Abstract
Hybridization is often considered maladaptive, but sometimes hybrids can invade new ecological niches and adapt to novel or stressful environments better than their parents. The genomic changes that occur following hybridization that facilitate genome resolution and/or adaptation are not well understood. Here, we examine hybrid genome evolution using experimental evolution of de novo interspecific hybrid yeast Saccharomyces cerevisiae × Saccharomyces uvarum and their parentals. We evolved these strains in nutrient-limited conditions for hundreds of generations and sequenced the resulting cultures identifying numerous point mutations, copy number changes, and loss of heterozygosity (LOH) events, including species-biased amplification of nutrient transporters. We focused on a particularly interesting example, in which we saw repeated LOH at the high-affinity phosphate transporter gene PHO84 in both intra- and interspecific hybrids. Using allele replacement methods, we tested the fitness of different alleles in hybrid and S. cerevisiae strain backgrounds and found that the LOH is indeed the result of selection on one allele over the other in both S. cerevisiae and the hybrids. This is an example where hybrid genome resolution is driven by positive selection on existing heterozygosity and demonstrates that even infrequent outcrossing may have lasting impacts on adaptation.
Keywords: hybrid, adaptation, loss of heterozygosity, experimental evolution, Saccharomyces uvarum, Saccharomyces cerevisiae
Introduction
Hybridization is now recognized as a common phenomenon across the tree of life. Historically however, the detection of hybrids has been difficult, and its incidence may be under-reported for both plants and animals, and almost certainly for certain eukaryotes like insects and fungi (Bullini 1994; Albertin and Marullo 2012). Its importance as an evolutionary force has thus been maligned, as hybrids appeared both rare and typically at a reduced fitness. In addition to potential postreproductive barriers, the hybrid is theorized to be ill-adapted to its environment and will also suffer minority cytotype disadvantage, because other hybrids are uncommon and backcrosses to parental species may be unfit (Mallet 2007). However, hybrids can have a variety of advantages over their parents, including heterozygote advantage, extreme phenotypic traits, and reproductive isolation (usually resulting from polyploidy), and can thus facilitate adaptation to novel or stressful conditions, invade unoccupied ecological niches, and even increase biodiversity.
Some hybridization events lead to new hybrid species (Rieseberg 1997; Nolte et al. 2005; Mavarez et al. 2006; Meyer et al. 2006; Soltis and Soltis 2009; Schumer et al. 2014), whereas most result in introgression from hybrid backcrosses to the more abundant parental species (Dowling et al. 1989; Taylor and Hebert 1993; Wayne 1993; Grant et al. 2005; Dasmahapatra et al. 2012). Hybridization introduces genetic variation into a population at orders of magnitude greater than what mutation alone can achieve, in a sense operating as a “multi-locus macro-mutation” (Grant and Grant 1994; Barton 2001; Mallet 2007; Abbott et al. 2013). Therefore, hybridization via introgression, polyploidy, or homoploid hybrid speciation may offer a rapid strategy for adaptation to changing environmental conditions. For example, in Darwin’s finches, adaptive introgression supplied the morphological variation that allowed the species to survive following an El Niño event (Grant and Grant 2010, 2002), and in ancient humans, introgression allowed adaptation to high altitudes (Huerta-Sanchez and Casey 2015), among other traits (Racimo et al. 2015). The most iconic example comes from the hybrid sunflower species Helianthus anomalus, Helianthus deserticola, and Helianthus paradoxus, from the parents Helianthus annuus and Helianthus petiolaris. These three hybrid species are locally adapted to extreme desert, salt marsh, and dune habitats, respectively, and show traits such as increased drought or salt tolerance relative to their parents (Heiser 1954; Rieseberg 1991; Schwarzbach et al. 2001; Rosenthal et al. 2002).
Agriculture and industry use both intra- and interspecific hybrids as a tool to increase yield or robustness, introduce resistance to pests, and create novel phenotype or flavor profiles. For example, plant breeders have crossed domesticated species to wild species to introduce resistance to a variety of pathogens in wheat, potato, and canola (Mason and Batley 2015), and almost all maize grown in the United States is grown from intraspecific hybrid seeds, which has increased yield and provided improved resistance to biotic and abiotic factors (Crow 1998). Vintners and brewers have created interspecific hybrids to select for traits such as lower acetic acid concentration (Bellon et al. 2015), and many incidental fungal hybrids have been discovered in brewing and industry, including Pichia sorbitophila (Louis et al. 2012), and various hybrids across the Saccharomyces clade (Gonzalez et al. 2006, 2008; Muller and McCusker 2009; Hittinger 2013; Bellon et al. 2015), most notably the lager-brewing yeast, Saccharomyces pastorianus (Tamai et al. 1998; Dunn and Sherlock 2008; Walther et al. 2014; Baker et al. 2015; Gibson and Liti 2015; Peris et al. 2016). It is presumed that the severe selection pressures exerted during industrial processes have selected for interspecific hybrid genomes that may be more able to cope with the extreme environments.
At the genomic level, hybridization induces chromosome loss/aneuploidy, chromosomal rearrangements, gene loss, changes in gene expression, changes in epigenetic modifications, transposable element mobilization, and large-scale loss of heterozygosity (LOH), in which the allele of one species is lost and the allele of the other species is retained and may even be duplicated via gene conversion or break-induced replication (Masly et al. 2006; Landry et al. 2007; Doyle et al. 2008; Michalak 2009; Ainouche and Jenczewski 2010; Albertin and Marullo 2012; Abbott et al. 2013; Soltis 2013; Borneman et al. 2014; Soltis et al. 2014). These extensive changes can result in a chimeric, stabilized hybrid, although the period of time for genome stabilization to occur can range dramatically (Soltis et al. 2014). It is unknown whether there are structural and functional biases in the ways in which genes/alleles are lost or modified. Both drift and selection influence the resolution of the hybrid genome, but their contributions are difficult to untangle.
Researchers have long been exploring the genetics of hybrid traits in the lab, particularly in agricultural crops, although this is often slowed by infertility and reduced viability in many interspecific hybrids (Perez-Prat and van Lookeren Campagne 2002; Hajjar and Hodgkin 2007; Ouyang et al. 2010). The Saccharomyces genus, which includes the budding yeast Saccharomyces cerevisiae, lends itself particularly well to experimental study. Many hybrids of this genus have been discovered in brewing, industrial, and natural environments; indeed, the genus itself is speculated to have been founded by the product of an ancient hybridization event (Hittinger 2013; Marcet-Houben and Gabaldon 2015; Barbosa et al. 2016; Leducq et al. 2016). Viable interspecific hybrids can be created de novo in the lab (Marinoni et al. 1999; Greig et al. 2002), and their ability to grow mitotically means that the catastrophic postzygotic barriers to speciation that generally doom other obligate sexually reproducing hybrids can be avoided. This experimental system allows us to observe evolution in real time in the laboratory environment, and the genetic and genomic tools available in this model genus facilitate characterization of the connection between genotype and phenotype, including fitness.
Previous work in our lab group has utilized experimental evolution to investigate adaptive events in haploid and homozygous diploid S. cerevisiae (Gresham et al. 2008; Payen et al. 2014; Sunshine et al. 2015). To investigate genome evolution post hybridization, we utilize an interspecific hybrid, S. cerevisiae × Saccharomyces uvarum, and its parentals: a homozygous diploid S. uvarum and an intraspecific hybrid S. cerevisiae GRF167 × S. cerevisiae S288C. This allows us to understand the impact of varying levels of heterozygosity on adaptation and genome evolution, ranging from none (S. uvarum and previous S. cerevisiae experiments), to intraspecific heterozygosity (S. cerevisiae GRF167 × S. cerevisiae S288C), to the most extreme case of interspecific hybrids. Saccharomycesuvarum is one of the most distantly related species of S. cerevisiae in the Saccharomyces clade, separated by 20 My and 20% sequence divergence at coding sites (Kellis et al. 2003; Cliften et al. 2006). Despite this extensive divergence, S. cerevisiae and S. uvarum are largely syntenic and create hybrids, though less than 1% of spores are viable (Greig 2009). The two species differ in their stress tolerances, for example, S. cerevisiae being more thermotolerant, S. uvarum being cryotolerant (Almeida et al. 2014). Previous evolution experiments using lab-derived hybrids have revealed novel and/or transgressive phenotypes for ammonium limitation, ethanol tolerance, and growth on xylose (Belloch et al. 2008; Wenger et al. 2010; Piotrowski et al. 2012; Dunn et al. 2013). Notably, Dunn et al. (2013) revealed several LOH events and a repeatable nonreciprocal translocation that produces a gene fusion at the high-affinity ammonium permease MEP2 after selection in ammonium limitation, offering insight into potential mutational events in the adaptation and/or stabilization of S. cerevisiae × S. uvarum hybrids.
Here, we evolved these hybrids and diploids in replicate in three nutrient-limited conditions for hundreds of generations. Using whole genome sequencing, we found whole chromosome aneuploidy, genome rearrangements, copy number variants, de novo point mutations, and LOH. We sought to determine how initial heterozygosity affects adaptation to novel conditions and explore whether neutral or selective forces are influencing the resolution of the hybrid genome over time. In particular, we investigated a reoccurring LOH event observed in both intra- and interspecific hybrids and found support for the hypothesis that LOH at this locus is due to selection.
Results
Experimental Evolution of Hybrid and Parental Species
An interspecific hybrid was created by crossing S. cerevisiae and S. uvarum (strains in supplementary table S1, Supplementary Material online) and evolved in continuous culture in the chemostat (Monod 1949; Novick and Szilard 1950a, 1950b). In parallel, homozygous diploid S. uvarum and heterozygous diploid S. cerevisiae (GRF167 × S288C) were also evolved. Each strain was grown in two or more replicate independent cultures under three different nutrient limitations—glucose, phosphate, and sulfate—for 85–557 generations (median 158) at 30 °C, except for S. uvarum, which was unable to achieve steady state in all conditions at 30 °C and so was evolved at 25 °C. The population sizes were largely similar across strains, species, and conditions.
Evolved clones were isolated from each population and subsequently competed individually against the appropriate green fluorescent protein (GFP)-tagged ancestor to gauge relative fitness. As expected, evolved hybrid and parental clones generally exhibit higher fitness than their unevolved ancestor, with typical relative fitness gains between 20% and 30% (tables 1 and 2). To explore whether these fitness gains are general or condition specific, we additionally competed each hybrid clone in the two nutrient-limited conditions in which the clone was not evolved. Results are variable, with some clones having negative or neutral fitness in the alternate conditions, suggesting condition-specific adaptation, and some clones experiencing fitness gains in multiple conditions, suggesting more general growth benefits (table 1). Only one clone exhibited fitness gains in all three nutrient environments, and no clones have a greater fitness gain in an alternate condition than the condition it was evolved in, signifying that clones are largely specifically adapted to the particular condition in which they were evolved.
Table 1.
