Abstract
Rationale: Vascular endothelial growth factor receptor (VEGFR) inhibition increases ceramides in lung structural cells of the alveolus, initiating apoptosis and alveolar destruction morphologically resembling emphysema. The effects of increased endogenous ceramides could be offset by sphingosine 1-phosphate (S1P), a prosurvival by-product of ceramide metabolism.
Objectives: The aims of our work were to investigate the sphingosine–S1P–S1P receptor axis in the VEGFR inhibition model of emphysema and to determine whether stimulation of S1P signaling is sufficient to functionally antagonize alveolar space enlargement.
Methods: Concurrent to VEGFR blockade in mice, S1P signaling augmentation was achieved via treatment with the S1P precursor sphingosine, S1P agonist FTY720, or S1P receptor-1 (S1PR1) agonist SEW2871. Outcomes included sphingosine kinase-1 RNA expression and activity, sphingolipid measurements by combined liquid chromatography–tandem mass spectrometry, immunoblotting for prosurvival signaling pathways, caspase-3 activity and terminal deoxynucleotidyltransferase–mediated dUTP nick end labeling assays, and airspace morphometry.
Measurements and Main Results: Consistent with previously reported de novo activation of ceramide synthesis, VEGFR inhibition triggered increases in lung ceramides, dihydroceramides, and dihydrosphingosine, but did not alter sphingosine kinase activity or S1P levels. Administration of sphingosine decreased the ceramide-to-S1P ratio in the lung and inhibited alveolar space enlargement, along with activation of prosurvival signaling pathways and decreased lung parenchyma cell apoptosis. Sphingosine significantly opposed ceramide-induced apoptosis in cultured lung endothelial cells, but not epithelial cells. FTY720 or SEW2871 recapitulated the protective effects of sphingosine on airspace enlargement concomitant with attenuation of VEGFR inhibitor–induced lung apoptosis.
Conclusions: Strategies aimed at augmenting the S1P–S1PR1 signaling may be effective in ameliorating the apoptotic mechanisms of emphysema development.
Keywords: ceramide, apoptosis, vascular endothelial growth factor, endothelial cells, chronic obstructive pulmonary disease
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Vascular endothelial growth factor (VEGF) receptor blockade leads to alveolar cell apoptosis and emphysema-like morphology in rats and mice. Ceramide up-regulation is a critical step in the alveolar destruction in this model of emphysema. We investigated whether strategies to activate the antiapoptotic signaling of sphingosine 1-phosphate (S1P), a prosurvival metabolite of ceramide, are sufficient to prevent airspace enlargement induced by VEGF receptor blockade.
What This Study Adds to the Field
This is the first study of S1P signaling in an emphysema model and reports that augmentation of an antiapoptotic signaling pathway that targets lung endothelial cells is sufficient to prevent airspace enlargement in an experimental emphysema model.
Emphysema is defined by destruction of alveolar walls causing abnormal permanent enlargement of the distal airspaces. There is currently no effective treatment to halt or to repair the alveolar wall enlargement in emphysema (1). Critical pathogenic processes and attractive therapeutic targets in emphysema include protease–antiprotease imbalance, oxidative stress, and apoptosis (2). Excessive apoptosis of lung microvascular endothelial cells may contribute to the paucity of lung tissue in emphysema (3). However, an indiscriminate apoptosis inhibitory approach in this disease may be detrimental to the homeostatic removal of other cell types. We asked whether enhancing primarily the prosurvival signaling of endothelial cells in a model of apoptosis-dependent emphysema is sufficient to prevent the development of airspace enlargement in mice.
Excessive alveolar structural cell apoptosis (i.e., of alveolar endothelial and epithelial cells) has been directly linked to alveolar–septal destruction and emphysema in various experimental animal models (2, 4–6), including the vascular endothelial growth factor receptor (VEGFR) blockade model of emphysema. In both mice and rats, VEGFR blockade causes endothelial cell apoptosis and oxidative stress in the absence of inflammation (attributed to inhibition of VEGFR on inflammatory cells), ultimately leading to emphysema (2, 5, 6). Ceramides, bioactive sphingolipid molecules that initiate signaling leading to apoptosis, senescence, and growth arrest (7), are up-regulated and necessary for the development of VEGFR blockade–induced airspace enlargement (8). However, excessive ceramide inhibition may deplete downstream prosurvival metabolites such as sphingosine 1-phosphate (S1P), causing apoptosis (8), because ceramide is catabolized to sphingosine, which in turn is phosphorylated by sphingosine kinase (SphK)-1 and SphK-2 to S1P (9–11).
S1P, primarily via S1P receptor-1 (S1PR1) expressed on endothelial cells, activates signal pathways involved primarily in cell survival, migration, and differentiation. It plays a key role in angiogenesis and maintenance of endothelial function (12, 13), ameliorating vascular inflammation in acute lung injury (14, 15). S1P is linked to suppression of apoptosis in several cell lines (16, 17) and organs such as ovary, where a proper balance between S1P and ceramide plays a key role in tissue homeostasis (16–18). The role of S1P in the homeostasis of lung parenchyma structures has not been elucidated.
The VEGFR inhibition model of emphysema develops in an apoptosis-dependent manner (6) with minimal inflammation, while preserving other crucial elements (apoptosis, oxidative stress, and alveolar septal and capillary destruction) that are involved in the pathogenesis of human emphysema (5, 6, 19, 20). Because S1P has been implicated in various immunologic responses, the absence of prominent inflammation in the VEGFR blockade model allows for a reductionistic investigation of the S1P effects on alveolar cell survival. Our data show that stimulation of the S1P–S1PR1 signaling axis was sufficient to counteract the deleterious effects of lung ceramide on airspace enlargement. Some of the results of these studies have been previously reported in the form of an abstract (21).
