Summary
A major question in neurobiology is whether myelin repair can restore neurological function following the course of a severe, progressive CNS demyelinating disease that induces axonal loss. In this study we used Theiler’s murine encephalomyelitis virus (TMEV) to induce a chronic progressive CNS demyelinating disease in mice that was immune-mediated and pathologically similar to human multiple sclerosis. Because immunosuppression of chronically TMEV-infected mice has been shown to enhance myelin repair, we first addressed the potential roles of CD4+ and CD8+ T cells in the inhibition of CNS remyelination during chronic disease. TMEV infection of susceptible PL/J mice deficient in CD4+ but not CD8+ T cells demonstrated a significant increase in severity of pathogenesis when compared with wild-type controls. This was characterized by enhanced demyelination, spinal cord atrophy, neurological deficits, and mortality. Interestingly, the PL/J CD4−/− mice that survived to the chronic stage of the disease had nearly complete spontaneous myelin repair mediated by both oligodendrocytes and infiltrating Schwann cells. Therefore, we next addressed whether this spontaneous myelin repair was associated with improved neurological function despite the increased pathology. Of interest, all surviving PL/J CD4−/− mice showed partial restoration of motor coordination and gait that coincided temporally with spontaneous myelin repair. Furthermore, functional recovery of motor coordination correlated strongly with the percentage of myelin repair mediated by Schwann cells, whereas restoration of hindlimb gait correlated with oligodendrocyte-mediated myelin repair. This is the first study to demonstrate that spontaneous remyelination correlates with partial restoration of neurological function during the course of a progressive, immune-mediated CNS demyelinating disease. Of greater importance, functional recovery occurred despite previous severe demyelination and spinal cord atrophy.
Keywords: picornavirus, neurodegeneration, glia, motor function, inflammation, axonal loss
Introduction
In the CNS, the myelin sheath insulates axons and facilitates rapid electrical signalling between neurones by saltatory conduction. Damage to the myelin sheath impairs electrical conduction in axons and is considered responsible for many of the functional abnormalities observed in human diseases such as multiple sclerosis, the most common demyelinating disease of the human CNS. Similarly, myelin repair is believed to contribute to the restoration of neurological function following acute attacks of inflammatory demyelination in multiple sclerosis. Because there is no cure for multiple sclerosis, the study of factors that contribute to remyelination and the restoration of function following inflammatory insult to the CNS is of great interest.
In human multiple sclerosis, remyelination can follow demyelination (Prineas and Connell, 1979; Hirano, 1989), may contribute to the slow recovery seen following some exacerbations of multiple sclerosis (McDonald, 1974) and is morphologically similar to that observed in experimental models (Hirano, 1989). In early multiple sclerosis lesions, significant myelin repair mediated by both oligodendrocytes (Prineas and Connell, 1979; Bruck et al., 1994) and Schwann cells (Ghatak et al., 1973) can occur. Oligodendrocytes can be found at higher density (Raine et al, 1981; Bruck et al., 1994; Ozawa et al., 1994) in lesions relative to periplaque white matter in patients with recent onset of multiple sclerosis (Raine et al., 1981). In contrast, lesions in patients who have had multiple sclerosis for many years demonstrate oligodendrocyte loss (Ozawa et al., 1994) and limited remyelination that is localized to the edges of inactive plaques (Perier and Gregoire, 1965; Suzuki et al., 1969), suggesting that recurrent episodes of inflammation may exhaust the ability of oligodendrocytes to regenerate myelin (Prineas et al., 1993). Depletion of oligodendrocytes or their progenitors is one explanation for the lack of myelin repair in chronic CNS demyelination (Ludwin, 1980; Prineas et al., 1984; Ozawa et al., 1994).
Several viruses have been shown to induce CNS demyelination in animals. Of these, Theiler’s virus provides the the best-studied model. Theiler’s murine encephalomyelitis virus (TMEV) induces a biphasic (Lipton, 1975), progressive CNS demyelinating disease that is pathologically similar to human multiple sclerosis when injected into susceptible strains of mice (Dal Canto and Lipton, 1975, 1977, 1979; Lipton and Dal Canto, 1976a; Rodriguez et al., 1987a, b). Mice that are resistant to TMEV infection develop acute encephalitis but clear virus from the CNS within 2–3 weeks and do not develop demyelination or neurological deficits. In susceptible mice, the immune system has been shown to contribute in part to the development of demyelination and neurological deficits (Lipton and Dal Canto, 1976b). The immune response appears to be directed against persistent virus as well as self-antigens within the CNS as a result of epitope spreading (Miller et al., 1997; Katz-Levy et al., 1999).
Although remyelination can occur following TMEV-induced demyelination, it is generally incomplete. By light microscopy, lesions from chronically infected susceptible SJL/J mice demonstrate minimal spontaneous myelin repair (Dal Canto and Lipton, 1975; Rodriguez and Lennon, 1990) despite a marked increase in proliferating cells of the oligodendrocyte lineage (Prayoonwiwat and Rodriguez, 1993). Immunosuppression of chronically infected mice with cyclophosphamide was reported to result in an eightfold increase in myelin repair compared with control mice (Rodriguez and Lindsley, 1992), suggesting that myelin repair may be a natural phenomenon, but is suppressed by a chronic inflammatory response to antigens present within the CNS.
In the present study, neuropathology (demyelination, remyelination and spinal cord atrophy) and objective measurements of neurological deficits were analysed in TMEV-infected susceptible PL/J mice lacking surface expression of CD4 or CD8 with the goal of testing two hypotheses. The first was to determine whether mice of a susceptible genotype with severe genetic immunodeficiencies had an increased potential for the development of myelin repair relative to immunocompetent controls. The second was to assess directly the role of spinal cord remyelination in the restoration of neurological function following a severe CNS demyelinating disease that is pathologically similar to human multiple sclerosis. In this study we confirmed (Murray et al., 1998) that deletion of CD4+ T cells, but not CD8+ T cells, resulted in a dramatic increase in myelin destruction, neurological deficits and disease mortality in susceptible PL/J mice. However, despite this severe pathology, surviving CD4-deficient mice had enhanced myelin repair when compared with wild-type or CD8-deficient mice. Importantly, these results demonstrate for the first time that myelin repair can occur in spite of severe neuropathology and spinal cord atrophy, and this remyelination is associated with partial restoration of neurological function.
Material and methods
Virus
The DA strain (Daniel’s) of Theiler’s virus was used in all experiments. The passage history of this virus has been described previously (Rodriguez et al., 1983). Animals were infected by intracerebral injection of 2 × 106 plaque-forming units of the DA strain of TMEV in a volume of 10 l. Age-matched PL/J CD4−/− mice were sham-infected intracranially with 10 μl of PBS (phosphate-buffered saline) and used as controls
Mice
Mice lacking surface expression of CD4 or CD8 were generated at the Ontario Cancer Institute (Fung-Leung et al., 1991; Rahemtulla et al., 1991; Yeung et al., 1994). Using homologous recombination in ES cells, we generated CD4−/− mice by interrupting the fifth exon of the L3T4 gene by the insertion of neomycin resistance gene sequences in the coding sequence (Rahemtulla et al., 1991). Similarly, CD8−/− mice were generated by disrupting the first exon of the LYT2 gene (Fung-Leung et al., 1991). Mice deficient in CD4 and CD8 were generated on a haplotype normally susceptible to TMEV-induced demyelination by crossing to PL/J strains for 8–10 generations (Murray et al., 1998). Once the crosses were completed, the mice were bred at the Mayo Clinic (Rochester, Minn., USA) animal facilities. Wild-type PL/J (+/+) mice were obtained from The Jackson Laboratories (Bar Harbor, Me., USA). Handling of all animals conformed to the requirements of the National Institutes of Health and Mayo Clinic Animal Care and Use Committee.