Mutations and Fitness of Evolved Hybrid Clones.
| Clone | Location | Gene(s) | Mutation | Species | Generations | Relative Fitness ± SE (condition) |
|---|---|---|---|---|---|---|
| Gh1 | chrXIII: 852028 | Intergenic | cer | 125 | 26.80 ± 0.98 (G); 0.35 ± 1.60 (S); −1.18 (P) | |
| chrII: 911866..917272 | HXT6/7 | CNV (amplification) | uva | |||
| Gh2 | chrIV: 111919 | SNF3 | Nonsynonymous: D114Y | cer | 100 | 28.17 ± 2.18 (G); 10.48 ± 0.78 (S); 11.23 (P) |
| chrIII: 51593 | GLK1 | Synonymous: T252T | cer | |||
| chrIV: 884801..912119 | 13 genes including IRC3 | LOH, CNV | uva lost | |||
| chrII: 912143..917470 | HXT6/7 | CNV (amplification) | uva | |||
| chrIV | 836 genes | CNV (amplification) | cer | |||
| Gh3 | chrII: 889421 | IRC3 | nonsynonymous: M333I | uva | 124 | 18.65 ± 0.47 (G); 17.68 ± 3.67 (S); −10.46 (P) |
| chrII: 912416..917778 | HXT6/7 | CNV (amplification) | uva | |||
| Ph1 | chrV: 269392 | Intergenic | cer | 103 | 29.18 ± 1.37 (P); −1.68 ± 0.78 (G); 0.08 ± 0.43 (S) | |
| chrXIV: 746688 | Intergenic | cer | ||||
| chrIV: 1055864 | MHR1 | Nonsynonymous: T218R | cer | |||
| chrIX | 241 genes | LOH, CNV | uva lost, cer amp | |||
| Ph2 | chrV: 432778 | GLC7 | Intron | cer | 124 | 25.34 ± 0.24 (P); 15.12 ± 4.66 (G); −2.45 ± 1.16 (S) |
| chrVII: 9524 | PDR11 | Nonsynonymous: L383* | uva | |||
| chrXVI: 232879 | MRPL40 | Nonsynonymous: V149E | uva | |||
| chrXIII: 194496 | YML037C | Nonsynonymous: P306S | uva | |||
| chrIV: 244399 | YDL114W | Nonsynonymous: G119C | uva | |||
| chr IV | 836 genes | CNV (amplification) | cer | |||
| Ph3 | chrIV: 1055864 | MHR1 | Nonsynonymous: T218R | cer | 167 | 30.03 ± 4.31 (P); 21.39 ± 6.25 (G); NA (S) |
| chrIX: 30830..33084 | YIL166C | CNV (amplification) | cer | |||
| chrXIII: 0..24562 | 10 genes including PHO84 | LOH, CNV | uva lost, cer amp | |||
| chrIV | 836 genes | CNV (amplification) | cer | |||
| Ph4 | chrVII: 555885 | RPL26B | Intron | cer | 131 | 27.02 ± 3.62 (P); 0.68 ± 4.10 (G); 20.46 ± 8.60 (S) |
| chrX: 246208 | PHS1 | Nonsynonymous: K206N | cer | |||
| chrXIII: 324121 | EIS1 | Nonsynonymous: E349* | uva | |||
| chrIII:0..82687 | 49 genes | LOH, CNV | cer lost | |||
| chrXIII:0..221753 | 112 genes, including PHO84 | LOH, CNV | uva lost, cer amp | |||
| Ph5 | chrXIII: 231731 | PPZ1 | Nonsynonymous: A63S | uva | 122 | 30.24 ± 8.32 (P); −8.20 ± 0.34 (G); 18.20 ± 2.91 (S) |
| chrXIII: 0..234112 | 120 genes, including PHO84 | LOH, CNV | uva lost, cer amp | |||
| chrIX:370117..439888 | 45 genes | LOH, CNV | cer lost | |||
| Ph6 | chrVII: 972813 | PFK1 | Nonsynonymous: G308S | cer | 111 | 25.52 ± 3.32 (P); 5.22 ± 2.81 (G); NA (S) |
| chrIV | 836 genes | CNV (amplification) | cer | |||
| Sh1 | chrII:511362..644974; 696397.. 813184 | 74 genes; 63 genes including SUL1 | LOH,CNV | cer lost; cer amp | 126 | 33.86 ± 4.60 (S); 3.81 ± 1.17 (G); −15.48 ± 8.55 (P) |
| chIV: 680386.. 866667; 866667.. 983774 | 104 genes; 63 genes | LOH, CNV | uva amp; uva lost | |||
| chrXVI: 847000.. 948066 | 49 genes | LOH, CNV | cer lost | |||
| Sh2 | chrVII: 936384 | MRPL9 | Nonsynonymous: D167G | cer | 268 | 19.64 ± 4.30 (S); −6.19 ± 1.21 (G); −1.43 ± 6.37 (P) |
| chrXVI: 572308 | ICL2 | Nonsynonymous: M247I | uva | |||
| chrVIII: 116661 | ERG11 | Nonsynonymous: S286C | uva | |||
| chrII:787389..813,184 | 11 genes including SUL1 | CNV (amplification) | cer | |||
| Sh3 | chrVI: 162998 | GCN20 | Nonsynonymous: D171Y | cer | 132 | 21.84 ± 1.53 (S); −6.07 ± 1.11 (G); 5.55 ± 4.81 (P) |
| chrXIV: 495890 | FKH2 | Synonymous: S418S | uva | |||
| chrII:786584..813,184 | 11 genes including SUL1 | CNV (amplification) | cer | |||
| Sh4 | chrXIV: 666675 | ARE2 | Nonsynonymous: I446T | cer | 285 | 27.19 ± 4.33 (S); −6.17 ± 0.51 (G); −20.55 ± 3.30 (P) |
| chrXV: 800832 | APC5 | 5’-upstream | cer | |||
| chrIV: 25917 | TRM3 | Synonymous: S201S | cer | |||
| chrV: 342563 | Intergenic | uva | ||||
| chrX: 769768 | SPO77 | Nonsynonymous: D418G | uva | |||
| chrX: 990873 | LEU3 | 5’-upstream | uva | |||
| chrXII: 192491 | Intergenic | uva | ||||
| chrXIV: 25138 | EGT2 | Synonymous: T168T | uva | |||
| chrII: 770311..813184 | 22 genes, including SUL1 | CNV (amplification) | cer | |||
| chrVIII | 321 genes | CNV (amplification) | uva | |||
| Sh5 | chrIV: 310881 | RXT3 | Nonsynonymous: P87T | uva | 263 | 46.52 ± 4.94 (S); 6.79 ± 1.35 (G); 3.72 ± 7.23 (P) |
| chrVIII: 16911 | Intergenic | uva | ||||
| chrII: 786040..813184 | 11 genes including SUL1 | CNV (amplification) | cer | |||
| Sh6 | chrV: 269392 | Intergenic | cer | 273 | 47.52 ± 3.69 (S); 2.60 ± 1.25 (G); 4.47 ± 6.04 (P) | |
| chrXIV: 746688 | Intergenic | cer | ||||
| chrIV: 413046 | Intergenic | uva | ||||
| chrII:778942..813,184 | 14 genes including SUL1 | CNV (amplification) | cer | |||
| Sh7 | chrII: 238875 | Intergenic | cer | 129 | 31.44 ± 0.49 (S); −1.87 ± 1.99 (G); 8.74 ± 9.22 (P) | |
| chrXVI: 490631 | SVL3 | Nonsynonymous: A245V | cer | |||
| chrXVI: 86106 | YPL245W | Nonsynonymous: A174D | cer | |||
| chrII: 273296 | Intergenic | uva | ||||
| chrII:737875..813184 | 42 genes, including SUL1 | CNV (amplification) | cer |
Point mutations, copy number variants (CNVs), and loss of heterozygosity events (LOH) are recorded for each evolved hybrid clone. Clones are identified by nutrient (G: glucose limitation, P: phosphate limitation, and S: sulfate limitation), an “h” denotes hybrid, and the number indicates its derivation from independent populations. Genes in underline have been found to have point mutations in prior experiments. Note that mutations in the S. uvarum genome use S. uvarum chromosomes and coordinates. All break points were called by visual inspection of sequencing reads and are thus approximate. Relative fitness is reported with standard error (SE) and the condition the clone was evolved in listed first, followed by the two alternative conditions; several clones are reported without SE due to technical difficulties with replicates.
Table 2.
Mutations and Fitness of Evolved Parental Clones.
| Clone | Location | Gene(s) | Mutation | Species | Generations | Relative Fitness ± SE |
|---|---|---|---|---|---|---|
| Gc1 | chrXIV:0..561000; 632250..784333 | 298 genes; 79 genes | CNV (amplification of chr 14L favoring GRF167; deletion of chr14R) | cer | 163 | 16.42 ± 3.42 |
| chrV:160000..576874 | 220 genes | LOH (favors GRF167) | ||||
| Gc2 | chrV:431750..576874 | 71 genes | CNV (amplification, favoring GRF167) | cer | 167 | 10.36 ± 0.58 |
| chrXV:710000..1091291 | 196 genes | LOH, CNV(monosomy, favoring S288C) | ||||
| Gu1 | chrXV | 597 genes | CNV (whole chromosome amplification) | uva | 468 | 18.03 ± 2.12 |
| chrII:911925..917281 | HXT6/7 | CNV (amplification) | ||||
| chrXV:385930 | NEL1 | Nonsynonymous: N129I | ||||
| chrII:911909 | Intergenic, part of the HXT6/7 amplification | |||||
| Gu2 | chrXV | 597 genes | CNV (whole chromosome amplification) | uva | 486 | 13.12 |
| chrII:911925..917281 | HXT6/7 | CNV (amplification) | ||||
| chrIV:100293 | RGT2 | Nonsynonymous: G107V | ||||
| chrV:42093 | FRD1 | Nonsynonymous: G128A | ||||
| chrII:917191 | HXT7 | Synonymous: H53H | ||||
| chrXI:155787 | Intergenic | |||||
| Pc1 | chrXIII:0..39000 (LOH); 0..196628 (CNV: 3 copies); 196628..373000 (CNV: 2 copies) | LOH: 15 genes including PHO84; CNV: 201 genes | LOH, CNV (amplification, favoring GRF167) | cer | 152 | 21.22 ± 0.81 |
| Pc2 | chrXIII:0..41100 (LOH); 0..196628 (CNV: 3 copies); 196628..373000 (CNV: 2 copies) | LOH: 16 genes including PHO84; CNV: 201 genes | LOH, CNV (amplification, favoring GRF167) | cer | 149 | 18.13 ± 1.03 |
| chrVIII:520349 | Intergenic | |||||
| Pc3 | chrXIII:0..39000 (LOH); 0..196628 (CNV: 3 copies); 196628..373000 (CNV: 2 copies) | LOH: 15 genes including PHO84; CNV: 201 genes | LOH, CNV (amplification, favoring GRF167) | cer | 127 | 19.49 |
| Pc4 | chrXIII:0..85500 (LOH); 0..196628 (CNV: 3 copies); 196628..373000 (CNV: 2 copies) | LOH: 40 genes including PHO84; CNV: 201 genes | LOH, CNV (amplification, favoring GRF167) | cer | 132 | 20.96 ± 1.41 |
| chrXII: 264000..1078177 | 437 genes | LOH (favoring S288C) | ||||
| chrXV:1023197 | PIP2 | Nonsynonymous: E6Q | ||||
| Pu1 | uva | 240 | −1.68 ± 1.10 | |||
| Pu2 | chrIX:14480 | YPS6 | 5’-upstream | uva | 234 | 21.30 ± 0.73 |
| chrIX: 225314 | SEC6 | Nonsynonymous: I184L | ||||
| chrXIII: 129567 | TCB3 | Nonsynonymous: E625G | ||||
| Sc1 | chrXIV:0..102000 (CNV: 3 copies); 632000..784333 (CNV: 1 copy); LOH: 100000..784333 | 48 genes; 79 genes; 367 genes | LOH, CNV (amplification of chr 14L; deletion of chr14R; LOH favoring S288C) | cer | 182 | 38.06 ± 1.75 |
| chrVIII:207967 | SMF2 | Nonsynonymous: W105S | ||||
| chrXIII:190000..196500 | RRN11, CAT2, VPS71 | LOH, CNV (deletion, favoring GRF167) | ||||
| chrII:787180..797350 | VBA1, SUL1, PCA1 | CNV (amplification) | ||||
| Sc2 | chrXII | 578 genes | CNV (whole chromosome amplification, favoring GRF167) | cer | 176 | 40.21 ± 1.33 |
| chrXII:692000..1078177 | 193 genes | LOH (favoring GRF167) | ||||
| chrII:773220..813184 | 18 genes including SUL1 | CNV (amplification) | ||||
| Sc3 | chrVI:94104 | FRS2 | Nonsynonymous: V303I | cer | 201 | 41.34 ± 6.77 |
| chrVIII:308903 | TRA1 | Nonsynonymous: V2048A | ||||
| chrXIV:232266 | POP1 | Nonsynonymous: S477* | ||||
| chrXV:291219 | TLG2 | Nonsynonymous: D286Y | ||||
| chrXV:30986 | HPF1 | Synonymous: T207T | ||||
| chrII:781800..792230 | 5 genes including BSD2 and SUL1 | CNV (amplification) | ||||
| Sc4 | chrII:275000..813184 | 289 genes | LOH (favoring GRF167) | cer | 190 | 31.25 ± 6.13 |
| chrII:788608..795833 | SUL1, PCA1 | CNV (amplification) | ||||
| chrXI:517650..666816 | 68 genes | CNV (amplification) | ||||
| chrXIII:190000..196500 | RRN11, CAT2, VPS71 | LOH, CNV (deletion, favoring GRF167) | ||||
| chrXIV:632000..784333 | 79 genes | LOH, CNV (deletion) | ||||
| chrXV: 336700..342000; 342000..1091291 | 2 genes; 384 genes | LOH (favoring GRF167; favoring S288C) | ||||
| chrIX:23367 | CSS1 | Nonsynonymous: D914N | ||||
| Su1 | chrX:177350..345680 | 96 genes including SUL2 | CNV (amplification) | uva | 557 | 21.8 ± 2.37 (Sanchez, et al. 2017) |
| chrXVI:466649 | DIG1 | Nonsynonymous: E49Q | ||||
| chrV:188548 | Intergenic | |||||
| Su2 | chrX:177350..345680 | 96 genes including SUL2 | CNV (amplification) | |||
| chrIV:803704 | KTR3 | 5’-upstream | ||||
| chrII:121779 | PIN4 | Nonsynonymous: N263S | ||||
| chrVII:165902 | MPT5 | Nonsynonymous: Q618K | ||||
| chrII:836169 | RSC3 | Synonymous: R4R | ||||
| chrIV:107948 | UFD2 | Synonymous: G691G | ||||
| chrIII:287618 | Intergenic |
Point mutations, copy number variants (CNV), and loss of heterozygosity events (LOH) are recorded for each evolved parental clone. Clones are identified by nutrient (G: glucose limitation, P: phosphate limitation, and S: sulfate limitation), by species (“c” denotes S. cerevisiae, “u” denotes S. uvarum), and the number indicates its derivation from independent populations. Note that mutations in the S. uvarum genome use S. uvarum chromosomes and coordinates. All break points were called by visual inspection of sequencing reads and are thus approximate. Relative fitness is reported with standard error (SE); several clones are reported without SE due to technical difficulties with replicates.
