Abstract
A gly388arg polymorphism (rs351855) in the transmembrane domain of the fibroblast growth factor receptor (FGFR4) is associated with increased risk, staging, and metastasis in several different types of cancer. To specifically assess the impact of the polymorphic FGFR4 in colorectal cancer (CRC), we engineered CRC cell lines with distinct endogenous expression patterns to overexpress either the FGFR4gly or FGFR4arg alleles. The biologic analyses revealed an oncogenic importance for both polymorphic alleles, but FGFR4gly was the stronger inducer of tumor growth, whereas FGFR4arg was the stronger inducer of migration. An evaluation of clinical specimens revealed that FGFR4 was upregulated in 20/71 patients independent of gly388arg status. There was no correlation between the presence of an FGFR4arg allele and CRC or polyp risk in 3,471 participants of the CORSA study. However, among 182 patients with CRC, FGFR4arg-carriers had a fivefold higher risk of tumors that were stage II or greater. Together, our results established that both allelic forms of FGFR4 exert an oncogenic impact and may serve equally well as therapeutic targets in CRC. One important implication of our findings is that FGFR4arg-carriers are at a higher risk for more aggressive tumors and therefore may profit from early detection measures.
Introduction
For the fibroblast growth factor receptor 4 (FGFR4), a polymorphism that causes a substitution of an arginine instead of a glycine in the transmembrane domain of the receptor (gly388arg; rs351855) has been described and has pathophysiologic impact in tumor development (1). Specifically, the FGFR4arg allele was found to be associated with increased cancer risk in the prostate (2) and with more aggressive tumors, metastasis, therapy resistance, and poor outcome in lung carcinomas (3), head and neck cancer (4, 5), breast cancer (1, 6), and melanomas (7). In the colon, 2 studies report an association of the FGFR4arg allele with tumor size and nodal status (1, 8) and with recurrence (8). Another study did not find a significant impact of the polymorphic alleles on colorectal cancer (CRC) pathophysiology (9). To date, no mechanistic studies that investigate the cell biologic impact of FGFR4 variants on malignant cell characteristics in CRC are available.
The cellular mechanism underlying FGFR4-effects on carcinogenesis in general is still poorly understood. FGF signaling plays essential roles in embryogenesis, development, and wound healing through activation of cell growth, survival, and cell migration (10–13). These effects have also been observed in malignant cells for several members of the FGF family (14–17). Consistent with this cell biologic pattern of FGF effects, therapy resistance conferred by FGFR4 in breast cancer cells was reported to involve expression of antiapoptotic genes (18). In addition, differential promigratory activity of the gly388 or arg388 forms of FGFR4 has been implicated in the formation of more aggressive breast tumors (19).
It is, therefore, the aim of this study to address the hypothesis that the gly388 and arg388 alleles of FGFR4 have differential impact on the malignant characteristics of CRC cells, thus causing an increased risk of CRC tumor progression and metastasis in individuals carrying an FGFR4arg allele. For this purpose, the biologic impact of FGFR4gly and FGFR4arg has been assessed using cell line models and the association of SNP rs351855 and expression with CRC risk and prognostic parameters has been analyzed in individuals participating in the CORSA study and CRC patients of hospitals in Vienna and Budapest.
Materials and Methods
Cell lines
SW480, SW620, HCT116, HT29, Colo201, T84, and Caco2 CRC cell lines were obtained from the American Type Culture Collection and kept under standard tissue culture conditions using Minimal Essential Medium containing 10% fetal calf serum (FCS). Cell lines were authenticated by analysis at the Genetic Resources Core Facility of the Johns Hopkins University.
Isolation of DNA and genotyping
DNA was extracted by standard protocols (Qiagen) and genotyping was conducted with ABI Prism 7500 Sequence Detection System (Applied Biosystems) using a TaqMan SNP-assay (Applied Biosystems 4351379). A detailed description can be found in Supplementary Materials.
Isolation of RNA and quantitative RT-PCR
Total RNA was isolated from subconfluent cultures or frozen colon tissue specimens using Trifast reagent according to the manufacturer's instructions (PeqLab). cDNA was synthesized using RevertAid MMuLV reverse transcriptase (Fermentas) and random hexamer primers (GE Healthcare).
TaqMan assays from Applied Biosystems were used to determine expression of FGFR4 (Hs00242558_m1) and GAPDH (Hs99999905_m1) mRNAs by quantitative RT-PCR (qRT-PCR). Expression in cell lines was calculated as an x-fold increase above the respective controls; expression in tumors was calculated as an x-fold change compared with the corresponding normal mucosa using GAPDH as control gene and the ΔΔCt method.
Reagents from the TaqMan Genotyping assay and a standard curve constructed with defined mixtures of pure FGFR4gly and FGFR4arg DNA were used on cDNA to assess the ratio of expressed alleles in heterozygous cells from the ΔCt between FGFR4arg and FGFR4gly (for details, see Supplementary Material).
Overexpression of FGFR4 in CRC cell lines
pcDNA3 plasmids expressing VSV-tagged forms of FGFR4gly or FGFR4arg that were kindly provided by A. Ullrich (Martinsried, Germany) were introduced into SW480, HCT116, and HT29 cells by lipofection with Transfectin (BioRad). Controls were transfected with GFP or the pcDNA3 vector and stable transfectants were selected in the presence of geneticin (G418).
Knockdown of gene expression
siRNAs specifically targeting FGFR4 were purchased from Ambion (Applied Biosystems) and transfected into 70% confluent cultures kept in medium containing 10% FCS using 3 μL siLentFect (BioRad) and 20 pmol of the siRNA per well in culture medium without serum. A scrambled siRNA without sequence homology to known human genes served as negative control. After 24 and 48 hours, RNA and protein were isolated to verify knockdown efficiency. Functional and growth assays were initiated 24 hours after transfection.
Protein isolation and Western blotting
To determine the impact of FGFR4 on intracellular signaling activity semiconfluent cultures of SW480 transfectants were starved and lysed for protein analysis 24 hours later. Cell membranes were prepared by cell lysis in Dounce buffer (10 mmol/L Tris HCl, 0.5 mmol/L MgCl2 protease and phosphatase inhibitors, pH7.6), followed by homogenization in tonicity restoration buffer (10 mmol/L Tris HCl, 0.5 mmol/L MgCl2, 0.6 mol/L NaCl and protease and phosphatase inhibitors, pH7.6) and ultracentrifugation (100,000 g, 90 minutes). Total protein extraction and Western blotting was conducted as described (17) using phosphospecific antibodies recognizing PLCγ, FRS2α, c-src, ERK, GSK3ß, and S6. A detailed list of the antibodies used can be found in Supplemental Materials (Supplementary Table S1). Bands were detected using second antibodies coupled to horseradish peroxidase and chemoluminescence staining reagents (GE Healthcare). Band intensity was quantified from the x-ray films using ImageQuant software (GE Healthcare).