Analysis of survival
Following resolution of the acute encephalitic phase of infection, mice were monitored weekly for the development of neurological deficits (including changes in general appearance and activity level or the development of spasticity or paralysis), and survival of mice during the chronic, demyelinating phase of the disease was recorded. Survival at various time points after infection was compared using the χ2 test (P < 0.05).
Rotarod assay
The Rotamex rotarod (Columbus Instruments, Columbus, OH, USA) measures balance, coordination and motor control and was used to assess neurological function. The rotarod consists of a suspended rod powered by a variable-speed motor capable of running at a fixed speed or accelerating at a constant rate. Mice were trained and tested as described previously (McGavern et al.,1999b). Prior to injection with PBS (sham) or TMEV, each mouse received 3 days of training using a constant-speed assay. This consisted of three 3-min trials over 3 days (12 r.p.m. on day 1, 13 r.p.m. on day 2 and 14 r.p.m. on day 3). Mice were then tested using an accelerated assay (initial speed of 10 r.p.m., accelerating at 10 r.p.m. per minute until the mouse fell off) to obtain a baseline measurement. Rotarod performance was subsequently measured at 25, 45, 90 and 180 days after intracerebral injection of PBS or virus. All subsequent trials consisted of 1 day of constant speed training (three 3-min trials at 14 r.p.m.) and 1 day of accelerated speed testing (three trials with an initial speed of 10 r.p.m., accelerating at 10 r.p.m. per minute until the mouse fell off). For the accelerated rotarod assays, the speed of rotation at the time of fall was recorded for each mouse. The data obtained from the rotarod were analysed by first subtracting the start speed from all individual measurements. The percentage decrease from baseline was then calculated for each mouse. Statistical differences between infected and sham-infected rotarod performances at each time point after infection were detected using Student’s t test (P < 0.05). Ratios of infected to sham-infected performance measurements were calculated by dividing the infected performances by the mean sham-infected performance at each time point. Statistical differences between the ratio of infected to sham-infected data for surviving PL/J CD4−/− mice at each time point after infection were calculated using one-way repeated measures analysis of variance (ANOVA). Pairwise comparisons were made using the Student–Newman–Keuls method (P < 0.05). Increases in rotarod performance for sham-infected and infected mice were calculated by dividing the 180-day rotarod performance by the 90-day rotarod performance. Statistically significant differences between these data were detected with the Mann–Whitney rank sum test (P < 0.05). Correlation coefficients between rotarod and pathological data were assessed with the Pearson product moment correlation (P < 0.05).
Footprint analysis
Forelimb and hindlimb length and width of stride were measured as described previously (McGavern et al., 1999b). Briefly, forelimb and hindlimb paws were painted with red and blue non-toxic paint (RoseArt Industries, Livingston, NJ, USA). Mice were then expected to walk for a minimum of five steps along a strip of white paper. Prints were digitized with a colour scanner and measured using a program written for the KS400 image analysis software (Kontron Elektronik, Munich, Germany). The program automatically calculated the length and width of stride for each measured step. Forelimb and hindlimb length and width measurements were quantified at the baseline and 25, 45, 90 and 180 days post-infection (d.p.i). The change in stride length or width from baseline (Δ) at various time points after infection was calculated by subtracting the baseline measurement from the measurement at the respective time point (25, 45, 90 or 180 d.p.i). Statistically significant differences between sham-infected mice, infected survivors and infected non-survivors at each time point after infection were detected by one-way ANOVA. Pairwise comparisons were done using the Student–Newman–Keuls method (P < 0.05). Correlation coefficients between footprint and pathological data were assessed with the Pearson product moment correlation (P < 0.05).
Quantitation of spinal cord demyelination and remyelination
Mice (n = 7–11 per strain per time point) were anaesthetized with 0.5 mg pentobarbital (intraperitoneally) and perfused by intracardiac puncture with Trump’s fixative (phosphate-buffered 4% formaldehyde with 1.0% glutaraldehyde, pH 7.2) (Rodriguez and David, 1985). Spinal cords were removed and cut into 1 mm blocks, and every third block was embedded in Araldite. Sections were stained with 4% paraphenylenediamine to visualize myelin. Areas (in mm2) of spinal cord white matter, demyelinating lesions and regions of myelin repair were calculated on transverse spinal cord sections (10 per mouse) using a Zeiss interactive digital analysis system and camera lucida attached to a Zeiss photomicroscope (Carl Zeiss, Thornwood, NY, USA) as described previously (McGavern et al., 1999a). All pathological studies were done in a blinded manner. For individual mice, the area of demyelinating lesions was divided by the total area of spinal cord white matter sampled and expressed as a percentage. Areas of oligodendrocyte- and Schwann cell-mediated remyelination were quantified separately as described (McGavern et al., 1999a). The area of oligodendrocyte or Schwann-cell mediated remyelination was divided by the area of lesions and expressed as a percentage. The total percentage of remyelination, which includes both oligodendrocyte and Schwann cell remyelination, was calculated in a similar manner. Oligodendrocyte-mediated remyelination was identified by abnormally thin myelin sheaths relative to axonal diameter, whereas remyelination mediated by Schwann cells demonstrated thick myelin sheaths, with one Schwann cell per axon. Oligodendrocyte- and Schwann cell-mediated remyelination was confirmed by electron microscopy. Because the purpose of this study was to investigate myelin repair, mice that demonstrated <0.4% demyelination at 90 or 180 days were excluded from analyses. This resulted in the exclusion of two of 58 mice. For parametric data, statistical differences between the percentages of demyelination or remyelination at different time points after infection were calculated by one-way ANOVA. Pairwise comparisons were made using the Student–Newman–Keuls method (P < 0.05). For non-parametric data, statistically significant differences were detected by one-way ANOVA (analysis of variance) on ranks. Pairwise comparisons were made with Dunn’s method (P < 0.05).
Spinal cord atrophy
Spinal cord atrophy was assessed as described previously (McGavern et al., 1999a, 2000). An Olympus Provis AX70 microscope fitted with a SPOT colour digital camera and a × 1.25 objective was used to digitize all spinal cord cross-sections cut for each mouse. The total cord area, grey matter area, posterior white matter area and the remaining anterior and lateral white matter area (which includes the anterior, lateral and anterolateral columns) were calculated after tracing the regions using a program written for the KS400 image analysis software. The cord sections were classified into one of three categories based on their location within the cord: C1–C7, C8–T11 and T12/13–L3. The data for all infected mice (n = 8–9 per time point) were then plotted as a percentage change from a group of sham-infected mice (n = 7) that had no pathological abnormalities in the spinal cord. Statistical comparisons between raw spinal cord area measurements were performed by one-way ANOVA. Pairwise comparisons were done with the Student–Newman–Keuls method (P < 0.05).
Immunocytochemistry for virus antigen
For immunoperoxidase studies, coronal spinal cord sections (five or six per mouse) from perfused animals were stored in 0.1 M phosphate buffer, rinsed in 0.1 M Tris buffer with 25 mM hydroxylamine (pH 7.4), treated with 10% dimethyl sulfoxide for 1 h, and quick-frozen in liquid nitrogen-chilled isopentane. Cryostat sections were incubated with a polyclonal rabbit antiserum to purified TMEV DA strain virions, which specifically reacts to all structural proteins of TMEV (Rodriguez et al., 1983). Slides were developed with the avidin–biotin immunoperoxidase system (Vector Laboratories, Burlingame, Calif., USA). Quantitative analysis was performed with a Zeiss interactive digital analysis system and camera lucida attached to a Zeiss photomicroscope. Spinal cord areas were traced to determine total area (mm2). The number of virus antigen-positive cells for each mouse was counted and expressed per mm2 of the spinal cord white matter. Statistical differences at each time point after infection were calculated by one-way ANOVA. Pairwise comparisons were made with the Student–Newman–Keuls method (P < 0.05).
