Abstract
Background
Chronic visceral pain is a defining symptom of many gastrointestinal disorders. The KV7 family (KV7.1–KV7.5) of voltage-gated potassium channels mediates the M current that regulates excitability in peripheral sensory nociceptors and central pain pathways. Here, we use a combination of immunohistochemistry, gut-nerve electrophysiological recordings in both mouse and human tissues, and single-cell qualitative real-time polymerase chain reaction of gut-projecting sensory neurons, to investigate the contribution of peripheral KV7 channels to visceral nociception.
Results
Immunohistochemical staining of mouse colon revealed labelling of KV7 subtypes (KV7.3 and KV7.5) with CGRP around intrinsic enteric neurons of the myenteric plexuses and within extrinsic sensory fibres along mesenteric blood vessels. Treatment with the KV7 opener retigabine almost completely abolished visceral afferent firing evoked by the algogen bradykinin, in agreement with significant co-expression of mRNA transcripts by single-cell qualitative real-time polymerase chain reaction for KCNQ subtypes and the B2 bradykinin receptor in retrogradely labelled extrinsic sensory neurons from the colon. Retigabine also attenuated responses to mechanical stimulation of the bowel following noxious distension (0–80 mmHg) in a concentration-dependent manner, whereas the KV7 blocker XE991 potentiated such responses. In human bowel tissues, KV7.3 and KV7.5 were expressed in neuronal varicosities co-labelled with synaptophysin and CGRP, and retigabine inhibited bradykinin-induced afferent activation in afferent recordings from human colon.
Conclusions
We show that KV7 channels contribute to the sensitivity of visceral sensory neurons to noxious chemical and mechanical stimuli in both mouse and human gut tissues. As such, peripherally restricted KV7 openers may represent a viable therapeutic modality for the treatment of gastrointestinal pathologies.
Keywords: Visceral pain, KV7, retigabine and nociception
Introduction
Chronic pain associated with visceral hypersensitivity to physiological stimuli is a hallmark of functional gastrointestinal (GI) disorders including irritable bowel syndrome (IBS). Persistent pain and decreased thresholds to bowel distension are a function of altered neuronal excitability of both sensitized peripheral sensory input and maladaptive central pain processing.1
The KV7 family (KV7.1–KV7.5) of voltage-gated potassium channels encoded by KCNQ1–5 are responsible for mediating the M current and can regulate excitability in a variety of central and peripheral neurons involved in pain pathways.2–4 At thresholds below action potential initiation and near resting membrane potential, KV7 channels have a significant influence on the resting membrane potential and contribute to a membrane-potential clamping effect that stabilizes neuronal excitability and acts to restrict repetitive firing.4 In acute inflammatory pain, Gq/11-coupled receptor-mediated attenuation of the M current can evoke depolarization and increased excitability of nociceptors.5 Thus, genetic or pharmacological inhibition of KV7 leads to increased action potential firing to excitatory stimuli, whereas KV7 openers, including the anticonvulsant drug retigabine, produce membrane hyperpolarization and reduced excitability.3,6,7 Retigabine is selective for KV7.2–7.5 over the cardiac KV7.1 subunit and is clinically efficacious in reducing partial seizures in epilepsies; however, significant centrally mediated adverse effects have restricted its more widespread utility both as an anticonvulsant and putative analgesic.8 In the periphery, enhanced KV7 channel activity has been identified as the main site of action for retigabine in the attenuation of somatic inflammatory pain as well as in bone cancer models where retigabine inhibits mechanical allodynia and thermal hyperalgesia.9,10 In addition to retigabine, tannic acid can also enhance the M current by acting on KV7.2/KV7.3 channels in small diameter neurons.11 Conversely, KV7 channel inhibitors act to sensitize peripheral neurons such as XE-991, which sensitizes C-fibres to noxious heat stimulation and induces chronic spontaneous activity of Aδ fibres, and camphor or menthol, which act on KV7.2/KV7.3 expressed on cutaneous nerve endings.12,13 Of particular relevance to this study is the action of inhibitory KV7 mediators under inflammatory conditions, with which visceral pain is often associated. For example, protease activated receptor 2 (PAR-2) has been implicated in mediating colonic visceral hypersensitivity and in IBS, which is characterized by abdominal pain.14,15 In rat nociceptive sensory neurons, PAR-2 inhibits the M current via phosphoinositide phospholipase (PLC) activation.16 Similarly, inhibition of Mas-related gene receptor D (MrgprD; which is known to be up-regulated during intestinal inflammation17), by its ligand ß-alanine also inhibits KV7.2/KV7.3 activity.18 As such, a peripherally restricted KV7 opener may represent a useful analgesic therapy for treating visceral pain associated with inflammation. Indeed when injected into the peritoneal cavity, retigabine reduces visceral pain behaviours in mice following intracolonic application of capsaicin;19 however, the exact site of action is still to be resolved. Moreover, following neuropathic injury, increased expression of repressor element 1-silencing transcription factor in dorsal root ganglia (DRG) down-regulates expression of KCNQ2 leading to neuropathic hyperalgesia.20 Selective ablation of KCNQ2 from the mouse forebrain results in visceral hyperalgesia suggesting that there may also be a contribution of CNS KV7 channels to visceral pain.21 Here, we sought to identify and confirm the role of peripheral KV7 channels in visceral nociception by studying nerve fibres innervating the GI tract of both mouse and human.
KV7 channel subtype expression is tissue specific, with KV7.1 highly expressed by cardiac myocytes22 as well as in epithelial23 and smooth muscle tissue,24 while KV7.2 to KV7.5 are found in the nervous system.25 In spinal sensory pathways, rodent small diameter transient receptor potential vanilloid subfamily type 1-positive nociceptors express KV7.2, KV7.3 and KV7.5,3 whilst retigabine can reduce the excitability of unmyelinated peripheral human axons.26 In the GI tract, KV7.2, KV7.3 and KV7.5 have all been identified in the smooth muscle of mouse colon suggesting a role in regulating gut motility.24 Whether such motor events are additionally mediated by KV7 expression within the enteric nervous system itself remains to be determined. With regard to the extrinsic innervation (vagal and spinal pathways) of the GI tract, KV7 channels regulate excitability in the predominantly non-nociceptive population of vagal sensory neurons that innervates the upper gut.27 However, the contribution of KV7 channels to the excitability of nociceptive sensory neurons innervating the colon is unknown.
Therefore, the aims of this study were to (1) determine the expression of KV7.3 and KV7.5 subtypes in mouse and human lower GI tract, (2) determine the expression of KV7 channel subtypes in DRG sensory neurons innervating the lower GI tract of mouse, (3) investigate the ability of KV7 channel opener retigabine to inhibit visceral afferent excitability to acute inflammatory and noxious stimuli (including bradykinin and mechanical distension) and (4) validate the ability of retigabine to attenuate human visceral afferent excitability using a novel human ex vivo model of visceral nociception.28
Materials and methods
Mouse and human tissues
Experiments were performed using male C57BL/6 mice (10–12 weeks of age) and in accordance with the UK Animal (Scientific Procedures) Act 1986. The collection and use of human tissues was performed with approval of the East London and The City HA Local Research Ethics Committee (NREC 09/H0704/2). Fresh, non-inflamed ileum (N = 4) and colon (N = 6 for immunohistochemistry and N = 6 for electrophysiology) were obtained under full written consent from 4 to 6 patients (four male, median age: 58, range: 33–67 years) undergoing surgery at Barts and The London NHS Trust and Whipps Cross University Hospitals (London, UK). All specimens were obtained from patients with non-obstructive tumours that were not occuring in the context of inflammatory bowel disease. Specimens were taken with the permission of the histopathologist following macroscopic examination and were a minimum of 10 cm away from tumour, resection margins or lymphatic drainage field.
