Abstract
Androgens and androgen receptors play essential roles in the development and progression of prostate cancer, a disease that claims roughly 28,000 lives annually. In addition to androgen biding, androgen receptor activity can be regulated via several post-translational modifications such as ubiquitination, acetylation, phosphorylation, methylation & SUMO-ylation. Off these modifications, phosphorylation has been the most extensively studied. Modification by phosphorylation can alter androgen receptor localization, protein stability and transcriptional activity, ultimately leading to changes in the biology of cancer cells and cancer progression. Understanding, role of phosphorylated androgen receptor species holds the key to identifying a potential therapeutic drug target for patients with prostate cancer and castrate resistant prostate cancer. Here, we present a brief review of recently discovered protein kinases phosphorylating AR, focusing on the functional role of phosphorylated androgen receptor species in prostate cancer and castrate resistant prostate cancer.
Keywords: LMTK2, MAPK, Lyn, Androgen Receptor, Castrate Resistant Prostate Cancer, Prostate Cancer, Kinases, Signaling
Introduction
Prostate cancer is one of the most common cancers amongst men. According to the Centers for Disease Control and Prevention, prostate cancer is the second leading cause of cancer-related deaths in men in the United States[1]. Indeed, in 2014 alone the National Cancer Institute recorded nearly a quarter of a million fresh cases of prostate cancer and ~30,000 deaths as a consequence of the disease[2]. Tumor growth, at least in its initial stages, is highly dependent upon androgen-mediated proliferative signaling mechanisms[3–5]. An observation that led Huggins, over 30 years ago, to conclude that androgen depletion could lead to prostate tumor remission[6]. These early ideas laid the foundation for Gonadotropin Releasing Hormone (GnRH) agonists, the current mainstay of initial pharmacological prostate cancer therapy. GnRH agonists work via the hypothalamic-pituitary-gonadal axis and lowers the amount of androgens synthesized by the testes, thus reducing or depleting circulating androgen levels. Although effective in the initial stages of prostate cancer, androgen ablation loses its efficacy, and most patients eventually progress to a so-called castrate-resistant prostate cancer (CRPC); also referred to as ‘hormone refractory’ or ‘androgen independent’ prostate cancer, a disease state which is yet to be fully understood[7–9]. Interestingly, studies using xenograft prostate tumor models have shown that tumors which emerged following androgen depletion therapy (categorized as CRPC), nonetheless expressed androgen receptor (AR)-regulated genes[10, 11]. Thus although clinically these cancer appear ‘androgen independent’ the AR still seems to play a role in cancer cell growth. These findings suggested that AR signaling pathways are still intact in CRPC. Indeed, recent studies have argued that receptor-mediated endocytosis of androgens may contribute to an efficient uptake of hormone despite a low circulating androgen level[12]. Critical to understanding how prostate cancer cells can grow with an apparent lack of androgens is an understanding of the molecular mechanisms by which modulation of AR signaling cascades can occur. This review will focus on kinase-dependent modulation of AR signaling, and will outline how understanding of kinase pathways could lead to potential new therapies.
Androgen and Androgen Receptor Signaling
The prostate is a walnut-sized gland found between the bladder and the penis, where its main function is to secrete a fluid that nourishes and protects sperm. During ejaculation, the prostate squeezes this fluid into the urethra and is expelled along with sperm. Androgens and their receptors play an important role in the development and maintenance of the prostate gland. The main circulating androgen in males is testosterone, which enters prostate cells predominantly via free diffusion, but also via endocytosis with the help of megalin, a multi-ligand endocytic receptor (megalin may be particularly important in CRPC states)[12]. Upon entering prostatic stromal and basal cells, testosterone is reduced to dihydrotestosterone (DHT) by the enzyme, 5-α reductase[13]. This conversion is necessary for complete prostate morphogenesis as evident by small or undetectable prostate glands in individuals lacking a functional 5-α reductase enzyme[14]. Androgens are also necessary for the initiation of prostate development. Thus, the prostate is absent in individuals with AR insensitivity due to mutations of AR, that alters androgen binding efficiency, as well as AR knockout mice and in testicular feminized mice (Tfm), which lack functional AR[15–18]. Interestingly, although AR plays a key role in the normal differentiation and maintenance of the prostate, AR also plays an essential part in driving malignant development of prostate cancer. Following the development of the prostate gland, AR continues to play an important role in promoting the survival of its secretory epithelia, the primary cell type transformed in prostate adenocarcinoma[19]. In a normal healthy prostate, cell death occurs each day at a rate of ~1–2%, but this is equally matched by the rate of cell proliferation, which, as noted, is dependent upon AR activity[20, 21]. So how does AR regulate cell proliferation? In the absence of androgens, AR is primarily present in an inactive state, bound to heat shock proteins (HSP-90, -70, -56) in the cell cytoplasm[22–24]. AR is a nuclear receptor that, upon activation by androgens, traffics to the nucleus and mediates transcription of androgen-responsive genes. AR protein consists of three major functional domains: The N-terminal domain, a DNA-binding domain (DBD) and a Ligand-binding domain (LBD) (Figure 1). Binding of androgen to its ligand-binding pocket on AR, results in a conformational change in the receptor leading to homo-dimerization, exposure of Nuclear Localization Signals (NLS) and the formation of new interactions with AR-coactivators. These modifications facilitate nuclear translocation of AR following which it binds to androgen response elements (ARE), which can be found in the promoter and enhancer regions of androgen responsive genes, including those known to promote cell proliferation e.g. Prostate-Specific Antigen (PSA) & Vascular Endothelial Growth Factor (VEGF). The AR transcriptional complex is thus able to modulate gene expression of these target genes and regulate cell proliferation.
