Abstract
Psoriasis vulgaris, a skin disease that is considered to be the result of a type 1 autoimmune response, provides an opportunity for studying the changes that occur in a target-diseased tissue during innovative immunotherapies. To gain a more comprehensive picture of the response to an approved biological therapy, we studied alfacept, which is a CD2 binding fusion protein. We examined T cells, dendritic cells (DCs), and expression of a number of inflammatory genes. In 22 patients, 55% demonstrated a clear histological remission of the disease, with a 73% reduction in lesional lymphocytes and a 79% decrease in infiltrating CD8+ cells. Only histological responders showed marked reductions in the tissue expression of inflammatory genes IFN-γ, signal transducer and activator of transcription 1, monokine induced by IFN-γ, inducible NO synthase, IL-8, and IL-23 subunits. Parallel decreases in CD83+ and CD11c+ DCs also were measured by immunohistochemistry. Because we observed that alefacept binds primarily to T cells and not DCs, we suggest that T cells are the primary target for therapy, but that DCs and a spectrum of type 1 inflammatory genes are coordinately suppressed.
Keywords: amevive, autoimmune disease, CD2
Psoriasis vulgaris is an inflammatory skin disease that affects 2–3% of people in the United States (1). Cellular alterations in the skin include marked hyperplasia of the epidermis, altered keratinocyte differentiation, angiogenesis, and marked infiltration of the skin by T lymphocytes, dendritic cells (DCs), and neutrophils. Psoriasis is considered to be an immune-mediated inflammatory or “autoimmune” disease much like Crohn's disease, rheumatoid arthritis, or juvenile-onset diabetes. Evidence includes identification of clonal populations of T cells in diseased skin (2–4) and reversal of psoriasiform epidermal hyperplasia as well as other pathologic alterations by T cell-targeted therapeutics. Also, psoriasis has erupted as a new disease in recipients of bone marrow transplants from psoriasis donors (5). In experimental xenograft models, “normal” skin of psoriasis patients has been converted to psoriasis by injection of superantigen-activated T cells (6), and spontaneous expansion of skin-resident T cells has produced psoriasis (7). T cells found in psoriasis lesions are strongly biased as type 1 T cells, with CD4+/Th1 cells predominant in the dermis of lesions and CD8+/Tc1 cells predominant in the epidermis (8). We have proposed a type 1 inflammatory pathway to explain the pathogenesis and maintenance of psoriasis (9), based on the genomic fingerprint of skin lesions (10–12). Potentially, the type 1 pathway is regulated in diseased skin by increased production of IL-23, which is a product of infiltrating myeloid DCs (13). In this pathway, signal transducer and activator of transcription 1 (STAT1) and >70 other genes regulated by IFN-γ induce downstream genes such as those encoding monokine induced by IFN-γ (MIG), IL-8, and inducible NO synthase (iNOS). These mediators may regulate influx of T cells, neutrophils, and vascular ectasia/local cellular injury, respectively.
Several immune-targeted biologic drugs recently have been approved for psoriasis. These agents include (i) alefacept, a lymphocyte function-associated antigen (LFA)-3Ig fusion protein that binds to CD2, (ii) efalizumab, a humanized LFA-1 antibody, and (iii) etanercept, a TNF receptor–Ig fusion protein that binds soluble TNF. The study of alefacept's mechanism is difficult, as this fusion protein contains the extracellular domain of CD58 (LFA-3), whose expression is limited to humans, whereas a cognate molecule (CD48) is expressed in lower species. Alefacept binds to CD2 through the LFA-3 domain and thus targets CD2-expressing cell populations (14). The major cell types that express CD2 are T lymphocytes and natural killer cells, but a small population of circulating CD14+ DCs are also CD2+ (15, 16). Despite identification of CD2 (E-rosette receptor) as the first stimulatory/costimulatory T cell surface protein (17, 18), surprisingly little is known about its regulatory function. Depending on the way CD2 is ligated, it can deliver a stimulatory or inhibitory signal or be involved in lymphocyte adhesion (19–23).