FGFR4 on the cell surface
Trypsinized cells were stained with a PE-coupled monoclonal antibody recognizing the N-terminus of FGFR4 (clone 4F&6D3; Biolegend). Control stains were done using a PE-coupled antimouse control antibody. Fluorescence-activated cell sorting (FACS) analysis was conducted on a FACS Calibur (Becton Dickinson).
Cell viability and growth assays
Cells were seeded at a density of 1 × 103 cells per well into 96-well plates for growth curves and 3 × 103 for knockdown experiments. Viability was determined by MTT assay (Easy4U; Biomedica).
DNA synthesis was determined by incubation with 3H-thymidine (1 μCi/mL) as described previously (14, 17).
For assessment of anchorage-independent growth, 5,000 cells/well were suspended in 0.25% agar prepared in RPMI medium containing 20% FCS and incubated for 2 to 3 weeks before counting the number of colonies microscopically.
Clonogenicity was determined from cells plated at a density of 100 or 200 cells/well onto 6-well plates in medium containing 10% FCS by staining with 0.01% of crystal violet solution to assess colony formation (14, 17).
Cell migration assay
Cells were seeded into 8-μm pore-size polyester tracketched membrane filters (BD-Falcon) in 24-well plates at a density of 0.5 × 105 cells/cm2. After a migration period of 24 and 48 hours for SW480 and HCT116 and 96 hours for HT29 cells, filters were removed and cells in the lower chamber were stained with crystal violet and colony number evaluated using Lucia software. Alternatively, migration was determined by scratch assay as described in ref. 20 with identical results.
Tumor growth and metastasis
SW480 cells selectively overexpressing FGFR4gly or FGFR4arg as well as control transfectants were suspended in serum-free medium at a density of 1 × 106 cells/50 μL and subcutaneously injected into the rear flanks of immunodeficient severe combined immunodeficient mice (SCID)/Balb/c recipient mice (female, aged 4 weeks, Harlan Winkelmann). Tumor formation was monitored by palpation and tumor size was determined using a Vernier caliper. Tumor volume was calculated using the formula (smaller diameter2 × larger diameter)/2. All experiments were conducted in quadruplicates and carried out according to the Austrian and FELASA guidelines for animal care and protection. Tissue sections of experimental tumors were analyzed by immunohistochemistry using antibodies directed against cytokeratin 20 and Ki67 as described previously (21).
Mouse lungs were prepared for immunohistochemistry and metastasizing tumor cells in lung sections were identified by their expression of Ki67. Metastasis was scored according to the number and size of metastatic foci as described in Supplemental Materials.
Hospital study population and tissue specimens
Tissue specimens were collected from patients undergoing surgery for CRC in hospitals in Vienna and Budapest (“hospital population”). Informed consent was obtained from all patients. Immediately after surgery, tissue specimens were frozen in liquid N2 until extraction of nucleic acids. All diagnostic information on tumor location, staging, and grading is available and the pattern of staging is given in Supplementary Table S2. Tumor tissue had a tumor cell content of at least 70% as judged from the histology of immediately adjacent tissue. The study had prior approval of the local ethics committees.
FGFR4 expression was analyzed from both the tumor and the adjacent normal mucosa by qRT-PCR. The rs351855 polymorphism was determined from the tumor tissue using the TaqMan Genotyping assay.
Population-based study population
Within a province-wide screening project in eastern Austria, Caucasian participants were recruited for the molecular epidemiology CRC study of Austria (CORSA). Participants with a positive fecal occult blood testing underwent colonoscopies and were asked to participate in the molecular epidemiology study. All subjects gave written informed consent. The study was approved by the Institutional Ethic Review Board. Details of the study population are described in refs. 22–24.
The control group (n = 1,794) consisted of participants that were free of polyps and CRC shown by colonoscopy. The adenoma group consisted of 1,330 and the CRC group of 178 patients who were newly diagnosed and previously untreated. CRC and polyp diagnosis was histologically confirmed and the adenoma group was classified in a high-risk (n = 292) and a low-risk (n = 1,038) subgroup based on the histology report. Sex, age, and nutrition have been shown to impact on CRC risk independent of most genetic variants (25, 26). The pattern of these confounding variables is summarized in Table 1.
Table 1. Characteristics of the CORSA study population.
| CRC patients n = 178 (5.3%) | High-risk adenoma patients n = 292 (9.0%) | Controls n = 1,794 (53.4%) | |
|---|---|---|---|
| Sex | |||
| Male | 55 (63.2) | 195 (66.8) | 810 (46.6) |
| Female | 32 (37.8) | 97 (33.2) | 928 (53.4) |
| Age, y | |||
| <50 | 8 (9.2) | 44 (15.1) | 353 (20.3) |
| 50–60 | 18 (20.7) | 60 (20.6) | 426 (24.5) |
| 60–70 | 24 (27.6) | 109 (37.3) | 550 (31.7) |
| 70–80 | 31 (35.6) | 77 (26.4) | 391 (22.5) |
| >80 | 6 (6.9) | 2 (0.7) | 18 (1.0) |
| Body mass index, kg/m2 | |||
| <19 | 1 (1.2) | 1 (0.3) | 7 (0.4) |
| 19–25 | 17 (19.5) | 49 (16.8) | 377 (21.7) |
| 25–30 | 35 (40.2) | 141 (48.3) | 729 (41.9) |
| >30 | 28 (32.2) | 95 (32.5) | 564 (32.5) |
| Missing | 6 (6.9) | 6 (2.1) | 61 (3.5) |
Statistical evaluation of data
The statistical analysis of CRC risk in relation to patient genotype is described in detail in refs. 22 and 23. Genotypic counts of controls were tested for Hardy–Weinberg equilibrium using a v2 test. Linkage disequilibrium statistics were computed using Haploview 4.0. Multiple logistic regressions were applied to compare individuals of the control group against the CRC group and the CRC + high-risk adenoma group. ORs and 95% confidence intervals (CI) were estimated using the software R Ver 2.6.2. All P values are 2-sided; P values less than 0.05 were considered to be statistically significant.
Power calculations were carried out with a calculator available from (27). Given the observed genotype distribution [50% homozygous wild type (gly/gly), 40% heterozygous (arg/gly), and 10% homozygous mutant (arg/arg)] ORs of 2.36 can be recognized with a power of 80% and a significance level α = 5% for carcinoma versus controls, arg/arg versus gly/gly. For carcinoma + high risk adenoma versus controls, arg/arg versus gly/gly the OR was 1.71.
Tissue expression data were analyzed by paired sample t test after obtaining a Gaussian distribution by transforming to log values. The relationship between SNP rs351855 and tumor stage was determined in comparison to stage I using contingency tables and Fisher exact or χ2 test using Graphpad-Prism. Tumor growth was analyzed by 2-way ANOVA and cell biologic results were analyzed by Student t test or Kruskal–Wallis test depending on the results of normality testing.