Figure 1. Androgen Receptor structure and phosphorylation sites.
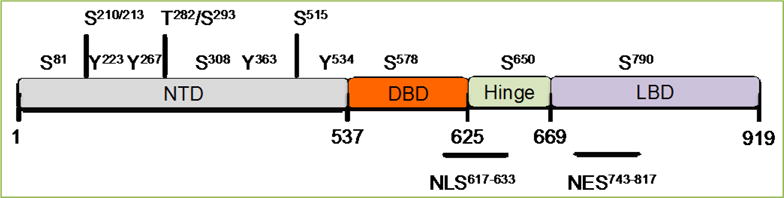
The location of the AR N-Terminal domain (NTD), DNA-binding domain (DBD), hinge region and C-terminal ligand-binding domain (LBD) are depicted. AR can be phosphorylated at Serine (S), threonine (T), and tyrosine (Y) residues by several kinases. Note the location for Nuclear Localization Signal (NLS) and Nuclear Exit Signal (NES).
In addition to androgen binding several post-translational modifications including phosphorylation, SUMO-ylation, ubiquitination, methylation, and acetylation have also been discovered to regulate AR activity[25]. Off these modifications, phosphorylation has been the most extensively studied. Van Laar et al first reported phosphorylation of AR[26, 27] in 1990. A two-fold increase in phosphorylation was observed after 30 min incubation of LNCaP, a androgen dependent prostate adenocarcinoma cells with 10nm R1881, a synthetic androgen analog. AR has phospho -serine, -threonine and -tyrosine sites on each of its major domains. Phosphorylation at many of these sites can regulate cell growth, AR-transcriptional activity and its sensitivity to androgens. For example, phosphorylation of AR by CDK1 and CDK9 can regulate the transcriptional activity of AR by regulating AR expression or transactivation respectively[28, 29]. Furthermore, kinases like LMTK2 and Lyn have also been implicated in manipulating AR signaling pathways and support the progression of prostate cancer to advanced androgen-independent stages of prostate cancer[30, 31]. Understanding, detailed molecular mechanisms and the role of the various phosphorylated AR species holds the key to identifying phosphorylation sites that could serve as a potential therapeutic drug targets for patients with prostate cancer and CRPC. In this article, we review a few of the recently discovered protein kinases regulating AR activity. Also, we also provide a list of several known kinases and their respective phosphorylation sites in AR (Table-1).
Table 1.
List of the known kinases phosphorylating AR and their respective phosphorylation sites in AR are indicated wherever possible. References are included. This table is not intended to be the comprehensive list, but rather to highlight a few well-studied kinases phosphorylating AR
| Sr. No | Kinase | Experimentally Determined Site | Function |
|---|---|---|---|
| 1 | CDK1 | S-81, S515 | CDK1 could increase AR expression.[29,60] |
| 2 | Lyn | Unknown | Lyn may enhance the AR-transcriptional activity[30]. |
| 3 | LMTK2 | Unknown | LMTK2 may negatively regulate the AR-transcriptional activity.[31] |
| 4 | MKK4/JNK | S-650 | Stress Kinase could negatively regulate the AR-transcriptional activity.[38] |
| 5 | MKK6/p38 | S-650 | Stress Kinase may negatively regulate the AR-transcriptional activity.[38] |
| 6 | Akt | S-210, S-790 | AKT may suppresses AR-induced apoptosis.[61] |
| 7 | Src | Y-534 | Src could promote the AR-transcriptional activity.[62] |
| 8 | CDK5 | S-81, S308 | CDK5 could increase AR stabilization and transactivation.[63,64] |
| 9 | CDK9 | S-81 | CDK9 could promote the AR-transcriptional activity.[28] |
| 10 | IKK1/IKK2 | Unknown | IKK1 could act as positive regulator of the AR activity, whereas, IKK2 could act as negative regulator.[65] |
| 11 | PIM-1 | S-213 | PIM-1 could act as a negative regulator of the AR-transcriptional activity.[66] |
| 12 | Aurora-A | T282 & S293 | Aurora-A may activate AR.[67] |
| 13 | Cyclin D3/CDK11P58 | S308 | Cyclin D3/CDK11p58 may negatively regulate AR function.[68] |
| 14 | CDK7 | S515 | TFIIH transcription factor via CDK7 may be required for AR transactivation.[69] |
| 15 | PKC | Unknown | PKC has been implicated as positive and negative regulator of AR-responsive PSA gene.[70,71] |
| 16 | PAK6 | S578 | PAK6 may promote ubiquitin-mediated AR degradation.[72] |
| 17 | Ack | Y267 & Y363 | Y267 phosphorylation may be critical for castration-resistant AR transactivation[73]. |
| 18 | Fer | Y223 | Fer-AR complexes may enhance PSA transcription[74]. |
| 19 | Gsk3β | Unknown | Gsk3β may inhibit AR-driven transcription[75]. |
| 20 | ZIP Kinase | Unknown | ZIP kinase may enhance the AR-mediated transactivation. ZIP kinase failed to phosphorylate AR in-vitro[76]. |
Stress Kinase Signaling
The mitogen-activated (MAP) kinase signaling pathways are some of the most highly conserved pathways amongst eukaryotes[32]. Of the four identified MAP kinases, c-Jun N-terminal kinase (JNK) and p38 MAP kinase are together referred to as stress-activated MAP kinases. Stress kinases get activated in response to a variety of environmental stresses including osmotic and oxidative stresses, heat shock, UV-irradiation, protein synthesis inhibitors and DNA-damaging agents[33–35]. Upon activation, stress kinases phosphorylate and activate several transcriptional factors such as ATF2 and Elk-1, which in turn regulate gene expression in response to cellular stress[36–38]. Importantly, stress kinases have not only been implicated in playing a role in tumor suppression but paradoxically, also in tumor formation and development. Since, stress kinases regulate several transcription factors, their role as tumor suppressors or activators may differ from one tumor microenvironment to another.