The soluble LFA-3Ig fusion protein (alefacept) was found to be an antagonist of T cell activation, leading to its original designation as LFA-3TIP (LFA-3 T cell inhibitory protein). Administration of alefacept to psoriasis patients significantly reduces the number of circulating CD4+ memory T cells, with little effect on naïve T cells and other leukocytes (24). A subset of treated patients (20–40%, depending on the outcome measure) shows significant clinical improvement in psoriasis after 12 weeks of alefacept adminstration (25–28), and some of these responding patients attain clearing of psoriasis lesions that can last for many months without further treatment. The basis for heterogeneous clinical responses to alefacept, as well as its ability to modulate pathologic cellular and inflammatory pathways in skin lesions, is largely unknown. Accordingly, we conducted a study to better elucidate how alefacept affects autoimmune inflammation through detailed analysis of its effect on skin-infiltrating leukocytes and disease-related type 1 inflammatory genes in responding and nonresponding psoriasis patients. We found that 55% of treated patients attain remission of disease activity in skin lesions based on pathological analysis. Responding patients show marked reductions in disease-associated T cell subsets, expression of proinflammatory genes, and CD11c+/CD83+ DC subsets, whereas nonresponders fail to show equivalent reductions. The impact of this agent on organized T cells and DC infiltrates may account for relatively long periods of remission attained in some patients. We have defined the molecular events and changes in both T cells and DCs during alefacept therapy, a T cell-targeted, immunosuppressive biologic agent. Alefacept is capable of reducing both DCs, T cells, and their inflammatory products, but the driving force for the agents' action seems to be the T cell, to which this LFA-3Ig fusion protein selectively binds.
Materials and Methods
Study Design. Twenty-two patients with moderate to severe psoriasis (19 males, 3 females, ages 29–68 years, median 49 years) were enrolled in this study, which was approved by The Rockefeller University Hospital Institutional Review Board. The study was powered to produce groups of at least six patients that could be designated as good versus poor responders (as defined below) to alefacept (Biogen). Major inclusion criteria were: involvement of psoriasis vulgaris of >10% body surface area, no systemic treatment for at least 4 weeks before entering the study, no significant infections or immunosuppression, and no significant renal, hepatic, or other medical disease. Patients were treated with 12 7.5-mg weekly i.v. doses of alefacept, and the effect of this fusion protein on lesion-infiltrating T cells was studied by using a combination of immunohistochemistry and RT-PCR analyses. Two patients withdrew because of nonresponse and did not have final biopsies.
Skin Samples. Skin punch biopsies were obtained at baseline [nonlesional (NL) and lesional] and 2, 6, and 13 weeks (lesional). These samples were cut in half: one piece was frozen in OCT compound (Sakura Finetechnical, Tokyo) and stored at –80°C for immunohistochemistry, and the remaining section was frozen directly in liquid nitrogen for RNA extraction and gene analysis. Histologic response (remission) of psoriatic lesions was defined by normalization of keratin 16 (K16) expression [negative in normal epidermis (29)], reduced epidermal hyperplasia, restoration of a granular layer, and orthokeratosis in week-13 biopsies. Other cases showed a range of outcomes that varied from some reduction in epidermal hyperplasia (but sustained K16 expression) to no improvement in hyperplasia. Eight patients were categorized as nonresponders based on histologic analysis, but we consider that 10 patients were nonresponders based on intent-to-treat.
Immunohistochemistry. Tissue sections were stained with hematoxylin (Fisher) and eosin (Shandon, Pittsburgh) and purified mouse anti-human mAbs to K16 (Sigma), CD3 and CD83 (Becton Dickinson), CD8 and CD11c (BD Pharmingen), and CD103 (BioDesign, Kennebunk, ME). Biotin-labeled horse anti-mouse antibody (Vector Laboratories) was amplified with avidin–biotin complex (Vector Laboratories) and developed with chromogen 3-amino-9-ethylcarbazole (Sigma Aldrich). Epidermal thickness measures were computed by using National Institutes of Health software (nih image 6.1), and positive cells were counted manually by using computer-assisted image analysis.
Peripheral Blood Samples. Peripheral blood draws were taken at baseline and week 13. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized samples by using standard Ficoll-Hypaque (Pharmacia Biotech) density gradient sedimentation. PBMCs were frozen in 10% DMSO (American Type Culture Collection) in RPMI medium 1640 (GIBCO/BRL) with 1 mM Hepes buffer (Sigma Aldrich), 0.1% gentamicin (GIBCO/BRL), and 5% normal human serum (C-Six Diagnostics, Germantown, WI), and stored at –80°C until required.
Peripheral Blood Mononuclear Cell (PBMC) Phenotype. PBMCs were stained for 15 min at room temperature with the following antibodies: CD45RO (phycoerythrin), CD4 (peridinin-chlorophyll-protein), and CD3 (allophycocyanin) (Becton Dickinson). Appropriate IgG isotype controls were also used (Becton Dickinson). Cells were washed with FACS wash [0.1% sodium azide (Sigma Aldrich), 2% FCS (GIBCO/BRL) in PBS] and resuspended in 1.3% formaldehyde (Fisher Scientific) in FACS wash. Samples were analyzed within 24 h with four-color staining by using a FACSCalibur Flow Cytometer and cellquest software after calibration with CaliBRITE beads and facscomp sotware (all Becton Dickinson).