Results
CD4+ T cells are critical for survival of TMEV- infected PL/J mice
Susceptibility to TMEV-induced demyelinating disease (TMEV-IDD) maps to the major histocompatibility complex (MHC) class I H-2D locus, the expression of Df,p,q,r,s or v conferring susceptibility in mice with an otherwise resistant C57BL/10 genetic background (Rodriguezes et al., 1986, 1987). It is therefore thought that PL/J mice are susceptible to TMEV-IDD in part because they lack the ability to mount a protective MHC class I-restricted immune response, and therefore only CD4+ T cells appear to play a protective role in PL/J mice (Murray et al., 1998). In the present study, we generated a time course for the study of myelin repair in immunocompromised mice by infecting wild-type, CD4-deficient and CD8-deficient PL/J mice for 45, 90 and 180 days. These time points represent a stage of early demyelination (45 days) (Rodriguez and David, 1985), a stage of late demyelination that precedes myelin repair (90 days) and a chronic stage suitable for the study of myelin repair (180 days).
Mortality in each group was recorded during the chronic stage (beginning 1 month after infection) of disease (Fig. 1A). Death during the chronic stage of disease was uncommon in wild-type mice and generally occurred between 90 and 180 d.p.i. Genetic deletion of CD4 dramatically reduced survival. In PL/J CD4−/− mice, survival was reduced to 84% by 2 months and to 36% by 6 months, compared with 100 and 85% survival in wild-type controls at the same time points. The decrease in survival due to deletion of CD4 was statistically significant 2 months after infection and all time points thereafter (P < 0.05). Consistent with the observation that susceptibility to TMEV-IDD is in part MHC class I-restricted, there were no statistically significant differences in survival between wild-type and CD8-deficient PL/J mice at any time point. This confirms our previous observations that, in mice of a susceptible haplotype, CD4+ T cells, but not CD8+ T cells, are required for protection against TMEV-induced neurological deficits (Murray et al., 1998).
Fig. 1.
Mortality is associated with increased viral load in PL/J CD4−/− mice. (A) A significant increase in mortality was observed 2 months after infection and all time points thereafter for PL/J CD4−/− mice compared with PL/J+/+ mice. No significant increase in mortality was observed in PL/J CD8−/− mice. Asterisks denote statistically significant differences using the χ2 test. (B) A significant increase in viral load was observed in PL/J CD4−/− mice at 45 d.p.i. when compared with PL/J+/+ and PL/J CD8−/− mice. Data are the mean number of virus antigen-positive cells per mm2 and the standard error of the mean for a total of three to six mice per group. Asterisks denote a statistically significant difference from PL/J+/+ mice at a given time point using one-way ANOVA (P < 0.05).
Viral load was increased in PL/J mice deficient in CD4+ T cells
In order to determine the relationship between virus persistence and mortality, we used immunocytochemistry to detect virus antigen in transverse spinal cord segments obtained from the mice that had been used for morphological studies. Virus antigen was detected in the spinal cord white matter of wild-type, CD8-deficient, and CD4-deficient PL/J mice at each time point (Fig. 1B), but not in resistant C57BL/6 control mice (data not shown). In wild-type PL/J mice, the number of virus antigen-positive cells at 45 days (P < 0.05). There was a statistically significant decline in the number of virus antigen-positive cells per mm2 by 90 days (P < 0.05) compared with 45 d.p.i. Virus persisted at 180 days, but the number of virus antigen-positive cells per mm2 was not statistically different from that at day 90. In addition, there were no statistically significant differences in virus antigen-positive cells per mm2 between wild-type and CD8-deficient PL/J at any of the time points tested. This is consistent with the hypothesis that susceptibility in this strain is due, at least in part, to lack of a TMEV-specific CTL response (Lin et al., 1998).
In contrast to immunocompetent animals, mice deficient in CD4+ T cells had a dramatic and statistically significant increase in virus antigen during the early stages of demyelination. At 45 days, there was a statistically significant increase in virus antigen in PL/J CD4−/− mice compared with wild-type controls and PL/J CD8−/− mice (P < 0.05). As in wild-type controls, there was a statistically significant decline in virus antigen between 45 and 90 d.p.i. (P < 0.05), such that by 90 days there were no significant differences in virus antigen between wild-type and surviving CD4-deficient PL/J mice. By 180 d.p.i. similar amounts of virus were observed in all strains of mice.
Extensive spinal cord demyelination was observed in CD4-deficient mice
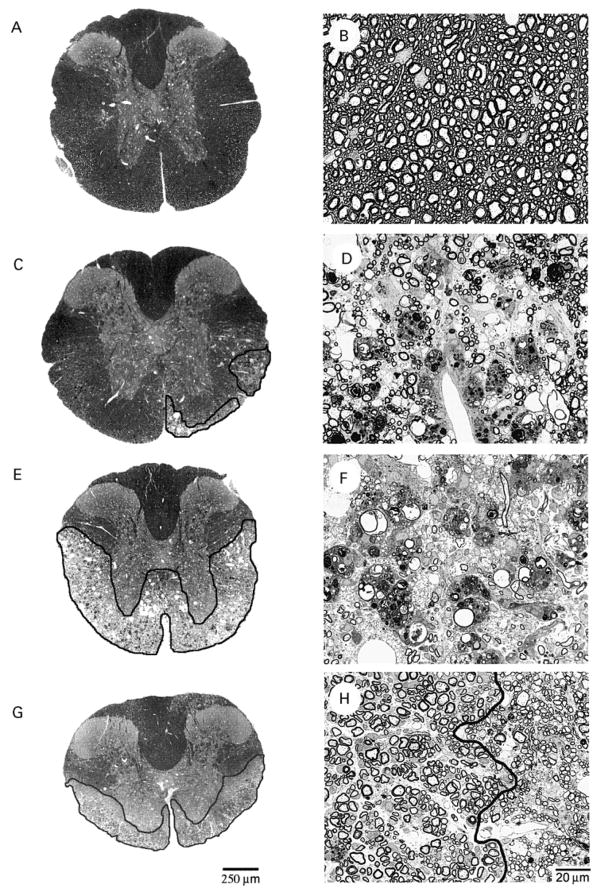
We have demonstrated previously that, 45 days after infection, both CD4+ and CD8+ T cells protect against demyelination in resistant C57BL/6 mice; however, only CD4+ T cells contribute to protection in susceptible PL/J mice (Murray et al., 1998). To assess pathology in chronically infected immunodeficient mice, we quantified demyelination in spinal cords from wild-type, CD4-deficient and CD8-deficient PL/J mice at 45, 90 and 180 d.p.i. (Figs 2 and 3A). In wild-type PL/J mice, demyelinating lesions encompassed 7% of the spinal cord white matter at 45 days (Figs 2C, 2D and 3A). Despite the observation that mortality increased to ~20% by 180 days, there was no significant increase in demyelination beyond the 45-day time point.
Fig. 2.