One of the roles of stress kinases in the prostate tumor milieu is to regulate AR signaling pathway (Figure 2). Gioeli et al. were first to show that the signaling from MAP kinase 4 & 6 (MKK4 & MKK6) could lead to JNK and/or P38 mediated phosphorylation of AR at serine-650[38]. The proximity of serine-650 to AR Nuclear Exit Signals (NES) suggested that such phosphorylation might regulate AR nuclear exit and a reduction in AR signaling. Supporting this hypothesis, nuclear-cytoplasmic shuttling assays demonstrated that stress kinases did indeed induce AR-S650 phosphorylation, which was required for AR nuclear exit. In contrast, inhibiting protein expression levels of MKK4 and MKK6 using siRNA in LNCaP cells resulted in a significant increase in AR transcriptional activity, as measured by PSA mRNA levels. Hence, in the context of AR signaling in prostate epithelia, stress kinase signaling appears to play a key role in tumor suppression. Further support for the role of stress-kinase signaling in tumor suppression comes from a systematic study that shows up-regulation of MKK4 and MKK6 protein levels in pre-neoplastic and neoplastic human prostate tissue, presumably in an attempt to limit AR signaling and tumor progression[39].
Figure 2. Protein kinases cross-talk with AR in prostate cancer cells.

Stress Kinases, LYN, PKA & LMTK2 interacts with androgen-androgen receptor signal axis and regulate the expression of downstream AR-dependent genes, hence regulating key cellular processes like proliferation, differentiation and apoptosis. In prostate epithelial cells, Lyn kinase promotes stability of AR protein by promoting binding of AR to HSP90. Stress kinases are able to regulate AR nuclear exit by phosphorylating AR at Serine-650. LMTK2 might be able to promote nuclear exit of ARS-650 species by inhibiting PP1C activity. Hence, the role of stress kinase and LMTK2 is that of a negative regulator of AR signaling. Furthermore, PKA through phosphatases can dephosphorylate AR, leading to increase in the AR-transcriptional activity. Green arrows for stimulatory effects while red arrows indicate inhibitory effects on AR activity respectively.
Lyn tyrosine Kinase
Lyn tyrosine kinase, a member of the SRC family of tyrosine kinase (SFK) plays an important role in regulating several epithelial and hematopoietic cellular events including cell survival, proliferation, differentiation and cell migration[40]. Lyn kinase, which was originally discovered in the context of hematopoietic cells, is also expressed in the prostatic epithelia of normal individuals and patients with and prostate cancer[41]. Interestingly, Goldenberg et al. in 2004 first showed that while the average calculated area of the largest cross-section for the prostate gland in control (Lyn +/+) mice was 6.74mm2 +/− 0.34 mm2, it was significantly reduced in Lyn-deficient mice (Lyn −/−) to 2.83 mm2 +/− 0.44m2. The study also observed diminution in the thickness of prostatic epithelia as well as in the complexity of the prostate ductal network in Lyn −/− mice compared to Lyn +/+. Together, these findings suggested a role for Lyn kinase in the development and physiology of the prostate epithelium[41].
In an effort to elucidate the mechanisms underlying the role of Lyn tyrosine kinase in prostate cancer, Zardan et al. found that while there was no significant difference in Lyn expression between normal prostate and primary androgen sensitive prostate tumors, Lyn expression was two fold higher in CRPC specimens when compared with primary prostate cancer specimens[30]. Furthermore, in-vitro studies of Lyn expression using prostate cancer cell lines, and in-vivo using CRPC xenograft mice models revealed that Lyn expression was up-regulated under androgen-deprivation conditions[30]. These findings suggest that Lyn expression in prostate cancer cells is regulated by androgen deprivation and is correlated with progression to the CRPC state. Moreover, overexpression of Lyn in androgen-deprived LNCaP cells resulted in a six-fold increase in AR signaling as measured by an AR-dependent luciferase reporter assay or quantitation of mRNA expression for PSA and the AR downstream gene FKBP51[30]. Collectively, the data suggest that not only is Lyn expression correlated with progression to CRPC, but also that, Lyn is a potent inducer of AR transcriptional activity, which is also associated with progression to the CRPC state.
