Abstract
Background:
A subset of patients have clinical internal and/or external knee rotational instability despite no apparent injury to the cruciate or collateral ligaments.
Purpose/Hypothesis:
The purpose of this study was to assess the effect of sequentially cutting the posterolateral, anterolateral, posteromedial, and anteromedial structures of the knee on rotational stability in the setting of intact cruciate and collateral ligaments. It was hypothesized that cutting of the iliotibial band (ITB), anterolateral ligament and lateral capsule (ALL/LC), posterior oblique ligament (POL), and posteromedial capsule (PMC) would significantly increase internal rotation, while sectioning of the anteromedial capsule (AMC) and the popliteus tendon and popliteofibular ligament (PLT/PFL) would lead to a significant increase in external knee rotation.
Study Design:
Controlled laboratory study.
Methods:
Ten pairs (N = 20) of cadaveric knees were assigned to 2 sequential cutting groups (group 1: posterolateral-to-posteromedial [PL → PM] and group 2: posteromedial-to-posterolateral [PM → PL]). Specimens were subjected to applied 5-N·m internal and external rotation torques at knee flexion angles of 0°, 30°, 60°, and 90° while intact and after each cut state. Rotational changes were measured and compared with the intact and previous cut states.
Results:
Sectioning of the ITB significantly increased internal rotation at 60° and 90° by 5.4° and 6.2° in group 1 (PL → PM) and 3.5° and 3.8° in group 2 (PM → PL). PLT/PFL complex sectioning significantly increased external rotation at 60° and 90° by 2.7° and 2.9° in group 1 (PL → PM). At 60° and 90° in group 2 (PM → PL), ALL/LC sectioning produced significant increases in internal rotation of 3.1° and 3.5°, respectively. In group 2 (PM → PL), POL sectioning produced a significant increase in internal rotation of 2.0° at 0°. AMC sectioning significantly increased external rotation at 30° to 90° of flexion with a magnitude of change of <1° in both groups 1 (PL → PM) and 2 (PM → PL).
Conclusion:
Collectively, the anterolateral corner structures provided primary internal rotation control of the knee from 60° to 90° of knee flexion in knees with intact cruciate and collateral ligaments. The ITB was the most significant primary stabilizer of internal rotation. The POL had a primary role for internal rotational stability at full extension. The PLT/PFL complex was a primary stabilizer for external rotation of the knee at 60° and 90°.
Clinical Relevance:
This study delineates the primary and secondary roles of the ITB, ALL/LC, POL, and PLT/PFL to rotatory stability of the knee and provides new information to understand knee rotational instabilities.
Keywords: rotational instability, anterolateral ligament, iliotibial band, popliteus tendon, popliteofibular ligament, posterior oblique ligament, knee ligaments
Rotational instability of the knee in the presence of intact cruciate and collateral ligaments is a complex clinical problem to accurately diagnose and treat. Although advances in anatomic and biomechanical understanding of the knee have led to improved treatment of ligament injuries, there is still a subset of patients who present with symptoms of rotational instability despite no obvious structural injury on physical examination or magnetic resonance imaging (MRI). Furthermore, some of the patients with intact cruciate and collateral ligaments may have sustained injuries to other structures that heal elongated, resulting in subtle laxity that can be difficult to detect on clinical examination. In the chronic phase, these structures may have a normal appearance on MRI. Patients may complain of rotational instability with limitations during athletic activity, especially during pivoting and lateral movements. The role of the cruciate and collateral ligaments in controlling knee rotation has been studied in detail.10,17,20,21,47,48 However, the role of other knee structures in controlling internal and external rotation of the knee in the face of these intact primary ligamentous stabilizers has not been well described.
Currently, there remains a lack of consensus on whether other ligament or capsular structures provide primary or secondary restraints to rotational laxity when the cruciate and collateral ligaments are intact. Delineation of these structures and their respective function will be a key component to the understanding of rotational instability in those patients and potentially in those with residual rotatory instability after “anatomic” ligament reconstructions.2,4,14,23,24,41 Therefore, the purpose of this study was to assess which anatomic structures have primary and secondary stabilization roles for knee internal and external rotation in the setting of intact cruciate and collateral ligaments. The effect of sequentially cutting the posterolateral, anterolateral, posteromedial, and anteromedial structures of the knee on rotational stability in the setting of intact cruciate and collateral ligaments was assessed in a biomechanical setup. It was hypothesized that sectioning of the iliotibial band (ITB), anterolateral ligament and lateral capsule (ALL/LC), posterior oblique ligament (POL), and the posteromedial capsule (PMC) would significantly increase internal rotation, while sectioning the anteromedial capsule (AMC) and the popliteus tendon and popliteofibular ligament (PLT/PFL) would lead to a significant increase in external knee rotation.
Methods
Specimen Preparation
Ten pairs (N = 20) of fresh-frozen, male cadaveric knee specimens (mean age, 57.8 years; range, 47-63 years) with no history of prior injury, surgery, anatomic abnormality, ligament instability, or osteoarthritis were included in this study. Institutional review board approval was not required because deidentified cadaveric specimens are exempt from review at our institution. The cadaveric specimens utilized in this study were donated to a tissue bank for the purpose of medical research and then purchased by our institution. All specimens were stored at −20°C and thawed at room temperature 24 hours prior to preparation. Once testing was completed, each specimen underwent diagnostic arthroscopy to confirm the absence of intra-articular pathology.
In preparation for potting, the tibial, fibular, and femoral diaphyses were cut 20 cm from the joint line. Sharp dissection to bone was performed, and all soft tissues were removed 10 cm distal and proximal to the joint line and the fibula was fixed to the tibia in its anatomic position. The tibia, fibula, and femur were potted in a cylindrical mold filled with poly methyl methacrylate (PMMA) (Fricke Dental International Inc). The skin and subcutaneous tissues were removed and all relevant structures were identified before the specimen was mounted in the robotic testing system. The knee was covered in gauze and kept moist with 0.9% saline at all times throughout preparation, setup, and biomechanical testing. During specimen preparation for each knee, range of motion (flexion-extension and internal-external rotation) was actively tested to detect and reduce the potential effect of joint stiffness and rigidity.
Robotic Testing Setup
Each knee was held in an inverted orientation, with the potted distal end secured in a custom-made fixture mounted onto a universal force/torque sensor (Delta F/T Transducer, ATI Industrial Automation) attached to the end effector of a 6-degrees-of-freedom robotic arm (Kuka KR-60-3, Kuka Robotics). The potted femur was then rigidly fixed onto a stationary pedestal (Figure 1).
Figure 1.

The robotic setup with the inverted right knee mounted in the robotic testing system. Tag stitches were placed to identify the structures to be sectioned before the knee was mounted. The skin and subcutaneous tissue were removed so that the structures could be easily identified, and the knee was covered in gauze and kept moist with 0.9% saline at all times throughout preparation, setup, and biomechanical testing.
