Over a century of effort of synthetic medicinal chemistry has not replaced natural products as essential components of our therapeutic arsenal. In fact, for some classes of drugs, such as the antibiotics, natural products and their semisynthetic derivatives dominate. Natural products can also have significant roles in pathology, e.g., by contributing to virulence during infection. The majority of natural products produced by microorganisms, marine organisms, and plants use one or a combination of three scaffold assembly plans: (i) nonribosomal peptide biosynthesis, (ii) polyketide assembly, and (iii) alkaloid biosynthesis. Whereas these scaffolds provide the first level of structural diversity, additional complexity and structural and functional expansion occur through a molecular toolkit of accessorizing proteins that are increasingly recognized as vital to our understanding and manipulation of new natural products and as potential targets for therapeutic agents (Fig. 1). In this issue of PNAS, Fischbach et al. (1) expand this toolkit with their characterization of IroB, a C-glycosyltransferase that modifies an iron-chelating siderophore that is a potential virulence factor in Salmonella.
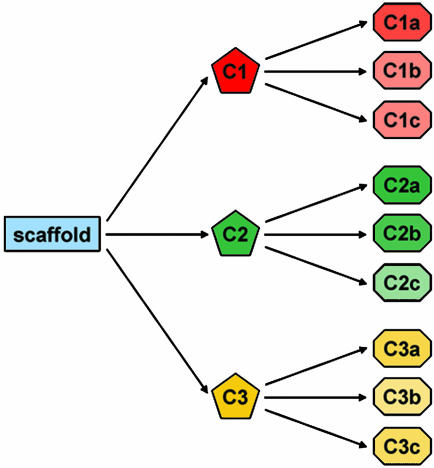
Fig. 1.
Diversity of natural products by means of biocombinatorial chemistry. Initially, biochemical modification of a scaffold results in a small number of structurally distinct compounds (C1, C2, and C3), followed by the production of more complex and unique compounds (C1a, C1b, C1c, etc.).
Microorganisms produce a plethora of natural products for diverse uses. These applications include protection from competing strains (2), chemical messengers for cell-cell communication (3), agents to facilitate colonization of a host (4), and gene regulation (5). The proven track record of natural products and their attributes as modulators of biological activity have made them agents of choice in lead discovery in the pharmaceutical industry and molecular biological research. At the same time, their structural and stereochemical complexity has made them often daunting from the perspective of standard structure/function studies. Developing a strategy whereby the biosynthetic systems responsible for their production could be manipulated to produce and accessorize these compounds is therefore very attractive.
IroB modifies an iron chelator that is a potential virulence factor in Salmonella.
The past decade has seen an explosion in our understanding of the mechanisms and strategies whereby many natural products are biosynthesized (6). In particular, the molecular organization, chemical strategies, mechanisms, and structures of the assembly line protein complexes that are responsible for the polyketide and nonribosomal peptide natural products have been elaborated in significant detail (7). Furthermore, the molecular blueprints that result in the formation of these scaffolds have been dissected in growing detail. It is now possible to reasonably predict the number (if not wholly the nature) of subunits in polyketide and peptide scaffold assembly. These scaffolds form the basis of some of the most important natural products we know of, including antibiotics that inhibit microbial cell growth, virulence factors that aid in infection, and other natural products that have assorted biological impact, from immune modulation to control of cell division. We are now at the stage where these assembly lines can be engineered, with reasonable expectation of success, to produce novel agents in the first steps of a biocombinatorial chemistry platform (Fig. 1).
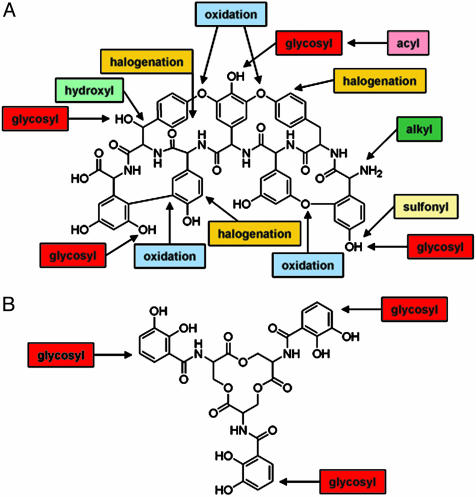
However, the biological effects of natural products depend on more than just their scaffolds. They are also accessorized by a number of tailoring reactions that greatly add to the chemical diversity of these agents. These reactions include modification by halogenation, oxidation and reduction, alkylation, acylation, sulfonation, and glycosylation (Fig. 2A). These reactions provide yet another order of chemical diversity and can have a dramatic impact on biological activity, bioavailability, and, for compounds destined to be drugs, pharmacology. Therefore, the next natural step in biocombinatorial chemistry is the elaboration of engineered scaffolds by a toolkit of tailoring enzymes to generate novel compounds.
Fig. 2.
Nature's tools for scaffold modification. (A) Accessorizing the glycopeptide antibiotic scaffold results in a variety of antibiotics with different properties. (B) Glycosylation of the enterobactin scaffold generates more efficient siderophores, namely salmochelins.
Knowledge of the chemical mechanisms of these reactions and of the structures and specificities of their protein catalysts is the next frontier in tailoring natural products and the engineering of new agents. Furthermore, for modifications that improve the virulence of microorganisms, these reactions can provide tractable targets for the development of new antimicrobial agents that block the activity of these small molecules. Because these catalysts tend to be highly specific to genera or even species of infectious organisms, such targets would provide a “smart bomb” approach to infectious disease control and leave off-target noninfectious commensal organisms intact, thereby reducing selective pressure for widespread drug resistance and avoiding unwanted side effects of antimicrobial therapy such as gastric problems and superinfection.
