Abstract
Cervical cancer is the fourth most common cause of cancer-related deaths in women worldwide. With the development of detection of precancerous lesions and preventive human papillomavirus (HPV) vaccination program, a survival improvement has been observed in these patients in developed countries, although disparities in accessibility to treatments exist across countries. While early-stage cervical cancer can be curable with surgery, prognosis of patients who recur remains poor, with limited treatment options. In this latter setting, recently, bevacizumab, an antiangiogenic monoclonal antibody targeting vascular endothelial growth factor (VEGF), has been shown to improve overall survival in combination with chemotherapy as compared with chemotherapy alone. No standard treatments exist beyond this treatment regimen. New effective treatments are therefore much needed in this setting. Immunotherapy has represented a breakthrough in recent years in oncology, with antitumor activity reported with immune-checkpoint inhibitors in a variety of tumor types. We discuss here the latest evidence and clinical usefulness of pembrolizumab, anti-PD-1 checkpoint inhibitor, in the treatment of advanced cervical cancer.
Keywords: cervical cancer, immune checkpoints, immunotherapy, pembrolizumab
Introduction
Cervical cancer is the fourth most common cause of cancer-related deaths in women worldwide.1 With availability and improvements in screening, which allows for the detection and removal of precancerous lesions, generalization of vaccination campaigns of women against human papillomavirus (HPV), and improvement of therapeutics available, a survival improvement has been observed in patients with cervical cancers over recent decades, mostly in developed countries where preventive strategies have been developed, and where access to most relevant and expensive therapeutics is facilitated. For example, in Eastern Europe, low survival rates are still observed, in comparison to Western Europe, suggesting insufficient screening/early diagnosis and also inadequate treatment options available.2 In developing countries, this contrast is even more critical, with around 527,600 new cases reported and 265,700 deaths worldwide in 2012.1
Early-stage cervical cancer can be cured with surgery, while concurrent chemoradiation is the treatment of choice for locally advanced stages. Patients with recurrent or metastatic cancers have, however, limited treatment options. The use of cisplatin-based chemotherapy regimens has been reported to be the most active choice in first-line treatment for advanced cervical cancer. Despite this, median overall survival of this patient population barely exceeds 1 year, with short-lived responses and rapid deterioration of quality of life.3 Recently, bevacizumab, a humanized monoclonal antibody targeting vascular endothelial growth factor (VEGF), defined as an antiangiogenic agent, has been approved in combination with chemotherapy as first-line therapy, with a statistically significant overall survival improvement as compared with chemotherapy alone.4 No validated treatment options exist beyond this first-line treatment regimen. Chemotherapy regimens in this situation are associated with substantial toxicity and poor efficacy with a median overall survival of 7 months.5 In several published phase II studies, evaluating single chemotherapy agents in the second-line setting, including topotecan, vinorelbine, gemcitabine, docetaxel and pemetrexed, the response rate ranged between 4.5% and 18%, with a median progression-free survival (PFS) and overall survival (OS) ranging approximately between 2–5 and 5–16 months, respectively. Cisplatin is still an option as a single agent in recurrent or metastatic cervical cancer with an overall response rate (ORR) ranging between 13% and 23%.6
New effective treatments are therefore much needed in this setting. Immunotherapy has represented a breakthrough in recent years in oncology, with antitumor activity reported with immune-checkpoint inhibitors in a variety of tumor types. We discuss here the latest evidence and clinical usefulness of pembrolizumab, anti-PD-1 checkpoint inhibitor, in the treatment of advanced cervical cancer.
Oncogenesis of cervical cancer
The majority of cervical cancers are associated with oncogenic HPV subtypes, high-risk type HPV-16 and HPV-18 being the most frequently involved in cervical cancer development.7 HPV belongs to the Papillomaviridae family with a nonenveloped, circular DNA genome that is covered with capsid proteins. HPV infections, such as HPV-16, are widespread in human population, and are commonly transmitted by sexual contact. Infection requires the availability of basal-layer cells that are able to proliferate, and occurs usually in microlesions of mucosa. The infected cell divides, and some of the progeny migrate into suprabasal differentiating cell layers, where viral genes are activated and capsid proteins are formed.7 HPV infections initially induce squamous intraepithelial lesions in women. The majority of these lesions will be cleared spontaneously in 6–12 months after appearance, in part by immunological intervention. The role of the immunologic system in the clearance of HPV is supported by the observed increased incidence and prolonged persistence of squamous intraepithelial lesions in immunosuppressed women.8,9 A small percentage of these intraepithelial lesions, however, will persist and progress to high-grade squamous intraepithelial lesions, carcinoma in situ, and to invasive carcinoma.
