Abstract
Purpose
Significant concerns exist regarding the potential for unwarranted behavior changes and the overuse of health care resources in response to direct-to-consumer personal genomic testing (PGT). However, little is known about customers’ behaviors after PGT.
Methods
Longitudinal surveys were given to new customers of 23andMe (Mountain View, CA) and Pathway Genomics (San Diego, CA). Survey data were linked to individual-level PGT results through a secure data transfer process.
Results
Of the 1,042 customers who completed baseline and 6-month surveys (response rate, 71.2%), 762 had complete cancer-related data and were analyzed. Most customers reported that learning about their genetic risk of cancers was a motivation for testing (colorectal, 88%; prostate, 95%; breast, 94%). No customers tested positive for pathogenic mutations in highly penetrant cancer susceptibility genes. A minority of individuals received elevated single nucleotide polymorphism-based PGT cancer risk estimates (colorectal, 24%; prostate, 24%; breast, 12%). At 6 months, customers who received elevated PGT cancer risk estimates were not significantly more likely to change their diet, exercise, or advanced planning behaviors or engage in cancer screening, compared with individuals at average or reduced risk. Men who received elevated PGT prostate cancer risk estimates changed their vitamin and supplement use more than those at average or reduced risk (22% v 7.6%, respectively; adjusted odds ratio, 3.41; 95% CI, 1.44 to 8.18). Predictors of 6-month behavior include baseline behavior (exercise, vitamin or supplement use, and screening), worse health status (diet and vitamin or supplement use), and older age (advanced planning, screening).
Conclusion
Most adults receiving elevated direct-to-consumer PGT single nucleotide polymorphism-based cancer risk estimates did not significantly change their diet, exercise, advanced care planning, or cancer screening behaviors.
INTRODUCTION
Although the vast majority of cancer genomic testing occurs within the health care system, direct-to-consumer (DTC) personal genomic testing (PGT) is an innovation that seeks to democratize access to genomic technologies and enhance efforts in cancer control. However, there are growing concerns about the potential for unwarranted health behavior change and the overuse of health care resources in the wake of PGT.1 These concerns often stem from the fact that the modest increases in cancer risk associated with single nucleotide polymorphism (SNP) based testing are not currently considered medically actionable.2,3 Critics of DTC-PGT have expressed concerns that customers may inappropriately alter their health behavior on the basis of highly uncertain genetic information that has little or no known clinical utility, that they may not receive proper guidance on health decisions, and that they may strain an already overburdened health care system if they pursue costly follow-up care based on PGT results.4-8 Early studies of PGT suggested that customers may rely on their physicians to help interpret results and recommend follow-up testing based on PGT data,9-11 and recent work suggests that some customers change health behaviors after testing.12-14 In contrast, others have found little evidence to suggest that customers significantly alter their health behaviors after PGT.15-18
The future of PGT remains an area of intense debate. For the time being, the US Food and Drug Administration has limited consumer access to the health component of some PGTs out of concern that such testing could have significant health consequences. In its warning letter to 23andMe (Mountain View, CA), the Food and Drug Administration expressed specific concerns about BRCA1/2-related cancer risk assessment given that such testing is considered a high-risk indication.19 In addition, the American Medical Association called for a ban on DTC advertising of prescription drugs and medical devices, citing concerns that DTC advertising may inflate demand.20 Although policymakers are actively debating the regulation of the genomic testing industry broadly,21-24 and of PGT specifically, regulatory decisions are significantly hampered by a lack of data that evaluate the effect of PGT on customer health behaviors and health care resource use.25-27
The Impact of Personal Genomics (PGen) Study is a prospective, longitudinal cohort study that was designed to examine the psychosocial, behavioral, and health outcomes related to DTC-PGT. The objective of the present analysis was to determine whether customers who received elevated SNP-based PGT cancer risk estimates were more likely to change their health-related (ie, diet, exercise, vitamin and supplement use, and advanced care planning) and cancer screening behaviors than customers who received average or reduced PGT cancer risk estimates. Because we were most interested in the use of relatively high-cost screening modalities, we evaluated mammography and colonoscopy for breast and colon cancer screening, respectively. Because imaging modalities and invasive procedures are not widely used for prostate cancer screening, we evaluated use of prostate-specific antigen (PSA) testing for prostate cancer. We hypothesized that customers who receive elevated PGT cancer risk estimates would not significantly alter their health-related behaviors but that they would be more likely to engage in cancer screening than consumers who receive average or reduced PGT cancer risk estimates. Secondary objectives were to describe individuals’ cancer-related motivations for PGT and to describe individual-level PGT cancer risk estimates.
METHODS
Study Design and Procedures
New customers of 23andMe28 and Pathway Genomics (San Diego, CA)29 were invited to enroll onto the PGen Study between March and July 2012. Participants were invited to complete three Web-based surveys at the following time points: at baseline (BL) before receiving results and 2 weeks and 6 months (6M) after viewing results. PGT results were returned to customers per standard company practice and then deidentified, linked to survey data, and provided to researchers. The PGen Study was approved by the Partners Human Research Committee and the University of Michigan Institutional Review Board. The study design30,31 and other findings have been reported previously.32-37
Study Measures
Participants from both companies received a single genetic risk estimate based on genotyping of multiple SNPs for breast (women only), prostate (men only), and colorectal cancer. Consistent with prior analyses of PGen data, a threshold relative risk (RR) level was selected to distinguish between elevated and nonelevated genetic risk, with results dichotomized into the following two categories: average or reduced genetic risk (23andMe RR < 1.2; two lowest Pathway categories) and elevated genetic risk (23andMe RR ≥ 1.2; three highest Pathway categories).35 The survey also queried participants about their interest in learning their cancer genetic risk and about BL cancer risk perceptions.38 At 6M, participants were asked about changes in their diet and exercise behaviors and use of vitamins and herbal supplements that were specifically motivated by their PGT results and about changes, or plans to make any changes, to advanced care planning (eg, creating a will, advance directives) as a result of learning their genetic information.39 Mammography, colonoscopy, and PSA testing were measured at BL and 6M.40 Additional details on the study measures are included in the Appendix (online only).
Statistical Analyses
Data from the BL and 6M surveys were analyzed. Participants were excluded if they reported any prior cancer diagnosis, reported prior genetic testing, and/or had missing data on demographic characteristics or PGT cancer risk estimates. We estimated whether participants’ BL health behaviors met published standards by comparing participants’ self-reported dietary and exercise behaviors with recommendations from the Centers for Disease Control and Prevention (CDC) and examined screening behaviors for participants younger and older than age 50 years (Appendix). We reported BL vitamin and/or supplement use as a dichotomous response (any use v no use).
Separate analyses were conducted for each behavioral outcome. Participants who affirmatively answered that they had made changes in their health behavior (eg, diet, exercise) that were specifically motivated by their PGT results were compared with those who did not make changes. We examined univariable associations between PGT risk estimates for each cancer and behaviors at 6M using the χ2 and Fisher’s exact tests. We examined unadjusted associations between participants’ genetic risk scores and their 6M behaviors according to whether the participant was above or below the recommended behavior level (or used vitamins or supplements) at BL. We then fit multivariable logistic regression models to examine the same associations, adjusting for all covariates of interest (ie, age, race, ethnicity, sex, education, employment, income, family history of cancer, insurance status, and health status) plus the BL behavior specific to the behavioral outcome (eg, BL fruit and vegetable consumption for 6M changes in diet, BL mammography for 6M mammography use) regardless of statistical significance. Additionally, because health-related behaviors may be influenced by the risk of developing diseases other than cancer, for these outcomes, we adjusted for participants’ PGT risks for type 2 diabetes, obesity, and heart disease. Finally, because interest in cancer risk information and cancer risk perception can be predictors of screening, we adjusted for these items in the models for the screening outcomes. In exploratory analyses, we evaluated the associations between genetic risk scores and screening at 6M for participants younger than age 50 years and in those age 50 years or older.
Given our sample size, the observed proportion of participants with elevated risk, and a one-sided type I error rate of 0.05, our study had power of 80% to detect absolute differences in excess of 20%, 12%, and 20% for changes motivated by being at elevated risk for breast, colorectal, or prostate cancer, respectively. No variable had ≥ 10% missing data. All statistical analyses were conducted using Stata version 13.1 (StataCorp, College Station, TX).