Analysis of Tissue mRNA Gene Expression. RNA was extracted from tissues frozen in liquid nitrogen by using the RNeasy Mini Kit (Qiagen, Valencia, CA). The primers and probes for TaqMan RT-PCR assays for K16, IFN-γ, STAT1, MIG, iNOS, IL-8, IL-12/IL-23p40, and IL-23p19 were generated by using the primer express algorithm, version 1.0, using published genetic sequences (National Center for Biotechnology Information/PubMed) for each gene (see Supporting Text, which is published as supporting information on the PNAS web site). All primers and probes were purchased from Applied Biosystems. The RT-PCR was performed by using EZ PCR Core Reagents (Applied Biosystems) according to the manufacturer's directions. The samples were amplified and quantified on an Applied Biosystems PRISM 7700 by using the following thermal cycler conditions: 2 min at 50°C; 30 min at 60°C; 5 min at 95°C; and 40 cycles of 15 sec at 95°C followed by 60 sec at 60°C. The human acidic ribosomal protein gene, a housekeeping gene, was used to normalize each sample and each gene. The data were analyzed, and samples were quantitated by the software provided with the Applied Biosystems PRISM 7700 (sequence detection systems, version 1.7). For gene analysis, there were 12 responders and five nonresponders: mRNA levels could not be measured in three patients because of unsatisfactory mRNA integrity, and two patients did not have final biopsies.
Statistical Analysis. Significance was accepted as P < 0.05. U statistics (U-score) were developed to rank patient responses by combining percent change in epidermal thickness and the presence or absence of K16 immunostaining into a response score (30). Changes in inflammatory cells or genes, either singly or in combinations of up to four at a time, were ranked by a similar U-score stratification method. Correlations between the response score and lesional cell counts or gene expression were then determined, and the r score is indicated for the given comparisons.
Results
Response of Psoriasis to Alefacept Administration. The patients demonstrated good clinical responses to alefacept, with 10/22 patients achieving an improvement of Psoriasis Area and Severity Index (PASI) >70%, with a mean overall reduction in PASI of 50%. As detailed further below, 12/22 patients were judged to have disease remission by histologic criteria after alefacept administration. In fact, patients often continued to improve after completing a course of therapy. Ten histological responders who were followed up for 12 weeks after treatment had a prolonged remission, with an average of 74% reduction in PASI at week 23.
To gauge the effect of alefacept on disease activity and T cell populations in psoriatic skin lesions, biopsies of an index skin lesion were taken at baseline and after 2, 6, or 13 weeks of treatment. Cryostat sections were analyzed for routine histopathology, K16 expression, and numbers of CD3+, CD8+, and CD103+ (epithelial homing) lymphocytes (Fig. 1A). Histologic remission of psoriatic lesions is a more objective and less blunt measure of response than assessment of PASI. Remission was defined as reduced epidermal hyperplasia, restoration of a granular layer and orthokeratosis, and normalization of K16 expression in week-13 biopsies. Twelve of 22 patients who met these criteria of disease reversal at week 13 are termed responders. The other cases, termed nonresponders, showed a range of outcomes that varied from some reduction in epidermal hyperplasia (but sustained K16 expression) to no improvement in hyperplasia.
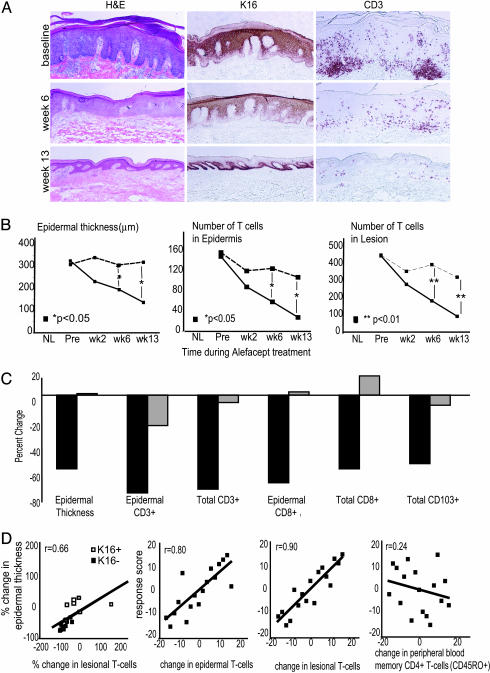
Fig. 1.
Routine histology and immunohistochemical analysis of skin biopsies before and during treatment with alefacept. (A) Example of one patient (a responder) showing routine hematoxylin/eosin stain with normalization of epidermal thickness and K16 staining with treatment, and corresponding reductions in CD3+ cells in the epidermis and dermis. (Magnifications: ×10.) (B) Mean values of epidermal thickness (μm), epidermal T cell (CD3+) number, and total (epidermal and dermal) T cell (CD3+) number per low-power field during treatment, with patients classified by response (see text) (solid line, responders; dashed line, nonresponders). *, P < 0.05; **, P < 0.01. (C) Percent decrease at week 13 compared with baseline in responders (black bars) and nonresponders (gray bars) for epidermal thickness, epidermal CD3+, total CD3+, epidermal CD8+, total CD8+, and total CD103+ cells. (D) Correlation plots with linear regression comparing changes in epidermal thickness and lesional T cells; response score and change in epidermal CD3+, lesional CD3+, and peripheral blood memory (CD45RO+) CD4+ T cells.
