Abstract
Iron fulfils a central role in many essential biochemical processes in human physiology, which makes proper processing of iron crucial. Although iron metabolism is subject to relatively strict physiological control, in recent years numerous disorders, such as cancer and neurodegenerative diseases, have been linked to deregulated iron homeostasis. Because of its involvement in the pathogenesis of these diseases, iron metabolism constitutes a promising and largely unexploited therapeutic target for the development of new pharmacological treatments. Several iron metabolism-targeted therapies are already under clinical evaluation for haematological disorders, and these and newly developed therapeutic agents will likely have substantial benefit in the clinical management of iron metabolism-associated diseases, for which few efficacious treatments are often available.
Iron is, after aluminium, the second most abundant metal on earth, and is an essential element for all forms of life on earth. Numerous enzymes involved in DNA replication, repair and translation rely on iron, often in the form of iron-sulphur (Fe-S) clusters, for proper functioning in animals, plants and fungi, as well as in organisms from the two prokaryotic domains of life, Bacteria and Archea1. The biological activity of iron lies, to a large extent, in its efficient electron transferring properties, enabling it to accept or donate electrons while switching between its ferrous bivalent (Fe(II), Fe2+), ferric trivalent (Fe(III), Fe3+) and its ferryl tetravalent (Fe(IV), Fe4+) states, thereby functioning as a catalysing cofactor in various biochemical reactions2. In vertebrates, the second main role of iron involves the oxygen-binding characteristic of porphyrin-complexed iron, better known as haem, which is crucial for the oxygen-carrying capacity of haemoglobin and myoglobin.
Taking into account these vital functions of iron in human physiology, it is clear that systemic or cellular disorders in iron metabolism may have serious consequences. At the systemic level, haem incorporated in haemoglobin (Hb) and myoglobin accounts for more than half of the approximately 4 grams of iron present in the human body, and by far the largest share of the total iron turnover is for haem production3. Consequently, an insufficient iron supply, unmet demand for iron, or substantial loss of iron will lead to a shortage of Hb, resulting in iron-deficiency anaemia4. Conversely, patients with red blood cell disorders such as β-thalassemia suffer from anaemia that is associated with malformed red blood cells that have a reduced life span due to dysfunctional β-globin expression and reduced Hb production5. In an attempt to compensate the chronic anaemia, these individuals produce large numbers of erythroid progenitors. This high erythroid activity is accompanied by a greatly increased iron demand, which promotes iron absorption and, in turn, causes serious comorbidity resulting from iron overloading.
At the cellular level, the presence of intracellular iron has a strong impact on the cellular redox status, contributing to oxidative stress in individual cells. Reactive oxygen species (ROS), such as superoxide (O2−) and hydrogen peroxide (H2O2), which are formed by a single and double univalent reduction of molecular oxygen (O2), respectively, are known to catalyse specific cellular redox reactions and are therefore involved in a number of signalling pathways. However, further reduction of relatively harmless H2O2 results in the formation of hydroxyl radicals (OH•) that are highly reactive, causing nonspecific oxidation and damage to nucleic acids, lipids and proteins6. Iron, as well as other metals, catalyses the formation of OH• from other ROS by Fenton chemistry7, which involves the oxidation of Fe(II) (to Fe(III)) and electron transfer to H2O2. The presence of superoxide further assists this process by promoting the reduction of Fe(III) to form Fe(II) (and O2) to complete the catalytic electron transport cycle of iron known as the Haber−Weiss reaction8.
As a consequence of its well-established roles in iron-deficiency anaemia and iron-loading anaemia, iron metabolism has historically remained within the scope of haematological pathologies. However, over the past decade, a range of ageing-related, non-haematological disorders has been associated with deregulated iron homeostasis as well. In this Review, we discuss iron metabolism as a target for the development of new therapeutics or drug delivery strategies in these diseases. We provide a systematic overview of the iron regulatory pathways and its key players, as well as the major pathophysiologies associated with dysfunctional iron homeostasis, and then review some the most promising iron metabolism-targeted therapeutics thus developed, which could provide new therapeutic options for these often difficult to treat disorders.
Physiology of iron metabolism
Systemic iron regulation − the hepcidin−ferroportin axis
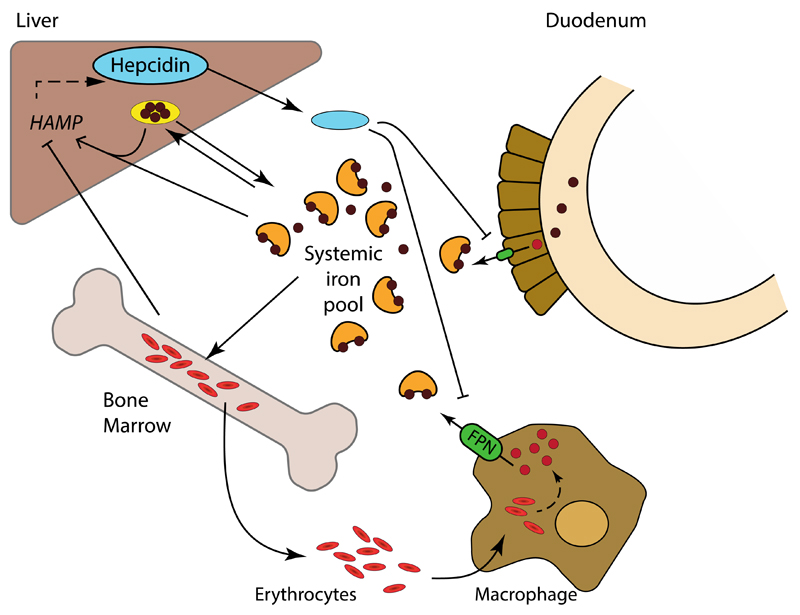
Hepcidin is a peptide comprising 25 amino acids that is encoded by the HAMP gene and named for its high expression in the liver9. Hepcidin was originally thought to be a peptide with moderate antimicrobial activity9,10, but it was soon recognized to be the master regulator of systemic iron metabolism11. Hepcidin regulates the systemic flux of iron by modulating the levels of ferroportin on the cell surface, the only known cellular exporter of unbound iron in vertebrates12. By directly binding to the extracellular domain of ferroportin, hepcidin induces endocytosis and degradation of the transmembrane protein, thereby preventing iron egress from the cell13. High levels of ferroportin are found in enterocytes in the duodenum (to transport absorbed iron), in hepatocytes (to transport stored iron), and in macrophages (to transport recycled iron), which together control systemic iron levels14–16. By reducing surface ferroportin, the expression of hepcidin limits the absorption, remobilization and recycling of iron, thereby reducing iron plasma levels (Figure 1).
Figure 1. Systemic iron metabolism.
Dietary iron is absorbed by duodenal enterocytes and added to the systemic iron pool upon export via ferroportin (FPN, green). The systemic iron pool, which under normal steady-state conditions is mainly bound to transferrin (orange), is primarily utilized for erythropoiesis. Excess systemic iron is stored as ferritin (yellow) in the liver, and to a lesser extent in other tissues. The iron utilized in senescent erythrocytes is recycled by macrophages and released, via ferroportin, back into the systemic iron pool. Ferroportin expression, and consequently systemic iron availability, is inhibited by hepcidin (blue), a peptide primarily produced by the liver. In turn, transcription of the gene encoding hepcidin, HAMP, is stimulated by the presence of iron and inhibited by erythropoietic demand.
With hepcidin as the central orchestrator, all major regulatory pathways of systemic iron homeostasis converge on HAMP transcription, mostly via SMAD signalling (Figure 2). As a result, alteration or genetic mutation of proteins that act within these pathways have been associated with a variety of disorders, including primary and secondary forms of iron overload, and chronic anaemia. Consequently, these proteins, together with ferroportin, are some of the most interesting therapeutic targets that could be exploited for a variety of disorders (Table 1).
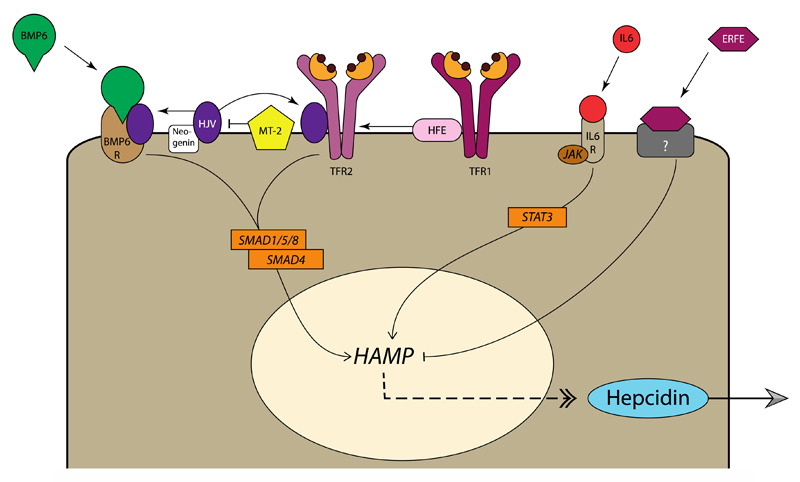
Figure 2. Regulation of hepcidin expression in hepatocytes.
Bone morphogenetic protein 6 (BMP6) is produced by non-parenchymal cells in the liver in response to iron stores and flux. Binding of BMP6 to the BMP receptor complex on hepatocytes, in conjunction with hemojuvelin (HJV) results in downstream signalling via SMAD1/5/8 and SMAD4, which in turn stimulates HAMP transcription and expression of hepcidin. HJV is stabilized by neogenin, and cleaved by matriptase 2 (MT-2), allowing for fine-tuning of BMP6 signalling. SMAD signalling is additionally regulated by transferrin receptor 2 (TfR2), upon association with human hemochromatosis protein (HFE) and HJV. HFE also interacts with TfR1, where it competes with holotransferrin. Consequently, when iron supply is high, HFE is displaced from TfR1 allowing it to associate with TfR2, thereby increasing hepcidin expression via SMAD signalling. Under inflammatory conditions, interleukin 6 (IL-6) and related cytokines bind IL-6 receptor, resulting in JAK1/2-STAT3 activation and hepcidin expression to restrict iron availability. Finally, developing erythroid cells confer their iron requirements via secretion of erythroferrone (ERFE), which inhibits hepcidin expression, via a currently unknown receptor and signalling pathway, to stimulate iron uptake under circumstances of high erythropoietic demand.
Table 1. Key players in systemic iron metabolism.
| Name | Gene | Characteristics | Function in systemic iron physiology | Dysfunctional phenotype | Refs |
|---|---|---|---|---|---|
|
Bone morphogenetic protein 6 (BMP6) |
BMP6 |
|
|
|
173,285–288 |
|
Erythroferrone (ERFE, also known as myonectin or CTRP15) |
FAM132B |
|
|
|
289,290 |
|
Ferroportin (FPN1, also known as SLC11A3, MTP1 or IREG1) |
SLC40A1 |
|
|
|
12,14–16,291–293 |
|
Hemojuvelin (HJV, also known as repulsive guidance molecule C; RGMc) |
HFE2 |
|
|
|
178,294–296 |
|
Hepcidin (also known as LEAP-1) |
HAMP |
|
|
|
9–11,13,297,298 |
| Human hemochromatosis protein (HFE, also known as HLA-H) | HFE |
|
|
|
296,299–302 |
|
Interleukin 6 receptor (IL-6R, also known as CD126) |
IL6R |
|
|
|
56,246,303,304 |
|
Matriptase 2 (MT-2) |
TMPRSS6 |
|
|
|
55,179,180,305,306 |
|
Neogenin |
NEO1 |
|
|
|
294,307,308 |
|
Transferrin receptor 2 (TfR2, also known as HFE3) |
TFR2 |
|
|
|
296,300,301,309–311 |
Cellular iron regulation
Cellular iron trafficking can be separated into three processes: intake, utilization and efflux (Figure 3, Table 2). There are four general pathways by which individual cells internalize iron. The most important pathway is centred around transferrin (Tf), an abundant plasma protein that acts a natural chelating agent with two high-affinity binding sites for ferric iron17. Tf fulfils several crucial physiological roles that include the solubilisation of relatively insoluble Fe(III), the prevention of iron-mediated redox toxicity, and the systemic trafficking of iron. Although under normal circumstances less than 0.1% of total body iron is Tf-bound, the high turnover of Tf ensures that each day 20-30 mg of iron is delivered to the bone marrow for erythropoiesis. Cellular internalization of transferrin-bound iron typically occurs after docking with the membrane-bound transferrin receptor 1 (TfR1), which has nanomolar affinity for iron-bound Tf, but not for apotransferrin (apoTf) (i.e. iron-free Tf) at physiological pH18. The Tf-TfR1 complex is internalized via clathrin-mediated endocytosis, and Fe(III) is liberated from Tf as a result of a drop in pH to 5.5. Subsequently, TfR1 and apoTf, which remain associated at this low pH, are recycled back to the cell surface, where the pH is physiological and apoTf is thus released19. In addition to the TfR-mediated uptake of transferrin, cells may also utilize an alternative, low-affinity route for transferrin internalization20.
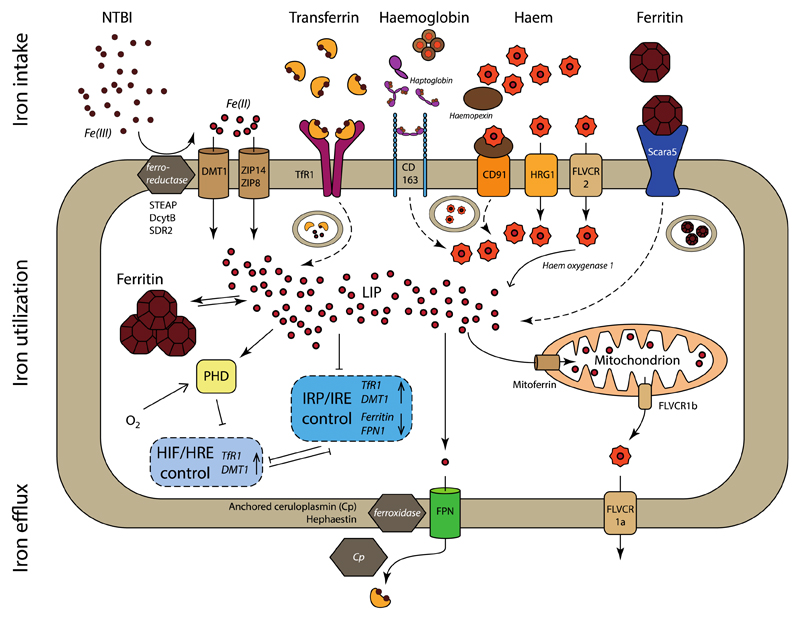
Figure 3. Cellular iron trafficking pathways.
Iron intake. Non-transferrin bound iron (NTBI) can be transported directly (solid arrow) into the cell by DMT1, ZIP14 and ZIP8, after reduction of Fe(III) to Fe(II) by ferrireductases STEAP, DcytB or SDR2. Transferrin-bound iron is taken up via binding to TfR1 and endocytosis (dashed arrow) of the receptor, release and reduction of Fe(III) and transport into the cytoplasm via DMT1. Haemoglobin associates with haptoglobin to allows endocytosis by CD163, upon which haemoglobin is degraded, and haem transported into the cytosol. Systemic haem is scavenged by complexation with haemopexin and endocytosis via CD91. Haem can be directly transported, also from the endosome, into the cytosol by HRG1 and FLVCR2, where it is processed by haem oxygenase 1 and iron released as Fe(II). Scara5 internalizes ferritin that consists of light chains, after which iron is liberated and transported to the cytosol. Ferritin composed of heavy chains can be endocytosed by TfR1 (not depicted here).
Iron utilization. The labile iron pool (LIP) comprises cytosolic Fe(II), which can be stored in cytoplasmic ferritin or utilized for biochemical processes. Most of these processes take place in the mitochondria, for which iron is supplied via mitoferrin, while FLVCR1b allows export of mitochondrially produced haem. The LIP controls the expression of TfR1, DMT1, ferritin and ferroportin via the posttranscriptional IRP/IRE regulatory system. Similarly, the transcriptional HIF/HRE regulatory system controls several intracellular processes, including the transcription of TfR1 and DMT1. HIF2α translation is inhibited by IRP1, while, conversely, IRP1 transcription is inhibited by HIF. HIF expression is regulated by PHD, which stimulates the degradation of HIFs under conditions of high oxygen.
Iron egress. Fe(II) is exported by ferroportin, followed by oxidation to Fe(III) by the ferroxidases hephaestin or ceruloplasmin, and subsequent binding to transferrin. Intracellular haem can be directly exported via FLVCR1a.
Not all pathways are present in all cells.
Table 2. Key players in cellular iron homeostasis.
| Name | Gene | Characteristics | Function in cellular iron physiology | Dysfunctional phenotype | Refs |
|---|---|---|---|---|---|
| Iron intake | |||||
|
Class A scavenger receptor 5 (Scara5, also known as TESR, NET33 or SR-A5) |
SCARA5 |
|
|
|
41,312,313 |
|
Low density lipoprotein receptor-related protein (LRP, also known as CD91 |
LRP1 |
|
|
|
37,314,315 |
|
CD163 (also known as M130, p155, haemoglobin scavenger receptor or SR-I1) |
CD163 |
|
|
|
35,36,316 |
|
Cytochrome b561, family including duodenal cytochrome b561 (Dcytb) and ferric chelate reductase 1 (also known as stromal cell-derived receptor 2; SDR2) |
CYBRD1 FRRS1 |
|
|
|
30,317–319 |
|
Divalent metal-ion transporter 1 (DMT1, also known as DCT1 or Nramp2) |
SLC11A2 |
|
|
|
21,27,194,320 |
|
Feline leukaemia virus, subgroup C, receptor 2 (FLVCR2, also known as SLC49A2) |
FLVCR2 |
|
|
|
39,321 |
|
Haem responsive gene-1 (HRG-1) |
SLC48A1 |
|
|
|
38,40,322 |
|
Six-transmembrane epithelial antigen of prostate (STEAP family proteins) |
STEAP1-4 |
|
|
|
31,32,323–325 |
|
Transferrin (Tf) |
TF |
|
|
|
17,18,326 |
|
Transferrin receptor 1 (TfR1, also known as CD71) |
TFRC |
|
|
|
18,19,42,78,327 |
|
Zrt- and Irt-like protein 8 (ZIP8) and ZIP14 |
SLC39A8 SLC39A14 |
|
|
|
22,23,29,208,328,329 |
| Iron utilization | |||||
|
Ferritin (Ftn) |
FTH1 (heavy chain), FTL (light chain) |
|
|
|
46,50,51,128,330 |
|
Haem oxygenase-1 (HO-1) |
HMOX1 |
|
|
|
331–333 |
|
Hypoxia-inducible factor (HIF) |
HIF1A (HIF1α) EPAS1 (HIF2α) HIF3A (HIF3α) |
|
|
|
209–213,334–336 |
|
Mitoferrin 1 and mitoferrin 2 |
SLC25A37 (mitoferrin 1) and SLC25A28 (mitoferrin 2) |
|
|
|
45,337 |
|
Prolyl-hydroxylase domain-containing protein (PHD, also known as HIF-PH) |
ELGN2 (PHD1) ELGN1 (PHD2) ELGN3 (PHD3 P4HTM (PHD4) |
|
|
|
216,217,219,338,339 |
| Iron efflux | |||||
|
Ceruloplasmin (Cp) |
CP |
|
|
|
49,340 |
|
Feline leukaemia virus, subgroup C, receptor 1 (FLVCR1a and FLVCR1b, also known as SLC49A1) |
FLVCR1 |
|
|
|
53,341–345 |
| Ferroportin | See Table 1 | ||||
|
Hephaestin (Hp) |
HEPH |
|
|
|
346–348 |
The second route by which cells can acquire iron is through the uptake of non-transferrin bound iron (NTBI), which can be regarded as ‘free iron’. Currently, three cellular membrane transporters have been shown to be involved in the uptake of NTBI: divalent metal-ion transporter 1 (DMT1)21, Zrt- and Irt-like protein 14 (ZIP14)22, and, most recently, ZIP823.