The predominant histologic subtype of invasive carcinoma is squamous cell carcinoma, accounting for 75% of cases, followed by adenocarcinomas representing between 20% and 24% of cases. Lymphomas and melanomas account for less than 1% of all cases.
Rationale for immunotherapy in cervical cancer
Cancer cells provide a diverse set of antigens that the immune system can use to distinguish those cells from their normal counterparts.10 This immune response, concerning T cells, is initiated through antigen recognition by the naïve T-cell receptor (TCR), via the antigen–peptide major-histocompatibility complex (MHC), and is regulated by a balance between costimulatory and inhibitory signals, called immune checkpoints.11 To be fully activated, T cells need a costimulatory antigen-dependent signal that occurs through the interaction between CD28 on T cells and B7-1 and B7-2 on the antigen-presenting cells (APC). Tumor cells can escape T-cell immune responses through inhibitory immune checkpoints.
Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), the first immune-checkpoint receptor to be clinically targeted, is expressed exclusively on T cells where it primarily regulates the amplitude of the early stages of T-cell activation, capable of downregulating T-cell activation, to prevent overstimulation of the immune system.11 CTLA-4 has much higher affinity with the B7 complex than CD28. By targeting the immune checkpoints that inhibit immune T-cell response, the purpose is to enhance endogenous antitumor immunity. The development of a lethal lymphoproliferative disorder and autoimmune phenotype in young CTLA4-deficient mice illustrated the pivotal role of CTLA-4 in immune homeostasis, and potentially highlighted the possible immune toxicity of CTLA-4 antibodies.12,13 In contrast to the severe pathologic characteristic of CTLA-4-deficient mice, transient CTLA-4 antibody blockade enhances antigen-specific T-cell responses with an acceptable toxicity profile.11,14 Preclinical findings encouraged the production and clinical testing of fully humanized CTLA-4 antibodies, such as ipilimumab.15
Programmed-cell-death protein 1 (PD-1), is another immune-checkpoint receptor, thought to be a more distal immune modulator than CTLA-4, whose major role is to limit the activity of effector T cells in peripheral tissues at the time of an inflammatory response to infection, to limit autoimmunity when effector T cells become activated, and that can induce a major immune resistance mechanism within the tumor microenvironment.11 PD-1 can be expressed on T cells when they become activated, and is highly expressed on regulatory T cells (TReg cells) where it may enhance their proliferation in the presence of a ligand, enhancing their immunosuppressive activity.11 PD-1 is also expressed on other non-T-lymphocytes subsets, such as B cells or natural killer cells. The two ligands for PD-1 are PD-1 ligand 1 (PD-L1) and PD-L2, the ligation of PD-1 to its ligand leading to a co-inhibitory signal in activated T cells.11 PD-1 is expressed on a large proportion of tumor-infiltrating lymphocytes (TILs) from many different tumor types, as PD-L1 has been reported to be the ligand that is commonly upregulated in many human cancers, promoting immune evasion of tumor cells, providing a good rationale for the development of antitumor therapies targeting the PD-1/PD-L1 pathway.16,17 In multivariate analysis, PD-L1 expression on tumor cells has been identified as an independent pejorative prognostic factor, the overall survival rate of melanoma patients whose tumors highly express PD-L1 being significantly lower than that of patients whose tumors poorly express PD-L1.18 Other reports in various tumor types have shown that PD-L1 expression correlates with poor prognosis, or shown no correlation with prognosis.19,20
There is a strong rationale supporting the development of immunotherapy in cervical cancer given the presence of a virus in its oncogenesis leading to antigens production (Figure 1). The presentation of viral antigens by APC activates naïve T cells to proliferate and differentiate into effector T cells, and therefore initiate an HPV-specific immune response, recognizing and eliminating virus-infected cells. Interestingly, a higher expression of PD-L1 has been described in virus-inducing cancers, and an upregulation of PD-1 and PD-L1 has been observed in high risk HPV-related cervical intraepithelial neoplasia.21–23
Figure 1.
