Abstract
Background:
Metastases represent a small percentage of the malignancies affecting the breast, and only 5% of melanomas originate from non-cutaneous sites. Multiple genetic aberrations have been associated with the development of melanocytic lesions, including BRAF V600E mutation. Mutations in PTEN gene have also been related to the pathogenesis of multiple malignancies.
Purpose/Method:
This is the case of a 28-year-old female who presented with a tender, palpable mass in the upper outer quadrant of the right breast. Ultrasound showed a 1-cm solid mass, initially diagnosed as invasive ductal carcinoma on biopsy. During pre-operative workup, a second mass was identified and biopsied. Immunohistochemical stains performed on the second mass biopsy demonstrated that the neoplastic cells were positive for cytokeratin AE1/3, pan-melanoma, tyrosinase, and SOX-10 and negative for CK7, CAM5.2, and GATA-3. Subsequent workup showed widespread metastatic disease involving the liver, lungs, bones, and brain. The brain metastasis tested positive for BRAF p.V600E and PTEN p.R130Efs*4 mutations. Thorough skin and eye examination did not reveal a primary melanoma.
Conclusion:
Only few reports have been published of melanoma presenting as a breast mass. This is an interesting case due to the clinical presentation, diagnostic challenges, and genetic mutations profile.
Keywords: Melanoma, carcinoma, breast, BRAF, PTEN
Introduction
It is estimated that there will be approximately 76,380 new diagnoses and 10,130 deaths due to melanoma in the United States during 2016.1 Although melanoma of the skin is frequently diagnosed among people in the sixth to seventh decade, 5.9% of the cases occur in patients aged 20–34 years.1 Metastasis of melanoma is more frequently located in the lungs, liver, central nervous system, and bones.2
Metastases to the breast represent less than 1.3% of breast malignancies.3,4 The most common primary tumors include hematologic malignancies, melanomas, and carcinomas of lungs and ovaries, among others.3,5,6 Even though melanomas metastasizing to the breast have been widely described, they typically present after a diagnosis of primary cutaneous melanoma. It is rare to diagnose melanoma presenting initially as a breast mass without a known previous lesion.
Multiple genetic alterations are associated with the development of melanoma. BRAF is the most commonly mutated gene present in approximately 37%–50% of melanomas.7,8 The most frequent BRAF mutation is a T > A transversion, a substitution at nucleotide 1799 that causes a valine to glutamic acid change in codon 600 at exon 15 (p.V600E). The V600E mutation accounts for 80% of BRAF mutations in melanoma.7,8 Neuroblastoma RAS viral oncogene homologue (NRAS) is the second most commonly mutated oncogene in melanoma present in 13%–25% of cases.7,9 Mutations in these two genes are considered drivers of melanoma development since they can be found with similar frequencies in benign melanocytic lesions.10 In addition, mutations in the telomerase reverse transcriptase (TERT) gene promoter have been identified in primary and metastatic melanoma with a frequency of 33% and 85%, respectively.11 Other genetic changes, including tumor suppressor gene alterations, can be found with lesser frequency.
Herein, we report the case of a female patient presenting with breast masses diagnosed as melanoma, with no evidence of cutaneous or ocular lesions. In addition, this neoplasm proved to be positive for concomitant BRAF and PTEN mutations.
Case report
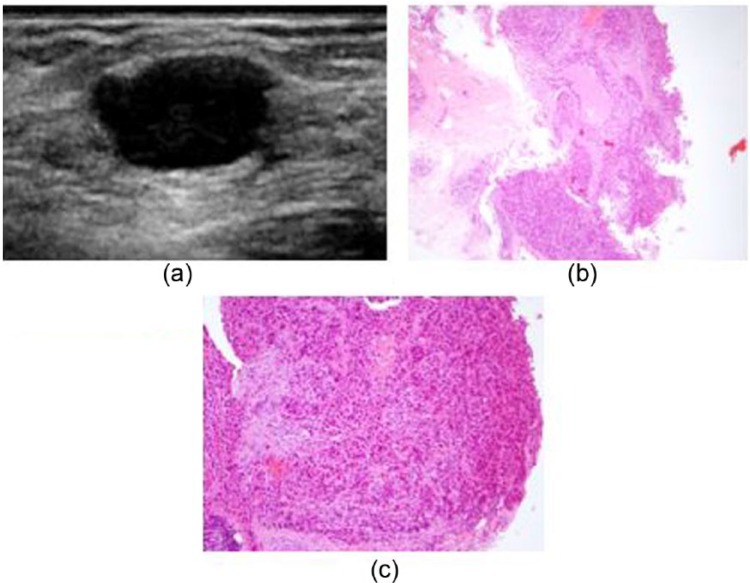
A 28-year-old Caucasian female presented to our institution with a 1-month history of a mildly tender, palpable lump in the upper outer quadrant of the right breast. The patient did not have any personal or family history of breast carcinoma. Her past medical history was only significant for prior biopsies of benign skin nevi. Targeted ultrasound of the area of palpable concern in the right breast upper outer quadrant demonstrated a corresponding 1-cm solid mass (Figure 1(a)). A core needle biopsy was performed. Histologically, tumor was characterized by sheets and nests of highly pleomorphic, mitotically active neoplastic cells infiltrating the breast parenchyma, with focal comedonecrosis (Figure 1(b) and (c)). An initial diagnosis of poorly differentiated breast carcinoma was made. Further studies showed the tumor to be negative estrogen receptor, progesterone receptor, and HER-2/neu oncogene, consistent with a triple-negative invasive ductal carcinoma.
