Abstract
The overexpression of P-glycoprotein (P-gp) causes resistance to chemotherapy in many tumor types. Here, we report intercellular transfer of functional P-gp from P-gp-positive to P-gp-negative cells in vitro and in vivo. The expression of acquired P-gp is transient in isolated cells but persists in the presence of P-gp-positive cells or under the selective pressure of colchicine. The intercellular transfer of functional P-gp occurs between different tumor cell types and results in increased drug resistance both in vitro and in vivo. Most importantly, the acquired resistance permits tumor cells to survive potentially toxic drug concentrations long enough to develop intrinsic P-gp-mediated resistance. P-gp transfer also occurs to putative components of tumor stroma, such as fibroblasts, raising the possibility that multidrug resistance could be conferred by resistant tumor cells to critical stromal elements within the tumor mass. This is the first report, to our knowledge, that a protein transferred between cells retains its function and confers a complex biologic property upon the recipient cell. These findings have important implications for proteomic analyses in tumor samples and resistance to cancer therapy.
Keywords: cell–cell communication, multidrug resistance phenotype
Cell–cell communication is an inherent feature of all life forms from bacteria to humans. A frequent result of cell–cell communication is collective population behavior that can potentially lead to complex phenotypes not observed in separate individual cells, e.g., in development or tumor formation (see, for example, ref. 1). Cell–cell communication can occur in multiple ways, including electrochemical coupling in synapses, molecule transfer through gap junctions, and coupling through biochemical ligand–receptor interactions, leading to paracrine signaling. Recently, there have been several studies suggesting that cells can also communicate by transferring membrane proteins, thus allowing transient transfer of new cell-surface proteins to the accepting cell. The implications of these findings for phenotypes of complex tissues or polyclonal tumors have not been explored so far.
Multidrug resistance (MDR) of cancer cells to chemotherapy is attributed in part to overexpression of various members of ATP-binding cassette proteins, most notably P-glycoprotein (P-gp) (encoded by the mdr-1 gene), and MDR-related protein (2, 3). A correlation between P-gp expression and poor treatment prognosis has been demonstrated in various malignancies (4, 5).
The present study was designed to determine whether intercellular transfer of P-gp-mediated MDR is possible and functionally relevant. Transfer of resistance between heterogeneous populations of cultured tumor cells has been previously observed. An increase in resistance of sensitive cell population to phenylalanine mustard was recognized as a result of coculturing in monolayer with their resistant counterparts (6). Similarly, in cell spheroids comprised of a mixture of sensitive and resistant cells, an increase in resistance of sensitive cell population to nitrosourea was observed (7). In both instances, a physical contact between sensitive and resistant cells was suggested to be essential for this transfer to occur. Increased resistance to melphalan was observed in vivo when sensitive and resistant tumors were grown in the same animal (8). Here, we investigate quantitatively P-gp transfer, its mechanism, and functional relevance. We demonstrate that, this transfer clearly does occur, and, under selective pressure, acquisition of extrinsic drug resistance can facilitate the acquisition of a stable drug-resistant phenotype.
Methods
Cell Lines. The cell lines used in the study were BE (2)-C human neuroblastoma cell line and its derivatives BE (2)-C/CHC(10′; IC50 to colchicine is 7 ng/ml), BE (2)-C/CHC(0.2), and BE (2)-C/CHC (1), developed by selection for resistance to 25, 500, and 2,500 nM colchicine, respectively (Sigma). The cells were grown as described in Supporting Methods, which is published as supporting information on the PNAS web site. Fibroblasts, NIH 3T3 cells, and G185 cells were a gift from P. Roepe (Georgetown University, Washington, DC); adenocarcinoma (MCF-7) and the corresponding mdr-1-transfected line BC19/3 were a gift from E. Bello-Reuss (University of Texas, Galveston); and Chinese hamster lung cell line DC-3F and its drug-resistant variant DC-3F/ADX were a gift of K. Scotto (Memorial Sloan–Kettering Cancer Center). Mouse embryonic fibroblasts at passage 4 were a kind gift of J. Elisseeff's laboratory (Johns Hopkins University). Human embryonic kidney 298T cells were a kind gift of I. Bossis (National Institutes of Health, Bethesda).
GFP Vector Construction and Retroviral Transduction. The GFP sequence was cloned into the NcoI and BamHI sites of the SFG vector (9), thus permitting constitutive GFP expression under the transcriptional control of the Moloney murine leukemia virus LTR. Recombinant particles pseudotyped with the vesicular stomatitis virus G glycoprotein were generated as described (10) and used without concentration to transduce subconfluent BE (2)-C cells in the presence of polybrene (Sigma) at 4 μg/ml. Transduction was highly efficient (>99%), thus obviating the need to sort positive cells. Expression remained stable under prolonged culture.
Transient and Stable Transfection with pMDR1-EGFP. Transfections were performed by using Lipofectomine 2000 (Invitrogen Life Technology, Carlsbad, CA), following the manufacturer's protocoll. Briefly, cells were ≈70–90% confluent before transfection. Each of ≈24 μg of pMDR1-EGFP (a kind gift by S. Simon, The Rockefeller University, New York) and 60 μl of Lipofectamine were diluted in 1.5 ml of DMEM, mixed, incubated for 20 min at room temperature, and added to the cells in a 10-cm culture dish. Media was changed 6 h after transfection and cells were harvested for flow cytometry 30 h after transfection. Equal initial numbers of sorted and untransfected cells were cocultured for ≈30 h at the confluence of ≈70%. The methods for creating stable transfection are described in Supporting Methods.