Immunohistochemistry
For sectioning studies, fresh mouse colon, as well as human ileum and colon, were post-fixed overnight (at 4℃) in 4% paraformaldehyde. Sections (10 µm) of cryoprotected tissue (30% sucrose w/v in 0.1 M phosphate-buffered saline (PBS)) were cut, incubated with blocking buffer (Dako, UK) for 1 h, before primary antibodies were applied overnight (4℃): KV7.3 (1:500, AP1-930, Thermo Scientific, UK), KV7.5 (1:500, PA1-941, Thermo Scientific, UK), CGRP (1:400, ab36001, Abcam, UK) and synaptophysin (1:200, MO776, Dako, UK). Tissues were washed (PBS; 3 × 5 min) and species-specific secondary antibodies conjugated to Alexa Fluor fluorescent dyes (1:400, Thermo Fisher Scientific, UK) applied for 1 h, before washing (PBS; 3 × 5 min), mounting (Vectashield® hard set mounting media, Vector Laboratories, USA) and coverslipping. For whole-mount studies, fresh mouse colon was cut open along the mesenteric border, pinned flat and fixed overnight (4℃) in 4% paraformaldehyde. Following fixation, mucosa was removed, washed (PBS; 3 × 10 min), blocked (blocking buffer; 1 h) and incubated with primary antibodies (KV7.3, KV7.5 and CGRP; all at 1:300) for 48 h at 4℃. Tissues were washed (PBS; 2 × 10 min), and species-specific secondary antibodies conjugated to Alexa Fluor fluorescent dyes (1:200, Thermo Fisher Scientific, UK) applied for 2 h. After a final wash (PBS; 3 × 10 min), tissues were mounted (50% glycerol in PBS) and coverslipped. Controls with no primary antibody were used in all experiments to check for non-specific secondary antibody binding. A Leica DM4000 epifluorescence microscope was used to visualize immunoreactivity of sections while whole mounts were examined with a Zeiss LSM710 confocal microscope. CGRP is commonly used as a marker of spinal sensory afferents29,30 and is a nociceptive marker in rodents.31 At present, there is no biochemical marker that can distinguish between intrinsic and extrinsic neurons in the GI tract. Intrinsic CGRP expression has been shown in some species where colchicine has been used to sequester CGRP,32 while others have demonstrated extrinsic CGRP33 and used it as a marker of extrinsic fibres showing co-labelling with TRPA134 and TRPV4.35
KCNQ subunit expression by single-cell qualitative real-time polymerase chain reaction in colonic sensory neurons
Sensory neurons innervating the distal colon of mouse were labelled with Fast Blue, individually harvested and collected for single-cell qualitative real-time polymerase chain reaction (qRT-PCR) of KCNQ subunits as previously described in literature.36 In brief, 6 to 8 injections (0.2 µl each) of retrograde neuronal tracer Fast Blue (2% in saline; polysciences GmbH) were injected into the wall of the distal colon under anesthetic (isoflurane; 4% induction and 1.5% maintenance) via laparotomy. Three to five days after recovery, thoracolumbar (TL; T10-L1) and lumbosacral (LS; L6-S1) DRG were removed and enzymatically dissociated as described previously in literature.36 Briefly, dissected ganglia were washed in PBS and incubated in Lebovitz L-15 Glutamax (Invitrogen) media containing 6 mg/ml bovine serum albumin (Sigma-Aldrich) and 1 mg/ml collagenase type 1 A (Sigma-Aldrich) for 15 min at 37℃ (in 5% CO2). Ganglia were subsequently incubated in L-15 media containing 1 mg/ml trypsin (Sigma-Aldrich) and 6 mg/ml bovine serum albumin for 30 min. After gentle trituration using fire-polished pipettes, dissociated cells were pelleted by centrifugation (350X g) and resuspended in L-15 Glutamax media containing 24 mM NaHCO3, 38 mM Glucose, 2% penicillin/streptomycin and 10% fetal bovine serum. Cells were plated onto poly-D-lysine/laminin-coated coverslips (BD Biosciences, UK) and incubated at 37℃ in 5% CO2. Individual cells were collected by pulled glass pipette within 24 h of plating. Glass tips containing single cells or no-template bath solution controls were collected in tubes containing 9 µl of preamplification mastermix (5 µl CellDirect 2x reaction buffer (Invitrogen, UK), 2.5 µl 0.2X primer/probe mix, 0.1 µl SUPERase-in (Ambion, USA) and 0.2 µl Superscript III Reverse Transcriptase/Platinum Taq mix (Invitrogen, UK)) in TE buffer (Applichem, Germany) and subjected to thermal cycling (50℃ for 30 min, 95℃ for 2 min, then 21 cycles of (95℃ for 15 s, 60℃ for 4 min); Bio-Rad C1000 Thermal Cycler) to produce cDNA products. Following dilution with 91 µl TE buffer, TaqMan quantitative polymerase chain assays were performed on the cDNA products for each gene of interest (TaqMan Assay ID: Bdkrb2, Mm00437788_s1; KCNQ1, Mm00434640_m1; KCNQ2, Mm00440080_m1; KCNQ3, Mm00548884_m1; KCNQ4, Mm01185500_m1 and KCNQ5, Mm01226041_m1; glyceraldehyde-3-phosphate dehydrogenase, Mm99999915_g1; Applied Biosystems, USA) using the following thermal cycle (50℃ for 2 min, 95℃ for 10 min, then 40 cycles of (95℃ for 15 s, 60℃ for 1 min); Bio-Rad C1000 Thermal Cycler and CFX96 Real-Time System). All single-cell reverse transcription polymerase chain reaction products expressed glyceraldehyde-3-phosphate dehydrogenase, the expression of which acted as an internal positive control, whilst bath control samples did not. The determination of gene expression was evaluated by quantification cycle (Cq) values less than the threshold of 35 being considered as positive. Overall, the expression of genes of interest was examined in 45 TL colonic sensory neurons (N = 3) and 45 LS colonic sensory neurons (N = 3).
Isolation of mouse and human colonic tissues
Mouse and human GI tissues were isolated, and electrophysiological activity of nerves innervating these tissues recorded using previously described methods.37,38 Human tissues removed during surgical resection, and in excess to histopathological assessment, were immediately placed in ice-cold carbogenated Krebs buffer (in mM: 124 NaCl, 4.8 KCl, 1.3 NaH2PO4, 2.5 CaCl2, 1.2 MgSO4.7H2O, 11.1 glucose and 25 NaHCO3) supplemented with nifedipine (10 µM) and atropine (10 µM) to block smooth muscle activity. In a bespoke recording chamber, the colonic wall was pinned mucosa-side down, the mesenteric blood vessels supplying the gut wall were identified and the surrounding mesentery dissected away. Mesenteric nerves running in close proximity to blood vessels were identified, dissected and isolated, and the tissue superfused with Krebs buffer (7–8 ml/min; 32℃–34℃). Mice were humanely killed by cervical dislocation of the neck, the distal colon gross dissected and removed along with all associated neurovasculature. In a recording chamber, the colon was superfused (7–8 ml/min; carbogenated Krebs buffer) and cannulated enabling luminal perfusion (0.1 ml/min; carbogenated Krebs buffer). In murine experiments, the Krebs buffer was additionally supplemented with 3 µM indomethacin to reduce endogenous prostanoid production. The lumbar splanchnic nerve was identified and isolated in preparation for electrophysiological recordings.
Extracellular electrophysiological recordings of mouse and human afferent fibre activity
In both mesenteric nerve recordings from human tissues and lumbar splanchnic nerve recordings from the distal colon of mouse, isolated nerves were drawn into a borosilicate glass suction electrode (Harvard Apparatus, UK) prefilled with Krebs buffer. Nerve activity was recorded on a Neurolog headstage (Digitimer Ltd, UK), amplified at a gain of 5000X, band pass filtered (100–2000 Hz) and data acquired (20 kHz sampling rate; micro1401, Cambridge Electronic Design, UK) to a desktop computer running Spike2 (Cambridge Electronic Design, UK) software. Action potentials were counted using an online spike processor (Digitimer Ltd, UK), and the threshold level for spike processing was set to the smallest identifiable spike (typically∼100 µV).
Electrophysiological protocols
For all electrophysiological experiments, a minimum of 15 min of baseline spontaneous activity was recorded prior to conducting any experimental protocol. In mouse distal colon recordings, four bradykinin (1 µM) bath superfusions (20 ml) were applied at ∼30 min intervals. Prior to, and in the presence of the third consecutive bradykinin application, retigabine (1 or 10 µM) or vehicle control (0.1% dimethyl sulfoxide (DMSO)) was applied by bath superfusion (20 ml volume). In separate experiments, phasic distension of the colon was performed by blocking the luminal out-flow and applying an elevated pressure head enabling rapid increases in intraluminal pressure (0–80 mmHg). Pain behaviours in vivo are evoked by such noxious pressures and have been previously used to investigate colonic nociceptor function.38 Specifically, repeat rapid phasic distensions (0–80 mmHg, 60 s at 9 min intervals) were applied until the response stabilized (typically after 5–6 distensions), at this point increasing concentrations of retigabine were superfused prior to, and during, every third phasic distension sequentially. In one set of experiments, responses to 0.1 µM, 1 µM and 10 µM retigabine were determined, and in a second set of experiments, responses to 30 µM and 100 µM retigabine were investigated. In separate phasic distension experiments, the KV7 blocker XE991 (10 µM) was superfused prior to, and during, the seventh phasic distension. In human tissues, the ability of bradykinin to repeatedly activate human colonic afferents was investigated.39 Bradykinin (1 µM) was applied, at an interval of 45 min, in a ‘step-up’ manner where bath superfusion of 10 ml of 1 µM bradykinin was supplemented with a direct application of 100 µM bradykinin (0.9 ml) to the recording chamber. In total, four sequential applications of bradykinin were made in parity with murine recording protocols. We next investigated the effect of retigabine on bradykinin-stimulated human colonic afferents. During the third repeat bradykinin application, retigabine (100 µM) was co-applied to the bath. Following the third bradykinin stimulation, 45 min of washout time was allowed before the fourth and final bradykinin stimulation.