METHODS
Animal Studies
Animal studies were approved by the Institutional Animal Care and Use Committee at Indiana University (Indianapolis, IN). The murine models of emphysema have been described previously (5, 8). We administered the specific VEGFR2 inhibitor SU5416 (20 mg/kg, subcutaneous; Calbiochem, Gibbstown, NJ) once, or its vehicle 0.5% carboxymethylcellulose (CMC; Sigma, St. Louis, MO) to male C57BL/6 mice (n = 5 per group; 3 mo old, 25 g; Jackson Laboratory, Bar Harbor, ME). d-erythro-Sphingosine (20 μg, intraperitoneal; Sigma), or FTY720 (0.1 mg/kg, intraperitoneal; Cayman Chemical, Ann Arbor, MI) (8, 14) was given daily for 5 days starting on the day of SU5416 administration, using ethanol (0.1%; intraperitoneal) as vehicle control. SEW2871 (20 mg/kg; Calbiochem) (22, 23) was similarly administered by gavage. Mice were harvested 4 weeks after SU5416 administration. Additional details are available in the online supplement and in Figure E1 (in the online supplement).
Sphingolipid Measurements
Ceramide, dihydroceramide, and S1P and dihydro-S1P were measured as described (24–26) and detailed in the online supplement.
RNA Extraction
RNA was extracted from the lungs of C57BL/6 mice injected with SU5416 and CMC controls, and from mice injected with the VEGFR-specific antibodies DC101 and MF1 (Imclone, New York, NY) (800 μg/mouse per injection, administered intraperitoneally, three times per week) and their controls (dialyzed rat IgG). This experiment has been described previously (27). Total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA), and the PureLink Micro-to-Midi total RNA purification (Invitrogen), as described in the online supplement.
Sphingosine Kinase-1 Quantitative Polymerase Chain Reaction
Exon-spanning primers specific for SphK1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed on the basis of published cDNA sequences: SphK-1, forward 5′-TGGGGCTATGACTTGGAAAG-3′, reverse 5′-CCAGGGAAGGTCCCTAAGAG-3′; for GAPDH, forward 5′-GCATTTGCTGATTGCACAGT-3′, reverse 5′-ACTGCCGCACTTTGTTCTCT-3′. Real-time polymerase chain reaction (PCR) was performed with the 7500 real-time PCR system (Applied Biosystems, Foster City, CA), as described in the online supplement.
Determination of Sphingosine Kinase-1 Enzymatic Activity
Lungs from DC101- and MF1- or SU5416-treated mice and their respective controls were homogenized via microultrasonic cell disrupter in assay buffer (9), followed by centrifugation at 100,000 × g for 20 minutes (28). SphK1 activity was measured in the supernatant, using d-sphingosine–coated phospholipid FlashPlates (PerkinElmer Life Sciences, Waltham, MA), as described in detail in the online supplement.
Cell Culture Experiments
Primary rat lung endothelial cells, kindly provided by T. Stevens (University of South Alabama, Mobile, AL) and rat lung epithelial cells (L2; American Type Culture Collection, Manassas, VA) were maintained in culture at 37°C in 5% CO2 and 95% air, up to passages 20 and 7, respectively. Cells were grown in Dulbecco's modified Eagle's high-glucose and Ham's F12 media, respectively, supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. Treatments with ceramide (polyethylene glycol--linked ceramide 16:0; Avanti Lipids, Alabaster, AL) or vehicle were performed under low-serum (2% fetal bovine serum) conditions.
Morphometric Analysis
Morphometric analysis of lung parenchyma with measurement of the mean linear intercept was performed as described (6).
Apoptosis
Caspase-3 activity in lung homogenates was measured using a fluorometric assay (Apo-ONE; Promega, Fitchburg, WI) as described (29).
Western Blotting
Protein were extracted from lung homogenates and Western blotting was performed with antibodies from Cell Signaling (Beverly, MA) unless otherwise noted: Akt (diluted 1:1,000), phospho-Akt (diluted 1:1,000), extracellular signal–regulated kinase-1/2 (ERK1/2, diluted 1:2,000), phospho-ERK1/2 (diluted 1:1,000), p38 (diluted 1:1,000), phospho-p38 (diluted 1:1,000), c-Jun N-terminal kinase (JNK, diluted 1:1,000), phospho-JNK (diluted 1:1,000), or vinculin (diluted 1:5,000; Calbiochem), as described in detail in the online supplement.
Immunohistochemistry
Terminal deoxynucleotidyltransferase–mediated dUTP nick end labeling (TUNEL) was performed with a terminal deoxynucleotidyltransferase apoptosis detection kit (Calbiochem). For colocalization studies, the TUNEL assay was performed in parallel with fluorescence immunohistochemistry for CD31 marker for endothelial cells as previously described (8). Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Microscopy was performed with a Nikon Eclipse (TE200S) inverted fluorescence system. Images of the parenchyma were captured in a blinded fashion and the total number of TUNEL-positive nuclei per high-power field (n = 3–6 fields; each field averaging 250 DAPI-stained nuclei) was recorded. A negative control for staining was performed to establish the threshold for nonspecific fluorescence.
Statistical Analysis
Statistical analysis was performed with SigmaStat (Systat Software Inc, Chicago, IL), using unpaired Student t test, analysis of variance, or Kruskal-Wallis one-way analysis of variance on ranks for morphometry analysis. A statistically significant difference was accepted at P < 0.05.