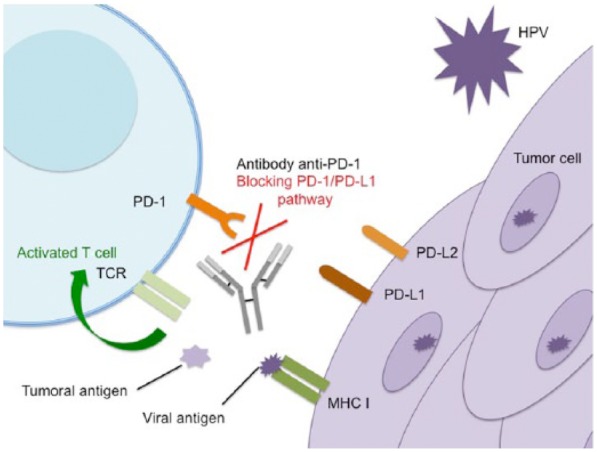
Mechanism of action of anti-programmed-cell-death-protein-1 antibody in cervical cancer.
HPV, human papillomavirus; MCH I, major histocompatibility complex I; TCR, T-cell receptor; PD-1, programmed-cell-death-protein 1; PD-L1, PD-1 ligand 1.
Neoantigens, such as cancer antigens or viral antigen in HPV-related cancer, are recognized by T cells via antigens-presenting cells, leading to T-cell activation. Immunotolerance in the tumoral microenvironment includes PD-1 overexpression on tumoral-infiltrating lymphocytes and PD-L1 overexpression on tumor cells, leading to the inhibition of activated effector T cells, via inhibitory checkpoint signals. By inhibiting the interaction between PD-1 and PD-L1, anti-PD-1/PD-L1 antibodies block the inhibitory checkpoint signals and restore endogenous antitumor immunity.
Clinical development of pembrolizumab
Pembrolizumab is a highly selective humanized monoclonal IgG4 kappa isotype antibody targeting PD-1. Pembrolizumab has been evaluated in a phase I trial in 135 patients with both pretreated and treatment-naïve advanced melanoma.24 The overall response rate (ORR) was 38% across all dose cohorts irrespective of prior treatment, with durable response of 11 months at median follow-up, and 81% of patients who had a response still receiving treatment at the time of data analysis. In terms of immune-related adverse events, pneumonitis was noted in 4% (all below grade 3), grade 3 or 4 transaminitis in 1%, grade 3 nephritis in 1%, grade 3 hyperthyroidism in 1%, grade 2 adrenal insufficiency in 1%, and hypothyroidism in 1%. Diarrhea was reported in 20% of the patients; however, only one case was grade 3. In a phase III study, pembrolizumab was shown to be more effective than ipilimumab in patients with recurrent and metastatic melanoma.25 Given those results, the US Food and Drug Administration (FDA) approved pembrolizumab in September 2014 first for the treatment of patients with advanced or unresectable melanoma, following treatment with ipilimumab, and eventually in the first-line setting. Pembrolizumab has also been approved by the FDA under the agency’s accelerated approval program in October 2015, for the treatment of patients with advanced non-small cell lung cancer (NSCLC) whose disease has progressed after platinum-based chemotherapy and whose tumors express PD-L1 using immunohistochemical analysis, with a cutoff of at least 1% of PD-L1 expression in tumor cells.26 More recently, pembrolizumab was approved, for the treatment of patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) with disease progression on or after platinum-containing chemotherapy, on the basis of tumor response rate and durability of response from the phase Ib study.27
Preliminary results from the phase Ib KEYNOTE-028 study, evaluating the safety and efficacy of pembrolizumab in patients with advanced solid tumors were presented at the American Society of Clinical Oncology (ASCO) conference in 2016.28 This multicohort phase I trial included an expansion cohort of patients with advanced cervical squamous cell cancer. Patients had to have an unresectable or metastatic cervical cancer, failed prior systemic therapy and standard treatment, an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0-1, and a PD-L1 expression in ≥1% of tumor or stroma cells by immunohistochemistry (IHC). Pembrolizumab was given at a dose of 10 mg/kg every 2 weeks for up to 24 months or until confirmed progression, intolerable toxicity, death, or withdrawal of consent. The primary endpoint was the overall response rate according to RECIST 1.1. A total of 24 patients with recurrent or metastatic cervical cancer were enrolled, with 15 patients having distant metastases (63%). Median age was 41.5 years. Most patients (96%) had received prior radiotherapy, and two or more prior lines of chemotherapy in this setting (63%). Nearly half of patients (42%) had previously received bevacizumab. The median follow-up duration was 48.9 weeks. Treatment-related adverse events occurred in 18 patients (75%), with pyrexia (n = 4) and rash (n = 3) occurring in ≥10% of patients. Five patients (21%) had a grade 3 adverse event related to treatment, including two rashes, one neutropenia, one proteinuria, and two of whom discontinued pembrolizumab; one for a colitis and the other for a Guillain-Barre syndrome. No grade 4 or 5 adverse events were reported. ORR was 17%, including long-lasting responses (mean duration of response, 26 weeks). Although median PFS was modest, median OS reached 9 months, which is substantial in this heavily pretreated patient population. In summary, pembrolizumab was well tolerated and showed promising antitumor activity in patients with PD-L1-positive recurrent or metastatic cervical squamous cell cancer. The clinical benefit of pembrolizumab in advanced cervical cancer is being further investigated in the phase II KEYNOTE-158 trial [ClinicalTrials.gov identifier: NCT02628067].