Pathology observed in TMEV-infected mice. Representative spinal cord cross sections (A, C, E and G) and enlarged white-matter fields (B, D, F and H) are shown for uninfected PL/J CD4−/− (A and B), 90-day-infected PL/J+/+ (C and D), 90-day-infected PL/J CD4−/− (E and F) and 180-day-infected PL/J CD4−/− mice (G and H). Normal spinal cord white matter is illustrated for an uninfected PL/J CD4−/− mouse (A and B). Note the absence of inflammation, intact axons and thick myelin sheaths. In wild-type PL/J mice, similar areas of demyelination were observed at 45, 90 and 180 d.p.i. (C and D). Note the loss of myelin sheaths, myelin ovoids and infiltrating macrophages in the enlarged white-matter field (D). Comparable areas of demyelination were found in TMEV-infected PL/J CD8−/− mice. Black outline highlights demyelinating spinal cord lesions. In contrast to PL/J+/+ and PL/J CD8−/− mice, lesions in TMEV-infected PL/J CD4−/− mice were very severe at 45 and 90 d.p.i. and encompassed nearly all of the lateral, anterolateral and anterior columns in some mice (E). Note that panel F is almost completely devoid of myelin. In PL/J CD4−/− mice that survived to 180 d.p.i., almost complete spontaneous remyelination (dark grey outline) was observed in spinal cord cross-sections that contained lesions (G). This remyelination was mediated by both oligodendrocytes and infiltrating Schwann cells. The black line in panel H separates spinal cord areas remyelinated by Schwann cells (left) versus oligodendrocytes (right). Scale bar = 20 μm for panels B, D, F and H.
Fig. 3.
Quantitation of spinal cord demyelination and remyelination in TMEV-infected mice. Areas of spinal cord demyelination (A) and remyelination (B) were quantified objectively for PL/J+/+, PL/J CD4−/− and PL/J CD8−/− mice at 45, 90 and 180 d.p.i. The area (mm2) of demyelination was divided by the area of spinal cord white matter and expressed as a percentage. The area of remyelination (oligodendrocyte-mediated and Schwann cell-mediated combined) was divided by the area of demyelination and expressed as a percentage. Data are represented as the mean percentage and standard error of the mean using 4–14 mice per group. Asterisks denote statistically significant differences from PL/J+/+ mice at a given point using one-way ANOVA (P < 0.05).
Genetic deletion of CD4 from PL/J mice resulted in a statistically significant increase in demyelination compared with wild-type controls and CD8-deficient mice at all time points tested (P < 0.05). In CD4-deficient PL/J mice, development of severe demyelination was rapid and exceeded 20% of the spinal cord white matter by 45 days (Figs 2E, 2F and 3A). This may explain the significant decrease in survival observed for these mice during the first 90 days of infection. Like wild-type PL/J mice, PL/J CD8−/− mice had no statistically significant increases in demyelination at 45, 90 and 180 d.p.i. The patterns and levels of demyelination were similar in CD8-deficient and wild-type controls at most time points after infection. A slight increase in demyelination was observed in PL/J CD8−/− mice at 90 d.p.i. when compared with wild-type PL/J mice (P < 0.05). However, the demyelination was not as severe as that observed in PL/J CD4−/− mice at this time point. Therefore, in PL/J mice CD4+ T cells provided a greater contribution to protection against TMEV-induced demyelination than did CD8+ T cells.
Significant spontaneous remyelination was observed in chronically infected CD4- deficient mice
To investigate the possibility of increased myelin repair in immunodeficient mice, we analysed lesions in spinal cords obtained from wild-type, CD4-deficient and CD8-deficient PL/J mice at 45, 90 and 180 d.p.i. (Fig. 3B). We quantified oligodendrocyte and Schwann cell-mediated remyelination independently in order to obtain an estimate of total spinal cord repair (see Material and methods) (McGavern et al., 1999a). In wild-type and CD8-deficient PL/J mice, a small amount of spontaneous remyelination was first detected at 45 days. At this time point, light microscopy demonstrated remyelination in only 4% of the demyelinated area in wild-type PL/J mice and 2% in PL/J CD8−/− mice. In each of these groups, myelin repair was progressive and had increased significantly by 180 days (P < 0.05). Although remyelination was not detected in PL/J CD4−/− mice at 45 days and was minimal at day 90 (4%), by 180 days myelin repair encompassed nearly 93% of the area of demyelinated lesions in the mice that survived. Approximately 82% of this spontaneous remyelination was mediated by oligodendrocytes and an additional 11% was mediated by Schwann cells (Fig. 2G and H). This extensive remyelination was significantly higher than that observed in wild-type PL/J and PL/J CD8−/− mice.
Remyelination and the restoration of neurological function in PL/J CD4−/− mice
The fact that surviving PL/J CD4−/− mice had substantial spontaneous remyelination following a severe progressive CNS demyelinating disease provided a useful model for further studies and allowed the design of a second experiment to determine definitively if myelin repair coincided with restoration of neurological function. PL/J CD4−/− mice were infected with TMEV (n = 48) and monitored at various time points after infection for signs of motor dysfunction using two independent assays of neurological function (rotarod and footprint analysis) (Fig. 4). The rotarod assesses the ability of mice to run on a rotating rod and requires balance, coordination and motor control, whereas footprint analysis measures gait abnormalities. Both assays are highly sensitive, objective and quantitative tools for measuring subtle changes in neurological function (McGavern et al., 1999b, 2000).
Fig. 4.
Assessment of neurological function in chronically infected PL/J CD4−/− mice. (A) Ratios of infected to sham-infected rotarod performances were calculated to facilitate comparisons between mice. Ratios equivalent to ≥1 (dotted line) signify that there was no difference between virus-infected and sham-infected (n = 10) PL/J CD4−/− mice. Rotarod performances were plotted for individual PL/J CD4−/− mice that died by 90 d.pi. (closed circles) or survived to 180 d.p.i. (open circles). TMEV-infected mice that survived to 180 d.p.i. were generally above the mean rotarod performance for the entire group (black lines) at 25, 45 and 90 d.p.i. Note the significant increase in rotarod performance between 90 and 180 d.p.i. for surviving (open circles) PL/J CD4−/− mice. Measurement of hindlimb length (B) and width (C) of stride using the same mice as those represented in panel A revealed a slower progression of gait abnormalities in TMEV-infected PL/J CD4−/− mice that survived to 180 d.p.i. when compared with those that died by 90 d.p.i. A greater negative number signifies a greater decrease in stride length or width from the baseline measurement. Reductions in the length and width of stride were less severe at 25 and 45 d.p.i. However, comparable reductions were observed at 90 d.p.i. A slight restoration in the hindlimb width of stride was also noted for surviving PL/J CD4−/− mice between 90 and 180 d.p.i. Data are represented as the mean change and standard error of the mean in hindlimb stride length or width from baseline.
Analysis of motor coordination by rotarod revealed a statistically significant (P = 0.04) decrease in performance in PL/J CD4−/− mice as early as 25 d.p.i. compared with sham-infected controls (n = 10) (Fig. 4A). During the chronic demyelinating phase of disease, there was a progressive decline in rotarod performance that was most severe at 90 d.p.i. At this time point, 77% of the mice (37 of 48) had succumbed to the disease and in the survivors rotarod performance was reduced by 87%. This temporal pattern of neurological dysfunction in PL/J CD4−/− was confirmed by measurements of hindlimb stride length (Fig. 4B) and width (Fig. 4C). Reductions in the hindlimb length and width of stride reached a maximum by 90 d.p.i. On the basis of the previous experiments, this time point coincided with maximum demyelination (28%), but minimal myelin repair (3%) by light microscopy (Fig. 3). Interestingly, although only 15% (7 of 48) of TMEV-infected PL/J CD4−/− mice survived to the 180-day time point, mice demonstrated a statistically significant improvement (P < 0.05) in rotarod performance compared with 90 d.p.i. However, rotarod performance did not return to the preinfection (baseline) level. The average fold-increase in rotarod performance between 90 and 180 d.p.i. for infected PL/J CD4−/− mice was 3.8 ± 1.2, which was statistically different (P < 0.001) from that for sham-infected controls (mean 1.2 ± 0.1). Additionally, the surviving mice also showed a slight restoration in the hindlimb width of stride that did not reach statistical significance (Fig. 4C).