Drugs
Stock concentrations of atropine (10 mM; ethanol), bradykinin (10 mM; H20), indomethacin (3 mM; DMSO), retigabine (100 mM; DMSO), XE991 (100 mM; DMSO) and nifedipine (10 mM; DMSO) were all purchased from Sigma Aldrich (UK) and prepared as described. All compounds were diluted to working concentrations in buffer on the day of experimentation.
Data analysis
In multi-unit experiments, peak changes of electrophysiological nerve activity were determined by subtracting baseline firing (3 min before drug application) from increases in nerve activity following nucleotide superfusion. The visualization of single-cell qRT-PCR expression data was created using R and the ggplot2 package.40 Statistical significance was set at P < 0.05, and data are displayed as mean ± SEM.
Results
KV7 subtypes are expressed in neurons and mesenteric fibres of mouse bowel tissues
GI function is regulated by a vast network of enteric neurons organized into two plexuses: the myenteric plexus located between the longitudinal and circular muscle layers, and the submucosal plexus located between the mucosa and the circular muscle. Afferent fibres of extrinsic spinal sensory neurons are also present in these tissues with projections to multiple layers of the bowel wall. The functional KV7 heteromultimers known to underpin neuronal M currents are combinations of KV7.2/KV7.3 and KV7.3/KV7.5.41–43 Thus, we first examined the expression of KV7.3 and KV7.5 in mouse colonic tissues.
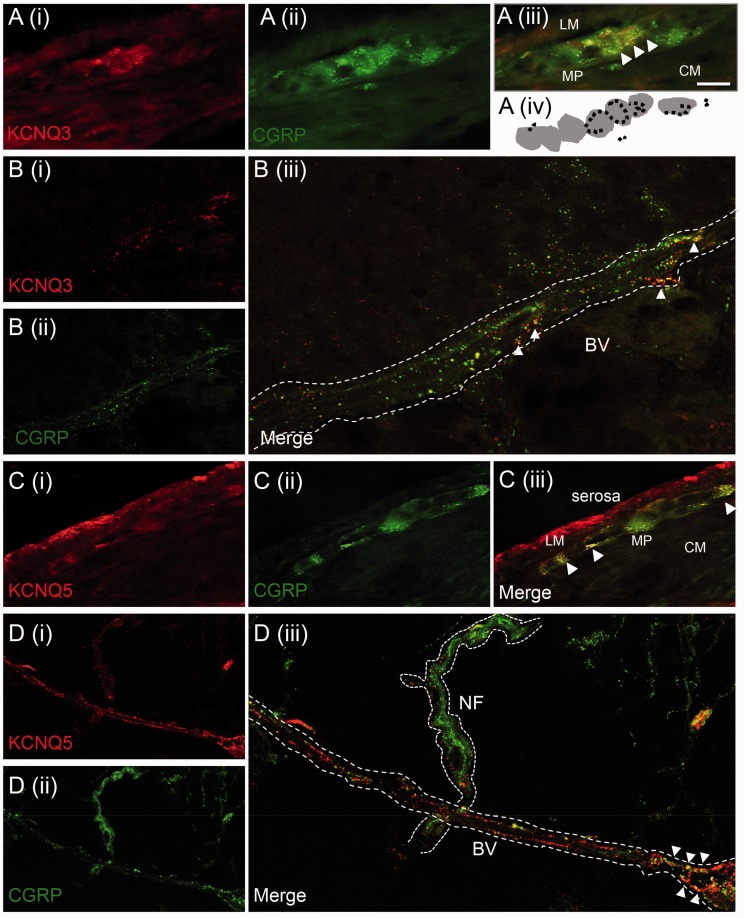
We hypothesized that KV7 channel subtypes were expressed by enteric neurons, and in extrinsic sensory fibres contributing to nociception. In order to investigate this, we performed whole-mount immunohistochemistry against multiple KV7 channel subtypes and the sensory neuronal marker CGRP, in separate layers of the mouse distal colon. Immunostaining revealed both KV7.3 and KV7.5 expression in the enteric neurons of the myenteric plexus, which co-localized with neuronal varicosities as identified by CGRP (Figure 1(a) and (c)). In the mesentery associated with the colon, extensive punctate expression of both KV7.3 and KV7.5 was observed along mesenteric blood vessels, which also co-expressed CGRP (Figure 1(b) and (d)). For both KV7 subtypes, examples of KV7-negative, CGRP-positive nerve fibres were also present (Figure 1(d)), suggesting heterogeneity of KV7 expression. Taken together, these observations identify KV7.3 and KV7.5 in both enteric neurons and extrinsic sensory fibres of the mouse distal colon. The expression of KV7 by extrinsic mesenteric fibres, which are important nociceptors in the gut,44 suggests that KV7 may be involved in visceral pain pathways.
Figure 1.
Expression of KV7 channels in mouse colon and mesenteric neurovasculature. (a) KV7.3 co-expression with CGRP-positive neurons within the myenteric plexus (MP) of mouse colon (tissue sections). Panel (iv) is a schematic representation of endings that co-label for CGRP and KV7.3 (in black) surrounding neuronal cell bodies (in grey) of the myenteric plexus. (b) In the mesentery associated with the colon, KV7.3 co-localizes with CGRP-positive neurons (white arrowheads) surrounding blood vessels (BV) in whole mounts. (c) KV7.5 is expressed in the serosal layer of mouse colon and co-localizes with CGRP-positive fibres in the myenteric plexus (tissue sections). (d) CGRP positive neurons that run alongside blood vessels found within the mesenteric attachment to the colon co-express KV7.5 (whole mounts). Punctate staining for KV7.5 is also found around CGRP positive nerve bundle (NF). All images taken at 40X, scale bar = 25 µm. Figures b(iii) and d(iii) are 4X of the original images, b(i) and d(i).
LM: longitudinal muscle; CM: circular muscle.
Expression of KCNQ transcripts in extrinsic sensory neurons innervating the mouse colon
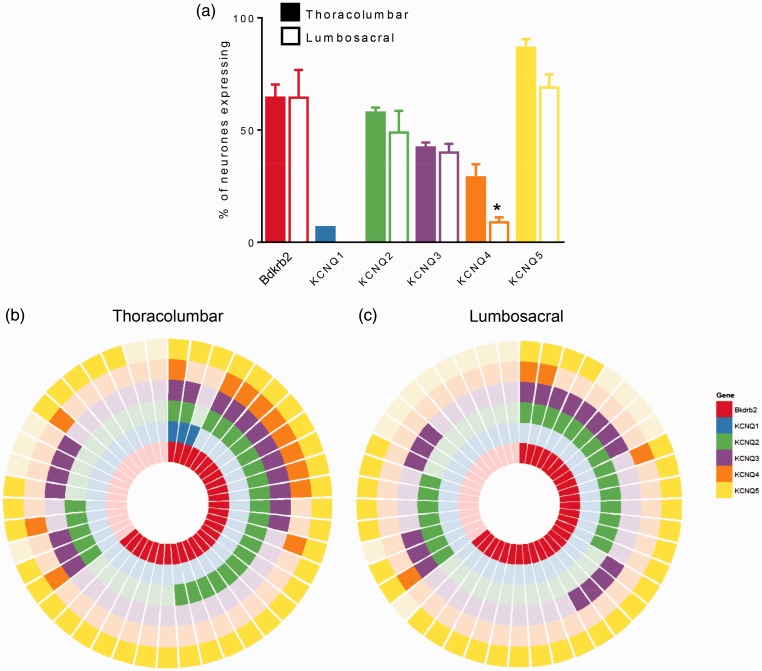
To confirm the expression of KV7 channel subtypes observed by immunohistochemistry in extrinsic sensory fibres innervating the distal colon, we performed single-cell qRT-PCR for KCNQ1–5 transcripts in DRG neurons retrogradely labelled from the mouse distal colon. We examined individual Fast Blue-positive sensory neurons harvested following primary culture of TL (T10-L1) and LS (L6-S1) DRG, corresponding to the splanchnic and pelvic neurons specifically innervating the distal colon, respectively.29 In total, 15 single cells were collected per spinal region (e.g., TL or LS) per mouse (N = 3). In colonic sensory neurons isolated from the TL region (representative of the splanchnic innervation), analysis of qRT-PCR products revealed greater expression of KCNQ5 transcripts (86.7 ± 3.8%) compared to KCNQ2 (57.8 ± 2.2%), KCNQ3 (42.2 ± 2.2%) and KCNQ4 (28.9 ± 5.9%; Figure 2(a)). KCNQ1 transcripts were observed infrequently (6.7 ± 0.0%) and were only present in one cell from each of the three animals labelled. By contrast, colonic pelvic sensory neurons isolated from LS DRG exhibited significantly lower expression of KCNQ4 transcripts (8.9 ± 2.2%; P < 0.05, unpaired t-test) compared to TL sensory neurons, whilst levels of KCNQ2 (48.9 ± 9.7%), KCNQ3 transcripts (40.0 ± 3.8%) and KCNQ5 (68.9 ± 5.9%; Figure 2(a)) did not significantly differ. No LS colonic sensory neuron expressed KCNQ1 transcripts (Figure 2(a) and (c)). Although KCNQ5 transcripts were present in the vast majority of TL and LS neurons investigated, the co-expression of KCNQ2 and KCNQ3 transcripts (as a proportion of all cells investigated) was greatest in TL (TL, 33.3 ± 3.8% vs. LS, 24.4 ± 2.2%). From this data, it is clear that KCNQ2, KCNQ3 and KCNQ5 are expressed in a large proportion of colonic sensory neurons; however, it is difficult to interpret whether differences in TL versus LS expression profiles are functionally meaningful. Overall, however, the high expression of KCNQ5 in extrinsic sensory neurons agrees with protein expression data described in Figure 1. Similarly, moderate expression of KCNQ3 follows the pattern of protein expressed observed in mouse colonic mesentery (Figure 1(b)).