PD-L1 expression in tumor cells has been the most studied biomarker for the prediction of treatment response to anti-PD1 or anti-PD-L1 therapies. PD-L1 diagnostic assays, based on immunohistochemistry on tumor biopsy specimens, are currently being used in the stratification of patients included in clinical trials that are evaluating anti-PD-1 or PD-L1 therapies; however, patients whose disease is PD-L1 negative can still achieve clinical benefit and objective responses from anti-PD1 or anti-PD-L1 therapies.29 The complexity to PD-L1 staining may be linked to the spatial and temporal heterogeneity in PD-L1 expression in tumor cells, the fact that different methods are used to measure PD-L1 expression in tumor only or including TILs, with different antibodies and staining protocols and also, that there are actually no defined standard thresholds for positive PD-L1 expression.29
PD-L2 has also been reported to be upregulated in certain tumor types, such as B-cell lymphomas and Hodgkin’s disease, pancreatic, esophageal or ovarian cancer.30,31 PD-L2 expression has been described to be correlated with a poorer prognosis in certain types of cancer, but it has not been shown to be correlated with treatment response to anti-PD-1 antibodies.32,33 Multiplexed-gene-expression profiling has also been used to develop immune-related gene-expression signatures. The NanoString nCounter technology allows analyses of the expression levels of up to 800 target genes including specific panels of immune-response genes. In different clinical trials evaluating pembrolizumab, different immune-response signatures have been evaluated on baseline tumor samples of patients including the 6-gene interferon-gamma (IFN-Υ) signature (IDO1, CXCL10, CXCL9, HLA-DRA, STAT1, IFNG), the 10-gene INF-Υ signature, and an expended-immune 28-gene signature, and correlated with overall response to anti-PD-1 treatment and OS in patients with various tumor types including advanced melanoma, HNSCC, anal canal, biliary tract, colorectal, esophageal and ovarian cancer. These results are consistent with the hypothesis that clinical responses to PD-1 blockade might occur in patients with a pre-existing interferon-mediated adaptive immune response.34–36
Immunotherapies beside pembrolizumab in cervical cancer
Signals of antitumor activity of ipilimumab have been reported in recurrent and metastatic cervical cancer in a phase I/II trial.37 After a run-in phase I safety cohort, a total of 42 patients were enrolled to received ipilimumab 10 mg/kg every 21 days for four cycles, followed with four cycles of maintenance therapy every 12 weeks for patients demonstrating radiologic response or stabilization. Toxicities were manageable. Grade 3 toxicities included diarrhea in four patients (10%) and colitis in three patients (7%). Of the 34 evaluable patients, three patients (9%) experienced an objective response. The median PFS was 2.5 months. Data regarding the expression of intratumoral biomarkers, pre and post treatment, such as CD3, CD4, PD-L1, or CD8+ cytotoxic T lymphocytes and FOXP3+-regulatory T cells, are pending.37
Several studies are ongoing evaluating immune-checkpoint inhibitors in the setting of advanced cervical cancer; selected ongoing trials are shown in (Table 1). An interesting approach is to combine immunotherapy with other types of treatments, as it is currently evaluated in a phase II study in combination with chemoradiation in advanced cervical cancer [ClinicalTrials.gov identifier: NCT02635360]. In this study, pembrolizumab will be administered concurrently, and following treatment with chemoradiation. This approach is interesting, since radiation therapy can kill malignant cells with ionizing radiation, releasing tumor antigens, creating a particularly immunogenic response that can initiate a relevant immune response in the irradiated tumor, but also in distant metastases (known as the abscopal effect), modulating tumor immunogenicity and enhancing both antigen presentation and cytokine production.38
Table 1.