Results
Impact of FGFR4 overexpression on malignant characteristics in vitro
SW480 and HCT116 cells are gly-homozygous cells expressing low and high levels of FGFR4, respectively. HT29 is gly/argheterozygous and expresses high levels of mainly FGFR4arg (for details, see Supplementary Fig. S1). These cells were transfected with FGFR4 expression vectors and clone pools were selected that stably overexpress a specific FGFR4 allele. In SW480 transfectants, overexpression was 3- to 4-fold on the RNA level and because of their low endogenous expression, the cells mainly expressed the transfected allele. FGFR4 at the cell membrane was increased 11-fold for FGFR4arg and 3-fold for FGFR4gly. In HCT116 that expressed higher levels of FGFR4gly, overexpression was only 60% and 25% for FGFR4arg and FGFR4gly, respectively, but FGFR4arg transfection shifted the arg/gly-ratio from 0:1 to 1:1. FGFR4 protein was increased 3-fold (FGFR4arg) and 1.6-fold (FGFR4gly). In the FGFR4arg expressing HT29 cells, RNA was upregulated 2.5- and 1.5-fold with FGFR4gly transfection shifting the arg/gly ratio to 1:1. Protein overexpression was 12-fold and 3.6-fold for FGFR4arg and FGFR4gly (details are shown in Supplementary Fig. S2). Impact of FGFR4 overexpression on logarithmic growth under standard culture conditions was only seen in SW480 cells (Supplementary Fig. S3).
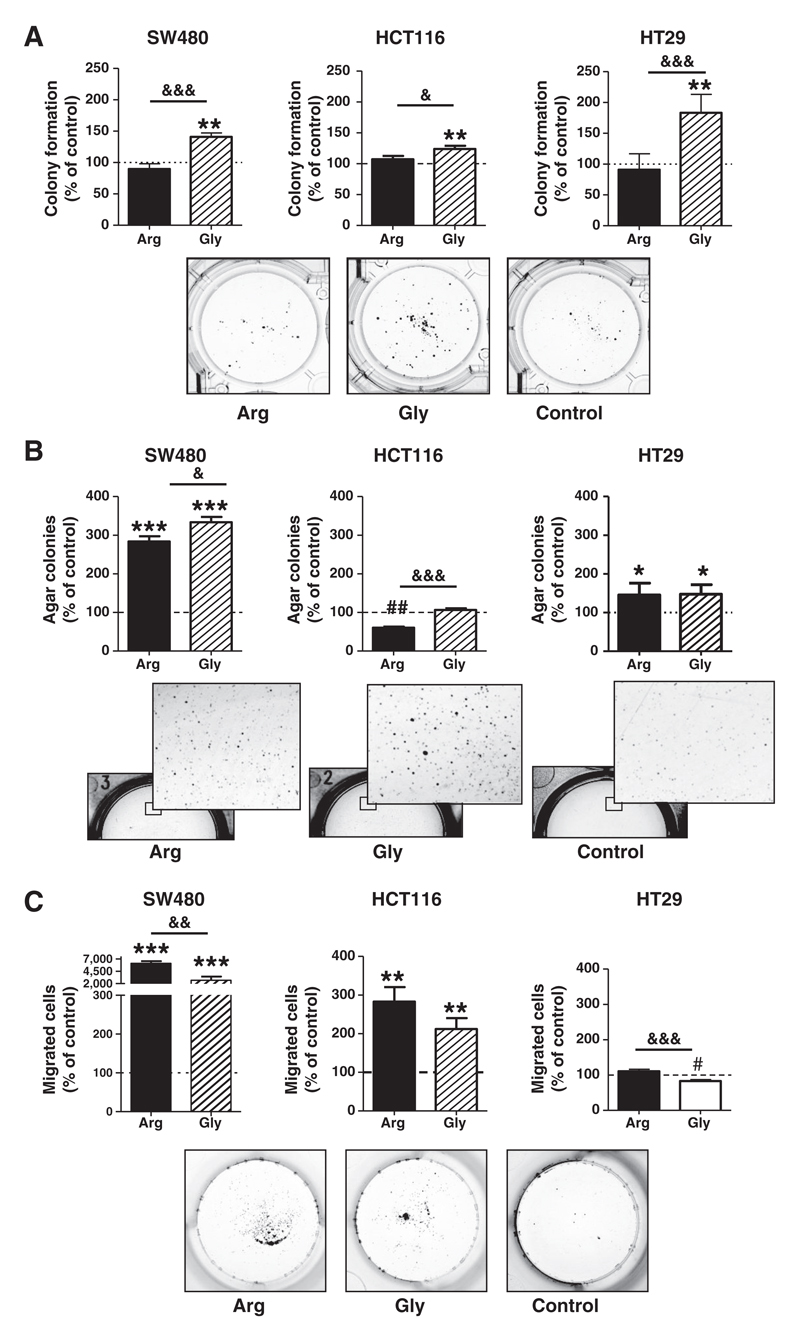
However, overexpression of FGFR4gly but not FGFR4arg enhanced the capacity to attach and form colonies in very low-density cultures by 20% to 60% in all 3 cell lines (Fig. 1A). Differential impact on anchorage-independent growth in soft agar was also observed depending on the endogenous FGFR4 expression level: in SW480 cells, growth stimulation was strong with both alleles and FGFR4gly was the stronger activator compared with FGFR4arg (gly vs. arg significantly different at P = 0.03). In HCT116, agar growth was not further increased by FGFR4gly and even decreased by transduction of FGFR4arg creating a highly significant difference between the 2 allelic forms. In contrast, in the FGFR4arg expressing HT29 cells agar growth was similarly stimulated by either FGFR4 allele (Fig. 1B).
Figure 1.
Impact of FGFR4 overexpression on tumor cell growth and migration in vitro. Stable transfectants overexpressing FGFR4arg or FGFR4gly in SW480, HCT116, or HT29 cells were used to assess growth and malignant characteristics in vitro. Stable transfectants with the pcDNA3 vector were used as controls. A, 100 and 200 cells were seeded/6-well, medium was changed after 24 hours, and the number of colonies counted after 10 days of growth. B, five thousand cells each were suspended in soft agar medium and plated in 6-well plates. The number of colonies was counted at a magnification of 10-fold after 3 weeks of growth. C, cell migration was determined by filter migration assay from 2 × 104 cells/24-well. Photographs of cultures show representative results from SW480 transfectants. Quantitative results from all cell lines were calculated as % of control, pooled from at least 3 independent experiments, and presented as mean ± SD. *, **, *** indicate an increase as compared with the control at P < 0.05, 0.01, and 0.001, respectively. ## indicates a decrease as compared with control at P < 0.01. &, &&, and &&& indicate a difference between the FGFR4gly and FGFR4arg groups at P < 0.05, 0.01, and 0.001, respectively.