Zardan et al. also suggested a mechanism through which Lyn might be regulating AR[30]. Their study found that in the absence of Lyn there was enhanced proteasome-mediated AR degradation. Treatment of Lyn knock down cells with the proteasome inhibitor MG-132 abrogated the AR protein degradation associated with loss of Lyn, and led to increased AR signaling. Thus an increase in Lyn expression, as seen in CRPC, could be expected to be associated with increased AR stability and hence increased AR signaling. Further investigation revealed that AR degradation associated with the loss of Lyn could be due the dissociation of AR from Hsp90, a molecular chaperone primarily responsible for stabilizing AR in its unbound state. The stability of AR might not be an issue in the presence of its ligand or AR activators, however in the androgen castrate condition, lack of ligand requires an increased stability of unbound AR and this may be achieved by increase in expression of Lyn, the precise event observed in CRPC tissues.
We now have a basic understanding of the role of Lyn kinase in prostate cancer. Apart from increasing the stability of ligand-unbound AR, it is possible that Lyn also supports AR transcriptional activity via phosphorylation events however this has yet to be investigated.
Protein Kinase A
Although more typically associated with acute, non-steroidal, signaling pathways, cAMP-dependent protein kinase (PKA) also modulates AR signaling pathways. Studies by Nazareth et al. showed that PKA activation can activate AR-dependent transcription in the absence of exogenous androgens; a finding abolished by the addition of PKA inhibitory peptide[42]. That the response to PKA activation was lost in the presence of the AR antagonists’ flutamide and bicalutamide indicated that AR was necessary to mediate the PKA effect. In addition, Sadar observed that treatment of LNCaP cells with the PKA activator forskolin, led to a dose- and time-dependent increase in PSA mRNA levels[43]. Again such responses were blocked by the AR-antagonist bicalutaminde. Intriguingly, Blok and colleagues[44, 45] reported that rather than increasing AR phosphorylation, PKA activation, in fact, led to a decrease in AR phosphorylation, specifically on residues Ser 641 and 654. Although unusual, decreased protein phosphorylation upon PKA activation is not without precedent. For example, ribosomal protein S6 and the retinoblastoma gene product (Rb) are dephosphorylated following PKA activation[46,47]. The rapid nature of the PKA dependent AR dephosphorylation suggests the actions of an ancillary phosphatase. Indeed the actions of a few phosphatases are known to be regulated by PKA activation. For example, the nuclear protein phosphatase-1 (PP-1N) is activated by PKA induced phosphorylation of nuclear inhibitor of protein phosphatase-1 (NIPP-1)[48]. Dephosphorylation of AR may involve this phosphatase, or another similarly regulated phosphatase. A potential reconciliation of the PKA data suggests that dephosphorylated non-liganded AR may be transcriptionally active (i.e., ligand independent activation). The discrepancy between the data of Blok and Nazareth, may unfortunately also reflect a cellular model difference (CV1 versus LNCaP) and differences in reporter genes (adenoviral-mediated DNA transfer versus endogenous genes).
Lemur Tyrosine Kinase-2
Lemur Tyrosine Kinase-2 (LMTK2), also known as BREK, KPI2, LMR2, AATYK2 and PPP1R100 is a member of the membrane-associated protein tyrosine kinase family[49, 50]. Although being classified as a tyrosine kinase by gene alignment, LMTK2 was found to phosphorylate only serine or threonine residues on protein substrates[51]. Despite being discovered a decade ago, researchers have just started focusing on the physiological importance of LMTK2. Renewed interest in this kinase, primarily stems from several Genome-Wide Analysis Studies (GWAS) showing a significant (P<0.0001) association between a genetic variant of LMTK2 with an intron 9 SNP and susceptibility to prostate cancer[52–55]. Furthermore, a comparative study of LMTK2 mRNA levels in tissue from patients prostate cancer and Benign Prostatic Hyperplasia (BPH) revealed a markedly reduced level of LMTK2 in prostate cancer patients[53]. Recently, using immunohistochemistry we demonstrated that LMTK2 protein levels are also down-regulated in human prostate cancer tissue in comparison to adjacent non-cancerous tissue or tissue from patients with BPH[31]. Furthermore, we identified AR to be a binding partner of LMTK2 in prostate cancer epithelial cells. Interestingly, while LMTK2 interacts with AR primarily in the cytoplasm of cells deprived of androgens, such interactions were also found in the nucleus of cells grown in the presence of synthetic androgen, R1881 suggesting that LMTK2 interacts with AR in presence and absence of androgens. Furthermore, prostate cancer cells transfected with shRNA against LMTK2 (LMTK2-KD) showed significantly higher AR activity in the presence and absence of ligand, as measured by mRNA and protein levels of several AR-dependent genes and an AR-dependent luciferase assay. Collectively, these data suggests that LMTK2 negatively regulates AR-transcriptional activity in prostate epithelia, and loss of LMTK2, as occurs in prostate cancer, can lead to an increase in AR activity. One of the important findings from this study was the role of LMTK2 in CRPC. FKBP51, an AR-dependent gene, which is also a positive feedback regulator of AR expression, is expressed two-fold higher in CRPC compared to primary tumors[56, 57]. LMTK2-KD prostate cancer cells deprived of androgen had significantly higher levels of FKBP51 in comparison to cells expressing normal levels of LMTK2. These results provide strong evidence of a role for LMTK2 in pathogenesis and progression of prostate cancer. In addition, cell viability data from the study argues for the strongest role of the decrease in LMTK2 in regards to androgen-independent growth in prostate cancer cells, androgen-dependent growth was also affected, although to a lesser degree.
