enantiomer and a rate constant of k(+) for the (+) enantiomer, ER may be expressed as a function of time (t) in the following relationship (23):
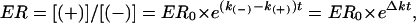 |
[1] |
where ER0 is the initial ER value, [(+)] is the concentration of the (+) enantiomer at time t, [(-)] is the concentration of the (-) enantiomer at time t, and Δk is the difference between k(-) and k(+), which reflects the rate at which ER deviates from ER0 over time. The above relationship can be further expressed in a linear form after logarithmic transformation of ER:
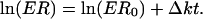 |
[2] |
Fitting the measured ER values in Fig. 3 to incubation time t using Eq. 2 did not yield a single straight line for any of the sediment–pesticide combinations (Fig. 5). The dependence of ln(ER) on t was better approximated with two separate lines of different slopes (i.e., Δk). It appears that the dependence of ER on time consisted of two phases: a rapidly increasing first phase that lasted 28–56 days and a slowly increasing second phase that extended till the end of incubation (Fig. 5). The Δk for the first phase was 5–12 times greater than that for the second phase for cis-bifenthrin, and the difference was 6–13 times for cis-permethrin. The reduced increasing rate at the later stage may be due to the fact that labile pesticides in the sediment were depleted from degradation during the initial phase and the remaining fraction became more resistant to biodegradation.
Fig. 5.
Regression of ER with incubation time after logarithmic transformation by using Eq. 2. Results are shown for cis-bifenthrin (a) and cis-permethrin (b). Vertical bars are standard errors of three replicates.
Ecotoxicological Implications. Occurrence of enantioselectivity in either degradation or toxicity alone would have limited environmental significance. For instance, if two enantiomers of a chiral compound have the same aquatic toxicity (i.e., nonenantioselective), changes in ER alone will not result in different effects on the organism, because the combined toxicity will remain unchanged in relation to time. Conversely, if enantioselectivity occurs only in toxicity but not in degradation, ER will remain unchanged over time, and the ecotoxicological effects are predictable from the racemate. Although previous studies showed that legacy chiral insecticides and some chiral herbicides or fungicides could undergo enantioselective degradation in the environment, the ecotoxicological significance was not revealed, because enantioselectivity in ecotoxicity was not simultaneously determined for these compounds. The lack of research may be attributable to the fact that these compounds have either long-term (e.g., chlorinated insecticides) or subtle (e.g., some herbicides and fungicides) ecotoxicological endpoints that are not easily measurable through short-term tests.
In this study, we demonstrated that enantioselectivity occurred concurrently in both aquatic toxicity and degradation for chiral compounds from the pyrethroid and organophosphate classes. This finding may have several important implications. First, as only specific enantiomers possess significant toxicity, the adverse ecotoxicological effects will closely depend on the environmental behavior of the toxicologically active enantiomer instead of on that of the racemate. Monitoring for the racemate concentration, as currently practiced, will give an inadequate or misleading basis for assessing the environmental risk of chiral contaminants. For instance, if the toxicologically active enantiomer is preferentially degraded, the use of racemate concentration will result in an overestimation of ecotoxicity. However, if the inactive enantiomer is selectively degraded, the use of racemate concentration will underestimate the ecotoxicological effect. Products enriched in biologically active enantiomers are increasingly used for achieving improved pest-control efficacy (12). For instance, enantiomer-enriched formulations of the SPs cyf luthrin, cypermethrin, and deltamethrin are currently in use (20). If the adverse ecological effects are mainly attributable to the enriched enantiomer, the environmental value of using enantiomer-enriched products must be questioned. However, if the adverse effects are caused only by or mostly by the excluded enantiomer, using enantiomer-enriched products may offer great environmental benefits. In addition, studies show that enantioselectivity may closely depend on environmental conditions and may vary significantly even for the same compound (4, 14, 24, 25). Further studies are needed to characterize interactions of environmental factors, such as soil/sediment properties, vegetation types, redox conditions, and microbial structures, with enantioselectivity in the behavior of chiral pesticides.
Both SPs and OPs have other modes of toxicological effects, but it is unknown whether enantioselectivity also occurs in those processes. For instance, because SPs have strong affinity for the sediment phase in a surface-water system, toxicity to sediment-dwelling organisms is of great importance, and enantioselectivity in sediment toxicity should be characterized. In addition to the acute lethality afforded by SPs, the U.S. Environmental Protection Agency has listed this insecticide class as a likely endocrine disrupter in humans and wildlife (26). Estrogenic and antiestrogenic effects from SP insecticides have been observed in terrestrial invertebrates and mammalian cell lines (27–29). Potential enantioselectivity in these chronic effects should be explored. Organophosphates commonly have a wide spectrum of activity for inhibiting cholinesterases in both mammals and invertebrates. However, it is unclear whether the toxicity is due to enantioselective inhibition of cholinesterases or alternative mechanisms. Because pyrethroids (or organophosphates) typically share the same mode of action, the enantioselectivity observed for the few insecticides in this study may be extrapolated to the other compounds in the same class. Given the widespread use of these insecticides, a more comprehensive understanding of the significance of enantioselectivity is imperative for improving risk assessment and regulation of these pesticides.
Author contributions: W.L. and J.G. designed research; W.L. performed research; W.L., J.G., D.S., and W.A.J. analyzed data; and J.G., D.S., and W.A.J. wrote the paper.
Abbreviations: SP, synthetic pyrethroid; OP, organophosphate; ER, enantiomer ratio; LC50, concentration that caused 50% mortality.