Mutations Are Recovered in Both Novel and Previously Observed Gene Targets in Interspecific Hybrids
To identify mutations in the evolved hybrids, we generated whole genome sequencing data for 16 clones from the end points of the evolution experiments (table 1). We thus captured data from a range of nutrient limitations (phosphate: 6; glucose: 3; sulfate: 7) and generations (100–285, median: 154). Each clone had an average of 2.4 point mutations, a number of which have been previously identified in prior S. cerevisiae evolution experiments. For example, a nonsynonymous mutation in the S. cerevisiae allele of the glucose-sensing gene SNF3 has been identified in glucose-limited experiments in S. cerevisiae (Kvitek and Sherlock 2013; Selmecki et al. 2015). To our knowledge, 20/27 coding point mutations are unique to these experiments (Payen et al. 2016).
In evolved parentals, we again sequenced one clone from the end point of each population. In total, we sequenced 16 clones, 6 from each of the 3 nutrients (2 S. uvarum diploids and 4 S. cerevisiae diploids), except in glucose limitation in which only 2 S. cerevisiae populations were sampled. The generations ranged from 234 to 557 (median: 477) in S. uvarum, with an average of 2.83 mutations per clone, and from 127 to 190 (median: 166.5) in S. cerevisiae, with an average of 0.9 point mutations per clone (table 2). This discrepancy in point mutations between S. cerevisiae and S. uvarum may be explained by differences in generation number.
With the limited number of samples we have from hybrid and parental clones, it is difficult to draw any conclusions regarding unique point mutations in hybrids. Furthermore, we have not tested the fitness of these mutations to prove they are adaptive. However, one class of mutations that may be of particular interest in hybrids are genomic mutations that may interact with the mitochondria, as previous work has shown that nuclear–mitochondria interactions can underlie hybrid incompatibility (Lee et al. 2008; Chou and Leu 2010; Meiklejohn et al. 2013). Other studies have found that only the S. cerevisiae mitochondria are retained in S. cerevisiae × S. uvarum hybrids (Antunovics et al. 2005), and we recapitulate these findings, potentially setting the stage for conflicting interactions between the S. uvarum nuclear genome and the foreign mitochondria. We observe several mitochondria-related mutations in hybrids in both S. cerevisiae and S. uvarum alleles. For example, one point mutation, a nonsynonymous mutation in the S. cerevisiae allele of the mitochondrial ribosomal protein gene MHR1, was seen in two separate clones independently evolved in phosphate limitation. This gene may be of particular interest as it was discovered in a previous screen as being haploproficient (increased fitness of 19%) in hybrids in which the S. cerevisiae allele is missing and the S. uvarum allele is retained (Lancaster S, Dunham MJ, unpublished data), suggesting that this mutation may alter or disable the S. cerevisiae protein in some way. Another example involves the gene IRC3, a helicase responsible for the maintenance of the mitochondrial genome, which has a nonsynonymous mutation in the S. uvarum allele in clone Gh3 and is deleted in clone Gh2, potentially suggesting that the S. uvarum allele is deleterious in the hybrid background. Although our sample size is small, 4/27 point mutations in hybrids are related to mitochondria function compared with 0/26 in parentals and may represent interesting targets for further exploration.
Copy Number Variants Frequently Involve the Amplification of Nutrient Transporters
Yeasts in both natural and artificial environments are known to frequently experience changes in copy number, ranging from single genes to whole chromosomes (Dunham et al. 2002; Gresham et al. 2008; Dunn et al. 2012; Kvitek and Sherlock 2013; Payen et al. 2014; Selmecki et al. 2015; Sunshine et al. 2015; Zhu et al. 2016). This holds true in our evolution experiments: We observe copy number changes across all genetic backgrounds (fig. 1; supplementary figs. S1–S3, Supplementary Material online). Clones were compared with array comparative genomic hybridization of populations to confirm that clones are representative of populations (see Materials and Methods). The evolved hybrid clones displayed an average of 1.5 copy number variants (CNVs) per clone (fig. 1, table 1; Supplementary fig. S3, Supplementary Material online), as defined by the number of segmental or whole chromosome amplifications/deletions (though it is likely that some of these CNVs were created in the same mutational event). The evolved S. cerevisiae clones had an average of 1.5 CNV per clone and the evolved S. uvarum clones had an average of 1 CNV per clone (table 2; supplementary figs. S1 and S2, Supplementary Material online). The most common event across nutrient limitations in the interspecific hybrids was an amplification of the S. cerevisiae copy of chromosome IV, which occurred in four independent hybrid clones (three in phosphate limitation, one in glucose limitation; supplementary fig. S3, Supplementary Material online). Several other characteristic rearrangements occurred in the evolved S. cerevisiae clones, including the amplification of the left arm of chromosome 14 accompanied by segmental monosomy of the right arm of chromosome 14, an event seen previously in other evolved populations (Dunham et al. 2002; Gresham et al. 2008; Sunshine et al. 2015). All copy number events in S. cerevisiae had break points at repetitive elements known as Ty elements, except those located on chrII, which may be mediated by another mechanism (Brewer et al. 2015). In contrast, copy number variants in the hybrid were rarely facilitated by repetitive elements, perhaps in part because S. uvarum has no full length Ty elements; however, why S. cerevisiae Ty elements and long terminal repeat (LTR) sequences from either genome were not utilized remains unknown.
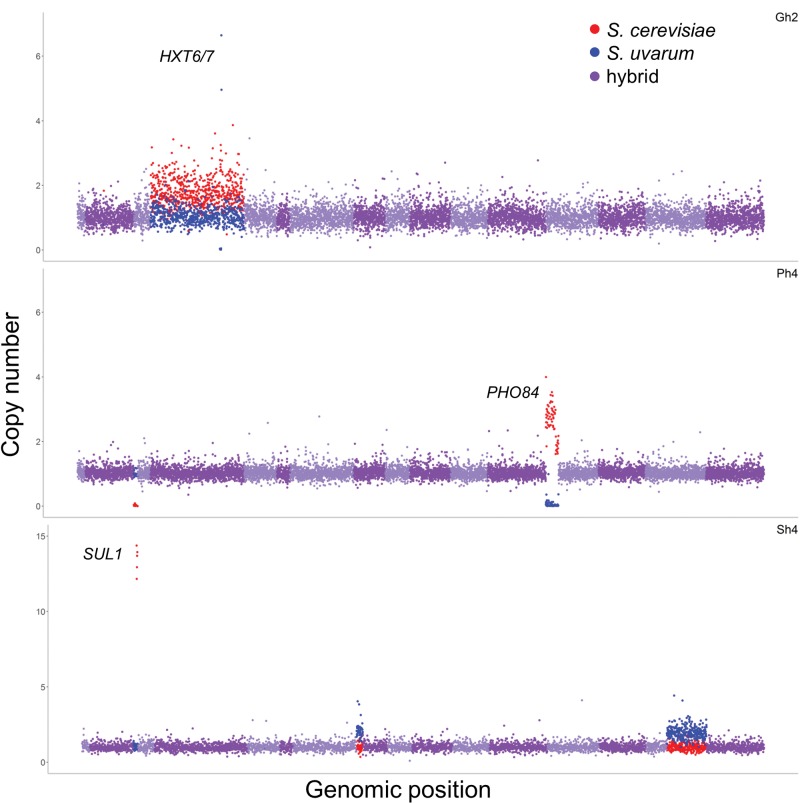
Fig. 1.
Evolved hybrids exhibit changes in copy number and loss of heterozygosity. Copy number variants are displayed for selected evolved hybrid clones from three nutrient-limited conditions: Gh2, glucose; Ph4, phosphate; and Sh4, sulfate. See additional figures in supplementary fig. S3, Supplementary Material online. Hybrid copy number, determined by normalized sequencing read depth per open reading frame (ORF), is plotted across the genome according to S. cerevisiae ORF coordinates to account for three reciprocal translocations between S. cerevisiae and S. uvarum. Chromosomes are plotted in alternating light and dark purple, red indicates a S. cerevisiae copy number variant, and blue indicates a S. uvarum copy number variant. Gh2 has a whole chromosome amplification of S. cerevisiae chrIV, a small segmental deletion of S. uvarum chrIV (non-copy neutral loss of heterozygosity), and an amplification of S. uvarum HXT6/7. Ph4 has a small segmental deletion of S. cerevisiae chrIII (non-copy neutral loss of heterozygosity) and an amplification of S. cerevisiae chrXIII with corresponding deletion of S. uvarum chrXIII (copy neutral loss of heterozygosity). Sh4 has an amplification of S. cerevisiae SUL1 and a whole chromosome amplification of S. uvarum chrVIII (note, there is a reciprocal translocation between chrVIII and chrXV in S. uvarum relative to S. cerevisiae). Note that Sh4 is plotted on a different scale. For specific coordinates of copy number variants, see table 1.
