Abstract
Background:
The main objective of the present systematic review is to identify potential risk factors for adverse drug reactions (ADRs) through prospective cohort studies in pediatric inpatients.
Methods:
The data search was done in the following electronic databases PubMed/MEDLINE; Scopus; LILACS and Web of Science from the earliest record until 31 May 2015. Two reviewers independently screened each study and one of them assessed the methodological quality according to the Newcastle–Ottawa scale for cohort studies. The data extraction was conducted according to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) initiative for cohort studies.
Results:
The only risk factor observed in all studies was the increase in the number of prescription drugs. However, other factors were identified, such as the increase in the length of stay or the number of low- or high-risk drugs prescribed, use of general anesthesia and oncological diagnosis. The cumulative incidence of ADR was 16.4% (95% confidence interval: 15.6 to 17.2). The main professional responsible for ADR identification was the pharmacist and the dominant category among the ADRs were gastrointestinal disorders. In addition, analgesics, antibacterial agents and corticosteroids were the drug classes commonly associated with ADRs. The methodology used in this study was tried to homogenize the data extracted; however, this was not sufficient to correct the discrepancies so it was not possible to perform a meta-analysis.
Conclusions:
The increase in the number of prescription drugs was the main risk factor in this population. However, additional studies are required to identify the risk factors for ADRs in pediatric inpatients.
Keywords: child, cohort studies, drug-related side effects and adverse reactions, hospitals, risk factors
Introduction
Adverse drug reactions (ADRs) are a public health problem, especially for pediatric inpatients. Therefore, the subject has become a study object in research and prevention programs worldwide.1–4 The World Health Organization (WHO) defines ADR as ‘a response to a drug that is noxious and unintended, and which occurs at doses normally used in man’.5 Thus, immune reactions, toxic effects, and abstinence symptoms are classic examples of ADRs.
When compared with adults, children respond differently to drugs. This is due to the immaturity of the immune system and the developmental pharmacology.6,7 In addition, the off-label use of drugs and the communication barriers between children and adults both potentiate the ADR risk in pediatric inpatients.8,9
Studies involving children and ADRs have been the subject of discussion in the scientific community. However, the different methodologies and the absence of systematic reviews or meta-analyses limit the understanding of risk factors for ADRs in pediatric inpatients.2 In addition, there are no systematic reviews assessing the quality of studies by means of validated instruments.
Thus, the identification of risk factors for ADRs can contribute to the clinical practice of health providers and programs of ADR prevention in children.10 This systematic review aimed to identify the risk factors for ADRs in pediatric inpatients in cohort studies.
Method
This systematic review of prospective cohort studies was conducted between June and December 2015, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA; Supplemental Table 9) guidelines.11 During the search, we used a review protocol, not available for access, written according to the PRISMA protocol guidelines.12
Eligibility criteria
Eligibility criteria are outlined in Table 1. Prospective cohort studies were included, with assessment of the risk factors for ADRs in children during hospital stay.
Table 1.
Eligibility criteria.
| Included studies | Criteria | Reasons |
|---|---|---|
| Population | Children aged under 18 admitted to the hospital | Absence of studies in this population |
| Exposure | Administration of any drug via any routes | n/a |
| Outcomes | Clinical event described as an adverse drug reaction or nonpreventable adverse drug event, occurring during hospitalization | n/a |
| Study design | Prospective cohort | Observational studies with higher level of evidence |
| Other criteria | Studies in English, Spanish and Portuguese, with an open access summary | n/a |
| Excluded studies | Criteria | Reasons |
| Studies that included outpatients | Outpatients have different characteristics to inpatients. The inclusion of these studies could influence the identification of risk factors for ADR in the hospital setting, implying biases. Among these characteristics, we can cite the lower number of prescribed drugs, the use of little or no injectable drug, the predominant use of low-risk drugs and the use of drugs that were previously prescribed and evaluated during hospitalization | |
| Studies related to a specific: (a) Drug (e.g. ifosfamide, carboplatin, ketamine, methotrexate); (b) Drug class (e.g. opiates); (c) Clinical condition (e.g. cancer patients, infants); (d) Adverse drug reaction |
Studies like these could cause ADR incidence variation and would not be representative of the hospitalized pediatric population |
ADR, Adverse Drug reaction; n/a, not applicable.
Search strategy
The electronic databases used were PubMed/MEDLINE; Scopus; LILACS and Web of Science. The main keywords used for the search were: ‘Child’, concerning the population; ‘Hospitals’, study setting; ‘Risk Factors’, exposure; ‘Drug-Related Side Effects and Adverse Reactions’, outcome; ‘Cohort Studies’, study type. To expand the search, we incorporated not only the indexed terms, but also its synonyms and subcategories (Supplemental Information Table 6).