Therapeutic Improvement Parallels T Cell Depletion in Psoriatic Skin Lesions. There was progressive epidermal thinning in responding patients during the treatment period, and this result was paralleled by progressive decreases in epidermal and total T cells (Fig. 1 A and B). In contrast, reductions in epidermal thickness were much smaller in nonresponders, and accompanying reductions in infiltrating T cells were also not as marked. Responding patients had consistent decreases in epidermal CD3+ (–77%), total CD3+ (–74%), epidermal CD8+ (–70%), total CD8+ (–60%), and total CD103+ (–55%) lymphocyte populations at week 13, whereas nonresponders showed slight changes (Fig. 1C).
Given that alefacept induced outcomes from little effect to complete disease resolution, we sought to examine the relationship between altered T cell infiltrates and histological response across the range of outcomes. Fig. 1D illustrates two approaches taken for this analysis. First, we related change in epidermal thickness to the change in number of T cells in biopsies (r = 0.66; Fig. 1D Left). K16 negative cases, or cases of disease resolution, were associated with the greatest change in lesional T cells and low T cell infiltrates. Second, we used a multivariate statistical tool that allows us to stratify response outcomes of this nonparametric and related data (30). A response score, which ranked patients' outcomes based on a combination of percent change in epidermal thickness and K16 expression, was correlated with lesional and circulating cell counts (Fig. 1D). In Fig. 1D Center, it can be appreciated that the response outcome is highly correlated with a change in T cells infiltrating the epidermis (r = 0.80) or a combination of epidermis and dermis (r = 0.90). In contrast, the response score was poorly correlated with changes in the number of memory CD4+ T cells in the peripheral circulation (r = 0.24, Fig. 1D Right) or with altered CD3+ T cells in the circulation (r = 0.21, data not shown). Hence, reductions in lesion-infiltrating T cells were more closely correlated with the ultimate therapeutic outcome than changes in circulating lymphocytes or memory CD4+ T cells.
Alefacept Modulates Proinflammatory Genes in Psoriasis. To better understand relationships between expression of proinflammatory (type 1) genes in skin lesions, disease activity, and the therapeutic mechanism of alefacept, we quantified expression of mRNA for a series of disease-related genes by using real-time RT-PCR (Fig. 2A). Genes chosen for analysis are all elements of the type 1 pathway proposed for the pathogenesis of psoriasis (10–12). As expected from prior work, we detected elevated expression of mRNA for K16 and several different inflammatory gene products in baseline psoriasis lesions compared with uninvolved skin (NL) from the same patients. Expression of mRNA for each of these gene products in posttreatment (week 13) lesions is shown for patients stratified as responders versus nonresponders by histological assessment (Fig. 2 A). Statistical analysis is for comparison within response group at baseline versus week 13. Consistent with histologic assessment, there was a marked reduction in K16 mRNA in responders (mean 87% reduction, P < 0.01), but much less reduction in K16 mRNA in the nonresponders. We measured a reduction in mRNA encoding IL-23 subunit p40 [shared with IL-12 (31)] and p19 in responding patients (P = 0.02 and 0.20, respectively). Furthermore, only responders had consistent reductions in mRNAs encoding IFN-γ (P = 0.12) and a series of downstream genes regulated through this cytokine, STAT1 (P = 0.18), iNOS (P = 0.003), and IL-19 (P = 0.19, data not shown) and the chemokines MIG (P = 0.02) and IL-8 (P = 0.01).
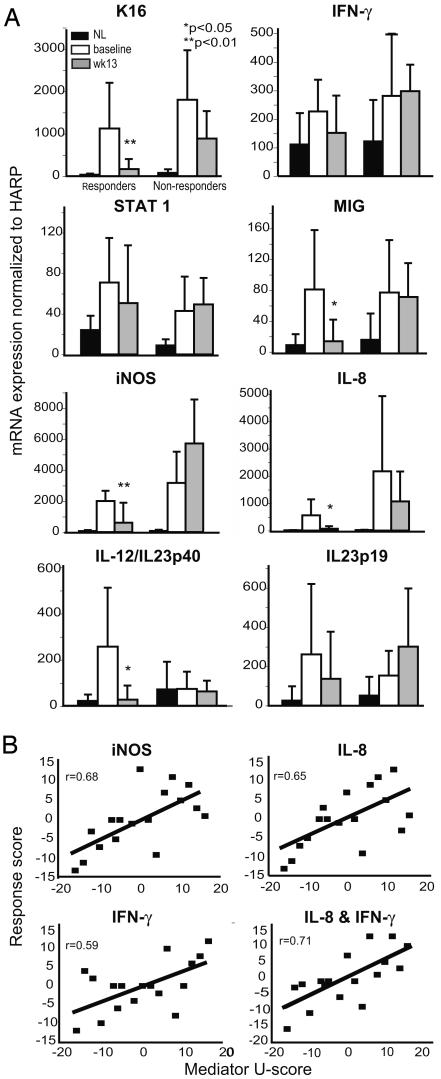
Fig. 2.