Because results from the functional assays were tabulated for individual mice, we were able to compare the neurological function in surviving mice and those that eventually succumbed to the disease at various time points after infection. Analysis of rotarod performances for PL/J CD4−/− mice that survived to 180 d.p.i. revealed that each of the survivors developed severe neurological deficits followed by partial functional improvement. At all time points, these mice generally performed better than the CD4-deficient mice that eventually succumbed to the disease (Fig. 4A). Hindlimb gait abnormalities were also less severe in the surviving mice during the first 45 days of infection (P < 0.05) (Fig. 4B and C). However, at 90 d.p.i. these gait abnormalities were similar to those observed in mice that eventually succumbed to the disease. This suggests that disease progression was slower in PL/J CD4−/− mice that survived to 180 d.p.i.
To determine if spontaneous remyelination was present in the surviving PL/J CD4−/− mice with functional improvement, both demyelination and remyelination were quantified in the spinal cords of these mice. The results revealed that ~18% of the spinal cord white matter was demyelinated and that remyelination accounted for 62% (40% oligodendrocyte-mediated and 22% Schwann cell-mediated) of the area in these lesions. Although the total extent of spinal cord remyelination was slightly lower than that observed in the previous experiment (Fig. 3B), the results confirmed that functional improvement on the rotarod coincided with spontaneous myelin repair.
Spontaneous remyelination correlates with functional improvement in surviving PL/J CD4−/− mice
To determine if the degree of spinal cord remyelination was related to the improvement in neurological function observed in surviving PL/J CD4−/− mice (shown in Fig. 4), we assessed correlative relationships between pathological and functional variables. When the percentage of spinal cord demyelination (assessed in surviving mice at 180 d.p.i.) was plotted against the ratio of infected to uninfected rotarod performances at 90 d.p.i., a strong negative correlation coefficient was obtained (r = −0.77, P = 0.040) (Fig. 5A). This is consistent with a previous study demonstrating that neurological function is related to the severity of spinal cord demyelination (McGavern et al., 2000). Interestingly, the percentage of total spinal cord demyelination did not correlate with the ratio of infected to uninfected rotarod performance at 180 d.p.i. (r = 0.05, P = 0.921) (Fig. 5B), suggesting that another variable was a better predictor of neurological function at this later time point.
Fig. 5.
Correlative relationships between pathological and functional variables in PL/J CD4−/− mice that survived to 180 d.pi. (A) A strong negative correlation coefficient (r = −0.77, P = 0.040) was obtained between the percentage of spinal cord demyelination at 180 d.p.i. and the ratio of infected to sham-infected rotarod performance at 90 d.p.i. (B) This linear relationship was lost when the percentage of spinal cord demyelination at 180 d.p.i. was plotted against the ratio of infected to sham-infected rotarod performance at 180 d.p.i. (r = 0.05, P = 0.921). (C) A strong positive correlation coefficient (r = 0.78, P = 0.040) was obtained between the percentage of Schwann cell remyelination and the fold-increase in rotarod performance between 90 and 180 d.p.i. The percentage of Schwann cell remyelination is an interaction variable that accounts for the interaction between the percentage of demyelination and Schwann cell remyelination for each mouse. The area of demyelination was divided by the area of spinal cord white matter and expressed as a percentage. The area of Schwann cell-mediated remyelination divided by the area of demyelination was then multiplied by this percentage. The resulting interaction variable was calculated because the percentages of both demyelination and Schwann cell remyelination correlate independently with the fold-increase in rotarod performance (and with each other). The interaction variable between the two increases the strength of the linear relationship with the fold-increase in rotarod performance. (D) A strong positive correlation coefficient (r = 0.82, P = 0.048) was obtained between the percentage of oligodendrocyte remyelination and the change (Δ) in hindlimb width between 90 and 180 d.p.i. Positive numbers denote restoration in the hindlimb width of stride between 90 and 180 d.p.i. One mouse was excluded from the data of panel D because it was unable to walk at 90 d.p.i. The percentage of oligodendrocyte remyelination was calculated by dividing the area of oligodendrocyte-mediated myelin repair by the area of demyelination and expressing the result as a percentage. An interaction variable was not calculated because demyelination and oligodendrocyte-mediated myelin repair are not related linearly. All correlation coefficients were calculated as the Pearson product moment correlation (P < 0.05). inf. = infected; uninf. = uninfected.
Because significant spontaneous remyelination (without a concomitant increase in demyelination) was observed in PL/J CD4−/− mice between 90 and 180 d.p.i., it was hypothesized that this remyelination was responsible for the lack of correlation between rotarod performance and spinal cord demyelination at 180 d.p.i. We therefore assessed correlative relationships between spinal cord remyelination and other pathological/functional variables. No significant correlative relationships were obtained between the total percentage of spinal cord remyelination (which includes both oligodendrocyte- and Schwann cell-mediated myelin repair) and pathological/functional variables. However, the percentage of spinal cord demyelination in 180 day-infected PL/J CD4−/− mice was found to correlate positively with the percentage of Schwann cell-mediated (r = 0.71, P = 0.073) but not oligodendrocyte-mediated (r = −0.19, P = 0.677) remyelination (plots not shown). These data suggest that increased spinal cord demyelination resulted in increased infiltration of Schwann cells from the periphery. Additionally, when the fold-increase in rotarod performance between 90 and 180 d.p.i. for PLJ CD4−/− mice was plotted against the percentage of Schwann cell remyelination (represented as an interaction variable; see legend of Fig. 5), a strong positive correlation was obtained (r = 0.78, P = 0.040) (Fig. 5C). This suggests that Schwann cell remyelination was important for the improvement in rotarod performance between these two time points. In contrast, no correlation was observed between the fold-increase in rotarod performance and the percentage of oligodendrocyte remyelination (r = 0.35, P = 0.440) (plot not shown). Finally, correlative relationships were assessed between spontaneous remyelination and the change (Δ) in hindlimb width of stride between 90 and 180 d.p.i. The percentage of oligodendrocyte-mediated (r = 0.82, P = 0.048) (Fig. 5D), but not Schwann cell-mediated (r = 0.40, P = 0.436) (plot not shown) remyelination correlated positively with the change in hindlimb width of stride between 90 and 180 d.p.i. These data suggest that oligodendrocyte-mediated remyelination contributed to the restoration in hindlimb stride width between 90 and 180 d.p.i.
Spinal cord atrophy was most severe in surviving PL/J CD4−/− mice
Having demonstrated significant spontaneous remyelination and functional improvement in surviving PL/J CD4−/−, we next assessed the severity of spinal cord injury in these mice by quantifying spinal cord atrophy (Fig. 6). We have demonstrated previously significant spinal cord atrophy in the combined lateral and anterior column area of susceptible SJL/L mice during the late, chronic stage of disease (192 d.p.i.) (McGavern et al., 2000). This spinal cord atrophy correlated almost perfectly with the loss of medium and large axons (r = 0.94) and with rotarod performance (r = 0.92) at this time point, demonstrating that severe spinal cord atrophy in the lateral and anterior columns is a powerful indicator of axonal loss and neurological dysfunction. In the present study, we hypothesized that spinal cord atrophy would be less severe in surviving PL/J CD4−/− mice than in all mice at earlier time points. Interestingly, the results contradicted this hypothesis by revealing that spinal cord atrophy was most severe in PL/J CD4−/− mice at 180 d.p.i. In fact, when total cord area was compared between PL/J CD4−/− at various time points after infection (Fig. 6A), a statistically significant (P < 0.05) reduction was observed only at 180 d.p.i. A 12% reduction was observed at the C1–C7 level of the spinal cord and a 25% reduction was observed from C8 to T11. When specific regions of the spinal cord were measured, no significant reduction in the posterior column or grey matter area was observed at any time point after infection, consistent with the observation that minimal or no pathology is observed in these spinal cord regions (Rivera-Quinones et al., 1998; McGavern et al., 2000). However, a substantial reduction (P < 0.05) in the combined lateral and anterior column area (Fig. 6B) at C1–C7 (24%), C8–T11 (42%) and T12/13–L3 (25%) was observed for PL/J CD4−/− mice at 180 d.p.i. These results indicate that functional improvement occurred despite severe spinal cord atrophy.