Selected ongoing trials of immune-checkpoint inhibitors in cervical cancer.
| Agent(s) | Target(s) | Phase | Indication | Clinical Trials.gov identifier |
|---|---|---|---|---|
| Pembrolizumab | PD-1 | II | Multiple types of advanced solid tumors | NCT02628067 |
| Pembrolizumab | PD-1 | II | Advanced cervical cancer, in combination with chemoradiation | NCT02635360 |
| Durvalumab Tremelimumab |
PD-L1 CTLA-4 |
I | Advanced solid tumors, including advanced cervical cancer | NCT01975831 |
| Atezolizumab Carboplatin Cyclophosphamide |
PD-L1 | Ib | Advanced breast cancer and gynecologic cancer | NCT02914470 |
| Ipilimumab | CTLA-4 | I | FIGO stage IB2/IIA with positive para-aortic lymph nodes only and stage IIB/IIIB/IVA with positive lymph nodes; cervical cancer following chemoradiation | NCT01711515 |
| Nivolumab Ipilimumab |
PD-1 CTLA-4 |
I/II | Neoadjuvant cohort and metastatic cohort in virus-associated cancers including HPV-induced cervical cancer | NCT02488759 |
| Nivolumab | PD-1 | II | HPV-16-positive incurable solid cancers, treatment naïve or in second line, including HPV-16-induced cervical cancer | NCT02426892 |
| Atezolizumab Bevacizumab |
PD-L1 | II | Persistent, recurrent or metastatic cervical cancer | NCT02921269 |
| Ipilimumab | CTLA-4 | II | Metastatic or recurrent HPV-related cervical cancer | NCT01693783 |
| Nivolumab | PD-1 | II | Persistent, recurrent or metastatic cervical cancer | NCT02257528 |
PD-L1, programmed-cell-death-protein 1 ligand 1; CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; FIGO, International Federation of Gynecology and Obstetrics; PD1, programmed-cell-death protein 1; HPV, human papillomavirus.
All listed trials were referenced from ClinicalTrials.gov, last accessed 16 December 2016.
