Abstract
Background
Lupus nephritis (LN) is a major complication of systemic lupus erythematosus (SLE). This study tested miR-146a and its target gene TRAF6 expression in LN patients and discussed their relationship with LN.
Material/Methods
One hundred twenty-eight LN patients and 30 healthy controls were enrolled in this study. MiR-146a and TRAF6 expression in peripheral blood mononuclear cells (PBMCs) were detected. Serum cytokines content was determined by ELISA. The diagnostic role of miR-146a and TRAF6 in LN activity was evaluated by ROC curve. The impact of miR-146a and TRAF6 on end-stage renal disease (ESRD) was compared by survival curve. The effect of miR-146a and TRAF6 on LN recurrence was analyzed.
Results
Compared with healthy controls, miR-146a expression was significantly reduced and TRAF6 was upregulated in LN patients. The expression was related to LN activity. MiR-146a expression was negatively correlated, whereas TRAF6 was positively correlated with serum IL-1β, IL-6, IL-8, and TNF-α activity. The area under the ROC curve (AUC) of miR-146a and TRAF6 on the diagnosis of LN was 0.821 and 0.897, respectively. The AUC of miR-146a and TRAF6 on LN activity differentiation was 0.921 and 0.872, respectively. Downregulation of miR-146a and upregulation of TRAF6 increased the incidence of ESRD progression. Downregulation of miR-146a and upregulation of TRAF6 elevated the possibility of recurrence within one year.
Conclusions
MiR-146a declined, while TRAF6 increased in LN patients compared with healthy controls. Their expression can be used to effectively differentiate LN and evaluate activity. MiR-146a reduction and TRAF6 upregulation increased the possibility of ESRD progress and recurrence within one year.
MeSH Keywords: Diethylpropion, Lupus Nephritis, TNF Receptor-Associated Factor 6
Background
Systemic lupus erythematosus (SLE) is a type of chronic autoimmune disease characterized by multiple organ damage, multiple autoantibodies formation, and numerous immune complex depositions with complicated etiological mechanism [1,2]. Kidney is the main injury organ involved in SLE. Lupus nephritis (LN) is one of the most serious complications of SLE, and it is defined as chronic kidney damage caused by the release of inflammatory mediators, inflammatory cells infiltration, and immune complex deposition [3–5]. About 30–50% of SLE patients suffered from LN [6,7]. SLE patients with LN are more likely to develop end-stage renal disease (ESRD) with high mortality and poor prognosis [8]. LN is one type of SLE complications, others immunity-related damage to the kidney include: hematuria, proteinuria, and renal insufficiency. The pathogenesis of LN is related to an imbalance in immunity, including immune complex formation, immune cells, and cytokines. Direct pathogenic factors include inflammatory mediators and inflammatory cells infiltration [9–12]. MicroRNAs (miRNAs), which are about 22–25 nucleotides in length, are a kind of non-coding single-stranded RNA molecule encoded by endogenous genes. They participate in gene regulation at the transcriptional level [13]. Each miRNA can regulate multiple target genes, while different miRNAs can also regulate the same target gene [14]. It is presumed that more than a third of targeted human genes are regulated by miRNAs [15]. MiRNA regulation is involved in a variety of biological processes, including individual growth and development, cell proliferation and apoptosis, cell metabolism, cell differentiation, and immune inflammatory response [16]. MiR-146a is a member of the miR-146 family that is located on the long arm of chromosome 5. MiR-146a is an immune and hematopoiesis related miRNA, participating in hematopoietic cell proliferation and differentiation, cell immune response, and release of inflammatory mediators [17]. One of its important functions involves regulating NF-κB signaling pathway activation and inflammatory cytokines release mediated by Toll-like receptor 4 (TRL4). It negatively regulates NF-κB signaling pathway and inflammatory reaction activation through targeting TRAF6, downstream of TLR4 [17]. MiR-146a level is significantly downregulated in the peripheral blood mononuclear cells (PBMC) of SLE, suggesting that it may participate in the pathogenesis of LN [18,19]. This study tested miR-146a and its target gene TRAF6 expression in LN patients and discusses their relationship with LN.