RESULTS
Effect of VEGFR Blockade on S1P Production and S1P Receptor Expression
We have previously demonstrated that ceramides are up-regulated in the lungs of mice and rats after VEGFR blockade, primarily because of increased synthesis via the de novo pathway during the initial 7 days after the administration of SU5416 (20 mg/kg) (8). These increases are concomitant with augmented alveolar cell apoptosis, both preceding the increase in airspace size, which peaks on Day 28 (8). Because S1P is a downstream metabolite of ceramide with opposing biological effects, we investigated whether S1P levels increased in parallel with increases in ceramides or whether there was any effect of VEGFR blockade on SphK1, the major regulator of S1P synthesis. Using tandem mass spectrometry, we measured the levels of S1P and dihydro-S1P at the time of ceramide elevation in the lungs of VEGFR-inhibited mice. On Day 7 after SU5416 administration, there was no significant increase in S1P levels in the lungs of VEGFR inhibitor–treated animals compared with vehicle-treated mice (Figure 1A). In turn, there was an increase in lung levels of dihydro-S1P, together with the expected elevations in ceramides and dihydroceramides in the VEGFR-inhibited mice (Figures 1B–1D). This pattern is consistent with increased de novo ceramide synthesis (demonstrated in Reference 8), which accounts for increased substrate availability for dihydro-S1P synthesis, combined with a lack of stimulation of SphK1 activity. To document the effect of VEGFR inhibition on SphK1, we measured SphK1 mRNA expression in mouse lungs by real-time PCR after either pharmacological or immunological (30) inhibition of VEGFR. Neither pharmacological inhibition of VEGFR with SU5416 (20 mg/kg), nor administration of VEGFR-specific antibodies DC101 and MF1 (800 μg/mouse three times weekly), altered lung SphK1 mRNA expression compared with their respective controls (relative expression of 1.9 ± 0.3 vs. 1.85 ± 0.2 in the CMC vehicle controls; and 1.84 ± 0.3 vs. 1.76 ± 0.1 in the IgG-injected controls, respectively). Similarly, when compared with control levels, lung SphK1 activity was not affected by either approach of VEGFR blockade with SU5416 (Figure 1E) or anti-VEGFR antibodies (data not shown). These results are consistent with the finding of an increased ceramide-to-S1P ratio 7 days after receiving VEGFR inhibitor (Figure 1F).
Figure 1.
(A–F) Ceramide and sphingosine 1-phosphate (S1P) expression in response to vascular endothelial growth factor receptor (VEGFR) inhibition. Sphingolipid expression (mean + SEM; n = 3–6) in the lung after administration of vehicle (Veh; carboxymethylcellulose, 0.5%) or the VEGFR inhibitor SU5416 (VEGFR-inh, 20 mg/kg; Day 7): (A) S1P levels, normalized by lipid inorganic phosphorus (Pi); (B) dihydro-S1P levels, P = 0.03; (C) ceramide levels, P = 0.001; (D) dihydro-ceramide lung levels, P = 0.001; (E) [33P]SPHK-1 activity in the lung; (F) ratio of ceramide to S1P in the lung, P = 0.02.
S1P Augmentation via Sphingosine Attenuates the Development of VEGFR Blockade–induced Emphysema
Because a homeostatic balance between ceramide and S1P may be essential to the maintenance of alveolar structures, and we documented an imbalance, that is, increased ceramide-to-S1P ratio, we reasoned that augmenting S1P signaling may be sufficient to attenuate the deleterious effects induced by ceramide in the lung.
To address our hypothesis that S1P supplementation will inhibit VEGFR inhibitor–induced lung injury, mice were administered SU5416 (20 mg/kg) followed by S1P augmentation during the first week of the 4 weeks of VEGFR inhibition (Figure E1), coinciding with the kinetics of ceramide-to-S1P ratio elevation. S1P augmentation was achieved by several approaches. Because S1P has a short half-life in the circulation (minutes), direct delivery of S1P is generally impractical. Indeed, after S1P administration (100 pmol; 40 μl via tail vein; or 0.5% ethanol vehicle; three times weekly), there was no significant elevation of S1P levels in the lung at 7 days (data not shown), whereas daily administrations of S1P (10 pmol or 50 pmol; 20 μl; or 0.1% ethanol vehicle; via retro-orbital sinus intravenous injection) for 5 days resulted in erratic S1P levels in the lung, measured 2 hours after the last S1P injection (e.g., whereas the 10-pmol dose elevated lung S1P levels by 80% [1.79-fold], 50 pmol did not). We therefore chose next to stimulate S1P signaling by systemic administration of d-erythro-sphingosine. Sphingosine is a direct precursor of S1P, requiring SphK1 or SphK2 for the conversion to S1P.
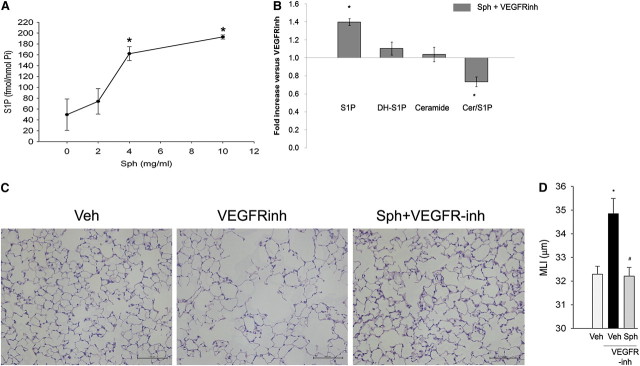
Systemic administration of sphingosine via daily intraperitoneal injection increased S1P levels in the lung in a dose-dependent manner on Day 5 (Figure 2A). In the presence of VEGFR blockade, sphingosine supplementation led to an increase in S1P levels and a decrease in the ceramide-to-S1P ratio, without a change in the ceramide or dihydo-S1P levels in the lung, compared with VEGFR inhibitor–treated lungs alone (Figure 2B). We next measured the effect of sphingosine administration on VEGFR inhibitor–induced alveolar enlargement. Compared with control lungs, the lungs of mice that were subjected to VEGFR inhibition had evidence of airspace enlargement (Figure 2C) measured by higher mean linear intercepts at 28 days (Figure 2D), consistent with the previous reports that defined this model of emphysema (8, 19). In contrast, mice that were subjected to VEGFR inhibition together with S1P supplementation in the form of d-erythro-sphingosine had no changes in mean linear intercepts compared with vehicle-treated control mice (Figures 2C and 2D).
Figure 2.
Effect of d-sphingosine supplementation on vascular endothelial growth factor receptor (VEGFR) inhibitor–induced airspace enlargement. (A) Sphingosine 1-phosphate (S1P) levels in the lung on Day 5 after administration of sphingosine (Sph) at the indicated concentrations (mean ± SEM; *P < 0.05; n = 2 or 3 per time point). (B) Changes in sphingolipids S1P, dihydro-S1P (DH-S1P), ceramide, and the ratio of ceramide to S1P in the lungs of mice receiving sphingosine supplementation after VEGFR inhibitor (VEGFR-inh) administration, compared with those animals that were treated with VEGFR inhibitor with vehicle (mean ± SEM; *P < 0.05; n = 5). (C) Representative micrographs of hematoxylin and eosin–stained sections of inflated and fixed alveolar lung tissue from mice treated with vehicle (Veh; 0.5% carboxymethylcellulose), VEGFR-inh (SU5416, 20 mg/kg, subcutaneous; Day 28), or d-sphingosine (Sph; 20 μg, intraperitoneal, daily on Days 1–5 after SU5416 administration). Scale bars, 100 μm. Note the airspace enlargement in the VEGFR inhibitor–treated lungs, which is reduced by treatment with Sph. (D) Morphometric measurements of the mean linear intercept (MLI), indicating airspace size in mice that were treated with Veh, VEGFR-inh, or Sph and VEGFR-inh (mean + SEM; *P < 0.05 vs. Veh; #P < 0.05 vs. VEGFR-inh; n = 5 per group; analysis of variance).