Mechanisms though which LMTK2 might mediate its effect on AR are not yet known. In fact, very little is known in terms of signaling molecules upstream to LMTK2. Recently, Christopher Miller’s group showed that CDK5/p35 phosphorylates LMTK2 at serine-1418, enabling LMTK2 to phosphorylate protein phosphatase 1 (PP1C) at threonine-320 and inactivate the phosphatase[58]. PP1C is an important phosphatase regulating a diverse array of function, including AR. PP1C can negatively regulate the AR-transcriptional activity by dephosphorylating AR at serine-650[59]. Hence, it is within the realm of possibility that LMTK2 might be regulating AR by regulating the activity of phosphatase. In terms of potential therapeutic target, small molecules that enhance the activity of LMTK2 can decrease AR-proliferative activity in patients with prostate cancer and more importantly with castrate resistant prostate cancer.
Concluding Remarks
The study of the pathways by which AR is regulated in the normal prostate as well as prostate cancer tissue has led to the discovery of several new pathways overlapping between the fields of endocrinology and oncology. Here, we have reviewed a few recently discovered kinase pathways, which play an important role in the development of prostate cancer and its progression to CRPC. Furthermore, we also provide a list of kinases that are known to regulate AR. Clearly, there is still a lot to understand about AR regulation through phosphorylation. Moreover, the requisite phosphatases required to reverse phosphorylation events have barely been elucidated. Upstream regulators for many kinases remain unknown and so does the full biological significance of AR phosphorylation in the context of normal and cancerous physiology of prostate epithelial cells. We do not believe that AR-kinase pathways are the only pathways leading to CRPC and, no doubt, further studies will reveal additional pathways. It is highly likely that not all the CRPC patients have dysregulation of the same pathways; hence an effective therapy of CRPC will require that therapy address each patient individually. Nonetheless, the plethora of kinases that impinge upon AR to regulate its signaling provide many potential novel therapeutic targets to treat the roughly 50% of the population that will develop some form of prostate abnormalities with age, and more specifically for those individuals who develop malignant CRPC.
Acknowledgments
This work was supported by the National Institutes of Health (1R01HL102208-01A1) and Cystic Fibrosis Foundation grants (BRADBU12XX0), awarded to Bradbury N.A.
Footnotes
Conflicting interests
The authors have declared that no competing interests exist.
References
- 1.United States Cancer Statistics (USCS) Centers for Disease Control and Prevention. 2012 [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W. SEER Cancer Statistics Review, 1975–2010. National Cancer Institute; Bethesda, MD: 2013. based on November 2012 SEER data submission, posted to the SEER web site. http//seercancergov/csr/1975_2010. [Google Scholar]
- 3.Dehm SM, Tindall DJ. Molecular regulation of androgen action in prostate cancer. J Cell Biochem. 2006;99:333–344. doi: 10.1002/jcb.20794. [DOI] [PubMed] [Google Scholar]
- 4.Roy AK, Lavrovsky Y, Song CS, Chen S, Jung MH, Velu NK, et al. Regulation of Androgen Action. Vitam Horm. 1998;55:309–352. doi: 10.1016/s0083-6729(08)60938-3. [DOI] [PubMed] [Google Scholar]
- 5.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 6.Huggins C, Stevens RE, Hodges CV. The effects of castration on advanced carcinoma of the prostate gland. JAMA Surg. 1941;43:209–223. [Google Scholar]
- 7.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 8.Smaletz O, Scher HI. Outcome predictions for patients with metastatic prostate cancer. Semin Oncol. 2002;20:155–163. doi: 10.1053/suro.2002.32938. [DOI] [PubMed] [Google Scholar]
- 9.Amler LC, Agus DB, LeDuc C, Sapinoso ML, Fox WD, Kern S, et al. Dysregulated expression of androgen-responsive and nonresponsive genes in the androgen-independent prostate cancer xenograft model CWR22-R. Cancer Res. 2000;60:6134–6141. [PubMed] [Google Scholar]
- 10.Sirotnak FM, She Y, Khokhar NZ, Hayes P, Gerald W, Scher HI. Microarray analysis of prostate cancer progression to reduced androgen dependence: Studies in unique models contrasts early and late molecular events. Mol Carcinog. 2004;41:150–163. doi: 10.1002/mc.20051. [DOI] [PubMed] [Google Scholar]
- 11.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 12.Hammes A, Andreassen TK, Spoelgen R, Raila J, Hubner N, Schulz H, et al. Role of endocytosis in cellular uptake of sex steroids. Cell. 2005;122:751–762. doi: 10.1016/j.cell.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal S, Thareja S, Verma A, Bhardwaj TR, Kumar M. An overview on 5alpha-reductase inhibitors. Steroids. 2010;75:109–153. doi: 10.1016/j.steroids.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Zhu YS, Imperato-McGinley JL. 5α-reductase isozymes and androgen actions in the prostate. Ann N Y Acad Sci. 2009;1155:43–56. doi: 10.1111/j.1749-6632.2009.04115.x. [DOI] [PubMed] [Google Scholar]