For many of these chemical processes, however, there is only rudimentary understanding of the basis of recognition of their substrates, their structures, or specificity. Harnessing the potential of biocombinatorial chemistry or targeting these reactions in therapeutic design will require a thorough understanding of the recognition rules of these accessorizing enzymes. One of the most important postscaffold assembly-accessorizing reactions is glycosylation. Glycosylated antibiotics include macrolides such as erythromycin, peptides such as vancomycin, and aminoglycosides such as gentamicin. Glycosylation is also important to natural-product anticancer agents such as doxorubicin, antiparasitic agents such as avermectin, and antifungal agents such as blasticidin-S. Most glycosyltransferase reactions occur through an NTP-glycosyl donor and a receptor nucleophilic hydroxyl or amino group (O- and N-glycosyl transfer, respectively). The O-glycosylation of natural products in particular has been well studied (8). C-glycosylation on the other hand is rare given the requirement for a nucleophilic center for sugar transfer. Nonetheless, there are a few C-glycosylated natural products known, including the antibiotics medermycin and granaticin and the anticancer agent urdamycin. Recently the salmochelins were reported as novel C-glucosyl-modified derivatives of the catecholate siderophore enterobactin (Fig. 2B) (9, 10).
Bacteria often find themselves in iron-deficient environments, especially during the infection process, and, as a result, synthesize iron-sequestering small-molecule natural products called siderophores. Pathogenic bacteria such as Escherichia coli and Salmonella enterica produce the catecholate siderophores enterobactin (also called enterochelin) and salmochelin to scavenge iron. This ability is necessary for their survival and proliferation in the iron-poor environment of tissues and cells, and, as a result, siderophores are often considered potential virulence factors. Salmochelins in particular seem to be superior siderophores, compared with enterobactin, in the presence of serum albumin; therefore, it has been suggested that salmochelins may play a significant role in some infections (10).
Fischbach et al. (1) have expressed, purified, and characterized IroB, the C-glycosyltransferase that accessorizes enterobactin to produce the better siderophore salmochelin (1). This is the first in vitro reconstitution of C-glycosyltransferase activity. They show, using purified IroB, enterobactin, and UDP-glucose, that C-glucosyl transfer occurs on successive 2,3-dihydroxybenzoyl rings at position 5, sequentially producing the mono-, di-, and tri-glucosyl enterobactins. The selective glucosylation exclusively at position 5, which is para to the phenol hydroxyl at position 2 of the 2,3-dihydroxybenzoyl group, suggests a reasonable means of generating the required carbanion for nucleophilic attack of the NDP-glucose donor. Fischbach et al. also show that the mono-, di-, and triglucosyl enterobactins can be separated and, in principle, that the biological activity of each of these molecules can now be assessed.
This research paves the way for the enzyme IroB and its orthologues to be considered in biocombinatorial strategies using appropriate phenolic substrates. Furthermore, given the link between C-glucosylation and virulence, inhibition of IroB could be considered a potential target in antibacterial drug design. Additionally, siderophores have been suggested as delivery vehicles in drug discovery (11). One could envision engineering IroB or another accessorizing enzyme to graft toxic glucosyl derivatives such as antibiotics onto siderophores to improve delivery and gain organism-specific selectivity. Regardless of the ultimate application of IroB, Fischbach et al. have added another reaction to the natural product accessorizing toolkit that further expands our knowledge of biochemical diversity and promises to contribute to the engineering of novel natural products (1).
See companion article on page 571.
References
- 1.Fischbach, M. A., Lin, H., Liu, D. R. & Walsh, C. T. (2005) Proc. Natl. Acad. Sci. USA 102, 571-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strohl, W. R. (1997) Biotechnology of Antibiotics, Drugs and the Pharmaceutical Sciences (Dekker, New York), 2nd Ed.
- 3.Smith, R. S. & Iglewski, B. H. (2003) Curr. Opin. Microbiol. 6, 56-60. [DOI] [PubMed] [Google Scholar]
- 4.Crosa, J. H. & Walsh, C. T. (2002) Microbiol. Mol. Biol. Rev. 66, 223-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nodwell, J. R., Yang, M., Kuo, D. & Losick, R. (1999) Genetics 151, 569-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khosla, C. & Keasling, J. D. (2003) Nat. Rev. Drug Discovery 2, 1019-1025. [DOI] [PubMed] [Google Scholar]
- 7.Walsh, C. T. (2004) Science 303, 1805-1810. [DOI] [PubMed] [Google Scholar]
- 8.Walsh, C., Freel Meyers, C. L. & Losey, H. C. (2003) J. Med. Chem. 46, 3425-3436. [DOI] [PubMed] [Google Scholar]
- 9.Hantke, K., Nicholson, G., Rabsch, W. & Winkelmann, G. (2003) Proc. Natl. Acad. Sci. USA 100, 3677-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bister, B., Bischoff, D., Nicholson, G. J., Valdebenito, M., Schneider, K., Winkelmann, G., Hantke, K. & Sussmuth, R. D. (2004) Biometals 17, 471-481. [DOI] [PubMed] [Google Scholar]
- 11.Roosenberg, J. M., II, Lin, Y. M., Lu, Y. & Miller, M. J. (2000) Curr. Med. Chem. 7, 159-197. [DOI] [PubMed] [Google Scholar]