Study selection
The study titles were grouped by databases in Microsoft Excel spreadsheets (Microsoft Corporation, Redmond, Washington). Two reviewers independently screened the titles, abstracts, and full-text reports to confirm for eligibility. Three categories were used in selecting the title and abstract: ‘yes’, ‘no’ and ‘maybe’. In the case of doubts (‘maybe’ category), the study was selected for evaluation of the full text, at this stage, disagreements were resolved by consensus between the two reviewers. One author (PHSA) screened the bibliographies of the included studies and reassessed the study selection for recovering articles missed in the original search (Supplemental Information Figure 2).
Data collection
The collected data was classified according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) initiative for cohort studies, also in Microsoft Excel. The STROBE compliance level was analyzed by one of the reviewers (PHSA) based on the report by Vandenbroucke et al.13 The suspected drug classes were categorized according to the Anatomical Therapeutic Chemical, and the ADRs according to Adverse Reaction Terminology, both from the WHO.
Quality evaluation of included studies
To decrease the risk of methodological biases, one of the reviewers (PHSA) estimated the quality of manuscripts according to the Newcastle–Ottawa scale for cohort studies14 and included in the quantitative summary only the studies over six stars.
Statistical analysis
Calculation of incidence of adverse drug reactions
Some of the included studies did not present the calculation of incidence of ADR and its confidence interval of 95%. Therefore, the ADR incidence and its confidence interval of 95% of these studies were calculated in the present systematic review. In the numerator, we used the patient number with ADRs and in the denominator, we used the patient number in the studies.
Adapted Forest graphic
The incidences were illustrated in Microsoft Excel by a graph similar to Forest. Instead of the odds ratio, we plotted the incidence measures in order to facilitate the visualization of these estimates.
Results
The characteristics of the included studies were outlined in Supplemental Information, Table 7 and 8.
Qualitative summary
Included studies
Seven studies were performed between 1999 and 2014 and included in the qualitative analysis.15–21 Among these publications, two included the ADRs between the drug-related problems (DRPs), defined by them as ‘an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes’.17,21 In addition, another five studies used the same concept of this study,15,16,18–20 although one of them used another conceptual aspect: nonpreventable adverse drug events.20 While the compliance level to STROBE, studies reached an average of 74.01% (range: 51.5–87.8%) (Supplemental Information Table 8).
Assessment of the methodological quality of the included studies
Only one study had its quality below six stars, thus was excluded from the quantitative summary15 (Supplemental Information Table 8). In relation to the selected item, one of the studies had no representative population of the pediatric community, since it was conducted in only one ward of a single public hospital and did not include intensive care unit (ICU) patients.16 At the same time, only one study did not describe if the data collection was realized by medical records, physical examination or structured interview.15 Furthermore, five studies did not describe the absence of the outcome before the study started.15–17,19,21 In relation to the outcomes, three publications did not describe the losses during follow up or did not report the inclusion and monitoring of the population.15,17,21 In addition, one of them used the spontaneous reporting with subsequent assessment of medical records to event confirmation.15
Study design
Three studies were multicenter and developed from the collaboration between the United Kingdom, Australia, Germany, China, Malaysia and Saudi Arabia, and were realized in 4 months (Supplemental Information Table 7 and 8).17,18,21 The other four studies were developed in 3–12 months and were performed in a single research center in Brazil16,20 or the United Kingdom.15,19 Five studies were conducted under the guidance of researchers from the United Kingdom.15,17–19,21 In addition, the only research dedicated exclusively to the ICU was the Brazilian study.20
Persons involved in adverse drug reaction identification
Five studies reported that the pharmacist was the professional responsible for ADR identification.16–19,21 Two studies included pediatricians18,19 and one included one nurse.19 Moreover, one study included a pediatrician as a second evaluator in the preventability assessment21 and another included a pediatrician in the ADR reassessment.15 Finally, one study did not describe the professional responsible.20
Methods for adverse drug reaction identification
Only one study used spontaneous reporting as an ADR identification method and did not use the medical record as a method.15 In general, the method used by the researchers included the assessment of (i) medical and nursing record; (ii) prescription and (iii) laboratory tests. In addition, one study included a structured interview method16 and another included an active search method based on triggers.20
Quantitative summary
In all results presenting this analysis, we excluded the study of Turner and collaborators15 for not having the expected quality level. Studies with incomplete data were excluded in each analysis.