To evaluate whether mice receiving d-erythro-sphingosine demonstrated a change in the survival signaling pathways that characterize the S1P-mediated effects, we measured the activation of the Akt and mitogen-activated protein kinase (MAPK) signaling pathway. The phosphorylation of Akt, measured by immunoblotting of lung lysates with a phospho-specific antibody and then normalized by total Akt levels, was decreased in the VEGFR inhibitor–treated mice compared with vehicle controls (Figure 3A). In contrast, d-erythro-sphingosine augmentation prevented the decrease in Akt phosphorylation induced by VEGFR blockade. All three major MAPK signaling pathways were down-regulated by VEGFR inhibition in the lung, whereas treatment with d-erythro-sphingosine significantly restored signaling of the ERK and p38 MAPK pathways, with variable effects on JNK1 activity (Figures 3B–3D).
Figure 3.
Effect of vascular endothelial growth factor receptor (VEGFR) inhibition and d-sphingosine supplementation on Akt and mitogen-activated protein kinase (MAPK) signaling pathways in the lung. Protein homogenates were isolated 28 days after administration of the VEGFR inhibitor SU5416 (VEGFR-inh; 20 mg/kg, once), with concomitant d-sphingosine (Sph; 20 μg/injection for 5 d) or its vehicle (Veh) and protein was used for immunoblotting (30 μg) with the respective antibodies, followed by densitometry. Each lane represents an individual animal. (A) Akt activation was measured as the ratio of phosphorylated Akt to total Akt, as detected by immunoblotting (right) (mean + SEM; *P < 0.05 vs. Veh; n = 4 or 5). (B) Extracellular signal–regulated kinase (ERK) (MAPK 42/44) activation was measured as the ratio of phosphorylated ERK to total ERK, as detected by immunoblotting (right) (mean + SEM; *P < 0.05 vs. Veh; #P < 0.05 vs. VEGFR-inh; analysis of variance [ANOVA]; n = 4–8). (C) p38 MAPK activation was measured as the ratio of phosphorylated p38 MAPK to total p38 MAPK, as detected by immunoblotting (right) (mean + SEM; *P < 0.05 vs. Veh; #P < 0.05 vs. VEGFR-inh; ANOVA; n = 4–8). (D) c-Jun N-terminal kinase-1 (JNK1) activation was measured as the ratio of phosphorylated JNK1 to total JNK1 (mean + SEM; *P < 0.05 vs. Veh; ANOVA; n = 3–8).
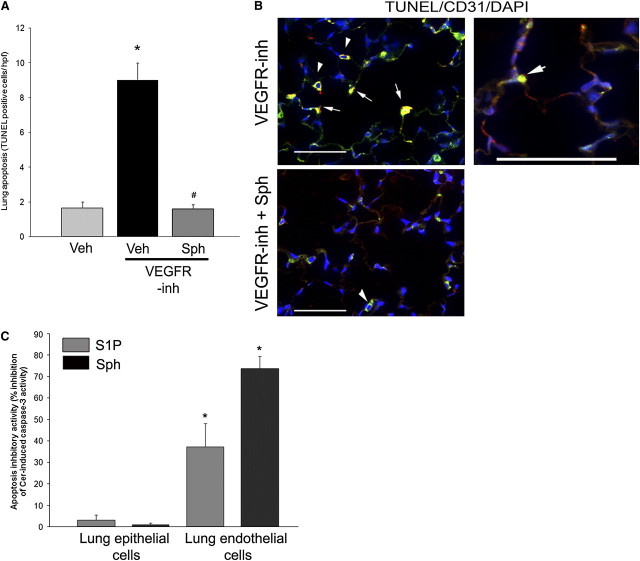
Modulatory effects of d-erythro-sphingosine treatment on the survival signaling pathways were associated, as expected, with an antiapoptotic effect, opposing VEFGR inhibitor–induced apoptosis in the lung parenchyma, as measured by TUNEL staining of alveolar structures (Figure 4A). To determine which alveolar cells were more likely to be rescued by S1P supplementation, we performed TUNEL staining of lung sections of VEGF-inhibited mice in conjunction with staining for CD31, a marker of endothelial cells, which were the alveolar cells predicted to undergo apoptosis on withdrawal of this trophic factor. Indeed, VEGFR inhibitor treatment was associated with TUNEL-positive nuclei of cells staining for CD31 (Figure 4B). In contrast, treatment with d-erythro-sphingosine markedly reduced the positive TUNEL staining in these cells (Figure 4B). To more specifically investigate the cell-specific antiapoptotic effect of d-erythro-sphingosine on alveolar epithelial and endothelial cells, we studied primary epithelial and endothelial lung cells isolated from rats. Murine epithelial and endothelial cells undergo apoptosis when treated with ceramide (24, 31). Rat epithelial or endothelial cells were simultaneously treated with ceramide (ceramide16:0; 10 mM) and either S1P or d-erythro-sphingosine, followed by quantification of apoptosis by caspase-3 activity of the cell lysates, normalized by protein concentration. S1P or d-erythro-sphingosine had a significantly higher inhibitory potency against ceramide-induced caspase-3 activity in endothelial cells compared with epithelial cells (Figure 4C).
Figure 4.