Mean tissue gene expression in NL and lesional skin before and after treatment with alefacept. (A) RT-PCR quantitation of K16, IFN-γ, STAT1, MIG, iNOS, IL-8, IL-12/IL-23p40, and IL-23p19 mRNA expression in skin biopsies in NL compared with lesional skin before (baseline) and after (week 13) treatment with alefacept in responders (n = 12) and nonresponders (n = 5). Ratio of gene-to-human acidic ribosomal protein (HARP) ×1,000 (standard deviation shown). *, P < 0.05; **, P < 0.01. (B) Correlation plots of response score against change in iNOS, IL-8, IFN-γ, and a composite score of IL-8 and IFN-γ.
To characterize how altered expression of individual inflammatory mediators relates to psoriasis disease activity, mRNA levels of these products were compared (one to four genes ranked as a U-score) with the response score at the end of treatment (week 13) (Fig. 2B). Expression of several genes, IFN-γ, iNOS, IL-8, MIG, IL-19, and IL-12/IL-23p40, correlated well with the response score (r values range from 0.56 to 0.68). Conversely, expression of STAT1 and IL-23p19 were less correlated with the response score (r = 0.19 and 0.48, respectively). The best correlations were iNOS (r = 0.68) and the combined expression of IFN-γ and IL-8 mRNAs (r = 0.71).
DCs Are Decreased in Psoriasis Lesions by Alefacept Treatment. In psoriasis there are increased subpopulations of epidermal and dermal DCs, predominantly immature DCs (CD11c+ CD14–), but also some mature DCs (CD11c+, CD83+) (unpublished work). We have determined that in psoriasis lesions, IL-23 (13) and iNOS (unpublished work) are synthesized mainly by CD11c+ myeloid DCs. As mRNAs for these products were strongly suppressed in responding patients, we performed immunohistochemcial staining for DCs on baseline and week-13 biopsies. In this analysis, CD11c was used to mark myeloid DCs and CD83 was used to mark activated/mature DCs (Fig. 3A). Compared with uninvolved skin, CD11c+ and CD83+ DCs are greatly increased in untreated psoriasis lesions (Fig. 3B). In responding patients (K16 negative), CD83+ cells decreased by a mean of 87% (P < 10–3) and CD11c+ cells decreased by a mean of 68% (P < 10–3) (Fig. 3B). Nonresponders had very little change in DC populations. Correlations of disease improvement (the response score) was highly correlated with altered numbers of CD11c+ cells (r = 0.71) or combined changes in CD11c+ and CD83+ DCs (r = 0.82), as shown in Fig. 3C. The decrease in DCs mirrored the decrease in T cells (Figs. 1 and 3). Overall, there was a high correlation between T cell and CD11c+ cell number at baseline (r = 0.728) and week 13 (r = 0.713) and percent change in T cells versus percent change in CD11c+ cells (r = 0.538).
Fig. 3.
Marked reduction in CD83+ and CD11c+ cells after treatment with alefacept. (A) Histomicrographs of CD11c and CD83 staining in psoriasis patients at baseline and week 13 of alefacept treatment. (Magnifications: ×10.) (B) Quantitation of CD11c (Left) and CD83 (Right) and positive cells in NL skin and psoriasis lesions before (baseline) and during treatment per low-power field is shown. Alefacept treatment in responders and nonresponders is shown. NL, black bars; baseline, white bars; wk13, gray bars. (C) Correlation plots of response score versus change in total CD11c+ cells (Left) and a composite score of change in epidermal CD83 and total CD11c (Right).
Alefacept Binds Predominantly to Lesional T Cells. In theory, alefacept could bind directly to leukocytes in psoriasis lesions expressing either CD2 or Fc receptors. CD2 has been detected on DCs (15), as well as T cells (14). The data shown in Fig. 4, which is published as supporting information on the PNAS web site, establish that alefacept, at a physiologically relevant concentration, binds mainly to T cells through CD2, with little or no direct binding to lesional DCs (which express high levels of FcγRII or CD32). This finding suggests that T cells are the direct cellular target of alefacept in diseased skin tissue.