Material and Methods
Main reagents and materials
RNA extraction reagent TRIzol and reverse transcription kit SuperScript® One-Step RT-PCR System were bought from Invitrogen. SYBR Green was from Takara. MiR-146a reverse transcription and PCR primer was from Ribobio. The PCR primer for TRAF6 was synthetized by Sangon. Rabbit anti-human TRAF6 antibody was from Abcam. ELISA kit was from eBioscience.
Clinical information
A total of 128 cases of LN patients, between January 2006 and September 2007, who were seen in nephrology unit of Linyi People’s Hospital were enrolled in the study. There were 16 males and 112 females with mean age of 40.6±8.2 years. No patients had received immunosuppressive treatment or immune modulators within three months. Patients with malignant tumors, acute or chronic infections, or other autoimmune diseases were excluded. Systemic lupus erythematosus diseases activity index (SLEDAI) was evaluated according to clinical manifestation and laboratory examination. Four points or less was considered the stationary phase (40 cases), while 5 points or higher was considered the active phase (88 cases). Another 30 cases of healthy volunteers were enrolled as controls, including 4 males and 26 females with mean age of 39.3±7.9 years. No statistical difference was observed in age and gender among the three groups (p>0.05). Peripheral blood was extracted from the participants to separate PBMC and serum. PBMCs were isolated using the density gradient centrifugation method. The blood, diluted with equal volume of PBS, was slowly added to 5 mL lymphocyte separation medium (Ficoll Paque). After centrifuging at 1,600 rpm at room temperature (RT) for 30 minutes, the fluid was washed with PBS, and 1 mL TrRIol was added, and then stored at −80°C. Anticoagulation was at 4 °C overnight and then centrifuged at 4,000 rpm for five minutes. The supernatant serum was stored at −80°C. The participants were followed for 19–121 months. ESRD was selected as the outcome indicator.
This study was pre-approved by the ethical committee of Linyi People’s Hospital. All participants signed the consent forms before being recruited into the study.
qRT-PCR
Total RNA was extracted from PBMCs using TRIzol. The cells were treated by TRIzol and extracted by chloroform. After isopropanol sediment and ethanol washing, total RNA was diluted in DEPC water. Then reverse transcription of the RNA to cDNA was done using SuperScript® One-Step RT-PCR System at 45°C for 30 minutes and 94°C for two minutes. The reverse transcription system contained 2 μg total RNA, 5 μL reaction mix (2×), 0.2 μL forward primer, 0.2 μL reverse primer, 0.2 μL RT/Platinum® Taq Mix, and ddH2O. The PCR primers used were as follows:
miR-146a PRT: 5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGC-3;
miR-146aPF: 5′-CAGTGCGTGTCGTGGAGT-3′,
miR-146aPR: 5′-GGGTGAGAACTGAATTCC-3′;
U6PRT: 5′-CGCTTCACGAATTTGCGTGTCAT-3;
U6PF: 5′-GCTTCGGCAGCACATATACTAAAAT-3′,
U6PR: 5′-CGCTTCACGAATTTGCGTGTCAT-3′;
TRAF6PF: 5′-ATGCGGCCATAGGTTCTGC-3′,
TRAF6PR: 5′-TCCTCAAGATGTCTCAGTTCCAT-3′;
β-actinPF: 5′-GAACCCTAAGGCCAAC-3′,
β-actinPR: 5′-TGTCACGCACGATTTCC-3′.
The PCR reaction system was composed of 5 μL of 2× SYBR Green Mixture, 1 μL forward and reverse primer at 2.0 μM, 1 μL cDNA, and 2 μL ddH2O. The reaction was performed at 95°C for 5 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 30 seconds. Each test was repeated three times.