Both FGFR4 alleles had strong impact on tumor cell migration in SW480 cells. With a 60-fold increase FGFR4arg was the more potent gene compared with FGFR4gly (25-fold; difference significant at P = 0.003). The impact was much weaker in HCT116 cells (2.8- and 2-fold, respectively, for FGFR4arg and FGFR4gly, and no significant difference between the 2 allelic forms). In HT29 cells that endogenously express high levels of FGFR4arg, no additional effect on cell migration was induced by FGFR4arg. FGFR4gly caused a 25% reduction of migration capacity (P = 0.0002 compared with control as well as with FGFR4arg; Fig. 1C).
Knockdown of FGFR4 expression
Knockdown of FGFR4 expression in the cell lines HCT116 and HT29 by lipofection with siRNA-oligonucleotides caused suppression of FGFR4 mRNA to 10% to 20% of control level (Supplementary Fig. S4). Shifts in the gly/arg ratio of HT29 cells were not observed. On the protein level for both cell lines, the cell population expressing FGFR4 on their cell surface was reduced to 20% to 40% of the controls. Suppression of FGFR4 expression reduced viability to 80% (P = 0.0005) of control in HT29 cells and to 35% (P = 0.029) in HCT116 (Fig. 2A). DNA synthesis was inhibited by 25% in both cell lines (Fig. 2B). Colony formation in low-density cultures was inhibited by 28% in HT29 (P = 0.005) and 16% in HCT116 cells (P = 0.029; Fig. 2C). Impact on cell migration was more pronounced in both cell lines with an inhibition of about 40% (P = 0.005 for HT29 and P = 0.031 for HCT116; Fig. 2D). An impact on anchorage-independent growth could not be detected in either cell line (data not shown).
Figure 2.
Impact of FGFR4 knockdown on clonogenicity and migration in vitro. siRNA oligonucleotides were introduced by lipofection into HCT116 and HT29 cells. Controls were transfected with scrambled control siRNA. A, five days after knockdown, cell viability was measured by MTT assay. B, 24 hours after knockdown, proliferation was determined by 3H-thymidine uptake. From parallel cultures, cells were harvested 24 hours after transfection and plated at 100 and 200 cells/6-well for colony formation assays (C) and 2 × 104 cells/filter for migration assays (D). Results were pooled from at least 3 independent experiments and presented as mean ± SD. # and ## indicate a decrease as compared with control at P < 0.05 and 0.01, respectively.
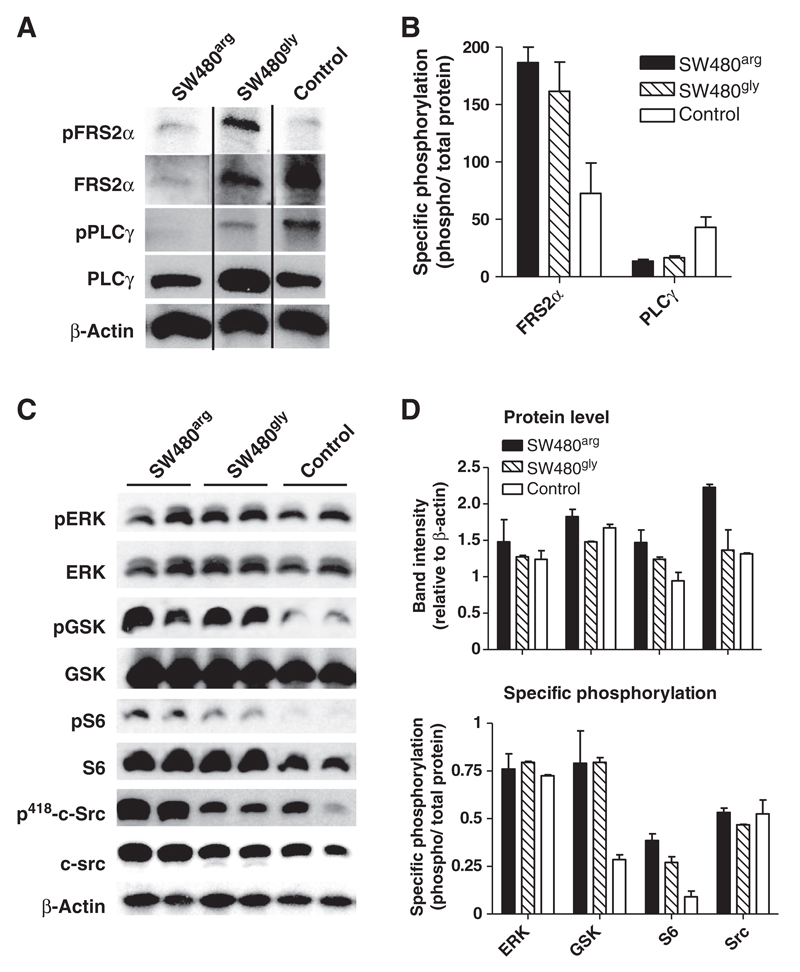
Impact on downstream signaling
Phosphorylation of the primary FGFR-target FRS2α was increased in FGFR4 overexpressing SW480 cells compared with the control (SW480co) even though the amount of FRS2α found in the particulate fraction was reduced. PLCγ phosphorylation was decreased in FGFR4 transfectants. The amount of FRS2α and PLCγ protein recruited to the membrane was distinctly higher in SW480gly than SW480arg, where-as specific phosphorylation of the signaling molecules was similar in SW480arg and SW480gly cells (Fig. 3A and B). In addition, c-src protein was upregulated in SW480arg but not SW480gly cells. The src protein was phosphorylated at tyrosine 418 indicating activation of kinase activity at a similar level in all transfectants (Fig. 3C and D). FGFR4-dependent phosphorylation of GSK3ß and S6 was observed—showing activation of survival pathways downstream of the phosphatidyl-inositol-3-kinase. Phosphorylation of ERK was not affected (Fig. 3C and D).
Figure 3.
FGFR4-dependent downstream signaling. A and B, semiconfluent cultures of SW480 transfectants were starved and cell membranes were prepared and extracted. Protein amount and phosphorylation of PLCγ and FRS2α was analyzed by Western blot analysis. C and D, from parallel cultures, protein lysates were prepared after 24 hours for analysis of signaling molecules using phospho-specific antibodies. Photographs of Western blot analysis show results of representative gel runs. Quantification was conducted from 2 independent experiments using duplicate samples.