In 1997, DMT1 was identified as a key mammalian metal-ion transporter that is responsible for the intestinal iron absorption of various cationic bivalent metals, such as Fe(II), but also Cd(II), Co(II), Cu(II), Pb(II), Mn(II), Ni(II) and Zn(II)21. Around the same time, the inheritable microcytic, hypochromic anaemia found in mk/mk mice and Belgrade (b) rats, which was already known to be associated with an impaired intestinal uptake and erythroid utilization of iron, was shown to originate from homozygous mutations in the gene encoding DMT1, Slc11a2 24,25. The critical role of DMT1 in the absorption of dietary non-haem iron has recently been confirmed using intestinal DMT1 knockout mice26, and in humans, homozygous or compound heterozygous mutations in the SLC11A2 gene have been associated with microcytic anaemia27. However, in contrast to the rodent models, patients with DMT1 mutations are overloaded with iron, rather than deficient. This effect is likely caused by a residual capacity for iron absorption, fulfilled either by the DMT1 mutant or by an alternative (haem) absorption pathway that is further stimulated by a regulatory feedback mechanism driven by the microcytic anaemia. The microcytic hypochromic anaemia in these patients, and in the mk/mk mice and Belgrade (b) rats, is therefore primarily related to the other main role of DMT1: the transport of Fe(II) through the endosomal membrane. As demonstrated in mk/mk mice, DMT1 co-localizes with TfR1 in the endosome to handle the cellular import of iron that is liberated from the Tf-TfR1 complex. DMT1 dysfunction particularly affects the developing red blood cells, as they require a substantial supply of iron and are heavily dependent on Tf-TfR1-mediated import28.
More recently, ZIP14 has been shown to fulfil an important role in the tissue accumulation of iron by NTBI. In genetic models of iron overloading in mice, ZIP14 deficiency reduced iron content in the liver and pancreas and provided some level of protection against hereditary hemochromatosis29. Each of the iron transporters only transport soluble Fe(II), and therefore require enzymes with ferroreductive activity to reduce Fe(III) prior to internalization. Family members of cytochrome b561, the best known of which is Dcytb, which is expressed in the duodenum30, as well as STEAP, which is found in erythroid progenitor cells31, have demonstrated such ferroreductive activity32. Recently, prion proteins present on the surface of neuronal cells have been shown to possess iron-reducing capacity as well, thereby assisting cellular uptake of NTBI in the brain33,34.
The third mechanism for iron internalization is the uptake of porphyrin-bound iron in the form of Hb and haem. The scavenger receptor CD163 is almost exclusively expressed by macrophages and is specialized to take in Hb in complex with the glycoprotein haptoglobin35,36. Similarly, CD91 orchestrates the uptake of haem that is in complex with the glycoprotein hemopexin37, and although CD91 is considered a promiscuous receptor recognising other ligands, it is highly expressed by CD163-positive macrophages and may therefore be an essential route for the clearance of iron that was incorporated into Hb and other haem-containing proteins. HRG138 and FLVCR239 are also haem-importing proteins, and although their role in cellular iron trafficking needs to be further elucidated, it has been shown that HRG1 in particular is involved in the transport of haem across the lysosomal membrane upon internalization and degradation of erythrocytes and haemoglobin by macrophages40.
The fourth general pathway for iron internalization consists of the uptake of the iron-storage protein ferritin by receptors that include Scara541, TfR142, and a currently uncharacterized human ferritin receptor that could be related to TIM-2, a well-characterised ferritin receptor in mice43,44.
Once inside the cell, iron can be utilized and stored. Mitoferrin 1 and 2 are specialized transporters that move iron into the mitochondria, where it assists with cellular respiration and is used for the synthesis of Fe-S clusters and haem45. Intracellular iron can be ‘stored’ in two forms: stably incorporated into ferritin, an iron-sequestering protein that can contain up to 4,500 iron atoms46, or as active unbound species, known as the labile iron pool (LIP). Because an excess of LIP may cause oxidative stress-related toxicity, iron is generally stored as cytosolic ferritin, with the liver functioning as the primary organ for systemic iron storage. The LIP is largely responsible for the regulation of cellular iron homeostasis through the iron regulatory protein (IRP)/iron-responsive element system, which post-transcriptionally modulates the translation mRNAs of several proteins that are involved in cellular iron trafficking, including DMT1, TfR1, ferritin and Fpn47,48.
The export of iron from cells is almost exclusively managed by ferroportin (Table 1). As a result, hepcidin regulates systemic iron uptake and mobilization by inducing the degradation of ferroportin as discussed above. The multi-copper oxidases ceruloplasmin (in serum and astrocytes), hephaestin (in the intestine, placenta, heart, brain and pancreatic β-cells), and zyklopen (in placenta), assist the export of iron by ferroportin by oxidizing Fe(II) to Fe(III), thereby allowing it to be loaded onto transferrin49. There are, however, other possible mechanisms for cellular export of complexed iron. These include the secretion of iron-containing ferritin by hepatocytes50, macrophages and kidney proximal tubule cells51, which is likely mediated by autophagy of cytoplasmic ferritin and subsequent release via the secretory-lysosomal pathway51,52. Another pathway is the secretion of haem by Feline leukaemia virus, subgroup C, receptor 1b (FLVCR1b), which on the one hand may function as an ‘emergency valve’ to prevent haem overload, especially in erythroid cells, and on the other hand as a potential route for macrophages to directly supply haem to developing erythroblasts53.
Iron and the pathophysiology of disease
Iron overload, iron deficiency and anaemia of inflammation and chronic disease
Iron overload is a major clinical challenge in management of hereditary hemochromatosis and haemoglobinopathy-related anaemia, also known as iron-loading anaemia. Primary iron overload, such as occurs in patients with hereditary hemochromatosis, is directly caused by disordered iron metabolism that is associated with genetic mutations in regulatory proteins. By contrast, secondary iron overload in patients with iron-loading anaemia is not intrinsically related to iron metabolism, but rather results from the anaemia that stimulates erythropoiesis and iron absorption. Moreover, the iron overload in severely anaemic patients is further aggravated by regular blood transfusions to that aim to improve haemoglobin levels. Tissues commonly affected by iron overload include the liver, spleen, heart, pancreas, and pituitary gland, and excess iron often eventually leads to complications such as liver failure, myocardial disease, diabetes mellitus and other metabolic disorders54.
Iron deficiency occurs when the systemic demand for iron surpasses its supply. As erythropoiesis is, in terms of iron requirements, the most important physiological process, iron deficiency will typically present as iron deficiency anaemia. Increasing or supplementing the dietary iron intake corrects iron deficiency in most cases, although in a rare inherited form of anaemia known as iron-refractory iron deficiency anaemia (IRIDA), mutations in TMPRSS6 lead to inappropriately high hepcidin expression and restricted intestinal iron absorption, so intravenous iron administration is often required55. A high hepcidin level is also one of the hallmarks in anaemia of inflammation and chronic disease (AI/ACD). Under conditions of chronic inflammation, such as inflammatory diseases and cancer, increased expression of IL-6 and structurally related cytokines lead to activation of the IL-6 receptor pathway, resulting in STAT3-mediated hepcidin expression and, consequently, limited iron absorption and availability56. Additionally, activin B, a member of the TGF-β superfamily that is expressed in the liver under inflammatory conditions, was shown to induce HAMP transcription via the SMAD1/5/8 signalling pathway57. Although the expression of hepcidin can be considered a useful defence mechanism against infections, as it limits the availability of iron to invading pathogens, the presence of AI/ACD in patients with chronic diseases is a common and substantial clinical problem that can negatively affect the recovery and quality of life in these patients58.
Iron and cellular redox status
The mitochondrial generation of ATP is highly dependent on the formation and presence of ROS, and mitochondria are a major source of cellular ROS. Mitochondrial iron homeostasis is therefore of crucial importance in the control of cellular redox and potential oxidative damage. For example, in Friedreich’s ataxia, a genetic disorder affecting the expression of the mitochondrial iron-chaperone frataxin, cardiomyopathy is a major complication that has been associated to mitochondrial iron overload. Additionally, complications secondary to hereditary hemochromatosis, including liver and cardiac toxicity, have been related to ROS-mediated damage and mitochondrial pathology59.
Ferroptosis is a recently described form of controlled non-apoptotic cell death that heavily depends on iron and ROS60. Ferroptosis has been associated with a diverse range of pathologies including neurological disorders61,62, ischaemia-reperfusion injury63,64, and cancer65,66. Although the ferroptotic pathway and its function still have several unknown features, peroxidation of lipids by cytosolic ferrous iron plays a central role. Ferroptosis can be induced by blocking the system Xc− cystine/glutamate antiporter, which limits glutathione production and thus reduces oxidative protection by glutathione peroxidase 4 67, while chelation of iron protects cells from ferroptosis60. The interplay between mitochondrial iron, ROS and ferroptosis has recently been reviewed in depth68–70, and will undoubtedly uncover key therapeutic targets that can be exploited to design novel therapies for diseases associated with abnormal ferroptotic activity.
Iron and immunity
The relationship between iron and the immune system is well established. As part of the innate immune system, macrophages are highly efficient phagocytes that fulfil a central role in the first-line defence against microorganisms. In parallel, they are responsible for iron recycling from red blood cells, and are thereby solely accountable for >95% of the iron supply needed for steady-state erythropoiesis. In addition, they have an iron-independent growth-supporting function in erythroid development71,72. Although these macrophage activities appear to be quite dissimilar, a connection between iron status and macrophage phenotype has been firmly demonstrated. First of all, multiple reports have shown that high intracellular iron levels stimulate the secretion of inflammatory cytokines such as TNF-α by macrophages73,74; the clinical significance of this has been illustrated by several preclinical studies. For example, intravenous administration of ferumoxytol iron oxide nanoparticles (also known as Feraheme; AMAG Pharmaceuticals), an FDA-approved for iron replacement therapy in chronic kidney disease (see also Box 1), significantly reduced tumour growth and suppressed liver metastasis in tumour-bearing mice, and was associated with a proinflammatory phenotype in tumour-associated macrophages75. Similarly, iron-overloaded macrophages in chronic venous ulcers have a strong proinflammatory phenotype, producing of TNF-α and ROS, thereby delaying wound healing and supporting further tissue damage76. Finally, unbound haem and iron can induce a switch in the phenotype of macrophages from growth-supporting to inflammatory, which can be prevented by iron chelation or scavenging of haem by hemopexin77. Because various complications in patients with iron-loading anaemias have a chronic inflammatory component, such as liver fibrosis, leg ulcers, pulmonary hypertension and musculoskeletal inflammation, it is tempting to hypothesize that iron-mediated inflammatory activation of macrophages has a role in the pathogenesis of these complications5.
Box 1. Iron oxide nanoparticles.
Over the past few years, an enormous variety of iron oxide nanoparticles have been developed for diagnostic and therapeutic applications, although few have reached the clinic and several have been withdrawn for various reasons251. Also known as superparamagnetic iron oxide nanoparticles (SPIONs), these sterically stabilised particles typically contain magnetite (Fe3O4) and maghemite (γFe2O3), and the resulting magnetic properties make them particularly interesting as magnetic resonance imaging-contrast agents. Due to their nanosized dimensions, iron oxide nanoparticles primarily localize in phagocytic cells, mostly macrophages252, and in the liver, spleen and lymphatic tissues, so iron oxide nanoparticles can be utilized for imaging these tissues. For example, Feraheme (ferumoxytol, AMAG Pharmaceuticals), which is clinically approved for iron replacement therapy in chronic kidney disease, has been investigated as marker for the presence of macrophages in solid tumours253, and has recently been shown to modulate the activity of these macrophages towards a proinflammatory, anti-tumoural phenotype75. There are several additional promising applications of SPIONs, such as using their magnetic properties and localisation tumours to create local hyperthermia by applying an alternating magnetic field (NanoTherm, MagForce AG), or using them as drug carriers that release their payload as a result of magnetically induced heating254. While SPIONs are generally considered non-toxic, it is important to realise that there is no physiological route of iron excretion from the human body, other than a passive intestinal loss that is typically limited to 2 mg iron per day. Therefore, intravenously administered iron oxide, which would be in the range of 400 mg iron (Feraheme, NCT01336803), for example, will add to the total iron pool and may, in parallel, cause an proinflammatory phenotypic switch in the macrophages clearing these nanoparticles75. Further evaluation regarding the long-term tolerability of SPIONs as routinely used imaging agents may be therefore warranted.
Iron homeostasis infuences adaptive immunity as well. For example, a form of immunodeficiency characterized by impaired development and function of lymphocytes has been attributed to mutations in the gene encoding TfR1, TFRC, which affects internalization of the surface receptor78. Although iron deficiency is the underlying cause of this disorder, illustrated by the reversal of defective lymphocyte proliferation by Fe(III) supplementation, interestingly, patients are only mildly anaemic.
Another connection between iron and immunity is related to the defence against invading pathogens. Microorganisms, as for all other forms of life, are highly dependent on the presence of iron to support growth and proliferation79. As a consequence, to limit infectivity of invading microorganisms, host organisms have developed strategies to restrict iron supply to pathogens. NTBI is considered an important source of iron for microbes, which some of them sequester by releasing iron-binding proteins known as siderophores. Capture of these siderophores, as well as NTBI, by macrophage-produced lipocalin-2 is therefore an effective defensive response to infection80,81. Another host mechanism to limit NTBI availability under inflammatory conditions is to block cellular iron egress by reducing ferroportin expression82. This inflammation-induced reduction of ferroportin is mainly driven by upregulation of hepcidin transcription via IL-6R-activated JAK/STAT3-signalling (Figure 2, Table 1), and via TGF-β1−TGF-β1R-mediated activation of SMAD signalling, which is distinct from BMP6-induced SMAD signalling83. Alternatively, ferroportin reduction is also controlled by hepcidin-independent mechanisms, such as signalling via Toll-like receptors 2 and 6, allowing for a rapid systemic response under inflammatory conditions84.
Iron and cancer
Changes in cellular redox status have a considerable role in malignant transformation and metastasis of cancer cells85, and consequently, many researchers have proposed and investigated the potential relationship between malignancies and iron86,87. Although several studies have indeed shown a correlation between the incidence and mortality of cancer and systemic iron levels, as measured by transferrin saturation or serum ferritin, this correlation is generally not strong, has shown conflicting outcomes, and it is often confounded by the presence of other pathology88,89. There is, however, clear evidence that deregulated iron homeostasis on a local — that is, microenvironmental and/or cellular — level is associated with tumour progression, as illustrated by changes in expression of iron-related genes in cancer cells (Figure 4), the levels of which are inversely correlated with patient survival90–92. In the context of tumour progression, one can envision that proliferating cancer cells, which require substantial amounts of iron to support their growth, will attempt to increase their iron intake, for example by increasing the expression of TfR193–95 and STEAP396,97; to modulate iron storage capacity by producing ferritin94,96; and to limit their iron export by downregulating ferroportin91,98–100. However, the additional intracellular oxidative stress that is accompanied by the increased presence of labile iron may also make them more sensitive to ferroptosis65,67 and hinder tumour metastasis85, illustrating the complex relationship between iron, ROS and cancer cell survival. Furthermore, although a connection between iron and cancer growth has been demonstrated in several forms of cancers, with breast cancer90,91,99–101, prostate cancer98,102,103 and glioblastoma as established examples94,104, it seems reasonable that many, but not all, cancer types will demonstrate a similar iron dependency.
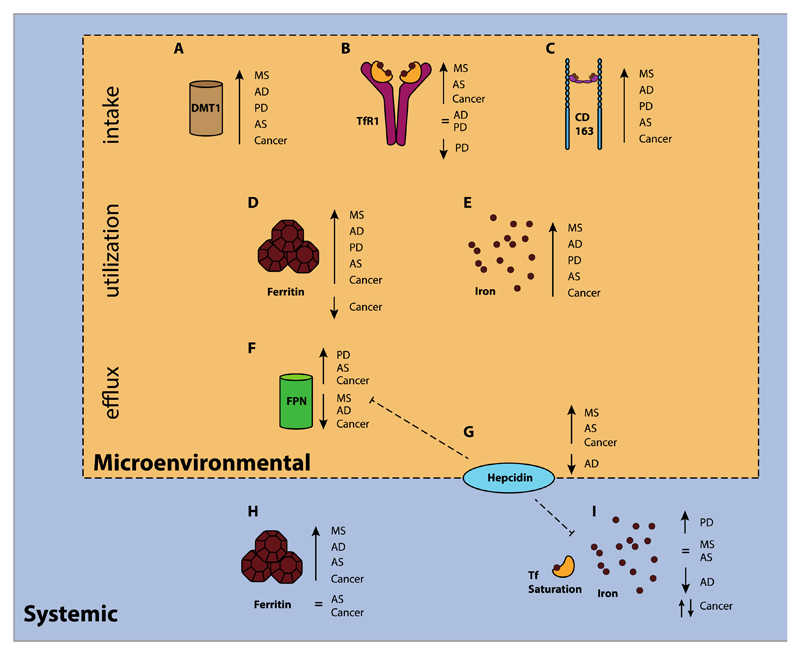
Figure 4. Up- and downregulation of several key players in non-haematological pathologies.
Schematic overview illustrating the microenvironmental and systemic changes in iron and iron metabolism-related proteins in selected pathologies in patients: multiple sclerosis (MS, sclerotic plaques), Alzheimer’s disease (AD, frontal cerebral cortex), Parkinson’s disease (PD, basal ganglia), atherosclerosis (AS, sclerotic plaques), and cancer (tumor tissue).
A. Upregulation of DMT1 has been observed in MS (preclinical)123, AD (preclinical)255, PD256, and cancer (colorectal)257. B. TfR1 upregulation has been found in MS258, atherosclerosis259, and cancer (colorectal257, breast95,101, glioblastoma94; normal TfR1 levels have been observed in AD260,261, while for PD normal levels262, and low levels have been reported263,264. C. Increased levels of the macrophage-associated scavenger receptor CD163 have been found in affected tissues of patients with MS265, AD266, PD266, AS267, and cancer (breast268, prostate269, glioblastoma270. D. Tissue iron stored as ferritin has found to be increased in MS271, AD272, PD264, AS259,273, and for cancer both high levels (glioblastoma)94 and low levels have been reported (breast)101. E. Similarly, microenvironmental iron deposits have been observed in MS271, AD271,272,274, PD256,264,274, AS273, and cancer (colorectal)257. F. Upregulation of FPN has been demonstrated in tissues of patients with PD264, AS273, and cancer (colorectal257, breast101), while low levels of FPN have been observed in MS (preclinical)123, AD120 and also in breast91 and prostate98 cancer. G. Hepcidin levels are not in all diseases consistent with (an inversed) FPN expression. High hepcidin concentrations have been found in MS (preclinical, tissue)123, AS (serum)275, breast cancer (tissue91,101 and serum276) and prostate cancer (tissue)98, whereas a low level has been observed in the tissues of patients with AD120. H. Increased systemic ferritin concentrations have found in the cerebrospinal fluid (CSF) of patients with MS277, AD127, and glioblastoma278, as well as in the serum of patients with AS279 and breast cancer276, although the levels of serum ferritin have also been found to be normal in patients with AS275 and non-skin cancers280. I. Systemic iron levels measured by iron concentration and transferrin saturation have been quite inconsistent: higher than average levels have been found in a meta analysis in PD281, normal levels have been found in MS282 and AS275, and low levels have been observed in AD283,284. With regard to cancer, the association with systemic iron levels is quite unclear: women with high levels of serum iron were at higher risk for developing non-skin cancer, while men were at lower risk280.