Antiapoptotic effects of d-sphingosine. (A) Lung apoptosis (number of terminal deoxynucleotidyltransferase–mediated dUTP nick end labeling [TUNEL]-positive cells per high-power lung field [hpf] measured on coded slides) was increased in response to vascular endothelial growth factor receptor inhibitor (VEGFR-inh), but not in mice supplemented with d-sphingosine (Sph) (mean + SEM; *P < 0.05 vs. vehicle [Veh]; #P < 0.05 vs. VEGFR-inh; Student's t test; n = 3–6). (B) Coimmunolocalization of TUNEL-positive apoptotic cells (green) with endothelial cell marker (CD31, red) in lungs treated with VEGFR-inh with or without Sph supplementation. Note TUNEL-positive nuclear staining of CD-31 decorated cells (arrows). Alveolar macrophages exhibited typical green autofluorescence (arrowheads). Scale bars, 50 μm. (C) Inhibitory activity of sphingosine 1-phosphate (S1P, 1 μM) or Sph (1 μM) cotreatments on ceramide-induced caspase-3 activity measured by kinetic fluorimetry (U/min) normalized by protein concentration in lysates of primary rat lung epithelial and endothelial cells. The inhibitory activity was calculated relative to the apoptotic activity induced by ceramide 16:0 (Cer, 10 μM, 24 h) treatment in the absence of S1P or Sph (mean + SEM; *P < 0.05 vs. inhibitory activity of the respective S1P agonist on epithelial cells; n = 3). DAPI = 4′,6-diamidino-2-phenylindole.
Together, these results suggested that sphingosine activated antiapoptotic S1P signaling, which typically occurs through engagement of its receptors.
S1P Augmentation via S1P Receptor Agonists Attenuates the Development of VEGFR Blockade–induced Emphysema
FTY720 is a sphingosine analog that is phosphorylated endogenously by SphK2 (32–34) to generate the active metabolite FTY720 phosphate, which activates S1P receptors 1, 3, 4, and 5 (12). SEW2871 is a selective S1PR1 receptor agonist (12). Treatment with either FTY720 or SEW2871 had a protective effect on VEGFR inhibitor–induced airspace enlargement measured by mean linear intercept (Figure 5A) of alveolar structures stained with hematoxylin and eosin (Figure 5B). We have previously reported that FTY720 (0.1 mg/kg) inhibited VEGFR inhibitor–induced apoptosis on Day 7 after SU5416 administration, which is the peak of apoptosis kinetics in this model (8). Interestingly, in contrast to FTY720, SEW2871-treated animals had a persistent decrease in apoptosis even 28 days after VEGFR inhibitor administration, as measured by caspase-3 activation in mouse lungs (Figure 5C). These antiapoptotic effects of SEW2871, but not FTY720, were associated with an elevation of Akt phosphorylation at this time point, measured by Western blotting and densitometry compared with the lungs of VEGFR inhibitor–treated animals. Similarly, SEW2871, but not FTY720, strongly opposed the inhibitory effect of VEGFR inhibitor on p38 MAPK (Figure 5E, panel ii) and had a modest stimulatory effect on ERK phosphorylation (Figure 5E, panel i) that did not reach statistical significance (P = 0.08). The negative effect of the VEGFR inhibitor on JNK activation was not opposed by the S1P agonists; moreover, FTY720 accentuated this inhibitory effect (Figure 5E, panel iii), possibly reflecting its wider range of effects on multiple cellular targets when compared with SEW2871.
Figure 5.
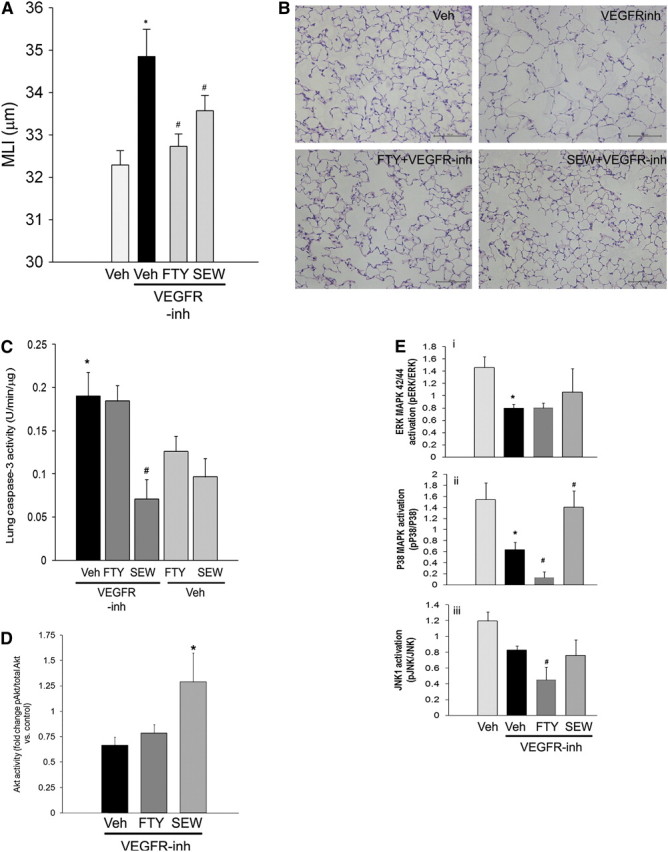
Effects of sphingosine 1-phosphate (S1P) receptor agonists on vascular endothelial growth factor receptor (VEGFR) blockade–induced airspace enlargement, apoptosis, and signaling. After administration of VEGFR inhibitor (VEGFR-inh) SU5416, with or without concomitant S1P receptor agonist FTY720 (0.1 mg/kg/day, daily, intraperitoneal, administered on Days 1–5 after SU5416) or SEW2871 (20 mg/kg, daily by gavage, administered on Days 1–5 after SU5416), airspace enlargement was quantified by mean linear intercept (MLI; Day 28 [A]) (mean + SEM, *P < 0.05 vs. control, #P < 0.05 vs. VEGFR-inh; n = 5). (B) Representative panels of hematoxylin and eosin–stained lung parenchyma. Scale bars, 100 μm. (C) Lung caspase-3 activity measured by kinetic fluorimetry in total lung lysates 28 days after VEGFR inhibitor administration. Note that SEW2871 significantly decreased VEGFR inhibitor–induced lung caspase-3 activity (mean + SEM; *P < 0.05 vs. control, #P < 0.05 vs. VEGFR-inh; n = 3–5). (D) Changes in lung Akt phosphorylation compared with controls measured by densitometry 28 days after VEGFR inhibitor administration (mean fold change vs. carboxymethylcellulose [CMC] control + SEM; #P < 0.05 vs. VEGFR-inh). (E) Mitogen-activated protein kinase (MAPK) activity in tissue protein (30 μg) immunoblotted with the respective antibodies and measured by densitometry of phospho- vs. total extracellular signal–regulated kinase (ERK; panel i), p38 MAPK (panel ii), or c-Jun N-terminal kinase-1 (JNK1) (panel iii).