Figure 2.
Expression of KCNQ and bradykinin receptor B2 (Bdkrb2) mRNA transcripts in mouse colon-projecting sensory neurones by single-cell qRT-PCR. (a) Proportion of thoracolumbar and lumbosacral colon-projecting sensory neurons expressing transcripts for Bdkrb2, KCNQ1, KCNQ2, KCNQ3, KCNQ4 and KCNQ5. *P < 0.05 versus TL, unpaired t-test. Co-expression analysis of Bdkrb2, KCNQ1, KCNQ2, KCNQ3, KCNQ4 and KCNQ5 transcripts in thoracolumbar (b) and lumbosacral (c) colonic sensory neurons. Each segment represents a single cell with a filled coloured block signifying positive expression.
Co-expression of KCNQ channel subtypes with bradykinin receptor subtype B2 in mouse colonic sensory neurons
The algogenic peptide bradykinin is an important endogenous inflammatory mediator that can activate pain pathways by binding two G-protein coupled receptor subtypes: B1 and B2. The acute effects of bradykinin are predominantly mediated by the constitutively expressed bradykinin B2 receptors45,46 which are capable of altering sensory neuronal excitability by multiple downstream intracellular pathways, including phosphorylation of TRP channels,47,48 increased Ca2+-activated Cl− conductance49 and suppression of Ca2+-dependent K+50,51 and M currents.52 In order to test the coupling of KV7 channels to bradykinin-evoked excitation in visceral sensory neurons, we first examined the co-expression of bradykinin receptor B2 (Bdkrb2) with KCNQ1–5 transcripts in single-cell qRT-PCR products. In TL colonic sensory neurons, Bdkrb2 mRNA transcripts were observed in 64.4 ± 5.9% of cells, and of these Bdkrb2-positive neurons, 72.9 ± 4.8% also expressed KCNQ2, 41.0 ± 2.3% expressed KCNQ3 transcripts and 100.0 ±0.0% expressed KCNQ5 transcripts (Figure 2(b)). Similar proportions of LS colonic sensory neurons expressed Bdkrb2 transcripts (64.4 ± 12.4%); however, co-expression with KCNQ2 (54.3 ± 7.2%), KCNQ3 (37.4 ± 10.4%) and KCNQ5 (85.6 ± 7.5%) transcripts were partially reduced compared to the TL populations (Figure 2(c)). Of both the TL and LS colonic sensory neurons that did express Bdkrb2 transcripts, 24% to 31% expressed transcripts for only one KCNQ subtype (exclusively KCNQ5), the rest of Bdkrb2-positive colonic sensory neurons co-expressed multiple KCNQ channel subtypes, principally KCNQ3 and KCNQ5.
Retigabine inhibits bradykinin-induced afferent activation in mouse colon
We next investigated whether the opening of KV7 channels was sufficient to alter acute chemosensitivity to bradykinin in peripheral terminals of sensory neurons innervating the distal colon. Bradykinin was the chosen chemical stimulus as activation of the B2 receptor on sensory neurons is known to induce store release of Ca2+ that then binds to KV7-associated calmodulin resulting in closure of KV7 channels and increased neuronal excitability.52 Specifically, multi-unit ex vivo extracellular electrophysiological recordings of the lumbar splanchnic nerve were made after cannulation of the mouse distal colon enabling stimulation by repeat bath superfusion of bradykinin. Application of bradykinin (1 µM) led to an increase in afferent firing to 22.3 ± 2.8 spikes/s above baseline discharge levels with a duration of approximately 20 min (N = 5). Repeat bradykinin applications were made at ∼30 min intervals. A significant desensitization in response to the second bradykinin (1 µM) response was observed (5.4 ± 1.21 spikes/s). Importantly, subsequent responses to the third (5.1 ± 0.9 spikes/s) and fourth (6.2 ± 1.9 spikes/s) bradykinin applications remained consistent in vehicle experiments (P = 0.54, N = 5, repeated measures (RM) analysis of variance (ANOVA); Figure 3(b)) enabling investigation of the effect of the KV7 channel opener retigabine on peripheral visceral afferent responses to bradykinin after the second application of bradykinin. Pre-incubation, and co-application, of 10 µM retigabine with 1 µM bradykinin abolished virtually all afferent firing (0.0 ±1.5 spikes/s; P < 0.05, N = 5, two-way RM ANOVA with Dunnett’s post hoc). This effect was completely reversible following washout with the response to subsequent superfusion of 1 µM bradykinin comparable to pre-retigabine responses (5.4 ± 0.7 spikes/s; Figure 3(b)). In the example trace shown in Figure 3(a), 10 µM retigabine was additionally superfused during the washout to the final bradykinin application, again completely inhibiting the elevated afferent firing. In separate experiments, the application of 1 µM retigabine also had an inhibitory effect on afferent firing to bradykinin; however, this was smaller and did not significantly differ from vehicle controls (4.0 ± 0.6 spikes/s; P > 0.05, N = 5, two-way RM ANOVA with Dunnett’s post hoc). Collectively, these data confirm that opening of KV7 reverses bradykinin-induced action potential firing in peripheral visceral afferent fibres and highlights the importance of KV7 channels in mechanisms underlying visceral inflammatory pain.
Figure 3.
Retigabine inhibits bradykinin-induced afferent firing in mouse lumbar splanchnic nerve recordings. (a) Example rate histogram with neurogram trace over time showing the effect of retigabine (10 µM) on afferent excitation evoked by repeat application of bradykinin (1 µM) at 30 min intervals. Repeat responses to bradykinin stabilized after initial desensitization following the first application. Retigabine was applied prior to, and during, the third bradykinin application. The black bars indicate the duration of compound superfusion into the bath. In this example only, retigabine (10 µM) was also applied during the wash from the fourth bradykinin application to fully confirm the ability to inhibit residual-evoked afferent excitation from bradykinin. Below, expanded traces showing action potential firing in the presence of bradykinin (left) and in the presence of both bradykinin and retigabine (right). Below far left, example single action potential. (b) Average change in peak firing rate to repeat application of bradykinin (1 µM) in the presence of either 1 µM or 10 µM retigabine or vehicle (0.1% DMSO) (applied during the third bradykinin). *P < 0.05 versus vehicle, repeated measures 2-way ANOVA with Dunnett’s post hoc.