Iron and neurodegenerative diseases
Iron homeostasis in the central nervous system (CNS) differs from that in other tissues105. Endothelial cells of the blood−brain barrier modulate iron influx into the CNS by importing iron from the systemic circulation via TfR1 and DMT1, and subsequently releasing iron via ferroportin at the basal side into the brain interstitium, forming a control mechanism reminiscent of the absorption of iron in the duodenum106,107. The presence of an additional layer of homeostatic regulation suggests that the brain may be spared from excessive iron accumulation in iron-overloaded patients, which is supported by the fact that large iron deposits in the brain are not typically found in patients with hemochromatosis108. Nevertheless, genetic mutations associated with an inherited form of hemochromatosis are overrepresented in patients with Parkinson’s disease (PD), parkinsonism and amyotrophic lateral sclerosis (ALS)109,110, indicating that cellular, rather than systemic, disturbances in brain iron homeostasis may have a role in the pathogenesis in these diseases.
Although progressive neurological disorders have been linked to disturbances in other metals such as copper (a hallmark of Wilson’s Disease, but also found in Alzheimer’s Disease (AD)111) and zinc (e.g. in PD112), many studies have implicated cellular iron overload and iron-induced oxidative stress in the development of PD, ALS, AD, Huntington’s disease, multiple sclerosis (MS) and several other neurodegenerative diseases (Figure 4)113,114. As an interesting example, prion protein (PrPC) has been shown to promote the reduction of Fe(III) to Fe(II), enabling its uptake into cells by ZIP14 and DMT133,34. However, the conversion of PrPC to its isoform PrPSc, which is implicated in neurological prion diseases such as Creutzfeldt–Jakob disease, is promoted by unbound iron115, and PrPSc can bind to ferritin to form an insoluble complex, thereby stimulating the cellular intake of iron and promoting the formation of additional PrPSc 116.
Astrocytes, glial cells in the CNS that have a function in the control of nutrient supply and brain remodelling, are thought to fulfil, in concert with endothelial cells of the blood–brain barrier, a critical role in regulating iron homeostasis in the brain106,117. Astrocytes produce hepcidin in response to inflammatory signalling and intracellular iron118, suggesting a control mechanism via ferroportin, similar to the regulation of systemic iron levels by hepatocytes. This regulatory feedback loop allows for local control over iron trafficking within the CNS, although the abundance of hepcidin in the brain interstitium suggests that at least some fraction of it is of systemic (likely hepatic) origin119. Low levels of ferroportin have been found in brains of patients with AD120, as well as in rat models of PD121 and ALS122. In a mouse model of MS, reduced expression of ferroportin and intracellular iron overload were specifically found in macrophage-like microglia, but not in astrocytes123. Moreover, iron-overloaded microglia can be found in active MS lesions and have a pro-inflammatory phenotype that inhibits CNS repair, thus contributing to disease progression124,125. Neurological dysfunctions are also found in patients with aceruloplasminaemia: these individuals lack functional ceruloplasmin, a protein that facilitates iron removal from tissues by oxidising exported Fe(II) to Fe(III), thereby promoting its association with transferrin. This genetic disease is characterized by a loss of neurons, particularly in the basal ganglia, and by astrocytes with an attenuated iron efflux capacity and iron-overload126. Finally, in a clinical prospective study, higher ferritin levels in the cerebrospinal fluid were found to be correlated with a lower cognitive function and brain structural changes in patients with AD, but this correlation seemed to also be present in individuals that were considered cognitively normal127. Interestingly, genetic mutations that affect the ferritin light chain, which probably makes the iron-storage protein ‘leaky’, lead to a heterogeneous neurological clinical presentation, known as neuroferritinopathy114,128. This raises the question of whether dysfunctional ferritin or malfunctions in its cellular processing may have some involvement in the pathogenesis of other neurodegenerative diseases129.
Iron and atherosclerosis
In atherosclerotic plaques, iron is present in much higher concentrations that it is in healthy tissues (Figure 4)130. The function of these iron deposits in atherosclerosis, and whether iron accumulation has a causative role or is a mere consequence of the pathogenic process, for example due to haemolysis, is still under debate. It seems reasonable to suggest that iron could be directly involved by catalysing the formation of oxidized low-density lipoprotein (LDL) in the arterial wall131, which is considered one of the hallmarks of atherosclerosis. However, in iron-overloaded patients with type 1 hemochromatosis the incidence of atherosclerosis is actually lower, rather than higher, than it is in the rest of the population132. Interestingly, the iron status of macrophages, which fulfil a key role in the development and progression of atherosclerotic plaques133,134, could explain this paradox, as macrophages of patients with this form of hemochromatosis contain less iron than do those of healthy individuals. Conversely, patients with β-thalassemia, a disorder that is accompanied by iron-loading especially of macrophages, indeed do have a higher risk of developing atherosclerosis, confirming the so-called ‘iron hypothesis’ in atherosclerosis135,136. A relationship between macrophages, iron and atherosclerosis could partially be owing to iron’s direct role in the oxidation of LDL by macrophages, as well as a phenotypic change in macrophages due to iron sequestration as described above. Nevertheless, various experimental studies that have investigated intracellular iron accumulation in macrophages as a driving mechanism for atherosclerosis have produced conflicting results, suggesting that iron takes part in a complex network involving several underlying mechanisms137–141.
Iron and other ageing-associated diseases
Besides cancer, neurodegenerative disorders and atherosclerosis, various other pathologies have been linked to iron homeostasis. In diseases such as type 2 diabetes mellitus142, osteoporosis143, osteoarthritis144, macular degeneration145, and liver fibrosis146, high levels of iron have been associated with disease severity, suggesting a possible role in pathogenesis or disease progression. Importantly, many of these disorders are considered chronic ageing-associated diseases of multifactorial aetiology, and are substantial causes of mortality and morbidity in the ageing Western society. This stresses the need for a thorough exploration of the role of iron dyshomeostasis in these diseases, as well the utility of targeting iron metabolism as a therapeutic approach or as a strategy to deliver pharmaceutical agents.
Iron metabolism as a therapeutic target
Therapeutics targeting systemic iron availability
The use of iron-chelating agents is a straightforward approach for limiting and redistributing systemic iron availability, and is therefore frequently used in the clinical management of primary or secondary iron overload. The currently available chelators are deferoxamine (Desferal, Novartis), deferiprone (Ferriprox, ApoPharma), and deferasirox (Exjade, Novartis). More than 50 years ago, desferoxamine (DFO) was the first chelator that showed clinical promise147. Iron chelation therapy was then rapidly demonstrated to be an effective strategy for mobilizing and removing iron via faecal and urinary excretion, illustrated by a marked decrease in mortality and iron-related complications in patients with thalassemia major148. Despite its success, DFO has poor bioavailability and requires parenteral administration that is often painful, and which should be executed slowly (up to 10h infusion) and regularly (5-7 times a week), making patient compliance a major limitation. In the following decades, the oral iron chelators deferiprone (DFP)149 and deferasirox (DFX)150 were developed and approved for the treatment of iron overload, improving iron chelation therapy patient satisfaction as compared to treatment with DFO151. Both DFO and DFX are currently recommended as first-line chelation therapy in iron overload, but the toxicity profile of these compounds, which include hypersensitivity reactions, liver dysfunction, renal dysfunction and neuronal hearing loss152, warrant further developments in iron chelation therapies. For example, in preclinical models of iron-loading anaemia, the administration of unmodified Tf, which can be considered the physiological iron chelator, has effectively redistributed iron to developing erythroid cells153.
In addition to iron overload disorders, iron chelation has also been extensively investigated as an adjuvant therapy in other disorders. In the 1990s, clinical trials of iron chelators in patients with neuroblastoma showed antitumor efficacy154,155, which encouraged further exploration of iron chelation therapy in cancer. Several novel chelating agents have been explored, of which one is currently under clinical development (DpC, Oncochel Therapeutics)156. In parallel, iron chelation has also shown clinical potential in neurodegenerative disorders, including multiple sclerosis157, Alzheimer’s158 and Parkinson’s disease159, thereby confirming the involvement of iron in the pathogenesis of these disorders.
Since the discovery of hepcidin in the beginning of this millennium, a substantial number of agents stimulating or blocking its expression or activity have been investigated (Table 3). Several short hepcidin derivatives have been designed as hepcidin-mimicking agents with an improved pharmacokinetic profiles, and are currently under development by Merganser Biotech (M012, phase 1)160, La Jolla Pharmaceutical Company (LJPC-401, phase 1, see Further information), and Protagonist Therapeutics (PTG-300, preclinical, see Further information). Interestingly, preclinical studies have not only demonstrated beneficial activity of hepcidin derivatives in several models for red blood cell and iron overload disorders, such as β-thalassemia, polycythaemia vera, and hemochromatosis161,162, but also as an antimicrobial agent, as it limits the availability of iron to certain types of microorganisms82. Conversely, therapeutics that bind to and inactivate hepcidin have been developed and studied for the treatment of anaemia of inflammation and chronic disease. Such hepcidin-binding agents include humanized monoclonal antibodies (LY2787106, discontinued after phase 1 in 2015, Eli Lilly163; and 12B9m, Amgen Inc164), an anticalin (PRS-080, Pieris Pharmaceutical)165 and a L-RNA spiegelmer (Lexaptepid pegol (NOX-H94), NOXXON Pharma)166,167.
Table 3. Therapeutic agents targeting iron metabolism under commercial development.
| Target | Name | Company | Type | Mechanism | Indication | Development status | Trial identifiersa |
|---|---|---|---|---|---|---|---|
| BMP6 receptor pathway | LY3113593 | Eli Lilly and Company | Humanized antibody | Capture of BMP6 to limit hepcidin expression | Anaemia | Phase 2 (lost through attrition 2016; see Further information) |
NCT02604160 NCT02144285 |
| FMX-8 | FerruMax Pharmaceuticals, Inc | HJV-Fc fusion protein | Capture of BMP6 to limit hepcidin expression | Anaemia | Phase 2 (terminated trials 2016) |
NCT02228655 NCT01873534 |
|
| Ferroportin | LY2928057 | Eli Lilly and Company | Humanized IgG4 antibody | Binding of ferroportin to prevent its degradation by hepcidin, thereby stabilizing ferroportin expression | Anaemia | Phase 2 (removed from pipeline 2016) |
NCT01991483 NCT01330953 |
| Hepcidin | M012 | Merganser Biotech Australia Pty Ltd. | Peptide | Hepcidin mimetic peptide that functions as a hepcidin agonist | β-thalassemia, low risk myelodysplastic syndrome, polycythaemia vera | Phase 1 | ACTRN12616000093482 |
| LJPC-401 | La Jolla Pharmaceutical Company | Peptide | Hepcidin mimetic peptide that functions as a hepcidin agonist | Iron overload | Phase 1 | ACTRN12616000132448 | |
| PTG-300 | Protagonist Therapeutics | Peptide | Hepcidin mimetic peptide that functions as a hepcidin agonist | Iron overload | preclinical | n.a. | |
| PRS-080 | Pieris Pharmaceuticals GmbH | Anticalin | Bioengineered lipocalin that captures hepcidin to prevent ferroportin degradation | Anaemia, functional iron deficiency | Phase 1b |
NCT02754167 NCT02340572 |
|
| NOX-H94 (lexaptepid pegol) | NOXXON Pharma AG | L-RNA spiegelmer | L-stereoisomeric RNA aptamer that captures hepcidin to prevent ferroportin degradation | Anaemia | Phase 2 |
NCT02079896 NCT01691040 NCT01522794 NCT01372137 |
|
| 12B9m | Amgen Inc | Human IgG2 antibody | Capture of hepcidin to prevent ferroportin degradation | Anaemia | Preclinical | n.a. | |
| Iron | Deferoxamine (Desferal) | Novartis | small molecule | Chelation of iron | Iron intoxication and overload | Marketed | |
| Deferasirox (Exjade) | Novartis | Small molecule | Chelation of iron | Iron overload | Marketed | ||
| Deferiprone (Ferriprox) | ApoPharma Inc. | Small molecule | Chelation of iron | Iron overload | Marketed | ||
| DpC | Oncochel Therapeutics | Small molecule | Chelation of iron | Advanced solid tumours | Phase 1 | NCT02688101 | |
| Matriptase-2 | IONIS-TMPRSS6-LRx | Ionis Pharmaceuticals | Antisense DNA oligo-nucleotide | Inhibition of MT-2 expression to promote hepcidin transcription | β-thalassemia | Preclinical | n.a. |
| ALN-TMP | Alnylam | siRNA | Inhibition of MT-2 expression to promote hepcidin transcription | β-thalassemia, iron overload | Preclinical | n.a. | |
| Prolyl hydroxylase | FG-4592 / ASP1517 (Roxadustat) | FibroGen Astellas Pharma AstraZeneca |
Small molecule | Inhibition of prolyl hydroxylases to stabilize HIFs | Anaemia in chronic kidney disease and end-stage renal disease | Phase 3 |
NCT02805374 NCT02780726 NCT02779764 NCT02780141 |
| GSK1278863 / GSK1278863A(Daprodustat) | GlaxoSmithKline | Small molecule | Inhibition of prolyl hydroxylases to stabilize HIFs | Anaemia in chronic kidney disease Wound healing |
Phase 3 |
NCT02879305 NCT02876835 NCT02829320 NCT02791763 |
|
| AKB-6548 (Vadadustat) | Procter & Gamble Akebia Therapeutics Mitsubishi Tanabe Pharma |
Small molecule | Inhibition of prolyl hydroxylases to stabilize HIFs | Anaemia in chronic kidney disease | Phase 3 |
NCT02892149 NCT02865850 NCT02680574 NCT02648347 |
|
| BAY85-3934 (Molidustat) | Bayer HealthCare AG | Small molecule | Inhibition of prolyl hydroxylases to stabilize HIFs | Anaemia in chronic kidney disease | Phase 2 |
NCT02312973 NCT02064426 NCT01975818 NCT02021409 |
|
| JTZ-951 | Japan Tobacco Inc. | Small molecule | Inhibition of prolyl hydroxylases to stabilize HIFs | Anaemia in chronic kidney disease | Phase 2 |
NCT02805244 JPRN-JapicCTI-152892 JPRN-JapicCTI-152891 JPRN-JapicCTI-152881 |
|
| ZYAN1 | Cadila Healthcare Ltd | Small molecule | Inhibition of prolyl hydroxylases to stabilize HIFs | Anaemia in chronic kidney disease | Phase 1 | CTRI/2016/02/006665 | |
| JNJ-42905343 | Johnson & Johnson | Small molecule | Inhibition of prolyl hydroxylases to stabilize HIFs | Anaemia, functional iron deficiency | Preclinical | n.a. | |
| Transferrin receptor 1 | MBP-426 | Mebiopharm Co., Ltd | Liposomal oxaliplatin functionalized with transferrin | Drug targeting to highly TfR1-expressing tumours | Gastric, gastro-oesophageal, and oesophageal adenocarcinoma | Phase 2 (last trial initiated 2009) |
NCT00964080 NCT00355888 |
| SGT-53 | SynerGene Therapeutics, Inc. | Liposomal plasmid DNA encoding p53 functionalized with TfR1-targeting single-chain variable fragment | Gene therapy to highly TfR1-expressing tumours and that crosses the blood-brain barrier | Solid tumours | Phase 2 |
NCT02354547 NCT02340156 NCT02340117 NCT00470613 |
|
| SGT-94 | SynerGene Therapeutics, Inc. | Liposomal plasmid DNA encoding RB94, functionalized with TfR1-targeting single-chain variable fragment | Gene therapy to highly TfR1-expressing tumours | Solid tumours | Phase 1 | NCT01517464 | |
Most recent clinical trials (4), ordered by date of registration on the WHO International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/Default.aspx)
A similar ferroportin-reducing effect can be achieved with agents that target ferroportin directly, blocking the binding of hepcidin and preventing internalization and degradation of the iron exporter protein. The structure of ferroportin has not yet been resolved, but the solved crystal structure of a bacterial homologue and modelling of human ferroportin strongly suggest that the transporter comprises 12 transmembrane domains with a central pore168,169, sharing structural features with the major facilitator superfamily (MFS) of transporters. The cys326 residue that is essential for the binding of hepcidin is likely located inside the central pore, which could indicate that binding of hepcidin could structurally block ferroportin function before internalization has taken place169,170. Fursultiamine, a small molecule also known as thiamine tetrahydrofurfuryl disulfide that targets the cys326 residue of ferroportin, indeed reduced hepcidin-mediated internalization of ferroportin and stimulated cellular export of iron, however its function in vivo is limited due to rapid conversion of fursultiamine into thiamine171. An antibody against ferroportin (LY2928057, Eli Lilly), which has been explored clinically, has had more success in vivo172. However, the largest challenge of any strategy directly targeting hepcidin or its receptor, ferroportin, is an underlying homeostatic feedback mechanism that may cause a rebound in hepcidin expression, thereby likely abolishing a large part of any long-term therapeutic effect164. This feedback loop may explain why the development of LY2928057 seems to have been discontinued upon completion of a phase 2 trial in 2015 (NCT01991483).
Due to the homeostatic control over hepcidin expression, approaches that interfere with the underlying regulatory pathways may therefore be more useful, although none has thus progressed past phase 2 of pharmaceutical development. Several strategies have inhibited hepcidin expression by modulating the BMP6/SMAD pathway. Capturing BMP6 to prevent its binding to the BMP6 receptor (BMP6R) has been investigated using an humanized antibody (LY3113593, Eli Lily, lost through attrition in 2016 (see Further information, NCT02604160), a fusion protein of hemojuvelin (HJV) and the Fc domain of IgG (FMX-8, phase 2 trials have been terminated in 2016 due an inability to recruit patients meeting eligibility criteria, FerruMax Pharmaceuticals173), or non-anticoagulant heparin-derivatives that bind BMP6 in preclinical studies174. A second strategy aims to reduce BMP6-induced hepcidin transcription downstream of the BMP6−BMP6R interaction, by blocking SMAD phosphorylation using dorsomorphin175 or its derivatives176, or by siRNA-induced degradation of mRNA encoding for TfR2 (ALN-HPN, discontinued for unknown reasons, Alnylam Pharmaceuticals Inc)177.