Western blot
Total protein was extracted and separated by SDS-PAGE. After transferred for 1.5 hours, the membrane was blocked by 5% skim milk at RT for 60 minutes. Next, the membrane was incubated in TRAF6 primary antibody (1: 500) at 4°C overnight and then incubated in secondary antibody at RT for 60 minutes (1: 5,000). Then the membrane was developed by ECL chemiluminiscence method and scanned. The band was analyzed by ImageJ.
ELISA
The 96-well plate was incubated by 100 μL coating antibody at 4°C overnight. After washed by PBS three times, the plate was blocked by 200 μL ELISA/ELISPOT Diluent at RT for 60 minutes. Next, 100 μL supernatant or gradient diluted standard substrate were added to each well and incubated at RT for two hours. Then after washed by Wash Buffer, detection antibody was added to the plate at RT for 60 minutes. Then 100 μL Avidin-HRP was added at RT for 30 minutes, and further treated by 100 μL TMB at RT for 15 minutes. At last, the plate was treated with 50 μL Stop Buffer and results read at 450 nm on a microplate reader.
Statistical analysis
All data analysis was performed on SPSS18.0. Measurement data was presented as mean ± standard deviation, while enumeration data was depicted as percentage. Measurement data was compared using the chi-square test. MiR-146a expression level was compared using the Mann-Whitney U rank-sum test. The relationship between miR-146a and TRAF6 was analyzed by Spearman rank correlation test. Survival curve was drawn by Kaplan-Meier method and compared by Log-rank test. Receiver operating characteristic (ROC) curve was established to analyze the diagnosis and differentiate value of miR-146a and TRAF6 on LN activity. A p<0.05 was considered statistically significant.
Results
MiR-146a and TRAF6 expression in the PBMCs of LN patients
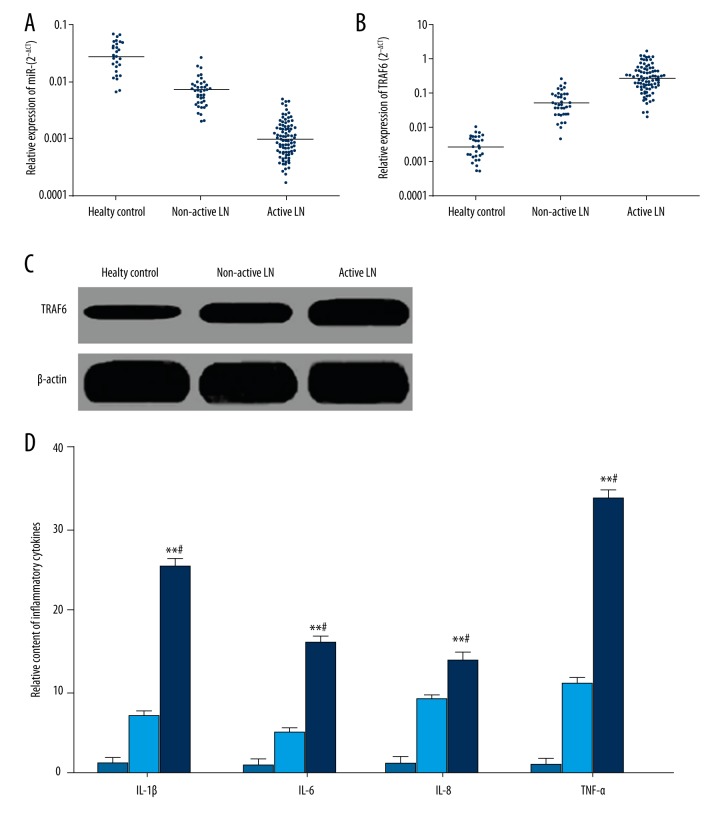
qRT-PCR revealed that compared with healthy controls, miR-146a level significantly declined, while TRAF6 mRNA and protein expression obviously were upregulated in the PBMCs of LN patients (Figure 1A–1C). Moreover, miR-146a level was markedly reduced, whereas TRAF6 mRNA and protein expression was significantly enhanced in the PBMCs from active LN patients compared with non-active LN patients (Figure 1A–1C). Correlation analysis demonstrated that miR-146a level was obviously negatively correlated with TRAF6 mRNA (r=−0.721, p=0.030), suggesting that miR-146a reduction in PBMCs of LN patients lead to TRAF6 upregulation. ELISA results demonstrated that serum IL-1β, IL-6, IL-8, and TNF-α levels in active LN patients were apparently higher than that in non-active patients and healthy controls (Figure 1D).