RESULTS
Participant Characteristics, PGT Results, and BL Behaviors
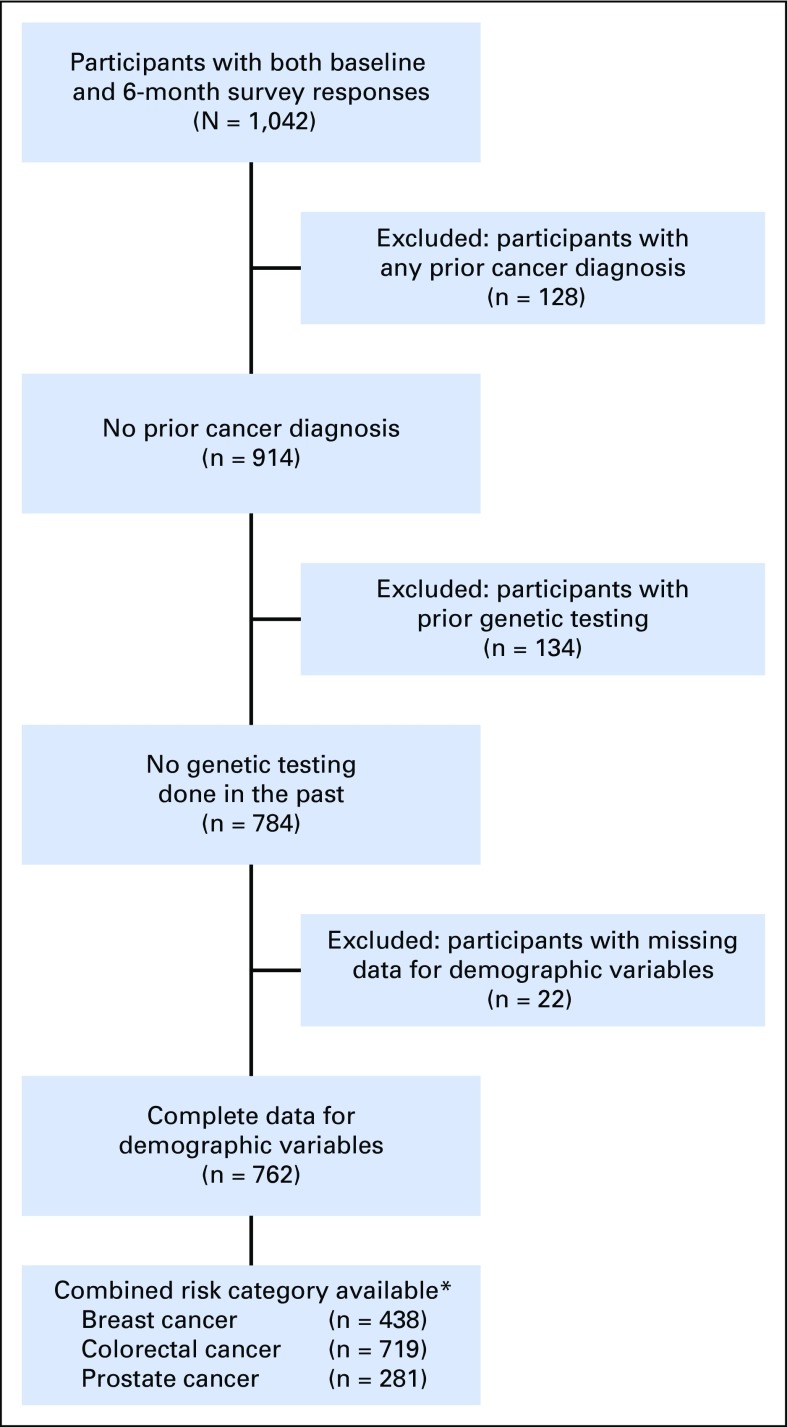
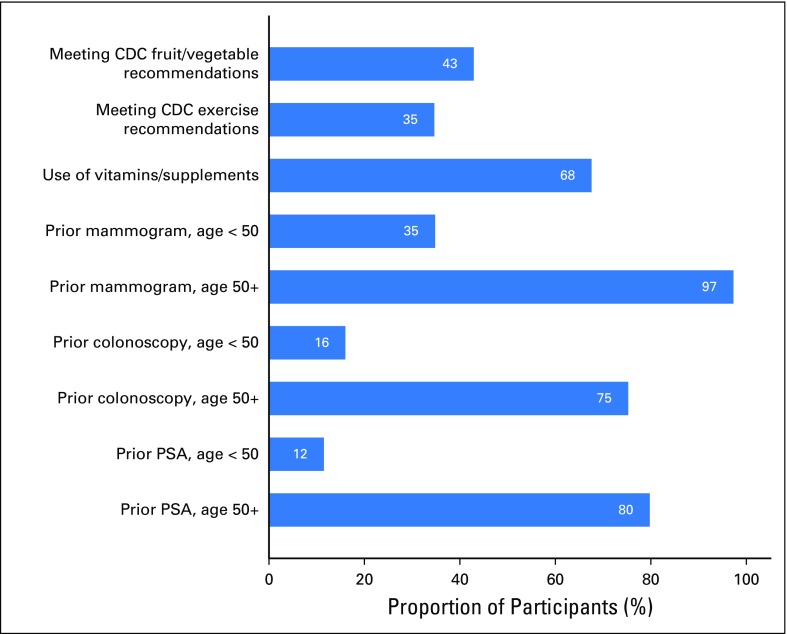
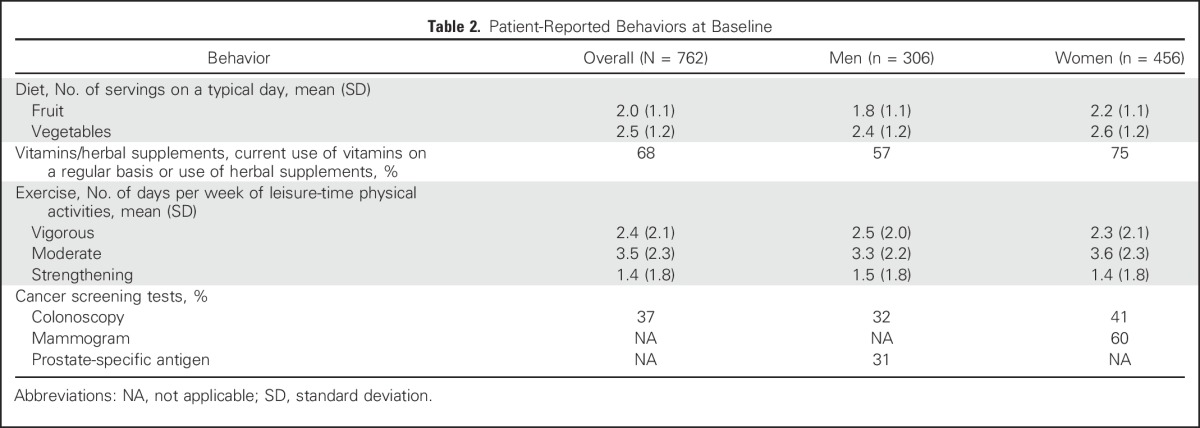
An enrollment summary is shown in Figure 1. Demographic and health characteristics and PGT risk estimates for the 762 participants included in at least one cancer screening behavior analysis are listed in Table 1. Self-reported BL behaviors are shown in Figure 2 and Table 2. At BL, 68% of participants used vitamins or supplements, 43% met CDC dietary recommendations, and 35% met CDC exercise recommendations. At BL, participants age 50 years and older reported high rates of past screening (mammogram, 97%; colonoscopy, 75%; PSA, 80%).
Fig 1.
Study enrollment. *Individuals may have received risk information for some but not all cancers. Missing data on breast and prostate risk are out of a total of 456 women and 306 men in the sample, respectively.
Table 1.