Adverse drug reactions incidence
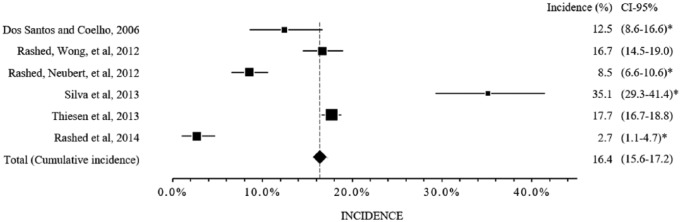
The ADR incidence varied from 2.7% to 35.1% (median 15.5%; interquartile interval 1–3, 9.5–17.4%]. If the included studies represented a single multicenter study, the cumulative incidence would be 16.4% (1281/7803, confidence interval 95%, 15.6 to 17.2) (Figure 1, Supplemental Information Table 8). It is also noteworthy that despite the attempt to homogenize the values, some work characteristics did not allow homogenization. The study site was one of the characteristics observed, for example. Only one study was conducted exclusively in the ICU.20 In addition, one study included only ‘defined’ and ‘probable’ ADRs, according to the algorithm by Naranjo,19 and five studies included not only the ‘defined’ and ‘probable’ ADRs, but also the ‘possible’ ADRs.16–18,20,21
Figure 1.
Incidences of the adverse drug reactions.
CI, confidence interval; *the confidence interval of 95% was calculated in the present systematic review from the data presented in the studies.
Related adverse drug reactions
All studies that used the same ADR concept of this review described the collected ADRs.16,18–20 However, one of them did not inform all the involved ADRs, so was not included in the analysis.16 Also, the two studies that used the concept of DRPs were not included.17,21 Based on the foregoing, the highlighted disorders were: (i) gastrointestinal; (ii) skin and appendages; (iii) metabolic and nutritional (Table 2).
Table 2.
Adverse drug reactions, according to the Adverse Reaction Terminology–World Health Organization (WHO–ART).
| System, organ | WHO–ART | Thiesen et al.19 |
Rashed et al.18 |
Silva et al.20 |
Total |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| n 1 | n1/1457* (%) | n 2 | n2/328 (%) | n 3 | n3/110 (%) | n | n/1895 (%) | CI, 95% | ||
| Gastrointestinal disorders | 0600 | 659 | 45.2 | 118 | 36.0 | 2 | 1.8 | 779 | 41.1 | 38.8–43.5 |
| Skin and appendage disorders | 0100 | 274 | 18.8 | 43 | 13.1 | 11 | 10.0 | 328 | 17.3 | 15.5–19.1 |
| Metabolic and nutritional disorders | 0800 | 37 | 2.5 | 30 | 9.1 | 57 | 51.8 | 124 | 6.5 | 5.4–7.7 |
| Cardiovascular disorders | 1000 | 33 | 2.3 | 50 | 15.2 | 16 | 14.5 | 99 | 5.2 | 4.2–6.3 |
| Psychiatric disorders | 0500 | 50 | 3.4 | 15 | 4.6 | 0 | 0.0 | 65 | 3.4 | 2.6–4.3 |
| Respiratory disorders | 1100 | 44 | 3.0 | 10 | 3.0 | 7 | 6.4 | 61 | 3.2 | 2.4–4.1 |
| Immune disorders and infections | 1830 | 51 | 3.5 | 7 | 2.1 | 1 | 0.9 | 59 | 3.1 | 2.3–3.9 |
| Urinary tract disorders | 1300 | 40 | 2.7 | 3 | 0.9 | 3 | 2.7 | 46 | 2.4 | 1.7–3.2 |
| Blood disorders | 1200 | 13 | 0.9 | 15 | 4.6 | 4 | 3.6 | 32 | 1.7 | 1.1–2.3 |
| Liver and biliary disorders | 0700 | 18 | 1.2 | 8 | 2.4 | 4 | 3.6 | 30 | 1.6 | 1.0–2.2 |
| Neurological disorders | 0400 | 15 | 1.0 | 10 | 3.0 | 2 | 1.8 | 27 | 1.4 | 0.9–2.0 |
| Body as a whole, general disorders | 1810 | 10 | 0.7 | 9 | 2.7 | 0 | 0.0 | 19 | 1.0 | 0.5–1.5 |
| Vascular, bleeding and clotting disorders | 1040 | 0 | 0.0 | 6 | 1.8 | 3 | 2.7 | 9 | 0.5 | 0.1–0.8 |
| Vision disorders | 0431 | 0 | 0.0 | 2 | 0.6 | 0 | 0.0 | 2 | 0.1 | 0.0–0.3 |
| Endocrine disorders | 0900 | 0 | 0.0 | 1 | 0.3 | 0 | 0.0 | 1 | 0.1 | 0.0–0.2 |
| Reproductive disorders | 1400 | 0 | 0.0 | 1 | 0.3 | 0 | 0.0 | 1 | 0.1 | 0.0–0.2 |
| Other reactions | – | 213 | 14.6 | 0 | 0.0 | 0 | 0.0 | 213 | 11.2 | 9.7–12.7 |
CI, confidence interval; ‘n’: sum of ‘n1’, ‘n2’ and ‘n3’.