Frequently in nutrient-limited evolution experiments, copy number variants involve amplification of the nutrient-specific transporter, and indeed, we also observed amplification of these transporters in many of the clones. In sulfate limitation, the S. cerevisiae allele of the high-affinity sulfate transporter gene SUL1 is amplified in 7/7 hybrid clones and 4/4 S. cerevisiae clones (fig. 1, tables 1 and 2; supplementary figs. S1 and S3, Supplementary Material online). Interestingly, SUL2 is the preferred sulfate transporter in S. uvarum (Sanchez et al. 2017) and was not observed to be amplified in the evolved hybrids (supplementary fig. S2 and table S2, Supplementary Material online). In glucose limitation, previous S. cerevisiae evolution experiments found frequent amplification of the high-affinity glucose transporter genes HXT6/7 (Brown et al. 1998; Dunham et al. 2002; Gresham et al. 2008; Kao and Sherlock 2008; Kvitek and Sherlock 2011). In our experiments, the S. uvarum alleles of the HXT6/7 transporters are amplified in 3/3 hybrid clones and both S. uvarum clones but are not amplified in evolved S. cerevisiae clones. This is suggestive that the S. uvarum HXT6/7 alleles confer a greater fitness advantage compared with S. cerevisiae; alternatively, the genomic context could be more permissive to amplification in S. uvarum (fig. 1, tables 1 and 2; supplementary figs. S1–S3, Supplementary Material online). Finally, in phosphate limitation, the S. cerevisiae copy of the high-affinity phosphate transporter gene PHO84 is amplified, and the S. uvarum allele is lost in 3/6 hybrid clones in an event known as LOH (figs. 1 and2, table 1; supplementary fig. 3, Supplementary Material online). Intriguingly, the evolved S. cerevisiae clones also display LOH and accompanied amplification favoring the allele derived from strain GRF167 over the S288C allele in 4/4 clones (fig. 2, table 2). All hybrid clones carry the “preferred” GRF167 S. cerevisiae allele, as this was the allele used to create the de novo hybrid.
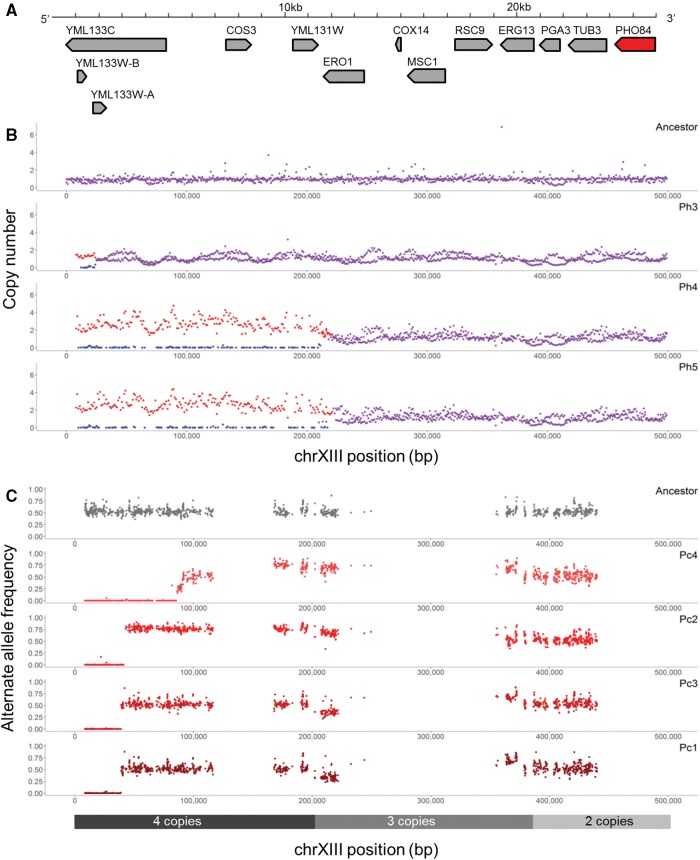
Fig. 2.
Repeated loss of heterozygosity at the PHO84 locus in intra- and interspecific hybrids. (A) The 25-kb region extending from the left telomere of chromosome XIII to the high-affinity phosphate transporter gene PHO84. (B) Copy number is plotted across part of chromosome XIII in the hybrid ancestor and three evolved hybrid clones in phosphate limitation (clone indicated in upper right corner). Red shows the S. cerevisiae allele, blue shows the S. uvarum allele, and purple shows where both species exhibit the same copy number. Note: 8 kb of telomere sequence is removed due to repetitive sequence. (C) Alternate allele frequency is plotted for a portion of chromosome XIII in the ancestor and four evolved S. cerevisiae clones in phosphate limitation (clone indicated in upper right corner). All evolved S. cerevisiae clones exhibit a loss of heterozygosity at the telomeric portion of chromosome XIII (loss of S288C, amplification of GRF167), as illustrated by an allele frequency of zero compared with the ancestor. Regions of heterozygosity are interspersed with regions of homozygosity, as one of the parents of the diploid was itself the product of a cross between strains FL100 and S288C, and the other parent was S288C. Regions of heterozygosity are due to FL100 haplotypes. S. cerevisiae copy number for the four evolved clones is shown below; the ancestor is diploid across the chromosome (also see table 2, supplementary fig. S1, Supplementary Material online).
Loss of Heterozygosity Is a Common Event in Heterozygous Evolving Populations
Selection on heterozygosity, as a LOH event could represent, is an underappreciated source of adaptation in microbial experimental evolution, as typical experiments evolve a haploid or homozygous diploid strain asexually and, as a result, have little variation to select upon. LOH is observed in natural and industrial hybrids (Albertin and Marullo 2012; Louis et al. 2012; Pryszcz et al. 2014; Wolfe 2015; Schroder et al. 2016), but here we document its occurrence in both intra- and interspecific hybrids in the laboratory as a result of short-term evolution (also see (Dunn et al. 2013; Burke et al. 2014)). LOH is observed across all nutrient conditions, with 13 independent LOH events detected in S. cerevisiae and 9 independent events documented in the hybrids (figs. 1 and2, tables 1 and 2; supplementary figs. S1 and S3, Supplementary Material online). It thus appears that this type of mutational event is both common and can occur over short evolutionary timescales.
The LOH event can result in copy-neutral (where one allele is lost and the other allele is amplified) or non-neutral chromosomal segments (where one allele is lost, rendering the strain hemizygous at that locus) and can favor the retention of either allele. In S. cerevisiae, there is a bias in resolution where LOH events favor retaining the GRF167 allele over the S288C allele (10/13 events, P = 0.0169; table 2; supplementary fig. S4, Supplementary Material online). One unique case in clone Sc4 has a small approximately 5 kb LOH event on chrXV favoring GRF167, which switches to favoring S288C for the rest of the chromosome. The retention of S. cerevisiae is slightly more common in the hybrids (5/9 events, P = 1.0; table 1; supplementary fig. S3, Supplementary Material online), though not as drastic as the observed genome resolution in the hybrid S. pastorianus, where LOH favors S. cerevisiae over S. eubayanus (Nakao et al. 2009). The size of the event ranges from approximately 25 kb to the whole chromosome level in the hybrids and from 5 kb to 540 kb in S. cerevisiae. Where LOH is accompanied by an amplification event, the LOH event always occurs first and does not share break points with the amplification event. Unlike many CNV events, most LOH events do not appear to be mediated by existing repetitive sequence such as a transposable element in the hybrid or S. cerevisiae and are most likely a product of break-induced replication or mitotic gene conversion, with break-induced replication as the favored method as all events extend to the telomere (Hoang et al. 2010). The exceptions are in hybrid clones Ph4, Ph5, and Sh1, where there is a non-copy neutral loss of S. cerevisiae mediated by a Ty element or LTR, and S. cerevisiae clones Sc1 and Sc4, where there is a 6.5-kb deletion of the S288C allele flanked by two Ty elements.
LOH events in hybrids could signify several ongoing processes in hybrid genome evolution: LOH regions may represent 1) loci with incompatibilities, 2) selection on existing variation, or 3) genetic drift eroding genomic segments. It is impossible to definitively rule out any of these hypotheses without further experimentation; however, there are several arguments disfavoring the incompatibility hypothesis. First, although our sample size is modest, failing to see repeated LOH events across nutrient conditions may indicate a lack of general incompatibility between species (although we cannot preclude condition-specific incompatibility, Hou et al. 2015; Piatkowska et al. 2013). This is consistent with previous studies in yeast, which suggest that classic Dobzhanksy–Muller incompatibilities are rare (Liti et al. 2006; Greig 2009; Hou et al. 2014). Furthermore, LOH events observed in evolved S. cerevisiae suggest that this mutation type is not unique to interspecific hybrids. Instead, repeated events within a particular condition, such as the repeated LOH at PHO84 in phosphate limitation or the 6.5 kb segment on chrXIII in sulfate limitation, suggest that these events are beneficial and are indeed selection on one allele over the other.
LOH Is Driven by Selection on One Allele
To test the hypothesis that LOH events provide a selective advantage, we used allele replacement, in which the allele of one species/strain is replaced with the allele of the other species/strain in an otherwise isogenic background. We tested this hypothesis using the most commonly seen LOH event, LOH at PHO84. Although the region extends from 25 kb to 234 kb in length in the hybrids and from 40 kb to 85 kb in S. cerevisiae and thus includes many genes, PHO84 was a prime candidate driving this event. PHO84 is one of only ten genes encompassed in the region extending from the telomere to the break point of the shortest LOH event and is included in every other LOH event on chromosome XIII (fig. 2). It is a high-affinity phosphate transporter responsible for inorganic phosphate uptake in high and low phosphate conditions (Wykoff and O'Shea 2001), and previous work identified a point mutation in PHO84 (an alanine to valine substitution at the 5ʹ end of the gene), which increased fitness by 23% in phosphate-limited conditions (Sunshine et al. 2015). Finally, prior work with other nutrient transporters has shown amplification of nutrient transporters to be a key event in adapting to nutrient-limited conditions.
We thus selected a region of approximately 2.5 kb encompassing the PHO84 ORF, its promoter, and 3ʹ-UTR (Nagalakshmi et al. 2008; Yassour et al. 2009; Cherry et al. 2012). We created allele replacement strains using the two alleles of S. cerevisiae in a S. cerevisiae diploid background; the two alleles are 99.1% identical in this region (supplementary fig. S5, Supplementary Material online) and each strain is identical to the ancestral strain used in our evolution experiments except at the PHO84 locus. The S. cerevisiae ancestor carries one copy of GRF167 (preferred) and one copy of S288C (“un-preferred”), so named due to which allele was retained and amplified in the evolved clones. To measure any resultant changes in fitness, we competed each strain individually against a fluorescent ancestral strain and measured which strain overtook the culture. Relative fitness is thus defined as the growth advantage per generation. Two copies of the un-preferred allele decreased relative fitness by −5.31% (±1.86), whereas two copies of the preferred allele increased relative fitness by 9.93% (±0.27). This displays an overall difference in relative fitness of 15.24% between the un-preferred and preferred alleles. By comparing the fitness of these allele replacement strains with the evolved clones (table 2), the allele replacement does not fully recapitulate the fitness gain observed in the evolved clone. One explanation is that the additional mutations present in the evolved strains also contribute to their total fitness. Another explanation could be the increased copy number of the PHO84 region that we see in these evolved clones. To further explore this fitness difference, we cloned the GRF167 allele onto a low copy number plasmid and transformed the allele replacement strain carrying two preferred S. cerevisiae alleles to simulate increased copy number of PHO84 and saw only a minimal fitness increase of 1.76% (±1.06; note all fitness competitions involving plasmids were done in conjunction with empty plasmids to take into account any fitness effects from the plasmid itself). This supports the conclusion that relative fitness gains in the evolved clone are largely due to the loss of the S288C allele, and selection and amplification of the GRF167 allele, with little additional benefit from further amplification. It could also be the case that co-amplification of other genes in the segment is required to attain the full benefit, as previously observed by the contribution of BSD2 to the SUL1 amplicon in sulfate limitation (Sunshine et al. 2015; Payen et al. 2016).