Next, the stylus tip of a portable measuring arm (Romer Absolute Arm, Hexagon Metrology; manufacturer-reported point repeatability of 0.025 mm) was used to define the knee joint coordinate system by collecting points at the medial- and lateral-most aspects of the tibial plateau, the medial and lateral femoral epicondyles, and along the tibial diaphysis.12,50 The coordinate system defined the knee joint center of rotation and the anterior-posterior, medial-lateral, and superior-inferior axes. Prior to testing, the knee was robotically subjected to a full passive path motion (0° to 120° of flexion) with minimal forces and torques on all axes. The native passive path of the knee in neutral rotation was recorded from full extension to 120° in 1° increments with minimized forces (<5 N) and torques (<0.5 N·m) in the remaining 5 degrees of freedom. A 1-N compressive load was applied along the axis of the tibial shaft to ensure tibiofemoral contact throughout testing. This robotic testing setup has been previously described and validated for knee joint kinematic testing.8,9
Biomechanical Testing
Based on previous anatomic studies and pilot tests, 6 key structures (3 medial and 3 lateral) were investigated by sequential cutting in the setting of intact cruciate and collateral ligaments.32,42,45,47,51 The medial structures sectioned were the PMC, the POL, and the AMC. The lateral structures sectioned were the PLT/PFL, the ALL/LC (mid-third lateral capsular ligament) and the ITB. During pilot testing, sectioning of the oblique posterior ligament, the lateral posterior capsule, and the medial posterior capsule did not produce observable increases in rotation. These structures were therefore not included in the final sectioning study.
The first knee of each matched pair was randomly assigned to 1 of 2 cutting sequences, and the contralateral knee was assigned to the alternate sequence. The cutting sequence for group 1 was from posterolateral-to-posteromedial (PL → PM): (1) PLT/ PFL, (2) ALL/LC, (3) ITB, (4) AMC, (5) POL, and (6) PMC. The cutting sequence for group 2 was from posteromedial-to-posterolateral (PM → PL): (1) PMC, (2) POL, (3) AMC, (4) ITB, (5) ALL/LC, and (6) PLT/PFL (Table 1).
TABLE 1.
Abbreviated and Fully Descriptive Names for States Within Each Sequential Cutting Ordera
| Group | Abbreviation | Full Description |
|---|---|---|
| Group 1: (PL → PM) | Intact | Intact |
| PLT/PFL | PLT/PFL Cut | |
| ALL/LC | PLT/PFL + ALL/LC Cut | |
| ITB | PLT/PFL + ALL/LC + ITB Cut | |
| AMC | PLT/PFL + ALL/LC + ITB + AMC Cut | |
| POL | PLT/PFL + ALL/LC + ITB + AMC + POL Cut | |
| PMC | PLT/PFL + ALL/LC + ITB + AMC + POL + PMC Cut | |
| Group 2: (PM→PL) | Intact | Intact |
| PMC | PMC Cut | |
| POL | PMC + POL Cut | |
| AMC | PMC + POL + AMC Cut | |
| ITB | PMC + POL + AMC + ITB Cut | |
| ALL/LC | PMC + POL + AMC + ITB + ALL/LC Cut | |
| PLT/PFL | PMC + POL + AMC + ITB + ALL/LC + PLT/PFL Cut |
aALL/LC, anterolateral ligament and lateral capsule (mid-third lateral capsular ligament); AMC, anteromedial capsule; ITB, iliotibial band; PFL, popliteofibular ligament; PL → PM, posterolateral-to-posteromedial; PLT, popliteus tendon; PM → PL, posteromedial-to-posterolateral; PMC, posteromedial capsule; POL, posterior oblique ligament.
Robotically simulated clinical examinations were performed according to previously reported protocols to assess for rotatory laxity.8 During pilot testing, a 10-N axial compressive load was initially used. This was found to be an excessive axial load affecting the magnitude of observed rotational changes with ligament sectioning and was therefore reduced to 1 N, which replicated the clinical setting of evaluating rotation with near-zero axial loads. Internal and external rotation torques of 5 N·m were performed at 0° (full extension), 30°, 60°, and 90° of knee flexion for the intact state and after each cut state. Prior to each testing state, the order of flexion angles was randomized to decrease the potential effects of repetitive testing. Data from the internal and external rotation torques applied during biomechanical testing were recorded as degrees of rotation about the axis of the tibial shaft from the center of the knee joint, defined by the 3-dimensional coordinate system.
Popliteus Tendon and Popliteofibular Ligament Sectioning
The PLT was identified and sectioned in the popliteal sulcus with the knee positioned at 90° of flexion through a 2-cm incision along the fibers of the ITB and a 2-cm vertical incision in the capsule.22 The PFL was identified with the knee in 30° of flexion and was cut at its attachment to the musculotendinous junction of the popliteus muscle.
Anterolateral Ligament and Lateral Capsule (Mid-third Lateral Capsular Ligament) Sectioning
With the knee flexed to 90°, the ALL/LC tibial attachment was identified midway between the center of Gerdy’s tubercle and the anterior margin of the fibular head, 9.5 mm distal to the joint line, as described by both Kennedy et al16 and Terry and LaPrade44 The ALL/LC was released from the anterior margin of the fibular collateral ligament extending 7 cm anteromedial to the center of the ALL tibial attachment (Figure 2). The ALL/LC was released as one unit. Careful attention was made to ensure that no injury to the lateral meniscus occurred.
Figure 2.
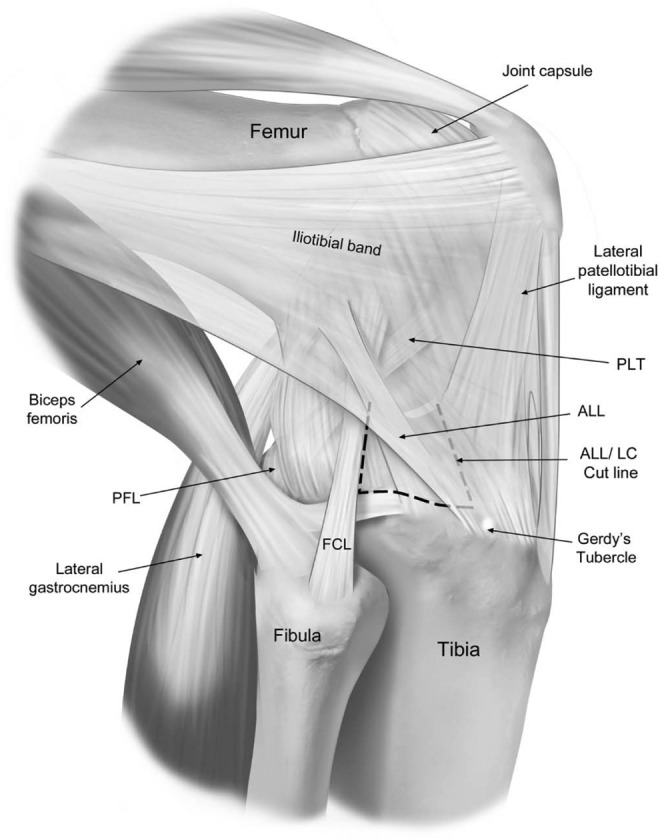
Lateral view of a right knee illustrating the anterolateral corner structures. The dashed line shows where the anterolateral ligament and lateral capsule (ALL/LC) were sectioned deep to the superficial layer of the iliotibial band (ghosted). The ALL/LC was released from the anterior margin of the fibular collateral ligament (FCL) extending 7 cm anteromedial to the center of ALL tibial attachment. The ALL/LC was released as one unit. PFL, popliteofibular ligament; PLT, popliteus tendon.