Sociodemographic and Risk Characteristics of Participants
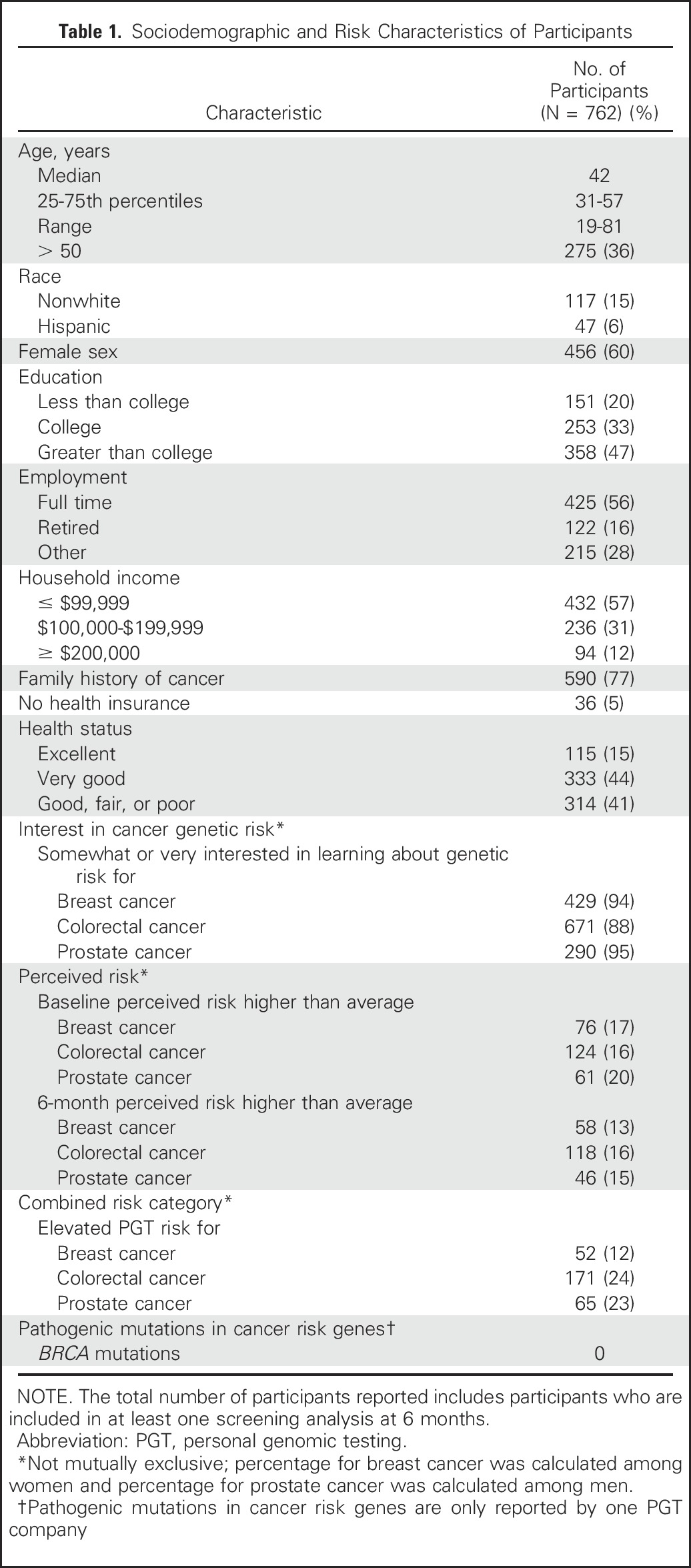
Fig 2.
Baseline behaviors according to compliance with Centers for Disease Control and Prevention (CDC) recommendations, proportion using vitamins or supplements, and cancer screening by age. PSA, prostate-specific antigen.
Table 2.
Patient-Reported Behaviors at Baseline
Health-Related Behaviors and Cancer Screening at 6M
At 6M, a minority of participants made changes in their diet (31%), exercise behavior (26%), advanced care planning behavior (6%), or use of vitamins/herbal supplements (21%) in response to PGT. Overall, screening since receiving PGT test results, as reported on the 6M survey, was 26% for mammography, 7% for colonoscopy, and 19% for PSA testing. Across all three cancers, participants who reported screening in the year before ordering PGT were the most likely to report screening at 6M. This trend was maintained after stratification by age (< v ≥ 50 years), with the exception of prostate cancer, where frequency counts were small. A small percentage of participants who reported no prior history of screening at BL reported screening at the 6M follow-up (mammography, 0.6%; colonoscopy, 2.0%; PSA, 2.5%) with slightly higher reported rates of colonoscopy (6.5%) and PSA testing (7.1%) in participants age 50 and older.
Associations Between Genetic Risk and Behavior at 6M
Results of univariable analyses between PGT risk scores and outcomes are listed in Tables 3-7. Six-month vitamin or supplement use significantly changed among men who were vitamin or supplement users at BL, and the use of PSA testing went up among men who had not reported PSA testing at BL.
Table 3.
Unadjusted Associations Between Genetic Risk Scores and Participants’ Diet at 6 Months, Overall and Separately by Behavior at Baseline
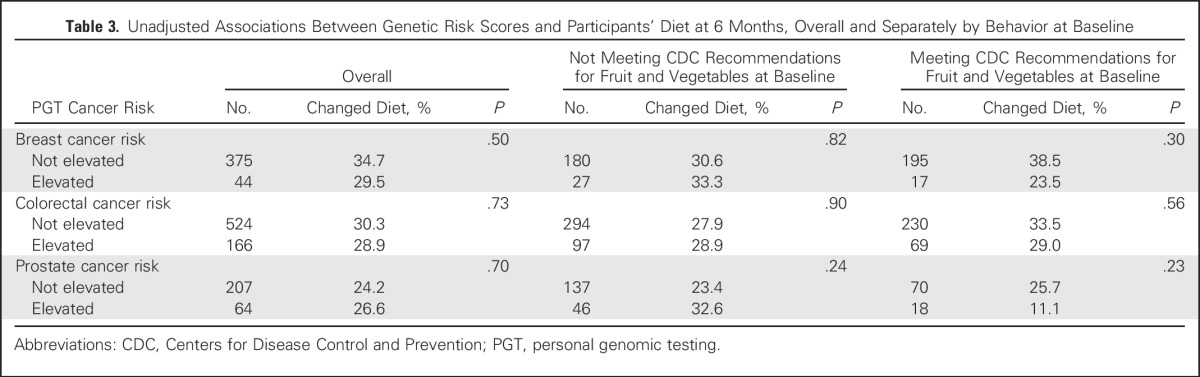
Table 7.
Unadjusted Associations Between Genetic Risk Scores and Participants’ Cancer-Specific Screening at 6 Months, Overall and Separately by Prior Screening
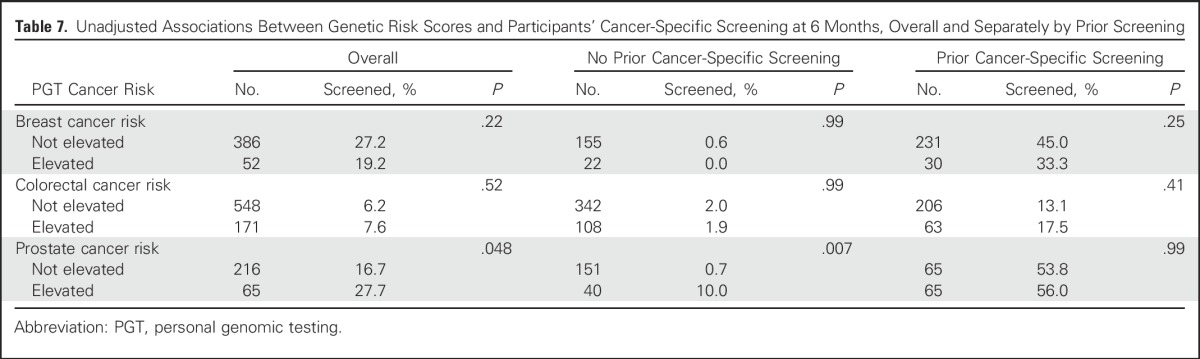
Table 4.
Unadjusted Associations Between Genetic Risk Scores and Participants’ Exercise Behavior at 6 Months, Overall and Separately by Behavior at Baseline
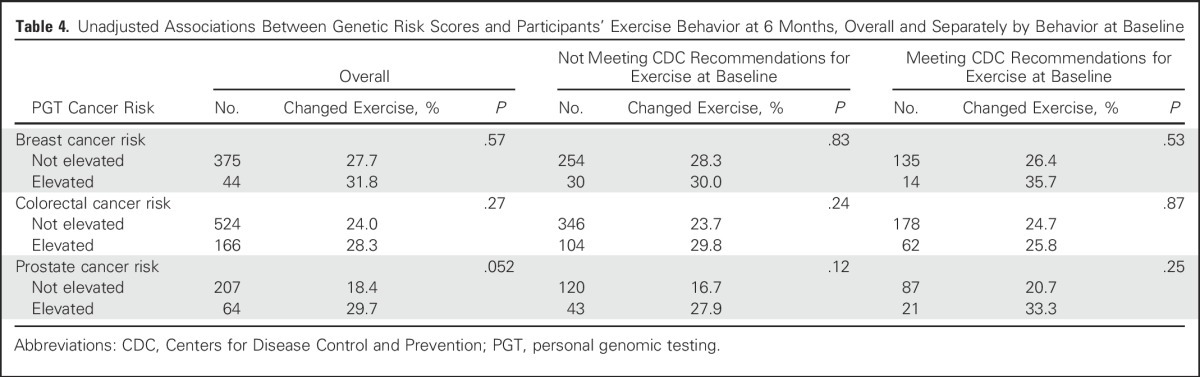
Table 5.