DISCUSSION
Our studies suggest that augmentation of S1P signaling in the VEGFR blockade model of emphysema results in attenuation of airspace enlargement and apoptosis, through reversal of the ceramide–S1P imbalance.
Previous studies in human promyelocytic cells (17) and female gonadal cells (16), and our studies in mouse lungs (8), have suggested that a balance between proapoptotic ceramide and antiapoptotic S1P is needed for homeostatic cellular survival. The current study built on our previous observation that VEGFR inhibition up-regulated lung ceramide through the de novo ceramide synthesis pathway (8). Whereas inhibition of ceramide synthesis by fumonisin B1 (FB1), a ceramide synthase inhibitor, led to attenuation of emphysema in VEGFR-inhibited lungs (8), unopposed inhibition of ceramide synthesis with FB1 in control lungs increased apoptosis and airspace enlargement (8). These deleterious effects of FB1 were previously reported in other organs, such as liver (35). We reasoned that these effects may be mediated by inhibition of downstream prosurvival sphingolipids (S1P). An excess of ceramide, in turn, amplifies the levels of oxidative stress and matrix proteolytic activity, and plays a central role in lung cell apoptosis and alveolar septal destruction (8). To functionally neutralize the excessive levels of ceramide in the lung, we aimed to up-regulate S1P signaling. S1P levels are regulated by three groups of enzymes: sphingosine kinase-1 and -2, which phosphorylate sphingosine to S1P, being the major enzymes involved in S1P synthesis (9, 28, 36); S1P lyase (37); and sphingosine phosphate phosphatases and lipid phosphate phosphatase-1 (11), which are involved in prompt S1P breakdown. Our studies indicate that VEGFR blockade does not inhibit SphK-1 activity and does not down-regulate S1P levels in the lung. Although additional effects of VEFGR blockade on the other enzymes regulating S1P were not further investigated in this work, the up-regulation of dihydro-S1P was consistent with activation of the de novo pathway of ceramide synthesis without concomitant activation of the S1P synthesis pathway. Dihdro-S1P, which differs from S1P by lacking the 4,5-trans double bond, is known to activate S1P receptors, while lacking the intracellular second-messenger properties of S1P (38). In addition, dihydro-S1P and S1P may have opposite signaling effects (39), including a loss of antiapoptotic effects of dihydro-S1P (38) Moreover, because SphK activity was not affected by the VEGFR inhibitor, we augmented S1P signaling by three approaches: increasing the substrate for SphK1 by administration of d-erythro-sphingosine (S1P precursor); generation of S1P signaling analog FTY720-P (32, 34), which was described as also inhibiting de novo ceramide biosynthesis (26, 40); or engaging S1P signaling in the lung via SEW2871, an S1PR1 agonist. All three approaches attenuated VEGFR blockade–induced airspace enlargement in mouse lungs.
Sphingosine administration significantly decreased the VEGFR inhibitor–induced ceramide-to-S1P ratio in the lung, concomitant with up-regulation of prosurvival Akt activation and inhibition of apoptosis in the lung. The antiapoptotic effect of sphingosine appeared to be affecting predominantly alveolar endothelial cells. Although S1P-independent effects of sphingosine were not ruled out by our experimental design, these results were consistent with augmentation of S1P signaling. Because the effect of S1P is mediated primarily via its receptors, we investigated the effect of specific S1P receptor agonists on VEGFR inhibitor–induced lung injury.
FTY720 is a sphingosine analog that is phosphorylated primarily by sphingosine kinase-2 to form its active metabolite FTY720 phosphate, which in turn activates the S1P receptors 1, 3, 4, and 5. Furthermore, important effects of FTY720 on the lung endothelium are S1P receptor independent (41) and FTY720 is also known to inhibit S1P lyase (42), to directly inhibit ceramide synthases, block ceramide generation, and up-regulate dihydro-S1P de novo biosynthesis (26, 40). Hence, FTY720 has pleiotropic effects on lymphocyte trafficking (32, 33, 43), endothelial vascular permeability (41, 44), heart rate, and myofibroblast differentiation (45), to name a few. These diverse effects may explain the dissimilar effects of FTY720 on major signaling pathway activation compared with sphingosine or SEW2871 and make it difficult to identify the precise mechanism by which FTY720-treated animals were protected from airspace enlargement in our model. For these reasons, we used SEW2871, a partial S1PR1 receptor agonist that leads to S1PR1 receptor internalization and recycling (23, 46). It has been shown to ameliorate ischemia–reperfusion injury in kidneys by decreasing lymphocyte numbers and proinflammatory cytokines, including tumor necrosis factor-α (22). The finding that SEW2871 blocks the development of emphysema after VEGFR inhibition suggests that S1PR1 plays a major role in the maintenance of alveolar septal integrity. S1PR1 is known to mediate the effects of S1P on endothelial cell differentiation, adherens junction formation, and maintenance of endothelial integrity (12), including inhibition of apoptosis (along with S1P3) via the Akt pathway. SEW2871 shared similar effects with sphingosine on signaling pathways, stimulating Akt, p38MAPK, and (modestly) ERK activities in the presence of the VEGFR inhibitor, supporting a shared pathway of lung apoptosis inhibition via S1PR1. Interestingly, S1PR1 is known to be up-regulated by VEGF (47), suggesting that, although we could not document a decrease in S1PR1 protein expression in response to VEGFR blockade, the inhibited VEGF function may have further weakened the effects of the endogenous S1P on S1PR1 signaling. The finding that S1PR1 agonist was sufficient to inhibit apoptosis and airspace enlargement in this model is encouraging, as this target would theoretically eliminate many of the side effects attributed to the other S1P receptors, such as tachycardia or hypotension (22, 23, 46).