Quantitative Flow Cytometry and FACS. The mAb MRK-16, directed against P-gp, was used in binding studies (a gift of T. Tsuruo, University of Tokyo, Tokyo). Quantitative flow cytometry assays were performed, following a slightly modified method of Ferrand et al. (11). Fluorescein and red fluorescent tagged phycoerythrin (PE)-conjugated goat anti-mouse Ab (Jackson ImmunoResearch) was used for MRK-16 labeling. The FACS experiments were performed with FACSVantage SE (Becton Dickinson). In the study of functionality of P-gp transfer, Rhodamine 123 (Sigma) was added to cells in the final concentration of 0.5 μg/ml, followed by incubation at 37°C for 1 h. In addition, in some experiments, cells were incubated with 100 μM verapamil for 6 h before Rhodamine 123 experiments. In all coincubation experiments, controls of mixing cell lines just before the experiment and by using irrelevant Abs were also performed.
RT-PCR. RT-PCR was performed by following the Noonan et al. (12) protocol. For positive control to ensure the efficiency of RNA extraction, β-actin specific sequence was amplified. A negative control was achieved by application of an irrelevant RNA template, pAW 109 (PerkinElmer). PCR was carried out in a GeneAmp PCR System 9600 (PerkinElmer).
Confocal Microscopy. For microscopy, cells were collected by treatment with trypsin, washed twice with PBS, and mounted on microscope slides. Immediately after this procedure, cells were imaged with a confocal Leica DM IBRE microscope. Digital images were obtained with Leica tcs software and stored in TIFF format.
Animal Experiments. Immunosuppressed BALB/cmice(20–25gof body weight) received s.c. injections of 107 cells to initiate xenograft tumor growth. When tumors reached 15–20 mm [≈2 weeks after injection, the mice were killed by carbon dioxide inhalation and tumors were excised for obtaining primary cell cultures by following the standard protocol (see, for example, ref. 13)]. The isolated cells (in suspension) were allowed to settle at the bottom of the flask and grow for 1–2 days. After this step, the cells were collected and quantitative fluorescence cytometry was performed as described.
Supporting Information. Further results are shown in Figs. 7–16, which are published as supporting information on the PNAS web site.
Results
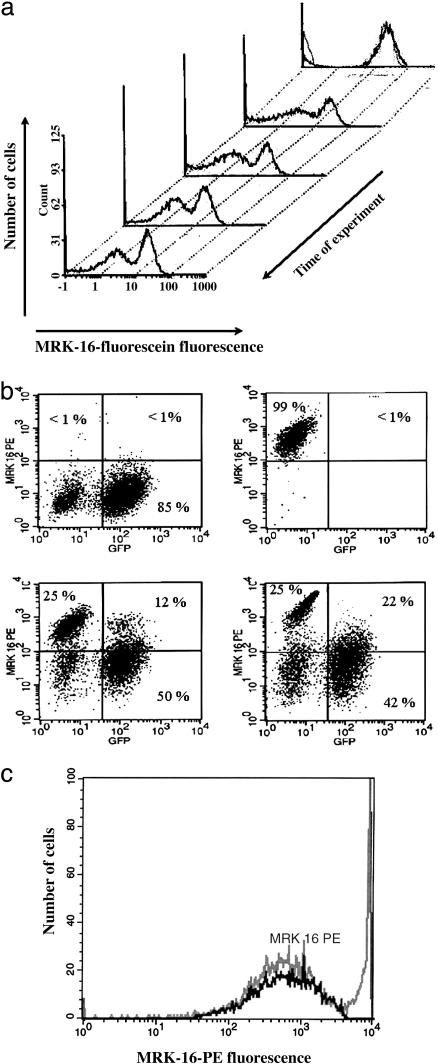
Characterization of Intercellular P-gp Transfer. To investigate whether coincubation of P-gp-positive cells with P-gp-negative cells affects their P-gp content, we analyzed mixtures of human neuroblastoma BE (2)-C cells with their MDR counterparts selected for resistance to colchicine. Two cell lines with intrinsic MDR: BE (2)-C/CHC (0.2) (IC50 ≈ 100 ng/ml) and BE (2)-C/CHC (1) (IC50 = 375 ng/ml) were cocultured with the parental sensitive line at equal proportions. Before the experiment, all of the intrinsically resistant cell lines exhibited stable P-gp expression levels when grown in the absence of any drug as a selection agent. P-gp expression in cocultures was measured by quantitative fluorescence cytometry using MRK-16 mAb and fluorescein-labeled secondary Ab, allowing simultaneous analysis of several cell subpopulations (Fig. 1a). Within several hours, the histogram peak of the sensitive subpopulation shifted toward new higher fluorescence values, reflecting an increased amount of P-gp and then continued to shift much more slowly over a period of 2–3 days. (see Supporting Methods for further quantification) A relatively stable position of the new subpopulation peak corresponded to ≈0.2 million molecules per cell, whereas P-gp in the parental BE (2)-C cell line grown separately was indistinguishable from 0.