Unadjusted Associations Between Genetic Risk Scores and Participants’ Use of Vitamins or Herbal Supplements at 6 Months, Overall and Separately by Use at Baseline
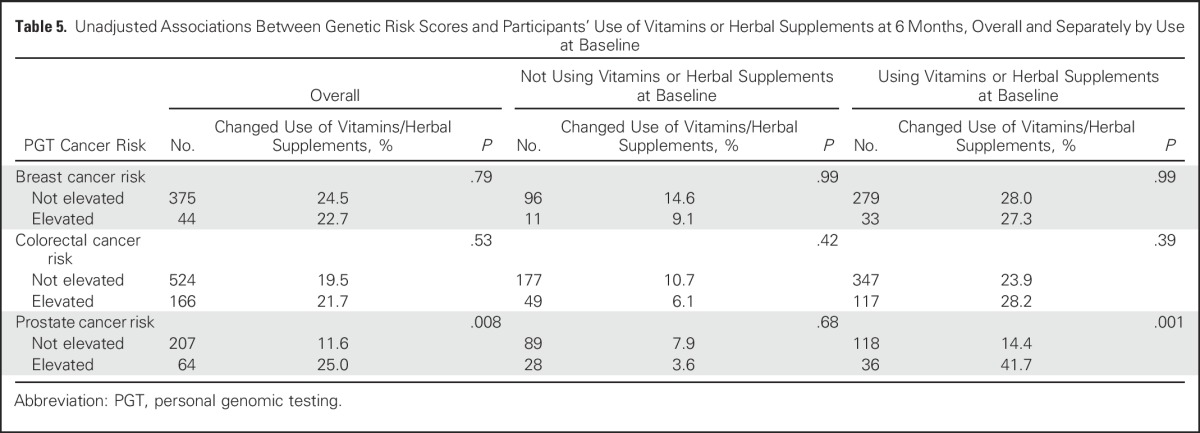
Table 6.
Unadjusted Associations Between Genetic Risk Scores and Participants’ Advance Planning at 6 Months
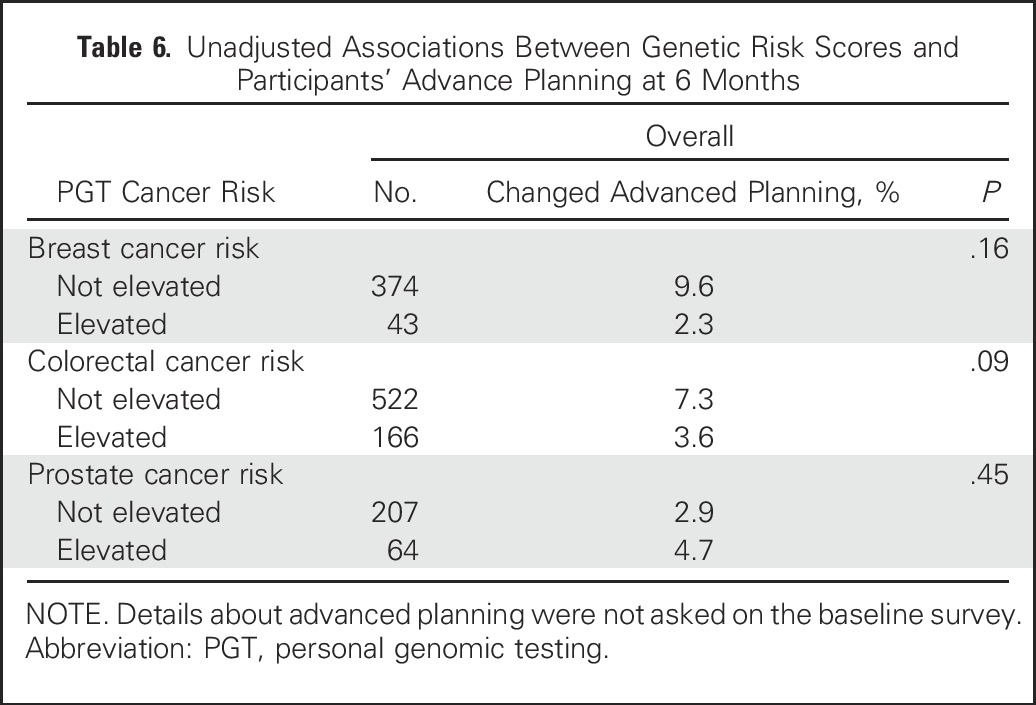
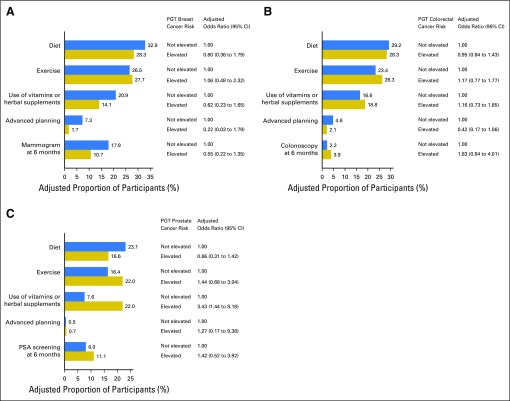
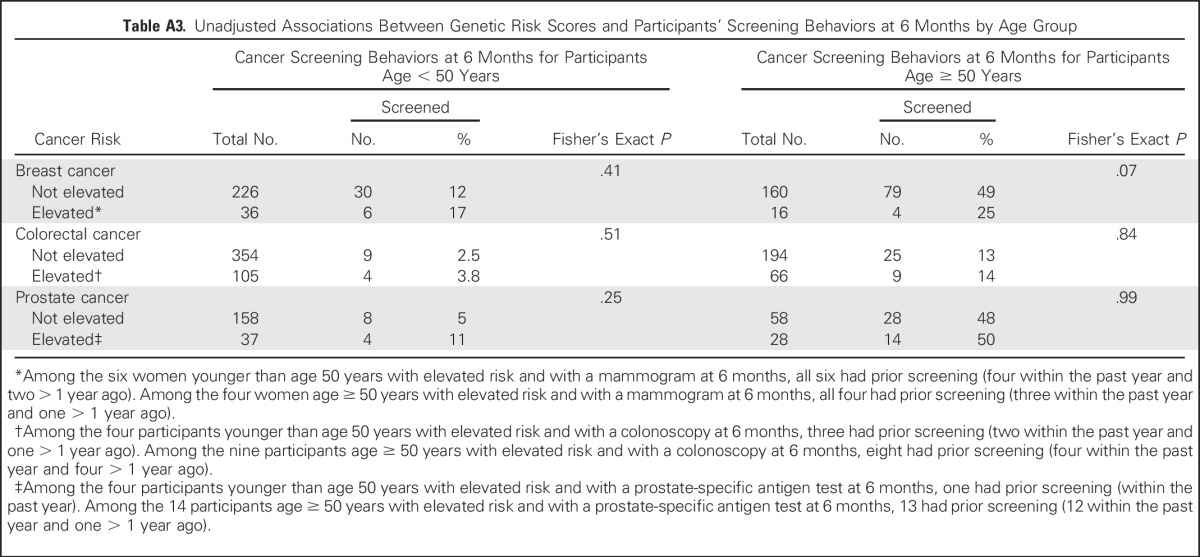
Figure 3 and Appendix Tables A1 and A2 (online only) present the multivariable logistic regression model results for 6M behaviors. Individuals with elevated cancer genetic risk scores were not significantly more likely to change their diet, exercise, use of vitamins or herbal supplements, or cancer screening behavior or engage in more advanced care planning than individuals who received average or reduced risk estimates, with one exception; men who had elevated PGT prostate cancer risk estimates were more likely to change their vitamin or herbal supplement use (22% of participants at elevated risk v 8% not at elevated risk; adjusted odds ratio, 3.43; 95% CI, 1.44 to 8.18). Other significant predictors of behavior change at 6M include BL behavior (eg, vigorous exercise; vitamin/supplement use; mammography, colonoscopy, and PSA testing), worse health status (for diet and vitamin or supplement use), and older age (for advanced planning and for mammography, PSA and colonoscopy). Finally, higher incomes were inversely associated with 6M changes in exercise, and women were more likely to report 6M changes in advanced care planning. Participants’ perception of elevated cancer risk at BL was a significant predictor only of colonoscopy use. Finally, we found no significant associations between elevated risk scores and 6M screening in participants younger than age 50 or age 50 and older (Appendix Table A3, online only).
Fig 3.