Iliotibial Band Sectioning
The borders of the ITB were identified with the knee positioned at 90° of flexion. The capsulo-osseous and superficial fibers of the ITB were sectioned off the surrounding area of Gerdy’s tubercle, 5 mm anteromedial from the center of the ALL and extending to the lateral border of the patellar tendon (Figure 3). Using dissection scissors, the ITB was released from the underlying capsule to the level of the joint line.
Figure 3.

Anterolateral view of a right knee showing the anterolateral and lateral structures. The iliotibial band (ITB) was sectioned 5 mm lateral from the center of the anterolateral ligament (ALL) extending to the lateral border of the patellar tendon (dashed line). The ITB was then released from the underlying capsule to the level of the joint line. FCL, fibular collateral ligament; PLT, popliteus tendon.
Anteromedial Capsule Tibial Attachment Sectioning
The AMC was identified at 90° of knee flexion and located between the medial border of the patellar tendon to the anterior margin of the superficial medial collateral ligament (sMCL). It was released from the tibia 15 mm distal to the joint line with an average cut length of 22.7 mm. The tibial attachment sites of the medial meniscus and anterior medial meniscus root were not disrupted. The deep medial collateral ligament (dMCL) was also left intact (Figure 4).
Figure 4.

Medial view of a right knee illustrating the medial structures. The anteromedial capsule (AMC) was cut from the medial border of the patellar tendon to the anterior margin of the superficial medial collateral ligament (sMCL), and 15 mm distal to the joint line (dashed line). The posteromedial capsule (PMC) was cut from the posterior margin of the posterior oblique ligament (POL) to the anterior border of the medial gastrocnemius tendon (MGT) (dashed line). AMT, adductor magnus tendon; MPFL, medial patellofemoral ligament; SM, semimembranosus; VMO, vastus medialis obliquus.
Posterior Oblique Ligament Sectioning
With the knee positioned at 30° of flexion, the central arm of the POL was identified adjacent to the posterior fibers of the superficial MCL, according to LaPrade et al.19 The POL, including the superficial POL, was sectioned 1 cm distal to its femoral attachment.
Posteromedial Capsule Sectioning
The PMC was identified with the knee in full extension between the posterior border of the POL and the anterior margin of the medial gastrocnemius tendon. The PMC was sectioned at the same level of the POL cut, 1 cm distal to its femoral attachment (Figure 4).
Statistical Analysis
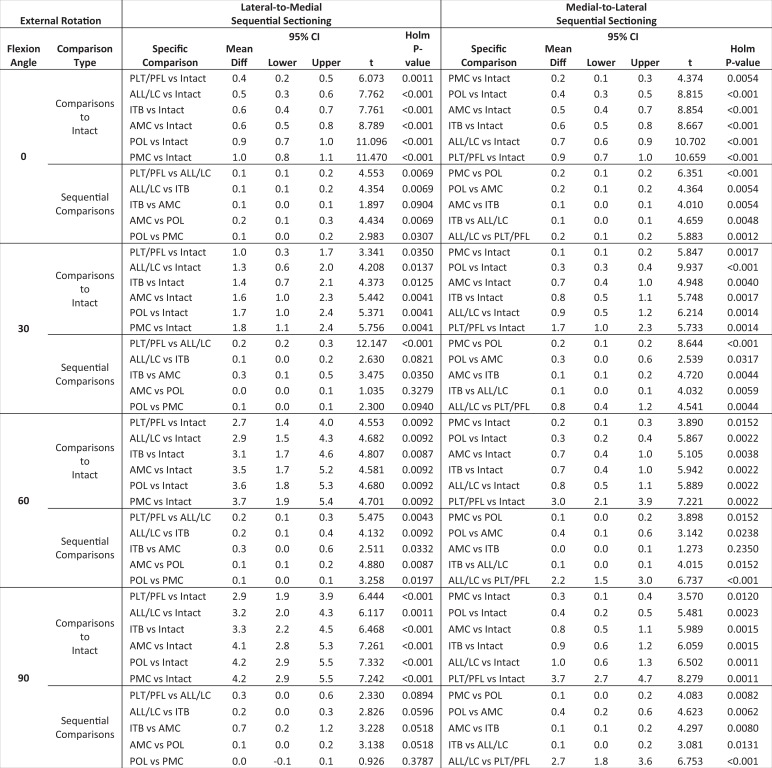
To address the primary hypothesis of this study, comparisons of external and internal rotation were made between the cut states. Initially, an attempt was made to model the flexion angle and cut state simultaneously using linear mixed-effects modeling; however, the state effects were dependent on the knee flexion angle in a complex manner. Thus, the analysis was reduced to multiple paired t tests. Within each combination of rotation type, flexion angle, and cutting order, paired t tests were conducted between the intact state and all subsequent cut states (6 comparisons) and also between sequential cut states (5 comparisons). The Holm-Bonferroni method was used to control the family-wise alpha error rate within each set of 11 comparisons. All statistical analyses and graphics were produced using the statistical package R version 3.2.4 (R Development Core Team, Vienna, Austria).34 Significance was assumed to be present for P < .05.
Results
All numerical data for group 1 (PL → PM) and group 2 (PM → PL) are presented in Tables 2 and 3, respectively. More detailed results and a comprehensive reporting of statistical analyses can be found in the Appendix.
TABLE 2.