Figure 1.
(a) Ultrasound demonstrated a solid mass at the site of palpable concern in the right breast upper outer quadrant. Core biopsy showed sheets of (b) high-grade neoplastic cells, with (c) focal comedonecrosis.
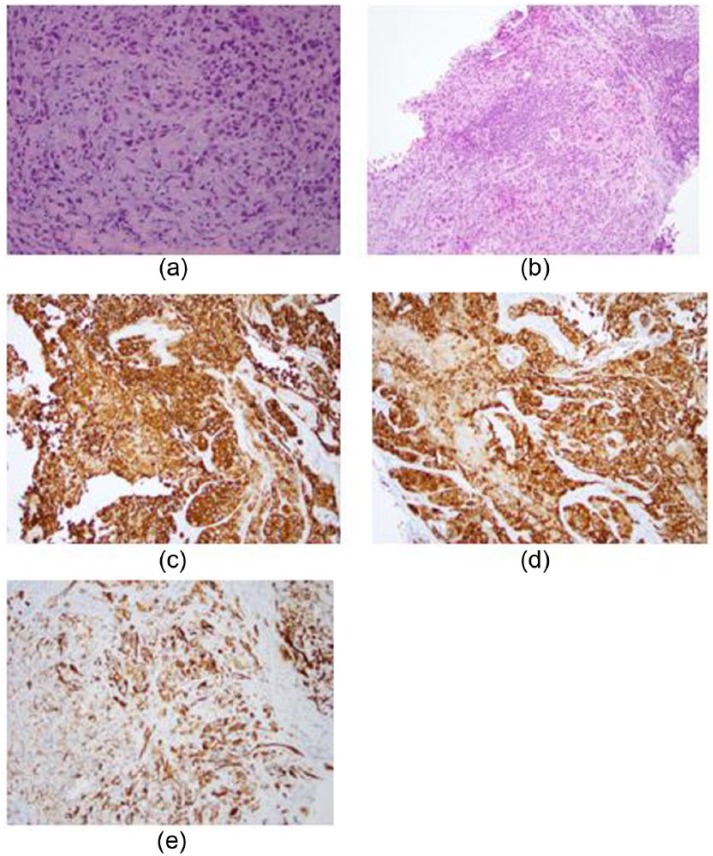
During pre-operative workup, an additional solid mass was identified in the inferior right breast. Core needle biopsy of this second mass showed cytologically similar neoplastic cells, with a prominent single-cell growth pattern. In addition, the tumor appeared to be well circumscribed with a thin rim of lymphoid tissue on one edge, suggestive of a replaced intra-parenchymal lymph node (Figure 2(a) and (b)). At this point, considering the patient’s age and multi-centricity of the disease, together with the somewhat unusual growth pattern of neoplastic cells in the second biopsy specimen, immunohistochemical stains were performed. The neoplastic cells were positive for cytokeratin AE1/3 (patchy), pan-melanoma, tyrosinase, and SOX-10 and negative for CK7, CAM5.2, and GATA-3 (Figure 2(c)–(e)). The same immunohistochemical panel was then performed on the tissue specimen from the first mass, with similar results. Therefore, the initial diagnosis of breast ductal carcinoma was revised to melanoma. In light of this diagnosis, the patient’s prior skin nevi biopsy results were reviewed and the absence of malignancy confirmed. In addition, thorough skin and eye examination failed to reveal any evidence of primary melanoma.