Fig. 6.
Spinal cord atrophy in chronically infected PL/J CD4−/− mice. Total spinal cord atrophy (A) and atrophy of the combined lateral, anterolateral and anterior column area (B) was most severe in PL/J CD4−/− mice infected for 180 days. Data are the mean change in spinal cord area from a group sham-infected PL/J CD4−/− mice, as percentage and standard error of the mean. Eight or nine mice were analysed per group. Spinal cord sections were compared at C1–C7, C8–T11 and T12/13–L3. Asterisks denote a statistically significant difference from sham-infected PL/J CD4−/− mice using a one-way ANOVA (P < 0.05).
Discussion
In this study we convincingly demonstrated that spontaneous myelin repair correlates with partial restoration of neurological function following severe inflammatory demyelination and spinal cord atrophy. In PL/J mice, deletion of CD4 resulted in increased early viral replication and nearly doubled the extent of demyelination. Augmentation of demyelination may have been due to a combination of direct lysis of infected cells (Rodriguez et al., 1983; Graves et al., 1986; Aubert et al., 1987; Rodriguez et al., 1988) and the residual antiviral immune response (Lipton and Dal Canto, 1976b). In any case, these mice demonstrated significantly increased morbidity and mortality when compared with wild-type mice. Furthermore, PL/J CD4−/− mice that survived to the late chronic stage of the disease (180 d.p.i.) developed severe spinal cord atrophy in the combined lateral and anterior column area (as high as 42% at C8–T11). In contrast to CD4-deficient mice, genetic deletion of CD8 did not alter disease pathogenesis or survival significantly. This is consistent with the observation that resistance and susceptibility to TMEV-induced demyelination maps to the class IMHC H2D locus (Rodriguez et al., 1986, 1987b), and CNS-infiltrating lymphocytes from susceptible strains of mice have impaired virus-specific cytotoxic lymphocyte responses (Lin et al., 1997, 1998, 1999). Therefore, because CD8+ T cells do not appear to contribute to the anti-viral immune response in mice with susceptible MHC haplotypes, it is not surprising that genetic deletion of CD8 had no major effect.
In spite of the severe demyelination and spinal cord atrophy, spontaneous remyelination was greatest in the CD4-deficient mice that survived to 180 d.p.i. compared with wild-type and PL/J CD8−/− mice. In these mice, remyelination encompassed nearly 73% (mean for all mice analysed) of the lesion area. This provided an interesting model for studying the role of myelin repair in functional improvement following severe spinal cord damage. Of interest, further studies revealed that coincident with the development of myelin repair, PL/J CD4−/− mice had a significant functional improvement on an objective test of motor coordination and slight restoration of the hindlimb width of stride. In support of the association between remyelination and functional improvement, strong positive correlation coefficients were found between Schwann cell-mediated remyelination and the fold-increase in rotarod performance (r = 0.78), and between oligodendrocyte-mediated remyelination and restoration of hindlimb stride width (r = 0.82). At present, it is unknown why Schwann cell remyelination correlated better with rotarod function and oligodendrocyte remyelination with the hindlimb width of stride. This may be related to the ability of the independent functional assays to measure different components of neurological function and to the anatomical location or calibre of spinal cord fibres remyelinated by Schwann cells versus oligodendrocytes (Dusart et al., 1992). Finally, the fact that neurological function never returned to baseline levels can be explained by the significant spinal cord atrophy that occurred during the course of this progressive demyelinating disease. We have demonstrated previously that < 10% of the lateral and anterior column spinal cord atrophy observed during the late, chronic stage of the disease in susceptible mice can be explained by axonal atrophy alone (Sathornsumetee et at., 2000). Therefore, severe spinal cord atrophy (to the degree observed in the present study) serves as a powerful indicator of axonal loss.
It remains to be determined why only PL/J CD4−/− mice showed almost complete spontaneous spinal cord remyelination during the late, chronic stage of the disease. It has been hypothesized that CD4-mediated activation of macrophages and microglia contributes to neuropathology (Clatch et al., 1986), and this process may be perpetuated by epitope spreading to myelin autoantigens (Miller et al., 1997; Katz-Levy et al., 1999). Localized inflammation (either antiviral or autoimmune) and phagocytosis of myelin and damaged axons may create an environment unfavourable to myelin regeneration. Although epitope spreading has not been studied in the PL/J strain, a role for CD4+ T cells in inhibiting myelin repair in susceptible mice (SJL background) is supported by studies of epitope spreading (Miller et al., 1997; Katz-Levy et al., 1999) and the fact that immunosuppression with cyclophosphamide or antibody depletion of CD4+ (but not CD8+) T cells enhances myelin repair (Rodriguez and Lindsley, 1992). However, indirect inhibition of myelin repair by CD4+ T cells may not explain the data obtained in the present study. According to this hypothesis, wild-type PL/J mice would be expected to develop chronic progressive demyelination and neurological deficits with a relative paucity of myelin repair. In contrast to the hypothesis, wild-type PL/J mice in the present study (with an intact CD4+ T cell-mediated immune response) developed remyelination in 31.2 ± 7.8% of the demyelinated spinal cord white matter, which is significantly more than that observed in susceptible SJL/J mice (4.1 ± 1.2%, n = 12) (P = 0.04). Furthermore, two of 10 wild-type PL/J mice had levels of spinal cord remyelination similar to that observed in CD4-deficient PL/J mice. These data suggest that factors associated with the genetic background of PL/J mice may contribute to the development of spontaneous remyelination (possibly by limiting viral load). In fact, we have previously observed minimal spontaneous remyelination in mice on the B6 × 129 background, which is deficient in MHC class II-CD4+ T cell interactions (Njenga et al., 1999). The lack of spontaneous myelin repair in B6 × 129 class II-deficient mice was associated with extremely high CNS viral loads throughout the course of disease. Thus, efficient control of virus (possibly related to background genes) may be critical for the development of spontaneous myelin repair.
An alternative hypothesis to CD4-mediated inhibition of myelin repair is that the CD4 deficiency placed selective pressure on infected PL/J mice, such that only mice which rapidly limited disease progression and engaged spontaneous myelin repair could survive to the chronic stage of the disease. Increased viral replication, demyelination, spinal cord atrophy and disease mortality was observed in PL/J CD4−/− mice. This resulted in a small proportion of PL/J CD4−/− mice surviving to the most chronic stage of disease. It is possible that only mice that controlled virus replication or interrupted immunopathogenesis relatively early in the course of the disease (at a time point optimal for the initiation of myelin repair) could survive to the chronic stage of the disease. This is supported by functional data demonstrating that disease progression was slower in PL/J CD4−/− mice that survived to 180 d.p.i. Although the mechanism by which individual mice in this group arrested immunopathogenesis is unclear, mice that failed to do so succumbed to the disease. According to the above hypothesis, similar to PL/J CD4−/− mice, a very low percentage of wild-type PL/J mice would be expected to arrest immunopathogenesis (following the same temporal profile as PL/J CD4−/− mice) and demonstrate extensive spontaneous remyelination. The data presented support this hypothesis in that a small percentage of wild-type PL/J mice showed high spontaneous remyelination and all but one of the PL/J CD4−/− mice that survived to 180 days demonstrated high remyelination.