- 15.Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ, et al. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987;8:338–362. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- 16.Lyon MF, Hawkes SG. X-linked gene for testicular feminization in the mouse. Nature. 1970;227:1217–1219. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- 17.Quigley CA, De Bellis A, Marschke KB, El-Awady MK, Wilson EM, French FS. Androgen receptor defects: Historical, clinical, and molecular perspectives. Endocr Rev. 1995;16:271–321. doi: 10.1210/edrv-16-3-271. [DOI] [PubMed] [Google Scholar]
- 18.Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A. 2002;99:13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Marzo AM, Nelson WG, Meeker AK, Coffey DS. Stem cell features of benign and malignant prostate epithelial cells. J Urol. 1998;160:2381–2392. doi: 10.1097/00005392-199812020-00004. [DOI] [PubMed] [Google Scholar]
- 20.Isaacs JT. Antagonistic effect of androgen on prostatic cell death. Prostate. 1984;5:545–557. doi: 10.1002/pros.2990050510. [DOI] [PubMed] [Google Scholar]
- 21.Berges RR, Vukanovic J, Epstein JI, CarMichel M, Cisek L, Johnson DE, et al. Implication of cell kinetic changes during the progression of human prostatic cancer. Clin Cancer Res. 1995;1:473–480. [PMC free article] [PubMed] [Google Scholar]
- 22.Veldscholte J, Berrevoets CA, Zegers ND, van der Kwast TH, Grootegoed JA, Mulder E. Hormone-induced dissociation of the androgen receptor-heat-shock protein complex: use of a new monoclonal antibody to distinguish transformed from nontransformed receptors. Biochemistry. 1992;31:7422–7430. doi: 10.1021/bi00147a029. [DOI] [PubMed] [Google Scholar]
- 23.He B, Bai S, Hnat AT, Kalman RI, Minges JT, Patterson C, et al. An androgen receptor NH2-terminal conserved motif interacts with the COOH terminus of the Hsp70-interacting protein (CHIP) J Biol Chem. 2004;279:30643–30653. doi: 10.1074/jbc.M403117200. [DOI] [PubMed] [Google Scholar]
- 24.Fang Y, Fliss AE, Robins DM, Caplan AJ. Hsp90 regulates androgen receptor hormone binding affinity in vivo. J Biol Chem. 1996;271:28697–28702. doi: 10.1074/jbc.271.45.28697. [DOI] [PubMed] [Google Scholar]
- 25.Van der Steen T, Tindall DJ, Huang H. Posttranslational modification of the androgen receptor in prostate cancer. Int J Mol Cell Med. 2013;14:14833–14859. doi: 10.3390/ijms140714833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Laar JH, Bolt-De Vries J, Zegers ND, Trapman J, Brinkmann AO. Androgen receptor heterogeneity and phosphorylation in human LNCaP cells. Biochem Biophys Res Commun. 1990;166:193–200. doi: 10.1016/0006-291x(90)91930-q. [DOI] [PubMed] [Google Scholar]
- 27.Van Laar JH, Berrevoets CA, Trapman J, Zegers ND, Brinkmann AO. Hormone-dependent androgen receptor phosphorylation is accompanied by receptor transformation in human lymph node carcinoma of the prostate cells. J Biol Chem. 1991;266:3734–3738. [PubMed] [Google Scholar]
- 28.Gordon V, Bhadel S, Wunderlich W, Zhang J, Ficarro SB, Mollah SA, et al. CDK9 regulates AR promoter selectivity and cell growth through serine 81 phosphorylation. Mol Endocrinol. 2010;24:2267–2280. doi: 10.1210/me.2010-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S, Xu Y, Yuan X, Bubley GJ, Balk SP. Androgen receptor phosphorylation and stabilization in prostate cancer by cyclin-dependent kinase 1. Proc Natl Acad Sci U S A. 2006;103:15969–15974. doi: 10.1073/pnas.0604193103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zardan1 A, Nip1 K, Thaper1 D, Toren1 P, Vahid1 S, Beraldi1 E, et al. Lyn tyrosine kinase regulates androgen receptor expression and activity in castrate-resistant prostate cancer. Oncogenesis. 2014;3:e115. doi: 10.1038/oncsis.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah K, Bradbury N. Lemur Tyrosine Kinase 2, a novel target in prostate cancer therapy. Oncotarget. 2015;6:14233–14246. doi: 10.18632/oncotarget.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 33.Whitmarsh AJ, Davis RJ. Role of mitogen-activated protein kinase kinase 4 in cancer. Oncogene. 2007;26:3172–3184. doi: 10.1038/sj.onc.1210410. [DOI] [PubMed] [Google Scholar]
- 34.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 35.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 36.Fuchs SY, Tappin I, Ronai Z. Stability of the ATF2 transcription factor is regulated by phosphorylation and dephosphorylation. J Biol Chem. 2000;275:12560–12564. doi: 10.1074/jbc.275.17.12560. [DOI] [PubMed] [Google Scholar]
- 37.Gille H, Strahl T, Shaw PE. Activation of ternary complex factor Elk-1 by stress-activated protein kinases. Curr Biol. 1995;5:1191–1200. doi: 10.1016/s0960-9822(95)00235-1. [DOI] [PubMed] [Google Scholar]