Although VEGFR blockade and the cigarette smoking models of emphysema share features such as ceramide up-regulation and alveolar cell apoptosis (8, 48), the presence of an inflammatory response and the irreversible nature of the airspace destruction are uniquely induced by cigarette smoke exposure. Future studies will need to address the effects of cigarette smoking on S1P levels and activity and the effectiveness of S1P supplementation against the onset and progression of emphysema in response to cigarette smoke. The results of the current study suggest S1PR1 receptor agonists may be useful therapeutic agents for ameliorating or arresting the progression of apoptosis in cells comprising the alveolar walls, in particular endothelial cells, which may ultimately protect against airspace enlargement in emphysema.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200906-0826OC on December 3, 2009
Conflict of Interest Statement: K.J.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.J.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.I.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.S.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.C.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.V.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. I.P. received $10,001–$50,000 from Quark Bio in industry-sponsored grants.
References
- 1.National Heart, Lung, and Blood Institute. The definition of emphysema: report of a National Heart, Lung, and Blood Institute, Division of Lung Diseases Workshop. Am Rev Respir Dis 1985;132:182–185. [DOI] [PubMed] [Google Scholar]
- 2.Tuder RM, Petrache I, Elias JA, Voelkel NF, Henson PM. Apoptosis and emphysema: the missing link. Am J Respir Cell Mol Biol 2003;28:551–554. [DOI] [PubMed] [Google Scholar]
- 3.Giordano RJ, Lahdenranta J, Zhen L, Chukwueke U, Petrache I, Langley RR, Fidler IJ, Pasqualini R, Tuder RM, Arap W. Targeted induction of lung endothelial cell apoptosis causes emphysema-like changes in the mouse. J Biol Chem 2008;283:29447–29460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoshiba K, Yokohori N, Nagai A. Alveolar wall apoptosis causes lung destruction and emphysematous changes. Am J Respir Cell Mol Biol 2003;28:555–562. [DOI] [PubMed] [Google Scholar]
- 5.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000;106:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuder RM, Zhen L, Cho CY, Taraseviciene-Stewart L, Kasahara Y, Salvemini D, Voelkel NF, Flores SC. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol 2003;29:88–97. [DOI] [PubMed] [Google Scholar]
- 7.Hannun YA, Obeid LM. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem 2002;277:25847–25850. [DOI] [PubMed] [Google Scholar]
- 8.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 2005;11:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukuda Y, Kihara A, Igarashi Y. Distribution of sphingosine kinase activity in mouse tissues: contribution of sphk1. Biochem Biophys Res Commun 2003;309:155–160. [DOI] [PubMed] [Google Scholar]
- 10.Bandhuvula P, Fyrst H, Saba JD. A rapid fluorescence assay for sphingosine-1-phosphate lyase enzyme activity. J Lipid Res 2007;48:2769–2778. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y, Kalari SK, Usatyuk PV, Gorshkova I, He D, Watkins T, Brindley DN, Sun C, Bittman R, Garcia JG, et al. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J Biol Chem 2007;282:14165–14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takuwa Y, Takuwa N, Sugimoto N. The Edg family G protein–coupled receptors for lysophospholipids: their signaling properties and biological activities. J Biochem 2002;131:767–771. [DOI] [PubMed] [Google Scholar]
- 13.Skoura A, Hla T. Lysophospholipid receptors in vertebrate development, physiology, and pathology. J Lipid Res 2009;50:S293–S298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 2004;169:1245–1251. [DOI] [PubMed] [Google Scholar]
- 15.McVerry BJ, Peng X, Hassoun PM, Sammani S, Simon BA, Garcia JG. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med 2004;170:987–993. [DOI] [PubMed] [Google Scholar]
- 16.Morita Y, Tilly JL. Sphingolipid regulation of female gonadal cell apoptosis. Ann N Y Acad Sci 2000;905:209–220. [DOI] [PubMed] [Google Scholar]
- 17.Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 1996;381:800–803. [DOI] [PubMed] [Google Scholar]
- 18.Payne SG, Milstien S, Spiegel S. Sphingosine-1-phosphate: dual messenger functions. FEBS Lett 2002;531:54–57. [DOI] [PubMed] [Google Scholar]
- 19.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med 2001;163:737–744. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro SD. Vascular atrophy and VEGFR-2 signaling: old theories of pulmonary emphysema meet new data. J Clin Invest 2000;106:1309–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diab KJGY, Adamowicz JJ, Kamocki K, Berdyshev E, Petrache I. S1P augmentation, an alveolar cell pro-survival therapeutic strategy in experimental emphysema [abstract]. Am J Respir Crit Care Med 2008;177:A241. [Google Scholar]
- 22.Lien YH, Yong KC, Cho C, Igarashi S, Lai LW. S1P1-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int 2006;69:1601–1608. [DOI] [PubMed] [Google Scholar]
- 23.Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem 2004;279:13839–13848. [DOI] [PubMed] [Google Scholar]
- 24.Medler TR, Petrusca DN, Lee PJ, Hubbard WC, Berdyshev EV, Skirball J, Kamocki K, Schuchman E, Tuder RM, Petrache I. Apoptotic sphingolipid signaling by ceramides in lung endothelial cells. Am J Respir Cell Mol Biol 2008;38:639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berdyshev EV, Gorshkova IA, Garcia JG, Natarajan V, Hubbard WC. Quantitative analysis of sphingoid base-1-phosphates as bisacetylated derivatives by liquid chromatography–tandem mass spectrometry. Anal Biochem 2005;339:129–136. [DOI] [PubMed] [Google Scholar]