The restoration of neurological function observed in surviving PL/J CD4−/− mice is supported by electrophysiological studies in animals that have demonstrated an association between remyelination and improved conduction properties (Smith et al., 1979; Smith et al., 1981; Felts and Smith, 1992; Honmou et al., 1996). Smith and colleagues demonstrated that, although conduction through the dorsal columns was impaired after focal demyelination in cats, it improved after progressive, oligodendrocyte-mediated myelin repair (Smith et al, 1979, 1981). Similarly, Felts and Smith reported in rats that remyelination of axons by Schwann cells improved conduction in the majority of axons. The authors also suggested that, because the refractory period of transmission was restored to nearly normal levels, remyelination of CNS axons by Schwann cells could contribute to the restoration of normal function (Felts and Smith, 1992). Transplantation of exogenous Schwann cells derived from neonatal rats has also been shown to result in remyehnation and the restoration of normal conduction in rats with focal dorsal column lesions (Honmou et al., 1996). These studies in concert demonstrate the potential for myelin repair to contribute to improved neurological function following an inflammatory demyelinating disease.
Recent research demonstrating a reduction in motor deficits following remyelination has complemented the previous electrophysiological studies. Jeffrey and Blakemore demonstrated that demyelination via intraspinal injection of rats with ethidium bromide resulted in impaired performance on a foot placement test of motor coordination (Jeffery and Blakemore, 1997). During the 5 weeks following injection, the rats developed spontaneous remyelination and resolution of the behavioural deficits; however, if remyelination was suppressed by exposure to X-irradiation, the improvement in functional recovery was abolished. Similarly, Jeffery and colleagues investigated whether transplantation of glial cell progenitors could promote behavioural recovery following focal demyelination (Jeffery et al., 1997). The authors concluded that myelin repair could restore neurological function, but axonal preservation was required. This conclusion is consistent with the partial restoration of neurological function following remyelination observed in surviving PL/J CD4−/− mice.
While the studies mentioned above have significantly advanced our understanding of the electrophysiological consequences of demyelination and remyelination, each study used a toxic model of demyelination that, unlike multiple sclerosis, is acute and non-progressive and occurs independently of an inflammatory response. In the present study, we demonstrated an improvement in neurological function following spontaneous remyelination during the course of a progressive, inflammatory CNS demyelinating disease that is pathologically similar to human multiple sclerosis. To our knowledge this is the first demonstration of an association between remyelination and restoration of neurological function in a model of chronic progressive immune-mediated demyelination. Importantly, this functional improvement occurred despite chronic viral persistence, severe demyelination and spinal cord atrophy. However, although our study suggests that neurological deficits induced by very severe CNS demyelination are partially reversible, axonal damage is irreversible and may prevent complete recovery following myelin repair.
Acknowledgments
We wish to thank Mabel L. Pierce and Michael Coenen for excellent technical assistance in tissue processing and the preparation of histological sections. We thank Dr Tak Mak for donation of CD4 and CD8-deficient mice and Alexandra Ho for breeding CD4−/− and CD8−/− mice onto the PL/J background. This work was supported by National Institutes of Health grants RO1 NS24180 and RO1 NS32129 and the generous contributions of Mr and Mrs Eugene Applebaum and Ms Kathryn Peterson. S.S. was supported by the Faculty of Medicine, Siriraj Hospital, Mahidol University, Thailand.
Abbreviations
- ANOVA
analysis of variance
- d.p.i
days post-infection
- IDD
induced demyelinating disease
- TMEV
Theiler’s murine encephalomyelitis virus
References
- Aubert C, Chamorro M, Brahic M. Identification of Theiler’s virus infected cells in the central nervous system of the mouse during demyelinating disease. Microb Pathog. 1987;3:319–26. doi: 10.1016/0882-4010(87)90002-7. [DOI] [PubMed] [Google Scholar]
- Bruck W, Schmied M, Suchanek G, Bruck Y, Breitschopf H, Poser S, et al. Oligodendrocytes in the early course of multiple sclerosis. Ann Neurol. 1994;35:65–73. doi: 10.1002/ana.410350111. [DOI] [PubMed] [Google Scholar]
- Clatch RJ, Lipton HL, Miller SD. Characterization of Theiler’s murine encephalomyelitis virus (TMEV)-specific delayed-type hypersensitivity responses in TMEV-induced demyelinating disease: correlation with clinical signs. J Immunol. 1986;136:920–7. [PubMed] [Google Scholar]
- Dal Canto MC, Lipton HL. Primary demyelination in Theiler’s virus infection. An ultrastructural study. Lab Invest. 1975;33:626–37. [PubMed] [Google Scholar]
- Dal Canto MC, Lipton HL. Multiple sclerosis. Animal model: Theiler’s virus infection in mice. Am J Pathol. 1977;88:497–500. [PMC free article] [PubMed] [Google Scholar]
- Dal Canto MC, Lipton HL. Recurrent demyelination in chronic central nervous system infection produced by Theiler’s murine encephalomyelitis virus. J Neurol Sci. 1979;42:391–405. doi: 10.1016/0022-510x(79)90172-2. [DOI] [PubMed] [Google Scholar]
- Dusart I, Marty S, Peschanski M. Demyelination, and remyelination by Schwann cells and oligodendrocytes after kainate-induced neuronal depletion in the central nervous system. Neuroscience. 1992;51:137–48. doi: 10.1016/0306-4522(92)90478-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felts PA, Smith KJ. Conduction properties of central nerve fibers remyelinated by Schwann cells. Brain Res. 1992;574:178–92. doi: 10.1016/0006-8993(92)90815-q. [DOI] [PubMed] [Google Scholar]
- Fung-Leung WP, Schilham MW, Rahemtulla A, Kundig TM, Vollenweider M, Potter J, et al. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991;65:443–9. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- Ghatak NR, Hirano A, Doron Y, Zimmerman HM. Remyelination in multiple sclerosis with peripheral type myelin. Arch Neurol. 1973;29:262–7. doi: 10.1001/archneur.1973.00490280074011. [DOI] [PubMed] [Google Scholar]
- Graves MC, Bologa L, Siegel L, Londe H. Theiler’s virus in brain cell cultures: lysis of neurons and oligodendrocytes and persistence in astrocytes and macrophages. J Neurosci Res. 1986;15:491–501. doi: 10.1002/jnr.490150406. [DOI] [PubMed] [Google Scholar]
- Hirano A. Review of the morphological aspects of remyelination [Review] Dev Neurosci. 1989;11:112–7. doi: 10.1159/000111892. [DOI] [PubMed] [Google Scholar]
- Honmou O, Felts PA, Waxman SG, Kocsis JD. Restoration of normal conduction properties in demyelinated spinal cord axons in the adult rat by transplantation of exogenous Schwann cells. J Neurosci. 1996;16:3199–208. doi: 10.1523/JNEUROSCI.16-10-03199.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery ND, Blakemore WF. Locomotor deficits induced by experimental spinal cord demyelination are abolished by spontaneous remyelination. Brain. 1997;120:27–37. doi: 10.1093/brain/120.1.27. [DOI] [PubMed] [Google Scholar]
- Katz-Levy Y, Neville KL, Girvin AM, Vanderlugt CL, Pope JG, Tan LJ, et al. Endogenous presentation of self myelin epitopes by CNS-resident APCs in Theiler’s virus-infected mice. J Clin Invest. 1999;104:599–610. doi: 10.1172/JCI7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Pease LR, Rodriguez M. Differential generation of class I H-2D- versus H-2K-restricted cytotoxicity against a demyelinating virus following central nervous system infection. Eur J Immunol. 1997;27:963–70. doi: 10.1002/eji.1830270424. [DOI] [PubMed] [Google Scholar]