Figure 2.
Biopsy of the second mass featuring diffusely infiltrating pleomorphic tumor cells (a) with a prominent single-cell growth pattern and (b) with a thin rim of lymphoid tissue suggestive of an underlying lymph node structure. (a and b) Diffuse staining with (c) pan-melanoma and (d) tyrosinase and patchy staining with (e) AE1/3 cytokeratin immunohistochemical stains.
Subsequent positron emission tomography–computed tomography (PET/CT) scan showed widespread metastatic disease to liver, lungs, and bones. Brain magnetic resonance imaging (MRI) demonstrated left temporal lobe metastasis, which was also histologically confirmed (Figure 2(b)). Genetic counseling was recommended, and the patient was tested with a gene panel for hereditary cancers, which was negative for germ line mutations. Targeted next-generation sequencing was performed, which revealed that the neoplastic cells harbored somatic BRAF p.V600E and PTEN p.R130Efs*4 mutations. Of note, TERT gene promoter mutation analysis was not performed.
In view of her advanced disease, the patient was enrolled in a clinical trial and was started on a combination of ipilimumab plus nivolumab, which was later changed to a combination of dabrafenib plus trametinib due to severe neurotoxicity (quadriparesis). This second chemotherapy course was complicated by severe neutropenia and hemorrhagic transformation of a temporal lobe lesion requiring craniotomy. Unfortunately, her disease continued to progress, and she died 8 months after the initial diagnosis.
Discussion
Malignant melanoma is known for its aggressive clinical behavior and propensity for metastasis. Approximately 5% of melanomas originate primarily from non-cutaneous sites.12 There have been several reports of melanomas arising from the skin of the breast.13–15 In retrospective studies, melanoma has been found to account for up to 38.5% of metastasis from solid neoplasms affecting the breast.6,16–18 However, only few cases have been described in the literature of melanoma presenting as a breast mass without evidence of a primary cutaneous lesion.
Biswas et al. reported the case of a 32-year-old female who presented with a 4-cm lower inner quadrant lump in the left breast. Diagnosis of melanoma was made by fine-needle aspiration biopsy.19 Physical examination failed to reveal any primary cutaneous lesions, and metastatic workup showed no evidence of systemic metastasis. The diagnosis was confirmed on wide local surgical excision. Only 1 out of 13 lymph nodes was positive for metastatic melanoma. The patient received radiotherapy and immunotherapy and was reported to be alive 9 months after surgery.19 Similar cases of melanoma affecting the breast without demonstrable cutaneous lesions or systemic metastasis have been described, predominantly in females aged 45–56 years old.12,20 No molecular studies were performed on any of these previously described cases.
Presentation of widespread metastatic melanoma as breast masses is very rare. Our case was originally diagnosed as poorly differentiated breast carcinoma, with completely different therapeutic implications. Expression of breast carcinoma tumor markers and therefore tumor molecular subtypes classification have been associated with important clinical and prognostic features. Triple-negative breast carcinomas have been identified as one of the factors associated with metastasis and shorter overall survival (hazard ratio = 2.24) in breast cancer patients.21 Triple-negative breast tumors have also been associated with black race and older age at diagnosis.21 Triple-negative carcinoma were recently reported to be diagnosed in 22.2% of patients younger than 35 years.22 It was also found that in the younger patients, triple-negative breast carcinoma cases may have a shorter disease-free survival but no significant difference in overall survival when compared to older patients.22
Another interesting fact in our case is the presence of concurrent BRAF and PTEN somatic mutations. PTEN is a tumor suppressor gene located in chromosome 10q23.23,24 It modulates cell cycle progression and survival by regulating phosphoinositide-3-kinase (PI3K) and the protein-SER/Thr kinase (AKT) signaling pathway.25,26 PTEN mutations have been described in advanced stages of glioblastomas, prostate, and breast carcinomas, among other neoplasms.27,28 This tumor suppressor gene has been implicated in the etiology of Cowden syndrome, which shows an inherited predisposition for breast and thyroid carcinomas in the affected patients.29 Mutations and deletions of PTEN have been reported in melanoma cell lines as well as uncultured melanomas. A higher incidence of PTEN mutations have been reported in melanoma cell lines than primary melanomas and metastatic melanoma biopsies, with a frequency of 27.6%, 7.3%, and 15.2%, respectively.30 Celibi et al.31 observed loss of heterozygosity of PTEN gene in 7 out of 21 (33%) melanoma cases.