Fig. 1.
Transfer of P-gp expression between resistant and sensitive variants of the BE (2)-C human neuroblastoma cell line. (a) Evolution of P-gp transfer with graphs showing histograms of a 50/50 mixture of sensitive and BE (2)-C/CHC (0.2) cells measured at 0 (3 h), 2, 4, 6, and 8 days after coincubation. MRK-16 Ab was used in a sandwich assay with fluorescein labeled secondary Abs. (b) Dependence of the transferred P-gp expression on the P-gp levels in resistant cells. Scatter histograms were obtained by gating cells according to PE and GFP emission spectra. One day cocultures of sensitive cells with BE (2)-C/CHC(0.2) (Left Lower) and BE (2)-C/CHC (1) (Right Lower) are compared with controls of pure sensitive (Left Upper) and pure BE (2)-C/CHC (0.2) (Right Upper) cells. (c) Coincidence of the AqMDR population (the first peak in the mixed population histogram, gray; see text) with the population gated for GFP expression (black). In these and all other experiments, medium was free of colchicine. Coincubation was for 10 days with BE (2)-C/CHC(0.2) cells. A control showing mixture of BE (2)-C/CHC (1) and BE (2)-C with no coincubation is shown in Fig. 16.
Although the appearance of a subpopulation with intermediate P-gp levels [henceforth termed acquired resistance MDR (AqMDR)] suggested intercellular transfer of P-gp expression to sensitive cells, the exact origin of the new cell population remained unclear. For instance, it was possible that these cells had formed from resistant cells through partial loss of P-gp mediated resistance. To address this issue, we marked sensitive cells by stable GFP transduction. Thus, their progeny could be distinguished from the progeny of resistant cells in further assays. A red fluorescent tagged PE was used for immunolabeling in a sandwich assay by using an anti-P-gp mAb MRK-16. Flow cytometry experiments revealed appearance of dually labeled cells, indicating that a majority of GFP-labeled sensitive cells do indeed start expressing elevated concentrations of P-gp (Fig. 1b). As is clear from the scatter diagrams of GFP and P-gp expression (e.g., Fig. 1b), there was a continuous variation of P-gp in AqMDR cells in the range defined by P-gp expression in sensitive and resistant cell lines used in the coculture. In contrast, there was an obvious separation of GFP-positive and -negative cells with a substantial gap in fluorescence intensity values. These observations suggested that the appearance of dually labeled cells is mediated by the transfer of P-gp to GFP-positive sensitive cells, rather than the transfer of GFP to resistant cells. In the following analysis, we define dually positive cell subpopulation to include only the cells with P-gp expression above the levels found in 99% of pure sensitive cells (the horizontal lines in Fig. 1b and other figures separating upper and lower quadrants; the dually positive cells are thus found in the upper right quadrants).
Further analysis revealed that the histogram of the GFP-expressing cell subpopulation represented a single peak that coincided in position with the peak of the AqMDR cell subpopulation seen previously (Fig. 1c). The time course of the appearance of the AqMDR and dually positive cells coincided (compare Fig. 1 a and c). In combination, the findings illustrated in Fig. 1 led us to conclude that cells in the AqMDR subpopulation are dually labeled, and, hence, the sensitive cells start acquiring P-gp expression when coincubated with intrinsically resistant cells.
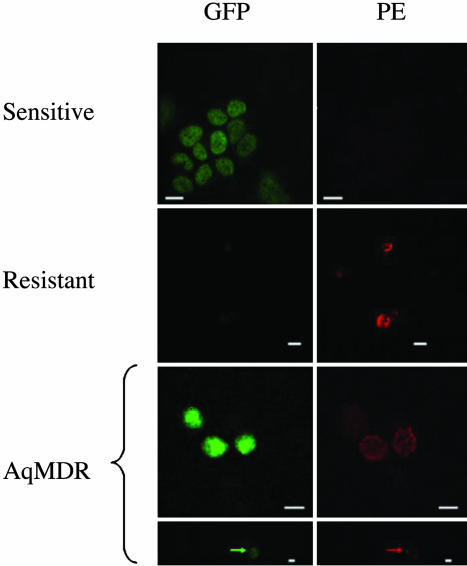
To eliminate the possibility that dually positive cells are, in fact, clumps of two or more cells carrying a single label, we studied the fixed cell mixtures by confocal microscopy. As shown in Fig. 2, we succeeded in identifying multiple individual dually positive cells (estimated to be 10–20% of the population). In many instances, P-gp was localized in bright membrane patches, in addition to fainter homogeneous membrane distribution. This finding was at variance with a more homogeneous membrane localization in GFP-negative resistant cells. These findings directly confirm the existence of individual AqMDR cells. Membrane localization of P-gp also suggested that it might be functional in these cells.
Fig. 2.