A hepcidin agonistic strategy comprises inhibition of matriptase 2 (MT-2)-induced cleavage of HJV. HJV is a coreceptor in the BMP6R complex that is required for iron sensing and hepcidin induction178, and MT-2 cleaves HJV, thereby suppressing BMP/SMAD-induced hepcidin expression179,180. MT-2 is a type II transmembrane serine protease that is structurally similar to matriptase, an endothelial enzyme associated with tumour development. Although a crystal structure of MT-2 is not yet available, modelling of the active domain has identified a number of residues that can be targeted, which allows the development of small-molecular inhibitors against MT-2181,182. Serum-stabilized antisense DNA (IONIS-TMPRSS6-LRx, Ionis Pharmaceuticals183,184) or liposomal siRNA (ALN-TMP, Alnylam Pharmaceuticals Inc185), block MT-2 mRNA translation, and have shown preclinical efficacy in animal models of β-thalassemia and iron overload. In addition, several physiological protease inhibitors have demonstrated specific MT-2-inhibiting activity 186, and some of them have a Kunitz domain that could be an important lead for further development of hepcidin-inducing peptides187.
The IL6-JAK1/2-STAT3 pathway is a second regulatory pathway that induces hepcidin expression. The isoflavone genistein promotes phosphorylation of STAT3, in addition to SMAD4, and thereby induces hepcidin expression in zebrafish, but its activity in a preclinical or clinical setting needs to be evaluated188. Conversely, inhibition of JAK by kinase inhibitors effectively reduces hepcidin expression in vitro (1,2,3,4,5,6-hexabromocyclohexane189) and in vivo (AG 490190), and similar effects have been obtained by disrupting STAT3 dimerization using a STAT3-binding peptide191.
Therapeutics targeting cellular iron trafficking
For many diseases disordered iron metabolism typically occurs only on a cellular level, whereas systemic iron homeostasis seems unaffected. Therapies that target cellular iron uptake, storage or efflux, may therefore be particularly promising. Several compounds target cellular uptake of iron, and DMT1 in particular has been a frequent target in preclinical studies. Structural modelling of human DMT1192,193, as well as the crystal structure of ScaDMT, a prokaryotic DMT1 homologue found in Staphylococcus capitis194, suggest that DMT1 is a 12 transmembrane protein of 2-fold symmetry and contains a hydrophobic core that coordinates the coupled transfer of a bivalent metal ion and a proton into the cytoplasm.
Several small molecule inhibitors of DMT1-mediated iron transport have been investigated. Ferristatin restricts cellular intake of free iron in vitro, but its efficacy in vivo has not yet been evaluated195. Although the inhibitory effect of ferristatin was at first attributed to degradation of TfR1196, subsequent work demonstrated that ferristatin directly inhibited activity of DMT1195. Similarly, ferristatin II is a structurally unrelated compound that has shown potent TfR1-inhibiting properties, mediated via DMT1 internalization, in vitro, as well as in vivo197,198. Interestingly, ferristatin II also induces hepcidin expression via JAK/STAT3 signalling in vivo199. However, the metabolisation of ferristatin II, also known as direct black 38 or chlorazol black, includes the formation of benzidine, which is classified by the WHO as Group 1 carcinogen (carcinogenic to humans)200, thereby limiting its translation for therapeutic purposes. The selenium-containing drug ebselen, which is known for its antioxidant activity that resembles glutathione peroxidase, strongly inhibits DMT1-mediated uptake of iron (IC50 ≈0.2 uM)201, so could have therapeutic potential in treating iron-related oxidative stress in a range of disorders, as has been shown preclinically for iron-induced cardiomyopathy202. Comparably potent DMT1 inhibition was achieved with pyrrolidine dithiobarbamate (PDTC, IC50 ≈1.5 μM) and Δ9-tetrahydrocannabinol (THC), which also have antioxidant activity201. Recent screening studies have identified a series of pyrazole derivatives and benzylisothioureas as potent inhibitors of DMT1 both in vitro (IC50 <1 μM) and in vivo (30-85% inhibition, 50 mg/kg, taken orally)203–205. A particularly interesting, but not yet explored strategy to limit iron overload may be the oral administration of a non-bioavailable DMT1 inhibitor, i.e. a compound that is not systemically absorbed and therefore likely has no systemic off-target effects, but would inhibit absorption of iron from the intestine. Although the capacity of the intestine to absorb haem-bound iron206 and nanosized particulate iron (such as the ferritin core)207, which bypass the DMT1 absorption pathway via unknown mechanisms, clearly forms a potential limitation of this approach, combining intestinal DMT1 inhibition with dietary restrictions could be a relatively safe therapeutic option in the treatment of primary or secondary iron overload.
The central role of ZIP14 in transport of NTBI into tissues that are typically affected by iron overload, especially the liver and pancreas29, makes the inhibition of ZIP14 an appealing strategy for the clinical management of iron overload. Similar to other members of the ZIP transporter family, ZIP14 is predicted to comprise eight transmembrane domains, which are all involved in the transport of bivalent metals208. Whether ZIP14 is a sufficiently druggable target still needs to be evaluated.
The hypoxia-inducible factor (HIF) transcription factors, which are composed of a heterodimerized α- and β-subunit, may be another very attractive target for interfering with iron metabolism209. HIFs modulate, by binding to a hypoxia response element (HRE) in the promoter of a target gene, the expression of a wide range of proteins that are involved in cellular oxygen status, glucose metabolism, angiogenesis and erythopoiesis. In parallel, the promoters of several genes encoding key iron trafficking proteins, namely DMT1210,211, DCytB211, TfR1212, and FPN213, especially those with roles in intestinal cells, also contain such HREs and have been shown to be susceptible to HIF regulation. As a consequence, inhibitors of HIFs, for example those that bind within the C-terminal PAS domains of HIF-2α to prevent HIF-2 heterodimerization214, may form an appealing strategy for limiting iron absorption and iron overload215. The oxygen-sensitive prolyl-4-hydroxylases (PHDs) are physiological intracellular inhibitors that regulate the post-translational expression of HIFs by promoting the hydroxylation of two proline residues in its α-subunit, labelling it for recognition by the VHL-E3-ubiquitin ligase complex and subsequent degradation216,217. PHD2, which seems to be most dominant of the three PHDs with respect to HIF regulation, is a homotrimeric protein that contains a coordinated Fe(II) and a hydrophobic pocket at its active site, and requires 2-oxoglutarate (2-OG) as a co-substrate218. By using 2-OG analogues as competitive substrates to pharmacologically inhibit PHDs, it is possible to stabilize HIF expression, which is a very promising strategy for promoting erythropoiesis in patients with anaemia219. Although the direct effect of HIF stabilization on the expression of erythropoietin is a key characteristic of this strategy, PHD inhibition also improves duodenal iron adsorption and utilization, as illustrated by an improved mean corpuscular volume (MCV) and mean corpuscular haemoglobin (MCH) of erythrocytes in a rat model for anaemia of inflammation and chronic disease220. Moreover, increased HIF-1 expression by inhibition of PHD2 improved neuronal survival in models of PD, likely due to an increased expression of the lysosomal proton pump ATP13A2, which reduced the cytosolic iron pool by improving lysosomal iron storage conditions221.
A substantial number of pharmaceutical companies, as well as one tobacco company, are actively pursuing the development of such inhibitors for the treatment of anaemia in chronic kidney disease and end-stage renal disease. Promising candidates that have already entered phase 3 clinical trials include FG-4592 (Roxadustat, Fibrogen/Astellas Pharma/AstraZeneca222), GSK1278863 (Daprodustat, GlaxoSmithKline223), and AKB-6548 (Vadadustat, Akebia Therapeutics224), and several others are currently being evaluated in a phase 2 (BAY85-3934, Molidustat, Bayer Pharma AG225; JTZ-951, Japan Tobacco226), phase 1 (DS-1093, Daiichi Sankyo (discontinued in 2016)); ZYAN1, Zydus Cadila227), or preclinical (JNJ-42905343, Johnson & Johnson220) setting.
Drug targeting strategies exploiting iron metabolism
Iron and iron-based materials have been widely researched for drug targeting and diagnostic applications, and iron oxide nanoparticles have especially received attention due to their superparamagnetic properties that can be used for therapeutic and diagnostic purposes (Box 1). The metabolism of iron can be utilized for the delivery of therapeutics to specific sites in the body by targeting iron-related proteins that are upregulated in certain tissues and pathological processes (Figure 5). Targeting TfR1 using drugs and drug delivery systems functionalized with transferrin, antibodies, antibody-derivatives, or TfR-targeted peptides, is a commonly employed and arguably the best-known strategy, as illustrated by its usage as a prototypical system for mechanistic studies of active targeting to cancer cells228. An impressive amount of different TfR-targeted delivery systems have been designed and evaluated for cancer therapy by exploiting the increased expression of TfR in some tumours, and this strategy has shown preclinical benefit when using a low-dose regimen229. At least three TfR-targeted systems are currently under clinical development (Table 3)(MBP-426, Mebiopharm; SGT-53, SGT-94, SynerGene Therapeutics)230,231.
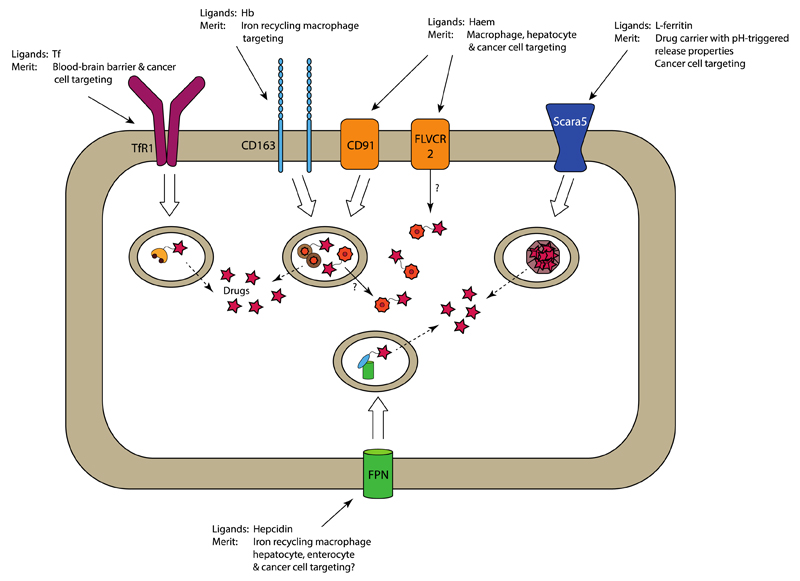
Figure 5. Proposed mechanisms for targeted drug delivery exploiting iron metabolism-associated cellular targets.
The natural ligand and the merits of its use as a delivery strategy are presented for each target. TfR1, CD163 and Scara5 allow endocytosis of drugs associated with their natural ligands. Subsequent lysosomal processing would then liberate the targeted drug, which then enters the cytosol by passive diffusion from the lysosome. This likely also occurs for haem-targeted constructs upon internalization by CD91, but possibly direct transport of the haem-linked drug into the cytosol by HRG1 and/or FLVCR2 could represent an alternative mechanism. Drug targeting to FPN, for example by using hepcidin, has not been explored until now, but forms an interesting approach for directing therapeutics to cell that overexpress FPN, such as some types of tumour cells. Tf, transferrin; Hb, haemoglobin; FPN, ferroportin
An elegant strategy that has been evaluated in preclinical cancer models is the application of transferrin-artemisinin conjugates as cytotoxic agents232. Artemisinin, discovered and developed by Tu Youyou, for which she was awarded the 2015 Nobel Prize in physiology and medicine, is a drug with antimalarial activity that is isolated from the herb Artemisia annua and contains an intramolecular peroxide bridge. Fe(II) is involved in the activation of artemisinin by cleavage of this endoperoxide bridge, resulting in the formation of reactive carbon-centred radicals and subsequent cytotoxicity233. By using transferrin as a targeting moiety, both iron and the inactive artemisinin can be delivered to TfR-overexpressing cancer cells; upon internalization of the complex the released Fe(III) is oxidized to Fe(II), which in turn reduces and activates artemisinin into its cytotoxic form232. However, the effect of such artemisinin-constructs on other tissues with high TfR1 expression, notably the bone marrow, has not yet been reported. A second notable application of TfR-targeting therapeutics is the transport of drugs and drug delivery systems across the blood−brain barrier. The endothelial cells of the blood−brain barrier transcytose TfR-transferrin complexes to the brain parenchyma to fulfil the iron requirements of the CNS. Exploiting this mechanism, transferrin and antibodies that target TfR can be used as a Trojan horse to deliver drug carriers and macromolecules to the brain, but future evaluation should demonstrate its practical potential in a clinical setting234,235.
Ferritin is a commonly used biomolecule for drug targeting, as it can efficiently encapsulate large amounts of drugs because of its ‘nanocage’ structure236. Ferritin can function as a ligand as well to target cells that highly express ferritin-internalizing receptors, such as Scara5 and TfR1237. Moreover, ferritin can be disassembled and reassembled by changing the pH to low or physiological, respectively, which facilitates drug loading and endows the protein with pH-triggered drug release properties, enabling discharge specifically upon uptake and lysosomal processing by the target cell238.
A relatively specific approach to target a subset of macrophages is the use of haemoglobin clearance mechanisms. The haemoglobin-scavenger receptor, CD163, is exclusively expressed by macrophages and monocytes that recycle iron35, and it can also be found in solid tumours239 and inflamed tissues240, for example, as these tissues have increased iron requirements. Consequently, a liposomal targeting strategy exploiting tissue-specific expression of CD163 using an anti-CD163 antibody has demonstrated effective macrophage targeting in vivo as compared to untargeted nanocarriers241.
The value of haem as a targeting ligand, which uses differences in cellular affinity for haem to specifically target subsets of cells, has not yet been fully explored. As in vitro studies have shown that PLGA nanoparticles functionalized with haematin, the oxidized Fe(III) form of haem, localize in tumour cells242, and haem-targeting improves the uptake of oligonucleotides in hepatocytes243, further evaluation of haem-targeting is certainly warranted.
Merits and challenges of targeting iron metabolism as therapeutic strategy
The majority of therapeutics that target iron metabolism has been developed in the context of haematological diseases and iron homeostasis disorders, such as iron deficiency and overload. However, considering the rapidly growing evidence implicating iron in a variety of other (often ageing-related) disorders, such as neurodegenerative diseases and atherosclerosis, further exploration of these therapeutics agents and their targets in other pathologies is certainly warranted. Although unbalanced iron trafficking should not be considered the sole cause, the chronic and inflammatory character of the underlying pathologic processes in iron metabolism-related diseases suggest that an iron-induced change in redox state within the affected tissues may form the missing link in the pathogenesis of several multifactorial disorders86,105,244. Importantly, apart from directly modulating the function of cells responsible for the condition, such as neurons and tumour cells, iron also impacts the inflammatory activity of local immune cells, especially macrophages134,245,246 (See also Box 1). Consequently, interfering with iron metabolism may be a promising therapeutic strategy in these disorders for which there are currently few effective treatments available. A clear additional advantage of such an approach is that it typically addresses pathologic pathways that are complementary to those targeted by other therapeutic approaches, allowing for additive or possibly even synergistic effects when used in a combination strategy.
However, the broad involvement of iron metabolism in many, rather diverse physiological and pathological processes potentially makes its use as a therapeutic target a double-edged sword. Although iron-targeted therapeutic interventions may effectively change the iron status in diseased tissues to treat a disorder, they also have an inherent risk of perturbing systemic iron homeostasis that may lead to substantial side effects, such as erythropoietic disorders. Using a targeted strategy that allows the delivery of therapeutics to specific tissues could limit such off-target activity.
In addition, most iron metabolism-related disorders are associated with cellular iron accumulation, which, due to the late onset of symptoms, is most likely a chronic process that takes several years to establish. Interfering with this process once iron-induced cellular damage has already occurred may not have a direct therapeutic effect, and prevention may be more efficacious in this context. Nevertheless, limiting further deterioration and modulating the inflammatory state within the affected tissue could certainly make iron metabolism-targeted therapies an effective adjuvant treatment124,247.
Conclusion and outlook
Over the past decade, a multitude of iron metabolism-targeting agents have been developed and clinically evaluated in diseases with an obvious iron-related background, such as anaemia, iron overload and iron-loading anaemia. However, alterations in iron metabolism have demonstrated involvement in a wide range of disorders, such as cancer, neurodegeneration and atherosclerosis, but also type 2 diabetes, osteoporosis, osteoarthritis, retinal disorders and liver fibrosis.
The chronic inflammatory and ageing-related character of many of these conditions suggests that a slowly changing intracellular iron level, causing a shift in cellular redox status that eventually may result in cell damage, is an underlying mechanism that could be exploited for the development of new therapeutic approaches. Further investigation is therefore required to explore current and newly developed therapies that target iron metabolism in a much broader context than haematological pathologies alone. Modulation of macrophage activity by targeting their role in iron recycling may be a particularly interesting approach. Similar to iron metabolism, the role of an altered macrophage phenotype has been increasingly implicated in various disorders248, and both concepts seem to be interconnected245. Targeted approaches — for example those using nanosized drug delivery systems that preferentially accumulate in phagocytic cells, to specifically interfere with iron trafficking in macrophages — would thereby enable therapeutic modulation of macrophage activity in cancer and chronic inflammatory disorders75,249,250. To achieve this, the cellular mechanisms governing the relationship between macrophage phenotype and its iron load need to be further elucidated. For example, recent work has demonstrated that internalization of unbound haem induces an inflammatory phenotype in macrophages, whereas using haem in complex with hemopexin, which is be scavenged by CD91, such a phenotypic switch was not observed77. This observation indicates that the pathway of internalization, rather than the absolute load of iron, has a dominant role in the influence of iron on the phenotype of macrophages, thereby providing valuable clues for the identification of new strategies for macrophage-modulating therapies.
Overall, owing to the substantial advances in our understanding of the role of iron in a range of diseases, it has become clear that targeting iron metabolism is a compelling new therapeutic strategy for these disorders. Although further research is still required to systematically explore the value of targeting the various iron-related proteins in individual pathologies, these efforts will almost certainly prove to be valuable in the quest for novel efficacious therapies for diseases associated with disturbed iron metabolism.
Key points.
-
-
Iron metabolism is a tightly regulated physiological process that has relatively low redundancy and its deregulation often leads to iron deficiency or iron overload.
-
-
Iron deficiency and iron overload are historically associated with erythroid disorders, but a deregulated iron metabolism has also been implicated in numerous ageing-related, non-haematological disorders including neurodegenerative disorders, atherosclerosis and cancer.
-
-
Intracellular iron is directly involved in the formation of reactive oxygen species that can cause cellular oxidative damage, and which are important for ferroptosis, a form of non-apoptotic cell death.
-
-
The internalization of iron by macrophages can modulate macrophage activity towards a pro-inflammatory phenotype, which is likely also dependent on the pathway of iron intake.
-
-
Agents that interfere with key regulators of iron metabolism and cellular iron trafficking represent a promising new class of therapeutic agents for various diseases, as they exploit pathological pathways that are complementary to those targeted by existing treatments.
-
-
Targeting therapeutics to diseased tissues that express high levels of transferrin receptor is a strategy that is used by several agents under clinical development, and extension of this strategy towards other iron metabolism-associated cellular transporters may be advantageous.
Acknowledgments
The authors wish to express their gratitude to K. Römhild for her assistance in the literature review. The authors’ work is supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska−Curie grant agreement No 707229 (ADHERE) (B.J.C), the German Research Foundation (DFG: La2937/1-2) (T.L), the European Research Council (ERC: StG-309495, PoC-680882) (T.L), the NIH grants R01 DK095112 and R01DK090554 (S.R.).
Glossary terms
- Microcytic, hypochromic anaemia
Low systemic Hb levels associated with a reduced Hb concentration inside each red blood cell, making them small (microcytic) and pale (hypochromic). This is most commonly caused by iron deficiency, but is also found in patients with red blood cell disorders such as thalassemia.