Retigabine inhibits visceral afferent sensitivity to noxious distension
Next we investigated the ability of KV7 channels to alter visceral mechanosensitivity in peripheral afferent terminals of the mouse distal colon. We applied repeat phasic distensions (0–80 mmHg for 60 s at an interval of 9 min; a pressure shown to activate all known mouse mechanosensitive nociceptors35), to determine the effect of the KV7 channel opener retigabine on afferent mechanoreceptors. Phasic distension led to an increase in splanchnic nerve afferent firing and rapid adaptation of the response during the 1-min distension (Figure 4(a)). Upon relief of the pressure, firing rates quickly returned to baseline levels. As with previous studies, tachyphylaxis of the response to repeat distension was observed over the subsequent distensions, with responses typically stabilizing by the fifth to sixth distension.53 Varying concentrations of retigabine (0.1–100 µM) were superfused before and during the seventh distension, leading to concentration-dependent inhibition of the peak firing rates to subsequent phasic distension (N = 4–6, Figure 4(b)). Although the response to the first distension directly after application of retigabine was inhibited, the maximal attenuation of response occurred by the third distension after application (e.g. ∼30 min post-retigabine; Figure 4(a)). At the concentration of retigabine (10 µM) where complete block of the bradykinin-evoked excitation occurred; Figure 3(a)), there was a 48.5 ± 3.2% inhibition of response to noxious distension. Application of the KV7 channel blocker XE991 (10 µM) led to a moderate increase (29.1 ± 9.2%) in response to noxious distension, highlighting the presence of a tonic KV7 current during non-inflamed states. In addition to regulating the response to phasic distension, there was a trend for decreased spontaneous discharge in the presence of retigabine (e.g. pre-retigabine, 3.89 ± 2.52 spikes/s vs. post-retigabine 10 µM, 0.87 ± 0.20 spikes/s, N = 6, P = 0.27, paired t-test), although this was not observable in all experiments due to very low spontaneous discharge in some preparations. By contrast, addition of XE991 (10 µM) tended to potentiate spontaneous discharge (pre-XE991, 3.70 ± 1.10 spikes/s vs. post-XE991 10 µM, 9.57 ± 2.51 spikes/s, N = 5, P = 0.05, paired t-test). These data suggest that KV7 channels are present on visceral nociceptors responsive to mechanical stimuli and that opening of KV7 channels is sufficient to make the afferent terminal less excitable by such stimuli.
Figure 4.
Effect of retigabine on visceral afferent mechanosensitivity to phasic noxious distension. (a) Example rate histogram, intraluminal pressure trace and raw neurogram trace to repeat phasic distension (60 s, at 9 min interval) to noxious pressures (0–80 mmHg). Retigabine was added after stabilization of the response to phasic distension (e.g., after the seventh distension) and leads to robust and reproducible reduction in spontaneous afferent activity and evoked responses to phasic distension. Maximum effects are observed during the third distension after application of retigabine began. In this example trace, the first three distensions are omitted, retigabine (10 µM) is applied prior to the seventh distension and distension continued for subsequent 60 mins during washout showing almost complete reversal of retigabine-dependent inhibition. (b) Example rate histogram, intraluminal pressure trace and raw neurogram trace to repeat phasic distension (60 s, at 9 min interval) to noxious pressures (0–80 mmHg) following application of XE991 (10 µM), which increases ectopic afferent activity and mechanically evoked afferent firing. In this example trace, the first three distensions are omitted and XE991 is applied prior to the seventh distension. (c) Average change in response from pre-application baseline to third distension after application of retigabine at varying concentrations (N = 4–6) or vehicle (0.03%–0.1% DMSO; N = 4) or XE991 (10 µM; N = 3).
KV7 channels are expressed in neurons of human bowel tissue
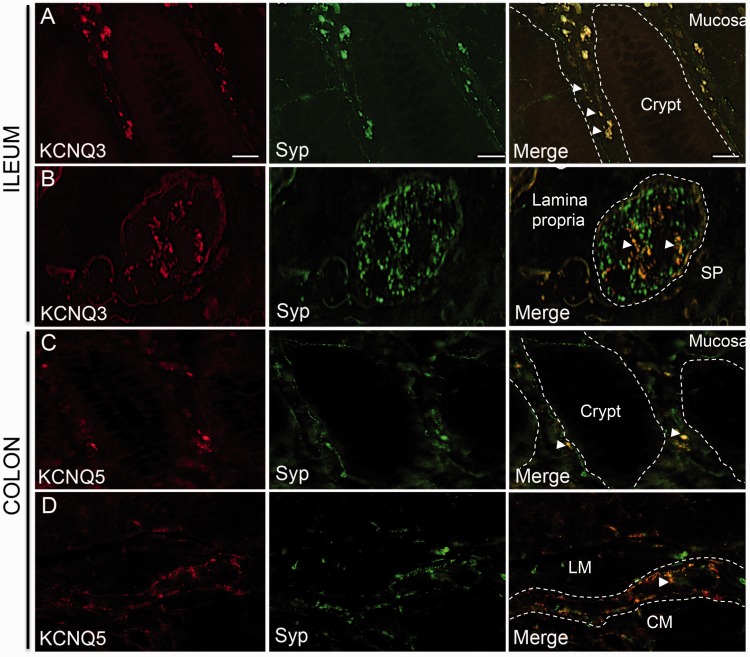
In order to investigate the contribution of KV channels to human GI function, we performed immunohistochemistry on human bowel tissues (ileum and colon). In normal human ileum, KV7.3 expression was found in the neuronal varicosities of the mucosal villi and showed significant co-expression with the neuronal marker synaptophysin (an integral membrane protein present in presynaptic vesicles of neurons;54 Figure 5(a)). Synaptophysin-positive nerve varicosities also co-labelled with KV7.3 in the intracellular spaces between villi (Figure 5(a)). In the submucosal plexus of human ileum, KV7.3 immunostaining was punctate and observed around the cell bodies rather than within the neurons themselves. Structures that stained positively for KV7.3 co-labelled with synaptophysin (Figure 5(b)) and CGRP (Figure 6(a)). In human colon, KV7.5 expression was observed in the lamina propria between mucosal crypts. Structures that positively stained for KV7.5 were identified as synaptophysin-positive fibres innervating the colonic mucosa (Figure 5(c)). Neuronal varicosities, identified by co-expression with synaptophysin, expressed KV7.5 in nerve fibres surrounding the colonic myenteric plexus (Figure 5(d)). KV7.5 also co-localized with CGRP in the myenteric plexus suggesting expression by sensory afferent pathways known to terminate at myenteric ganglia.55 Collectively, these data identify significant expression of KV7 channels subtypes in both intrinsic and extrinsic neuronal populations of the human GI tract.
Figure 5.
Co-localization of KV7 channels with synaptophysin in human bowel tissues. (a) KV7.3 is expressed in mucosa of human ileum, alongside synaptophysin (Syp)-positive nerve fibres running between mucosal crypts (as outlined by dotted lines). (b) KV7.3 co-localizes with a sub-population of synaptophysin-positive neurons in sub-mucosal ganglia (outlined by dotted lines) of ileum. (c) In human colonic mucosa, some synaptophysin-positive neurons around crypts (outlined by dotted lines) express KV7.5. (d) KV7.5 is expressed around the myenteric plexus (outlined by dotted lines) of human colon and co-express with synaptophysin surrounding the plexus (white arrow); 20X objective used on a and c, 40X objective used on b and d. Scale bar: a and c = 20 µm; b and d = 40 µm.
Figure 6.
Co-localization of KV7 channels with CGRP neurons in human bowel tissues. (a) Sub-mucosal plexus (SP) showing KV7.3 expressed on CGRP-positive neurons (white arrows) in human ileum. (b) KV7.5 is expressed on CGRP-positive fibres (white arrows) within the myenteric plexus (MP) of human colon. All images taken using 40X objective. Scale bar = 40 µm.
Retigabine inhibits bradykinin-induced afferent activation in human colon
In order to further understand the role of KV7 channels in human spontaneous inflammatory pain and to translate our murine findings into man, we utilized a model of human visceral afferent activity and a similar experimental paradigm as above.28 Using surgically resected bowel tissues, we recorded mesenteric nerve activity to repeat application of bradykinin. A concentration of 1 µM bradykinin was used as this has previously been shown robustly excite human visceral afferent fibres in ex vivo electrophysiological recordings.28,37 Bath superfusion of 1 µM bradykinin and a bolus of 10 ml bradykinin applied directly to the nerve under investigation led to a robust increase in human mesenteric afferent firing averaging 20 min in duration (Figure 7(a)). Following a washout interval of 45 min, a subsequent repeat bradykinin concentration was applied, although the response was partly diminished, it did not significantly differ from the first (first bradykinin, 2.14 ± 0.30 spikes/s vs. second bradykinin, 1.40 ± 0.36 spikes/s, P = 0.13, N = 7, paired t-test, Figure 7(a)).
Figure 7.
Retigabine inhibits bradykinin-evoked afferent activation in human colon. (a) Representative recording showing human mesenteric afferent excitation to bradykinin (1 µM); an effect that is inhibited in the presence of KV7 channel opener retigabine. Upon washout of retigabine, bradykinin-evoked afferent excitation is recovered. Top, example rate histogram; middle, raw neurogram traces and bottom, expanded traces showing human action potential firing during the application of bradykinin under different conditions. (b) Average response to bradykinin before, during and after the addition of retigabine (N = 6). *P < 0.05 versus control, one-way ANOVA with Bonferroni post hoc.