To understand the fitness effects of LOH and amplification in the hybrids, we generated hybrid strains with varying numbers of S. cerevisiae GRF167 PHO84 alleles. Unfortunately, we were unable to obtain a successful strain carrying the preferred S. cerevisiae allele in a S. uvarum background; however, we were able to generate a S. uvarum PHO84 knockout strain, thus creating a hybrid with one copy of S. cerevisiae PHO84. When combined with a low copy plasmid carrying the same allele, this strain effectively has two or more copies of S. cerevisiae PHO84 in a hybrid background. This hybrid strain can serve as a proxy for the LOH event observed in the evolution experiments and has a relative fitness gain of 25.57% (±2.88). The ancestral hybrid with the same plasmid (effectively 1 S. uvarum allele and 2 or more S. cerevisiae alleles) has a relative fitness gain of 12.53% (±1.31). The difference between these two hybrids suggests that while amplification is beneficial, the highest fitness is achieved with the loss of the S. uvarum allele (P = 0.0061).
Together, these results support the conclusion that the S. cerevisiae GRF167 allele is preferred over the S288C allele and that S. cerevisiae alleles are preferred over the S. uvarum allele in the hybrid and, hence, that the LOH events seen in both intra- and interspecific hybrids are the product of selection.
Discussion
In summary, we sought to understand forces underlying genome stabilization and evolution in interspecific and intraspecific hybrids as they adapt to novel environments. We evolved and sequenced clones from 16 hybrid populations and 16 parental populations to reveal a variety of mutational events conferring adaptation to 3 nutrient-limited conditions. Of particular note, we find LOH in both evolved intraspecific and interspecific hybrid clones in all nutrient environments, potentially signifying areas where selection has acted on preexisting variation present in the ancestral clone. We used an allele replacement strategy to test this hypothesis for a commonly repeated LOH event and show that selection is indeed driving the homogenization of the genome at this locus. Although other studies in natural, industrial, and lab-evolved isolates have observed LOH, we present the first empirical test of the causal evolutionary forces influencing these events. This work can begin to help us understand past hybridization events and subsequent genome resolution in hybrids in natural and artificial systems.
Similarities and Differences between Intra- and Interspecific Hybrids
Although our sample size is modest, we observe several interesting trends when comparing S. uvarum clones (homozygous), S. cerevisiae clones (intraspecific hybrid and previously published homozygous), and interspecific hybrid clones. First, theory and previous research predict the interspecific hybrid may experience more genome instability in the form of chromosomal rearrangements and CNV events (Xiong et al. 2011; Lloyd et al. 2014; Chester et al. 2015; Mason and Batley 2015). Instead, in our work, the interspecific hybrid and S. cerevisiae clones experience the same number of CNV events (both 1.5 CNVs/clone). However, the mechanism of CNV formation seems to differ between the hybrid and S. cerevisiae clones. Whereas S. cerevisiae CNV break points typically occur at transposable elements in our study, and previous studies (Dunham et al. 2002; Gresham et al. 2008; Fedoroff 2012), the interspecific hybrid CNVs do not utilize transposable elements, although they obviously share the same sequence background as the S. cerevisiae clones (albeit in one copy). Whether this difference is due to the absence of full-length transposable elements in S. uvarum is unknown, but this could potentially explain the lower number of CNV events in S. uvarum clones (1 CNV/clone) and presents an intriguing direction for future study.
The Predictability of Evolution
We now have many examples of predictable evolution in natural systems (Losos et al. 1998; Rundle et al. 2000; Elmer and Meyer 2011; Conte et al. 2012; Jones et al. 2012; Martin and Orgogozo 2013; Wessinger and Rausher 2014), and in laboratory experimental evolution, in which there often appears to be a limited number of high fitness pathways that strains follow when adapting to a particular condition (Ferea et al. 1999; Woods et al. 2006; Gresham et al. 2008; Burke et al. 2010; Salverda et al. 2011; Kawecki et al. 2012; Kvitek and Sherlock 2013; Lang and Desai 2014). For example, it is well established that amplifications of nutrient transporters are drivers of adaptation in evolution in nutrient-limited conditions. Previous work in our group has particularly focused on the amplification of the high-affinity sulfate transporter gene SUL1 in sulfate-limited conditions, which occurs in almost every sulfate-limited evolution experiment and confers a fitness advantage of as much as 40% compared with the unevolved ancestor strain. The amplification of phosphate transporters has been markedly less common, and thus drivers of adaptation in this condition have been less clear. Gresham et al. (2008) identified a whole chromosome amplification of chrXIII in one population. In a follow-up study, Sunshine et al. (2015) found whole or partial amplification of chrXIII in 3/8 populations. A genome-wide screen for segmental amplifications found a slight increase in fitness for a small telomeric segment of chromosome XIII, and a A49V point mutation in PHO84 was observed to increase fitness by 23%. However, screens by Payen et al. (2016) showed that although PHO84 is recurrently mutated in various experiments, it showed no benefit when amplified or deleted in phosphate-limited conditions. Finally, additional evolution experiments recapitulated the point mutation seen in Sunshine et al. (2015) in 24/32 populations and saw amplification of PHO84 in 8/32 populations (Miller A, Dunham MJ, unpublished data). It is important to note that all these experiments used a strain background derived from S288C or CEN.PK, both of which carry the same (un-preferred) PHO84 allele.
In our work, we observed amplification of the S. cerevisiae GRF167 allele of PHO84 in 4/4 S. cerevisiae clones from 4 populations and 3/6 hybrid clones from 6 populations. This amplification was always preceded by the loss of the S288C allele in S. cerevisiae clones, and the LOH break points are never shared with the amplification break points. There is a 15% fitness difference between carrying two copies of the S288C allele of PHO84 compared with carrying two copies of the GRF167 allele of PHO84, and additional copies of the GRF167 allele do not provide substantial further fitness gains. The two alleles differ by several noncoding changes, and three nonsynonymous substitutions: a mutation from glutamic acid to aspartic acid (E229D), leucine to proline (L259P), and leucine to glutamine (L556Q), the latter two of which are considered “nonconservative” protein mutations due to changes in hydrophobicity and structure (supplementary fig. S5, Supplementary Material online). Intriguingly, the L259P mutation has actually been previously identified as being responsible for resistance to tetrachloroisophthalonitrile and partial resistance to pentachlorophenol in a QTL study of small-molecule drugs (Perlstein et al. 2007). Indeed, Perlstein et al. found proline at residue 259 to be conserved across fungal species, and even in orthologous human xenobiotic transporters, likely because proline-induced kinks in transmembrane spans have been shown to be essential to protein function (Cordes et al. 2002; Perlstein et al. 2007). It thus appears that amplification of PHO84 has been less predictable, as the S288C allele does not confer a fitness advantage unless mutated, per Sunshine et al. (2015). Together, these results imply that strain background can constrain adaptive pathways.
In hybrids, the amplification of the S. cerevisiae segment occurred in conjunction with the loss of the S. uvarum allele. Hybrid strains with the LOH had a 25.57% relative fitness gain, whereas hybrid strains with amplification of the S. cerevisiae PHO84 allele without the LOH had a 12.53% fitness gain. Thus, LOH confers an additional 13.04% fitness gain, showing that selection for LOH has a larger impact on fitness than amplification alone. Note that S. uvarum does have proline at residue 259, like the preferred GRF167 allele, and differs from both S. cerevisiae alleles at the other two coding substitutions (229N and 556K), but the amino acid and noncoding divergence is too high to speculate what substitutions are responsible for the selection of the GRF167 allele (supplementary fig. S6, Supplementary Material online). Why the loss of one allele is more beneficial remains unclear, as PHO84 is thought to function as a monomer (Bun-Ya et al. 1991), but it may be due to competition for cell wall space or negative interactions with other genes in the PHO pathway (Mouillon and Persson 2006).
The infusion of variation created by hybridization provides new templates for selection to act upon, which can be more important than either point mutations or copy number variants alone. Our work shows that outcrossing need not be common to have long-lasting effects on adaptation. This implication is particularly relevant in yeast, where outcrossing may occur quite rarely followed by thousands of asexual generations (Ruderfer et al. 2006; Greig and Leu 2009; Liti 2015).
Applications to Other Hybrids and Cancer
The observation that LOH occurs in hybrid genomes is increasingly documented (Louis et al. 2012; Borneman et al. 2014; Soltis et al. 2014; Marcet-Houben and Gabaldon 2015; Pryszcz et al. 2014; Schroder et al. 2016), although the reason(s) for this type of mutation has been unresolved. As most examples stem from allopolyploid events that occurred millions of years ago, understanding why LOH is important in hybrid genome evolution is difficult. Cancer cells are also known to experience LOH, sometimes involved in the inactivation of tumor suppressor genes, leaving only one copy of the gene that may be mutated or silenced (Thiagalingam et al. 2001; Tuna et al. 2009; Lapunzina and Monk 2011). Data support the conclusion that LOH events are selected for during tumor development, as many LOH events involve specific chromosomal segments (Thiagalingam et al. 2001), although the underlying molecular and genetic reasons for selection is an open debate (Ryland et al. 2015).
Here, we experimentally demonstrate that LOH can occur in homoploid interspecific hybrids as well as in intraspecific hybrids. These events occur within a few hundred generations and are common mutations, more common on average in the intraspecific hybrid (1.3 events/clone) than the interspecific hybrid (0.56 events/clone). Interestingly, the LOH events do not share break points with the CNV events in S. cerevisiae; instead, they appear to occur independently and to precede any subsequent amplification (amplification occurs following 9/13 LOH events). Competition assays with the PHO84 locus provide support that LOH itself may be more beneficial than amplification or at least increase the selective benefit of amplification events. The observation that LOH in intraspecific hybrids occurs independently from copy number change provides different opportunities for adaptation to novel conditions.
Other cases of LOH, like the copy number neutral events observed in Ph4, Ph5, and Sh4, all of which favor the retention of the S. uvarum allele (supplementary fig. S3, Supplementary Material online), may be due to hybrid incompatibility within a particular protein complex, other epistatic interactions (Piatkowska et al. 2013), or neutral processes. We furthermore discover examples where one species allele appears to be preferred over the other without LOH, such as the repeated amplification of the S. uvarum high-affinity glucose transporters HXT6/7. When one species allele is amplified and the other is not amplified, one explanation is that the local sequence context can permit or deny amplification. In the case of HXT6/7, previous experiments in S. cerevisiae have shown that amplification of HXT6/7 is quite common (Brown et al. 1998; Dunham et al. 2002; Gresham et al. 2008; Kao and Sherlock 2008; Kvitek and Sherlock 2011), thus suggesting that when given a choice between this locus in S. cerevisiae or S. uvarum in the hybrid, the preferred allele is indeed S. uvarum, though more subtle differences in rate cannot yet be ruled out. A similar scenario is observed with S. cerevisiae SUL1 (Sanchez et al. 2017). Together, our results show that the heterozygosity supplied by hybridization is an important contributor to adaptive routes explored by populations as they adapt to novel conditions.
Although we cannot generalize our results from the PHO84 locus across the many other LOH events discovered in our hybrids and S. cerevisiae, in the future, we can use similar methodology to explore whether positive selection always drives LOH or whether other explanations such as incompatibility resolution contribute as well. Future experiments might also utilize a high throughput method to explore segmental LOH in hybrids at a genome-wide scale, similar to ongoing experiments at the gene level (Lancaster S, Dunham MJ, unpublished data). Although our sample size is moderate, this is a novel and necessary step in understanding forces underlying hybrid genome stabilization and highlighting an underappreciated mechanism of hybrid adaptation.