Another approach is the combination of immunotherapy with other classes of agents, such as anti-angiogenic agents. Bevacizumab is currently evaluated in combination with atezolizumab, a humanized antibody targeting PD-L1 in advanced cervical cancer in a phase II trial [ClinicalTrials.gov identifier: NCT02921269]. Anti-angiogenic treatment could potentially enhance antitumor effect by an enhanced antitumor immune response by increasing intratumoral T-cell infiltration, reducing the number of myeloid-derived suppressor cells, and reversing the imbalance of VEGF–VEGF receptor (VEGFR) signaling pathway present in the tumor microenvironment with the induction of the maturation of immature blood vessels, and therefore reducing the high interstitial pressure that could limit influx of immune cells in the tumor.39,40
Another interesting perspective is the combination of two different types of immunotherapies. As an example, durvalumab, an anti-PD-L1 antibody is being combined with tremelimumab, an anti-CTLA-4 antibody, in a phase I study [ClinicalTrials.gov identifier: NCT01975831], since promising antitumor activity has been reported in metastatic melanoma, with the combination of ipilimumab, an anti-CTLA-4 antibody, with nivolumab, an anti-PD-1 antibody, being more efficient in terms of overall survival than ipilimumab alone.41 Dose reductions of ipilimumab were needed in order to safely combine these two immune-checkpoint inhibitors.41
Other means of immune therapy are currently being developed in cervical cancer, especially in HPV-induced cervical cancer, typically anticancer therapeutic vaccines, that rely on cell-mediated immunity against HPV-infected cells, with the HPV-produced oncoproteins E6 and E7 used as the selected antigens.21,42 This vaccination is capable of inducing an immune response against cells already infected with HPV, including those with pre-invasive or invasive cervical lesions. Several approaches are currently being investigated, including live-vector-attenuated vaccines, such as ADXS11-001, which is a bacterial–vector vaccine targeting the oncoprotein E7, currently evaluated in ongoing phase II trials in nonresponding or recurrent cervical cancers [ClinicalTrials.gov identifiers: NCT02164461 and NCT01266460]. Preliminary results from a phase II trial, in patients with recurrent or refractory cervical cancer treated with ADXS11-001 [1 × 109 colony forming units (CFUs)] with or without cisplatin treatment showed an overall disease control rate of 43% (six complete responses, six partial responses, and 35 with stable disease) with median duration of response being 10.5 months in both treatment groups, and a 36% 12-months’ survival. The addition of cisplatin to ADXS11-001 did not significantly improve survival outcomes or tumor response. This study also showed a good safety profile; the majority of patients experienced a grade 2 adverse event during treatment, most commonly fever, and flu-like symptoms.43 Preliminary results of another study evaluating ADX-001 in patients with persistent/recurrent metastatic cervical cancer, confirmed the favorable safety profile of ADX-001 and 1/26 complete response [ClinicalTrials.gov identifier: NCT01266460].44 ADX-001 is currently being evaluated in a phase III trial, administered following chemoradiation as adjuvant treatment for high-risk locally advanced cervical cancer [ClinicalTrials.gov identifier: NCT02853604].
Another approach involves the ISA101 peptide vaccine, currently evaluated in a phase I/II trial in combination with standard of care chemotherapy (carboplatin and paclitaxel with or without bevacizumab) in women with HPV-positive advanced or recurrent cervical cancer who have no curative treatment options [ClinicalTrials.gov identifier: NCT02128126].
Adoptive T-cell therapy has shown promising results in melanoma and is currently evaluated in patients with HPV-induced metastatic cervical cancer, who had previously received platinum-based chemotherapy. An objective response (two complete responses, one partial response) was reported in three out of nine patients, with a complete response lasting 22 months.21,45 This strategy consists of transferring autologous tumor-reactive T cells, developed from cultured HPV TILs from HPV-induced cervical cancers. The T-cell cultures are initiated from fragments of metastatic tumors expanded ex vivo and tested for reactivity against HPV-16 or HPV-18 E6 and E7 oncoproteins. Cells are then selected for patient administration based on HPV–oncoprotein reactivity, rapid growth, high T-cell purity, and high frequency of CD8+ T cells. This strategy necessitates administration of a nonmyeloablative lymphocyte-depleting preparative chemotherapy regimen, such as the combination of cyclophosphamide and fludarabine. No adverse events secondary to TIL infusion were reported, whereas grade 3 and 4 adverse events were attributed to the cytotoxic chemotherapy regimen. Interestingly, the HPV reactivity of T cells in the infusion product correlated positively with clinical response, as well as the frequency of HPV-reactive T cells in peripheral blood 1 month after treatment.45
In the field of adoptive T-cell therapy, chimeric antigen receptors (CARs) T-cell therapy seems to be a promising approach as well, with impressive antitumor activity in relapsed and refractory acute lymphoblastic leukemia treated with CAR T cells targeting the B-cell-specific antigen CD19.46 After isolation of T cells from patients, T cells are engineered ex vivo to express a specific CAR at their surface, in order to identify and eliminate malignant cells through tumor-specific antigen recognition. These autologous T cells are then readministered to patients. A CAR is a recombinant receptor construct composed of an extracellular antigen-recognition domain, derived from a monoclonal antibody fragment (scFv), linked to intracellular signaling domains of the T-cell receptor complex. T cells engineered to express such CARs engage an antigen on a tumor cell through the extracellular antibody domain, thereby activating the T cells and stimulating a potent cytotoxic response.46 Some challenges include the safety profile (cytokine-release syndrome) that is sometimes poor, the ex vivo cell culture system used to manufacture large quantities of engineered T cells, and the gene transfer technologies used to engineer T cells to express CARs.46
A phase I study is currently recruiting patients with refractory/metastatic HPV-related cancers, to evaluate the safety of CAR T-cell therapy, using autologous T cells that are isolated from a patient, genetically engineered in vitro with a TCR-targeting HPV-16 E7 (E7 TCR) that displays specific reactivity against HPV-16+ target cells. Cells are then reinjected to the same patient; this treatment is being evaluated in combination with or without pembrolizumab [ClinicalTrials.gov identifier: NCT02858310].