Figure 1.
MiR-146a and TRAF6 expression in LN patients. (A) qRT-PCR detection of miR-146a expression in PBMCs. (B) qRT-PCR detection of TRAF6 mRNA expression in PBMCs. (C) Western blot detection of TRAF6 protein expression in PBMCs. (D) ELISA detection of inflammatory cytokines in serum.
LN patients were further divided into high-level and low-level groups based on the median of miR-146a and TRAF6 mRNA expression. The ratio of miR-146a low expression and TRAF6 mRNA overexpression in active LN patients was obviously higher than that in non-active LN patients (p=0.004 and 0.006, respectively) (Table 1). MiR-146a and TRAF6 expression showed no significant relationship with age and gender (p>0.05) (Table 1). Correlation analysis showed that miR-146a was negatively correlated, whereas TRAF6 was positively correlated with serum IL-1β, IL-6, IL-8, and TNF-α content (Table 2).
Table 1.
The relationship between clinical characteristics and miR-146a/TRAF6 expression in PBMCs in LN patients.
| Clinical characteristics | Cases | miR-146a | χ2 | P | TRAF6 | χ2 | P | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Overex-pression | Low expression | Overex-pression | Low expression | |||||||
| Age (year) | ≤30 | 56 | 36 | 20 | 0.278 | 0.598 | 30 | 26 | 0.755 | 0.385 |
| >30 | 72 | 43 | 29 | 33 | 39 | |||||
| Gender | Male | 16 | 9 | 7 | 1.854 | 0.173 | 6 | 10 | 0.079 | 0.778 |
| Female | 112 | 85 | 27 | 38 | 74 | |||||
| Activity | Yes | 40 | 12 | 28 | 7.271 | 0.007 | 30 | 10 | 11.972 | <0.001 |
| No | 88 | 49 | 39 | 37 | 51 | |||||
Table 2.
Correlation analysis of miR-146a/TRAF6 with IL-1β, IL-6, IL-8, and TNF-α.
| miR-146a | TRAF6 | |||
|---|---|---|---|---|
| r | P | r | P | |
| IL-1β | −0.654 | 0.022 | 0.711 | 0.016 |
| IL-6 | −0.591 | 0.041 | 0.680 | 0.028 |
| IL-8 | −0.701 | 0.019 | 0.645 | 0.034 |
| TNF-α | −0.623 | 0.027 | 0.733 | 0.011 |
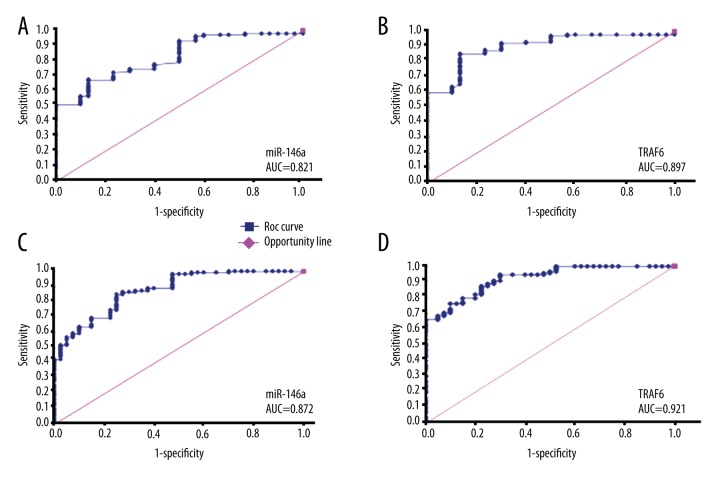
The diagnostic value of miR-146a and TRAF6 on LN and its activity
ROC curve was constructed to calculate the diagnostic value of miR-146a and TRAF6 on LN and its activity. The results showed that the area under the curve (AUC) of miR-146a and TRAF6 on diagnosis of LN was 0.821 and 0.897, respectively (Figure 2A, 2B). The AUC of TRAF6 on LN activity differentiation was 0.921, which was slightly higher than that of miR-146a at 0.872 (Figure 2C, 2D).