Direct demonstration of P-gp transfer through imaging by confocal microscopy. Controls of pure BE (2)-C/CHC (1) (first row) and sensitive (second row) cells compared with dually labeled (AqMDR, 8 days 50:50 ratio coculture) cells (third and fourth rows). The arrows indicate a local “bright spot” of high P-gp-related membrane fluorescence. (Scale bars, 20 μm.)
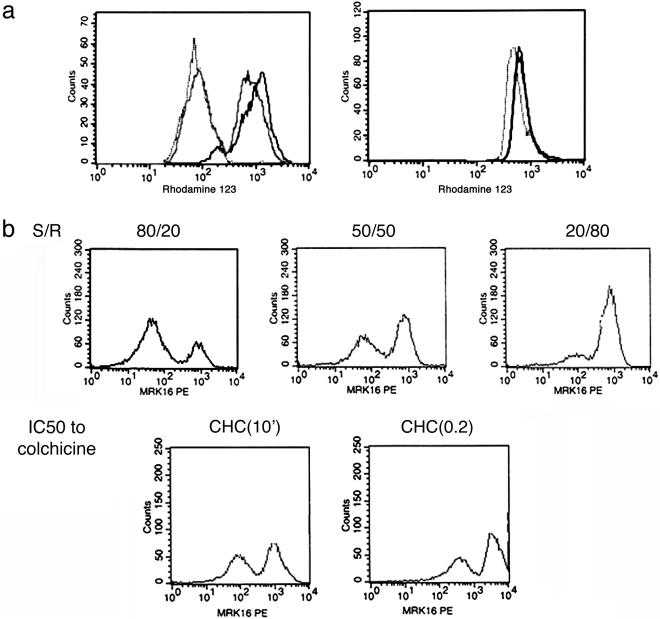
To verify that the transferred protein was functional, we performed a series of flow cytometry experiments with a fluorescent P-gp substrate Rhodamine123, commonly used as an indicator of the MDR phenotype. As expected, the histogram of the mixed population displayed two peaks, with the higher concentration peak (corresponding to a more sensitive subpopulation) shifted significantly compared with that of the pure sensitive population (Fig. 3a). This shift was abolished by the effect of a known P-gp inhibitor, verapamil. Thus, both P-gp labeling and dye extrusion assays indicated that cell coincubation results in a functional P-gp transfer from P-gp positive cells to P-gp negative cells, resulting in a significant resistance phenotype. Below, we provide additional evidence of functionality of the transfer.
Fig. 3.
Characterization of P-gp transfer. (a) Transferred P-gp is functional, as judged by the change in the Rhodamine 123 extrusion in coculture (gray line) versus BE (2)-C (rightmost black solid line showing concentration without mixing for reference) and BE (2)-CHC(0.2) (leftmost dotted line) cell lines (Left) and the same experiment in the presence of verapamil (Right). In Left, the most resistant subset has a very low peak of rhodamine, but even the most sensitive subset in the mixture shows a peak that has a definite shift toward less rhodamine retention, which is consistent with functioning P-gp transfer. The effect is virtually abolished by verapamil. (b) The extent of P-gp transfer depends on the relative proportions [sensitive/resistant ratio (S/R):80/20; 50/50; 20/80 ratios of BE (2)-C and BE (2)-C/CHC(10′) lines] and levels of P-gp expression in the resistant line [BE (2)-C/CHC(10′) less resistant versus BE (2)-C/CHC(0.2) more resistant]. The peak MDR expression in the AqMDR peak is higher when sensitive cells are incubated with more resistant cells, showing a kind of dose effect. The controls for these experiments are in Fig. 11.
Finally, coincubation of sensitive cells with cells of P-gp expression levels or with the same resistant cell line in different relative proportions resulted in the expected outcome: the more resistant cells are present, and, the higher their resistance, the more P-gp is transferred (Fig. 3b). However, even at relatively low levels, the P-gp content of AqMDR cells was still significantly higher than in pure sensitive cells.
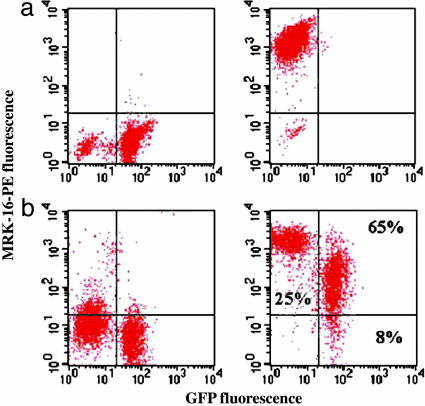
P-gp Transfer Occurs in Neuroblastoma in Vivo and in Multiple Cell Lines in Vitro. Because in vivo conditions may present tumor cells with complex and changing environments, potentially influencing interaction between sensitive and resistant cells, we investigated whether P-gp transfer also occurred in vivo, by growing tumors from mixed populations as well as pure sensitive and resistant cell controls. The grown tumors were excised, and cells were extracted, purified, and analyzed for P-gp expression. We found that the relative proportions of resistant and AqMDR cells had not changed throughout tumor growth. We also found that the levels of P-gp in AqMDR cells within tumors had dramatically increased compared with those found in the cells grown in cell culture experiments (Fig. 4) coming to the levels of P-gp content close to those in resistant cells. These findings suggest that intercellular protein transfer does occur in vivo and that the efficiency of this transfer may increase in the more physiological tumor tissue environment.