The described value, 1457, which represents the sum of all n1 values, does not match the adverse drug reactions total number, definite and probable, described in the study (1446); however, this error occurred in the publication and therefore, the result presented was kept.
Suspected drug classes involved in adverse drug reactions
Among the four studies, that used the same ADR concept of this review,16,18–20 two of them described the suspected drugs involved in the ADRs (Table 3).19,20 One study showed the suspected drug classes, without describing the incidence.16 Another study neither described the suspected drug nor the involved drug classes.18 Therefore, it was not included in Table 3. Similarly, the two studies that used the concept of DRPs were not included,17,21 in order to maintain the consistency of the results. Based on the foregoing, the main drug classes highlighted in the two studies were analgesics (N02), antibacterials for systemic use (J01), corticosteroids for systemic use (H02), drugs for obstructive airway diseases (R03), and diuretics (C03). It is noteworthy that in these studies, the drug numbers are greater than the ADR number.
Table 3.
Suspected drug classes involved in adverse drug reactions, according to the Anatomical Therapeutic Chemical–World Health Organization (WHO–ATC).
| Thiesen et al.19 | |||
|---|---|---|---|
| ATC level | WHO–ATC | n | n/3022 (%) |
| Anesthetics | N01 | 957 | 31.67 |
| Analgesics | N02 | 707 | 23.40 |
| Antineoplastic agents | L01 | 384 | 12.71 |
| Antibacterials for systemic use | J01 | 307 | 10.16 |
| Cough and cold preparations* | R05 | 144 | 4.77 |
| Corticosteroids for systemic use | H02 | 61 | 2.02 |
| Drugs for obstructive airway diseases | R03 | 58 | 1.92 |
| Antiemetics and antinauseants | A04 | 51 | 1.69 |
| Psycholeptics | N05 | 42 | 1.39 |
| Diuretics | C03 | 41 | 1.36 |
| Others | – | 270 | 8.93 |
| Silva et al.20 | |||
| ATC level | WHO–ATC | n | n/165 (%) |
| Antibacterials for systemic use | J01 | 41 | 24.85 |
| Diuretics | C03 | 24 | 14.55 |
| Antiepileptics | N03 | 23 | 13.94 |
| Analgesics | N02 | 17$ | 10.30 |
| Corticosteroids for systemic use | H02 | 18 | 10.91 |
| Antihypertensives | C02 | 9 | 5.45 |
| Drugs for obstructive airway diseases | R03 | 8 | 4.85 |
| Drugs for acid-related disorders | A02 | 3 | 1.82 |
| Immunosuppressants | L04 | 4 | 2.42 |
| Cardiac therapy | C01 | 5 | 3.03 |
| Others | – | 18 | 10.91 |
This item corresponds only to codeine, classified by the WHO–ATC as ‘cough and cold preparations’, but classified as an analgesic by the study.
Sum of analgesics and sedatives, according to the authors.
Risk factors
Despite using the prospective cohort method, no study applied the relative risk calculation; instead, the hazard ratio and odds ratio were used. It is worth pointing out that one study described relative risk values wrongly, since the reported values were the result of the odds ratio calculation.16
The absence of descriptive measures, grouped by exposed and unexposed, during the odds ratio calculation was observed in all manuscripts. This made impossible the calculation of relative risk and metaregression. Moreover, authors of one study observed the absence of confidence intervals in the factors without imminent risk.20
Finally, the statistic calculations for risk factor detection varied. Studies used odds ratio,16–18,20,21 hazard ratio19 and slope coefficient20 as well as univariate,16–19,21 bivariate19 or multivariate analysis16–21.
Adverse drug reactions
Four publications were included in this evaluation.16,18–20 Interpretation of results in one of the studies was difficult since the data were not shown in tables or graphs. In this case, a combination of analyses between age and number of drugs was not fully understood. Furthermore, the presentation of abbreviations without definition, such as ‘R2’, further hampered data interpretation.20 Due to the heterogeneity of the statistical analysis and data presentation, as well as the different age groups (0–16 years, 0–18 years or no description) and reference values used in the uni- or multivariate analysis, it was not possible to perform a further analysis. For example, in some publications the odds ratio calculation was employed as a reference for either the female gender17,18,21 or the male gender.16,19,20 Therefore, the only statistically significant variable in all publications was the increase in the number of prescription drugs, sometimes associated with a number greater than five drugs (Table 4). Nevertheless, one of the studies used no drug administration as a benchmark in the univariate and multivariate analysis.18 At this point, the author compared a risk zero variable with another that had imminent risk.
Table 4.