Figure 2.
ROC curve analysis of miR-145a and TRAF6 on LN diagnosis. (A) miR-146a identification of LN patients from healthy controls. (B) TRAF6 identification of LN patients from healthy controls. (C) miR-146a differentiation of LN activity. (D) TRAF6 differentiation of LN activity.
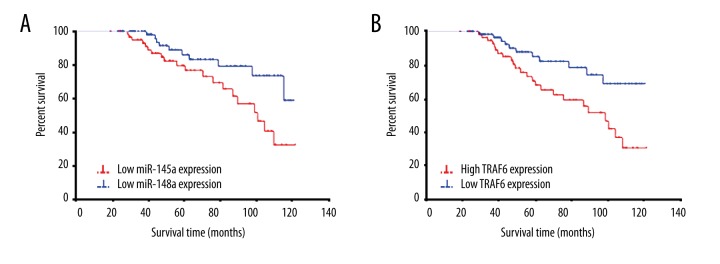
The impact of miR-146a and TRAF6 expression on ESRD progression
ESRD progression was considered as the outcome indicator. The survival curve of miR-146a downregulation was obviously steeper than that of miR-146a overexpression, indicating higher incidence of ESRD progression (χ2=5.050, p=0.025) (Figure 3A). The survival curve of TRAF6 upregulation was significantly steeper than that of TRAF6 low expression, suggesting higher incidence of ESRD progression (χ2=6.511, p=0.011) (Figure 3B).
Figure 3.
The relationship between miR-146a/TRAF6 expression and ESRD progression. (A) Survival curve analysis of miR-146a impact on ESRD progression. (B) Survival curve analysis of TRAF6 impact on ESRD progression.
The impact of miR-146a and TRAF6 expression on LN activity
The LN patients in the active phase were selected and divided into a high expression group and a low expression group according to miR-146a and TRAF6 mRNA expression levels. As shown in Table 3, the recurrence rate within one year in the miR-146a downregulation group was apparently higher than that of the high level group (χ2=5.066, p=0.024), while the ratio in the TRAF6 upregulation group was obviously lower than that of the low level group (χ2=4.526, p=0.033).
Table 3.
The impact of miR-146a and TRAF6 expression on LN activity.