Fig. 4.
Intercellular P-gp transfer occurs in vivo. Cells extracted from tumors grown in immunodeficient mice were isolated and analyzed by flow cytometry. (a) Results shown are for tumors grown from BE (2)-C (a Left), BE (2)-C/CHC (1) (a Right), and AqMDR cells from a 50/50 ratio after 2 days of coculture. (b Left) A negative control performed with same cells by using a nonspecific mAb with MRK-16 isotype. (b Right) More than 65% of the cells are in the right upper quadrant, indicating both GFP and P-gp, attesting to efficient transfer. The cells injected had scatter plot profiles qualitatively identical to Fig. 1b.
Our further studies have also confirmed that protein transfer occurs in cell types other than human neuroblastoma. In particular, we have successfully observed P-gp transfer between human adenocarcinoma (MCF-7) and the corresponding mdr-1-transfected line BC19/3. On the other hand, we failed to observe P-gp transfer between NIH 3T3 fibroblasts and the corresponding mdr-1-transfected line G185, or Chinese Hamster lung cell line DC-3F, and its drug-resistant variant, DC-3F/ADX. However, all of the cells studied, including fibroblasts, were excellent donors of P-gp in cocultures with different cell types (Table 1), suggesting that intercellular protein transfer might be important in interactions between various drug-resistant and -sensitive cell types, both of cancerous and normal origin. Importantly, we have also observed P-gp transfer from BE (2)-C/CHC(0.2) cells to embryonic mouse fibroblasts (Supporting Methods) that provided further support for the nongenetic nature of P-gp transfer, because the MRK-16 Ab used in the experiment is specific to human, but not murine, P-gp.
Table 1. Transfer of P-gp in cocultured cells of different origin.
| Acceptor
|
||||
|---|---|---|---|---|
| Donor | BE(2)-C | DC-3F | MCF-7 | NIH 3T3 |
| BE(2)-C/CHC | Yes | Yes | N/A | Yes |
| DC3F/ADX | No | No | N/A | N/A |
| BC 19/3 | Yes | N/A | Yes | N/A |
| G185 | Yes | N/A | N/A | No |
Protein transfer determination is based on whether the corresponding shift for P-gp acceptor cells peak on a flow cytometry histogram exceeds the mean of the control peak for the same line plus 2 SD. N/A, not available.
As a control of the Ab (MRK-16 specifically recognizes human P-gp), we tested for P-gp transfer between Chinese hamster lung cell line DC-3F, and its drug-resistant variant, DC-3F/ADX. As expected, no significant transfer was detected. Additionally, we have confirmed that cells transiently transfected [BE (2)-C, Fig. 13] and stably (human embryonic kidney 293T cells, Fig. 14) transfected with mdr-1-EGFP containing plasmid can donate the resulting P-gp-EGFP fusion protein to BE (2)-C cells.
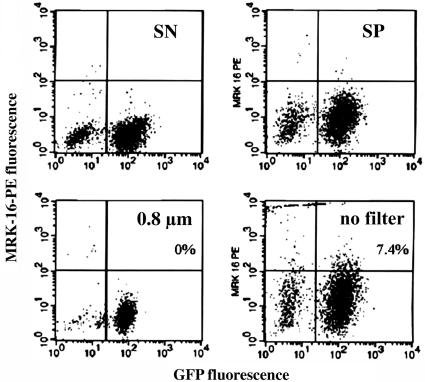
P-gp Transfer Is Unstable and Does Not Occur Through Small Microparticles. We next explored whether extracellular (paracrine) signaling mediates P-gp expression transfer. Sensitive cells were divided into two groups to be incubated for several days in filtered or unfiltered supernatant medium removed from resistant cell culture. The pore size of the filter used (0.8 μm) was large enough to allow passage of various organic compounds and possibly small membrane microparticles (normally 0.1–1 μm in diameter), but not cells. We observed transfer of P-gp expression when unfiltered but not filtered medium was used (Fig. 5), suggesting that the transfer mechanism was not mediated by paracrine signaling through extracellular medium, but by a process requiring either cell-to-cell contact or exchange of relatively large (>0.8 μm) membrane microparticles. This was confirmed by the examination of the growth of sensitive and resistant cells in two chambers separated by a 0.2-μm filter allowing exchange of the growth medium. No transfer of P-gp expression was observed in this experiment (data not shown). Co-incubation of sensitive and resistant cells in the presence of 5 μM forskolin, an agent known to increase development of gap junctions (GJ) (14) did not result in additional transfer of P-gp expression (data not shown), suggesting that P-gp transfer is not mediated by GJ. We also confirmed that the AqMDR cells have the same size as BE (2)-C-sensitive and -resistant cells, providing evidence against potential cell fusion (Fig. 15).
Fig. 5.
P-gp transfer is mediated by large microparticles or cell-to-cell contact. The control of sensitive cells labeled with P-gp nonspecific (SN) and P-gp specific (MRK-16, SP) Abs grown with no medium transfer as compared with sensitive cells labeled with specific Ab grown for 1 day in unfiltered or filtered (0.8 μm) medium transferred from BE (2)-C/CHC (1) cells. Virtual absence of resistant cells in the unfiltered medium is evident, as shown in Right Upper (in contrast to the scatter plot of the cell line from which the unfiltered medium was obtained, shown in Fig. 1b Right Upper).