Conclusions
The mutation events we observe in our experimentally evolved hybrids are in many ways quite representative of mutations observed in ancient hybrid genomes, suggesting that hybrid genome stabilization and adaptation can occur quite rapidly (within several hundred generations). Furthermore, our results illustrate that the infusion of variation introduced by hybridization at both the intra- and inter-species level can increase fitness by providing choices of alleles for selection to act upon, even when sexual reproduction is rare. This may be particularly important for leveraging existing variation for agricultural and industrial processes and as climate change potentially increases natural hybridization (Kelly et al. 2010; Hoffmann and Sgro 2011; Muhlfeld et al. 2014).
Materials and Methods
Strains
A list of strains used in this study is included in supplementary table S1, Supplementary Material online. All interspecific hybrids were created by crossing a ura3 LYS2 haploid parent to a URA3 lys2 haploid parent of the other mating type, plating on media lacking both uracil and lysine, and selecting for prototrophs. The S. cerevisiae strain background, known as “GRF167”, is itself a cross between FL100 and the genomic type strain S288C (data not shown). GRF167 was chosen as a strain background for simultaneous work investigating transposable elements during experimental evolution, which will be addressed in a future study.
Evolution Experiments
Continuous cultures were established using media and conditions previously described (Gresham et al. 2008; Sanchez et al. 2017). Detailed protocols and media recipes are available at http://dunham.gs.washington.edu/protocols.shtml (last accessed March 6, 2017). Samples were taken daily and measured for optical density at 600 nm and cell count; microscopy was performed to check for contamination; and archival glycerol stocks were made daily. An experiment was terminated when contamination, growth in tubing, or clumping appeared (number of generations at the end point for each population are presented in tables 1 and 2). Samples from each end point population were colony purified to yield two clones for further study.
Array Comparative Genomic Hybridization
Populations from the end point of each evolution were analyzed for copy number changes using array comparative genomic hybridization following the protocol used in Sanchez et al. (2017).
Sequencing
DNA was extracted from overnight cultures using the Hoffman–Winston protocol (Hoffman and Winston 1987) and cleaned using the Clean & Concentrator kit (Zymo Research). Nextera libraries were prepared following the Nextera library kit protocol and sequenced using paired end 150 bp reads on the Illumina NextSeq 500 machine (sequencing coverage in supplementary table S2, Supplementary Material online). The reference genomes used were S. cerevisiae v3 (Engel et al. 2014), S. uvarum (Scannell et al. 2011), and a hybrid reference genome created by concatenating the two genomes. Sequence was aligned to the appropriate reference genome using bwa v0.6.2 (Li and Durbin 2009) and mutations were called using GATK (McKenna et al. 2010) and samtools 0.1.19 (Li et al. 2009). Mutations in evolved clones were filtered in comparison with the ancestor to obtain de novo mutations. All mutations were first visually inspected using Integrative Genomics Viewer (Robinson et al. 2011). Subsequently, point mutations in the hybrids were confirmed with Sanger sequencing (supplementary table S3, Supplementary Material online). Copy number variants were visualized using DNAcopy for S. cerevisiae and S. uvarum (Seshan and Olshen 2016). LOH events were called based on sequencing coverage in the hybrids and by identifying homozygous variant calls in S. cerevisiae. All break points were called by visual inspection of sequencing reads and are thus approximate.
Fitness Assays
The pairwise competition experiments were performed in 20 ml chemostats (Miller and Dunham 2013). Each competitor strain was cultured individually until steady state was reached and then was mixed 50:50 with a GFP-tagged ancestor. Each competition was conducted in at least two biological replicates for approximately 15 generations after mixing. Samples were collected and analyzed twice daily. The proportion of GFP+ cells in the population was detected using a BD Accuri C6 flow cytometer (BD Biosciences). The data were plotted with ln [dark cells/GFP+ cells] versus generations. The relative fitness coefficient was determined from the slope of the linear region.
Strain Construction
Allele replacements for the PHO84 locus were done following the protocol of the Caudy lab with further modifications described here. The native locus was replaced with Kluyveromyces lactis URA3. The pho84Δ::URA3 strain was grown overnight in 5 ml of C-URA media, then inoculated in a flask of 100 ml yeast extract peptone dextrose (YPD) and grown to an optical density of 0.6–0.8. Cells were washed then aliquoted. 275 µl of transformation mix (35 µl 1 M lithium acetate, 240 µl of 50% 3500 polyethylene glycol), 10 µl of salmon sperm, and approximately 3 µg of polymerase chain reaction product were added to the cell pellet. It was incubated at 37 °C (S. uvarum) or 42 °C (S. cerevisiae) for 45 min, then plated to YPD. It was replica plated to 5-fluoroorotic acid the following day, and colonies were tested for the gain of the appropriate species allele. The GRF167 allele was cloned into the pIL37 plasmid using Gibson assembly (Gibson et al. 2009). Correct assembly was verified by Sanger sequencing. All primers used can be found in supplementary table S3, Supplementary Material online.
Supplementary Material
Supplementary data are available at Molecular Biology and Evolution online.
Supplementary Material
Acknowledgments
We thank Noah Hanson and Erica Alcantara for technical assistance. We thank Monica Sanchez and three anonymous reviewers for helpful comments on this manuscript. Joseph Schacherer kindly confirmed the contribution of FL100 to the GRF167 strain background. Thanks to Yixian Zheng and Doug Koshland for contributing to the initial experimental design, creating yeast strains, and purchasing the oligonucleotides used for the microarrays. This work was supported by the National Science Foundation (grant number 1516330) and the National Institutes of Health (grant number R01 GM094306). M.D. is a Senior Fellow in the Genetic Networks program at the Canadian Institute for Advanced Research and a Rita Allen Foundation Scholar. C.S.H. was supported in part by National Institutes of Health (grant number T32 HG00035). This work was also supported by the National Institutes of Health (grant number P50 GM071508) to the Lewis-Sigler Institute and from the Howard Hughes Medical Institute to Doug Koshland and Yixian Zheng. Sequencing read data have been deposited at the NCBI under BioProject accession PRJNA374049. Microarray data are deposited in the Gene Expression Omnibus (GEO) repository under accession numbers GSE95086 and GSE87401 and in the Princeton Microarray Database.
References
- Abbott R, Albach D, Ansell S, Arntzen JW, Baird SJ, Bierne N, Boughman J, Brelsford A, Buerkle CA, Buggs R, et al. 2013. Hybridization and speciation. J Evol Biol. 26:229–246. [DOI] [PubMed] [Google Scholar]
- Ainouche ML, Jenczewski E.. 2010. Focus on polyploidy. New Phytol. 186:1–4. [DOI] [PubMed] [Google Scholar]
- Albertin W, Marullo P.. 2012. Polyploidy in fungi: evolution after whole-genome duplication. Proc Biol Sci. 279:2497–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida P, Goncalves C, Teixeira S, Libkind D, Bontrager M, Masneuf-Pomarede I, Albertin W, Durrens P, Sherman DJ, Marullo P, et al. 2014. A Gondwanan imprint on global diversity and domestication of wine and cider yeast Saccharomyces uvarum. Nat Commun. 5:4044.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunovics Z, Nguyen HV, Gaillardin C, Sipiczki M.. 2005. Gradual genome stabilisation by progressive reduction of the Saccharomyces uvarum genome in an interspecific hybrid with Saccharomyces cerevisiae. FEMS Yeast Res. 5:1141–1150. [DOI] [PubMed] [Google Scholar]
- Baker E, Wang B, Bellora N, Peris D, Hulfachor AB, Koshalek JA, Adams M, Libkind D, Hittinger CT.. 2015. The Genome sequence of Saccharomyces eubayanus and the domestication of lager-brewing yeasts. Mol Biol Evol. 32:2818–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa R, Almeida P, Safar SVB, Santos RO, Morais PB, Nielly-Thibault L, Leducq JB, Landry CR, Goncalves P, Rosa CA, Sampaio JP.. 2016. Evidence of natural hybridization in Brazilian wild lineages of Saccharomyces cerevisiae. Genome Biol Evol. 8:317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton NH. 2001. The role of hybridization in evolution. Mol Ecol. 10:551–568. [DOI] [PubMed] [Google Scholar]
- Belloch C, Orlic S, Barrio E, Querol A.. 2008. Fermentative stress adaptation of hybrids within the Saccharomyces sensu stricto complex. Int J Food Microbiol. 122:188–195. [DOI] [PubMed] [Google Scholar]
- Bellon JR, Yang F, Day MP, Inglis DL, Chambers PJ.. 2015. Designing and creating Saccharomyces interspecific hybrids for improved, industry relevant, phenotypes. Appl Microbiol Biotechnol. 99:8597–8609. [DOI] [PubMed] [Google Scholar]
- Borneman AR, Zeppel R, Chambers PJ, Curtin CD.. 2014. Insights into the Dekkera bruxellensis genomic landscape: comparative genomics reveals variations in ploidy and nutrient utilisation potential amongst wine isolates. PLoS Genet. 10:e1004161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer BJ, Payen C, Di Rienzi SC, Higgins MM, Ong G, Dunham MJ, Raghuraman MK.. 2015. Origin-dependent inverted-repeat amplification: tests of a model for inverted DNA amplification. PLoS Genet. 11:e1005699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Todd KM, Rosenzweig RF.. 1998. Multiple duplications of yeast hexose transport genes in response to selection in a glucose-limited environment. Mol Biol Evol. 15:931–942. [DOI] [PubMed] [Google Scholar]
- Bullini L. 1994. Origin and evolution of animal hybrid species. Trends Ecol Evol. 9:422–426. [DOI] [PubMed] [Google Scholar]
- Bun-Ya M, Nishimura M, Harashima S, Oshima Y.. 1991. The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol Cell Biol. 11:3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MK, Dunham JP, Shahrestani P, Thornton KR, Rose MR, Long AD.. 2010. Genome-wide analysis of a long-term evolution experiment with Drosophila. Nature 467:587–590. [DOI] [PubMed] [Google Scholar]
- Burke MK, Liti G, Long AD.. 2014. Standing genetic variation drives repeatable experimental evolution in outcrossing populations of Saccharomyces cerevisiae. Mol Biol Evol. 31:3228–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JM, Hong EL, Amundsen C, Balakrishnan R, Binkley G, Chan ET, Christie KR, Costanzo MC, Dwight SS, Engel SR, et al. 2012. Saccharomyces genome database: the genomics resource of budding yeast. Nucleic Acids Res. 40:D700–D705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester M, Riley RK, Soltis PS, Soltis DE.. 2015. Patterns of chromosomal variation in natural populations of the neoallotetraploid Tragopogon mirus (Asteraceae). Heredity (Edinb) 114:309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou JY, Leu JY.. 2010. Speciation through cytonuclear incompatibility: insights from yeast and implications for higher eukaryotes. Bioessays 32:401–411. [DOI] [PubMed] [Google Scholar]