| Indicator | Low expression group | High expression group | χ2 | P value | ||
|---|---|---|---|---|---|---|
| Recurrence within one year | Yes | No | Yes | No | ||
| miR-146a | 15 | 29 | 6 | 38 | 5.066 | 0.024 |
| TRAF6 | 8 | 36 | 17 | 27 | 4.526 | 0.033 |
Discussion
SLE is a type of autoimmune disease with complicated etiology mechanism. It is featured as multiple autoantibody formation and immune complex deposition in the viscera, thus mediating chronic inflammation and inflammatory injury, leading to systemic multiple organs damage [1,2]. The kidney is the main affected organ, and also one of the most serious complications of SLE. About 20% of such patients can further develop ESRD within 10 years [20]. The clinical manifestations of the kidney injury include urinary proteinuria, erythrocyturia, leukocyturia, cylindruria, glomerular filtration function decline, and renal tubular dysfunction. LN-induced glomerular damage is the most common type of secondary chronic glomerular disease, which may affect the prognosis of SLE directly. The whole course of SLE often alternates between active and resting stage. Progressive renal function decline and renal failure in the active stage is the main cause of death in SLE [21]. It has been shown that the possibility of LN concurrence in SLE patients was 30–50%, and the extent of the renal lesions caused a direct effect on the prognosis of SLE [6,7]. Thus, an in-depth understanding of the pathogenesis of LN and a search for related biomarkers has important practical significance to the understanding of the severity of LN lesions, judging LN activity, evaluating prognosis, and guiding treatment. In recent years, more and more studies have focused on the pathogenesis of LN and related biomarkers, aiming to improve the prediction of LN activity and the sensitivity and specificity of disease progression [22].
Inflammatory factor is one of the important conditions of LN. Immune complex deposition may induce a large number of inflammatory cytokines in the initial disease stage, resulting in lupus glomerulonephritis. IL-6 is a kind of inflammatory factor mainly secreted by T cells and macrophages, which plays an important role in activating T cells and B cells [22]. Cash et al. [23] demonstrated that IL-6 knockout significantly alleviate the symptoms of proteinuria, hematuria, and lymphocyte infiltration in LN mice. Yoshida et al. [24] reported that IL-6 receptor antagonist obviously reduced proteinuria in LN mice. Tsai et al. [25] found that IL-6 and IL-8 content in the urine of active LN patients were markedly higher than that in non-active LN patients and healthy controls. Yung et al. [26] confirmed that anti-DNA antibody may induce glomerular mesangial and renal tubular epithelial cells secreting IL-1β, IL-6, and TNF-α, thus amplifying inflammatory response. All of the aforementioned results suggest a role for inflammation factors IL-1β, IL-6, IL-8, and TNFα in LN. MiR-146a is a member of miR-146 family that is located on the long arm of chromosome 5. MiR-146a plays an important role in the targeted inhibition of TRAF6 that mediates TRL4 and NF-κB [17]. It can negatively regulate immune inflammation to protect immune response from excessive activation by inhibiting TRAF6 gene expression, suppressing TLR4 mediated NF-κB signaling pathway activation, and restraining IL-1β, IL-6, IL-8, and TNF-α expression and secretion [17]. Tang et al. [18] revealed that the miR-146a levels in the PBMCs of SLE patients were obviously downregulated, whereas low expression of miR-146a mediated high reactivity of PBMCs from SLE patients to INF-I, thus promoting SLE oncomes. Luo et al. [19] showed that the gene polymorphism of the binding site rs57095329 of the miR-146a promoter region transcription factor Ets-1 mediated low expression of miR-146a, while the locus gene polymorphisms increased the risk of SLE. Given the described role of miR-146a in immune regulation, this study aimed to investigate miR-146a and its target gene TRAF6 expression in LN and related mechanism.