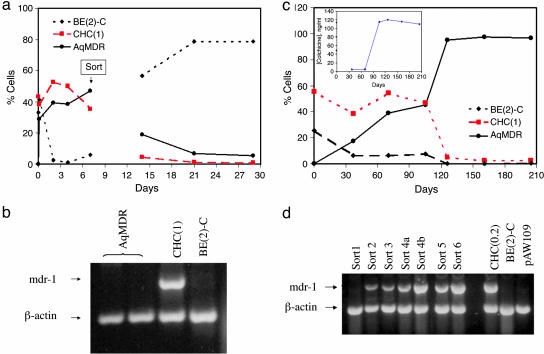
To further study the AqMDR cell population, we performed FACS experiments, resulting in separation of AqMDR from sensitive and resistant cells. After each sort, we assayed AqMDR by flow cytometry, RT-PCR, and measurement of their resistance to colchicine (Fig. 6). The sorted AqMDR had significantly increased levels of the P-gp expression and colchicine resistance (Fig. 6a). No significant increase in mdr-1 mRNA was detected (Fig. 6b), in agreement with the proposed nongenetic mechanism of P-gp transfer. Both P-gp expression and resistance levels decreased to the values found in the sensitive cells within 2 weeks after FACS. This decrease was reversed, if, after sorting, the AqMDR cells were repeatedly exposed to colchicine at levels toxic to sensitive cells and sorted again (see below). We thus conclude that the acquired P-gp expression in the AqMDR cell population alone is unstable, and the presence of either resistant cells or selective pressure is required for stabilization of P-gp-mediated resistance in these cells.
Fig. 6.
Evolution of resistance transfer before and after FACS of AqMDR cells. (a) Percentage of BE (2)-C (♦), BE (2)-C/CHC (1) (▪) and AqMDR (•) cells as determined by flow cytometry. The sort occurred on the ninth day after coculture initiation as indicated. See Fig. 8 for simulation of this experiment. Note that because of the gap in time between the sort and the first subsequent measurement, a significant reduction in the P-gp content may have occurred. (b) An RT-PCR experiment showing mdr-1 levels in the indicated cell lines. The experiments with AqMDR were performed for 8 days (left lane) and 12 days (right lane) of coculture followed by FACS. (c and d) Transfer of resistance allows sensitive cells to survive cytotoxic drug concentrations and ultimately develop intrinsic resistance. BE (2)-C and BE (2)-C/CHC (1) cells were cocultured (in a 50:50 ratio) for 10 days and then dually labeled (AqMDR) cells were separated from the rest of the cells by FACS. Postsort AqMDR cells were then grown in colchicine-free medium for 7–10 days and treated with 5 ng/ml colchicine [close to an IC50 of sensitive cells (4.5 ng/ml)] medium for 1–2 weeks. This sequence was repeated six times at the time intervals indicated. P-gp transfer measured by flow cytometry and colchicine resistance (c Inset) and RT-PCR data (d) are shown. In a, BE (2)-C (♦), BE (2)-C/CHC (1) (▪), and AqMDR (•) cells are shown. The lanes in d correspond to experiments performed after each indicated sort (a data point in c) and the controls of sensitive and resistant cells and irrelevant RNA from pAW109 plasmid.
P-gp Transfer Slows Cell Growth. The data presented in Figs. 1 a and d and 4a indicated that coincubation of sensitive and resistant cells results in stabilization of relative fractions of sensitive, resistant, and AqMDR cells. Consistently, a moderate decrease in the fraction of resistant cells was observed, whereas the fractions of sensitive and AqMDR cells changed reciprocally, apparently with two time constants (hours and days). In Supporting Methods, we present evidence for slower growth of P-gp expressing cells, including AqMDR compared with P-gp-negative cells, and present a mathematical model of P-gp transfer obtained as a result. The reasons for cell proliferation dependence on P-gp are not clear, although an inverse relationship between the degree of resistance and the rate of cell proliferation is commonly reported (15). A similar moderate decrease in the growth rate has been also observed in cells transfected with the mdr-1 gene (16). We hypothesize that intercellular transfer of P-gp may slow the growth of AqMDR cells and stabilize the relative proportions of intrinsically resistant, AqMDR cells and sensitive cells in coculture. In the absence of the transfer, sensitive cells are expected to rapidly displace resistant cells due to the growth-rate advantage.