Conclusion
Preliminary results from a phase I clinical study showed that pembrolizumab was well tolerated and showed potential antitumor activity in patients with PD-L1-positive heavily pretreated recurrent or metastatic cervical cancer. Pembrolizumab is currently being evaluated in the locally advanced setting in combination with radiation therapy given a strong biological rationale. However, response to pembrolizumab alone remains low, explaining the current evaluation of drug combinations with pembrolizumab, as well as strong efforts to identify predictive biomarkers or signatures of efficacy. Besides immune-checkpoint inhibitors, various therapeutic HPV vaccines are currently being developed, along with adoptive T-cell therapy in the treatment of HPV-related cervical cancer, showing some promising results in eliciting T-cell-mediated HPV-c.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Christophe Le Tourneau has participated in scientific advisory boards from MSD and received honoraria for this.
Contributor Information
Edith Borcoman, Department of Medical Oncology, Institut Curie, Paris & Saint-Cloud, France.
Christophe Le Tourneau, Department of Medical Oncology, Institut Curie, INSERM U900 Research unit, 35 rue Dailly, 92210 Saint-Cloud, France.
References
- 1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Sant M, Chirlaque Lopez MD, Agresti R, et al. Survival of women with cancers of breast and genital organs in Europe 1999–2007: results of the EUROCARE-5 study. Euro J Cancer 2015; pii: S0959-8049(15)00702-9. [DOI] [PubMed] [Google Scholar]
- 3. Monk BJ, Sill MW, McMeekin DS, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 2009; 27: 4649–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tewari KS, Sill MW, Long HJ, III, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 2014; 370: 734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tewari KS, Monk BJ. New strategies in advanced cervical cancer: from angiogenesis blockade to immunotherapy. Clin Cancer Res 2014; 20: 5349–5358. [DOI] [PubMed] [Google Scholar]
- 6. Boussios S, Seraj E, Zarkavelis G, et al. Management of patients with recurrent/advanced cervical cancer beyond first line platinum regimens: where do we stand? A literature review. Crit Rev Oncol Hematol 2016; 108: 164–174. [DOI] [PubMed] [Google Scholar]
- 7. Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2002; 2: 342–350. [DOI] [PubMed] [Google Scholar]
- 8. Zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst 2000; 92: 690–698. [DOI] [PubMed] [Google Scholar]
- 9. Petry KU, Scheffel D, Bode U, et al. Cellular immunodeficiency enhances the progression of human papillomavirus-associated cervical lesions. Int J Cancer 1994; 15: 836–840. [DOI] [PubMed] [Google Scholar]
- 10. Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 2008; 8: 467–477. [DOI] [PubMed] [Google Scholar]
- 11. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 1995; 270: 985–988. [DOI] [PubMed] [Google Scholar]
- 13. Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995; 3: 541–547. [DOI] [PubMed] [Google Scholar]
- 14. Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA 2003; 100: 4712–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ohaegbulam KC, Assal A, Lazar-Molnar E, et al. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med 2015; 21: 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8: 793–800. [DOI] [PubMed] [Google Scholar]
- 18. Hino R, Kabashima K, Kato Y, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010; 116: 1757–1766. [DOI] [PubMed] [Google Scholar]
- 19. Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005; 11: 2947–2953. [DOI] [PubMed] [Google Scholar]
- 20. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crafton SM, Salani R. Beyond chemotherapy: an overview and review of targeted therapy in cervical cancer. Clin Ther 2016; 38: 449–458. [DOI] [PubMed] [Google Scholar]