Discussion
The study of end-organ inflammation in human T cell-mediated diseases, e.g., rheumatoid arthritis, inflammatory bowel disease, diabetes, or multiple sclerosis, is often severely limited by direct access to the affected tissue during disease flares or therapeutic modulation. Psoriasis vulgaris shares many immune features with these other diseases, but affected skin tissue is readily accessible for histopathologic and molecular analysis during pharmacologic manipulation of disease activity. Biologic agents provide a means to selectively antagonize one part of a complex inflammatory cascade in humans and thus help us to better understand cellular and molecular factors that produce pathogenic inflammation in psoriasis and related diseases. We have studied a disease-remitting antipsoriasis therapy examining lesional histology, DC and T cell phenotyping, and mRNA analyses. Genes chosen for study all are in the proposed type 1 pathway, based on increased expression in psoriasis (9–12).
Patients responding to alefacept show consistent reductions in the number of T cells infiltrating diseased skin (particularly CD8+ and CD103+ subsets infiltrating the epidermis), reductions in CD11c+ and CD83+ DCs, and marked reductions in several key inflammatory molecules such as IL-23 and iNOS that are linked to these cell types. In addition, the chemokines MIG and IL-8, which are most likely synthesized by other cell types, e.g., keratinocytes and neutrophils, are strongly suppressed by alefacept treatment in responding patients. Thus overall, response to alefacept is slow but durable (24) and involves both DC and T cells, and there is a loss of organized dermal infiltrates of DCs and T cells. The durability of response to alefacept treatment should be emphasized; our histological responders maintained a significant mean reduction in PASI. This reduction may be caused by depletion of activated clones of disease causing memory T cells in tissues. Alternatively, there may be a relative increase in regulatory (CD4+ CD25+) T cells via CD2 stimulation (32).
There are several possibilities to explain psoriasis pathogenesis and successful response to treatment. We note that end products of the type 1 pathway (iNOS, IL-8, MIG) were strongly and consistently suppressed in responding patients (P < 0.05 for all genes). The proposed upstream inducers of these genes, IFN-γ and STAT1 (9), were also decreased in responding patients, but the magnitude of suppression was lower. Hence, smaller changes in these genes may control larger reductions in downstream genes in a biologically meaningful fashion. Partial attenuation of the type 1 pathway by alefacept would also explain the lower magnitude of reduction of IFN-γ and STAT1. Alternatively, there may be a second distinct pathway involving DCs, IL-23, and iNOS (13) (also strongly suppressed in responders). This system may not be exclusive of the type 1 pathway, and in fact these two mechanisms may be related. Cytokine products of activated, type 1 T cells, i.e., IFN-γ and TNF, induce expression of end or “effector” inflammatory products (iNOS, TNF-α); IL-23 is an important T cell-activating cytokine.
Our study provides only a partial explanation of the basis for response versus nonresponse of psoriasis in different patients to alefacept (24). Nonresponse is related to a lesser ability of alefacept to effect T cell and DC reductions (and related expression of inflammatory genes) in a subset of patients. We were unable to identify any significant differences in CD2 expression on T cells or the ability of labeled alefacept to bind to T cells across the range of patients studied (data not shown). We did note, however, that some nonresponders had quantitatively more T cells in skin lesions (because of the very large surface area affected) and that intermediate degrees of improvement were seen with partial T cell reductions in these patients (data not shown). Hence, use of larger doses of alefacept or treatment for longer periods might convert some nonresponders to responders, but more work is required to fully understand the basis of the response dichotomy to alefacept.
Overall, there is a related decrease in both T cells and CD11c+ cells. The specific mechanism of action of alefacept and primary consequence of blockade of CD2 remain to be determined. Alefacept binds to lesional T cells, mainly via CD2 (Fig. 4). Furthermore, this binding interaction is proportional to the level of expression of CD2. Activated/memory T cells (CD8 > CD4) express the highest levels of CD2 and thus bind the highest levels of alefacept (14, 33). Binding of alefacept to CD2 on T cells has the potential to decrease cell activation to other stimuli (19) or induce cell lysis via bridging with natural killer cells or other cell types expressing Fc receptors (34, 35). The results of this study are consistent with a T cell-depleting mechanism, because T cells were eliminated mostly from skin lesions of responding patients, and parallel increases of T cells in the circulation were not measured (data not shown). A relative increase in regulatory T cells may occur at the tissue level, as disease-causing effector T cells are removed. The mechanism by which DCs are diminished in psoriasis lesions is also not certain. Some circulating myeloid DCs or precursors have been found to express CD2 (at relatively low levels) (15, 16), so it is also possible that alefacept could have some direct effect on these cells in the circulation. The effect on DCs could also be mediated indirectly through T cell depletion, as both activation-induced cytokines (IFN-γ and TNF), chemokines, and activation-induced surface molecules, e.g., CD40L, stimulate the activation/maturation of myeloid-derived DCs (36, 37).