Risk factors associated with adverse drug reactions or nonpreventable adverse drug events.
| Risk factor | Rashed et al.18 |
Thiesen et al.19 |
Silva et al.20 |
Dos Santos and Coelho16 |
|---|---|---|---|---|
| Multivariate analysis |
Multivariate analysis |
Bivariate/multivariate analysis |
Multivariate analysis |
|
| (OR; CI 95%; p) | (HR; CI 95%; p) | (OR; CI 95%; p) | (OR; CI 95%; p) | |
| Age | 11–18-years old (2.1; 1.1–3.8; 0.02) |
Increasing age (1.06; 1.04–1.07; <0.001) |
<48 months (2.1; 1.19–3.72; 0.01) |
– |
| Gender | – | – | – | Male (2.83; 1.19–6.73; <0.05) |
| Prescription drugs | Number of low-risk drugs prescribed (≥1) (2.3; 1.4–4.0; 0.002) |
Increase in the number of prescription drugs (1.25; 1.22–1.28; <0.001) |
Increase in the number of prescription drugs (≥5)*
(2.19; 1.14–4.20, 0.01) |
Number of drugs administered (6–10) (7.45; 1.88–29.65; <0.001) |
| Number of low-risk drugs prescribed (5–10) (4.7; 2.4–9.3; <0.001) |
Received a GA (6.38; 5.30–7.68; <0.001) |
Number of drugs administered (≥11) (20.33; 3.57–115.73; <0.05) |
||
| Number of low-risk drugs prescribed (>10) (11.5; 3.6–36.3; <0.001) |
– | – | – | |
| Number of high-risk drugs prescribed (≥2) (2.4; 1.0–5.6; 0.04) |
– | – | – | |
| Number of high-risk drugs prescribed (>3) (6.5; 2.7–16.0; <0.001) |
– | – | – | |
| Length of stay | – | – | Increase in length of stay*
(slope coefficient: 2.75; p = 0.001) |
– |
| Diagnoses | D50–D89 (2.3; 1.0–5.1; 0.04) |
Oncology (1.89; 1.36–2.63; <0.001) |
– | – |
| G00–G99 (2.3; 1.3–4.2; 0.006) |
– | – | – | |
| P00–P96 (2.6; 1.0–6.5; 0.04) |
– | – | – | |
| Combinations | – | – | Age < 48 months + increase in the number of drugs (≥5) (2.05; 1.18–3.57; 0.01) |
– |
CI, confidence interval; GA, general anesthetic; D50–D89, diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism; G00–G99, diseases of the nervous system; HR, hazard ratio; OR, odds ratio; P00–P96, certain conditions originating in the perinatal period; p, significance level.
Variables related to the bivariate analysis.
Drug-related problems
Two publications were included in this evaluation, because they integrated the ADRs among DRPs.17,21 As observed in the ADRs, the studies inferred the increase in the number of prescription drugs a risk factor for DRP, greater than or equal to five drugs, in both statistical models (uni- or multivariate). However, other discussed risk factors were both different among them and among the analysis type (Table 5).
Table 5.
Risk factors associated with drug-related problems.
| Risk factor | Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| Rashed et al.21 |
Rashed et al.17 |
Rashed et al.21 |
Rashed et al.17 |
|||||
| OR (CI 95%) | p | OR (CI 95%) | p | OR (CI 95%) | p | OR (CI 95%) | p | |
| Gender | ||||||||
| Female | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| Male | 0.8 (0.5–1.4) | 0.42 | 1.0 (0.7–1.3) | 0.80 | 0.7 (0.4–1.3) | 0.26 | 0.9 (0.6–1.2) | 0.33 |
| Age | ||||||||
| 0–1 month | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| >1 month to ≤2 years | 0.7 (0.3–1.5) | 0.30 | 1.3 (0.8–2.0) | 0.28 | 1.3 (0.5–3.8) | 0.60 | 1.3 (0.8–2.1) | 0.33 |
| >2 to ≤6 years | 0.6 (0.3–1.3) | 0.21 | 1.3 (0.8–2.0) | 0.35 | 1.8 (0.5–6.8) | 0.36 | 1.4 (0.8–2.4) | 0.19 |
| >6 to ≤12 years | 0.9 (0.4–2.0) | 0.74 | 1.6 (1.0–2.6) | 0.06 | 2.1 (0.5–7.8) | 0.28 | 1.9 (1.1–3.3) | 0.01 |
| >12 to ≤18 years | 0.9 (0.4–2.0) | 0.82 | 1.4 (0.8–2.5) | 0.24 | 2.5 (0.7–8.7) | 0.16 | 1.5 (0.8–2.8) | 0.20 |
| Diagnoses | ||||||||
| A00–B99 (yes/no) | 3.0 (1.1–7.7) | 0.03 | – | – | 3.2 (1.2–8.9) | 0.02 | – | – |
| D50–D89 (yes/no) | – | – | 2.2 (1.1–4.3) | 0.02 | – | – | 1.6 (0.8–3.4) | 0.20 |
| J00–J99 (yes/no) | – | – | 1.8 (1.0–3.1) | 0.04 | – | – | 1.4 (0.8–2.6) | 0.26 |
| Number of prescription drugs (≥5 versus <5) | 1.9 (1.1–3.4) | 0.02 | 3.0 (2.2–4.2) | <0.001 | 2.2 (1.3–4.0) | 0.006 | 2.4 (1.7–3.4) | <0.001 |
| Admission type | ||||||||
| Emergency | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| Scheduled | 2.0 (1.1–3.7) | 0.02 | 1.2 (0.8–1.8) | 0.40 | 2.0 (0.9–4.1) | 0.07 | 2.6 (1.7–4.1) | <0.001 |
| Transferred | 1.8 (0.8–3.9) | 0.14 | 2.1 (1.5–2.9) | <0.001 | 1.5 (0.6–3.4) | 0.38 | 4.2 (2.9–6.1) | <0.001 |
CI, confidence interval; OR, odds ratio; p, significance level.