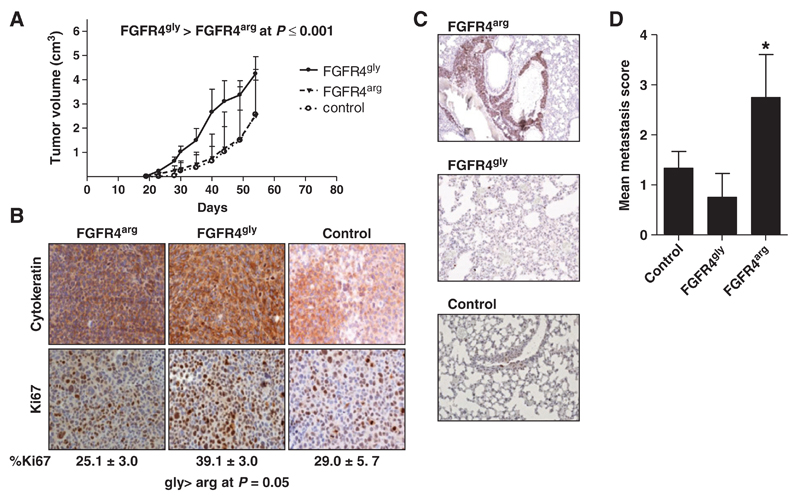
Tumor growth in vivo
As the results of the in vitro growth, assays strongly indicated a differential protumorigenic impact of FGFR4 overexpression, SW480gly, SW480arg, and SW480co cells were injected s.c. into SCID mice to assess local tumor growth and metastasis. All 3 cell lines grew to local tumors consisting mainly of cytokeratin 20 expressing, poorly differentiated cells. The FGFR4gly gene significantly enhanced tumor growth as compared with the SW480co (P = 0.0002), whereas the FGFR4arg variant had no impact. The fraction of proliferating Ki67-positive cells was similar in SW480co and SW480arg tumors (29.0 ± 5.7% and 25.1 ± 3.1%, respectively), but increased in the SW480gly tumors (39.1 ± 3.0%; P = 0.05; Fig. 4A and B).
Figure 4.
Impact of FGFR4 overexpression on tumor growth and metastasis in vivo. 106 cells each of SW480gly, SW480arg, and SW480co cells were injected s.c. into SCID mice. A and B, tumor growth was monitored and the mice sacrificed when tumor size reached 5 cm3 or after 9 weeks, whichever came first. The tumors were analyzed by immunohistochemistry using antibodies recognizing cytokeratin 20 and Ki67. C and D, lungs were isolated from tumor-bearing mice and fixed with formalin. Serial sections were stained using antibodies recognizing Ki67 to detect tumor cells that were scored according to the criteria given in Supplementary Materials.
To assess metastasis, lungs from tumor-bearing mice were analyzed in serial sections stained with an antibody detecting Ki67 that is not expressed in normal lung tissue. The only metastatic lesion large enough to be observed macroscopically was found in the lung of an animal from the FGFR4arg group. The other lungs obtained from the SW480arg group contained large clusters of tumor cells (>10 cells), whereas only single tumor cells or small clusters were found in the lungs of SW480gly and control animals. The number and size of metastatic colonies for each mouse were scored showing a higher average score for the SW480arg than for control or SW480gly tumors (Fig. 4C and D; P = 0.05).
FGFR4 expression and SNP rs351855 in human CRC
FGFR4 gene expression was determined from paired tissue specimen obtained from patients undergoing surgery for CRC (hospital population). FGFR4 was found overexpressed 2-fold or more as compared with normal mucosa in a subgroup of 20/71 (28%) of these specimens. The range of relative expression levels varied from 0.04 to 33.76 resulting in a mean of 2.73 ± 0.69 (increased above control at P = 0.015). However, expression level did not correlate with either histopathological parameters or the patients' SNP rs351855.
FGFR4 allele distribution was analyzed from the genomic DNA of 3,471 participants of the CORSA study. The genotype distribution in controls was in Hardy–Weinberg equilibrium. Multiple logistic regression was applied to compare individuals of the control group against 2 different case groups: the CRC group and the combined CRC + high-risk adenoma group (Table 1). The prevalence of homozygous arg/arg genotype in the control population was 8.1%, whereas it was 11.8% and 8.5% in the CRC and CRC + high-risk adenoma group, respectively, which was not statistically different from the control population. This resulted in a relative CRC risk of 1.42 (95% CI, 0.68–2.93) for developing CRC and 1.03 (95% CI, 0.77–1.36) for developing CRC or a high-risk adenomatous polyp conferred by SNP rs351855 (Table 2). Both were not statistically different from risk of the homozygous gly/gly population.
Table 2. FGFR4 (rs351855) genotype distribution and CRC risk.
| CRC |
CRC + high-risk adenomas |
||||||
|---|---|---|---|---|---|---|---|
| Genotype | Control | Cases | OR (95% CI) | Significance level | Cases | OR (95% CI) | Significance level |
| gly/gly | 802 | 42 | 1.0 | n.s. | 190 | 1.00 | n.s. |
| gly/arg | 723 | 33 | 0.87 (0.54–1.40) | n.s. | 148 | 0.86 (0.67–1.09) | n.s. |
| arg/arg | 135 | 10 | 1.41 (0.68–2.93) | n.s. | 25 | 0.81 (0.51–1.28) | n.s. |
NOTE: Genotype was analyzed from blood samples of participants of the CORSA study and analyzed in relation to CRC and high-risk polyp diagnosis.
Abbreviation: n.s., not significant.
Histopathologic parameters were available for 55 of the CRC patients in the CORSA study population. As this was not sufficient for a meaningful analysis, further genotype information was obtained from 122 tissue specimens of the hospital population. Because of the different settings for the diagnosis, the tumor stage distribution was different for the 2 CRC groups. Specifically, the percentage of stage I tumors was lower in the hospital population (3.9% vs. 26.8% in the CORSA screening study; Supplementary Table S2). Additional details can be found in Supplementary Materials.
On the basis of the strong promigratory impact of FGFR4arg even in an FGFR4gly background, the study populations were grouped into patients with a gly/gly genotype and arg carriers consisting of both heterozygous and arg-homozygous patients. To determine whether the different stage distribution of the CORSA and hospital populations affects, the association of SNP rs351855 with tumor stage, the data for both populations were analyzed separately with similar results (Table 3). In the hospital population, all stage I tumors were obtained from gly-homozygous patients and the fraction of arg carriers increased with tumor stage, resulting in a 19.4-fold risk (95% CI, 1.10–359.00 by Fisher exact test) for tumors stage II or higher. The main difference in the CORSA population was the presence of 33% FGFR4arg hetero- and homozygous individuals in the stage I group, whereas the fraction of FGFR4arg genotypes in stage II was lower compared with the hospital population (52% vs. 62%). No differences in allele distribution between the 2 populations were seen for stages III and IV. Because of the small number of cases in the CORSA population, no significant results could be obtained. However, the trend of increasing fraction of arg carriers with higher tumor stage was similar to the hospital population. In the combined hospital and CORSA population, patients carrying the arg allele had a 5-fold increased risk of tumor stage II or higher already at diagnosis (95% CI, 1.75–14.60 by Fisher exact test). SNP rs351855 did not correlate with any other parameter including tumor size and grade (data not shown).