References
- 1.Koeller, K. M. & Wong, C. H. (2001) Nature 409, 232-240. [DOI] [PubMed] [Google Scholar]
- 2.Yoon, T. P. & Jacobsen, E. N. (2003) Science 299, 1691-1693. [DOI] [PubMed] [Google Scholar]
- 3.Williams, A. (1996) Pestic. Sci. 46, 3-9. [Google Scholar]
- 4.Lewis, D. L., Garrison, A. W., Wommack, K. E., Whittemore, A., Steudler, P. & Melillo, J. (1999) Nature 401, 898-901. [DOI] [PubMed] [Google Scholar]
- 5.Huhnerfuss, H., Kallenborn, R., Konig, W. A. & Rimkus, G. (1992) Organohalogen Comp. 10, 97-100. [Google Scholar]
- 6.Falconer, R. L., Bidleman, T. F., Gregor, D. J., Semkin, R. & Teixeira, C. (1995) Environ. Sci. Technol. 29, 1297-1302. [DOI] [PubMed] [Google Scholar]
- 7.Vetter, W. & Schurig, V. (1997) J. Chromatogr. A 774, 143-175. [DOI] [PubMed] [Google Scholar]
- 8.Buser, H. R. & Muller, M. D. (1998) Environ. Sci. Technol. 32, 626-633. [Google Scholar]
- 9.Bidleman, T. F. & Falconer, R. L. (1999) Environ. Sci. Technol. 33, 2299-2301. [DOI] [PubMed] [Google Scholar]
- 10.Wiberg, K., Letcher, R. J., Sandau, C. D., Norstrom, R. J., Tysklind, M. & Bidleman, T. F. (2000) Environ. Sci. Technol. 34, 2668-2674. [Google Scholar]
- 11.Vetter, W. (2001) Food Rev. Int. 17, 113-182. [Google Scholar]
- 12.Hegeman, W. J. & Laane, R.W. (2002) Rev. Environ. Contam. Toxicol. 173, 85-116. [PubMed] [Google Scholar]
- 13.Moller, K., Bretzke, C., Huhnerfuss, H., Kallenborn, R., Kinkel, J. N., Kopf, J. & Rimkus, G. (1994) Angew. Chem. 33, 882-884. [Google Scholar]
- 14.Buerge, I. J., Poiger, T., Muller, M. D. & Buser, H. R. (2003) Environ. Sci. Technol. 37, 2668-2674. [DOI] [PubMed] [Google Scholar]
- 15.Mokrey, L. E. & Hoagland, K. D. (1989) Environ. Toxicol. Chem. 9, 1045-1051. [Google Scholar]
- 16.Werner, I., Deanovic, L. A., Hinton, D. E., Henderson, J. D., de Oliveira, G. H., Wilson, B. W., Krueger, W., Wallender, W. W., Oliver, M. N. & Zalom, F. G. (2002) Bull. Environ. Contam. Toxicol. 68, 29-36. [DOI] [PubMed] [Google Scholar]
- 17.Anderson, B. S., Hunt, J. W., Phillips, B. M., Nicely P. A., de Vlaming, V., Connor, V., Richard, N. & Tjeerdema, R. S. (2003) Environ. Pollut. 124, 523-532. [DOI] [PubMed] [Google Scholar]
- 18.Weston, D. P., You, J. C. & Lydy, M. J. (2004) Environ. Sci. Technol. 38, 2752-2759. [DOI] [PubMed] [Google Scholar]
- 19.Chamberlain, K., Matsuo, N., Kaneko, H. & Khambay, B. P. S. (1998) in Chirality in Agrochemicals, eds. Kurihara, N. & Miyamoto, J. (Wiley, New York), pp. 9-84.
- 20.Sasaki, M. (1998) in Chirality in Agrochemicals, eds. Kurihara, N. & Miyamoto, J. (Wiley, New York), pp. 85-139.
- 21.Rodriguez, O. P., Muth, G. W., Berkman, C. E., Kim, K. & Thompson, C.M. (1997) Bull. Environ. Contam. Toxicol. 58, 171-176. [DOI] [PubMed] [Google Scholar]
- 22.Weber, C. I. (1995) Report 600/4–90/027F (U.S. Environmental Protection Agency, Cincinnati).
- 23.Buser, H. R., Muller, M. D., Poiger, T. & Balmer, M. E. (2002) Environ. Sci. Technol. 36, 221-226. [DOI] [PubMed] [Google Scholar]
- 24.Muller, M. D. & Buser, H. R. (1995) Environ. Sci. Technol. 29, 2031-2037. [DOI] [PubMed] [Google Scholar]
- 25.Monkiedje, A., Spiteller, M. & Bester, K. (2003) Environ. Sci. Technol. 37, 707-712. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Environmental Protection Agency (1996) Report EPA/630/R-96/012 (U.S. Environmental Protection Agency, Washington, DC).
- 27.Taylor, D., Chinzei, Y., Ito, K., Higuchi, N. & Ando, K. (1991) J. Med. Entomol. 28, 322-329. [DOI] [PubMed] [Google Scholar]
- 28.Go, V., Garey, J., Wolff, M. S. & Pogo, B. G. T. (1999) Environ. Health Perspect. 107, 173-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyler, C. R., Beresford, N., van der Woning, M., Sumpter, J. P. & Thorpe, K. (2000) Environ. Toxicol. Chem. 19, 801-809. [Google Scholar]