BRAF is a proto-oncogene located in chromosome 7q34 that helps transmit extracellular signals to the cell nucleus as part of the RAS/mitogen-activated protein kinase (MAPK) pathway.32 This pathway controls several important cell functions such as cell growth and division. BRAF mutational activation is detected in more than 50% of patients affected by melanoma and is one of the earliest and most common genetic alterations in its development.8 BRAF mutations are also detected in benign nevi and other non-malignant melanocytic lesions.33 Besides the RAS/MAPK pathway, PI3K, Rho GTPases, JAK-STAT, and WNT/B-catenin pathways are also contributors to melanoma progression.10
Malignant progression of BRAF p.V600E–expressing melanocytes is frequently promoted by silencing of the tumor suppressor PTEN.34 Dankort et al.35 demonstrated that the induction of BRAF p.V600E expression in mice, combined with PTEN tumor suppressor gene silencing, elicited development of melanomas and also predisposed to metastasis. This phenotype was not observed in BRAF p.V600E–induced mice in the absence of PTEN alterations.
Our case is interesting due to the unusual clinical presentation (a breast mass in a woman with no other known primary malignancies) and diagnostic challenge it posed from the diagnostic standpoint (sheets of non-pigmented epithelioid cells with focal comedonecrosis). These unusual histologic features were further compounded by keratin positivity of the neoplastic cells, which is known to occur in only approximately 5% of melanomas.36 Furthermore, targeted next-generation sequencing analysis showed concurrent a BRAF and PTEN mutation profile, making the case even more interesting and unique. Only few reports have been published of malignant melanomas presenting as a breast mass, and to our knowledge, none has shown concurrent BRAF and PTEN mutations.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
References
- 1. Howlader N, Noone AM, Krapcho M, et al. (eds). SEER cancer statistics review, 1975–2013. Bethesda, MD: National Cancer Institute, http://seer.cancer.gov/csr/1975_2013/ [Google Scholar]
- 2. De Vries E, Bray F, Coebergh JW, et al. Malignant melanoma:introduction. In: LeBoit PE, Burg G, Weedon D, et al. (eds) World Health Organization classification of tumors: pathology and genetics skin tumors. Lyon: IARC Press, 2006, p. 52. [Google Scholar]
- 3. Lee A, Sahin A. Metastases of extramammary malignancies to the breast. In: Lakhani SR, Ellis IO, Schnitt SJ, et al. (eds) World Health Organization classification of tumors of the breast. 4th ed. Lyon: IARC Press, 2012, p. 162. [Google Scholar]
- 4. Alvarado Cabrero I, Carrera Alvarez M, Perez Montiel D, et al. Metastasis to the breast. Eur J Surg Oncol 2003; 29: 854. [DOI] [PubMed] [Google Scholar]
- 5. Alva S, Shetty-Alva N. An update of tumor metastasis to the breast data. Arch Surg 1999; 134: 450. [DOI] [PubMed] [Google Scholar]
- 6. Williams SA, Ehlers RA, Hunt KK, et al. Metastases to the breast from non-breast solid neoplasms: presentations and determinants of survival. Cancer 2007; 110: 731–737. [DOI] [PubMed] [Google Scholar]
- 7. My Cancer Genome. Molecular profiling of melanoma, https://www.mycancergenome.org/content/disease/melanoma/ (accessed 15 September 2016).