To investigate the role of KV7 in human visceral afferent sensitivity to bradykinin, we applied 100 µM retigabine in the presence of the third bradykinin application. This led to a significant decrease of the afferent response, reducing both the duration of the overall response (991 ± 522 s vs. retigabine, 178 ± 102 s) and change in peak firing (pre-retigabine, 1.40 ± 0.36 spikes/s vs. retigabine, 0.36 ± 0.23 spikes/s, P < 0.05, N = 7, one-way ANOVA with Dunnett’s post hoc) compared to the response to bradykinin prior to retigabine being applied. Following the washout period of 45 min, bradykinin was again applied to the bath and a partial recovery in response to levels of afferent activation comparable to control applications was observed (retigabine vs. post-retigabine, 0.89 ± 0.17 spikes/s, P > 0.05, N = 7, one-way ANOVA with Dunnett’s post hoc, Figure 7(b)). Collectively, these findings demonstrate a functional role for KV7 channels in modulating the sensitivity of peripheral terminals of visceral sensory neurons innervating the human lower GI tract.
Discussion
Here we demonstrate that KV7 channel subtypes are expressed by gut-projecting sensory neurons and that modulation of these channels can greatly alter afferent sensitivity to mechanical and inflammatory stimuli. We have shown in mouse that opening KV7 channels attenuates visceral afferent sensitivity to noxious distension of the colon and to the algogenic inflammatory mediator bradykinin; the latter an effect that translates to human visceral afferents. Conversely, blockade of KV7 channels results in hypersensitivity to mechanical stimuli. As such, KV7/M-channel activity represents an integral regulator of visceral afferent sensitivity downstream of multiple transduction mechanisms and is likely to contribute to dampening of peripheral pain pathways from visceral organs in humans.
The molecular constituents of the neuronal M current are heteromultimeric combinations of KV7 channel subtypes, principally KV7.2, KV7.3 and KV7.5. As a slowly deactivating, non-inactivating sub-threshold current, the M current exerts a stabilizing influence on neuronal excitability, with small changes in currents capable of significant alterations in excitability.3,56 In the central nervous system, mutations in KCNQ2 and KCNQ3 can manifest as familial neonatal epilepsies with unprovoked partial or generalized convulsions.57,58 Peripherally, KV7 channels are expressed by sensory neurons of the DRG3 and contribute to the regulation of nociceptor excitability in models of both inflammatory and neuropathic pain.3,9,16,52,59 Whilst its role in peripheral pain pathways is well established, the contribution of KV7 in peripheral visceral nociception and the site of action of retigabine however remains largely unknown. Here we show in mouse GI tissues that KV7 channel subunits (KV7.3 and KV7.5) are expressed in extrinsic sensory afferents (as identified by the presence of CGRP) associated with blood vessels in the colonic mesentery. Such vascular mesenteric afferents are important nociceptors known to transduce noxious stimuli including ischaemia, inflammation and traction of the mesentery.44,60 We also confirm the presence of mRNA transcripts for those subunits contributing to the M current, including KCNQ2, KCNQ3 and KCNQ5, in the soma of individual sensory neurons innervating the distal colon via either the lumbar splanchnic or pelvic nerves. By using the potent algogen bradykinin in rodent ex vivo recordings of visceral afferent activity, we show that the KV7/M-channel opener retigabine could abolish bradykinin-evoked responses thus supporting the KCNQ expression observed by single-cell qRT-PCR and immunohistochemistry in visceral nociceptors innervating the colon.
We observed significant co-expression of mRNA transcripts for B2 (Brdrb2), the primary receptor responsible for bradykinin sensitivity in visceral afferents,28,45,46 with KCNQ/M current relevant subtypes: KCNQ2, KCNQ3 and KCNQ5. Bradykinin is algogenic in human blister base applications61 and evokes nocifensive behaviours in rodents such as licking and paw lifting.62–64 B2 receptor-mediated activation undergoes tachyphylaxis or desensitization63,65,66 via NO-cGMP pathway and receptor down regulation,67–69 in agreement with our observations following sequential bradykinin application. Indeed, bradykinin acts to depolarize and sensitize sensory nociceptors via multiple downstream signalling cascades including PKA- and PKC-dependant phosphorylation of TRPA1,47 PKC-mediated phosphorylation of transient receptor potential vanilloid subfamily type 1,48 reduced Ca2+-dependent K+ conductance50,51 and also increased Ca2+ activated Cl− conductance.49 The latter mechanism contributing to membrane depolarization in sensory neurons due to elevated Cl− concentrations driven by constitutive activity of Na-K-Cl co-transporter.70,71 In addition, bradykinin inhibits M currents in sensory neurons via a PLC-IP3-Ca2+ pathway.52 In the present study, the ability of retigabine to completely suppress bradykinin-evoked firing would suggest that KV7 modulation is a critical mechanism by which visceral afferent sensitivity may be regulated; however, it is likely that multiple rheostatic mechanisms act in consort to enable neuronal depolarization. Indeed, opening of M channels, blockade of Ca2+ activated Cl− channels and genetic ablation of the persistent sodium channel NaV1.9 are all capable of attenuating nocifensive behaviours to intraplantar injection of bradykinin.52,62 As such, it is highly likely that KV7 channels represent one mechanism by which visceral afferents regulate firing and by which retigabine is able to reduce visceral pain behaviours in mice evoked by algogens capsaicin19 or acetic acid;21 mirroring recordings from the saphenous nerve where retigabine blocks responses to inflammatory mediators.59
Whilst bradykinin-mediated excitation is clearly closely coupled to M current inhibition, M current modulation is a downstream effector that is utilized by multiple extracellular signalling pathways to alter sensory neuronal sensitivity to stimuli. Receptors, such as purinergic P2Y1,2, histamine H1, bradykinin B2 and substance P NK1 are all capable of inducing PLC cascades, which are likely to lead to inhibition of M current in sensory neurons16 and that have well-characterized roles in potentiating visceral pain mechanisms.72,73 Indeed, activation of PAR-2 by exogenous proteases and mrgprD by β-alanine both inhibit M current causing depolarization leading to nociception.16,18
We also observe a strong modulatory effect of KV7 on mechanosensitivity in visceral afferents with retigabine capable of inhibiting both spontaneous discharge and evoked firing during phasic distension of the distal colon in a concentration-dependent manner. In contrast to saphenous recordings, peripheral nerve damage was not required for retigabine to attenuate responses to mechanical stimuli.59 Application of XE991 led to increased spontaneous discharge and also increased (∼30%) firing to distension in naïve animals, suggesting the presence of a background, and therefore suppressant, KV7 current in these preparations. Whilst slowly adapting (C and Aδ) mechanoreceptors innervating the paw have been shown to be resistant to KV7 block by XE991, rapidly-adapting (Aβ) mechanoreceptors may alter their firing characteristics to release a volley of action potential in response to mechanical probe.59 Linopirdine, a generic KV7 channel blocker74 potentiates excitability of rapidly adapting mechanoreceptors and D-hair (Aδ) receptors to mechanical probe; the former in a KV7.4-dependent manner.75 This heterogeneity in KV7 function in afferents innervating the paw seems to be less relevant to those innervating the viscera where fibres are typically less diverse in conduction velocity and fibre ending type.76,77 KV7 is likely to not only be able to modulate neuronal responses to chemical and mechanical stimuli but also to do so across the vast majority of afferents innervating the distal colon; a concept supported by the significant expression of KV7 observed by single-cell quantitative polymerase chain. The presence of KCNQ4 mRNA transcripts in some TL versus LS neurons could imply a role in mechanosensitivity given its association with touch sensation in mice and humans,78 although further studies are required to confirm this. Multiple ion channels have been associated with mechanotransduction in visceral afferents, including TRP34,35 and ASIC channels.79,80 We show that M-channel openers are capable of inhibiting depolarizing mechanical stimuli in a process that is likely to be independent of secondary intracellular signalling cascades.
In order to translate our findings to humans, we confirmed expression of KV7.3 and KV7.5 in GI tissues. Specifically, both were observed co-localizing with synaptic vesicle membrane protein synaptophysin and the neuropeptide CGRP. Synaptophysin is found within the submucosal and myenteric plexi, longitudinal and circular muscle and co-localizes with the pan-neuronal marker PGP9.5.81 Therefore, co-labelling of KV7 with synaptophysin and CGRP in human ileum and colon indicates expression within neuronal populations either extrinsically or intrinsically innervating the GI tract. Interestingly, the presence of KV7 in the mucosa and submucosal plexus of the ileum is also suggestive of a role in chemosensing and possibly in response to inflammatory stimuli. Whilst the GI smooth muscle of the colon expresses KV7 channels, we observed minimal labelling in either the circular or longitudinal muscle layers.24 Nevertheless, we cannot totally exclude the possibility that the inhibitory effects of retigabine are at least partially driven by direct activity of KV7 on smooth muscle. Using afferent recordings in human gut,28,37 we confirm that peripherally expressed KV7 channels can modulate human colonic afferent firing to noxious stimuli. In parity to mouse recordings discussed above, responses to repeat applications of bradykinin to the serosal surface of the colon were significantly attenuated in the presence of retigabine. These build on previous findings showing that retigabine, by increasing KV7 channel conductance, can alter excitability in isolated human sural nerve by confirming that KV7 opening can counteract disease-relevant stimuli applied to the native tissue with the endogenous nerve transduction architecture in place.26
In conclusion, we show that KV7 is expressed, and that opening of KV7 channels can inhibit chemical and mechanical responsiveness in mouse and human colonic afferents. We also confirm expression of KCNQ mRNA in gut-projecting DRG sensory neurons from both splanchnic and pelvic extrinsic pathways innervating the distal colon. As such KV7 channels contribute to modulating the excitability of afferents innervating the distal colon and are likely to be a key mechanism in acute excitation to inflammatory mediators such as bradykinin or increased sensitivity to normally innocuous mechanical stimuli in pathologies where visceral hypersensitivity is a principal symptom, such as IBS and inflammatory bowel disease. Our data support the hypothesis that a peripherally restricted KV7 channel opener capable of limiting centrally mediated side effects may represent a therapeutic opportunity for GI disease, especially where inflammation is an important component. As such, the data presented here offer promising evidence for the usefulness of KV7 channels as novel GI targets.