Table 3. FGFR4 (rs351855) genotype distribution and CRC tumor stage.
| Stage | gly/gly- | arg/arg gly/arg | arg/arg, gly/arg (% of total) |
OR | (95%CI) | Significance level |
|---|---|---|---|---|---|---|
| All patients | ||||||
| I | 15 | 5 | 25 | |||
| II | 33 | 49 | 59 | 4.5 | 1.48–13.44 | ** |
| III | 19 | 40 | 67 | 6.7 | 2.10–21.16 | *** |
| IV | 9 | 12 | 57 | 4.0 | 1.06–15.14 | * |
| ≥ II | 61 | 101 | 62 | 5.1 | 1.75–14.60 | ** |
| Hospital patients only | ||||||
| I | 5 | 0 | 0 | |||
| II | 23 | 38 | 62 | 18.0 | 0.95–341.20 | ** |
| III | 15 | 32 | 68 | 23.1 | 1.20–444.40 | ** |
| IV | 6 | 8 | 57 | 14.4 | 0.67–310.10 | * |
| ≥ II | 44 | 78 | 64 | 19.4 | 1.05–359.40 | ** |
| CORSA patients only | ||||||
| I | 10 | 5 | 33 | |||
| II | 10 | 11 | 52 | 2.2 | 0.56–8.69 | n.s. |
| III | 4 | 8 | 66 | 4.0 | 0.80–20.02 | n.s. |
| IV | 3 | 4 | 57 | 2.7 | 0.42–16.83 | n.s. |
| ≥ II | 17 | 23 | 57 | 2,1 | 0.81–5.28 | n.s. |
NOTE: Genotype information was obtained from blood samples 57 of patients with CRC in the CORSA population and from tissue of 127 patients at hospitals in Vienna and Budapest. *, **, and *** represent differences to stage I at P ≤ 0.05, P ≤ 0.01, and P ≤ 0.001, respectively.
Abbreviation: n.s., not significant.
Discussion
The gly388arg polymorphism of FGFR4 has been described as a predisposing and/or prognostic factor for malignancies of the lung (28, 29), the prostate (30), the head and neck (4), the breast (6), the liver (31), and for melanomas (7). For CRC, there are 3 prior reports: Bange and colleagues have shown a correlation of the FGFR4arg allele with tumor size and metastasis (1) and Gordon and colleagues described a correlation with recurrence after chemoradiation (8). In contrast, Spinola and colleagues did not observe any association of the SNP rs351855 with either risk or prognostic parameters (9).
In this study, we have constructed moderately FGFR4 over-expressing cell lines for analysis of the cellular mechanisms induced by the 2 polymorphic forms of FGFR4. We have used 3 different cell lines SW480, HCT116, and HT29 that represent 3 possible variations of endogenous FGFR4 expression background–low expression, high FGFR4gly expression, and high FGFR4arg expression—resulting in 3 sets of isogenic human cell lines. Using an assay panel informative about malignant growth and cell migration, we observed that both FGFR4 alleles stimulated anchorage-independent growth as well as cell migration in SW480 cells that have low endogenous FGFR4 expression. Comparison of the 2 alleles revealed that FGFR4gly was the stronger stimulator of malignant cell growth, whereas FGFR4arg was the stronger activator of cell migration. The differential impact was more obvious in the cell lines with high endogenous FGFR4 expression. In the FGFR4gly background of HCT116 cells FGFR4gly had no additional effect on malignant growth but still stimulated cell migration, whereas FGFR4arg stimulated cell migration, but inhibited anchorage-independent growth. In contrast, in the FGFR4arg expressing HT29 cells both alleles increased malignant growth, whereas no additional effect of FGFR4arg and inhibition by FGFR4gly was observed for cell migration. This indicates that both polymorphic alleles are capable of counteracting the main activity of the respective other form. This agrees with reports on inhibition of cell migration by FGFR4gly in breast cancer cells by Bange and colleagues (1). However, this only occurred in a high FGFR4arg background, whereas FGFR4gly was fully capable of stimulating cell migration in a low FGFR4 background in CRC cells.
The most consistent difference between the FGFR4gly and FGFR4arg alleles was observed in colony formation assays where FGFR4arg did not stimulate any of the cell lines used. The assay determines the combined effect of cell attachment and growth potential so that both the weaker impact of FGFR4arg on growth and viability and the reduction in cell attachment inherent in the ability to better migrate should contribute to this effect.
SW480 transfectants were used for tumorigenicity studies in vivo, because they mainly express the transfected FGFR4 allele. After xenotransplantation under the skin of SCID mice, SW480gly cells grew to larger tumors locally, whereas SW480arg cells had a higher tendency to metastasize confirming the differential oncogenic effect of FGFR4 polymorphic alleles in vivo.
Analysis of human tissue specimen supports the same conclusions: overall expression of FGFR4 in human tumor specimens was elevated about 2-fold because of very strong upregulation (up to 30-fold) in a 28% subgroup of patients. Expression level was not related to histopathologic parameters or FGFR4 genotype, however. Both polymorphic alleles were affected in a similar way indicating that both alleles have a similar potential of tumorigenic impact in CRC.
With regard to downstream signaling activity, both polymorphic forms of FGFR4 activated FRS2α and survival signaling downstream of PI3K as indicated by increased phosphorylation of the Akt-substrate GSK3β and of S6. Differential activity was seen with the primary receptor substrates PLCγ and FRS2α that were better recruited into the signaling complex by FGFR4gly, and for c-src that was increased by FGFR4arg. The latter effect has been correlated with FGFR-dependent migration signaling in previous studies. Association of c-src activation with higher receptor stability and extended signaling activity with induction of cell migration has been described for N-CAM–mediated induction of cell migration in HeLa cells (32).
We actually did observe higher mRNA levels in CRC cell lines carrying an arg allele than in gly/gly homozygous cell lines, similar to observations in breast cancer cell lines (1). On the protein level, the differences of expression between FGFR4gly and FGFR4arg were still clearer: FGFR4 protein on the cell membrane was increased 4- to 12-fold in FGFR4arg transfectants, but only 1.6- to 4-fold in FGFR4gly cells. This strongly suggests higher stability of the FGFR4arg protein as compared with FGFR4gly similar to observations in prostate cancer where increased stability and sustained phosphorylation of FGFR4arg has been discussed as an essential contribution to the metastatic phenotype (33).
In the absence of exogenously added ligands, activation of FGFR4-dependent signaling has to come from autocrine growth factors in the culture supernatant. For the SW480 cells, this will mainly be FGF18 that is a survival and migration factor in CRC cells (17, 20) and a strong ligand for FGFR4 (34). Stronger membrane recruitment and activation of FRS2α by FGFR4gly explains the stronger growth impact of the receptor on tumor cell growth by a higher activity of canonical survival signaling (35, 36). Activation of downstream survival signaling is similar in both transfectants suggesting a different pathway is used that still needs to be elucidated.