Discussion
The lack of drug attribution to ADRs due to the absence of other information sources that can clarify this association is a bias commonly found in retrospective studies, especially the drug utilization studies.22,23 Therefore, prospective studies are more advantageous than retrospective. Nevertheless, it is intriguing that the main method of the studies included in this review has been similar to the retrospective studies. Studies like these, while claimed as prospective, may contain information biases similar to retrospective studies.
With regard to risk factors associated with ADRs, such as those highlighted by Smyth and collaborators,2 we found evidence that the increase in the number of prescription drugs is a predictor of ADRs. This may be due to the additional risk of an ADR when receiving several drugs, to drug–drug or drug–disease interactions, and to greater susceptibility of prescribing and administration errors during the hospital stay.
The results of this review suggest that pharmacists are primarily responsible for these studies. This professional category seems to have an important role in the ADR notification, mainly in the countries that participate in the ADR monitoring program of the WHO.24 Moreover, their training, focused on the rational use of medicines, may have influenced this result.
By comparing the total of drugs involved in the ADRs and the total ADRs included in the studies, we note that the drug total number exceeded the ADR total number. We suspect that for each ADR, there was more than one drug involved. Despite being a controversial fact, we assume that the causality scale proposed by Naranjo cannot be effectively conclusive, although widely recognized by the scientific community.25 Thus, this algorithmic scale may likely identify a suspected drug, giving a margin for personal inferences of the researcher.
Regarding the methodologies, different methods were conducted, although similar objectives have been observed. There were differences in (i) causality assessment; (ii) clinical setting; (iii) age group; (iv) implementation period; (v) terms used to define ADR; (vi) the drug or ADR classification models. Thus, a methodological standardization of ADR risk assessment studies would be necessary in children during hospitalization.
In relation to ADR incidence, there was large variation in the results (amplitude 32.4%). The value of the incidence pooled estimate calculated by the review (16.4%) was 1.72 times higher than the value of the pooled estimate of ADRs occurring during hospitalization in pediatric patients reported by another review, also based on prospective studies [9.53% (95% confidence interval 6.81–12.26)].26
Some variations in the methodology affected this amplitude. We observed that the ADR incidence was inferior in the studies which included these events between the DRPs.17,21 The search for DRPs in these studies was wide and included five other events in addition to ADRs. Thus, the focus on ADRs may have been smaller. At the same time, they were multicenter studies. Studies like these may incorporate observer bias, due to the complexity of the training. In this case, each researcher should have offered more or less focus on one of the six DRPs.22 Conversely, we observed a greater ADR incidence in a study conducted in the ICU.20 Thus, the incidence may have been influenced by the polypharmacy and greater number of high-risk drugs used.27
With regards the incidence values, the use of causality-defined instruments for ADRs, and the inclusion of ADRs classified as ‘possible’ affected the results of the present systematic review. Studies that included ‘possible’ ADRs made their calculations from three classifications (‘defined’, ‘probable’ and ‘possible’),16–18,20,21 unlike those that included only two (‘definite’ and ‘probable’)19 or those that included any suspicious reaction investigated.15 However, the ADR incidences varied by study site, use of the Naranjo instrument and the ADR inclusions among DRPs.
In addition, the care delivered in different health systems may have influenced in part the variation in incidence. Some of these hospitals could have more effective services engaged in ADR prevention. However, it was not possible to evaluate this influence due to the methodological diversity of the included studies since other factors, which were described in the previous paragraphs, seem to have had more influence on the ADR incidence. Moreover, it was not possible to evaluate the influence of the number of hospital beds on the incidence, since it was not possible to observe a pattern between the included studies.
On the other hand, the absence of an item of STROBE (i.e. item 14b) made it impossible to carry out metaregression and classification of risk factors by means of risk categorization. Despite the good quality of studies used in the quantitative synthesis, no publication has exposed the descriptive characteristics in numbers, grouped by exposed and unexposed groups for all risk factors analyzed. In addition, some factors considered as a risk in some studies were not statistically associated with ADRs in others.