P-gp Transfer Can Protect Sensitive Cells and Lead to Formation of Permanently Resistant Cells. As a consequence of P-gp transfer, AqMDR cells may survive exposure to levels of chemotherapeutic drugs normally toxic to sensitive cells. To investigate this possibility, we exposed the sorted AqMDR cells to several rounds of incubation with colchicine alternating with additional sorting (Fig. 6 c and d). The concentration of colchicine applied (5 ng/ml) exceeds the IC50 of the sensitive BE (2)-C cells (4.5 ng/ml) and normally leads to an almost complete growth inhibition and subsequent death of BE (2)-C cells. The evolution of mdr-1 mRNA, P-gp expression, and drug resistance was then followed for 4months (Fig. 6c). We observed a gradual increase in the levels of mdr-1 mRNA correlated to an increase in P-gp expression. The level of resistance also increased, although in a more discrete manner, indicating occurrence of a singular event, possibly mdr-1 gene duplication. Throughout the experiment, during exposure to colchicine, cell growth was not visibly inhibited and cell mortality was not increased, indicating a complete protection of AqMDR cells by the transferred P-gp and later endogenously expressed P-gp. After the experiment, the P-gp expression and the level of resistance remained constant, indicating that a stable resistant line had been selected.
Discussion
In this report, we present evidence for intercellular transfer of P-gp expression between drug-resistant and -sensitive human cancer cells. This transfer results in an increased P-gp-mediated MDR in previously drug-sensitive cells that allowed them to survive normally toxic levels of a chemotherapeutic drug, and, ultimately, acquire a permanent resistance phenotype.
Transfer of chemokine receptor CCR5 between CCR5-positive and -negative cells has been described (17). It has been suggested that this transfer occurs by means of membrane microparticles, small (0.1–2 μm) membrane vesicles released into extracellular medium by a variety of cell types. In other instances, formation of a tight contact between cells such as the immune synapse may be required for protein transfer (18–20). Exchange of membrane microparticles may also be a part of this process (18, 21, 25). Finally, cell fusion (thought to be common in cancer cells) may lead to sharing of proteins between adjacent cells (22, 23). Our data seem to agree best with a contact mediated transfer, based on the absence of transfer in the filtered medium experiments, unaltered size of AqMDR cells, and instability of the transferred protein in the sorted cells; the results argue against microparticle or fusion-mediated transfer.
Transfer of P-gp results in the phenotype commonly observed in cells transfected with the mdr-1 gene, implying intact functionality of the transferred protein. In particular, the cells acquire elevated levels of resistance to a chemotherapeutic drug and lower rates of proliferation. Indeed, clonal heterogeneity of human tumors often results in the coexistence of several cell subpopulations exhibiting different MDR levels (24, 25). Our results indicate that intercellular transfer of P-gp may protect cancer cells by both increasing the overall resistance in a tumor and stabilizing relative proportions of various cell subpopulations in tumor growth. Resistant cells that might have been displaced from the tumor due to growth-rate disadvantage can now be retained, whereas relatively sensitive cells become protected due to increased P-gp expression. We suggest, therefore, that precluding transfer of P-gp and other MDR-producing membrane transporters can increase efficacy of cancer chemotherapy. Further investigation of the mechanisms of intercellular P-gp transfer is thus warranted.
Our findings add P-gp to the growing list of proteins that can be transferred from one eukaryotic cell to another. As far as we are aware, this is the initial direct observation that the transferred protein is actually functional and can confer a complex biologic property to the recipient cell, namely MDR. In the current paper, we described a situation in which the functional protein transfer results in conferring a competitive advantage on the recipient cell, based on the biologic function of the transferred protein.
Intercellular P-gp transfer has implications for interpreting the link between protein expression and gene expression within complex tissues, such as tumors. Thus, at the individual cell level, it is possible to detect protein expression, and an associated phenotype that is not the consequence of gene expression within the same cell.
In solid tumors, an increase in the proportion of tumors expressing P-gp has been observed in metastatic tumors from patients that have undergone multiple cycles of chemotherapy. It is a common observation that there is an increase in P-gp expression in the endothelial cells within the tumor mass at these tumor sites (26). This observation has usually been interpreted to mean that mdr-1 gene is being expressed in the endothelial cells as a result of the selective pressure from chemotherapy. An alternative hypothesis is suggested by our data, namely, that intercellular transfer from resistant tumor cells could occur to stroma tissues, including endothelial cells within the tumor mass, as a mechanism for protection of essential cell types that support in vivo growth. Such protection may be particularly valuable at the time of new vessel formation at sites within the tumor mass where chemotherapy might otherwise damage these tissues.
Acknowledgments
We thank Ms. Joanne Green, A. Pathan, T. Delohey, P. Anderson, H. Gallardo, M. Menon, and R. Pittman for their expert assistance and Dr. J. L. Biedler (Memorial Sloan–Kettering Cancer Center) for the cell lines used in the present work. This work was supported by Department of Energy Grant DE-FGO2-86ER60407, National Institutes of Health Grants CA-59350 and CA08748, and the Whitaker Foundation (to A.L.).
Author contributions: A.L. and S.L. designed research; A.L., B.M.M., X.N., G.K., L.V., D.W., and D.P. performed research; M.S. contributed new reagents/analytic tools; A.L., B.M.M., X.N., G.K., L.V., and D.P. analyzed data; A.L., D.W., and S.L. wrote the paper; M.S. provided a valuable critique; and S.L. provided detailed editing and a response to the critique.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: P-gp, P-glycoprotein; MDR, multidrug resistance; AqMDR, acquired resistance MDR; PE, phycoerythrin.