- Hemochromatosis
Hemochromatosis is a family of hereditary diseases that are characterised by genetic mutations affecting an iron metabolism-regulating protein and resulting in excessive iron absorption. Since there is no physiological mechanism for iron excretion, this will eventually lead to iron overload.
- Anticalin
Technology developed by Pieris Pharmaceuticals that modifies lipocalins, which are natural extracellular proteins that bind and transport a variety of ligands, to make therapeutics that target a specific molecule.
- Spiegelmer
Technology developed by NOXXON Pharma that uses l-RNA aptamers as therapeutic agents that bind a specific molecule. l-RNA, the stereoisomeric form of physiological d-RNA, is not sensitive to nuclease activity and therefore has improved biological stability.
- Kunitz domain
A Kunitz domain, or Kunitz-type protease inhibitor domain, is the active domain of various protease inhibitors. Their relatively short peptide sequence of around 60 amino acids makes them an interesting lead for the development new biopharmaceutical protease inhibitors.
Biographies
Author biographies
Bart J. Crielaard
Bart Crielaard is an assistant professor at the Zernike Institute for Advanced Materials, University of Groningen, The Netherlands. After finishing his medical training, he further specialized in Biomedical Engineering and obtained a PhD degree in Pharmaceutics from Utrecht University, The Netherlands. During his PhD and his postdoctoral fellowship at Weill Cornell Medical College, U.S.A., he developed a particular interest in macrophage biology and iron metabolism, which could be exploited for therapeutic purposes. In his current role as a group leader at the University of Groningen, he is currently investigating this and other strategies for the development of new therapeutics.
Twan Lammers
Twan Lammers obtained a DSc degree in Radiation Oncology from Heidelberg University, Germany, in 2008 and a PhD degree in Pharmaceutics from Utrecht University, The Netherlands, in 2009. In the same year, he started the Nanomedicine and Theranostics group at the Institute for Experimental Molecular Imaging RWTH Aachen University, Germany. In 2014, he was promoted to full professor at RWTH Aachen. Since 2012, he has also worked as a part-time assistant professor at the Department of Targeted Therapeutics at the University of Twente. His primary research interests include drug targeting to tumors, image-guided drug delivery and tumor-targeted combination therapies.
Stefano Rivella
Stefano Rivella is Professor of Pediatrics with tenure at the Children’s Hospital of Philadelphia and University of Pennsylvania, U.S.A., and holds the Kwame Ohene-Frempong Chair on Sickle Cell Anemia. He characterized the role of seminal factors contributing to morbidity and mortality in various haematological disorders, including hepcidin and ferroportin, which control iron absorption and organ iron distribution; and macrophages, which play an important role in iron recycling, inflammation as well as erythroid proliferation and maturation. Based on these studies, he contributed to the development of novel therapeutics presently in clinical and preclinical trials.
Footnotes
Competing financial interests
S.R. has restricted stocks in Merganser Biotech. S.R. is a consultant for Novartis Pharmaceuticals, Bayer Healthcare, Merganser Biotech and Keryx Pharmaceuticals. S.R. is a member of scientific advisory board of Merganser Biotech and Ionis Pharmaceuticals. T.L. is a member of scientific advisory board of Cristal Therapeutics B.V‥
Further information
Protagonist’s pipeline. http://www.protagonist-inc.com/pipeline-main.php
La Jolla Pharmaceutical LJPC-401 Phase 1 Results and Development Update. http://lajollapharmaceutical.com/wp-content/uploads/2016/09/LJPC-401-Phase-1-Results-and-Development-Update-9.7.16.pdf
Eli Lilly’s clinical pipeline. https://www.lilly.com/_assets/SiteCollectionDocuments/Pipeline/Clinical-Development-Pipeline/index.html
References
- 1.Rouault TA. Iron-sulfur proteins hiding in plain sight. Nat Chem Biol. 2015;11:442–445. doi: 10.1038/nchembio.1843. [DOI] [PubMed] [Google Scholar]
- 2.Hohenberger J, Ray K, Meyer K. The biology and chemistry of high-valent iron-oxo and iron-nitrido complexes. Nat Commun. 2012;3:720. doi: 10.1038/ncomms1718. [DOI] [PubMed] [Google Scholar]
- 3.Ganz T. Systemic Iron Homeostasis. Physiol Rev. 2013;93:1721–1741. doi: 10.1152/physrev.00008.2013. [DOI] [PubMed] [Google Scholar]
- 4.Camaschella C. Iron-Deficiency Anemia. N Engl J Med. 2015;372:1832–1843. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- 5.Rivella S. β-thalassemias: paradigmatic diseases for scientific discoveries and development of innovative therapies. Haematologica. 2015;100:418–430. doi: 10.3324/haematol.2014.114827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 7.Fenton HJH. Oxidation of tartaric acid in presence of iron. J Chem Soc Trans. 1894;65:899–910. [Google Scholar]
- 8.Haber F, Weiss J. Über die Katalyse des Hydroperoxydes. Naturwissenschaften. 1932;20:948–950. [Google Scholar]
- 9.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a Urinary Antimicrobial Peptide Synthesized in the Liver. J Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 10.Krause A, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity1. FEBS Lett. 2000;480:147–150. doi: 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 11.Nicolas G, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donovan A, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 13.Nemeth E, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 14.McKie AT, et al. A Novel Duodenal Iron-Regulated Transporter, IREG1, Implicated in the Basolateral Transfer of Iron to the Circulation. Mol Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 15.Donovan A, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Abboud S, Haile DJ. A Novel Mammalian Iron-regulated Protein Involved in Intracellular Iron Metabolism. J Biol Chem. 2000;275:19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 17.Aisen P, Leibman A, Zweier J. Stoichiometric and site characteristics of the binding of iron to human transferrin. J Biol Chem. 1978;253:1930–1937. [PubMed] [Google Scholar]
- 18.Fotticchia I, et al. Energetics of ligand-receptor binding affinity on endothelial cells: An in vitro model. Colloids Surf B Biointerfaces. 2016;144:250–256. doi: 10.1016/j.colsurfb.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Dautry-Varsat A, Ciechanover A, Lodish HF. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983;80:2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trinder D, Zak O, Aisen P. Transferrin receptor-independent uptake of differic transferrin by human hepatoma cells with antisense inhibition of receptor expression. Hepatology. 1996;23:1512–1520. doi: 10.1053/jhep.1996.v23.pm0008675172. [DOI] [PubMed] [Google Scholar]
- 21.Gunshin H, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 22.Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci. 2006;103:13612–13617. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C-Y, et al. ZIP8 Is an Iron and Zinc Transporter Whose Cell-surface Expression Is Up-regulated by Cellular Iron Loading. J Biol Chem. 2012;287:34032–34043. doi: 10.1074/jbc.M112.367284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleming MD, et al. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- 25.Fleming MD, et al. Nramp2 is mutated in the anemic Belgrade (b) rat: Evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shawki A, et al. Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. Am J Physiol - Gastrointest Liver Physiol. 2015;309:G635–G647. doi: 10.1152/ajpgi.00160.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mims MP, et al. Identification of a human mutation of DMT1 in a patient with microcytic anemia and iron overload. Blood. 2005;105:1337–1342. doi: 10.1182/blood-2004-07-2966. [DOI] [PubMed] [Google Scholar]
- 28.Canonne-Hergaux F, Zhang A-S, Ponka P, Gros P. Characterization of the iron transporter DMT1 (NRAMP2/DCT1) in red blood cells of normal and anemic mk/mkmice. Blood. 2001;98:3823–3830. doi: 10.1182/blood.v98.13.3823. [DOI] [PubMed] [Google Scholar]
- 29.Jenkitkasemwong S, et al. SLC39A14 Is Required for the Development of Hepatocellular Iron Overload in Murine Models of Hereditary Hemochromatosis. Cell Metab. 2015;22:138–150. doi: 10.1016/j.cmet.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKie AT, et al. An Iron-Regulated Ferric Reductase Associated with the Absorption of Dietary Iron. Science. 2001;291:1755–1759. doi: 10.1126/science.1057206. [DOI] [PubMed] [Google Scholar]
- 31.Ohgami RS, et al. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet. 2005;37:1264–1269. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohgami RS, Campagna DR, McDonald A, Fleming MD. The Steap proteins are metalloreductases. Blood. 2006;108:1388–1394. doi: 10.1182/blood-2006-02-003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tripathi AK, et al. Prion protein functions as a ferrireductase partner for ZIP14 and DMT1. Free Radic Biol Med. 2015;84:322–330. doi: 10.1016/j.freeradbiomed.2015.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh A, et al. Prion protein regulates iron transport by functioning as a ferrireductase. J Alzheimers Dis JAD. 2013;35:541–552. doi: 10.3233/JAD-130218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kristiansen M, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 36.Andersen CBF, et al. Structure of the haptoglobin-haemoglobin complex. Nature. 2012;489:456–459. doi: 10.1038/nature11369. [DOI] [PubMed] [Google Scholar]
- 37.Hvidberg V, et al. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106:2572–2579. doi: 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- 38.Rajagopal A, et al. Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature. 2008;453:1127–1131. doi: 10.1038/nature06934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duffy SP, et al. The Fowler Syndrome-Associated Protein FLVCR2 Is an Importer of Heme. Mol Cell Biol. 2010;30:5318–5324. doi: 10.1128/MCB.00690-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White C, et al. HRG1 Is Essential for Heme Transport from the Phagolysosome of Macrophages during Erythrophagocytosis. Cell Metab. 2013;17:261–270. doi: 10.1016/j.cmet.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li JY, et al. Scara5 Is a Ferritin Receptor Mediating Non-Transferrin Iron Delivery. Dev Cell. 2009;16:35–46. doi: 10.1016/j.devcel.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L, et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc Natl Acad Sci U S A. 2010;107:3505–3510. doi: 10.1073/pnas.0913192107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moss D, Powell LW, Arosio P, Halliday JW. Characterization of the ferritin receptors of human T lymphoid (MOLT-4) cells. J Lab Clin Med. 1992;119:273–279. [PubMed] [Google Scholar]
- 44.Chen TT, et al. TIM-2 is expressed on B cells and in liver and kidney and is a receptor for H-ferritin endocytosis. J Exp Med. 2005;202:955–965. doi: 10.1084/jem.20042433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw GC, et al. Mitoferrin is essential for erythroid iron assimilation. Nature. 2006;440:96–100. doi: 10.1038/nature04512. [DOI] [PubMed] [Google Scholar]
- 46.Bou-Abdallah F. The iron redox and hydrolysis chemistry of the ferritins. Biochim Biophys Acta BBA - Gen Subj. 2010;1800:719–731. doi: 10.1016/j.bbagen.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 47.Leibold EA, Munro HN. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5’ untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci. 1988;85:2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rouault TA, Hentze MW, Caughman SW, Harford JB, Klausner RD. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. 1988;241:1207–1210. doi: 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- 49.Vashchenko G, MacGillivray RTA. Multi-Copper Oxidases and Human Iron Metabolism. Nutrients. 2013;5:2289–2313. doi: 10.3390/nu5072289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosh S, Hevi S, Chuck SL. Regulated secretion of glycosylated human ferritin from hepatocytes. Blood. 2004;103:2369–2376. doi: 10.1182/blood-2003-09-3050. [DOI] [PubMed] [Google Scholar]
- 51.Cohen LA, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574–1584. doi: 10.1182/blood-2009-11-253815. [DOI] [PubMed] [Google Scholar]
- 52.Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509:105–109. doi: 10.1038/nature13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keel SB, et al. A Heme Export Protein Is Required for Red Blood Cell Differentiation and Iron Homeostasis. Science. 2008;319:825–828. doi: 10.1126/science.1151133. [DOI] [PubMed] [Google Scholar]
- 54.Taher AT, et al. Overview on practices in thalassemia intermedia management aiming for lowering complication rates across a region of endemicity: the OPTIMAL CARE study. Blood. 2010;115:1886–1892. doi: 10.1182/blood-2009-09-243154. [DOI] [PubMed] [Google Scholar]
- 55.Finberg KE, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA) Nat Genet. 2008;40:569–571. doi: 10.1038/ng.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gardenghi S, et al. Distinct roles for hepcidin and interleukin-6 in the recovery from anemia in mice injected with heat-killed Brucella abortus. Blood. 2014;123:1137–1145. doi: 10.1182/blood-2013-08-521625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Canali S, et al. Activin B Induces Noncanonical SMAD1/5/8 Signaling via BMP Type I Receptors in Hepatocytes: Evidence for a Role in Hepcidin Induction by Inflammation in Male Mice. Endocrinology. 2016;157:1146–1162. doi: 10.1210/en.2015-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiss G, Schett G. Anaemia in inflammatory rheumatic diseases. Nat Rev Rheumatol. 2013;9:205–215. doi: 10.1038/nrrheum.2012.183. [DOI] [PubMed] [Google Scholar]
- 59.Eaton JW, Qian M. Molecular bases of cellular iron toxicity. Free Radic Biol Med. 2002;32:833–840. doi: 10.1016/s0891-5849(02)00772-4. [DOI] [PubMed] [Google Scholar]
- 60.Dixon SJ, et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skouta R, et al. Ferrostatins Inhibit Oxidative Lipid Damage and Cell Death in Diverse Disease Models. J Am Chem Soc. 2014;136:4551–4556. doi: 10.1021/ja411006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kang Y, Tiziani S, Park G, Kaul M, Paternostro G. Cellular protection using Flt3 and PI3Kα inhibitors demonstrates multiple mechanisms of oxidative glutamate toxicity. Nat Commun. 2014;5:3672. doi: 10.1038/ncomms4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Friedmann Angeli JP, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Linkermann A, et al. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci. 2014;111:16836–16841. doi: 10.1073/pnas.1415518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang L, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun X, et al. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene. 2015;34:5617–5625. doi: 10.1038/onc.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang WS, et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 69.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 2014;14:709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Conrad M, Angeli JPF, Vandenabeele P, Stockwell BR. Regulated necrosis: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2016;15:348–366. doi: 10.1038/nrd.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chow A, et al. CD169+ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med. 2013;19:429–436. doi: 10.1038/nm.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramos P, et al. Macrophages support pathological erythropoiesis in polycythemia vera and β-thalassemia. Nat Med. 2013;19:437–445. doi: 10.1038/nm.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.She H, et al. Iron activates NF-κB in Kupffer cells. Am J Physiol - Gastrointest Liver Physiol. 2002;283:G719–G726. doi: 10.1152/ajpgi.00108.2002. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Z, et al. Ferroportin1 deficiency in mouse macrophages impairs iron homeostasis and inflammatory responses. Blood. 2011;118:1912–1922. doi: 10.1182/blood-2011-01-330324. [DOI] [PubMed] [Google Scholar]
- 75.Zanganeh S, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016 doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sindrilaru A, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vinchi F, et al. Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease. Blood. 2016;127:473–486. doi: 10.1182/blood-2015-08-663245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jabara HH, et al. A missense mutation in TFRC, encoding transferrin receptor 1, causes combined immunodeficiency. Nat Genet. 2016;48:74–78. doi: 10.1038/ng.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wandersman C, Delepelaire P. Bacterial Iron Sources: From Siderophores to Hemophores. Annu Rev Microbiol. 2004;58:611–647. doi: 10.1146/annurev.micro.58.030603.123811. [DOI] [PubMed] [Google Scholar]
- 80.Bao G, et al. Iron traffics in circulation bound to a siderocalin (Ngal)–catechol complex. Nat Chem Biol. 2010;6:602–609. doi: 10.1038/nchembio.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Flo TH, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 82.Arezes J, et al. Hepcidin-Induced Hypoferremia Is a Critical Host Defense Mechanism against the Siderophilic Bacterium Vibrio vulnificus. Cell Host Microbe. 2015;17:47–57. doi: 10.1016/j.chom.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen S, et al. Transforming Growth Factor β1 (TGF-β1) Activates Hepcidin mRNA Expression in Hepatocytes. J Biol Chem. 2016;291:13160–13174. doi: 10.1074/jbc.M115.691543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guida C, et al. A novel inflammatory pathway mediating rapid hepcidin-independent hypoferremia. Blood. 2015;125:2265–2275. doi: 10.1182/blood-2014-08-595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Piskounova E, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–191. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013;13:342–355. doi: 10.1038/nrc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bystrom LM, Rivella S. Cancer cells with irons in the fire. Free Radic Biol Med. 2015;79:337–342. doi: 10.1016/j.freeradbiomed.2014.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim K-S, Son H-G, Hong N-S, Lee D-H. Associations of Serum Ferritin and Transferrin Saturation With All-cause, Cancer, and Cardiovascular Disease Mortality: Third National Health and Nutrition Examination Survey Follow-up Study. J Prev Med Pub Health. 2012;45:196–203. doi: 10.3961/jpmph.2012.45.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ashmore JH, Rogers CJ, Kelleher SL, Lesko SM, Hartman TJ. Dietary Iron and Colorectal Cancer Risk: A Review of Human Population Studies. Crit Rev Food Sci Nutr. 2016;56:1012–1020. doi: 10.1080/10408398.2012.749208. [DOI] [PubMed] [Google Scholar]