- Cliften PF, Fulton RS, Wilson RK, Johnston M.. 2006. After the duplication: gene loss and adaptation in Saccharomyces genomes. Genetics 172:863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte GL, Arnegard ME, Peichel CL, Schluter D.. 2012. The probability of genetic parallelism and convergence in natural populations. Proc Biol Sci. 279:5039–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes FS, Bright JN, Sansom MS.. 2002. Proline-induced distortions of transmembrane helices. J Mol Biol. 323:951–960. [DOI] [PubMed] [Google Scholar]
- Crow JF. 1998. 90 years ago: the beginning of hybrid maize. Genetics 148:923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasmahapatra KK, Walters JR, Briscoe AD, Davey JW, Whibley A, Nadeau NJ, Zimin AV, Hughes DST, Ferguson LC, Martin SH, et al. 2012. Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 487:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling TE, Smith GR, Brown WM.. 1989. Reproductive isolation and introgression between Notropis-Cornutus and Notropis-Chrysocephalus (Family Cyprinidae)—comparison of morphology, allozymes, and mitochondrial-DNA. Evolution 43:620–634. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Flagel LE, Paterson AH, Rapp RA, Soltis DE, Soltis PS, Wendel JF.. 2008. Evolutionary genetics of genome merger and doubling in plants. Annu Rev Genet. 42:443–461. [DOI] [PubMed] [Google Scholar]
- Dunham MJ, Badrane H, Ferea T, Adams J, Brown PO, Rosenzweig F, Botstein D.. 2002. Characteristic genome rearrangements in experimental evolution of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 99:16144–16149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B, Paulish T, Stanbery A, Piotrowski J, Koniges G, Kroll E, Louis EJ, Liti G, Sherlock G, Rosenzweig F.. 2013. Recurrent rearrangement during adaptive evolution in an interspecific yeast hybrid suggests a model for rapid introgression. PLoS Genet. 9:e1003366.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B, Richter C, Kvitek DJ, Pugh T, Sherlock G.. 2012. Analysis of the Saccharomyces cerevisiae pan-genome reveals a pool of copy number variants distributed in diverse yeast strains from differing industrial environments. Genome Res. 22:908–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B, Sherlock G.. 2008. Reconstruction of the genome origins and evolution of the hybrid lager yeast Saccharomyces pastorianus. Genome Res. 18:1610–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer KR, Meyer A.. 2011. Adaptation in the age of ecological genomics: insights from parallelism and convergence. Trends Ecol Evol. 26:298–306. [DOI] [PubMed] [Google Scholar]
- Engel SR, Dietrich FS, Fisk DG, Binkley G, Balakrishnan R, Costanzo MC, Dwight SS, Hitz BC, Karra K, Nash RS, et al. 2014. The reference genome sequence of Saccharomyces cerevisiae: then and now. G3 (Bethesda) 4:389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff NV. 2012. Presidential address. Transposable elements, epigenetics, and genome evolution. Science 338:758–767. [DOI] [PubMed] [Google Scholar]
- Ferea TL, Botstein D, Brown PO, Rosenzweig RF.. 1999. Systematic changes in gene expression patterns following adaptive evolution in yeast. Proc Natl Acad Sci U S A. 96:9721–9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson B, Liti G.. 2015. Saccharomyces pastorianus: genomic insights inspiring innovation for industry. Yeast 32:17–27. [DOI] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, Smith HO.. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–5. [DOI] [PubMed] [Google Scholar]
- Gonzalez SS, Barrio E, Gafner J, Querol A.. 2006. Natural hybrids from Saccharomyces cerevisiae, Saccharomyces bayanus and Saccharomyces kudriavzevii in wine fermentations. FEMS Yeast Res. 6:1221–1234. [DOI] [PubMed] [Google Scholar]
- Gonzalez SS, Barrio E, Querol A.. 2008. Molecular characterization of new natural hybrids of Saccharomyces cerevisiae and S. kudriavzevii in brewing. Appl Environ Microbiol. 74:2314–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant PR, Grant BR.. 1994. Phenotypic and genetic consequences of hybridization in Darwin's finches. Evolution 48:297–316. [DOI] [PubMed] [Google Scholar]
- Grant PR, Grant BR.. 2002. Unpredictable evolution in a 30-year study of Darwin's finches. Science 296:707–711. [DOI] [PubMed] [Google Scholar]
- Grant PR, Grant BR.. 2010. Natural selection, speciation and Darwin's finches. Proc Calif Acad Sci. 61:245–260. [Google Scholar]
- Grant PR, Grant BR, Petren K.. 2005. Hybridization in the recent past. Am Nat. 166:56–67. [DOI] [PubMed] [Google Scholar]
- Greig D. 2009. Reproductive isolation in Saccharomyces. Heredity (Edinb) 102:39–44. [DOI] [PubMed] [Google Scholar]
- Greig D, Leu JY.. 2009. Natural history of budding yeast. Current Biol. 19:R886–R890. [DOI] [PubMed] [Google Scholar]
- Greig D, Louis EJ, Borts RH, Travisano M.. 2002. Hybrid speciation in experimental populations of yeast. Science 298:1773–1775. [DOI] [PubMed] [Google Scholar]
- Gresham D, Desai MM, Tucker CM, Jenq HT, Pai DA, Ward A, DeSevo CG, Botstein D, Dunham MJ.. 2008. The repertoire and dynamics of evolutionary adaptations to controlled nutrient-limited environments in yeast. PLoS Genet. 4:e1000303.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar R, Hodgkin T.. 2007. The use of wild relatives in crop improvement: a survey of developments over the last 20 years. Euphytica 156:1–13. [Google Scholar]
- Heiser CB. 1954. Variation and subspeciation in the common sunflower, Helianthus annuus. Am Midl Nat. 51:287–305. [Google Scholar]
- Hittinger CT. 2013. Saccharomyces diversity and evolution: a budding model genus. Trends Genet. 29:309–317. [DOI] [PubMed] [Google Scholar]
- Hoang ML, Tan FJ, Lai DC, Celniker SE, Hoskins RA, Dunham MJ, Zheng Y, Koshland D.. 2010. Competitive repair by naturally dispersed repetitive DNA during non-allelic homologous recombination. PLoS Genet. 6:e1001228.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA, Sgro CM.. 2011. Climate change and evolutionary adaptation. Nature 470:479–485. [DOI] [PubMed] [Google Scholar]
- Hoffman CS, Winston F.. 1987. A 10-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57:267–272. [DOI] [PubMed] [Google Scholar]
- Hou J, Friedrich A, de Montigny J, Schacherer J.. 2014. Chromosomal rearrangements as a major mechanism in the onset of reproductive isolation in Saccharomyces cerevisiae. Curr Biol. 24:1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Friedrich A, Gounot JS, Schacherer J.. 2015. Comprehensive survey of condition-specific reproductive isolation reveals genetic incompatibility in yeast. Nat Commun. 6:7214.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta-Sanchez E, Casey FP.. 2015. Archaic inheritance: supporting high-altitude life in Tibet. J Appl Physiol (1985). 119:1129–1134. [DOI] [PubMed] [Google Scholar]
- Jones FC, Grabherr MG, Chan YF, Russell P, Mauceli E, Johnson J, Swofford R, Pirun M, Zody MC, White S, et al. 2012. The genomic basis of adaptive evolution in threespine sticklebacks. Nature 484:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao KC, Sherlock G.. 2008. Molecular characterization of clonal interference during adaptive evolution in asexual populations of Saccharomyces cerevisiae. Nat Genet. 40:1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawecki TJ, Lenski RE, Ebert D, Hollis B, Olivieri I, Whitlock MC.. 2012. Experimental evolution. Trends Ecol Evol. 27:547–560. [DOI] [PubMed] [Google Scholar]
- Kellis M, Patterson N, Endrizzi M, Birren B, Lander ES.. 2003. Sequencing and comparison of yeast species to identify genes and regulatory elements. Nature 423:241–254. [DOI] [PubMed] [Google Scholar]
- Kelly B, Whiteley A, Tallmon D.. 2010. The Arctic melting pot. Nature 468:891–891. [DOI] [PubMed] [Google Scholar]
- Kvitek DJ, Sherlock G.. 2011. Reciprocal sign epistasis between frequently experimentally evolved adaptive mutations causes a rugged fitness landscape. PLoS Genet. 7:e1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvitek DJ, Sherlock G.. 2013. Whole genome, whole population sequencing reveals that loss of signaling networks is the major adaptive strategy in a constant environment. PLoS Genet. 9:e1003972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry CR, Hartl DL, Ranz JM.. 2007. Genome clashes in hybrids: insights from gene expression. Heredity (Edinb). 99:483–493. [DOI] [PubMed] [Google Scholar]
- Lang GI, Desai MM.. 2014. The spectrum of adaptive mutations in experimental evolution. Genomics 104:412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapunzina P, Monk D.. 2011. The consequences of uniparental disomy and copy number neutral loss-of-heterozygosity during human development and cancer. Biol Cell 103:303–317. [DOI] [PubMed] [Google Scholar]
- Leducq JB, Nielly-Thibault L, Charron G, Eberlein C, Verta JP, Samani P, Sylvester K, Hittinger CT, Bell G, Landry CR.. 2016. Speciation driven by hybridization and chromosomal plasticity in a wild yeast. Nat Microbiol. 1:15003. [DOI] [PubMed] [Google Scholar]
- Lee HY, Chou JY, Cheong L, Chang NH, Yang SY, Leu JY.. 2008. Incompatibility of nuclear and mitochondrial genomes causes hybrid sterility between two yeast species. Cell 135:1065–1073. [DOI] [PubMed] [Google Scholar]
- Li H, Durbin R.. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R.. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liti G. 2015. The fascinating and secret wild life of the budding yeast S. cerevisiae. Elife 4:e05835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liti G, Barton DB, Louis EJ.. 2006. Sequence diversity, reproductive isolation and species concepts in Saccharomyces. Genetics 174:839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd AH, Ranoux M, Vautrin S, Glover N, Fourment J, Charif D, Choulet F, Lassalle G, Marande W, Tran J, et al. 2014. Meiotic gene evolution: can you teach a new dog new tricks? Mol Biol Evol. 31:1724–1727. [DOI] [PubMed] [Google Scholar]
- Losos JB, Jackman TR, Larson A, de Queiroz K, Rodriguez-Schettino L.. 1998. Contingency and determinism in replicated adaptive radiations of island lizards. Science 279:2115–2118. [DOI] [PubMed] [Google Scholar]
- Louis VL, Despons L, Friedrich A, Martin T, Durrens P, Casaregola S, Neuveglise C, Fairhead C, Marck C, Cruz JA, et al. 2012. Pichia sorbitophila, an interspecies yeast hybrid, reveals early steps of genome resolution after polyploidization. G3 (Bethesda) 2:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet J. 2007. Hybrid speciation. Nature 446:279–283. [DOI] [PubMed] [Google Scholar]
- Marcet-Houben M, Gabaldon T.. 2015. Beyond the whole-genome duplication: phylogenetic evidence for an ancient interspecies hybridization in the baker's yeast lineage. PLoS Biol. 13:e1002220.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinoni G, Manuel M, Petersen RF, Hvidtfeldt J, Sulo P, Piskur J.. 1999. Horizontal transfer of genetic material among Saccharomyces yeasts. J Bacteriol. 181:6488–6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Orgogozo V.. 2013. The loci of repeated evolution: a catalog of genetic hotspots of phenotypic variation. Evolution 67:1235–1250. [DOI] [PubMed] [Google Scholar]
- Masly JP, Jones CD, Noor MAF, Locke J, Orr HA.. 2006. Gene transposition as a cause of hybrid sterility in Drosophila. Science 313:1448–1450. [DOI] [PubMed] [Google Scholar]
- Mason AS, Batley J.. 2015. Creating new interspecific hybrid and polyploid crops. Trends Biotechnol. 33:436–441. [DOI] [PubMed] [Google Scholar]