Posterolateral-to-Posteromedial (Group 1) Sequential Sectioning: Responses to 5-N·m Internal and External Rotation Torques at Various Flexion Anglesa
| Testing State | Internal Rotation, deg | External Rotation, deg | ||||||
|---|---|---|---|---|---|---|---|---|
| 0° | 30° | 60° | 90° | 0° | 30° | 60° | 90° | |
| Intact | 8.5 ± 2.5 | 17.5 ± 4.5 | 18.0 ± 5.0 | 16.6 ± 3.6 | 12.7 ± 2.7 | 17.7 ± 4.6 | 16.8 ± 4.5 | 18.0 ± 3.4 |
| PLT/PFL | 8.6 ± 2.5 | 17.6 ± 4.7 | 18.5 ± 5.0 | 17.1 ± 3.8 | 13.1 ± 2.8 | 18.7 ± 4.5 | 19.5 ± 4.1 | 20.9 ± 3.8 |
| ALL/LC | 8.8 ± 2.6 | 18.1 ± 4.9 | 18.9 ± 5.1 | 17.6 ± 3.8 | 13.2 ± 2.8 | 18.9 ± 4.5 | 19.7 ± 4.1 | 21.2 ± 4.1 |
| ITB | 8.9 ± 2.7 | 19.4 ± 5.0 | 24.3 ± 4.9 | 23.8 ± 3.6 | 13.3 ± 2.8 | 19.0 ± 4.5 | 19.9 ± 4.2 | 21.4 ± 4.0 |
| AMC | 8.9 ± 2.7 | 19.6 ± 5.1 | 24.4 ± 5.0 | 24.3 ± 3.7 | 13.4 ± 2.8 | 19.3 ± 4.4 | 20.3 ± 4.1 | 22.1 ± 3.9 |
| POL | 10.8 ± 2.4 | 21.2 ± 4.7 | 25.2 ± 5.1 | 24.6 ± 3.9 | 13.6 ± 2.8 | 19.3 ± 4.4 | 20.4 ± 4.1 | 22.2 ± 3.9 |
| PMC | 11.3 ± 2.5 | 21.3 ± 4.7 | 25.3 ± 5.1 | 24.7 ± 4.0 | 13.7 ± 2.9 | 19.4 ± 4.4 | 20.5 ± 4.1 | 22.3 ± 3.9 |
aAll data are presented as mean ± SD. ALL/LC, anterolateral ligament and capsule; AMC, anteromedial capsule; ITB, iliotibial band; PLT/PFL, popliteus tendon and popliteofibular ligament; PMC, posteromedial capsule; POL, posterior oblique ligament.
TABLE 3.
Posteromedial-to-Posterolateral (Group 2) Sequential Sectioning: Responses to 5-N·m Internal and External Rotation Torques at Various Flexion Anglesa
| Testing State | Internal Rotation, deg | External Rotation, deg | ||||||
|---|---|---|---|---|---|---|---|---|
| 0° | 30° | 60° | 90° | 0° | 30° | 60° | 90° | |
| Intact | 9.1 ± 2.0 | 17.8 ± 4.5 | 18.1 ± 4.9 | 16.4 ± 2.8 | 12.7 ± 3.0 | 17.5 ± 5.3 | 17.5 ± 5.3 | 18.9 ± 3.9 |
| PMC | 9.3 ± 2.3 | 17.9 ± 4.5 | 18.3 ± 4.9 | 16.6 ± 2.8 | 13.0 ± 3.1 | 17.6 ± 5.3 | 17.7 ± 5.2 | 19.2 ± 3.9 |
| POL | 11.3 ± 2.4 | 18.9 ± 4.7 | 18.5 ± 5.0 | 16.7 ± 2.9 | 13.1 ± 3.1 | 17.8 ± 5.2 | 17.8 ± 5.2 | 19.3 ± 3.9 |
| AMC | 11.4 ± 2.5 | 18.9 ± 4.7 | 18.6 ± 5.0 | 16.9 ± 2.9 | 13.3 ± 3.1 | 18.1 ± 5.4 | 18.2 ± 5.4 | 19.7 ± 4.1 |
| ITB | 11.5 ± 2.5 | 20.0 ± 4.7 | 22.1 ± 5.4 | 20.7 ± 3.6 | 13.3 ± 3.2 | 18.3 ± 5.4 | 18.2 ± 5.4 | 19.8 ± 4.2 |
| ALL/LC | 12.0 ± 2.9 | 21.4 ± 4.5 | 25.2 ± 5.0 | 24.3 ± 3.5 | 13.4 ± 3.1 | 18.3 ± 5.4 | 18.3 ± 5.4 | 19.9 ± 4.1 |
| PLT/PFL | 12.1 ± 2.9 | 21.6 ± 4.5 | 25.3 ± 5.1 | 24.5 ± 3.5 | 13.6 ± 3.2 | 19.2 ± 5.6 | 20.5 ± 5.5 | 22.6 ± 4.3 |
aAll data are presented as mean ± SD. ALL/LC, anterolateral ligament and capsule; AMC, anteromedial capsule; ITB, iliotibial band; PLT/PFL, popliteus tendon and popliteofibular ligament; PMC, posteromedial capsule; POL, posterior oblique ligament.
Validation Analysis
For the intact and fully sectioned states, the same knee condition existed regardless of cutting order, and thus equitable comparisons could be made between paired specimens. There was no significant difference (P > .25 in all cases) in internal or external rotation between paired knee specimens randomized into group 1 (PL → PM) or group 2 (PM → PL) at all flexion angles for the intact state and the fully sectioned state. Differences observed between the paired knees can therefore be attributed to the sectioning sequence and are not due to variability between the paired knees.
Internal Rotation
Iliotibial Band
In group 1 (PL → PM), release of the ITB after sectioning of the PLT/PFL and the ALL/LC complexes caused an increase in internal rotation of 5.4° at 60° (95% CI, 3.1°-7.6°, P = .002) and 6.2° at 90° (95% CI, 3.8°-8.6°, P = .001) (Table 2, Figure 5). In group 2 (PM → PL), release of the ITB after sectioning of the medial structures (PMC, POL, and AMC) resulted in a significant increase in internal rotation of 3.5° at 60° (95% CI, 1.5°-5.4°, P = .01) and 3.8° at 90° (95% CI, 2.1°-5.6°, P = .003) (Table 3, Figure 6). During sequential cutting in group 1 and group 2, sectioning of the ITB produced significant changes in internal rotation (P < .05) at 0° and 30°; however, the magnitude of change was <1° (Tables 2 and 3, Figures 5 and 6).
Figure 5.

Changes in internal rotation from the intact state during a 5-N·m internal rotation torque when sequentially cutting structures in group 1 (PL → PM). ALL/LC, anterolateral ligament and capsule; ITB, iliotibial band; AMC, anteromedial capsule; PL → PM, posterolateral-to-posteromedial; PLT/PFL, popliteus tendon and popliteofibular ligament; PMC, posteromedial capsule; POL, posterior oblique ligament. *Significantly different compared with previous cut state (P < .05).
Figure 6.