- 8. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417: 949–954. [DOI] [PubMed] [Google Scholar]
- 9. Ball NJ, Yohn JJ, Morelli JG, et al. RAS mutations in human melanoma: a marker of malignant progression. J Invest Dermatol 1994; 102(3): 285–290. [DOI] [PubMed] [Google Scholar]
- 10. Timar J, Vizkeleti L, Doma V, et al. Genetic progression of malignant melanoma. Cancer Metastasis Rev 2016; 35: 93–107. [DOI] [PubMed] [Google Scholar]
- 11. Horn S, Figl A, Rachakonda PS, et al. TERT promoter mutations in familial and sporadic melanoma. Science 2013; 339: 959–961. [DOI] [PubMed] [Google Scholar]
- 12. Kim TY, Chae MK, Kim HH, et al. A case of malignant melanoma presenting as a breast mass. J Korean Breast Cancer Soc 2003; 6: 35. [Google Scholar]
- 13. Bernardo MM, Mascarenhas MJ, Lopes DP. Primary malignant melanoma of the breast. Acta Med Port 1980; 2: 39–43. [PubMed] [Google Scholar]
- 14. Papachristou DN, Kinne DW, Rosen PP, et al. Cutaneous melanoma of the breast. Surgery 1979; 85: 322–328. [PubMed] [Google Scholar]
- 15. Roses DF, Harris MN, Stern JS, et al. Cutaneous melanoma of the breast. Ann Surg 1979; 189: 112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akcay MN. Metastatic disease in the breast. Breast 2002; 11: 526–528. [DOI] [PubMed] [Google Scholar]
- 17. Cangiarella J, Symmans WF, Cohen JM, et al. Malignant melanoma metastatic to the breast: a report of seven cases diagnosed by fine-needle aspiration cytology. Cancer 1998; 84: 160–162. [DOI] [PubMed] [Google Scholar]
- 18. Bacchi CE, Wludarski SC, Ambaye AB, et al. Metastatic melanoma presenting as an isolated breast tumor: a study of 20 cases with emphasis on several primary mimickers. Arch Pathol Lab Med 2013; 137(1): 41–49. [DOI] [PubMed] [Google Scholar]
- 19. Biswas A, Goyal S, Jain A, et al. Primary amelanotic melanoma of the breast: combating a rare cancer. Breast Cancer 2014; 21: 236–240. [DOI] [PubMed] [Google Scholar]
- 20. Yeom YK, Cha JH, Kim HH, et al. Primary melanoma of the breast: a case report with imaging findings. J Korean Radiol Soc 2015; 73: 287–291. [Google Scholar]
- 21. Leone BA, Vallejo CT, Romero AO, et al. Prognostic impact of metastatic pattern in stage IV breast cancer at initial diagnosis. Breast Cancer Res Treat 2017; 161(3): 537–548. [DOI] [PubMed] [Google Scholar]
- 22. Liu Y, Xin T, Huang DY, et al. Prognosis in very young women with triple-negative breast cancer: retrospective study of 216 cases. Med Oncol 2014; 31(12): 222. [DOI] [PubMed] [Google Scholar]
- 23. Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997; 275: 1943–1947. [DOI] [PubMed] [Google Scholar]
- 24. Steck PA, Pershouse MA, Jasser SA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 1997; 15: 356–362. [DOI] [PubMed] [Google Scholar]
- 25. Myers MP, Pass I, Batty IH, et al. The lipid phosphatase activity of PTEN is critical for its tumour suppressor function. Proc Natl Acad Sci USA 1998; 95: 13513–13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu X, Senechal K, Neshat MS, et al. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA 1998; 95: 15587–15591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cairns P, Okami K, Halachmi S, et al. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res 1997; 57: 4997–5000. [PubMed] [Google Scholar]
- 28. Rhei E, Kang L, Bogomolniy F, et al. Mutation analysis of the putative tumor suppressor gene PTEN/MMAC1 in primary breast carcinomas. Cancer Res 1997; 57: 3657–3659. [PubMed] [Google Scholar]
- 29. Dahia PL, Marsh DJ, Zheng Z, et al. Somatic deletions and mutations in the Cowden disease gene, PTEN, in sporadic thyroid tumors. Cancer Res 1997; 57: 4710–4713. [PubMed] [Google Scholar]
- 30. Aguissa-Toure AH. Genetic alterations of PTEN in human melanoma. Cell Mol Life Sci 2012; 69(9): 1475–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Celibi JT, Shendrik I, Silvers DN, et al. Identification of PTEN mutations in metastatic melanoma specimens. J Med Genet 2000; 37: 653–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. U.S. National Library of Medicine. BRAF: genetics home reference, https://ghr.nlm.nih.gov/gene/BRAF#location (accessed 15 September 2016).
- 33. Pollock PM, Harper UL, Hansen KS, et al. High frequency of BRAF mutations in nevi. Nat Genet 2003; 33: 19–20. [DOI] [PubMed] [Google Scholar]
- 34. Silva JM, Bulman C, McMahon M. BRAFV600E cooperates with PI3K signaling, independent of AKT, to regulate melanoma cell proliferation. Mol Cancer Res 2014; 12: 447–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dankort D, Curley DP, Cartlidge RA, et al. BrafV600E cooperates with Pten loss to induce metastatic melanoma. Nat Genet 2009; 41(5): 544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Korabiowska M, Fischer G, Steinacker A, et al. Cytokeratin positivity in paraffin-embedded malignant melanomas: comparative study of KL1, A4 and Lu5 Antibodies. Anticancer Res 2004; 24: 3203–3207. [PubMed] [Google Scholar]