References
- 1.Darland, D. C. & D'Amore, P. A. (2001) Curr. Top. Dev. Biol. 52, 107–149. [DOI] [PubMed] [Google Scholar]
- 2.Ambudkar, S. V., Dey, S., Hrycyna, C. A., Ramachandra, M., Pastan, I. & Gottesman, M. M. (1999) Annu. Rev. Pharmacol. Toxicol. 39, 361–398. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy, W. T. (1996) Annu. Rev. Pharmacol. Toxicol. 36, 161–183. [DOI] [PubMed] [Google Scholar]
- 4.Trock, B. J., Leonessa, F. & Clarke, R. (1997) J. Natl. Cancer Inst. 89, 917–931. [DOI] [PubMed] [Google Scholar]
- 5.Dunne, B. M., Mc Namara, M., Clynes, M., Schering, S. G., Larkin, A. M., Moran, E., Barnes, C. & Kennedy, S. M. (1998) Hum. Pathol. 29, 594–598. [DOI] [PubMed] [Google Scholar]
- 6.Frankfurt, O. S., Seckinger, D. & Sugarbaker, E. V. (1991) Cancer Res. 51, 1190–1195. [PubMed] [Google Scholar]
- 7.Tofilon, P. J., Buckley, N. & Deen, D. F. (1984) Science 226, 862–864. [DOI] [PubMed] [Google Scholar]
- 8.Miller, B. E., Machemer, T., Lehotan, M. & Heppner, G. H. (1991) Cancer Res. 51, 4378–4387. [PubMed] [Google Scholar]
- 9.Riviere, I., Brose, K. & Mulligan, R. C. (1995) Proc. Natl. Acad. Sci. USA 92, 6733–6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallardo, H. F., Tan, C., Ory, D. & Sadelain, M. (1997) Blood 90, 952–957. [PubMed] [Google Scholar]
- 11.Ferrand, V. L., Montero Julian, F. A., Chauvet, M. M., Hirn, M. H. & Bourdeaux, M. J. (1996) Cytometry 23, 120–125. [DOI] [PubMed] [Google Scholar]
- 12.Noonan, K. E., Beck, C., Holzmayer, T. A., Chin, J. E., Wunder, J. S., Audrulis, I. L., Gazdar, A. F., William, C. L., Griffith, B., Hoff, D. D. V. & Roninson, I. B. (1990) Proc. Natl. Acad. Sci. USA 87, 7160–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freshney, R. I. (1983) Culture of Animal Cells: A Manual of Basic Technique (Liss, New York).
- 14.Dowling-Warriner, C. V. & Trosko, J. E. (2000) Neuroscience 95, 859–868. [DOI] [PubMed] [Google Scholar]
- 15.Wosikowski, K., Schuurhuis, D., Kops, G. J., Saceda, M. & Bates, S. E. (1997) Clin. Cancer Res. 3, 2405–2414. [PubMed] [Google Scholar]
- 16.Santai, C. T., Fritz, F. & Roepe, P. D. (1999) Biochemistry 38, 4227–4234. [DOI] [PubMed] [Google Scholar]
- 17.Mack, M., Kleinschmidt, A., Brühl, H., Klier, C., Nelson, P. J., Cihak, J., Plachý, J., Stangassinger, M., Erfle, V. & Schlöndorff, D. (2000) Nat. Med. 6, 769–775. [DOI] [PubMed] [Google Scholar]
- 18.Carlin, L. M., Eleme, K., McCann, F. E. & Davis, D. M. (2001) J. Exp. Med. 194, 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, J. F., Yang, Y., Sepulveda, H., Shi, W., Hwang, I., Peterson, P. A., Jackson, M. R., Sprent, J & Cai, Z. (1999) Science 286, 952–954. [DOI] [PubMed] [Google Scholar]
- 20.Zitvogel, L., Regnault, A., Lozier, A., Wolfers, J., Flament, C., Tenza, D., Ricciardi-Castagnoli, P., Raposo, G. & Amigorena, S. (1998) Nat. Med. 4, 594–600. [DOI] [PubMed] [Google Scholar]
- 21.Wolfers, J., Lozier, A., Raposo, G., Regnault, A., Théry, C., Masurier, C., Flament, C., Pouzieux, S., Faure, F., Tursz, T., et al. (2001) Nat. Med. 7, 297–303. [DOI] [PubMed] [Google Scholar]
- 22.Kerbel, R. S., Lagarde, A. E., Dennis, J. W. & Donaghue, T. P. (1983) Mol. Cell. Biol. 3, 523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heppner, G. H. & Miller, F. R. (1998) Int. Rev. Cytol. 177, 1–56. [DOI] [PubMed] [Google Scholar]
- 24.Heenan, M., O'Driscoll, L., Cleary, I., Connolly, L. & Clynes, M. (1997) Int. J. Cancer 7 1, 907–915. [DOI] [PubMed] [Google Scholar]
- 25.Knaust, E., Porwit-MacDonald, A., Gruber, A., Xu, D. & Peterson, C. (2000) Haematologica 85, 124–132. [PubMed] [Google Scholar]
- 26.Petrylak, D. P., Scher, H. I., Reuter, V., O'Brien, J. P. & Cordon-Cardo, C. (1994) Ann. Oncol. 5, 835–840. [DOI] [PubMed] [Google Scholar]