Changes in internal rotation from the intact state during a 5-N·m internal rotation torque when sequentially cutting structures in group 2 (PM → PL). ALL/LC, anterolateral ligament and capsule; AMC, anteromedial capsule; ITB, iliotibial band; PLT/PFL, popliteus tendon and popliteofibular ligament; PM → PL, posteromedial-to-posterolateral; PMC, posteromedial capsule; POL, posterior oblique ligament. *Significantly different compared with previous cut state (P < .05).
Anterolateral Ligament and Lateral Capsule (Mid-third Lateral Capsular Ligament)
Sectioning of the ALL/LC in group 2 resulted in significant increases in internal rotation of 3.1° at 60° of knee flexion (95% CI, 1.5°-4.7°, P = .008) and 3.5° at 90° (95% CI, 2.0°-5.1°, P = .003). At 0° and 30°, cutting of the ALL/LC resulted in significant changes of <1.5° of internal rotation (P < .05) (Table 3, Figure 6). Sectioning of the ALL/LC in group 1 produced significant (P < .05) increases of ≤0.5° of internal rotation throughout flexion angles from 0° to 90° (Table 2, Figure 5).
Posterior Oblique Ligament
Sectioning of the POL in group 2 produced a significant increase in internal rotation of 2.0° at 0° of knee flexion (95% CI, 1.5°-2.5°, P < .001). Significant (P < .05) changes of ≤1° were observed at flexion angles 30° through 90° (Table 3, Figure 6). Sectioning of the POL in group 1 produced significant (P < .05) internal rotation increases of <2° at 0° and 30° of flexion (Table 2, Figure 5).
Posteromedial Capsule
In group 1, sectioning of the PMC resulted in significant (P < .05) increases of <0.7° in internal rotation at 0°, 30°, and 60° (Table 2, Figure 5). In group 2, sectioning of the PMC produced significant (P < .05) increases in internal rotation of <0.3° at 30°, 60°, and 90° of knee flexion (Table 3, Figure 6).
Anteromedial Capsule
In group 2, significant (P < .05) changes of <0.3° of internal rotation were observed at flexion angles 0° through 90° when the AMC was sectioned (Table 3, Figure 6). In group 1, significant (P < .05) increases of <0.5° in internal rotation at 30° and 90° of knee flexion were observed when the AMC was sectioned (Table 2, Figure 5).
Popliteus Tendon and Popliteofibular Ligament
In both group 1 and group 2, sectioning of the PLT/PFL produced significant (P < .05) increases in internal rotation of ≤0.5° throughout flexion angles 0° to 90° (Tables 2 and 3, Figures 5 and 6).
External Rotation
Popliteus Tendon and Popliteofibular Ligament
Sectioning of the PLT/PFL complex in group 1 (PL → PM) produced significant increases in external rotation of 2.7° at 60° (95% CI, 1.4°-4.0°, P = .009) and 2.9° at 90° (95% CI, 1.9°-3.9°, P < .001). At 0° and 30°, significant (P < .05) changes of ≤1° were observed (Table 2, Figure 7). In group 2 (PM → PL), sectioning of the PLT/PFL complex increased external rotation significantly by 2.2° at 60° (95% CI, 1.5°-3.0°, P < .001) and 2.7° at 90° (95% CI, 1.8°-3.6°, P < .001). Significant increases of <1° occurred at 0° and 30° (Table 3, Figure 8).
Figure 7.

Changes in external rotation from the intact state during a 5-N·m external rotation torque when sequentially cutting structures in group 1 (PL → PM). ALL/LC, anterolateral ligament and capsule; AMC, anteromedial capsule; ITB, iliotibial band; PL→PM, posterolateral-to-posteromedial; PLT/PFL, popliteus tendon and popliteofibular ligament; PMC, posteromedial capsule; POL, posterior oblique ligament. *Significantly different compared with previous cut state (P < .05).
Figure 8.
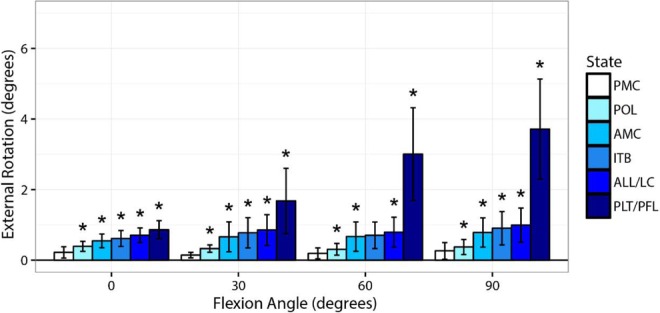
Changes in external rotation from the intact state during a 5-N·m external rotation torque when sequentially cutting structures in group 2 (PM → PL). ALL/LC, anterolateral ligament and capsule; AMC, anteromedial capsule; ITB, iliotibial band; PLT/PFL, popliteus tendon and popliteofibular ligament; PM → PL, posteromedial-to-posterolateral; PMC, posteromedial capsule; POL, posterior oblique ligament. *Significantly different compared with previous cut state (P < .05).
Anteromedial Capsule
In group 2, significant (P < .05) increases of <0.5° of external rotation were observed when the AMC was sectioned from flexion angles 0° through 90° (Table 3, Figure 8). Sectioning of the AMC in group 1 produced significant (P < .05) increases of <0.5° at 30° and 60° of knee flexion (Table 2, Figure 7).
Posteromedial Capsule
In group 2, sectioning of the PMC resulted in significant (P < .05) increases of <0.5° of external rotation at knee flexion angles of 0° through 90° (Table 3, Figure 8). In group 1, sectioning of the PMC produced significant external rotation (P < .05) increases of 0.1° at 0° and 60° (Table 2, Figure 7).
Posterior Oblique Ligament
In group 2, sectioning of the POL produced significant (P < .05) increases in external rotation of <0.3° at flexion angles 0° through 90° (Table 3, Figure 8). In group 1, sectioning of the POL produced significant (P < .05) increases in external rotation of <0.3° at flexion angles of 0° and 60° (Table 2, Figure 7).
Iliotibial Band
In group 2, sectioning of the ITB resulted in significant (P < .05) external rotation increases of <0.3° at 0°, 30°, and 90° of knee flexion (Table 3, Figure 8). In group 1, sectioning of the ITB resulted in significant (P < .05) external rotation increases of <0.3° at 0° and 60° of knee flexion (Table 2, Figure 7).
Anterolateral Ligament and Lateral Capsule (Mid-third Lateral Capsular Ligament)
Sectioning of the ALL/LC complex produced significant (P < .05) increases in external rotation of <0.3° from 0° through 90° in group 2 (Table 3, Figure 8). In group 1, sectioning of the ALL/LC complex produced significant (P < .05) increases in external rotation of <0.3° at 0° through 60° of knee flexion (Table 2, Figure 7).