Our study results showed that compared with healthy controls, miR-146a levels significantly declined, while TRAF6 mRNA and protein expression obviously was upregulated in the PBMCs of LN patients. Moreover, it was associated with activity, indicating that miR-146a downregulation lead to TRAF6 elevation and may be involved in the pathogenesis of LN. Furthermore, serum IL-1β, IL-6, IL-8, and TNF-α content in active LN patients were obviously higher than in non-active LN patients and healthy controls, which was similar to results of Tsai et al. [25]. In addition, miR-146a levels in PBMCs of LN patients were significantly negatively correlated with inflammatory cytokines content, while TRAF6 presented a positive correlation. This suggests that miR-146a and TRAF6 may participate in promoting the release of IL-1β, IL-6, IL-8, and TNF-α, and thus take part in LN. In view of the significant difference in miR-146a and TRAF6 expression between LN patients and healthy controls, we explored the diagnostic role of miR-146a and TRAF on LN and its activity. It was shown that miR-146a and TRAF6 present relatively high diagnostic values for LN and differentiate values for LN activity. TRAF6 demonstrated higher accuracy for LN diagnosis and LN activity differentiation, which may be due to TRAF6 acting as a direct factor affecting the activity of the NF-κB pathway and the release of inflammatory mediators, whereas miR-146a only played an indirect role. Detection of miR-146a and TRAF6 expression is of great significance for LN diagnosis and treatment. Since LN is only one of the complications of SLE, it makes it ideal to apply survival and death to evaluate SLE survival and prognosis. Therefore, this study chose ESRD as the primary outcome to evaluate the impact of miR-146a and TRAF6 on LN. It was shown that miR-146a downregulation and TRAF6 upregulation increased the incidence of ESRD progression. In addition, miR-146a downregulation and TRAF6 upregulation elevated the possibility of recurrence within one year.
Conclusions
Compared with healthy controls, miR-146a declined, while TRAF6 increased in the PBMCs of LN patients. Their expression can be used to effectively differentiate LN and evaluate activity. MiR-146a reduction and TRAF6 upregulation increased the possibility of ESRD progress and recurrence within one year.
Footnotes
Disclosure of conflict of interest
None.
Source of support: Departmental sources
References
- 1.Vasugi Z, Danda D. Systemic lupus erythematosis with antiphospholipid antibody syndrome: A mimic of Buerger’s disease. J Postgrad Med. 2006;52:132–33. [PubMed] [Google Scholar]
- 2.Calamia KT, Balabanova M. Vasculitis in systemic lupus erythematosis. Clin Dermatol. 2004;22:148–56. doi: 10.1016/j.clindermatol.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Sakakibara R, Uchiyama T, Yoshiyama M, et al. Urinary dysfunction in patients with systemic lupus erythematosis. Neurourol Urodyn. 2003;22:593–96. doi: 10.1002/nau.10126. [DOI] [PubMed] [Google Scholar]
- 4.Wolf BJ, Spainhour JC, Arthur JM, et al. Development of biomarker models to predict outcomes in lupus nephritis. Arthritis Rheumatol. 2016;68(8):1955–63. doi: 10.1002/art.39623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel M, Oni L, Midgley A, et al. Increased concentration of plasma TNFR1 and TNFR2 in paediatric lupus nephritis. Lupus. 2016;25(9):1040–44. doi: 10.1177/0961203316631634. [DOI] [PubMed] [Google Scholar]
- 6.Moroni G, Raffiotta F, Ponticelli C. Remission and withdrawal of therapy in lupus nephritis. J Nephrol. 2016;29(4):559–65. doi: 10.1007/s40620-016-0313-6. [DOI] [PubMed] [Google Scholar]