- 38.Gioeli D, Black BE, Gordon V, Spencer A, Kesler CT, Eblen ST, et al. Stress kinase signaling regulates androgen receptor phosphorylation, transcription, and localization. Mol Endocrinol. 2006;20:503–515. doi: 10.1210/me.2005-0351. [DOI] [PubMed] [Google Scholar]
- 39.Lotan TL, Lyon M, Huo D, Taxy JB, Brendler C, Foster BA. Up-regulation of MKK4, MKK6 and MKK7 during prostate cancer progression: an important role for SAPK signalling in prostatic neoplasia. Cancer Res. 2007;212:386–394. doi: 10.1002/path.2194. [DOI] [PubMed] [Google Scholar]
- 40.Bolen JB, Rowley RB, Spana C, Tsygankov AY. The Src family of tyrosine protein kinases in hemopoietic signal transduction. FASEB J. 1992;6:3403–3409. doi: 10.1096/fasebj.6.15.1281458. [DOI] [PubMed] [Google Scholar]
- 41.Goldenberg-Furmanov M, Stein I, Pikarsky E, Rubin H, Kasem S, Wygoda M, et al. Lyn Is a Target Gene for Prostate Cancer: Sequence-Based Inhibition Induces Regression of Human Tumor Xenografts. Cancer Res. 2004;64:1058–1066. doi: 10.1158/0008-5472.can-03-2420. [DOI] [PubMed] [Google Scholar]
- 42.Nazareth LV, Weigel NL. Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem. 1996;271:19900–19907. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- 43.Sadar MD. Androgen-independent induction of prostate-specific antigen gene expression via cross-talk between the androgen receptor and protein kinase A signal transduction pathways. J Biol Chem. 1999;274:7777–7783. doi: 10.1074/jbc.274.12.7777. [DOI] [PubMed] [Google Scholar]
- 44.Blok LJ, de Ruiter PE, Brinkmann AO. Androgen receptor phosphorylation. Endocr Res. 1996;22:197–219. doi: 10.3109/07435809609030508. [DOI] [PubMed] [Google Scholar]
- 45.Blok LJ, De Ruiter PE, Brinkrnann AO. Forskolin-induced dephosphorylation of the androgen receptor impairs ligand binding. Biochemistry. 1998;37:3850–3857. doi: 10.1021/bi9724422. [DOI] [PubMed] [Google Scholar]
- 46.Christoffersen J, Smeland EB, Stokke T, Taskén K, Andersson KB, Blomhoff HK. Retinoblastoma protein is rapidly dephosphorylated by elevated cyclic adenosine monophosphate levels in human B-lymphoid cells. Cancer Res. 1994;54:2245–2250. [PubMed] [Google Scholar]
- 47.Hara-Yokoyama M, Sugiya H, Furuyama S, Wang JH, Yokoyama N. Dephosphorylation of ribosomal protein S6 phosphorylated via the cAMP-mediated signaling pathway in rat parotid gland: effect of okadaic acid and Zn2+ Biochem Mol Biol Int. 1994;34:1177–1187. [PubMed] [Google Scholar]
- 48.Beullens M, Van Eynde A, Bollen M, Stalmans W. Inactivation of nuclear inhibitory polypeptides of protein phosphatase-1 (NIPP-1) by protein kinase A. J Biol Chem. 1993;268:13172–13177. [PubMed] [Google Scholar]
- 49.Nixon A, Jia Y, White C, Bradbury NA. Determination of the membrane topology of lemur tyrosine kinase 2 (LMTK2) by fluorescence protease protection. Am J Physiol Cell Physiol. 2013;304:C164–C169. doi: 10.1152/ajpcell.00288.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang BD. A novel transmembrane Ser/Thr kinase complexes with protein phosphatase-1. J Biol Chem. 2002;277:49605–49612. doi: 10.1074/jbc.M209335200. [DOI] [PubMed] [Google Scholar]
- 51.Wang H, Brautigan DL. Peptide microarray analysis of substrate specificity of the transmembrane Ser/Thr kinase KPI-2 reveals reactivity with cystic fibrosis transmembrane conductance regulator and phosphorylase. Mol Cell Proteomics. 2006;5:2124–2130. doi: 10.1074/mcp.M600188-MCP200. [DOI] [PubMed] [Google Scholar]
- 52.Shui IM, Lindström S, Kibel AS, Berndt SI, Campa D, Gerke T, et al. Prostate cancer (PCa) risk variants and risk of fatal PCa in the national cancer institute breast and prostate cancer cohort consortium. Eur Urol. 2014;65:1069–1075. doi: 10.1016/j.eururo.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harries LW, Perry JRB, McCullagh P, Crundwell M. Alterations in LMTK2, MSMB and HNF1B gene expression are associated with the development of prostate cancer. BMC Cancer. 2010;10:315. doi: 10.1186/1471-2407-10-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eeles RA, Kote-Jarai Z, Giles GG, Olama AA, Guy M, Jugurnauth SK, et al. Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet. 2008;40:316–321. doi: 10.1038/ng.90. [DOI] [PubMed] [Google Scholar]
- 55.Guy M, Kote-Jarai Z, Giles GG, Al Olama AA, Jugurnauth SK, Mulholland S, et al. Identification of new genetic risk factors for prostate cancer. Asian J Androl. 2009;11:49–55. doi: 10.1038/aja.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Febbo PG, Lowenberg M, Thorner AR, Brown M, Loda M, Golub TR. Androgen mediated regulation and functional implications of fkbp51 expression in prostate cancer. J Urol. 2005;173:1772–1777. doi: 10.1097/01.ju.0000155845.44729.ba. [DOI] [PubMed] [Google Scholar]
- 57.Ni L, Yang CS, Gioeli D, Frierson H, Toft DO, Paschal BM. FKBP51 promotes assembly of the Hsp90 chaperone complex and regulates androgen receptor signaling in prostate cancer cells. Mol Cell Biol. 2010;30:1243–1253. doi: 10.1128/MCB.01891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manser C, Vagnoni A, Guillot F, Davies J, Miller CCJ. Cdk5/p35 phosphorylates lemur tyrosine kinase-2 to regulate protein phosphatase-1C phosphorylation and activity. J Neurochem. 2012;121:343–348. doi: 10.1111/j.1471-4159.2012.07650.x. [DOI] [PubMed] [Google Scholar]