Discussion
The most important finding of this study was that in the setting of intact cruciate and collateral ligaments, the ITB was a significant primary stabilizer for internal rotation of the knee at high flexion angles (60°-90°). The ALL/LC complex was found to have a significant role in controlling internal rotation at high flexion angles; however, it was less substantial than the ITB. With the knee in full extension (0°), the POL was a primary stabilizer for internal rotation. In addition, the PLT/PFL complex was a significant primary stabilizer of external rotation of the tibia at high flexion angles (60°-90°). Furthermore, the posteromedial and anteromedial capsules had small, yet significant, contributions to rotational stability.
Identifying rotational instability in a clinical setting can be challenging because subtle changes are often difficult to detect during physical examination. Although sometimes small, these increases in rotation can impair knee function and reduce patient quality of life. A recent study of in vivo 3-dimensional gait kinematics between anterior cruciate ligament (ACL)–deficient knees and ACL-intact knees reported a significant increase of 1.4° ± 0.2° in internal rotation in the ACL deficient group.39 The ACL-deficient knee patients were noncopers and unable to return to their premorbid level of sports play or activity. An amplitude of greater than 2° was therefore believed to be a clinically relevant amount of rotational instability in this study.
The magnitude of the increase in internal rotation after release of the ITB correlated with the intact versus cut state of the ALL/LC complex. Increased internal rotation was found for a combined release of the ITB and ALL/LC complex compared with an isolated ITB release. Sectioning of the ALL/LC produced an amplitude of >2° of internal rotation change only when the ITB had been previously sectioned. This intricate interaction between the ITB and the ALL/LC indicates a contribution of both structures for internal rotational control. However, the magnitude of rotational changes observed when sectioning the ALL/LC prior to the ITB (group 1 [PL → PM]) was <2°, indicating the ALL/LC complex was a less important secondary stabilizer of internal rotation compared with the ITB.
The importance of the ITB in controlling internal rotation has been previously reported.13,18,40,45,49 Lutz et al27 reported that the “Kaplan fibers” (attachment of the ITB to the distal femur) acted as a stabilizing ligament, holding the distal portion of the ITB against the lateral epicondyle and increasing tension during internal rotation. Avulsions of the proximal attachment of the ITB have also been reported in patients suffering from increased anterolateral rotational instability of the knee.6
An injury to the anterolateral corner (ALC) structures results in what has historically been referred to as anterolateral rotatory instability (ALRI). ALRI has been defined as a combined anterior translational and internal rotational movement of the tibia.5,13,26 According to some studies, the ALC structures are frequently injured during ACL tears.1,3,7 Wroble et al49 reported a significant increase in internal rotation at knee flexion angles of 30° to 90° after sectioning the entire ALC but made no distinction as to the individual contributions of each structure. Terry and LaPrade43 demonstrated a correlation between increased anterior translation with the knee at 25° of flexion on the Lachman test and injury to the biceps–capsulo-osseous iliotibial tract confluence.
Traditionally, the ACL has been considered the main structure for internal rotatory stability of the knee at lower flexion angles.15,25,28,49,52 The results of this study demonstrate the significant role of the ITB in internal rotatory stability of ACL-intact knees. Furthermore, it outlines the interplay between the ALC structures (ALL/LC complex and the ITB) in limiting internal rotation of the knee.
The role of the ALL in rotational control of the knee is not without controversy. Studies by Rasmussen et al,36 Sonnery-Cottet et al,40 and Parsons et al32 have reported the ALL to have a significant role in controlling internal rotation with a deficient ACL. Nitri et al31 reported that in a combined ACL and ALL injury, the only means of restoring rotatory stability was to reconstruct both structures and not just the ACL in isolation. However, not all studies in the literature are in line with those findings. Kittl et al18 reported only a minor contribution of the ALL on internal rotation stability with a deficient ACL. In an anatomic and biomechanical study by Rahnemai-Azar et al,35 the anterolateral capsule was reported to be lacking any structural and biomechanical properties of a ligament, posing questions about the existence of the ALL as a distinct structure (vs solely a capsular thickening). Terry et al45 and Yamamoto et al51 reported that the ITB and not the ACL was the key structure in controlling the pivot shift. In addition, lateral extra-articular tenodesis procedures have been reported to improve the pivot-shift grade in biomechanical and clinical studies,7,30,38 which supports the role of the anterolateral structures in controlling internal rotation of the tibia. The role of individual capsular structures in providing rotatory stability of the knee had not been investigated thoroughly prior to the present study.
The PLT/PFL was a primary stabilizer of the knee in external rotation at higher flexion angles (60°-90°). This has been reported previously22,29,33,46 and confirmed in this study. Sectioning of the AMC and PMC (Table 3) demonstrated significant changes in external rotation; however, the magnitudes of these changes were <1°. The POL contributed significantly to restraining internal rotation of the tibia near full extension, confirming results reported in previous studies.11,47 The PMC did not contribute significantly to rotational stability in the present study. In contrast, Robinson et al37 reported a significant increase in internal rotation laxity at 0° and 15° after sectioning the PMC; however, the PMC in their study included what is termed the POL in the present study.
We acknowledge some limitations in this study. This was a cadaveric study with a relatively limited number of samples. The sequences of sectioning may overestimate each individual component’s stability because of the interplay between the structures that cannot be separated. As a cadaveric study, we were not able to assess the contribution and functional stability of muscles. Release of the AMC did not include complete release of the meniscotibial attachment to the medial meniscus anteriorly and subsequently might have limited the magnitude of change with its release. Furthermore, the magnitude of rotation that can cause symptoms and thus be clinically significant is still unclear. Currently there is limited literature on this subject, and using the 3-dimensional kinematics in the absence of axial compression forces might overestimate the effect of these injuries.
Conclusion
Collectively, the anterolateral corner structures had a primary role in internal rotational control of the knee from 60° to 90° of knee flexion in knees with intact cruciate and collateral ligaments. The ITB was the most significant primary stabilizer for internal rotation, while the ALL/LC complex had a significant secondary role. The POL had a primary role for internal rotational stability at full extension, while the PLT/PFL complex was a primary stabilizer for external rotation of the knee at higher flexion angles (60° and 90°).
Appendix
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: R.F.L. is a consultant for Arthrex, Ossur, and Smith & Nephew; receives royalties from Arthrex and Smith & Nephew; and receives research support from Arthrex, Ossur, and Smith & Nephew. J.M.S. receives royalties from Arthrex, Tornier, and Zimmer-Biomet; receives other financial/material support from Smith & Nephew ; receives research support from Synthes and Zimmer-Biomet; is a paid consultant for Tornier, Zimmer-Biomet, and Wright Medical Technology; is a paid presenter for Tornier, Zimmer-Biomet, and Wright Medical Technology; and has stock/stock options in Tornier. G.M. has received research grants from South-Eastern Norway Health Authorities (Helse Sør-Øst) and Arthrex.