Acknowledgements
The authors thank Prof Charles H Knowles (National Centre for Bowel Research, Blizard Institute, Queen Mary University of London) for allowing us access to surgical resection tissue.
Author Contributions
Madusha Peiris and James RF Hockley conceived, designed and conducted experiments. David E Reed conducted electrophysiology experiments. L Ashley Blackshaw, David C Bulmer and Ewan St. John Smith supervised the project and assisted with data interpretation. All authors wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by an EFIC-Grunenthal grant awarded to Madusha Peiris, CAG/CIHR/CCFR Fellowship to David E Reed, Rosetrees Postdoctoral Grant (A1296) to James RF Hockley/Ewan St. John Smith, Medical Research Council Grant (G0900907) to David C Bulmer and a Wellcome Trust University Award to L Ashley Blackshaw.
References
- 1.Knowles CH, Aziz Q. Basic and clinical aspects of gastrointestinal pain. Pain 2009; 141: 191–209. [DOI] [PubMed] [Google Scholar]
- 2.Rivera-Arconada I, Lopez-Garcia JA. Retigabine-induced population primary afferent hyperpolarisation in vitro. Neuropharmacology 2006; 51: 756–763. [DOI] [PubMed] [Google Scholar]
- 3.Passmore GM, Selyanko AA, Mistry M, et al. KCNQ/M currents in sensory neurons: significance for pain therapy. J Neurosci 2003; 23: 7227–7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol 2009; 156: 1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du X, Hao H, Gigout S, et al. Control of somatic membrane potential in nociceptive neurons and its implications for peripheral nociceptive transmission. Pain 2014; 155: 2306–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tatulian L, Delmas P, Abogadie FC, et al. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J Neurosci 2001; 21: 5535–5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otto JF, Yang Y, Frankel WN, et al. A spontaneous mutation involving Kcnq2 (Kv7.2) reduces M-current density and spike frequency adaptation in mouse CA1 neurons. J Neurosci 2006; 26: 2053–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacob S, Nair AB. An updated overview on therapeutic drug monitoring of recent antiepileptic drugs. Drugs R D 2016; 16: 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi H, Iwata M, Tsuchimori N, et al. Activation of peripheral KCNQ channels attenuates inflammatory pain. Mol Pain 2014; 10: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Q, Fang D, Liu M, et al. Suppression of KCNQ/M (Kv7) potassium channels in dorsal root ganglion neurons contributes to the development of bone cancer pain in a rat model. Pain 2013; 154: 434–448. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Zhang H, Zhou N, et al. Tannic acid modulates excitability of sensory neurons and nociceptive behavior and the ionic mechanism. Eur J Pharmacol 2015; 764: 633–642. [DOI] [PubMed] [Google Scholar]
- 12.Passmore GM, Reilly JM, Thakur M, et al. Functional significance of M-type potassium channels in nociceptive cutaneous sensory endings. Front Mol Neurosci 2012; 5: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vetter I, Hein A, Sattler S, et al. Amplified cold transduction in native nociceptors by M-channel inhibition. J Neurosci 2013; 33: 16627–16641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coelho AM, Vergnolle N, Guiard B, et al. Proteinases and proteinase-activated receptor 2: a possible role to promote visceral hyperalgesia in rats. Gastroenterology 2002; 122: 1035–1047. [DOI] [PubMed] [Google Scholar]
- 15.Kayssi A, Amadesi S, Bautista F, et al. Mechanisms of protease-activated receptor 2-evoked hyperexcitability of nociceptive neurons innervating the mouse colon. J Physiol 2007; 580: 977–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linley JE, Rose K, Patil M, et al. Inhibition of M current in sensory neurons by exogenous proteases: a signaling pathway mediating inflammatory nociception. J Neurosci 2008; 28: 11240–11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avula LR, Buckinx R, Alpaerts K, et al. The effect of inflammation on the expression and distribution of the MAS-related gene receptors MrgE and MrgF in the murine ileum. Histochem Cell Biol 2011; 136: 569–585. [DOI] [PubMed] [Google Scholar]
- 18.Crozier RA, Ajit SK, Kaftan EJ, et al. MrgD activation inhibits KCNQ/M-currents and contributes to enhanced neuronal excitability. J Neurosci 2007; 27: 4492–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirano K, Kuratani K, Fujiyoshi M, et al. Kv7.2-7.5 voltage-gated potassium channel (KCNQ2-5) opener, retigabine, reduces capsaicin-induced visceral pain in mice. Neurosci Lett 2007; 413: 159–162. [DOI] [PubMed] [Google Scholar]
- 20.Rose K, Ooi L, Dalle C, et al. Transcriptional repression of the M channel subunit Kv7.2 in chronic nerve injury. Pain 2011; 152: 742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bi Y, Chen H, Su J, et al. Visceral hyperalgesia induced by forebrain-specific suppression of native Kv7/KCNQ/M-current in mice. Mol Pain 2011; 7: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong XZ, Harhun MI, Olesen SP, et al. Participation of KCNQ (Kv7) potassium channels in myogenic control of cerebral arterial diameter. J Physiol 2010; 588: 3277–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dedek K, Waldegger S. Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflugers Arch 2001; 442: 896–902. [DOI] [PubMed] [Google Scholar]
- 24.Jepps TA, Greenwood IA, Moffatt JD, et al. Molecular and functional characterization of Kv7 K+ channel in murine gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol 2009; 297: G107–G115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci 2000; 1: 21–30. [DOI] [PubMed] [Google Scholar]
- 26.Lang PM, Fleckenstein J, Passmore GM, et al. Retigabine reduces the excitability of unmyelinated peripheral human axons. Neuropharmacology 2008; 54: 1271–1278. [DOI] [PubMed] [Google Scholar]
- 27.Wladyka CL, Kunze DL. KCNQ/M-currents contribute to the resting membrane potential in rat visceral sensory neurons. J Physiol 2006; 575: 175–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGuire C, Boundouki G, Hockley JR, et al. Ex vivo study of human visceral nociceptors. Gut. Epub ahead of print 21 September 2016. doi: 10.1136/gutjnl-2016-311629. PMID: 27654583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson DR, McNaughton PA, Evans ML, et al. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil 2004; 16: 113–124. [DOI] [PubMed] [Google Scholar]
- 30.Green T, Dockray GJ. Calcitonin gene-related peptide and substance P in afferents to the upper gastrointestinal tract in the rat. Neurosci Lett 1987; 76: 151–156. [DOI] [PubMed] [Google Scholar]
- 31.Christianson JA, McIlwrath SL, Koerber HR, et al. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience 2006; 140: 247–257. [DOI] [PubMed] [Google Scholar]
- 32.Mitsui R. Characterisation of calcitonin gene-related peptide-immunoreactive neurons in the myenteric plexus of rat colon. Cell Tissue Res 2009; 337: 37–43. [DOI] [PubMed] [Google Scholar]
- 33.Su HC, Bishop AE, Power RF, et al. Dual intrinsic and extrinsic origins of CGRP- and NPY-immunoreactive nerves of rat gut and pancreas. J Neurosci 1987; 7: 2674–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brierley SM, Jones RCW, Gebhart GF, et al. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 2004; 127: 166–178. [DOI] [PubMed] [Google Scholar]
- 35.Brierley SM, Page AJ, Hughes PA, et al. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology 2008; 134: 2059–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hockley JRF, Tranter MM, McGuire C, et al. P2Y receptors sensitize mouse and human colonic nociceptors. J Neurosci 2016; 36: 2364–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peiris M, Bulmer DC, Baker MD, et al. Human visceral afferent recordings: preliminary report. Gut 2011; 60: 204–208. [DOI] [PubMed] [Google Scholar]
- 38.Hockley JR, Boundouki G, Cibert-Goton V, et al. Multiple roles for NaV1.9 in the activation of visceral afferents by noxious inflammatory, mechanical, and human disease-derived stimuli. Pain 2014; 155: 1962–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci 2005; 6: 850–862. [DOI] [PubMed] [Google Scholar]
- 40.Wickham H. Ggplot2: elegant graphics for data analysis. New York: Springer, 2009, p.viii, 212.