Despite these findings, more studies are still needed to identify the risk factors for ADRs in pediatric inpatients; mainly studies including the assessment of off-label and unlicensed prescribing, as observed by Impicciatore et al. in another review.26
Difficulties encountered by this review
The presentation form of data in publications sometimes affected evaluation. Data arranged in graphics, as suggested by STROBE, were more enlightening than data presented in written form. In addition, some publications showed dissonant data, making its interpretation difficult. In general, these errors did not alter the results presented, as the risk factor.
Benefits
This systematic review was instigated by reanalyzing the data described in the manuscripts. Moreover, it makes use of a comprehensive search strategy based on the synonyms and subcategories.
Limitations/correction bias
The results of this study should be interpreted with consideration to its limitations. This systematic review searched prospective cohort studies in children, with evaluation of the risk factors for ADRs occurring during the hospital stay, so it should not be extrapolated to the general pediatric population. Despite the heterogeneity of publications, the methodology we chose in this study attempted to homogenize the presented results. There was a reclassification of categories and new calculations were performed from the data reported by the authors. However, this was not sufficient to correct such discrepancies. Some studies had to be removed from the assessment during quantitative synthesis, and others could not be corrected, being described in accordance with the publication. Although the studies have included children of different ages, the present study and another two systematic reviews did not identify the immaturity of child development as a risk factor for ADR in hospitals.2,26 In the present review, only one study identified the age less than 48 months as a risk factor.20 However, other studies identified an increase of age or older ages.17–19,21 This may represent a limitation of this review. However, a better approach on the subject is necessary, since there are differences in the results presented in the studies. In addition, the search method may not be the most suitable. If we consider that each type of ADR has specific risk factors, maybe the best methodology to be employed would be the assessment of the 10 most common drug-ADR combinations in the pediatric inpatient population followed by an assessment of the risk factors common across these combinations.
Conclusion
The increase in the number of prescription drugs favors the occurrence of ADRs in pediatric inpatients. Although the quality of these studies was generally good, it is still necessary that authors should follow guidelines such as STROBE with regard to the presentation of the results, mainly in relation to exposed and nonexposed groups. Moreover, it is necessary to use validated international classifications for defining ADRs, drug class, and causality. The methodology for the search of the records appears to be effective, but can suffer from information bias as a retrospective study, leaving the researcher to incorporate new search strategies such as triggers, clinical follow up in bed, and evaluation of laboratory tests.
Supplementary Material
Acknowledgments
This work was supported by a Fellowship of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) awarded to PHSA. We furthermore thank Alfredo Dias de Oliveira Filho, Francilene Amaral da Silva, Lincoln Marques Cavalcante Santos and Saravanan Shanmugam for helpful comments on our manuscript.
Footnotes
Funding: All phases ofthis study were supported by a Fellowship of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) awarded to Paulo Henrique Santos Andrade.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Paulo Henrique Santos Andrade, Laboratório de Ensino e Pesquisa em Farmácia Social (LEPFS), Departamento de Farmácia, Universidade Federal de Sergipe, Av. Marechal Rondon, S/n – Jardim Rosa Elze, São Cristóvão – SE, Brazil.
Adriano da Silva Santos, Universidade Federal de Sergipe, Aracaju, Sergipe, Brazil.
Carlos Adriano Santos Souza, Universidade Federal de Sergipe, Aracaju, Sergipe, Brazil.
Iza Maria Fraga Lobo, Hospital Universitário de Sergipe, Aracaju, Sergipe, Brazil.
Wellington Barros da Silva, Universidade Federal de Sergipe, Aracaju, Sergipe, Brazil.