- 90.Miller LD, et al. An Iron Regulatory Gene Signature Predicts Outcome in Breast Cancer. Cancer Res. 2011;71:6728–6737. doi: 10.1158/0008-5472.CAN-11-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pinnix ZK, et al. Ferroportin and Iron Regulation in Breast Cancer Progression and Prognosis. Sci Transl Med. 2010;2:43ra56. doi: 10.1126/scisignal.3001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Habashy HO, et al. Transferrin receptor (CD71) is a marker of poor prognosis in breast cancer and can predict response to tamoxifen. Breast Cancer Res Treat. 2009;119:283–293. doi: 10.1007/s10549-009-0345-x. [DOI] [PubMed] [Google Scholar]
- 93.Jeong SM, Hwang S, Seong RH. Transferrin receptor regulates pancreatic cancer growth by modulating mitochondrial respiration and ROS generation. Biochem Biophys Res Commun. 2016;471:373–379. doi: 10.1016/j.bbrc.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 94.Schonberg DL, et al. Preferential Iron Trafficking Characterizes Glioblastoma Stem-like Cells. Cancer Cell. 2015;28:441–455. doi: 10.1016/j.ccell.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Högemann-Savellano D, et al. The Transferrin Receptor: A Potential Molecular Imaging Marker for Human Cancer. Neoplasia. 2003;5:495–506. doi: 10.1016/s1476-5586(03)80034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Isobe T, et al. Human STEAP3 maintains tumor growth under hypoferric condition. Exp Cell Res. 2011;317:2582–2591. doi: 10.1016/j.yexcr.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 97.Savci-Heijink CD, Halfwerk H, Koster J, Vijver MJ. A novel gene expression signature for bone metastasis in breast carcinomas. Breast Cancer Res Treat. 2016;156:249–259. doi: 10.1007/s10549-016-3741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tesfay L, et al. Hepcidin regulation in prostate and its disruption in prostate cancer. Cancer Res. 2015;75:2254–2263. doi: 10.1158/0008-5472.CAN-14-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen Y, et al. Disordered signaling governing ferroportin transcription favors breast cancer growth. Cell Signal. 2015;27:168–176. doi: 10.1016/j.cellsig.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 100.Zhang S, et al. Disordered hepcidin−ferroportin signaling promotes breast cancer growth. Cell Signal. 2014;26:2539–2550. doi: 10.1016/j.cellsig.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 101.Marques O, et al. Local iron homeostasis in the breast ductal carcinoma microenvironment. BMC Cancer. 2016;16:187. doi: 10.1186/s12885-016-2228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sanders AJ, et al. Genetic upregulation of matriptase-2 reduces the aggressiveness of prostate cancer cells in vitro and in vivo and affects FAK and paxillin localisation. J Cell Physiol. 2008;216:780–789. doi: 10.1002/jcp.21460. [DOI] [PubMed] [Google Scholar]
- 103.Chen Y, et al. Myeloid zinc-finger 1 (MZF-1) suppresses prostate tumor growth through enforcing ferroportin-conducted iron egress. Oncogene. 2015;34:3839–3847. doi: 10.1038/onc.2014.310. [DOI] [PubMed] [Google Scholar]
- 104.Calzolari A, et al. Transferrin Receptor 2 Is Frequently and Highly Expressed in Glioblastomas. Transl Oncol. 2010;3:123–134. doi: 10.1593/tlo.09274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014;13:1045–1060. doi: 10.1016/S1474-4422(14)70117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Simpson IA, et al. A Novel Model for Brain Iron Uptake: Introducing the Concept of Regulation. J Cereb Blood Flow Metab. 2015;35:48–57. doi: 10.1038/jcbfm.2014.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McCarthy RC, Kosman DJ. Mechanisms and regulation of iron trafficking across the capillary endothelial cells of the blood-brain barrier. Front Mol Neurosci. 2015;8:31. doi: 10.3389/fnmol.2015.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nielsen JE, Jensen LN, Krabbe K. Hereditary haemochromatosis: a case of iron accumulation in the basal ganglia associated with a parkinsonian syndrome. J Neurol Neurosurg Psychiatry. 1995;59:318–321. doi: 10.1136/jnnp.59.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dekker MCJ, et al. Mutations in the hemochromatosis gene (HFE), Parkinson’s disease and parkinsonism. Neurosci Lett. 2003;348:117–119. doi: 10.1016/s0304-3940(03)00713-4. [DOI] [PubMed] [Google Scholar]
- 110.Wang X-S, et al. Increased incidence of the Hfe mutation in amyotrophic lateral sclerosis and related cellular consequences. J Neurol Sci. 2004;227:27–33. doi: 10.1016/j.jns.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 111.Bucossi S, et al. Association of K832R and R952K SNPs of Wilson’s disease gene with Alzheimer’s disease. J Alzheimers Dis JAD. 2012;29:913–919. doi: 10.3233/JAD-2012-111997. [DOI] [PubMed] [Google Scholar]
- 112.Kong SMY, et al. Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes α-Synuclein externalization via exosomes. Hum Mol Genet. 2014;23:2816–2833. doi: 10.1093/hmg/ddu099. [DOI] [PubMed] [Google Scholar]
- 113.Kell DB. Towards a unifying, systems biology understanding of large-scale cellular death and destruction caused by poorly liganded iron: Parkinson’s, Huntington’s, Alzheimer’s, prions, bactericides, chemical toxicology and others as examples. Arch Toxicol. 2010;84:825–889. doi: 10.1007/s00204-010-0577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rouault TA. Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:551–564. doi: 10.1038/nrn3453. [DOI] [PubMed] [Google Scholar]
- 115.Singh N, Das D, Singh A, Mohan ML. Prion protein and metal interaction: physiological and pathological implications. Curr Issues Mol Biol. 2010;12:99–107. [PMC free article] [PubMed] [Google Scholar]
- 116.Singh A, et al. Abnormal Brain Iron Homeostasis in Human and Animal Prion Disorders. PLOS Pathog. 2009;5:e1000336. doi: 10.1371/journal.ppat.1000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dringen R, Bishop GM, Koeppe M, Dang TN, Robinson SR. The Pivotal Role of Astrocytes in the Metabolism of Iron in the Brain. Neurochem Res. 2007;32:1884–1890. doi: 10.1007/s11064-007-9375-0. [DOI] [PubMed] [Google Scholar]
- 118.Urrutia P, et al. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J Neurochem. 2013;126:541–549. doi: 10.1111/jnc.12244. [DOI] [PubMed] [Google Scholar]
- 119.Raha-Chowdhury R, et al. Expression and cellular localization of hepcidin mRNA and protein in normal rat brain. BMC Neurosci. 2015;16:24. doi: 10.1186/s12868-015-0161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Raha AA, Vaishnav RA, Friedland RP, Bomford A, Raha-Chowdhury R. The systemic iron-regulatory proteins hepcidin and ferroportin are reduced in the brain in Alzheimer’s disease. Acta Neuropathol Commun. 2013;1:55. doi: 10.1186/2051-5960-1-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang J, Jiang H, Xie J-X. Ferroportin1 and hephaestin are involved in the nigral iron accumulation of 6-OHDA-lesioned rats. Eur J Neurosci. 2007;25:2766–2772. doi: 10.1111/j.1460-9568.2007.05515.x. [DOI] [PubMed] [Google Scholar]
- 122.Halon M, et al. Changes in skeletal muscle iron metabolism outpace amyotrophic lateral sclerosis onset in transgenic rats bearing the G93A hmSOD1 gene mutation. Free Radic Res. 2014;48:1363–1370. doi: 10.3109/10715762.2014.955484. [DOI] [PubMed] [Google Scholar]
- 123.Zarruk JG, et al. Expression of iron homeostasis proteins in the spinal cord in experimental autoimmune encephalomyelitis and their implications for iron accumulation. Neurobiol Dis. 2015;81:93–107. doi: 10.1016/j.nbd.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 124.Kroner A, et al. TNF and Increased Intracellular Iron Alter Macrophage Polarization to a Detrimental M1 Phenotype in the Injured Spinal Cord. Neuron. 2014;83:1098–1116. doi: 10.1016/j.neuron.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 125.Mehta V, et al. Iron Is a Sensitive Biomarker for Inflammation in Multiple Sclerosis Lesions. PLOS ONE. 2013;8:e57573. doi: 10.1371/journal.pone.0057573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jeong SY, David S. Glycosylphosphatidylinositol-anchored Ceruloplasmin Is Required for Iron Efflux from Cells in the Central Nervous System. J Biol Chem. 2003;278:27144–27148. doi: 10.1074/jbc.M301988200. [DOI] [PubMed] [Google Scholar]
- 127.Ayton S, Faux NG, Bush AI, Alzheimer’s Disease Neuroimaging Initiative Ferritin levels in the cerebrospinal fluid predict Alzheimer/’s disease outcomes and are regulated by APOE. Nat Commun. 2015;6:6760. doi: 10.1038/ncomms7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baraibar MA, Barbeito AG, Muhoberac BB, Vidal R. A mutant light-chain ferritin that causes neurodegeneration has enhanced propensity toward oxidative damage. Free Radic Biol Med. 2012;52:1692–1697. doi: 10.1016/j.freeradbiomed.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Biasiotto G, Lorenzo DD, Archetti S, Zanella I. Iron and Neurodegeneration: Is Ferritinophagy the Link? Mol Neurobiol. 2016;53:5542–5574. doi: 10.1007/s12035-015-9473-y. [DOI] [PubMed] [Google Scholar]
- 130.Stadler N, Lindner RA, Davies MJ. Direct Detection and Quantification of Transition Metal Ions in Human Atherosclerotic Plaques: Evidence for the Presence of Elevated Levels of Iron and Copper. Arterioscler Thromb Vasc Biol. 2004;24:949–954. doi: 10.1161/01.ATV.0000124892.90999.cb. [DOI] [PubMed] [Google Scholar]
- 131.Satchell L, Leake DS. Oxidation of Low-Density Lipoprotein by Iron at Lysosomal pH: Implications for Atherosclerosis. Biochemistry (Mosc.) 2012;51:3767–3775. doi: 10.1021/bi2017975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sullivan JL. Do Hemochromatosis Mutations Protect Against Iron-Mediated Atherogenesis? Circ Cardiovasc Genet. 2009;2:652–657. doi: 10.1161/CIRCGENETICS.109.906230. [DOI] [PubMed] [Google Scholar]
- 133.Robbins CS, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol. 2015;12:10–17. doi: 10.1038/nrcardio.2014.173. [DOI] [PubMed] [Google Scholar]
- 135.Gursel O, et al. Premature Atherosclerosis in Children With β-Thalassemia Major. J Pediatr Hematol Oncol. 2012;34:630–634. doi: 10.1097/MPH.0b013e3182707f4d. [DOI] [PubMed] [Google Scholar]
- 136.Cheung YF, Chow PC, Chan GC, Ha SY. Carotid intima-media thickness is increased and related to arterial stiffening in patients with beta-thalassaemia major. Br J Haematol. 2006;135:732–734. doi: 10.1111/j.1365-2141.2006.06349.x. [DOI] [PubMed] [Google Scholar]
- 137.Li JJ, et al. Hepcidin Destabilizes Atherosclerotic Plaque Via Overactivating Macrophages After Erythrophagocytosis. Arterioscler Thromb Vasc Biol. 2012;32:1158–1166. doi: 10.1161/ATVBAHA.112.246108. [DOI] [PubMed] [Google Scholar]
- 138.Kautz L, et al. Testing the Iron Hypothesis in a Mouse Model of Atherosclerosis. Cell Rep. 2013;5:1436–1442. doi: 10.1016/j.celrep.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mehta NU, et al. Apolipoprotein E−/− Mice Lacking Hemopexin Develop Increased Atherosclerosis via Mechanisms That Include Oxidative Stress and Altered Macrophage Function. Arterioscler Thromb Vasc Biol. 2016;36:1152–1163. doi: 10.1161/ATVBAHA.115.306991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Boyle JJ, et al. Activating Transcription Factor 1 Directs Mhem Atheroprotective Macrophages Through Coordinated Iron Handling and Foam Cell Protection. Circ Res. 2012;110:20–33. doi: 10.1161/CIRCRESAHA.111.247577. [DOI] [PubMed] [Google Scholar]
- 141.Bories G, et al. Liver X Receptor Activation Stimulates Iron Export in Human Alternative Macrophages. Circ Res. 2013;113:1196–1205. doi: 10.1161/CIRCRESAHA.113.301656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fernández-Real JM, McClain D, Manco M. Mechanisms Linking Glucose Homeostasis and Iron Metabolism Toward the Onset and Progression of Type 2 Diabetes. Diabetes Care. 2015;38:2169–2176. doi: 10.2337/dc14-3082. [DOI] [PubMed] [Google Scholar]
- 143.Kim B-J, et al. Iron overload accelerates bone loss in healthy postmenopausal women and middle-aged men: A 3-year retrospective longitudinal study. J Bone Miner Res. 2012;27:2279–2290. doi: 10.1002/jbmr.1692. [DOI] [PubMed] [Google Scholar]
- 144.Camacho A, et al. Iron overload in a murine model of hereditary hemochromatosis is associated with accelerated progression of osteoarthritis under mechanical stress. Osteoarthritis Cartilage. 2016;24:494–502. doi: 10.1016/j.joca.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 145.Dunaief JL, et al. Macular Degeneration in a Patient with Aceruloplasminemia, a Disease Associated with Retinal Iron Overload. Ophthalmology. 2005;112:1062–1065. doi: 10.1016/j.ophtha.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 146.Fargion S, Valenti L, Fracanzani AL. Beyond hereditary hemochromatosis: New insights into the relationship between iron overload and chronic liver diseases. Dig Liver Dis. 2011;43:89–95. doi: 10.1016/j.dld.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 147.Keberle H. The Biochemistry of Desferrioxamine and its Relation to Iron Metabolism. Ann N Y Acad Sci. 1964;119:758–768. doi: 10.1111/j.1749-6632.1965.tb54077.x. [DOI] [PubMed] [Google Scholar]
- 148.Borgna-Pignatti C, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–1193. [PubMed] [Google Scholar]
- 149.Olivieri NF, et al. Iron-Chelation Therapy with Oral Deferiprone in Patients with Thalassemia Major. N Engl J Med. 1995;332:918–922. doi: 10.1056/NEJM199504063321404. [DOI] [PubMed] [Google Scholar]
- 150.Nick H, et al. Development of Tridentate Iron Chelators: From Desferrithiocin to ICL670. Curr Med Chem. 2003;10:1065–1076. doi: 10.2174/0929867033457610. [DOI] [PubMed] [Google Scholar]
- 151.Porter J, et al. Health-Related Quality of Life, Treatment Satisfaction, Adherence and Persistence in β-Thalassemia and Myelodysplastic Syndrome Patients with Iron Overload Receiving Deferasirox: Results from the EPIC Clinical Trial. Anemia. 2012;2012:e297641. doi: 10.1155/2012/297641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Borgna-Pignatti C, Marsella M. Iron Chelation in Thalassemia Major. Clin Ther. 2015;37:2866–2877. doi: 10.1016/j.clinthera.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 153.Li H, et al. Transferrin therapy ameliorates disease in β-thalassemic mice. Nat Med. 2010;16:177–182. doi: 10.1038/nm.2073. [DOI] [PubMed] [Google Scholar]
- 154.Donfrancesco A, et al. Effects of a Single Course of Deferoxamine in Neuroblastoma Patients. Cancer Res. 1990;50:4929–4930. [PubMed] [Google Scholar]
- 155.Donfrancesco A, et al. Deferoxamine followed by cyclophosphamide, etoposide, carboplatin, thiotepa, induction regimen in advanced neuroblastoma: Preliminary results. Eur J Cancer. 1995;31:612–615. doi: 10.1016/0959-8049(95)00068-t. [DOI] [PubMed] [Google Scholar]
- 156.Kovacevic Z, Chikhani S, Lovejoy DB, Richardson DR. Novel Thiosemicarbazone Iron Chelators Induce Up-Regulation and Phosphorylation of the Metastasis Suppressor N-myc Down-Stream Regulated Gene 1: A New Strategy for the Treatment of Pancreatic Cancer. Mol Pharmacol. 2011;80:598–609. doi: 10.1124/mol.111.073627. [DOI] [PubMed] [Google Scholar]
- 157.Lynch SG, Peters K, LeVine SM. Desferrioxamine in chronic progressive Multiple Sclerosis: a pilot study. Mult Scler. 1996;2:157–160. doi: 10.1177/135245859600200306. [DOI] [PubMed] [Google Scholar]
- 158.McLachlan DRC, et al. Intramuscular desferrioxamine in patients with Alzheimer’s disease. The Lancet. 1991;337:1304–1308. doi: 10.1016/0140-6736(91)92978-b. [DOI] [PubMed] [Google Scholar]
- 159.Devos D, et al. Targeting Chelatable Iron as a Therapeutic Modality in Parkinson’s Disease. Antioxid Redox Signal. 2013;21:195–210. doi: 10.1089/ars.2013.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Preza GC, et al. Minihepcidins are rationally designed small peptides that mimic hepcidin activity in mice and may be useful for the treatment of iron overload. J Clin Invest. 2011;121:4880–4888. doi: 10.1172/JCI57693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Casu C, et al. Minihepcidin peptides as disease modifiers in mice affected by β-thalassemia and polycythemia vera. Blood. 2016;128:265–276. doi: 10.1182/blood-2015-10-676742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ramos E, et al. Minihepcidins prevent iron overload in a hepcidin-deficient mouse model of severe hemochromatosis. Blood. 2012;120:3829–3836. doi: 10.1182/blood-2012-07-440743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vadhan-Raj S, et al. Phase 1 Study of a Hepcidin Antagonist, LY2787106, in Cancer-Associated Anemia. Blood. 2015;126:537–537. [Google Scholar]
- 164.Cooke KS, et al. A fully human anti-hepcidin antibody modulates iron metabolism in both mice and nonhuman primates. Blood. 2013;122:3054–3061. doi: 10.1182/blood-2013-06-505792. [DOI] [PubMed] [Google Scholar]
- 165.Hohlbaum A, et al. Iron mobilization and pharmacodynic marker measurements in non-human primates following administration of PRS-080, a novel and highly specific anti-hepcidin therapeutic. Am J Hematol. 2013;88:E41. [Google Scholar]
- 166.van Eijk LT, et al. Effect of the antihepcidin Spiegelmer lexaptepid on inflammation-induced decrease in serum iron in humans. Blood. 2014;124:2643–2646. doi: 10.1182/blood-2014-03-559484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Boyce M, et al. Safety, pharmacokinetics and pharmacodynamics of the anti-hepcidin Spiegelmer lexaptepid pegol in healthy subjects. Br J Pharmacol. 2016;173:1580–1588. doi: 10.1111/bph.13433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Le Gac G, et al. Structure-Function Analysis of the Human Ferroportin Iron Exporter (SLC40A1): Effect of Hemochromatosis Type 4 Disease Mutations and Identification of Critical Residues. Hum Mutat. 2013;34:1371–1380. doi: 10.1002/humu.22369. [DOI] [PubMed] [Google Scholar]
- 169.Taniguchi R, et al. Outward- and inward-facing structures of a putative bacterial transition-metal transporter with homology to ferroportin. Nat Commun. 2015;6:8545. doi: 10.1038/ncomms9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fernandes A, et al. The molecular basis of hepcidin-resistant hereditary hemochromatosis. Blood. 2009;114:437–443. doi: 10.1182/blood-2008-03-146134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fung E, et al. High-Throughput Screening of Small Molecules Identifies Hepcidin Antagonists. Mol Pharmacol. 2013;83:681–690. doi: 10.1124/mol.112.083428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Leung D, et al. LY2928057, An Antibody Targeting Ferroportin, Is a Potent Inhibitor Of Hepcidin Activity and Increases Iron Mobilization In Normal Cynomolgus Monkeys. Blood. 2013;122:3433–3433. [Google Scholar]
- 173.Andriopoulos B, Jr, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Poli M, et al. Glycol-split nonanticoagulant heparins are inhibitors of hepcidin expression in vitro and in vivo. Blood. 2014;123:1564–1573. doi: 10.1182/blood-2013-07-515221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yu PB, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cuny GD, et al. Structure–activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett. 2008;18:4388–4392. doi: 10.1016/j.bmcl.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Akinc A, et al. Targeting the Hepcidin Pathway with RNAi Therapeutics for the Treatment of Anemia. Blood. 2011;118:688–688. [Google Scholar]