- Mavarez J, Salazar CA, Bermingham E, Salcedo C, Jiggins CD, Linares M.. 2006. Speciation by hybridization in Heliconius butterflies. Nature 441:868–871. [DOI] [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, et al. 2010. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20:1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiklejohn CD, Holmbeck MA, Siddiq MA, Abt DN, Rand DM, Montooth KL.. 2013. An Incompatibility between a mitochondrial tRNA and its nuclear-encoded tRNA synthetase compromises development and fitness in Drosophila. PLoS Genet. 9:e1003238.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Salzburger W, Schartl M.. 2006. Hybrid origin of a swordtail species (Teleostei: Xiphophorus clemenciae) driven by sexual selection. Mol Ecol. 15:721–730. [DOI] [PubMed] [Google Scholar]
- Michalak P. 2009. Epigenetic, transposon and small RNA determinants of hybrid dysfunctions. Heredity (Edinb). 102:45–50. [DOI] [PubMed] [Google Scholar]
- Miller AW, Dunham MJ.. 2013. Design and use of multiplexed chemostat arrays. J Vis Exp. 72:e50262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod J. 1949. The growth of bacterial cultures. Annu Rev Microbiol. 3:371–394. [Google Scholar]
- Mouillon JM, Persson BL.. 2006. New aspects on phosphate sensing and signalling in Saccharomyces cerevisiae. FEMS Yeast Res. 6:171–176. [DOI] [PubMed] [Google Scholar]
- Muhlfeld CC, Kovach RP, Jones LA, Al-Chokhachy R, Boyer MC, Leary RF, Lowe WH, Luikart G, Allendorf FW.. 2014. Invasive hybridization in a threatened species is accelerated by climate change. Nat Clim Change 4:620–624. [Google Scholar]
- Muller LAH, McCusker JH.. 2009. A multispecies-based taxonomic microarray reveals interspecies hybridization and introgression in Saccharomyces cerevisiae. FEMS Yeast Res. 9:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagalakshmi U, Wang Z, Waern K, Shou C, Raha D, Gerstein M, Snyder M.. 2008. The transcriptional landscape of the yeast genome defined by RNA sequencing. Science 320:1344–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao Y, Kanamori T, Itoh T, Kodama Y, Rainieri S, Nakamura N, Shimonaga T, Hattori M, Ashikari T.. 2009. Genome sequence of the lager brewing yeast, an interspecies hybrid. DNA Res. 16:115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte AW, Freyhof J, Stemshorn KC, Tautz D.. 2005. An invasive lineage of sculpins, Cottus sp (Pisces, Teleostei) in the Rhine with new habitat adaptations has originated from hybridization between old phylogeographic groups. Proc Biol Sci. 272:2379–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick A, Szilard L.. 1950a. Description of the chemostat. Science 112:715–716. [DOI] [PubMed] [Google Scholar]
- Novick A, Szilard L.. 1950b. Experiments with the Chemostat on spontaneous mutations of bacteria. Proc Natl Acad Sci U S A. 36:708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YD, Liu YG, Zhang QF.. 2010. Hybrid sterility in plant: stories from rice. Curr Opin Plant Biol. 13:186–192. [DOI] [PubMed] [Google Scholar]
- Payen C, Di Rienzi SC, Ong GT, Pogachar JL, Sanchez JC, Sunshine AB, Raghuraman MK, Brewer BJ, Dunham MJ.. 2014. The dynamics of diverse segmental amplifications in populations of Saccharomyces cerevisiae adapting to strong selection. G3 (Bethesda) 4:399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payen C, Sunshine AB, Ong GT, Pogachar JL, Zhao W, Dunham MJ.. 2016. High-throughput identification of adaptive mutations in experimentally evolved yeast populations. PLoS Genet. 12:e1006339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Prat E, van Lookeren Campagne MM.. 2002. Hybrid seed production and the challenge of propagating male-sterile plants. Trends Plant Sci. 7:199–203. [DOI] [PubMed] [Google Scholar]
- Peris D, Langdon QK, Moriarty RV, Sylvester K, Bontrager M, Charron G, Leducq JB, Landry CR, Libkind D, Hittinger CT.. 2016. Complex ancestries of lager-brewing hybrids were shaped by standing variation in the wild yeast Saccharomyces eubayanus. PLoS Genet. 12:e1006155.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein EO, Ruderfer DM, Roberts DC, Schreiber SL, Kruglyak L.. 2007. Genetic basis of individual differences in the response to small-molecule drugs in yeast. Nat Genet. 39:496–502. [DOI] [PubMed] [Google Scholar]
- Piatkowska EM, Naseeb S, Knight D, Delneri D.. 2013. Chimeric protein complexes in hybrid species generate novel phenotypes. PLoS Genet. 9:e1003836.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski JS, Nagarajan S, Kroll E, Stanbery A, Chiotti KE, Kruckeberg AL, Dunn B, Sherlock G, Rosenzweig F.. 2012. Different selective pressures lead to different genomic outcomes as newly-formed hybrid yeasts evolve. BMC Evol Biol. 12:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryszcz LP, Nemeth T, Gacser A, Gabaldon T.. 2014. Genome comparison of Candida orthopsilosis clinical strains reveals the existence of hybrids between two distinct subspecies. Genome Biol Evol. 6:1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racimo F, Sankararaman S, Nielsen R, Huerta-Sanchez E.. 2015. Evidence for archaic adaptive introgression in humans. Nat Rev Genet. 16:359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieseberg LH. 1991. Homoploid reticulate evolution in Helianthus (Asteraceae)—evidence from ribosomal genes. Am J Bot. 78:1218–1237. [Google Scholar]
- Rieseberg LH. 1997. Hybrid origins of plant species. Annu Rev Ecol Systemat. 28:359–389. [Google Scholar]
- Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP.. 2011. Integrative genomics viewer. Nat Biotechnol. 29:24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal DM, Schwarzbach AE, Donovan LA, Raymond O, Rieseberg LH.. 2002. Phenotypic differentiation between three ancient hybrid taxa and their parental species. Int J Plant Sci. 163:387–398. [Google Scholar]
- Ruderfer DM, Pratt SC, Seidel HS, Kruglyak L.. 2006. Population genomic analysis of outcrossing and recombination in yeast. Nat Genet. 38:1077–1081. [DOI] [PubMed] [Google Scholar]
- Rundle HD, Nagel L, Boughman JW, Schluter D.. 2000. Natural selection and parallel speciation in sympatric sticklebacks. Science 287:306–308. [DOI] [PubMed] [Google Scholar]
- Ryland GL, Doyle MA, Goode D, Boyle SE, Choong DY, Rowley SM, Li J, Bowtell DD, Tothill RW, Campbell IG, et al. 2015. Australian Ovarian Cancer Study Group, Loss of heterozygosity: what is it good for? BMC Med Genomics 8:45.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salverda MLM, Dellus E, Gorter FA, Debets AJM, van der Oost J, Hoekstra RF, Tawfik DS., de Visser JAGM. 2011. Initial mutations direct alternative pathways of protein evolution. PLoS Genet. 7:e1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MR, Miller AW, Liachko I, Sunshine AB, Lynch B, Huang M, Alcantara E, DeSevo CG, Pai DA, Tucker CM, et al. 2017. Differential paralog divergence modulates genome evolution across yeast species. PLoS Genet. 132:e1006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannell DR, Zill OA, Rokas A, Payen C, Dunham MJ, Eisen MB, Rine J, Johnston M, Hittinger CT.. 2011. The awesome power of yeast evolutionary genetics: new genome sequences and strain resources for the Saccharomyces sensu stricto genus. G3 (Bethesda) 1:11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder MS, Martinez de San Vicente K, Prandini TH, Hammel S, Higgins DG, Bagagli E, Wolfe KH, Butler G.. 2016. Multiple origins of the pathogenic yeast Candida orthopsilosis by separate hybridizations between two parental species. PLoS Genet. 12:e1006404.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumer M, Rosenthal GG, Andolfatto P.. 2014. How common is homoploid hybrid speciation? Evolution 68:1553–1560. [DOI] [PubMed] [Google Scholar]
- Schwarzbach AE, Donovan LA, Rieseberg LH.. 2001. Transgressive character expression in a hybrid sunflower species. Am J Bot. 88:270–277. [PubMed] [Google Scholar]
- Selmecki AM, Maruvka YE, Richmond PA, Guillet M, Shoresh N, Sorenson AL, De S, Kishony R, Michor F, Dowell R, et al. 2015. Polyploidy can drive rapid adaptation in yeast. Nature 519:349–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshan VE, Olshen A.. 2016. DNAcopy: DNA copy number data analysis. Version R package version 1.46.0.
- Soltis DE, Visger CJ, Soltis PS.. 2014. The polyploidy revolution then…and now: Stebbins revisited. Am J Bot. 101:1057–1078. [DOI] [PubMed] [Google Scholar]
- Soltis PS. 2013. Hybridization, speciation and novelty. J Evol Biol. 26:291–293. [DOI] [PubMed] [Google Scholar]
- Soltis PS, Soltis DE.. 2009. The role of hybridization in plant speciation. Annu Rev Plant Biol. 60:561–588. [DOI] [PubMed] [Google Scholar]
- Sunshine AB, Payen C, Ong GT, Liachko I, Tan KM, Dunham MJ.. 2015. The fitness consequences of aneuploidy are driven by condition-dependent gene effects. PLoS Biol. 13:e1002155.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai Y, Momma T, Yoshimoto H, Kaneko Y.. 1998. Co-existence of two types of chromosome in the bottom fermenting yeast, Saccharomyces pastorianus. Yeast 14:923–933. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Hebert PDN.. 1993. Habitat-dependent hybrid parentage and differential introgression between neighboringly sympatric Daphnia species. Proc Natl Acad Sci U S A. 90:7079–7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagalingam S, Laken S, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B, Lengauer C.. 2001. Mechanisms underlying losses of heterozygosity in human colorectal cancers. Proc Natl Acad Sci U S A. 98:2698–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuna M, Knuutila S, Mills GB.. 2009. Uniparental disomy in cancer. Trends Mol Med. 15:120–128. [DOI] [PubMed] [Google Scholar]
- Walther A, Hesselbart A, Wendland J.. 2014. Genome sequence of Saccharomyces carlsbergensis, the world's first pure culture lager yeast. G3 (Bethesda) 4:783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne RK. 1993. Molecular evolution of the dog family. Trends Genet. 9:218–224. [DOI] [PubMed] [Google Scholar]
- Wenger JW, Schwartz K, Sherlock G.. 2010. Bulk segregant analysis by high-throughput sequencing reveals a novel xylose utilization gene from Saccharomyces cerevisiae. PLoS Genet. 6:e1000942.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessinger CA, Rausher MD.. 2014. Predictability and irreversibility of genetic changes associated with flower color evolution in Penstemon barbatus. Evolution 68:1058–1070. [DOI] [PubMed] [Google Scholar]
- Wolfe KH. 2015. Origin of the yeast whole-genome duplication. PLoS Biol. 13:e1002221.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods R, Schneider D, Winkworth CL, Riley MA, Lenski RE.. 2006. Tests of parallel molecular evolution in a long-term experiment with Escherichia coli. Proc Natl Acad Sci U S A. 103:9107–9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykoff DD, O'Shea EK.. 2001. Phosphate transport and sensing in Saccharomyces cerevisiae. Genetics 159:1491–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z, Gaeta RT, Pires JC.. 2011. Homoeologous shuffling and chromosome compensation maintain genome balance in resynthesized allopolyploid Brassica napus. Proc Natl Acad Sci U S A. 108:7908–7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassour M, Kapian T, Fraser HB, Levin JZ, Pfiffner J, Adiconis X, Schroth G, Luo SJ, Khrebtukova I, Gnirke A, et al. 2009. Ab initio construction of a eukaryotic transcriptome by massively parallel mRNA sequencing. Proc Natl Acad Sci U S A. 106:3264–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YO, Sherlock G, Petrov DA.. 2016. Whole genome analysis of 132 clinical Saccharomyces cerevisiae strains reveals extensive ploidy variation. G3 (Bethesda) doi:10.1534/g3.116.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.