References
- 1. Henriksen K, Battles JB, Marks ES, et al. (eds). Advances in Patient Safety: From Research to Implementation (Volume 3: Implementation Issues). Rockville, MD: Agency for Healthcare Research and Quality (US), 2005. February. [PubMed] [Google Scholar]
- 2. Smyth RMD, Gargon E, Kirkham J, et al. Adverse drug reactions in children—a systematic review. PLoS One 2012; 7: e24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ACSQHC. Literature review: medication safety in Australia. Sydney: Australian Commission on Safety and Quality in Health Care, http://www.safetyandquality.gov.au/wp-content/uploads/2014/02/Literature-Review-Medication-Safety-in-Australia-2013.pdf (2013, accessed 10 March 2016). [Google Scholar]
- 4. ANVISA. Anexo 3: Protocolo de Segurança na Prescrição, uso e Administração de Medicamentos. Brasília: Agência Nacional de Vigilância Sanitária, http://www20.anvisa.gov.br/segurancadopaciente/index.php/publicacoes/item/seguranca-na-prescricao-uso-e-administracao-de-medicamentos (2013, accessed 10 March 2016). [Google Scholar]
- 5. WHO. International drug monitoring: the role of national centres. Report of a WHO Meeting Geneva: World Health Organization, Report No.: 498, http://who-umc.org/graphics/24756.pdf (1972, accessed 10 March 2016) [PubMed] [Google Scholar]
- 6. Fernandez E, Perez R, Hernandez A, et al. Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutic 2011; 3: 53–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahmood I. Developmental pharmacology: impact on pharmacokinetics and pharmacodynamics of drugs. In: Mahmood I, Burckart G. (eds) Fundamentals of pediatric drug dosing. Cham: Springer International Publishing, 2016. [Google Scholar]
- 8. Levy FH. Technology and pediatric patient safety: what to target is the dilemma. J Pediatr 2008; 152: 153–155. [DOI] [PubMed] [Google Scholar]
- 9. Bellis JR, Kirkham JJ, Nunn AJ, et al. Adverse drug reactions and off-label and unlicensed medicines in children: a prospective cohort study of unplanned admissions to a paediatric hospital. Br J Clin Pharmacol 2014; 77: 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. AHRQ. Reducing and preventing adverse drug events to decrease Hospital costs: research in action. Rockville, MD: Agency for Healthcare Research and Quality, http://archive.ahrq.gov/research/findings/factsheets/errors-safety/aderia/ade.html (2001, accessed 10 March 2016). [Google Scholar]
- 11. Urrútia G, Bonfill X. Declaración PRISMA: una propuesta para mejorar la publicación de revisiones sistemáticas y metaanálisis. Med Clin (Barc) 2010; 135: 507–511. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Epidemiology 2007; 18: 805–835. [DOI] [PubMed] [Google Scholar]
- 14. Wells GA, Shea B, O’Connell D, et al. Newcastle-Ottawa quality assessment form for cohort studies. E17–E18, http://www.ncbi.nlm.nih.gov/books/NBK115843/bin/appe-fm3.pdf (2014, accessed 10 March 2016).
- 15. Turner S, Nunn AJ, Fielding K, et al. Adverse drug reactions to unlicensed and off-label drugs on paediatric wards: a prospective study. Acta Paediatr 1999; 88: 965–968. [DOI] [PubMed] [Google Scholar]
- 16. dos Santos DB, Coelho HLL. Adverse drug reactions in hospitalized children in Fortaleza, Brazil. Pharmacoepidemiol Drug Saf 2006; 15: 635–640. [DOI] [PubMed] [Google Scholar]
- 17. Rashed AN, Neubert A, Tomlin S, et al. Epidemiology and potential associated risk factors of drug-related problems in hospitalised children in the United Kingdom and Saudi Arabia. Eur J Clin Pharmacol 2012; 68: 1657–1666. [DOI] [PubMed] [Google Scholar]
- 18. Rashed AN, Wong IC, Cranswick N, et al. Risk factors associated with adverse drug reactions in hospitalised children: international multicentre study. Eur J Clin Pharmacol 2012; 68: 801–810. [DOI] [PubMed] [Google Scholar]
- 19. Thiesen S, Conroy EJ, Bellis JR, et al. Incidence, characteristics and risk factors of adverse drug reactions in hospitalized children–a prospective observational cohort study of 6,601 admissions. BMC Med 2013; 11: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silva DC, Araujo OR, Arduini RG, et al. Adverse drug events in a paediatric intensive care unit: a prospective cohort. Br Med J Open 2013; 3 pii: e001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rashed AN, Wilton L, Lo CC, et al. Epidemiology and potential risk factors of drug-related problems in Hong Kong paediatric wards. Br J Clin Pharmacol 2014; 77: 873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mann CJ. Observational research methods. Research design II: cohort, cross sectional, and case-control studies. Emerg Med J 2003; 20: 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions. Drug Saf 2009; 32: 19–31. [DOI] [PubMed] [Google Scholar]
- 24. van Grootheest K, Olsson S, Couper M, et al. Pharmacists’ role in reporting adverse drug reactions in an international perspective. Pharmacoepidemiol Drug Saf 2004; 13: 457–464. [DOI] [PubMed] [Google Scholar]
- 25. Zaki SA. Adverse drug reaction and causality assessment scales. Lung India 2011; 28: 152–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Impicciatore P, Choonara I, Clarkson A, et al. Incidence of adverse drug reactions in paediatric in/out-patients: a systematic review and meta-analysis of prospective studies. Br J Clin Pharmacol 2001; 52: 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Biswal S, Mishra P, Malhotra S, et al. Drug utilization pattern in the intensive care unit of a tertiary care hospital. J Clin Pharmacol 2006; 46: 945–951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.