- 59.Chen S, Kesler CT, Paschal BM, Balk SP. Androgen receptor phosphorylation and activity are regulated by an association with protein phosphatase 1. J Biol Chem. 2009;284:25576–25584. doi: 10.1074/jbc.M109.043133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willder JM, Heng SJ, McCall P, Adams CE, Tannahill C, Fyffe G, et al. Androgen receptor phosphorylation at serine 515 by Cdk1 predicts biochemical relapse in prostate cancer patients. Br J Cancer. 2013;108:139–148. doi: 10.1038/bjc.2012.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin HK, Yeh S, Kang HY, Chang C. Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc Natl Acad Sci U S A. 2001;98:7200–7205. doi: 10.1073/pnas.121173298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo Z, Dai B, Jiang T, Xu K, Xie Y, Kim O, et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10:309–319. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 63.Hsu FN, Chen MC, Chiang MC, Lin E, Lee YT, Huang PH, et al. Regulation of androgen receptor and prostate cancer growth by cyclin-dependent kinase 5. J Biol Chem. 2011;286:33141–33149. doi: 10.1074/jbc.M111.252080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lindqvist J, Julia, Susumu YI, Elin T, Marjo M, Mika R, et al. Cyclin-dependent kinase 5 acts as a critical determinant of AKT-dependent proliferation and regulates differential gene expression by the androgen receptor in prostate cancer cells. Mol Biol Cell. 2015;26:1971–1984. doi: 10.1091/mbc.E14-12-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jain G, Voogdt C, Tobias A, Spindler KD, Möller P, Cronauer MV, et al. IκB kinases modulate the activity of the androgen receptor in prostate carcinoma cell lines. Neoplasia. 2012;14:178–189. doi: 10.1593/neo.111444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ha S, Iqbal NJ, Mita P, Ruoff R, Gerald WL, Lepor H, et al. Phosphorylation of the androgen receptor by PIM1 in hormone refractory prostate cancer. Oncogene. 2013;32:3992–4000. doi: 10.1038/onc.2012.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shu SK, Liu Q, Coppola D, Cheng JQ. Phosphorylation and activation of androgen receptor by Aurora-A. J Biol Chem. 2010;285:33045–33053. doi: 10.1074/jbc.M110.121129. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Zong H, Chi Y, Wang Y, Yang Y, Zhang L, Chen H, et al. Cyclin D3/CDK11p58 complex is involved in the repression of androgen receptor. Mol Cell Biol. 2007;27:7125–7142. doi: 10.1128/MCB.01753-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chymkowitch P, Le May N, Charneau P, Compe E, Egly JM. The phosphorylation of the androgen receptor by TFIIH directs the ubiquitin/proteasome process. EMBO J. 2011;30:468–479. doi: 10.1038/emboj.2010.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Ruiter PE, Teuwen R, Trapman J, Dijkema R, Brinkmann AO. Synergism between androgens and protein kinase-C on androgen-regulated gene expression. Mol Cell Endocrinol. 1995;110:R1–R6. doi: 10.1016/0303-7207(95)03534-e. [DOI] [PubMed] [Google Scholar]
- 71.Andrews PE, Young CY, Montgomery BT, Tindall DJ. Tumor-promoting phorbol ester down-regulates the androgen induction of prostate-specific antigen in a human prostatic adenocarcinoma cell line. Cancer Res. 1992;52:1525–1529. [PubMed] [Google Scholar]
- 72.Liu T, Li Y, Gu H, Zhu G, Li J, Cao L, et al. P21-Activated kinase 6 (PAK6) inhibits prostate cancer growth via phosphorylation of androgen receptor and tumorigenic E3 ligase murine double minute-2 (MDM2) J Biol Chem. 2013;288:3359–3369. doi: 10.1074/jbc.M112.384289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mahajan K, Coppola D, Rawal B, Chen YA, Lawrence HR, Engelman RW, et al. Ack1 mediated androgen receptor phosphorylation modulates radiation resistance in castration resistant prostate cancer. J Biol Chem. 2012;287:22112–22122. doi: 10.1074/jbc.M112.357384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rocha J, Zouanat FZ, Zoubeidi A, Hamel L, Benidir T, Scarlata E, et al. The Fer tyrosine kinase acts as a downstream interleukin-6 effector of androgen receptor activation in prostate cancer. Mol Cell Endocrinol. 2013;381:140–149. doi: 10.1016/j.mce.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 75.Salas TR, Kim J, Vakar-Lopez F, Sabichi AL, Troncoso P, Jenster G, et al. Glycogen Synthase Kinase-3{beta} Is Involved in the Phosphorylation and Suppression of Androgen Receptor Activity. J Biol Chem. 2004;279:19191–19200. doi: 10.1074/jbc.M309560200. [DOI] [PubMed] [Google Scholar]
- 76.Leister P, Felten A, Chasan AI, Scheidtmann KH. ZIP kinase plays a crucial role in androgen receptor-mediated transcription. Oncogene. 2008;27:3292–3300. doi: 10.1038/sj.onc.1210995. [DOI] [PubMed] [Google Scholar]