Behavioral and cancer screening changes specifically motivated by personal genomic testing (PGT) risk for (A) breast cancer, (B) colorectal cancer, and (C) prostate cancer. Adjusted proportions and odds ratios from multivariable logistic regression are shown. All models are adjusted for age, race, ethnicity, sex, education, employment, household income, family history of cancer, health insurance, health status, and baseline behavior. Baseline behavior was defined by participant reports of fruit and vegetable consumption (diet); number of days of leisure-time physical activity (exercise); use of vitamins or herbal supplements; and mammography, colonoscopy, or prostate-specific antigen (PSA) testing within the year before testing. Of note, there was no baseline item that specifically pertained to advanced planning. All models except for the mammography, colonoscopy, or PSA outcome are adjusted further for PGT risk for type 2 diabetes, obesity, and coronary heart disease. The model for the mammography outcome is adjusted further for interest in genetic risk for breast cancer and baseline perceived risk for breast cancer. The model for the colonoscopy outcome is adjusted further for interest in genetic risk for colorectal cancer and baseline perceived risk for colorectal cancer. The model for the PSA outcome is adjusted further for interest in genetic risk for prostate cancer and baseline perceived risk for prostate cancer.
DISCUSSION
This study uses a longitudinal design to examine the impact of return of DTC-PGT cancer risk test results from two prominent PGT companies on study participants’ cancer-related behaviors. Consistent with our hypothesis, most PGT customers did not alter their health-related behaviors in the wake of PGT cancer results, with one exception; men who received elevated PGT prostate cancer risk estimates were significantly more likely to change their vitamin or supplement use than men who received average or reduced risk estimates. Counter to our hypothesis, however, we found that individuals who received elevated PGT cancer risk estimates did not have higher cancer screening rates at 6 months than individuals who received average or reduced PGT cancer risk estimates. It should be noted that our ability to detect changes in cancer screening in our sample of PGT customers was limited, particularly for those older than age 50 years, because customers tended to be high users of cancer screening at BL. In contrast, our ability to detect changes in dietary and exercise behavior was greater given that only 35% to 45% of participants reported BL behaviors that were consistent with CDC recommendations.
Although it is not possible to generalize the results of this study to all Americans, it is important to study early adopters of PGT as a first step in understanding how direct access to genetics may or may not affect health-related behaviors and health care use. Our data advance the field by addressing the questions of whether customers will change their health-related behaviors or use cancer screening after receiving PGT results. The provision of DTC-PGT SNP risk estimates to consumers remains controversial because the clinical implications of low effect size risk variants are uncertain and the use of SNP data to independently predict cancer risk is limited.2,3,41,42 Other studies have found that participants report visiting providers and altering their health behaviors in the wake of testing12-14; however, recent review articles suggest that the effect of PGT on personal health behaviors is minimal.4,27,43 Our data confirm and extend the cancer-related findings from the Scripps study,15,16 in that neither that study nor ours found significant associations between the return of individuals’ condition-specific genetic risk estimates and health-related behaviors or cancer-related screening. Our data also contribute to the evolving body of literature that indicates that individuals infrequently alter their risk behaviors after the receipt of genetic risk estimates. Recent meta-analyses of studies investigating DNA-based risk estimate testing find no changes in physical activity, smoking, diet, medication or supplement use, or other unintended adverse effects of testing.44,45
The association between the receipt of elevated PGT prostate cancer risk estimates and the use of dietary supplements among men is notable given the conflicting data about the relative benefits and harms of vitamin and supplement use for prostate cancer prevention and management.46-51 In fact, the American College of Preventive Medicine recently recommended against the use of supplements (ie, multivitamins, vitamin E, and β-carotene) for cancer prevention.52 Our findings are also consistent with data from the Risk Evaluation and Education for Alzheimer’s Disease (REVEAL) study in which investigators found that 16% of participants reported a change in dietary supplement use (eg, vitamins E, gingko biloba) after undergoing genetic risk assessment for Alzheimer disease.53 Notably, individuals who had at least one copy of the allele that confers an elevated risk of Alzheimer disease (ie, apolipoprotein E ε4) had an odds of supplement use 4.75 times the odds of individuals without the elevated risk allele. However, given that our survey asked specifically about changes in vitamin or supplement use related to PGT testing, we are unable to determine whether vitamin or supplement use increased or decreased. Additionally, customers tended to be high vitamin and supplement users at BL, and the changes in vitamin and supplement use after PGT tended to be greatest among BL users. Given the growing nutraceutical industry in the United States and the paucity of regulation of dietary supplements, findings such as these raise questions about how PGT and clinic-based genetic testing might be contributing to the growth of this industry and highlight the need for studies that specifically focus on the use of nutraceuticals after genomic testing.
Variation across studies in regard to the effect of PGT on health-related behavior may be attributed to multiple factors. Changes in screening behaviors in the wake of PGT may be less common than lifestyle changes given that providers often play a gatekeeping role when it comes to accessing medical technologies. Differences in cancer risk perception may be another factor, because multiple studies have shown that perceived risk is often predictive of cancer screening behavior.54 Kaufman et al12 found that PGT customers who considered themselves to be at high risk for colon cancer were more likely to discuss PGT results with a physician, change their diet, and increase their physical activity. Carere et al35 evaluated perceived cancer risk among the broader PGen Study cohort and found that, with the exception of perceptions for lung cancer risk, consumers who received an elevated PGT risk result had modest mean positive changes in their risk perception. Among our participants, colon cancer risk perception before testing was an independent predictor of colonoscopy use after PGT. Another factor that may contribute to variation in customer behavior after testing is customer PGT result comprehension. A separate PGen Study analysis found that customers generally interpreted PGT risk estimates correctly; however, cancer risk estimates were not specifically evaluated.33 Kaufman et al12 found that the majority of PGT customers interpret test results correctly when presented with hypothetical scenarios, and the Multiplex Initiative found that most individuals who received testing recalled what had been reported.18 Other studies demonstrate that individuals in the general population often misinterpret PGT test results when presented with hypothetical scenarios.55 Finally, heterogeneity in study populations may influence PGT use and post-PGT behavior. Early adopter populations (such as those explored in our study and others12,15) may be higher health care users in general, especially compared with a more diverse sample such as the Multiplex population.18 Additional research is needed to determine whether there are specific customer populations that will be more likely to alter their behavior or use more health resources after PGT.
Our findings should be interpreted in the context of a few limitations. First, although we specifically intended to study current PGT users and the PGen Study sample is demographically representative of the DTC-PGT user population,31 our sample is not representative of the general population. Unlike the general population, a large proportion of participants had previously received cancer screening, with 91% of participants older than age 50 years reporting having been screened in the past (mammography, 97%; colonoscopy, 76%; PSA, 79%). For comparison, in 2010, the national estimate of adults age 50 to 75 years receiving a colonoscopy was 54.9%.56 It is unclear how PGT would influence the cancer screening behaviors of those who do not meet the recommended rates of screening. Second, the study included 6 months of follow-up, but observing behavior changes may take longer, especially for screening behaviors that require a provider order and behaviors that are recommended on an annual or less frequent basis. Third, only limited clinical data were collected. For instance, clinical factors that may be associated with screening recommendations (eg, having received radiation) were not captured. Additionally, the survey did not ask about cancer screening through other modalities, such as flexible sigmoidoscopy, or other potential confounders (eg, physician ambivalence toward PGT test results). Finally, we had limited power to detect greater behavior change among participants at elevated risk compared with participants at average or reduced risk for some outcomes. Nonetheless, our findings may not have differed substantially had we had greater power; among our participants, for example, mammography use was actually lower among the elevated risk group.
In summary, our study found that adults receiving elevated SNP-based cancer risk estimates from PGT did not significantly alter their diet, exercise, or advanced care planning behavior and were not more likely to engage in cancer screening than adults receiving average or reduced risk estimates. Given the fact that SNP-based risk estimates have limited clinical use, patients need to be prepared for the ambiguities inherent in PGT, and providers need to be prepared to counsel patients about such testing. If PGT expands to additional clinical settings and larger populations, future research will need to assess its association with cancer-related behaviors in broader populations and health care resource use that may or may not accrue as a result.
ACKNOWLEDGMENT
Members of the Impact of Personal Genomics Study Group at the time of publication are as follows: Robert C. Green, Joel B. Krier, Caroline M. Weipert, Margaret H. Helm, Sarah S. Kalia, Kurt D. Christensen, Lisa S. Lehmann, Harvard Medical School and Brigham and Women’s Hospital; Deanna Alexis Carere, Peter Kraft, Harvard T.H. Chan School of Public Health; J. Scott Roberts, Mack T. Ruffin IV, Lan Q. Le, Jenny Ostergren, Wendy R. Uhlmann, Mick P. Couper, University of Michigan; Joanna L. Mountain, Amy K. Kiefer, 23andMe; Tanya A. Moreno, Glenn Braunstein, Pathway Genomics; Scott D. Crawford, Survey Sciences Group; L. Adrienne Cupples, Clara A. Chen, Catharine Wang, Boston University; Stacy W. Gray, Dana-Farber Cancer Institute; Barbara A. Koenig, University of California San Francisco; Kimberly Kaphingst, University of Utah; and Sarah Gollust, University of Minnesota.