- 22. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015; 348: 69–74. [DOI] [PubMed] [Google Scholar]
- 23. Yang W, Song Y, Lu YL, et al. Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology 2013; 139: 513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369: 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372: 2521–2532. [DOI] [PubMed] [Google Scholar]
- 26. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372: 2018–2028. [DOI] [PubMed] [Google Scholar]
- 27. Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicenter, phase 1b trial. Lancet Oncol 2016; 17: 956–965. [DOI] [PubMed] [Google Scholar]
- 28. Frenel JS, Le Tourneau C, Bert H, et al. Pembrolizumab in patients with advanced cervical squamous cell cancer: preliminary results from the phase Ib KEYNOTE-028 study. J Clin Oncol 2016; 34(Suppl.): abstract 5515. [Google Scholar]
- 29. Gibney GT, Weiner LM, Atkins MB, et al. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016; 17: e542–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med 2003; 198: 851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 2015; 14: 561–584. [DOI] [PubMed] [Google Scholar]
- 32. Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 2007; 13: 2151–2157. [DOI] [PubMed] [Google Scholar]
- 33. Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014; 20: 5064–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ribas A, Robert C, Hodi FS, et al. Association of response to programmed death receptor 1 (PD-1) blockade with pembrolizumab (MK-3475) with an interferon-inflammatory immune gene signature. J Clin Oncol 2015: 33(Suppl.): abstract 3001. [Google Scholar]
- 35. Man Chow LQ, Mehra R, Haddad RI, et al. Biomarkers and response to pembrolizumab (pembro) in recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J Clin Oncol 2016; 34(Suppl.): abstract 6010. [Google Scholar]
- 36. Piha-Paul SA, Bennouna J, Albright A, et al. T-cell inflamed phenotype gene expression signatures to predict clinical benefit from pembrolizumab across multiple tumor types. J Clin Oncol 2016; 34(Suppl.): abstract 1536. [Google Scholar]
- 37. Lheureux S, O.Butler M, Clarke B, et al. A phase I/II study of ipilimumab in women with metastatic or recurrent cervical carcinoma: a study of the Princess Margaret and Chicago N01 Consortia. J Clin Oncol 2015; 33(Suppl.): abstract 3061. [Google Scholar]
- 38. Bernier J. Immuno-oncology: allying forces of radio- and immune-therapy to enhance cancer cell killing. Crit Rev Oncol Hematol 2016; 108: 97–108. [DOI] [PubMed] [Google Scholar]
- 39. Du Four S, K Maenhout S, P Niclou S, et al. Combined VEGFR and CTLA-4 blockade increases the antigen-presenting function of intratumoral DCs and reduces the suppressive capacity of intratumoral MDSCs. Am J Cancer Res 2016; 6: 2514–2531. [PMC free article] [PubMed] [Google Scholar]
- 40. Shrimali RK, Yu Z, Theoret MR, et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res 2010; 70: 6171–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Welters MJ, van der Sluis TC, van Meir H, et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci Transl Med 2016; 8: 334ra52. [DOI] [PubMed] [Google Scholar]
- 43. Basu P, Mehta AO, Jain MM, et al. ADXS11-001 immunotherapy targeting HPV-E7: final results from a phase 2 study in Indian women with recurrent cervical cancer. J Clin Oncol 2014; 32(Suppl. 5): abstract 5610. [Google Scholar]
- 44. Huh W, Dizon D, Powell MA, et al. ADXS11-001 immunotherapy in squamous or non-squamous persistent/recurrent metastatic cervical cancer: results from stage I of the phase II GOG/NRG0265 study (NCT01266460). J Clin Oncol 2016; 34(Suppl.): abstract 5516. [Google Scholar]
- 45. Stevanovic S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol 2015; 33; 1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maude SL, Teachey DT, Porter DL, et al. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 2015; 125: 4017–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]