Supplementary Material
Acknowledgments
This research was supported in part by General Clinical Research Center Grant M01-RR00102 from the National Center for Research Resources at the National Institutes of Health. J.G.K. is supported by National Institutes of Health Grants R01 AI-49572 and AI-49832. Partial support for this study came from an unrestricted grant from Biogen.
Author contributions: M.A.L., T.K., and J.G.K. designed research; F.C., E.L., T.K., P.G., M.S.-W., I.C., A.K., I.N., and J.G.K. performed research; F.C., M.A.L., S.-L.L., E.L., T.K., M.S.-W., K.M.W., and J.G.K. analyzed data; E.L. and K.M.W. contributed new reagents/analytic tools; and F.C., M.A.L., S.-L.L., and J.G.K. wrote the paper.
Abbreviations: LFA, lymphocyte function-associated antigen; STAT1, signal transducer and activator of transcription 1; iNOS, inducible NO synthase; MIG, monokine induced by IFN-γ; K16, keratin 16; DC, dendritic cell; PASI, Psoriasis Area and Severity Index; NL, nonlesional.
References
- 1.Krueger, J. G. (2002) J. Am. Acad. Dermatol. 46, 1–23. [DOI] [PubMed] [Google Scholar]
- 2.Chang, J. C., Smith, L. R., Froning, K. J., Schwabe, B. J., Laxer, J. A., Caralli, L. L., Kurland, H. H., Karasek, M. A., Wilkinson, D. I., Carlo, D. J. & Brestoff, S. W. (1994) Proc. Natl. Acad. Sci. USA 91, 9282–9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prinz, J. C., Grob, B., Vollmer, S., Trommler, P., Strobel, I., Meurer, M. & Plewig, G. (1994) Eur. J. Immunol. 24, 593–598. [DOI] [PubMed] [Google Scholar]
- 4.Lin, W.-J., Norris, D. A., Achziger, M., Kotzin, B. L. & Tomkinson, B. (2001) J. Invest. Dermatol. 117, 1546–1553. [DOI] [PubMed] [Google Scholar]
- 5.Snowden, J. A. & Heaton, D. C. (1997) Br. J. Dermatol. 137, 130–132. [PubMed] [Google Scholar]
- 6.Wrone-Smith, T. & Nickoloff, B. J. (1996) J. Clin. Invest. 98, 1878–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyman, O., Hefti, H. P., Conrad, C., Nickoloff, B. J., Suter, M. & Nestle, F. O. (2004) J. Exp. Med. 199, 731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Austin, L. M., Ozawa, M., Kikuchi, T., Walters, I. B. & Krueger, J. G. (1999) J. Invest. Dermatol. 113, 752–759. [DOI] [PubMed] [Google Scholar]
- 9.Lew, W., Bowcock, A. M. & Krueger, J. G. (2004) Trends Immunol. 25, 295–305. [DOI] [PubMed] [Google Scholar]
- 10.Zhou, X., Krueger, J. G., Kao, M. C., Lee, E., Du, F., Menter, A., Wong, W. H. & Bowcock, A. M. (2003) Physiol. Genomics 13, 69–78. [DOI] [PubMed] [Google Scholar]
- 11.Oestreicher, J. L., Walters, I. B., Kikuchi, T., Gilleaudeau, P., Surette, J., Schwertschlag, U., Dorner, A. J., Krueger, J. G. & Trepicchio, W. L. (2001) Pharmacogenomics J. 1, 272–287. [DOI] [PubMed] [Google Scholar]
- 12.Bowcock, A. M., Shannon, W., Du, F., Duncan, J., Cao, K., Aftergut, K., Catier, J., Fernandez-Vina, M. A. & Menter, A. (2001) Hum. Mol. Genet. 10, 1793–1805. [DOI] [PubMed] [Google Scholar]
- 13.Lee, E., Trepicchio, W. L., Oestreicher, J. L., Pittman, D., Wang, F., Chamian, F., Dhodapkar, M. & Krueger, J. G. (2004) J. Exp. Med. 199, 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dustin, M. L. & Springer, T. A. (1991) Annu. Rev. Immunol. 9, 27–66. [DOI] [PubMed] [Google Scholar]
- 15.Crawford, K., Gabuzda, D., Pantazopoulos, V., Xu, J., Clement, C., Reinherz, E. & Alper, C. A. (1999) J. Immunol. 163, 5920–5928. [PubMed] [Google Scholar]