Appendix
Supplemental Methods
Study Measures
Genetic risk estimates.
Participants from both companies received a single genetic risk estimate based on genotyping of multiple single nucleotide polymorphisms for breast (women only), prostate (men only), and colorectal cancer. 23andMe (Mountain View, CA) customers were presented with a relative risk (RR) for each cancer, whereas Pathway Genomics (San Diego, CA) customers received results on a five-category scale corresponding to increasing RR of cancer (eg, Learn More). To harmonize genetic risk information across companies, a threshold RR level was selected to distinguish elevated from nonelevated genetic risk, and results were dichotomized into the following two categories: average or reduced genetic risk (23andMe RR < 1.2; two lowest Pathway categories) and elevated genetic risk (23andMe RR ≥ 1.2; three highest Pathway categories).
Interest in learning cancer genetic risk.
Interest in learning cancer genetic risk was assessed using a single item (three categories from “not at all interested” to “very interested”).
Cancer risk perceptions.
At baseline, participants were asked to rate their chances of developing breast cancer (women only), prostate cancer (men only), and colorectal cancer “compared to the average [man or woman] of [the same] age.” Responses were recorded on a five-point scale ranging from “much lower than average” to “much higher than average”;34 alternatively, participants could select “I have been diagnosed with this condition.”
Lifestyle behavior, supplement use, advanced care planning behaviors.
At 6 months, participants were asked about changes in their diet and exercise and use of vitamins or herbal supplements that were specifically motivated by their personal genomic testing results and about changes, or plans to make any changes, to advanced care planning (eg, creating a will, advance directives) as a result of learning their genetic information.35
Screening behaviors.
Mammography, colonoscopy, and prostate-specific antigen testing were measured at baseline and 6 months using questions from the 2011 Behavioral Risk Factor Surveillance System Questionnaire,36 modified to reflect a 6-month window of interest.
Use of vitamins and herbal supplements.
At baseline, participants were asked “Are you currently taking any vitamins on a regular basis (most days)?” and “Are you currently taking any herbal supplements?” Response options were “yes” or “no.”
Comparisons of Participants’ Self-Reported Behaviors With Published Standards
Dietary recommendations.
To estimate whether participants’ baseline dietary behaviors met published standards, we compared their self-reported behaviors with 2010 recommendations from the Centers for Disease Control and Prevention (http://www.choosemyplate.gov/fruit and http://www.choosemyplate.gov/vegetables). For diet, we equated a serving (the unit of measurement included in the survey items) with 1 cup and rounded up where necessary. For example, if the recommendation was 1.5 cups per day, then we required the participant to report having two or more servings per day.
-
Recommendations for fruit intake by age and sex
Women age 19 to 30 years = 2 cups per day
Women older than age 30 years = 1.5 cups per day (round up to two servings)
Men = 2 cups per day
-
Recommendations for vegetable intake by age and sex
Women age 19 to 50 years = 2.5 cups per day (round up to three servings)
Women older than age 50 years = 2 cups per day
Men = 3 or 2.5 cups per day, depending on age (round up to three servings)
Exercise recommendations.
To estimate whether participants’ baseline exercise behaviors met published standards, we compared their self-reported behaviors with 2008 recommendations from the Centers for Disease Control and Prevention (http://www.cdc.gov/physicalactivity/basics/index.htm). For exercise, we calculated an equivalent mix of moderate and vigorous activity as (2 × vigorous + moderate) exceeding the threshold set for moderate activity. Recommendations for exercise by age were as follows:
-
Age 18 to 64 years (1 or 2 or 3)
150 minutes per week of moderate-intensity aerobic activity plus muscle-strengthening activities on 2 or more days per week
75 minutes per week of vigorous-intensity aerobic activity plus muscle-strengthening activities on 2 or more days per week
An equivalent mix of moderate- and vigorous-intensity aerobic activity plus muscle-strengthening activities on 2 or more days per week
-
Age 65 years or older (1 or 2 or 3)
300 minutes per week of moderate-intensity aerobic activity plus muscle-strengthening activities on 2 or more days per week
150 minutes a week of vigorous-intensity aerobic activity plus muscle-strengthening activities on 2 or more days per week
An equivalent mix of moderate- and vigorous-intensity aerobic activity plus muscle-strengthening activities on 2 or more days per week
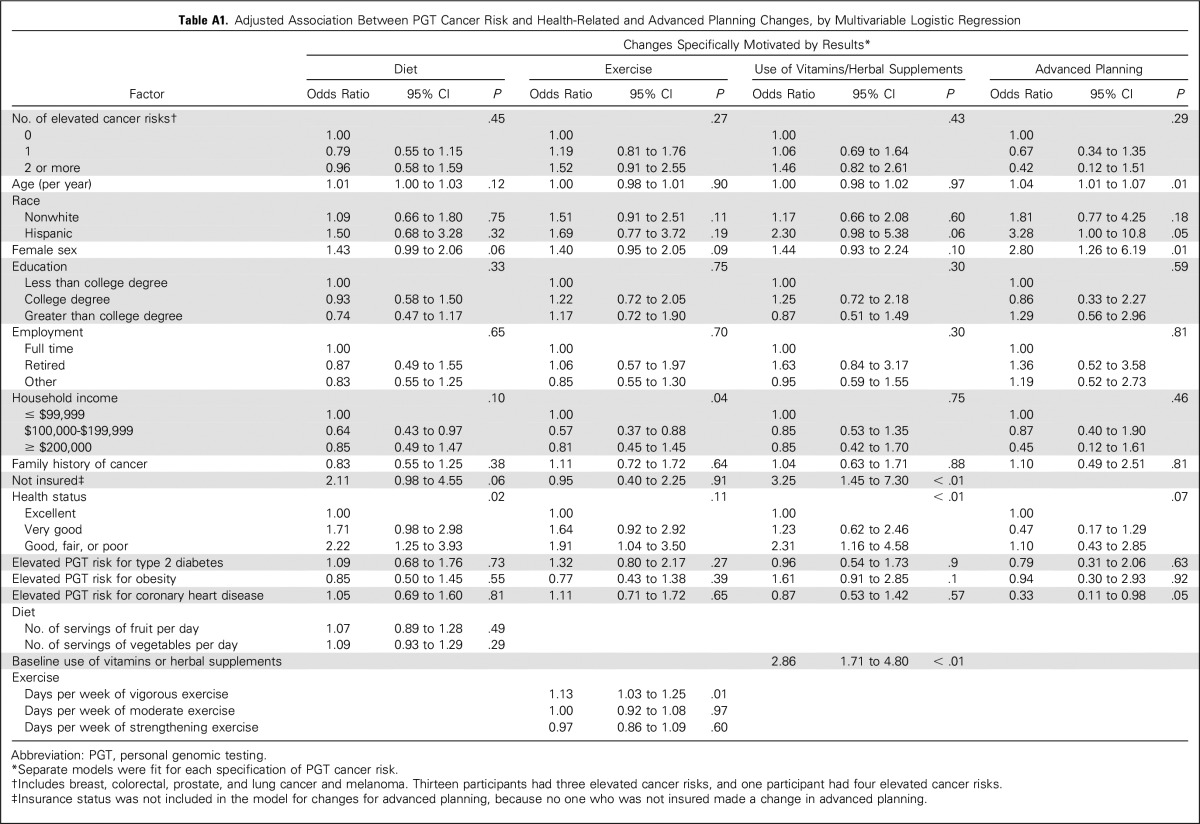
Table A1.
Adjusted Association Between PGT Cancer Risk and Health-Related and Advanced Planning Changes, by Multivariable Logistic Regression
Table A2.