Ethical approval for this study was waived by the Steadman Philippon Research Institute.
References
- 1. Amirault JD, Cameron JC, MacIntosh DL, Marks P. Chronic anterior cruciate ligament deficiency. Long-term results of MacIntosh’s lateral substitution reconstruction. J Bone Joint Surg Br. 1988;70:622–624. [DOI] [PubMed] [Google Scholar]
- 2. Chambat P, Guier C, Sonnery-Cottet B, Fayard JM, Thaunat M. The evolution of ACL reconstruction over the last fifty years. Int Orthop. 2013;37:181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Claes S, Bartholomeeusen S, Bellemans J. High prevalence of anterolateral ligament abnormalities in magnetic resonance images of anterior cruciate ligament–injured knees. Acta Orthop Belg. 2014;80:45–49. [PubMed] [Google Scholar]
- 4. Crawford SN, Waterman BR, Lubowitz JH. Long-term failure of anterior cruciate ligament reconstruction. Arthroscopy. 2013;29:1566–1571. [DOI] [PubMed] [Google Scholar]
- 5. Ellison AE. Distal iliotibial-band transfer for anterolateral rotatory instability of the knee. J Bone Joint Surg Am. 1979;61:330–337. [PubMed] [Google Scholar]
- 6. Fleming RE, Jr, Blatz DJ, McCarroll JR. Lateral reconstruction for anterolateral rotatory instability of the knee. Am J Sports Med. 1983;11:303–307. [DOI] [PubMed] [Google Scholar]
- 7. Gibson M, Mikosz R, Reider B, Andriacchi T. Analysis of the Muller anterolateral femorotibial ligament reconstruction using a computerized knee model. Am J Sports Med. 1986;14:371–375. [DOI] [PubMed] [Google Scholar]
- 8. Goldsmith MT, Jansson KS, Smith SD, Engebretsen L, LaPrade RF, Wijdicks CA. Biomechanical comparison of anatomic single- and double-bundle anterior cruciate ligament reconstructions: an in vitro study. Am J Sports Med. 2013;41:1595–1604. [DOI] [PubMed] [Google Scholar]
- 9. Goldsmith MT, Smith SD, Jansson KS, LaPrade RF, Wijdicks CA. Characterization of robotic system passive path repeatability during specimen removal and reinstallation for in vitro knee joint testing. Med Eng Phys. 2014;36:1331–1337. [DOI] [PubMed] [Google Scholar]
- 10. Griffith CJ, LaPrade RF, Johansen S, Armitage B, Wijdicks C, Engebretsen L. Medial knee injury, part 1: static function of the individual components of the main medial knee structures. Am J Sports Med. 2009;37:1762–1770. [DOI] [PubMed] [Google Scholar]
- 11. Griffith CJ, Wijdicks CA, LaPrade RF, Armitage BM, Johansen S, Engebretsen L. Force measurements on the posterior oblique ligament and superficial medial collateral ligament proximal and distal divisions to applied loads. Am J Sports Med. 2009;37:140–148. [DOI] [PubMed] [Google Scholar]
- 12. Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105:136–144. [DOI] [PubMed] [Google Scholar]
- 13. Hughston JC, Andrews JR, Cross MJ, Moschi A. Classification of knee ligament instabilities. Part II. The lateral compartment. J Bone Joint Surg Am. 1976;58:173–179. [PubMed] [Google Scholar]
- 14. Inderhaug E, Strand T, Fischer-Bredenbeck C, Solheim E. Long-term results after reconstruction of the ACL with hamstrings autograft and transtibial femoral drilling. Knee Surg Sports Traumatol Arthrosc. 2013;21:2004–2010. [DOI] [PubMed] [Google Scholar]
- 15. Kanamori A, Woo SL, Ma CB, et al. The forces in the anterior cruciate ligament and knee kinematics during a simulated pivot shift test: a human cadaveric study using robotic technology. Arthroscopy. 2000;16:633–639. [DOI] [PubMed] [Google Scholar]
- 16. Kennedy MI, Claes S, Fuso FA, et al. The anterolateral ligament: an anatomic, radiographic, and biomechanical analysis. Am J Sports Med. 2015;43:1606–1615. [DOI] [PubMed] [Google Scholar]
- 17. Kennedy NI, Wijdicks CA, Goldsmith MT, et al. Kinematic analysis of the posterior cruciate ligament, part 1: the individual and collective function of the anterolateral and posteromedial bundles. Am J Sports Med. 2013;41:2828–2838. [DOI] [PubMed] [Google Scholar]
- 18. Kittl C, El-Daou H, Athwal KK, et al. The role of the anterolateral structures and the ACL in controlling laxity of the intact and ACL-deficient knee. Am J Sports Med. 2016;44:345–354. [DOI] [PubMed] [Google Scholar]
- 19. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89:2000–2010. [DOI] [PubMed] [Google Scholar]
- 20. LaPrade RF, Muench C, Wentorf F, Lewis JL. The effect of injury to the posterolateral structures of the knee on force in a posterior cruciate ligament graft: a biomechanical study. Am J Sports Med. 2002;30:233–238. [DOI] [PubMed] [Google Scholar]
- 21. LaPrade RF, Resig S, Wentorf F, Lewis JL. The effects of grade III posterolateral knee complex injuries on anterior cruciate ligament graft force. A biomechanical analysis. Am J Sports Med. 1999;27:469–475. [DOI] [PubMed] [Google Scholar]
- 22. LaPrade RF, Tso A, Wentorf FA. Force measurements on the fibular collateral ligament, popliteofibular ligament, and popliteus tendon to applied loads. Am J Sports Med. 2004;32:1695–1701. [DOI] [PubMed] [Google Scholar]
- 23. Leys T, Salmon L, Waller A, Linklater J, Pinczewski L. Clinical results and risk factors for reinjury 15 years after anterior cruciate ligament reconstruction: a prospective study of hamstring and patellar tendon grafts. Am J Sports Med. 2012;40:595–605. [DOI] [PubMed] [Google Scholar]
- 24. Lie DT, Bull AM, Amis AA. Persistence of the mini pivot shift after anatomically placed anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2007;457:203–209. [DOI] [PubMed] [Google Scholar]
- 25. Lipke JM, Janecki CJ, Nelson CL, et al. The role of incompetence of the anterior cruciate and lateral ligaments in anterolateral and anteromedial instability. A biomechanical study of cadaver knees. J Bone Joint Surg Am. 1981;63:954–960. [PubMed] [Google Scholar]
- 26. Losee RE, Johnson TR, Southwick WO. Anterior subluxation of the lateral tibial plateau. A diagnostic test and operative repair. J Bone Joint Surg Am. 1978;60:1015–1030. [PubMed] [Google Scholar]