The CORSA study population consists of 3,471 participants. As it was conducted within the context of a screening program, it provided a very well defined control population consisting of those participants that were found free of both polyps and cancer by colonoscopy. A disadvantage of the early-detection setting is the low incidence of CRC. Only 2.8% of the participants had already developed cancer and the staging was generally lower than in the hospital-based CRC group. Incidence of high-risk adenomas among the CORSA participants was 14% and overall adenoma incidence was 42%. On the basis of the power analysis for the cohort, an OR of 2.36 for the arg/arg group could have been determined with a significance level of P = 0.05. On the basis of the multistep nature of CRC, adenoma incidence can be regarded as representative of tumor initiation (37, 38). Therefore, we extended analysis to the larger group of CRC + high-risk adenomas that allowed recognition of an OR = 1.71 for the arg/arg patients. Actual differences in ORs observed in our study were too small to be considered significant.
In contrast, significant correlation was observed within the CRC group between the presence of an FGFR4arg allele in the patients' genome and higher tumor stage but not with tumor size or grade. In the hospital population for patients carrying the arg allele, the relative risk of their tumor being stage II or higher was 19-fold. All CRC patients with stage I tumors in the hospital population were FGFR4gly homozygous. In the CORSA population, the number of stage I tumors was much higher because of the diagnosis in the context of a screening program and also included 33% FGFR4arg carriers. This difference and the small number of patients in the CORSA population prevented observation of a significant effect of FGFR4arg on tumor stage. However, a similar trend toward increased frequency of FGFR4arg carriers in higher tumor stages was observed as in the hospital population. Addition of the CORSA patients in the overall analysis lowered the extent of the impact FGFR4arg had on the risk of higher stage tumors but did not abolish it. This further supports a role for the polymorphic allele in tumor progression and an invasive phenotype. These results are in agreement with the studies of Bange and colleagues (1) and Gordon (8) who reported aspects of more aggressive tumor behavior. It contradicts Spinola's report of no impact of FGFR4 on CRC (9). Like ours, all previous studies report on patient cohorts smaller than 200.
In summary, the results obtained from the functional characterization of our cell line models show oncogenic effects in CRC for both polymorphic forms of FGFR4. Consequently, both FGFR4 forms are suitable candidate therapeutic targets in CRC. In addition, results on allele distribution in CRC patients that need to be tested in a larger population indicate that the FGFR4arg allele may serve as a prognostic marker for more aggressive tumors and that FGFR4arg carriers may profit from CRC screening and early detection.
Supplementary Material
Acknowledgments
The authors thank X. Hudec and K. Bernhart for expert technical assistance.
Grant Support
This work was supported by the Austrian National Bank (Project 12684), the Austrian Science Foundation (P 19920, P23693), and OTKA MOB 80325 to B. Hegedus.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
Conception and design: M. Grusch, W. Berger, B. Marian
Development of methodology: A. Gsur, B. Grasl-Kraupp, M. Grusch, B. Marian
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): C. Heinzle, A. Gsur, M. Hunjadi, Z. Erdem, S. Stättner, J. Karner, M. Klimpfinger, F.F. Wrba, A. Reti, B. Hegedus, K. Holzmann, W. Berger
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): C. Heinzle, A. Baierl, B. Grasl-Kraupp, K. Holzmann, W. Berger, B. Marian
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): A. Gsur, M. Klimpfinger, F.F. Wrba, K. Holzmann
Writing, review, and/or revision of the manuscript: C. Heinzle, C. Gauglhofer, S. Stättner, J. Karner, M. Klimpfinger, F.F. Wrba, B. Grasl-Kraupp, M. Grusch, W. Berger, B. Marian
Study supervision: B. Marian
References
- 1.Bange J, Prechtl D, Cheburkin Y, Specht K, Harbeck N, Schmitt M, et al. Cancer progression and tumor cell motility are associated with the FGFR4 Arg(388) allele. Cancer Res. 2002;62:840–7. [PubMed] [Google Scholar]
- 2.Ma Z, Tsuchiya N, Yuasa T, Inoue T, Kumazawa T, Narita S, et al. Polymorphisms of fibroblast growth factor receptor 4 have association with the development of prostate cancer and benign prostatic hyper-plasia and the progression of prostate cancer in a Japanese population. Int J Cancer. 2008;123:2574–9. doi: 10.1002/ijc.23578. [DOI] [PubMed] [Google Scholar]
- 3.Spinola M, Leoni V, Pignatiello C, Conti B, Ravagnani F, Pastorino U, et al. Functional FGFR4 Gly388Arg polymorphism predicts prognosis in lung adenocarcinoma patients. J Clin Oncol. 2005;23:7307–11. doi: 10.1200/JCO.2005.17.350. [DOI] [PubMed] [Google Scholar]
- 4.da Costa Andrade VC, Parise O, Jr, Hors CP, de Melo Martins PC, Silva AP, Garicochea B. The fibroblast growth factor receptor 4 (FGFR4) Arg388 allele correlates with survival in head and neck squamous cell carcinoma. Exp Mol Pathol. 2007;82:53–7. doi: 10.1016/j.yexmp.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Streit S, Bange J, Fichtner A, Ihrler S, Issing W, Ullrich A. Involvement of the FGFR4 Arg388 allele in head and neck squamous cell carcinoma. Int J Cancer. 2004;111:213–7. doi: 10.1002/ijc.20204. [DOI] [PubMed] [Google Scholar]
- 6.Thussbas C, Nahrig J, Streit S, Bange J, Kriner M, Kates R, et al. FGFR4 Arg388 allele is associated with resistance to adjuvant therapy in primary breast cancer. J Clin Oncol. 2006;24:3747–55. doi: 10.1200/JCO.2005.04.8587. [DOI] [PubMed] [Google Scholar]
- 7.Streit S, Mestel DS, Schmidt M, Ullrich A, Berking C. FGFR4 Arg388 allele correlates with tumour thickness and FGFR4 protein expression with survival of melanoma patients. Br J Cancer. 2006;94:1879–86. doi: 10.1038/sj.bjc.6603181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon MA, Gil J, Lu B, Zhang W, Yang D, Yun J, et al. Genomic profiling associated with recurrence in patients with rectal cancer treated with chemoradiation. Pharmacogenomics. 2006;7:67–88. doi: 10.2217/14622416.7.1.67. [DOI] [PubMed] [Google Scholar]
- 9.Spinola M, Leoni VP, Tanuma J, Pettinicchio A, Frattini M, Signoroni S, et al. FGFR4 Gly388Arg polymorphism and prognosis of breast and colorectal cancer. Oncol Rep. 2005;14:415–9. [PubMed] [Google Scholar]
- 10.Itoh N. The Fgf families in humans, mice, and zebrafish: their evolutional processes and roles in development, metabolism, and disease. Biol Pharm Bull. 2007;30:1819–25. doi: 10.1248/bpb.30.1819. [DOI] [PubMed] [Google Scholar]