- Lin X, Pease LR, Murray PD, Rodriguez M. Theiler’s virus infection of genetically susceptible mice induces central nervous system-infiltrating CTLs with no apparent viral or major myelin antigenic specificity. J Immunol. 1998;160:5661–8. [PubMed] [Google Scholar]
- Lin X, Roos RP, Pease LR, Wettstein P, Rodriguez M. A Theiler’s virus alternatively initiated protein inhibits the generation of H-2K-restricted virus-specific cytotoxicity. J Immunol. 1999;162:17–24. [PubMed] [Google Scholar]
- Lipton HL. Theiler’s virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11:1147–55. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton HL, Dal Canto MC. Chronic neurologic disease in Theiler’s virus infection of SJL/J mice. J Neurol Sci. 1976a;30:201–7. doi: 10.1016/0022-510x(76)90267-7. [DOI] [PubMed] [Google Scholar]
- Lipton HL, Dal Canto MC. Theiler’s virus-induced demyelination: prevention by immunosuppression. Science. 1976b;192:62–4. doi: 10.1126/science.176726. [DOI] [PubMed] [Google Scholar]
- Ludwin SK. Chronic demyelination inhibits remyelination in the central nervous system. An analysis of contributing factors. Lab Invest. 1980;43:382–7. [PubMed] [Google Scholar]
- McDonald WI. Pathophysiology in multiple sclerosis [Review] Brain. 1974;97:179–96. doi: 10.1093/brain/97.1.179. [DOI] [PubMed] [Google Scholar]
- McGavern DB, Murray PD, Rodriguez M. Quantitation of spinal cord demyelination, remyelination, atrophy, and axonal loss in a model of progressive neurologic injury. J Neurosci Res. 1999a;58:492–504. doi: 10.1002/(sici)1097-4547(19991115)58:4<492::aid-jnr3>3.0.co;2-p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavern DB, Zoecklein L, Drescher KM, Rodriguez M. Quantitative assessment of neurologic deficits in a chronic progressive murine model of CNS demyelination. Exp Neurol. 1999b;158:171–81. doi: 10.1006/exnr.1999.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavern DB, Murray PD, Rivera-Quinones C, Schmelzer JD, Low PA, Rodriguez M. Axonal loss results in spinal cord atrophy, electrophysiological abnormalities and neurological deficits following demyelination in a chronic inflammatory model of multiple sclerosis. Brain. 2000;123:519–31. doi: 10.1093/brain/123.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SD, Vanderlugt CL, Begolka WS, Pao W, Yauch RL, Neville KL, et al. Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–6. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- Murray PD, Pavelko KD, Leibowitz J, Lin X, Rodriguez M. CD4(+) and CD8(+) T cells make discrete contributions to demyelination and neurologic disease in a viral model of multiple sclerosis. J Virol. 1998;72:7320–9. doi: 10.1128/jvi.72.9.7320-7329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njenga MK, Murray PD, McGavern D, Lin X, Drescher KM, Rodriguez M. Absence of spontaneous central nervous system remyelination in class II-deficient mice infected with Theiler’s virus. J Neuropathol Exp Neurol. 1999;58:78–91. doi: 10.1097/00005072-199901000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K, Suchanek G, Breitschopf H, Bruck W, Budka H, Jellinger K, et al. Patterns of oligodendroglia pathology in multiple sclerosis. Brain. 1994;117:1311–22. doi: 10.1093/brain/117.6.1311. [DOI] [PubMed] [Google Scholar]
- Perier O, Gregoire A. Electron microscopic features of multiple sclerosis lesions. Brain. 1965;88:937–52. doi: 10.1093/brain/88.5.937. [DOI] [PubMed] [Google Scholar]
- Prayoonwiwat N, Rodriguez M. The potential for oligodendrocyte proliferation during demyelinating disease. J Neuropathol Exp Neurol. 1993;52:55–63. doi: 10.1097/00005072-199301000-00007. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Connell F. Remyelination in multiple sclerosis. Ann Neurol. 1979;5:22–31. doi: 10.1002/ana.410050105. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Kwon EE, Cho ES, Sharer LR. Continual breakdown and regeneration of myelin in progressive multiple sclerosis plaques. Ann N Y Acad Sci. 1984;436:11–32. doi: 10.1111/j.1749-6632.1984.tb14773.x. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Barnard RO, Revesz T, Kwon EE, Sharer L, Cho ES. Multiple sclerosis. Pathology of recurrent lesions. Brain. 1993;116:681–93. doi: 10.1093/brain/116.3.681. [DOI] [PubMed] [Google Scholar]
- Rahemtulla A, Fung-Leung WP, Schilham MW, Kundig TM, Sambhara SR, Narendran A, et al. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991;353:180–4. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- Raine CS, Scheinberg L, Waltz JM. Multiple sclerosis. Oligodendrocyte survival and proliferation in an active established lesion. Lab Invest. 1981;45:534–46. [PubMed] [Google Scholar]
- Rivera-Quinones C, McGavern D, Schmelzer JD, Hunter SF, Low PA, Rodriguez M. Absence of neurological deficits following extensive demyelination in a class I-deficient murine model of multiple sclerosis. Nat Med. 1998;4:187–93. doi: 10.1038/nm0298-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, David CS. Demyelination induced by Theiler’s virus: influence of the H-2 haplotype. J Immunol. 1985;135:2145–8. [PubMed] [Google Scholar]
- Rodriguez M, Lennon VA. Immunoglobulins promote remyelination in the central nervous system. Ann Neurol. 1990;27:12–17. doi: 10.1002/ana.410270104. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Lindsley MD. Immunosuppression promotes CNS remyelination in chronic virus-induced demyelinating disease. Neurology. 1992;42:348–57. doi: 10.1212/wnl.42.2.348. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Leibowitz JL, Lampert PW. Persistent infection of oligodendrocytes in Theiler’s virus-induced encephalomyelitis. Ann Neurol. 1983;13:426–33. doi: 10.1002/ana.410130409. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Leibowitz J, David CS. Susceptibility to Theiler’s virus-induced demyelination: mapping of the gene within the H-2D region. J Exp Med. 1986;163:620–31. doi: 10.1084/jem.163.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, David CS, Pease LR. The contribution of MHC gene products to demyelination by Theiler’s virus. In: David CS, editor. H-2 antigens. New York: Plenum Publishing; 1987a. pp. 747–56. [Google Scholar]
- Rodriguez M, Oleszak E, Leibowitz J. Theiler’s murine encephalomyelitis: a model of demyelination and persistence of virus [Review] Crit Rev Immunol. 1987b;7:325–65. [PubMed] [Google Scholar]
- Rodriguez M, Siegel LM, Hovanec-Burns D, Bologa L, Graves MC. Theiler’s virus-associated antigens on the surface of cultured glial cells. Virology. 1988;166:463–74. doi: 10.1016/0042-6822(88)90517-x. [DOI] [PubMed] [Google Scholar]
- Sathornsumetee S, McGavern DB, Ure DR, Rodriguez M. Quantitative ultrastructural analysis of a single spinal cord demyelinated lesion predicts total lesion load, axonal loss, and neurological dysfunction in a murine model of multiple sclerosis. Am J Pathol. 2000;157:1365–76. doi: 10.1016/S0002-9440(10)64650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EJ, Blakemore WF, McDonald WI. Central remyelination restores secure conduction. Nature. 1979;280:395–6. doi: 10.1038/280395a0. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Blakemore WF, McDonald WI. The restoration of conduction by central remyelination. Brain. 1981;104:383–404. doi: 10.1093/brain/104.2.383. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Andrews JM, Waltz JM, Terry RD. Ultrastructural studies of multiple sclerosis. Lab Invest. 1969;20:444–54. [PubMed] [Google Scholar]
- Yeung RS, Penninger J, Mak TW. T-cell development and function in gene-knockout mice [Review] Curr Opin Immunol. 1994;6:298–307. doi: 10.1016/0952-7915(94)90105-8. [DOI] [PubMed] [Google Scholar]