- 16.Di Pucchio, T., Lapenta, C., Santini, S. M., Logozzi, M., Parlato, S. & Belardelli, F. (2003) Eur. J. Immunol. 33, 358–367. [DOI] [PubMed] [Google Scholar]
- 17.Suthanthiran, M. (1987) Transplant. Proc. 19, 336–337. [PubMed] [Google Scholar]
- 18.Selvaraj, P., Plunkett, M. L., Dustin, M., Sanders, M. E., Shaw, S. & Springer, T. A. (1987) Nature 326, 400–403. [DOI] [PubMed] [Google Scholar]
- 19.Miller, G. T., Hochman, P. S., Meier, W., Tizard, R., Bixler, S. A., Rosa, M. D. & Wallner, B. P. (1993) J. Exp. Med. 178, 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lattine, D., De La Parra, B., Nizet, Y., Cornet, A., Giovino-Barry, V., Monroy, R., White-Schart, M. E. & Bazin, H. (1995) Int. Immunol. 8, 1113–1119. [DOI] [PubMed] [Google Scholar]
- 21.Dumont, C., Deas, O., Mollereau, B., Hebib, C., Giovino-Barry, V., Bernard, A., Hirsch, F., Charpentier, B. & Senik, A. (1998) J. Immunol. 160, 3797–3804. [PubMed] [Google Scholar]
- 22.Bacchmann, M. F. (1999) J. Immunol. 190, 1383–1391. [Google Scholar]
- 23.van Kooyk, Y., van de Weil-van Kemenade, P., Weder, P., Kuijers, T. W. & Figdor, C. G. (1989) Nature 342, 811–812. [DOI] [PubMed] [Google Scholar]
- 24.Gordon, K. B., Vaishnaw, A. K., O'Gorman, J., Haney, J. & Menter, A. (2003) Arch. Dermatol. 139, 1563–1570. [DOI] [PubMed] [Google Scholar]
- 25.Ellis, C. N. & Krueger, G. G. (2001) N. Engl. J. Med. 345, 248–255. [DOI] [PubMed] [Google Scholar]
- 26.Krueger, G. G., Papp, K. A., Stough, D. B., Loven, K. H., Gulliver, W. P. & Ellis, C. N. (2002) J. Am. Acad. Dermatol. 47, 821–833. [DOI] [PubMed] [Google Scholar]
- 27.Lebwohl, M. G., Christophers, E., Langley, R., Ortonne, J. P., Roberts, J. & Griffiths, C. E. M. (2003) Arch. Dermatol. 139, 719–727. [DOI] [PubMed] [Google Scholar]
- 28.Goedkoop, A. Y., de Rie, M. A., Picavet, D. I., Kraan, M. C., Dinant, H. J., van Kuijk, A. W., Tak, P. P., Bos, J. D. & Teunissen, M. B. (2004) Arch. Dermatol. Res. 295, 465–473. [DOI] [PubMed] [Google Scholar]
- 29.Leigh, I. M., Navsaria, H., Purkis, P. E., McKay, I. A., Bowden, P. E. & Riddle, P. N. (1995) Br. J. Dermatol. 133, 501–511. [DOI] [PubMed] [Google Scholar]
- 30.Wittkowski, K. M., Lee, E., Nussbaum, R., Chamian, F. N. & Krueger, J. G. (2004) Stat. Med. 23, 1579–1792. [DOI] [PubMed] [Google Scholar]
- 31.Kopp, T., Lenz, P., Bello-Fernandez, C., Kastelein, R. A., Kupper, T. S. & Stingl, G. (2003) J. Immunol. 170, 5438–5444. [DOI] [PubMed] [Google Scholar]
- 32.Wakkach, A., Cottrez, F. & Groux, H. (2001) J. Immunol. 167, 3107–3113. [DOI] [PubMed] [Google Scholar]
- 33.Sanders, M. E., Makgoba, M. W., Sharrow, S. O., Stephany, D., Springer, T. A., Young, H. A. & Shaw, S. (1988) J. Immunol. 140, 1401–1407. [PubMed] [Google Scholar]
- 34.Majeau, G. R., Meier, W., Jimmo, B., Kioussis, D. & Hochman, P. S. (1994) J. Immunol. 152, 2753–2767. [PubMed] [Google Scholar]
- 35.da Silva, A. J., Brickelmaier, M., Majeau, G. R., Li, Z., Su, L., Hsu, Y.-M. & Hochman, P. S. (2002) J. Immunol. 168, 4462–4471. [DOI] [PubMed] [Google Scholar]
- 36.Vieira, P. L., de Jong, E. C., Wierenga, E. A., Kapsenberg, M. L. & Kalinski, P. (2000) J. Immunol. 164, 4507–4512. [DOI] [PubMed] [Google Scholar]
- 37.Koya, R. C., Kasahara, N., Favaro, P. M., Lau, R., Ta, H. Q., Weber, J. S. & Stripecke, R. (2003) J. Immunother. 26, 451–460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.