- 11.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–29. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 12.Kubota Y, Ito K. Chemotactic migration of mesencephalic neural crest cells in the mouse. Dev Dyn. 2000;217:170–9. doi: 10.1002/(SICI)1097-0177(200002)217:2<170::AID-DVDY4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Heinzle C, Sutterluty H, Grusch M, Grasl-Kraupp B, Berger W, Marian B. Targeting fibroblast-growth-factor-receptor-dependent signaling for cancer therapy. Expert Opin Ther Targets. 2011;15:829–46. doi: 10.1517/14728222.2011.566217. [DOI] [PubMed] [Google Scholar]
- 14.Allerstorfer S, Sonvilla G, Fischer H, Spiegl-Kreinecker S, Gauglhofer C, Setinek U, et al. FGF5 as an oncogenic factor in human glioblastoma multiforme: autocrine and paracrine activities. Oncogene. 2008;27:4180–90. doi: 10.1038/onc.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gauglhofer C, Sagmeister S, Schrottmaier W, Fischer C, Rodgarkia-Dara C, Mohr T, et al. Up-regulation of the fibroblast growth factor 8 subfamily in human hepatocellular carcinoma for cell survival and neoangiogenesis. Hepatology. 2011;53:854–64. doi: 10.1002/hep.24099. [DOI] [PubMed] [Google Scholar]
- 16.Metzner T, Held G, Bedeir A, Peter-Vörösmarty B, Ghassemi S, Heinzle C, et al. Fibroblast growth factor receptors as therapeutic targets in human melanoma: synergism with BRAF inhibition. J Invest Dermatol. 2011;131:2087–95. doi: 10.1038/jid.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonvilla G, Allerstorfer S, Stattner S, Karner J, Klimpfinger M, Fischer H, et al. FGF18 in Colorectal tumour cells: autocrine and paracrine effects. Carcinogenesis. 2008;29:15–24. doi: 10.1093/carcin/bgm202. [DOI] [PubMed] [Google Scholar]
- 18.Roidl A, Berger H-J, Kumar S, Bange J, Knyazev P, Ullrich A. Resistance to chemotherapy is associated with fibroblast growth factor receptor 4 upregulation. Clin Cancer Res. 2009;15:2058–66. doi: 10.1158/1078-0432.CCR-08-0890. [DOI] [PubMed] [Google Scholar]
- 19.Stadler CR, Knyazev P, Bange J, Ullrich A. FGFR4 GLY388 isotype suppresses motility of MDA-MB-231 breast cancer cells by EDG-2 gene repression. Cell Signal. 2006;18:783–94. doi: 10.1016/j.cellsig.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Sonvilla G, Allerstorfer S, Heinzle C, Stattner S, Karner J, Klimpfinger M, et al. Fibroblast growth factor receptor 3-IIIc mediates colorectal cancer growth and migration. Br J Cancer. 2010;102:1145–56. doi: 10.1038/sj.bjc.6605596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger W, Setinek U, Hollaus P, Zidek T, Steiner E, Elbling L, et al. Multidrug resistance markers P-glycoprotein, multidrug resistance protein 1, and lung resistance protein in non-small cell lung cancer: prognostic implications. J Cancer Res Clin Oncol. 2005;131:355–63. doi: 10.1007/s00432-004-0653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feik E, Baierl A, Hieger B, Führlinger G, Pentz A, Stättner S, et al. Association of IGF1 and IGFBP3 polymorphisms with colorectal polyps and colorectal cancer risk. Cancer Causes Control. 2010;21:91–97. doi: 10.1007/s10552-009-9438-4. [DOI] [PubMed] [Google Scholar]
- 23.Hofer P, Baierl A, Feik E, Führlinger G, Leeb G, Mach K, et al. MNS16A tandem repeats minisatellite of human telomerase gene: a risk factor for colorectal cancer. Carcinogenesis. 2011;32:866–71. doi: 10.1093/carcin/bgr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gsur A, Bernhart K, Baierl A, Feik E, Führlinger G, Hofer P, et al. No association of XRCC1 polymorphisms Arg194Trp and Arg399Gln with colorectal cancer risk. Cancer Epidemiol. 2011;35:e38–e41. doi: 10.1016/j.canep.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125:171–80. doi: 10.1002/ijc.24343. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs ET, Thompson PA, Martinez ME. Diet, gender, and colorectal neoplasia. J Clin Gastroenterol. 2007;41:731–46. doi: 10.1097/MCG.0b013e3180338e56. [DOI] [PubMed] [Google Scholar]
- 27.Dimidenko E. Sample size determination for logistic regression revisited. Stat Med. 2007;26:3385–97. doi: 10.1002/sim.2771. [DOI] [PubMed] [Google Scholar]
- 28.Falvella FS, Frullanti E, Galvan A, Spinola M, Noci S, De Cecco L, et al. FGFR4 Gly388Arg polymorphism may affect the clinical stage of patients with lung cancer by modulating the transcriptional profile of normal lung. Int J Cancer. 2009;124:2880–5. doi: 10.1002/ijc.24302. [DOI] [PubMed] [Google Scholar]
- 29.Matakidou A, El Galta R, Rudd MF, Webb EL, Bridle H, Eisen T, et al. Further observations on the relationship between the FGFR4 Gly388Arg polymorphism and lung cancer prognosis. Br J Cancer. 2007;96:1904–7. doi: 10.1038/sj.bjc.6603816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Stockton DW, Ittmann M. The fibroblast growth factor receptor-4 Arg388 allele is associated with prostate cancer initiation and progression. Clin Cancer Res. 2004;10:6169–78. doi: 10.1158/1078-0432.CCR-04-0408. [DOI] [PubMed] [Google Scholar]
- 31.Ho HK, Pok S, Streit S, Ruhe JE, Hart S, Lim KS, et al. Fibroblast growth factor receptor 4 regulates proliferation, anti-apoptosis and alpha-fetoprotein secretion during hepatocellular carcinoma progression and represents a potential target for therapeutic intervention. J Hepatol. 2009;50:118–27. doi: 10.1016/j.jhep.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Francavilla C, Cattaneo P, Berezin V, Bock E, Ami D, de Marco A, et al. The binding of NCAM to FGFR1 induces a specific cellular response mediated by receptor trafficking. J Cell Biol. 2009;187:1101–16. doi: 10.1083/jcb.200903030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Yu W, Cai Y, Ren C, Ittmann MM. Altered fibroblast growth factor receptor 4 stability promotes prostate cancer progression. Neoplasia. 2008;10:847–56. doi: 10.1593/neo.08450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family: the complete mammalian fgf family. J Biol Chem. 2006;281:15694–700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acevedo VD, Ittmann M, Spencer DM. Paths of FGFR-driven tumorigenesis. Cell Cycle. 2009;8:580–8. doi: 10.4161/cc.8.4.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knights V, Cook SJ. De-regulated FGF receptors as therapeutic targets in cancer. Pharmacol Ther. 2010;125:105–17. doi: 10.1016/j.pharmthera.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Fearon ER, Jones PA. Progressing toward a molecular description of colorectal cancer development. Faseb J. 1992;6:2783–90. doi: 10.1096/fasebj.6.10.1321771. [DOI] [PubMed] [Google Scholar]
- 38.Muto T, Bussey H, Morson B. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251–70. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.