- 178.Babitt JL, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 179.Silvestri L, et al. The Serine Protease Matriptase-2 (TMPRSS6) Inhibits Hepcidin Activation by Cleaving Membrane Hemojuvelin. Cell Metab. 2008;8:502–511. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Du X, et al. The Serine Protease TMPRSS6 Is Required to Sense Iron Deficiency. Science. 2008;320:1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Maurer E, et al. Insights into Matriptase-2 Substrate Binding and Inhibition Mechanisms by Analyzing Active-Site-Mutated Variants. ChemMedChem. 2012;7:68–72. doi: 10.1002/cmdc.201100350. [DOI] [PubMed] [Google Scholar]
- 182.Sisay MT, et al. Identification of the First Low-Molecular-Weight Inhibitors of Matriptase-2. J Med Chem. 2010;53:5523–5535. doi: 10.1021/jm100183e. [DOI] [PubMed] [Google Scholar]
- 183.Guo S, et al. Reducing TMPRSS6 ameliorates hemochromatosis and β-thalassemia in mice. J Clin Invest. 2013;123:1531–1541. doi: 10.1172/JCI66969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Casu C, et al. Combination of Tmprss6- ASO and the iron chelator deferiprone improves erythropoiesis and reduces iron overload in a mouse model of beta-thalassemia intermedia. Haematologica. 2016;101:e8–e11. doi: 10.3324/haematol.2015.133348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schmidt PJ, et al. An RNAi therapeutic targeting Tmprss6 decreases iron overload in Hfe−/− mice and ameliorates anemia and iron overload in murine β-thalassemia intermedia. Blood. 2013;121:1200–1208. doi: 10.1182/blood-2012-09-453977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Béliveau F, Désilets A, Leduc R. Probing the substrate specificities of matriptase, matriptase-2, hepsin and DESC1 with internally quenched fluorescent peptides. FEBS J. 2009;276:2213–2226. doi: 10.1111/j.1742-4658.2009.06950.x. [DOI] [PubMed] [Google Scholar]
- 187.Beckmann A-M, et al. En Route to New Therapeutic Options for Iron Overload Diseases: Matriptase-2 as a Target for Kunitz-Type Inhibitors. ChemBioChem. 2016;17:595–604. doi: 10.1002/cbic.201500651. [DOI] [PubMed] [Google Scholar]
- 188.Zhen AW, et al. The small molecule, genistein, increases hepcidin expression in human hepatocytes. Hepatology. 2013;58:1315–1325. doi: 10.1002/hep.26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chung B, Matak P, McKie AT, Sharp P. Leptin Increases the Expression of the Iron Regulatory Hormone Hepcidin in HuH7 Human Hepatoma Cells. J Nutr. 2007;137:2366–2370. doi: 10.1093/jn/137.11.2366. [DOI] [PubMed] [Google Scholar]
- 190.Zhang S-P. AG490: An inhibitor of hepcidin expression in vivo. World J Gastroenterol. 2011;17:5032. doi: 10.3748/wjg.v17.i45.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fatih N, et al. Natural and synthetic STAT3 inhibitors reduce hepcidin expression in differentiated mouse hepatocytes expressing the active phosphorylated STAT3 form. J Mol Med. 2010;88:477–486. doi: 10.1007/s00109-009-0588-3. [DOI] [PubMed] [Google Scholar]
- 192.Czachorowski M, Lam-Yuk-Tseung S, Cellier M, Gros P. Transmembrane Topology of the Mammalian Slc11a2 Iron Transporter. Biochemistry (Mosc) 2009;48:8422–8434. doi: 10.1021/bi900606y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang D, Song Y, Li J, Wang C, Li F. Structure and metal ion binding of the first transmembrane domain of DMT1. Biochim Biophys Acta BBA - Biomembr. 2011;1808:1639–1644. doi: 10.1016/j.bbamem.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 194.Ehrnstorfer IA, Geertsma ER, Pardon E, Steyaert J, Dutzler R. Crystal structure of a SLC11 (NRAMP) transporter reveals the basis for transition-metal ion transport. Nat Struct Mol Biol. 2014;21:990–996. doi: 10.1038/nsmb.2904. [DOI] [PubMed] [Google Scholar]
- 195.Buckett PD, Wessling-Resnick M. Small molecule inhibitors of divalent metal transporter-1. Am J Physiol - Gastrointest Liver Physiol. 2009;296:G798–G804. doi: 10.1152/ajpgi.90342.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Horonchik L, Wessling-Resnick M. The Small-Molecule Iron Transport Inhibitor Ferristatin/NSC306711 Promotes Degradation of the Transferrin Receptor. Chem Biol. 2008;15:647–653. doi: 10.1016/j.chembiol.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Byrne SL, et al. Ferristatin II Promotes Degradation of Transferrin Receptor-1 In Vitro and In Vivo. PLOS ONE. 2013;8:e70199. doi: 10.1371/journal.pone.0070199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yanatori I, Yasui Y, Noguchi Y, Kishi F. Inhibition of iron uptake by ferristatin II is exerted through internalization of DMT1 at the plasma membrane. Cell Biol Int. 2015;39:427–434. doi: 10.1002/cbin.10403. [DOI] [PubMed] [Google Scholar]
- 199.Alkhateeb AA, et al. The small molecule ferristatin II induces hepatic hepcidin expression in vivo and in vitro. Am J Physiol - Gastrointest Liver Physiol. 2015;308:G1019–G1026. doi: 10.1152/ajpgi.00324.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. A review of human carcinogens Part F: Chemical agents and related occupations. 100F. World Health Organization; 2012. pp. 53–64. [PMC free article] [PubMed] [Google Scholar]
- 201.Wetli HA, Buckett PD, Wessling-Resnick M. Small-Molecule Screening Identifies the Selanazal Drug Ebselen as a Potent Inhibitor of DMT1-Mediated Iron Uptake. Chem Biol. 2006;13:965–972. doi: 10.1016/j.chembiol.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Davis MT, Bartfay WJ. Ebselen Decreases Oxygen Free Radical Production and Iron Concentrations in the Hearts of Chronically Iron-Overloaded Mice. Biol Res Nurs. 2004;6:37–45. doi: 10.1177/1099800403261350. [DOI] [PubMed] [Google Scholar]
- 203.Cadieux JA, et al. Synthesis and biological evaluation of substituted pyrazoles as blockers of divalent metal transporter 1 (DMT1) Bioorg Med Chem Lett. 2012;22:90–95. doi: 10.1016/j.bmcl.2011.11.069. [DOI] [PubMed] [Google Scholar]
- 204.Zhang Z, et al. Discovery of benzylisothioureas as potent divalent metal transporter 1 (DMT1) inhibitors. Bioorg Med Chem Lett. 2012;22:5108–5113. doi: 10.1016/j.bmcl.2012.05.129. [DOI] [PubMed] [Google Scholar]
- 205.Montalbetti N, et al. Discovery and characterization of a novel non-competitive inhibitor of the divalent metal transporter DMT1/SLC11A2. BioChem Pharmacol. 2015;96:216–224. doi: 10.1016/j.bcp.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 206.Span K, et al. A novel oral iron-complex formulation: Encapsulation of hemin in polymeric micelles and its in vitro absorption. Eur J Pharm Biopharm. 2016;108:226–234. doi: 10.1016/j.ejpb.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 207.Latunde-Dada GO, et al. A Nanoparticulate Ferritin-Core Mimetic Is Well Taken Up by HuTu 80 Duodenal Cells and Its Absorption in Mice Is Regulated by Body Iron. J Nutr. 2014;144:1896–1902. doi: 10.3945/jn.114.201715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Taylor KM, Morgan HE, Johnson A, Nicholson RI. Structure–function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Lett. 2005;579:427–432. doi: 10.1016/j.febslet.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 209.Peyssonnaux C, et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117:1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Qian Z-M, et al. Divalent metal transporter 1 is a hypoxia-inducible gene. J Cell Physiol. 2011;226:1596–1603. doi: 10.1002/jcp.22485. [DOI] [PubMed] [Google Scholar]
- 211.Shah YM, Matsubara T, Ito S, Yim S-H, Gonzalez FJ. Intestinal Hypoxia-Inducible Transcription Factors Are Essential for Iron Absorption following Iron Deficiency. Cell Metab. 2009;9:152–164. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tacchini L, Bianchi L, Bernelli-Zazzera A, Cairo G. Transferrin receptor induction by hypoxia. HIF-1-mediated transcriptional activation and cell-specific post-transcriptional regulation. J Biol Chem. 1999;274:24142–24146. doi: 10.1074/jbc.274.34.24142. [DOI] [PubMed] [Google Scholar]
- 213.Taylor M, et al. Hypoxia-Inducible Factor-2α Mediates the Adaptive Increase of Intestinal Ferroportin During Iron Deficiency in Mice. Gastroenterology. 2011;140:2044–2055. doi: 10.1053/j.gastro.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Scheuermann TH, et al. Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat Chem Biol. 2013;9:271–276. doi: 10.1038/nchembio.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Scheuermann TH, et al. Isoform-Selective and Stereoselective Inhibition of Hypoxia Inducible Factor-2. J Med Chem. 2015;58:5930–5941. doi: 10.1021/acs.jmedchem.5b00529. [DOI] [PubMed] [Google Scholar]
- 216.Bruick RK, McKnight SL. A Conserved Family of Prolyl-4-Hydroxylases That Modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 217.Epstein ACR, et al. C. elegans EGL-9 and Mammalian Homologs Define a Family of Dioxygenases that Regulate HIF by Prolyl Hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 218.McDonough MA, et al. Cellular oxygen sensing: Crystal structure of hypoxia-inducible factor prolyl hydroxylase (PHD2) Proc Natl Acad Sci. 2006;103:9814–9819. doi: 10.1073/pnas.0601283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Maxwell PH, Eckardt K-U. HIF prolyl hydroxylase inhibitors for the treatment of renal anaemia and beyond. Nat Rev Nephrol. 2016;12:157–168. doi: 10.1038/nrneph.2015.193. [DOI] [PubMed] [Google Scholar]
- 220.Barrett TD, et al. Prolyl hydroxylase inhibition corrects functional iron deficiency and inflammation-induced anaemia in rats. Br J Pharmacol. 2015;172:4078–4088. doi: 10.1111/bph.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Rajagopalan S, Rane A, Chinta SJ, Andersen JK. Regulation of ATP13A2 via PHD2-HIF1α Signaling Is Critical for Cellular Iron Homeostasis: Implications for Parkinson’s Disease. J Neurosci. 2016;36:1086–1095. doi: 10.1523/JNEUROSCI.3117-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Provenzano R, et al. Roxadustat (FG-4592) Versus Epoetin Alfa for Anemia in Patients Receiving Maintenance Hemodialysis: A Phase 2, Randomized, 6- to 19-Week, Open-Label, Active-Comparator, Dose-Ranging, Safety and Exploratory Efficacy Study. Am J Kidney Dis. 2016;67:912–924. doi: 10.1053/j.ajkd.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 223.Brigandi RA, et al. A Novel Hypoxia-Inducible Factor–Prolyl Hydroxylase Inhibitor (GSK1278863) for Anemia in CKD: A 28-Day, Phase 2A Randomized Trial. Am J Kidney Dis. 2016;67:861–871. doi: 10.1053/j.ajkd.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 224.Hartman CS, et al. AKB-6548, a new hypoxia-inducible factor prolyl hydroxylase inhibitor increases hemoglobin while decreasing ferritin in a 28-day, phase 2a dose escalation study in stage 3 and 4 chronic kidney disease patients with anemia. Proceedings of the American Society of Nephrology Kidney Week. 2011 [Google Scholar]
- 225.Boettcher MF, et al. First-in-man study with BAY 85-3934—a new oral selective HIF-PH inhibitor for the treatment of renal anemia. J Am Soc Nephrol. 2013;24:347A. [Google Scholar]
- 226.Akizawa T, Hanaki K, Arai M. JTZ-951, an Oral Novel Hif-Phd Inhibitor, Elevates Hemoglobin in Japanese Anemic Patients with Chronic Kidney Disease Not on Dialysis. Nephrol Dial Transplant. 2015;30:iii196. [Google Scholar]
- 227.Jain M, et al. Pharmacological Characterization of ZYAN1, a Novel Prolyl Hydroxylase Inhibitor for the Treatment of Anemia. Drug Res. 2015;66:107–112. doi: 10.1055/s-0035-1554630. [DOI] [PubMed] [Google Scholar]
- 228.Salvati A, et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol. 2013;8:137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 229.Singh R, et al. Dose-Dependent Therapeutic Distinction between Active and Passive Targeting Revealed Using Transferrin-Coated PGMA Nanoparticles. Small. 2016;12:351–359. doi: 10.1002/smll.201502730. [DOI] [PubMed] [Google Scholar]
- 230.van der Meel R, Vehmeijer LJC, Kok RJ, Storm G, van Gaal EVB. Ligand-targeted particulate nanomedicines undergoing clinical evaluation: Current status. Adv Drug Deliv Rev. 2013;65:1284–1298. doi: 10.1016/j.addr.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 231.Senzer N, et al. Phase I Study of a Systemically Delivered p53 Nanoparticle in Advanced Solid Tumors. Mol Ther. 2013;21:1096–1103. doi: 10.1038/mt.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhang H, et al. Transferrin-mediated fullerenes nanoparticles as Fe2+-dependent drug vehicles for synergistic anti-tumor efficacy. Biomaterials. 2015;37:353–366. doi: 10.1016/j.biomaterials.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 233.O’Neill PM, Barton VE, Ward SA. The Molecular Mechanism of Action of Artemisinin—The Debate Continues. Molecules. 2010;15:1705–1721. doi: 10.3390/molecules15031705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wiley DT, Webster P, Gale A, Davis ME. Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor. Proc Natl Acad Sci. 2013;110:8662–8667. doi: 10.1073/pnas.1307152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sehlin D, et al. Antibody-based PET imaging of amyloid beta in mouse models of Alzheimer/’s disease. Nat Commun. 2016;7:10759. doi: 10.1038/ncomms10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jutz G, van Rijn P, Santos Miranda B, Böker A. Ferritin: A Versatile Building Block for Bionanotechnology. Chem Rev. 2015;115:1653–1701. doi: 10.1021/cr400011b. [DOI] [PubMed] [Google Scholar]
- 237.Crich SG, et al. Targeting ferritin receptors for the selective delivery of imaging and therapeutic agents to breast cancer cells. Nanoscale. 2015;7:6527–6533. doi: 10.1039/c5nr00352k. [DOI] [PubMed] [Google Scholar]
- 238.Lei Y, et al. Targeted tumor delivery and controlled release of neuronal drugs with ferritin nanoparticles to regulate pancreatic cancer progression. J Controlled Release. 2016;232:131–142. doi: 10.1016/j.jconrel.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 239.Hu H, et al. The M2 phenotype of tumor-associated macrophages in the stroma confers a poor prognosis in pancreatic cancer. Tumor Biol. 2016;37:8657–8664. doi: 10.1007/s13277-015-4741-z. [DOI] [PubMed] [Google Scholar]
- 240.Van Gorp H, Delputte PL, Nauwynck HJ. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol Immunol. 2010;47:1650–1660. doi: 10.1016/j.molimm.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 241.Etzerodt A, et al. Efficient intracellular drug-targeting of macrophages using stealth liposomes directed to the hemoglobin scavenger receptor CD163. J Controlled Release. 2012;160:72–80. doi: 10.1016/j.jconrel.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 242.Amin ML, Kim D, Kim S. Development of hematin conjugated PLGA nanoparticle for selective cancer targeting. Eur J Pharm Sci. 2016;91:138–143. doi: 10.1016/j.ejps.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 243.Takle GB, et al. Delivery of Oligoribonucleotides to Human Hepatoma Cells Using Cationic Lipid Particles Conjugated to Ferric Protoporphyrin IX (Heme) Antisense Nucleic Acid Drug Dev. 1997;7:177–185. doi: 10.1089/oli.1.1997.7.177. [DOI] [PubMed] [Google Scholar]
- 244.Kell DB. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genomics. 2009;2:2. doi: 10.1186/1755-8794-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Nairz M, et al. ‘Ride on the ferrous wheel’ – The cycle of iron in macrophages in health and disease. Immunobiology. 2015;220:280–294. doi: 10.1016/j.imbio.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 246.Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol. 2015;15:500–510. doi: 10.1038/nri3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Miron VE, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Biswas SK, Chittezhath M, Shalova IN, Lim J-Y. Macrophage polarization and plasticity in health and disease. Immunol Res. 2012;53:11–24. doi: 10.1007/s12026-012-8291-9. [DOI] [PubMed] [Google Scholar]
- 249.Bronte V, Murray PJ. Understanding Local Macrophage Phenotypes In Disease: Modulating macrophage function to treat cancer. Nat Med. 2015;21:117–119. doi: 10.1038/nm.3794. [DOI] [PubMed] [Google Scholar]
- 250.Crielaard BJ, et al. Macrophages and liposomes in inflammatory disease: Friends or foes? Int J Pharm. 2011;416:499–506. doi: 10.1016/j.ijpharm.2010.12.045. [DOI] [PubMed] [Google Scholar]
- 251.Kiessling F, Mertens ME, Grimm J, Lammers T. Nanoparticles for Imaging: Top or Flop? Radiology. 2014;273:10–28. doi: 10.1148/radiol.14131520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lartigue L, et al. Biodegradation of Iron Oxide Nanocubes: High-Resolution In Situ Monitoring. ACS Nano. 2013;7:3939–3952. doi: 10.1021/nn305719y. [DOI] [PubMed] [Google Scholar]
- 253.Daldrup-Link HE, et al. MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles. Clin Cancer Res Off J Am Assoc Cancer Res. 2011;17:5695–5704. doi: 10.1158/1078-0432.CCR-10-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Hervault A, et al. Doxorubicin loaded dual pH- and thermo-responsive magnetic nanocarrier for combined magnetic hyperthermia and targeted controlled drug delivery applications. Nanoscale. 2016;8:12152–12161. doi: 10.1039/c5nr07773g. [DOI] [PubMed] [Google Scholar]
- 255.Xian-hui D, et al. Age-related changes of brain iron load changes in the frontal cortex in APPswe/PS1ΔE9 transgenic mouse model of Alzheimer’s disease. J Trace Elem Med Biol. 2015;30:118–123. doi: 10.1016/j.jtemb.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 256.Salazar J, et al. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson’s disease. Proc Natl Acad Sci. 2008;105:18578–18583. doi: 10.1073/pnas.0804373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Brookes MJ, et al. Modulation of iron transport proteins in human colorectal carcinogenesis. Gut. 2006;55:1449–1460. doi: 10.1136/gut.2006.094060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hulet SW, Powers S, Connor JR. Distribution of transferrin and ferritin binding in normal and multiple sclerotic human brains. J Neurol Sci. 1999;165:48–55. doi: 10.1016/s0022-510x(99)00077-5. [DOI] [PubMed] [Google Scholar]
- 259.Li W, Xu L-H, Forssell C, Sullivan JL, Yuan X-M. Overexpression of Transferrin Receptor and Ferritin Related to Clinical Symptoms and Destabilization of Human Carotid Plaques. Exp Biol Med. 2008;233:818–826. doi: 10.3181/0711-RM-320. [DOI] [PubMed] [Google Scholar]
- 260.Jefferies WA, et al. Reactive microglia specifically associated with amyloid plaques in Alzheimer’s disease brain tissue express melanotransferrin. Brain Res. 1996;712:122–126. doi: 10.1016/0006-8993(95)01407-1. [DOI] [PubMed] [Google Scholar]
- 261.Kalaria RN, Sromek SM, Grahovac I, Harik SI. Transferrin receptors of rat and human brain and cerebral microvessels and their status in Alzheimer’s disease. Brain Res. 1992;585:87–93. doi: 10.1016/0006-8993(92)91193-i. [DOI] [PubMed] [Google Scholar]