Participant Characteristics Associated With Cancer Screening at 6 Months: Multivariable Logistic Regression
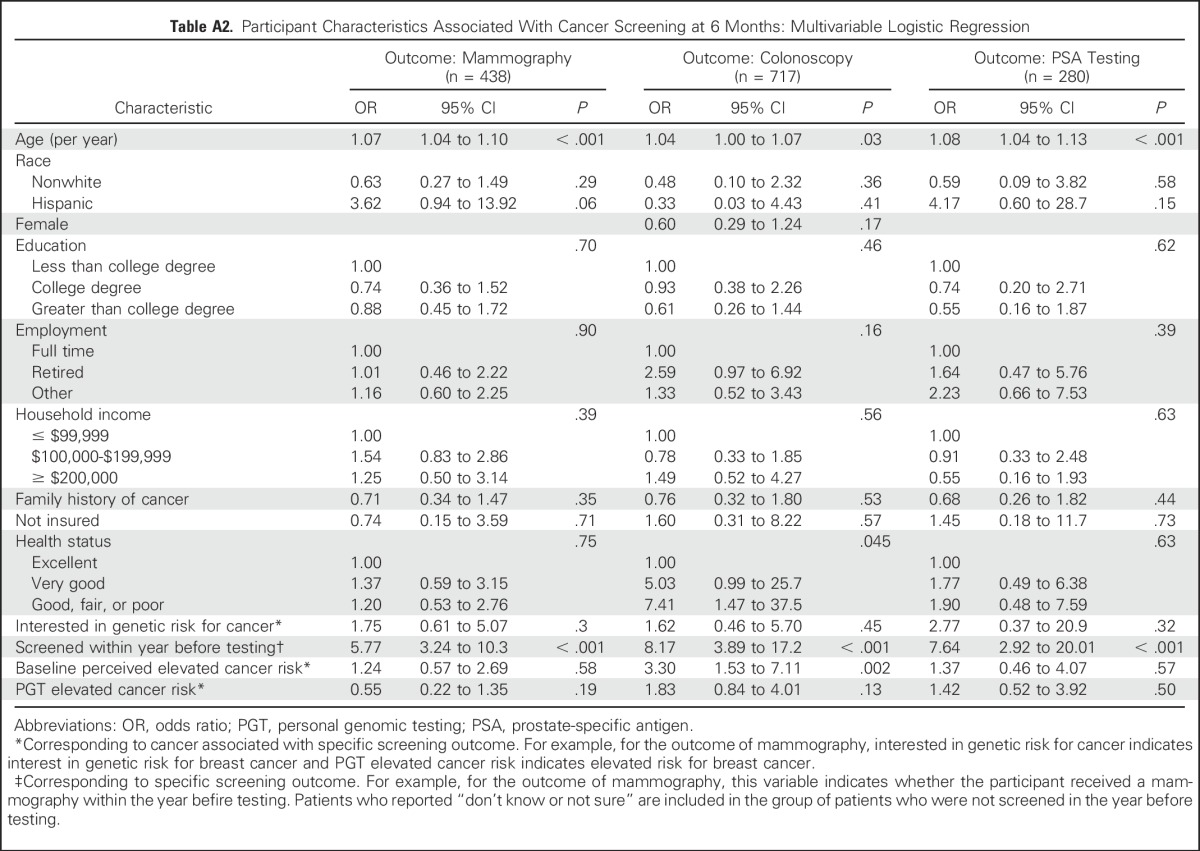
Table A3.
Unadjusted Associations Between Genetic Risk Scores and Participants’ Screening Behaviors at 6 Months by Age Group
Footnotes
Supported by the National Institutes of Health (NIH) National Human Genome Research Institute (Grant No. R01-HG005092). S.W.G. is supported by National Human Genome Research Institute (Grant No. U01HG006492) and by the American Cancer Society (Grant No. 120529-MRSG-11-006-01-CPPB). D.A.C. was supported by a Canadian Institutes of Health Research Doctoral Foreign Study Award when these analyses were performed. C.W. is supported by Grant No. NIH K07CA131103. R.C.G. is supported by Grants No. U01-HG006500, U19-HD077671, U01-HG008685, and U41-HG006834.
Written on behalf of the Impact of Personal Genomics (PGen) Study Group. A list of PGen Study Group members is provided in the Acknowledgment.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the National Institutes of Health, or the Canadian Institutes of Health Research.
AUTHOR CONTRIBUTIONS
Conception and design: Stacy W. Gray, Sarah E. Gollust, Deanna Alexis Carere, Mack T. Ruffin IV, Catharine Wang, J. Scott Roberts, Robert C. Green
Administrative support: Sarah S. Kalia
Collection and assembly of data: Deanna Alexis Carere, Sarah S. Kalia, J. Scott Roberts, Robert C. Green
Data analysis and interpretation: Stacy W. Gray, Sarah E. Gollust, Deanna Alexis Carere, Clara A. Chen, Angel Cronin, Huma Q. Rana, Mack T. Ruffin IV, Catharine Wang, Robert C. Green
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Personal Genomic Testing for Cancer Risk: Results From the Impact of Personal Genomics Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Stacy W. Gray
No relationship to disclose
Sarah E. Gollust
No relationship to disclose
Deanna Alexis Carere
Travel, Accommodations, Expenses: 23andMe
Clara A. Chen
No relationship to disclose
Angel Cronin
No relationship to disclose
Sarah S. Kalia
Consulting or Advisory Role: Genome Medical, Recombine
Huma Q. Rana
No relationship to disclose
Mack T. Ruffin IV
No relationship to disclose
Catharine Wang
No relationship to disclose
J. Scott Roberts
No relationship to disclose
Robert C. Green
Honoraria: Illumina
Consulting or Advisory Role: Invitae, Prudential, AIA, Roche, Helix
REFERENCES
- 1.McGuire AL, Burke W. An unwelcome side effect of direct-to-consumer personal genome testing: raiding the medical commons. JAMA. 2008;300:2669–2671. doi: 10.1001/jama.2008.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stadler ZK, Thom P, Robson ME, et al. Genome-wide association studies of cancer. J Clin Oncol. 2010;28:4255–4267. doi: 10.1200/JCO.2009.25.7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robson ME, Storm CD, Weitzel J, et al. American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28:893–901. doi: 10.1200/JCO.2009.27.0660. [DOI] [PubMed] [Google Scholar]
- 4.Bellcross CA, Page PZ, Meaney-Delman D. Direct-to-consumer personal genome testing and cancer risk prediction. Cancer J. 2012;18:293–302. doi: 10.1097/PPO.0b013e3182610e38. [DOI] [PubMed] [Google Scholar]
- 5.Gollust SE, Hull SC, Wilfond BS. Limitations of direct-to-consumer advertising for clinical genetic testing. JAMA. 2002;288:1762–1767. doi: 10.1001/jama.288.14.1762. [DOI] [PubMed] [Google Scholar]
- 6.Murray MF. Why we should care about what you get for “only $99” from a personal genomic service. Ann Intern Med. 2014;160:507–508. doi: 10.7326/M13-2804. [DOI] [PubMed] [Google Scholar]
- 7.Caulfield T, McGuire AL. Direct-to-consumer genetic testing: Perceptions, problems, and policy responses. Annu Rev Med. 2012;63:23–33. doi: 10.1146/annurev-med-062110-123753. [DOI] [PubMed] [Google Scholar]
- 8.Gray S, Olopade OI. Direct-to-consumer marketing of genetic tests for cancer: Buyer beware. J Clin Oncol. 2003;21:3191–3193. doi: 10.1200/JCO.2003.12.069. [DOI] [PubMed] [Google Scholar]
- 9.van der Wouden CH, Carere DA, Maitland-van der Zee AH, et al. Consumer perceptions of interactions with primary care providers after direct-to-consumer personal genomic testing. Ann Intern Med. 2016;164:513–522. doi: 10.7326/M15-0995. [DOI] [PubMed] [Google Scholar]
- 10.McGuire AL, Diaz CM, Wang T, et al. Social networkers’ attitudes toward direct-to-consumer personal genome testing. Am J Bioeth. 2009;9:3–10. doi: 10.1080/15265160902928209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gollust SE, Gordon ES, Zayac C, et al. Motivations and perceptions of early adopters of personalized genomics: Perspectives from research participants. Public Health Genomics. 2012;15:22–30. doi: 10.1159/000327296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufman DJ, Bollinger JM, Dvoskin RL, et al. Risky business: Risk perception and the use of medical services among customers of DTC personal genetic testing. J Genet Couns. 2012;21:413–422. doi: 10.1007/s10897-012-9483-0. [DOI] [PubMed] [Google Scholar]
- 13.Bloss CS, Schork NJ, Topol EJ. Direct-to-consumer pharmacogenomic testing is associated with increased physician utilisation. J Med Genet. 2014;51:83–89. doi: 10.1136/jmedgenet-2013-101909. [DOI] [PubMed] [Google Scholar]
- 14.Egglestone C, Morris A, O’Brien A. Effect of direct-to-consumer genetic tests on health behaviour and anxiety: A survey of consumers and potential consumers. J Genet Couns. 2013;22:565–575. doi: 10.1007/s10897-013-9582-6. [DOI] [PubMed] [Google Scholar]
- 15.Bloss CS, Schork NJ, Topol EJ. Effect of direct-to-consumer genomewide profiling to assess disease risk. N Engl J Med. 2011;364:524–534. doi: 10.1056/NEJMoa1011893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bloss CS, Wineinger NE, Darst BF, et al. Impact of direct-to-consumer genomic testing at long term follow-up. J Med Genet. 2013;50:393–400. doi: 10.1136/jmedgenet-2012-101207. [DOI] [PubMed] [Google Scholar]
- 17.Reid RJ, McBride CM, Alford SH, et al. Association between health-service use and multiplex genetic testing. Genet Med. 2012;14:852–859. doi: 10.1038/gim.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaphingst KA, McBride CM, Wade C, et al. Patients’ understanding of and responses to multiplex genetic susceptibility test results. Genet Med. 2012;14:681–687. doi: 10.1038/gim.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutierrez A: 23andMe, Inc. FDA Warning Letter, 11/22/2013. http://www.fda.gov/iceci/enforcementactions/warningletters/2013/ucm376296.htm
- 20.American Medical Association: AMA Calls for Ban on Direct to Consumer Advertising of Prescription Drugs and Medical Devices. https://www.ama-assn.org/content/ama-calls-ban-direct-consumer-advertising-prescription-drugs-and-medical-devices
- 21.US Food and Drug Administration: Draft guidance for industry, Food and Drug Administration staff and clinical laboratories: FDA notification and medical device reporting for laboratory developed tests (LDTs). http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM416684.pdf
- 22.Evans JP, Watson MS. Genetic testing and FDA regulation: Overregulation threatens the emergence of genomic medicine. JAMA. 2015;313:669–670. doi: 10.1001/jama.2014.18145. [DOI] [PubMed] [Google Scholar]