- 26.Berdyshev EV, Gorshkova I, Skobeleva A, Bittman R, Lu X, Dudek SM, Mirzapoiazova T, Garcia JG, Natarajan V. FTY720 inhibits ceramide synthases and up-regulates dihydrosphingosine 1-phosphate formation in human lung endothelial cells. J Biol Chem 2009;284:5467–5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrache I, Fijalkowska I, Zhen L, Medler TR, Brown E, Cruz P, Choe KH, Taraseviciene-Stewart L, Scerbavicius R, Shapiro L, et al. A novel antiapoptotic role for α1-antitrypsin in the prevention of pulmonary emphysema. Am J Respir Crit Care Med 2006;173:1222–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin ZQ, Goetzl EJ, Karliner JS. Sphingosine kinase activation mediates ischemic preconditioning in murine heart. Circulation 2004;110:1980–1989. [DOI] [PubMed] [Google Scholar]
- 29.Niles AL, Moravec RA, Riss TL. Caspase activity assays. Methods Mol Biol 2008;414:137–150. [DOI] [PubMed] [Google Scholar]
- 30.Petrache I, Fijalkowska I, Zhen L, Medler TR, Brown E, Cruz P, Choe KH, Taraseviciene-Stewart L, Scerbavicius R, Shapiro L, et al. A novel anti-apoptotic role for α-1 antitrypsin in the prevention of pulmonary emphysema. Am J Respir Crit Care Med 2006;173:1222–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teichgraber V, Ulrich M, Endlich N, Riethmuller J, Wilker B, De Oliveira-Munding CC, van Heeckeren AM, Barr ML, von Kurthy G, Schmid KW, et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med 2008;14:382–391. [DOI] [PubMed] [Google Scholar]
- 32.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 2002;277:21453–21457. [DOI] [PubMed] [Google Scholar]
- 33.Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein–coupled receptors. FASEB J 2004;18:551–553. [DOI] [PubMed] [Google Scholar]
- 34.Meno-Tetang GM, Li H, Mis S, Pyszczynski N, Heining P, Lowe P, Jusko WJ. Physiologically based pharmacokinetic modeling of FTY720 (2-amino-2[2-(4-octylphenyl)ethyl]propane-1,3-diol hydrochloride) in rats after oral and intravenous doses. Drug Metab Dispos 2006;34:1480–1487. [DOI] [PubMed] [Google Scholar]
- 35.He Q, Suzuki H, Sharma N, Sharma RP. Ceramide synthase inhibition by fumonisin B1 treatment activates sphingolipid-metabolizing systems in mouse liver. Toxicol Sci 2006;94:388–397. [DOI] [PubMed] [Google Scholar]
- 36.Thompson CR, Iyer SS, Melrose N, VanOosten R, Johnson K, Pitson SM, Obeid LM, Kusner DJ. Sphingosine kinase 1 (SK1) is recruited to nascent phagosomes in human macrophages: Inhibition of SK1 translocation by Mycobacterium tuberculosis. J Immunol 2005;174:3551–3561. [DOI] [PubMed] [Google Scholar]
- 37.Kariya Y, Kihara A, Ikeda M, Kikuchi F, Nakamura S, Hashimoto S, Choi CH, Lee YM, Igarashi Y. Products by the sphingosine kinase/sphingosine 1-phosphate (S1P) lyase pathway but not S1P stimulate mitogenesis. Genes Cells 2005;10:605–615. [DOI] [PubMed] [Google Scholar]
- 38.Spiegel S, Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem 2002;277:25851–25854. [DOI] [PubMed] [Google Scholar]
- 39.Bu S, Kapanadze B, Hsu T, Trojanowska M. Opposite effects of dihydrosphingosine 1-phosphate and sphingosine 1-phosphate on transforming growth factor-β/Smad signaling are mediated through the PTEN/PPM1A-dependent pathway. J Biol Chem 2008;283:19593–19602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lahiri S, Park H, Laviad EL, Lu X, Bittman R, Futerman AH. Ceramide synthesis is modulated by the sphingosine analog FTY720 via a mixture of uncompetitive and non-competitive inhibition in an acyl CoA chain length-dependent manner. J Biol Chem 2009;284:16090–16098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dudek SM, Camp SM, Chiang ET, Singleton PA, Usatyuk PV, Zhao Y, Natarajan V, Garcia JG. Pulmonary endothelial cell barrier enhancement by FTY720 does not require the S1P1 receptor. Cell Signal 2007;19:1754–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bandhuvula P, Tam YY, Oskouian B, Saba JD. The immune modulator FTY720 inhibits sphingosine-1-phosphate lyase activity. J Biol Chem 2005;280:33697–33700. [DOI] [PubMed] [Google Scholar]
- 43.Idzko M, Hammad H, van Nimwegen M, Kool M, Muller T, Soullie T, Willart MA, Hijdra D, Hoogsteden HC, Lambrecht BN. Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J Clin Invest 2006;116:2935–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu HB, Cui NQ, Wang Q, Li DH, Xue XP. Sphingosine-1-phosphate and its analogue FTY720 diminish acute pulmonary injury in rats with acute necrotizing pancreatitis. Pancreas 2008;36:e10–e15. [DOI] [PubMed] [Google Scholar]
- 45.Keller CD, Rivera Gil P, Tolle M, van der Giet M, Chun J, Radeke HH, Schafer-Korting M, Kleuser B. Immunomodulator FTY720 induces myofibroblast differentiation via the lysophospholipid receptor S1P3 and Smad3 signaling. Am J Pathol 2007;170:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jo E, Sanna MG, Gonzalez-Cabrera PJ, Thangada S, Tigyi G, Osborne DA, Hla T, Parrill AL, Rosen H. S1P1-selective in vivo–active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem Biol 2005;12:703–715. [DOI] [PubMed] [Google Scholar]
- 47.Igarashi J, Erwin PA, Dantas AP, Chen H, Michel T. VEGF induces S1P1 receptors in endothelial cells: implications for cross-talk between sphingolipid and growth factor receptors. Proc Natl Acad Sci USA 2003;100:10664–10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petrache I, Medler TR, Richter AT, Kamocki K, Chukwueke U, Zhen L, Gu Y, Adamowicz J, Schweitzer KS, Hubbard WC, et al. Superoxide dismutase protects against apoptosis and alveolar enlargement induced by ceramide. Am J Physiol Lung Cell Mol Physiol 2008;295:L44–L53. [DOI] [PMC free article] [PubMed] [Google Scholar]