- 27. Lutz C, Sonnery-Cottet B, Niglis L, Freychet B, Clavert P, Imbert P. Behavior of the anterolateral structures of the knee during internal rotation. Orthop Traumatol Surg Res. 2015;101:523–528. [DOI] [PubMed] [Google Scholar]
- 28. Markolf KL, Park S, Jackson SR, McAllister DR. Anterior-posterior and rotatory stability of single and double-bundle anterior cruciate ligament reconstructions. J Bone Joint Surg Am. 2009;91:107–118. [DOI] [PubMed] [Google Scholar]
- 29. Maynard MJ, Deng X, Wickiewicz TL, Warren RF. The popliteofibular ligament. Rediscovery of a key element in posterolateral stability. Am J Sports Med. 1996;24:311–316. [DOI] [PubMed] [Google Scholar]
- 30. Monaco E, Ferretti A, Labianca L, et al. Navigated knee kinematics after cutting of the ACL and its secondary restraint. Knee Surg Sports Traumatol Arthrosc. 2012;20:870–877. [DOI] [PubMed] [Google Scholar]
- 31. Nitri M, Rasmussen MT, Williams BT, et al. An in vitro robotic assessment of the anterolateral ligament, part 2: anterolateral ligament reconstruction combined with anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44:593–601. [DOI] [PubMed] [Google Scholar]
- 32. Parsons EM, Gee AO, Spiekerman C, Cavanagh PR. The biomechanical function of the anterolateral ligament of the knee: response. Am J Sports Med. 2015;43:NP22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pasque C, Noyes FR, Gibbons M, Levy M, Grood E. The role of the popliteofibular ligament and the tendon of popliteus in providing stability in the human knee. J Bone Joint Surg Br. 2003;85:292–298. [DOI] [PubMed] [Google Scholar]
- 34. R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 35. Rahnemai-Azar AA, Miller RM, Guenther D, et al. Structural properties of the anterolateral capsule and iliotibial band of the knee. Am J Sports Med. 2016;44:892–897. [DOI] [PubMed] [Google Scholar]
- 36. Rasmussen MT, Nitri M, Williams BT, et al. An in vitro robotic assessment of the anterolateral ligament, part 1: secondary role of the anterolateral ligament in the setting of an anterior cruciate ligament injury. Am J Sports Med. 2016;44:585–592. [DOI] [PubMed] [Google Scholar]
- 37. Robinson JR, Bull AM, Thomas RR, Amis AA. The role of the medial collateral ligament and posteromedial capsule in controlling knee laxity. Am J Sports Med. 2006;34:1815–1823. [DOI] [PubMed] [Google Scholar]
- 38. Samuelson M, Draganich LF, Zhou X, Krumins P, Reider B. The effects of knee reconstruction on combined anterior cruciate ligament and anterolateral capsular deficiencies. Am J Sports Med. 1996;24:492–497. [DOI] [PubMed] [Google Scholar]
- 39. Shabani B, Bytyqi D, Lustig S, Cheze L, Bytyqi C, Neyret P. Gait changes of the ACL-deficient knee 3D kinematic assessment. Knee Surg Sports Traumatol Arthrosc. 2015;23:3259–3265. [DOI] [PubMed] [Google Scholar]
- 40. Sonnery-Cottet B, Lutz C, Daggett M, et al. The involvement of the anterolateral ligament in rotational control of the knee. Am J Sports Med. 2016;44:1209–1214. [DOI] [PubMed] [Google Scholar]
- 41. Suomalainen P, Jarvela T, Paakkala A, Kannus P, Jarvinen M. Double-bundle versus single-bundle anterior cruciate ligament reconstruction: a prospective randomized study with 5-year results. Am J Sports Med. 2012;40:1511–1518. [DOI] [PubMed] [Google Scholar]
- 42. Terry GC, Hughston JC, Norwood LA. The anatomy of the iliopatellar band and iliotibial tract. Am J Sports Med. 1986;14:39–45. [DOI] [PubMed] [Google Scholar]
- 43. Terry GC, LaPrade RF. The biceps femoris muscle complex at the knee. Its anatomy and injury patterns associated with acute anterolateral-anteromedial rotatory instability. Am J Sports Med. 1996;24:2–8. [DOI] [PubMed] [Google Scholar]
- 44. Terry GC, LaPrade RF. The posterolateral aspect of the knee. Anatomy and surgical approach. Am J Sports Med. 1996;24:732–739. [DOI] [PubMed] [Google Scholar]
- 45. Terry GC, Norwood LA, Hughston JC, Caldwell KM. How iliotibial tract injuries of the knee combine with acute anterior cruciate ligament tears to influence abnormal anterior tibial displacement. Am J Sports Med. 1993;21:55–60. [DOI] [PubMed] [Google Scholar]
- 46. Veltri DM, Deng XH, Torzilli PA, Maynard MJ, Warren RF. The role of the popliteofibular ligament in stability of the human knee. A biomechanical study. Am J Sports Med. 1996;24:19–27. [DOI] [PubMed] [Google Scholar]
- 47. Wijdicks CA, Griffith CJ, LaPrade RF, et al. Medial knee injury, part 2: load sharing between the posterior oblique ligament and superficial medial collateral ligament. Am J Sports Med. 2009;37:1771–1776. [DOI] [PubMed] [Google Scholar]
- 48. Wijdicks CA, Kennedy NI, Goldsmith MT, et al. Kinematic analysis of the posterior cruciate ligament, part 2: a comparison of anatomic single- versus double-bundle reconstruction. Am J Sports Med. 2013;41:2839–2848. [DOI] [PubMed] [Google Scholar]
- 49. Wroble RR, Grood ES, Cummings JS, Henderson JM, Noyes FR. The role of the lateral extraarticular restraints in the anterior cruciate ligament-deficient knee. Am J Sports Med. 1993;21:257–262. [DOI] [PubMed] [Google Scholar]
- 50. Wu G, Cavanagh PR. ISB recommendations for standardization in the reporting of kinematic data. J Biomech. 1995;28:1257–1261. [DOI] [PubMed] [Google Scholar]
- 51. Yamamoto Y, Hsu WH, Fisk JA, Van Scyoc AH, Miura K, Woo SL. Effect of the iliotibial band on knee biomechanics during a simulated pivot shift test. J Orthop Res. 2006;24:967–973. [DOI] [PubMed] [Google Scholar]
- 52. Zantop T, Herbort M, Raschke MJ, Fu FH, Petersen W. The role of the anteromedial and posterolateral bundles of the anterior cruciate ligament in anterior tibial translation and internal rotation. Am J Sports Med. 2007;35:223–227. [DOI] [PubMed] [Google Scholar]