- 262.Faucheux BA, et al. Distribution of 125I-Ferrotransferrin Binding Sites in the Mesencephalon of Control Subjects and Patients with Parkinson’s Disease. J Neurochem. 1993;60:2338–2341. doi: 10.1111/j.1471-4159.1993.tb03527.x. [DOI] [PubMed] [Google Scholar]
- 263.Morris CM, Candy JM, Omar S, Bloxham CA, Edwardson JA. Transferrin receptors in the Parkinsonian midbrain. Neuropathol Appl Neurobiol. 1994;20:468–472. doi: 10.1111/j.1365-2990.1994.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 264.Visanji NP, et al. Iron deficiency in parkinsonism: region-specific iron dysregulation in Parkinson’s disease and multiple system atrophy. J Park Dis. 2013;3:523–537. doi: 10.3233/JPD-130197. [DOI] [PubMed] [Google Scholar]
- 265.Zhang Z, et al. Parenchymal accumulation of CD163+ macrophages/microglia in multiple sclerosis brains. J Neuroimmunol. 2011;237:73–79. doi: 10.1016/j.jneuroim.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 266.Pey P, Pearce RK, Kalaitzakis ME, Griffin WST, Gentleman SM. Phenotypic profile of alternative activation marker CD163 is different in Alzheimer’s and Parkinson’s disease. Acta Neuropathol Commun. 2014;2:21. doi: 10.1186/2051-5960-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Boyle JJ, et al. Coronary Intraplaque Hemorrhage Evokes a Novel Atheroprotective Macrophage Phenotype. Am J Pathol. 2009;174:1097–1108. doi: 10.2353/ajpath.2009.080431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Tiainen S, et al. High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology. 2015;66:873–883. doi: 10.1111/his.12607. [DOI] [PubMed] [Google Scholar]
- 269.Lundholm M, et al. Secreted Factors from Colorectal and Prostate Cancer Cells Skew the Immune Response in Opposite Directions. Sci Rep. 2015;5:15651. doi: 10.1038/srep15651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Mignogna C, et al. A reappraisal of macrophage polarization in glioblastoma: Histopathological and immunohistochemical findings and review of the literature. Pathol - Res Pract. 2016;212:491–499. doi: 10.1016/j.prp.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 271.LeVine SM. Iron deposits in multiple sclerosis and Alzheimer’s disease brains. Brain Res. 1997;760:298–303. doi: 10.1016/s0006-8993(97)00470-8. [DOI] [PubMed] [Google Scholar]
- 272.Connor JR, Menzies SL, St Martin SM, Mufson EJ. A histochemical study of iron, transferrin, and ferritin in Alzheimer’s diseased brains. J Neurosci Res. 1992;31:75–83. doi: 10.1002/jnr.490310111. [DOI] [PubMed] [Google Scholar]
- 273.Ji J, et al. Low expression of ferroxidases is implicated in the iron retention in human atherosclerotic plaques. Biochem Biophys Res Commun. 2015;464:1134–1138. doi: 10.1016/j.bbrc.2015.07.091. [DOI] [PubMed] [Google Scholar]
- 274.Loeffler DA, et al. Transferrin and Iron in Normal, Alzheimer’s Disease, and Parkinson’s Disease Brain Regions. J Neurochem. 1995;65:710–716. doi: 10.1046/j.1471-4159.1995.65020710.x. [DOI] [PubMed] [Google Scholar]
- 275.Galesloot TE, et al. Serum hepcidin is associated with presence of plaque in postmenopausal women of a general population. Arterioscler Thromb Vasc Biol. 2014;34:446–456. doi: 10.1161/ATVBAHA.113.302381. [DOI] [PubMed] [Google Scholar]
- 276.Orlandi R, et al. Hepcidin and ferritin blood level as noninvasive tools for predicting breast cancer. Ann Oncol. 2014;25:352–357. doi: 10.1093/annonc/mdt490. [DOI] [PubMed] [Google Scholar]
- 277.LeVine SM, et al. Ferritin, transferrin and iron concentrations in the cerebrospinal fluid of multiple sclerosis patients. Brain Res. 1999;821:511–515. doi: 10.1016/s0006-8993(98)01360-2. [DOI] [PubMed] [Google Scholar]
- 278.Sato Y, et al. Cerebrospinal fluid ferritin in glioblastoma: Evidence for tumor synthesis. J Neurooncol. 1998;40:47–50. doi: 10.1023/a:1006078521790. [DOI] [PubMed] [Google Scholar]
- 279.Sung K-C, et al. Ferritin Is Independently Associated With the Presence of Coronary Artery Calcium in 12 033 Men. Arterioscler Thromb Vasc Biol. 2012;32:2525–2530. doi: 10.1161/ATVBAHA.112.253088. [DOI] [PubMed] [Google Scholar]
- 280.Chua AC, Knuiman MW, Trinder D, Divitini ML, Olynyk JK. Higher concentrations of serum iron and transferrin saturation but not serum ferritin are associated with cancer outcomes. Am J Clin Nutr. 2016;104:736–742. doi: 10.3945/ajcn.115.129411. [DOI] [PubMed] [Google Scholar]
- 281.Wang R, Liu Z, Yan L. Serum iron levels and Parkinson’s disease risk: evidence from a meta-analysis. Int J Clin Exp Med. 2016;9:3167–3172. [Google Scholar]
- 282.Valberg LS, Flanagan PR, Kertesz A, Ebers GC. Abnormalities in Iron Metabolism in Multiple Sclerosis. Can J Neurol Sci. 1989;16:184–186. doi: 10.1017/s0317167100028869. [DOI] [PubMed] [Google Scholar]
- 283.Faux NG, et al. An anemia of Alzheimer’s disease. Mol Psychiatry. 2014;19:1227–1234. doi: 10.1038/mp.2013.178. [DOI] [PubMed] [Google Scholar]
- 284.Hare DJ, et al. Decreased Plasma Iron in Alzheimer’s Disease Is Due to Transferrin Desaturation. ACS Chem Neurosci. 2015;6:398–402. doi: 10.1021/cn5003557. [DOI] [PubMed] [Google Scholar]
- 285.Meynard D, et al. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41:478–481. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 286.Feng Q, Migas MC, Waheed A, Britton RS, Fleming RE. Ferritin upregulates hepatic expression of bone morphogenetic protein 6 and hepcidin in mice. Am J Physiol - Gastrointest Liver Physiol. 2012;302:G1397–G1404. doi: 10.1152/ajpgi.00020.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Rausa M, et al. Bmp6 Expression in Murine Liver Non Parenchymal Cells: A Mechanism to Control their High Iron Exporter Activity and Protect Hepatocytes from Iron Overload? PLOS ONE. 2015;10:e0122696. doi: 10.1371/journal.pone.0122696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Daher R, et al. Heterozygous Mutations in BMP6 Pro-peptide Lead to Inappropriate Hepcidin Synthesis and Moderate Iron Overload in Humans. Gastroenterology. 2016;150:672–683.e4. doi: 10.1053/j.gastro.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 289.Kautz L, et al. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46:678–684. doi: 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kautz L, Jung G, Nemeth E, Ganz T. Erythroferrone contributes to recovery from anemia of inflammation. Blood. 2014;124:2569–2574. doi: 10.1182/blood-2014-06-584607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Montosi G, et al. Autosomal-dominant hemochromatosis is associated with a mutation in the ferroportin (SLC11A3) gene. J Clin Invest. 2001;108:619–623. doi: 10.1172/JCI13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Njajou OT, et al. A mutation in SLC11A3 is associated with autosomal dominant hemochromatosis. Nat Genet. 2001;28:213–214. doi: 10.1038/90038. [DOI] [PubMed] [Google Scholar]
- 293.Wallace DF, Clark RM, Harley HAJ, Subramaniam VN. Autosomal dominant iron overload due to a novel mutation of ferroportin1 associated with parenchymal iron loading and cirrhosis. J Hepatol. 2004;40:710–713. doi: 10.1016/j.jhep.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 294.Healey EG, et al. Repulsive guidance molecule is a structural bridge between neogenin and bone morphogenetic protein. Nat Struct Mol Biol. 2015;22:458–465. doi: 10.1038/nsmb.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Papanikolaou G, et al. Mutations in HFE2 cause iron overload in chromosome 1q–linked juvenile hemochromatosis. Nat Genet. 2004;36:77–82. doi: 10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 296.D’Alessio F, Hentze MW, Muckenthaler MU. The hemochromatosis proteins HFE, TfR2, and HJV form a membrane-associated protein complex for hepcidin regulation. J Hepatol. 2012;57:1052–1060. doi: 10.1016/j.jhep.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 297.Roetto A, et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. 2003;33:21. doi: 10.1038/ng1053. [DOI] [PubMed] [Google Scholar]
- 298.Vaiopoulos G, et al. Arthropathy in juvenile hemochromatosis. Arthritis Rheum. 2003;48:227–230. doi: 10.1002/art.10755. [DOI] [PubMed] [Google Scholar]
- 299.Feder JN, et al. A novel MHC class I–like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 300.Wallace DF, et al. Combined deletion of Hfe and transferrin receptor 2 in mice leads to marked dysregulation of hepcidin and iron overload. Hepatology. 2009;50:1992–2000. doi: 10.1002/hep.23198. [DOI] [PubMed] [Google Scholar]
- 301.Latour C, et al. Differing impact of the deletion of hemochromatosis-associated molecules HFE and transferrin receptor-2 on the iron phenotype of mice lacking bone morphogenetic protein 6 or hemojuvelin. Hepatology. 2016;63:126–137. doi: 10.1002/hep.28254. [DOI] [PubMed] [Google Scholar]
- 302.Schmidt PJ, Toran PT, Giannetti AM, Bjorkman PJ, Andrews NC. The Transferrin Receptor Modulates Hfe-Dependent Regulation of Hepcidin Expression. Cell Metab. 2008;7:205–214. doi: 10.1016/j.cmet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Nemeth E, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Boulanger MJ, Chow D, Brevnova EE, Garcia KC. Hexameric Structure and Assembly of the Interleukin-6/IL-6 α-Receptor/gp130 Complex. Science. 2003;300:2101–2104. doi: 10.1126/science.1083901. [DOI] [PubMed] [Google Scholar]
- 305.Meynard D, et al. Regulation of TMPRSS6 by BMP6 and iron in human cells and mice. Blood. 2011;118:747–756. doi: 10.1182/blood-2011-04-348698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Nai A, et al. Limiting hepatic Bmp-Smad signaling by matriptase-2 is required for erythropoietin-mediated hepcidin suppression in mice. Blood. 2016 doi: 10.1182/blood-2015-11-681494. blood-2015-11-681494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Bell CH, et al. Structure of the Repulsive Guidance Molecule (RGM)–Neogenin Signaling Hub. Science. 2013;341:77–80. doi: 10.1126/science.1232322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Zhao N, et al. Neogenin Facilitates the Induction of Hepcidin Expression by Hemojuvelin in the Liver. J Biol Chem. 2016;291:12322–12335. doi: 10.1074/jbc.M116.721191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kawabata H, et al. Molecular Cloning of Transferrin Receptor 2. A new member of the transferrin receptor-like family. J Biol Chem. 1999;274:20826–20832. doi: 10.1074/jbc.274.30.20826. [DOI] [PubMed] [Google Scholar]
- 310.Camaschella C, et al. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet. 2000;25:14–15. doi: 10.1038/75534. [DOI] [PubMed] [Google Scholar]
- 311.Pagani A, et al. Regulation of cell surface transferrin receptor-2 by iron-dependent cleavage and release of a soluble form. Haematologica. 2015;100:458–465. doi: 10.3324/haematol.2014.118521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Jiang Y, Oliver P, Davies KE, Platt N. Identification and Characterization of Murine SCARA5, a Novel Class A Scavenger Receptor That Is Expressed by Populations of Epithelial Cells. J Biol Chem. 2006;281:11834–11845. doi: 10.1074/jbc.M507599200. [DOI] [PubMed] [Google Scholar]
- 313.Ojala JRM, Pikkarainen T, Elmberger G, Tryggvason K. Progressive Reactive Lymphoid Connective Tissue Disease and Development of Autoantibodies in Scavenger Receptor A5–Deficient Mice. Am J Pathol. 2013;182:1681–1695. doi: 10.1016/j.ajpath.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 314.Herz J, Clouthier DE, Hammer RE. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell. 1992;71:411–421. doi: 10.1016/0092-8674(92)90511-a. [DOI] [PubMed] [Google Scholar]
- 315.Overton CD, Yancey PG, Major AS, Linton MF, Fazio S. Deletion of Macrophage LDL Receptor–Related Protein Increases Atherogenesis in the Mouse. Circ Res. 2007;100:670–677. doi: 10.1161/01.RES.0000260204.40510.aa. [DOI] [PubMed] [Google Scholar]
- 316.Philippidis P, et al. Hemoglobin Scavenger Receptor CD163 Mediates Interleukin-10 Release and Heme Oxygenase-1 Synthesis Antiinflammatory Monocyte-Macrophage Responses In Vitro, in Resolving Skin Blisters In Vivo, and After Cardiopulmonary Bypass Surgery. Circ Res. 2004;94:119–126. doi: 10.1161/01.RES.0000109414.78907.F9. [DOI] [PubMed] [Google Scholar]
- 317.Vargas JD, et al. Stromal cell-derived receptor 2 and cytochrome b561 are functional ferric reductases. Biochim Biophys Acta. 2003;1651:116–123. doi: 10.1016/s1570-9639(03)00242-5. [DOI] [PubMed] [Google Scholar]
- 318.Gunshin H, et al. Cybrd1 (duodenal cytochrome b) is not necessary for dietary iron absorption in mice. Blood. 2005;106:2879–2883. doi: 10.1182/blood-2005-02-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Choi J, et al. Duodenal Reductase Activity and Spleen Iron Stores Are Reduced and Erythropoiesis Is Abnormal in Dcytb Knockout Mice Exposed to Hypoxic Conditions. J Nutr. 2012;142:1929–1934. doi: 10.3945/jn.112.160358. [DOI] [PubMed] [Google Scholar]
- 320.Gunshin H, et al. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115:1258–1266. doi: 10.1172/JCI24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Kvarnung M, et al. Mutations in FLVCR2 associated with Fowler syndrome and survival beyond infancy. Clin Genet. 2016;89:99–103. doi: 10.1111/cge.12565. [DOI] [PubMed] [Google Scholar]
- 322.Delaby C, et al. Subcellular Localization of Iron and Heme Metabolism Related Proteins at Early Stages of Erythrophagocytosis. PLOS ONE. 2012;7:e42199. doi: 10.1371/journal.pone.0042199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Gomes IM, Maia CJ, Santos CR. STEAP Proteins: From Structure to Applications in Cancer Therapy. Mol Cancer Res. 2012;10:573–587. doi: 10.1158/1541-7786.MCR-11-0281. [DOI] [PubMed] [Google Scholar]
- 324.Grandchamp B, et al. A novel type of congenital hypochromic anemia associated with a nonsense mutation in the STEAP3/TSAP6 gene. Blood. 2011;118:6660–6666. doi: 10.1182/blood-2011-01-329011. [DOI] [PubMed] [Google Scholar]
- 325.Liu D, et al. Human STEAP3 mutations with no phenotypic red cell changes. Blood. 2016;127:1067–1071. doi: 10.1182/blood-2015-09-670174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Knisely AS, Gelbart T, Beutler E. Molecular characterization of a third case of human atransferrinemia. Blood. 2004;104:2607–2607. doi: 10.1182/blood-2004-05-1751. [DOI] [PubMed] [Google Scholar]
- 327.Levy JE, Jin O, Fujiwara Y, Kuo F, Andrews N. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat Genet. 1999;21:396–399. doi: 10.1038/7727. [DOI] [PubMed] [Google Scholar]
- 328.Hojyo S, et al. The Zinc Transporter SLC39A14/ZIP14 Controls G-Protein Coupled Receptor-Mediated Signaling Required for Systemic Growth. PLOS ONE. 2011;6:e18059. doi: 10.1371/journal.pone.0018059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Ji C, Kosman DJ. Molecular mechanisms of non-transferrin-bound and transferring-bound iron uptake in primary hippocampal neurons. J Neurochem. 2015;133:668–683. doi: 10.1111/jnc.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Cozzi A, et al. Human L-ferritin deficiency is characterized by idiopathic generalized seizures and atypical restless leg syndrome. J Exp Med. 2013;210:1779–1791. doi: 10.1084/jem.20130315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Kovtunovych G, Eckhaus MA, Ghosh MC, Ollivierre-Wilson H, Rouault TA. Dysfunction of the heme recycling system in heme oxygenase 1–deficient mice: effects on macrophage viability and tissue iron distribution. Blood. 2010;116:6054–6062. doi: 10.1182/blood-2010-03-272138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Kawashima A, Oda Y, Yachie A, Koizumi S, Nakanishi I. Heme oxygenase–1 deficiency: The first autopsy case. Hum Pathol. 2002;33:125–130. doi: 10.1053/hupa.2002.30217. [DOI] [PubMed] [Google Scholar]
- 334.Scortegagna M, et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1-/- mice. Nat Genet. 2003;35:331–340. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 335.Zhuang Z, et al. Somatic HIF2A Gain-of-Function Mutations in Paraganglioma with Polycythemia. N Engl J Med. 2012;367:922–930. doi: 10.1056/NEJMoa1205119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Sanchez M, Galy B, Muckenthaler MU, Hentze MW. Iron-regulatory proteins limit hypoxia-inducible factor-2α expression in iron deficiency. Nat Struct Mol Biol. 2007;14:420–426. doi: 10.1038/nsmb1222. [DOI] [PubMed] [Google Scholar]
- 337.Troadec M-B, et al. Targeted deletion of the mouse Mitoferrin1 gene: from anemia to protoporphyria. Blood. 2011;117:5494–5502. doi: 10.1182/blood-2010-11-319483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Percy MJ, et al. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc Natl Acad Sci U S A. 2006;103:654–659. doi: 10.1073/pnas.0508423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Takeda K, et al. Placental but Not Heart Defects Are Associated with Elevated Hypoxia-Inducible Factor α Levels in Mice Lacking Prolyl Hydroxylase Domain Protein 2. Mol Cell Biol. 2006;26:8336–8346. doi: 10.1128/MCB.00425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Kono S. Aceruloplasminemia. Curr Drug Targets. 2012;13:1190–1199. doi: 10.2174/138945012802002320. [DOI] [PubMed] [Google Scholar]
- 341.Quigley JG, et al. Identification of a Human Heme Exporter that Is Essential for Erythropoiesis. Cell. 2004;118:757–766. doi: 10.1016/j.cell.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 342.Byon JCH, Chen J, Doty RT, Abkowitz JL. FLVCR is necessary for erythroid maturation, may contribute to platelet maturation, but is dispensable for normal hematopoietic stem cell function. Blood. 2013;122:2903–2910. doi: 10.1182/blood-2012-10-465104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Chiabrando D, et al. The mitochondrial heme exporter FLVCR1b mediates erythroid differentiation. J Clin Invest. 2012;122:4569–4579. doi: 10.1172/JCI62422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Rey MA, et al. Enhanced alternative splicing of the FLVCR1 gene in Diamond Blackfan anemia disrupts FLVCR1 expression and function that are critical for erythropoiesis. Haematologica. 2008;93:1617–1626. doi: 10.3324/haematol.13359. [DOI] [PubMed] [Google Scholar]
- 345.Philip M, et al. Heme Exporter FLVCR Is Required for T Cell Development and Peripheral Survival. J Immunol. 2015;194:1677–1685. doi: 10.4049/jimmunol.1402172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Vulpe CD, et al. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet. 1999;21:195–199. doi: 10.1038/5979. [DOI] [PubMed] [Google Scholar]
- 347.Griffiths TAM, Mauk AG, MacGillivray RTA. Recombinant Expression and Functional Characterization of Human Hephaestin: A Multicopper Oxidase with Ferroxidase Activity. Biochemistry (Mosc.) 2005;44:14725–14731. doi: 10.1021/bi051559k. [DOI] [PubMed] [Google Scholar]
- 348.Hudson DM, et al. Human hephaestin expression is not limited to enterocytes of the gastrointestinal tract but is also found in the antrum, the enteric nervous system, and pancreatic β-cells. Am J Physiol - Gastrointest Liver Physiol. 2010;298:G425–G432. doi: 10.1152/ajpgi.00453.2009. [DOI] [PubMed] [Google Scholar]