- 41.Wallace RH, Wang DW, Singh R, et al. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta1 subunit gene SCN1B. Nat Genet 1998; 19: 366–370. [DOI] [PubMed] [Google Scholar]
- 42.Schroeder BC, Hechenberger M, Weinreich F, et al. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J Biol Chem 2000; 275: 24089–24095. [DOI] [PubMed] [Google Scholar]
- 43.Roche JP, Westenbroek R, Sorom AJ, et al. Antibodies and a cysteine-modifying reagent show correspondence of M current in neurons to KCNQ2 and KCNQ3 K+ channels. Br J Pharmacol 2002; 137: 1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song X, Chen BN, Zagorodnyuk VP, et al. Identification of medium/high-threshold extrinsic mechanosensitive afferent nerves to the gastrointestinal tract. Gastroenterology 2009; 137: 274–284, 84 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brierley SM, Jones RC, Xu L, et al. Activation of splanchnic and pelvic colonic afferents by bradykinin in mice. Neurogastroenterol Motil 2005; 17: 854–862. [DOI] [PubMed] [Google Scholar]
- 46.Maubach KA, Grundy D. The role of prostaglandins in the bradykinin-induced activation of serosal afferents of the rat jejunum in vitro. J Physiol 1999; 515: 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang S, Dai Y, Fukuoka T, et al. Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: a molecular mechanism of inflammatory pain. Brain 2008; 131: 1241–1251. [DOI] [PubMed] [Google Scholar]
- 48.Lee SY, Lee JH, Kang KK, et al. Sensitization of vanilloid receptor involves an increase in the phosphorylated form of the channel. Arch Pharm Res 2005; 28: 405–412. [DOI] [PubMed] [Google Scholar]
- 49.Oh EJ, Weinreich D. Bradykinin decreases K(+) and increases Cl(-) conductances in vagal afferent neurones of the guinea pig. J Physiol 2004; 558: 513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinreich D. Bradykinin inhibits a slow spike afterhyperpolarization in visceral sensory neurons. Eur J Pharmacol 1986; 132: 61–63. [DOI] [PubMed] [Google Scholar]
- 51.Undem BJ, Weinreich D. Electrophysiological properties and chemosensitivity of guinea pig nodose ganglion neurons in vitro. J Auton Nerv Syst 1993; 44: 17–33. [DOI] [PubMed] [Google Scholar]
- 52.Liu B, Linley JE, Du X, et al. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl- channels. J Clin Invest 2010; 120: 1240–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hockley JR, Boundouki G, Cibert-Goton V, et al. Multiple roles for NaV1.9 in the activation of visceral afferents by noxious inflammatory, mechanical, and human disease-derived stimuli. Pain 2014; 155: 1962–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moll R, Franke WW, Schiller DL, et al. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982; 31: 11–24. [DOI] [PubMed] [Google Scholar]
- 55.Lynn PA, Olsson C, Zagorodnyuk V, et al. Rectal intraganglionic laminar endings are transduction sites of extrinsic mechanoreceptors in the guinea pig rectum. Gastroenterology 2003; 125: 786–794. [DOI] [PubMed] [Google Scholar]
- 56.King CH, Lancaster E, Salomon D, et al. Kv7.2 regulates the function of peripheral sensory neurons. J Comp Neurol 2014; 522: 3262–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gardiner M, Lehesjoki AE. Genetics of the epilepsies. Curr Opin Neurol 2000; 13: 157–164. [DOI] [PubMed] [Google Scholar]
- 58.Hughes S, Marsh SJ, Tinker A, et al. PIP(2)-dependent inhibition of M-type (Kv7.2/7.3) potassium channels: direct on-line assessment of PIP(2) depletion by Gq-coupled receptors in single living neurons. Pflug Arch Eur J Phy 2007; 455: 115–124. [DOI] [PubMed] [Google Scholar]
- 59.Roza C, Lopez-Garcia JA. Retigabine, the specific KCNQ channel opener, blocks ectopic discharges in axotomized sensory fibres. Pain 2008; 138: 537–545. [DOI] [PubMed] [Google Scholar]
- 60.Brookes SJ, Spencer NJ, Costa M, et al. Extrinsic primary afferent signalling in the gut. Nat Rev Gastroenterol Hepatol 2013; 10: 286–296. [DOI] [PubMed] [Google Scholar]
- 61.Armstrong D, Jepson JB, Keele CA, et al. Pain-producing substance in human inflammatory exudates and plasma. J Physiol 1957; 135: 350–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amaya F, Wang H, Costigan M, et al. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci 2006; 26: 12852–12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferreira J, da Silva GL, Calixto JB. Contribution of vanilloid receptors to the overt nociception induced by B2 kinin receptor activation in mice. Br J Pharmacol 2004; 141: 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Griesbacher T, Amann R, Sametz W, et al. The nonpeptide B2 receptor antagonist FR173657: inhibition of effects of bradykinin related to its role in nociception. Br J Pharmacol 1998; 124: 1328–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beck PW, Handwerker HO. Bradykinin and serotonin effects on various types of cutaneous nerve fibers. Pflugers Arch 1974; 347: 209–222. [DOI] [PubMed] [Google Scholar]
- 66.Burgess GM, Mullaney I, McNeill M, et al. Second messengers involved in the mechanism of action of bradykinin in sensory neurons in culture. J Neurosci 1989; 9: 3314–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McGehee DS, Goy MF, Oxford GS. Involvement of the nitric oxide-cyclic GMP pathway in the desensitization of bradykinin responses of cultured rat sensory neurons. Neuron 1992; 9: 315–324. [DOI] [PubMed] [Google Scholar]
- 68.Bauer MB, Simmons ML, Murphy S, et al. Bradykinin and capsaicin stimulate cyclic GMP production in cultured rat dorsal root ganglion neurons via a nitrosyl intermediate. J Neurosci Res 1993; 36: 280–289. [DOI] [PubMed] [Google Scholar]
- 69.Harvey JS, Burgess GM. Cyclic GMP regulates activation of phosphoinositidase C by bradykinin in sensory neurons. Biochem J 1996; 316: 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Plotkin MD, Kaplan MR, Peterson LN, et al. Expression of the Na(+)-K(+)-2Cl- cotransporter BSC2 in the nervous system. Am J Physiol 1997; 272: C173–C183. [DOI] [PubMed] [Google Scholar]
- 71.Sung KW, Kirby M, McDonald MP, et al. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci 2000; 20: 7531–7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blackshaw LA. Transient receptor potential cation channels in visceral sensory pathways. Br J Pharmacol 2014; 171: 2528–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burnstock G. Purinergic receptors and pain. Curr Pharm Des 2009; 15: 1717–1735. [DOI] [PubMed] [Google Scholar]
- 74.Søgaard R, Ljungstrøm T, Pedersen KA, et al. KCNQ4 channels expressed in mammalian cells: functional characteristics and pharmacology. Am J Physiol Cell Physiol 2001; 280: C859–C866. [DOI] [PubMed] [Google Scholar]
- 75.Heidenreich M, Lechner SG, Vardanyan V, et al. KCNQ4 K(+) channels tune mechanoreceptors for normal touch sensation in mouse and man. Nat Neurosci 2011; 15: 138–145. [DOI] [PubMed] [Google Scholar]
- 76.Janig W, Koltzenburg M. Receptive properties of sacral primary afferent neurons supplying the colon. J Neurophysiol 1991; 65: 1067–1077. [DOI] [PubMed] [Google Scholar]
- 77.Sengupta JN, Gebhart GF. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol 1994; 71: 2046–2060. [DOI] [PubMed] [Google Scholar]
- 78.Heidenreich M, Lechner SG, Vardanyan V, et al. KCNQ4 K(+) channels tune mechanoreceptors for normal touch sensation in mouse and man. Nat Neurosci 2011; 15: 138–145. [DOI] [PubMed] [Google Scholar]
- 79.Page AJ, Brierley SM, Martin CM, et al. Acid sensing ion channels 2 and 3 are required for inhibition of visceral nociceptors by benzamil. Pain 2007; 133: 150–160. [DOI] [PubMed] [Google Scholar]
- 80.Page AJ, Brierley SM, Martin CM, et al. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut 2005; 54: 1408–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bottner M, Harde J, Barrenschee M, et al. GDNF induces synaptic vesicle markers in enteric neurons. Neurosci Res 2013; 77: 128–136. [DOI] [PubMed] [Google Scholar]