- 23.Green RC, Farahany NA. Regulation: The FDA is overcautious on consumer genomics. Nature. 2014;505:286–287. doi: 10.1038/505286a. [DOI] [PubMed] [Google Scholar]
- 24.Evans BJ, Burke W, Jarvik GP. The FDA and genomic tests: Getting regulation right. N Engl J Med. 2015;372:2258–2264. doi: 10.1056/NEJMsr1501194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldsmith L, Jackson L, O’Connor A, et al. Direct-to-consumer genomic testing: Systematic review of the literature on user perspectives. Eur J Hum Genet. 2012;20:811–816. doi: 10.1038/ejhg.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bloss CS, Darst BF, Topol EJ, et al. Direct-to-consumer personalized genomic testing. Hum Mol Genet. 2011;20:R132–R141. doi: 10.1093/hmg/ddr349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts JS, Ostergren J. Direct-to-consumer genetic testing and personal genomics services: A review of recent empirical studies. Curr Genet Med Rep. 2013;1:182–200. doi: 10.1007/s40142-013-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.23andMe: 23andMe homepage. https://www.23andme.com/
- 29.Pathway Genomics: Pathway Genomics homepage. https://www.pathway.com/
- 30.Lehmann LS, Kaufman DJ, Sharp RR, et al. Navigating a research partnership between academia and industry to assess the impact of personalized genetic testing. Genet Med. 2012;14:268–273. doi: 10.1038/gim.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carere DA, Couper MP, Crawford SD, et al. Design, methods, and participant characteristics of the Impact of Personal Genomics (PGen) Study, a prospective cohort study of direct-to-consumer personal genomic testing customers. Genome Med. 2014;6:96. doi: 10.1186/s13073-014-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carere DA, Kraft P, Kaphingst KA, et al: Consumers report lower confidence in their genetics knowledge following direct-to-consumer personal genomic testing. Genet Med 18:65-72, 2016 [DOI] [PMC free article] [PubMed]
- 33.Ostergren JE, Gornick MC, Carere DA, et al. How well do customers of direct-to-consumer personal genomic testing services comprehend genetic test results? Findings from the Impact of Personal Genomics Study. Public Health Genomics. 2015;18:216–224. doi: 10.1159/000431250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meisel SF, Carere DA, Wardle J, et al. Explaining, not just predicting, drives interest in personal genomics. Genome Med. 2015;7:74. doi: 10.1186/s13073-015-0188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carere DA, VanderWeele T, Moreno TA, et al. The impact of direct-to-consumer personal genomic testing on perceived risk of breast, prostate, colorectal, and lung cancer: Findings from the PGen study. BMC Med Genomics. 2015;8:63. doi: 10.1186/s12920-015-0140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baptista NM, Christensen KD, Carere DA, et al. Adopting genetics: Motivations and outcomes of personal genomic testing in adult adoptees. Genet Med. 2016;18:924–932. doi: 10.1038/gim.2015.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreiger JL, Murray F, Roberts JS, et al: The impact of personal genomics on risk perceptions and medical decision-making. Nat Biotechnol 34:912-918, 2016 [DOI] [PubMed]
- 38.Wang C, O’Neill SM, Rothrock N, et al. Comparison of risk perceptions and beliefs across common chronic diseases. Prev Med. 2009;48:197–202. doi: 10.1016/j.ypmed.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med. 2009;361:245–254. doi: 10.1056/NEJMoa0809578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention: Behavioral Risk Factor Surveillance System Survey Questionnaire. http://www.cdc.gov/brfss/annual_data/pdf-ques/2011brfss.pdf
- 41.Stadler ZK, Schrader KA, Vijai J, et al. Cancer genomics and inherited risk. J Clin Oncol. 2014;32:687–698. doi: 10.1200/JCO.2013.49.7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krier J, Barfield R, Green RC, et al. Reclassification of genetic-based risk predictions as GWAS data accumulate. Genome Med. 2016;8:20. doi: 10.1186/s13073-016-0272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saukko P. State of play in direct-to-consumer genetic testing for lifestyle-related diseases: Market, marketing content, user experiences and regulation. Proc Nutr Soc. 2013;72:53–60. doi: 10.1017/S0029665112002960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marteau TM, French DP, Griffin SJ, et al. Effects of communicating DNA-based disease risk estimates on risk-reducing behaviours. Cochrane Database Syst Rev. 2010;(10):CD007275. doi: 10.1002/14651858.CD007275.pub2. [DOI] [PubMed] [Google Scholar]
- 45.Hollands GJ, French DP, Griffin SJ, et al. The impact of communicating genetic risks of disease on risk-reducing health behaviour: Systematic review with meta-analysis. BMJ. 2016;352:i1102. doi: 10.1136/bmj.i1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin P-H, Aronson W, Freedland SJ. Nutrition, dietary interventions and prostate cancer: The latest evidence. BMC Med. 2015;13:3. doi: 10.1186/s12916-014-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein EA, Thompson IM, Jr,, Tangen CM, et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hackshaw-McGeagh LE, Perry RE, Leach VA, et al. A systematic review of dietary, nutritional, and physical activity interventions for the prevention of prostate cancer progression and mortality. Cancer Causes Control. 2015;26:1521–1550. doi: 10.1007/s10552-015-0659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Golabek T, Bukowczan J, Sobczynski R, et al. The role of micronutrients in the risk of urinary tract cancer. Arch Med Sci. 2016;12:436–447. doi: 10.5114/aoms.2016.59271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fortmann SP, Burda BU, Senger CA, et al: Vitamin, Mineral, and Multivitamin Supplements for the Primary Prevention of Cardiovascular Disease and Cancer: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Evidence Report No. 108. AHRQ Publication No. 14-05199-EF-1. Rockville, MD, Agency for Healthcare Research and Quality, 2013 [PubMed]
- 51.Wang Z, Fan J, Liu M, et al. Nutraceuticals for prostate cancer chemoprevention: From molecular mechanisms to clinical application. Expert Opin Investig Drugs. 2013;22:1613–1626. doi: 10.1517/13543784.2013.833183. [DOI] [PubMed] [Google Scholar]
- 52.Livingston CJ, Freeman RJ, Mohammad A, et al: Choosing Wisely® in preventive medicine: The American College of Preventive Medicine's top 5 list of recommendations. Am J Prev Med 51:141-149, 2016 [DOI] [PubMed]
- 53.Vernarelli JA, Roberts JS, Hiraki S, et al. Effect of Alzheimer disease genetic risk disclosure on dietary supplement use. Am J Clin Nutr. 2010;91:1402–1407. doi: 10.3945/ajcn.2009.28981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katapodi MC, Lee KA, Facione NC, et al. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: A meta-analytic review. Prev Med. 2004;38:388–402. doi: 10.1016/j.ypmed.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 55.Leighton JW, Valverde K, Bernhardt BA. The general public’s understanding and perception of direct-to-consumer genetic test results. Public Health Genomics. 2012;15:11–21. doi: 10.1159/000327159. [DOI] [PubMed] [Google Scholar]
- 56.National Center for Health Statistics: Health, United States, 2013: With special feature on prescription drugs. http://www.cdc.gov/nchs/data/hus/hus13.pdf [PubMed]