- 7.Besouw MT, Vande Walle JG, Ilias MI, et al. Pediatric lupus nephritis presenting with terminal renal failure. Acta Clin Belg. 2016 doi: 10.1080/17843286.2016.1159383. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.de Zubiria Salgado A, Herrera-Diaz C. Lupus nephritis: An overview of recent findings. Autoimmune Dis. 2012;2012:849684. doi: 10.1155/2012/849684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koga T, Ichinose K, Tsokos GC. T cells and IL-17 in lupus nephritis. Clin Immunol. 2016 doi: 10.1016/j.clim.2016.04.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szymczak M, Kuzniar J, Kopec W, et al. Increased granulocyte heparanase activity in neutrophils from patients with lupus nephritis and idiopathic membranous nephropathy. Arch Immunol Ther Exp (Warsz) 2016 doi: 10.1007/s00005-016-0396-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosalka J, Jakiela B, Musial J. Changes of memory B- and T-cell subsets in lupus nephritis patients. Folia Histochem Cytobiol. 2016;54:32–41. doi: 10.5603/FHC.a2016.0005. [DOI] [PubMed] [Google Scholar]
- 12.Heinemann K, Wilde B, Hoerning A, et al. Decreased IL-10(+) regulatory B cells (Bregs) in lupus nephritis patients. Scand J Rheumatol. 2016;45:312–16. doi: 10.3109/03009742.2015.1126346. [DOI] [PubMed] [Google Scholar]
- 13.Deng P, Chen L, Liu Z, et al. MicroRNA-150 inhibits the activation of cardiac fibroblasts by regulating c-Myb. Cell Physiol Biochem. 2016;38:2103–22. doi: 10.1159/000445568. [DOI] [PubMed] [Google Scholar]
- 14.Hamam R, Ali AM, Alsaleh KA, et al. microRNA expression profiling on individual breast cancer patients identifies novel panel of circulating microRNA for early detection. Sci Rep. 2016;6:25997. doi: 10.1038/srep25997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Q, Wang H, Singh A, Shou F. Expression and function of microRNA-497 in human osteosarcoma. Mol Med Rep. 2016;14(1):439–45. doi: 10.3892/mmr.2016.5256. [DOI] [PubMed] [Google Scholar]
- 16.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 17.Boldin MP, Taganov KD, Rao DS, et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med. 2011;208:1189–201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang Y, Luo X, Cui H, et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009;60:1065–75. doi: 10.1002/art.24436. [DOI] [PubMed] [Google Scholar]
- 19.Luo X, Yang W, Ye DQ, et al. A functional variant in microRNA-146a promoter modulates its expression and confers disease risk for systemic lupus erythematosus. PLoS Genet. 2011;7:e1002128. doi: 10.1371/journal.pgen.1002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sexton DJ, Reule S, Solid C, et al. ESRD from lupus nephritis in the United States, 1995–2010. Clin J Am Soc Nephrol. 2015;10:251–59. doi: 10.2215/CJN.02350314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivera F, Fulladosa X, Poveda R, et al. Spanish Group for the Study of Glomerular Disease (GLOSEN) Mycophenolate as induction therapy in lupus nephritis with renal function impairment. Am J Nephrol. 2012;35:424–33. doi: 10.1159/000337916. [DOI] [PubMed] [Google Scholar]
- 22.Misra R, Gupta R. Biomarkers in lupus nephritis. Int J Rheum Dis. 2015;18:219–32. doi: 10.1111/1756-185X.12602. [DOI] [PubMed] [Google Scholar]
- 23.Cash H, Relle M, Menke J, et al. Interleukin 6 (IL-6) deficiency delays lupus nephritis in MRL-Faslpr mice: The IL-6 pathway as a new therapeutic target in treatment of autoimmune kidney disease in systemic lupus erythematosus. J Rheumatol. 2010;37:60–70. doi: 10.3899/jrheum.090194. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida H, Hashizume M, Suzuki M, Mihara M. Induction of high-dose tolerance to the rat anti-mouse IL-6 receptor antibody in NZB/NZW F1 mice. Rheumatol Int. 2011;31:1445–49. doi: 10.1007/s00296-010-1500-8. [DOI] [PubMed] [Google Scholar]
- 25.Tsai CY, Wu TH, Yu CL, et al. Increased excretions of beta2-microglobulin, IL-6, and IL-8 and decreased excretion of Tamm-Horsfall glycoprotein in urine of patients with active lupus nephritis. Nephron. 2000;85:207–14. doi: 10.1159/000045663. [DOI] [PubMed] [Google Scholar]
- 26.Yung S, Tsang RC, Leung JK, Chan TM. Increased mesangial cell hyaluronan expression in lupus nephritis is mediated by anti-DNA antibody-induced IL-1beta. Kidney Int. 2006;69:272–80. doi: 10.1038/sj.ki.5000042. [DOI] [PubMed] [Google Scholar]