Abstract
Presynaptic GABAB receptors (GABABRs) are highly expressed in dorsal root ganglion neurons and spinal cord dorsal horn. GABABRs located in superficial dorsal horn play an important antinociceptive role, by acting at both pre- and postsynaptic sites. GABABRs expressed in deep dorsal horn could be involved in the processing of touch sensation and possibly in the generation of tactile allodynia in chronic pain. The objective of this study was to characterize the morphological and functional properties of GABABRs expressed on Aβ fibers projecting to lamina III/IV and to understand their role in modulating excitatory synaptic transmission. We performed high-resolution electron microscopic analysis, showing that GABAB2 subunit is expressed on 71.9% of terminals in rat lamina III-IV. These terminals were engaged in axodendritic synapses and, for the 46%, also expressed glutamate immunoreactivity. Monosynaptic excitatory postsynaptic currents, evoked by Aβ fiber stimulation and recorded from lamina III/IV neurons in spinal cord slices, were strongly depressed by application of baclofen (0.1–2.5 µM), acting as a presynaptic modulator. Application of the GABABR antagonist CGP 55845 caused, in a subpopulation of neurons, the potentiation of the first of two excitatory postsynaptic currents recorded with the paired-pulse protocol, showing that GABABRs are endogenously activated. A decrease in the paired-pulse ratio accompanied the effect of CGP 55845, implying the involvement of presynaptic GABABRs. CGP 55845 facilitated only the first excitatory postsynaptic current also during a train of four consecutive stimuli applied to Aβ fibers. These results suggest that GABABRs tonically inhibit glutamate release from Aβ fibers at a subset of synapses in deep dorsal horn. This modulation specifically affects only the early phase of synaptic excitation in lamina III-IV neurons.
Keywords: Presynaptic inhibition, dorsal horn, GABAB receptors, mechanoreception
Introduction
The superficial (I-II) and deep (III-IV) laminae of the dorsal horn of the spinal cord are under strong inhibitory control by the γ-aminobutyric acid (GABA). GABA, the main inhibitory transmitter in the central nervous system (CNS), modulates neurotransmitter release and neuronal excitability by acting on two types of specific receptors referred to as GABAA receptors (GABAARs) and GABAB receptors (GABABRs) that may have both pre- and postsynaptic localizations. In the dorsal horn, GABAARs and GABABRs may be localized at the level of the primary afferent terminals originating from the dorsal root ganglion (DRG) neurons, the cell bodies and processes of certain interneurons, and the terminals of descending fibers from supraspinal centers. Anatomically, the distribution of GABABRs in these compartments of the dorsal horn neuropil has been widely investigated,1–8 and functionally, it was demonstrated that presynaptic GABABRs exert an important modulatory role on nociceptive transmission.9 In laminae I-II, electrophysiological studies performed in rat demonstrated that the exogenous or endogenous activation of presynaptic GABABRs inhibits the release of peptides and glutamate from the primary afferent terminals of Aδ and C types5,10–14 and decreases the release of GABA and glycine from the spinal inhibitory interneurons.14–16 The depression of neurotransmitter release mediated by GABABRs derives from the concurrent inhibition of presynaptic calcium channels17–19 and release machinery downstream Ca2+ entry into the nerve terminals.20
Investigators paid comparatively little attention to the contribution of GABABRs to the elaboration of tactile information in laminae III-IV. In a previous study in rat, we showed that the synapses between low-threshold myelinated A fibers and lamina III/IV neurons are regulated by endogenous GABA acting on presynaptic GABAARs, and that these receptors are activated during the repetitive stimulation of the A fibers, thus modulating glutamate release and neuron excitability.21 Our observations were in line with the notion that the large size DRG neurons, which give rise to the A fibers, express the GABAARs at their terminals in laminae III-IV.22 Notably, GABABRs are also expressed on large diameter DRG neurons and central terminals of the myelinated A fibers.5,23 Thus, it seems possible that this family of GABARs also intervenes in the modulation of mechanosensation. In keeping with this possibility, activation of GABABRs by the specific agonist baclofen depresses the excitatory postsynaptic currents (EPSCs) evoked by tactile stimuli in vivo.24
By combined morphological and electrophysiological approaches, we investigated the subcellular localization and functional properties of the GABABRs expressed on Aβ fibers and their role in modulating the excitability of lamina III/IV neurons in the rat spinal cord. By recording from an in vitro spinal cord slice preparation, we show here that presynaptic GABABRs expressed on Aβ fibers are endogenously activated and contribute to regulate glutamate release during primary afferent stimulation.
Methods
Animals
The Italian Ministry of Health approved all experiments that were conducted on young adult rats (P20-P60) in accordance with the Guide for the Care and Use of Laboratory Animals and the European Union and Italian regulations on animal welfare. Three rats were used for electron microscopy preparations; 70 rats were employed in electrophysiological experiments.
Electron microscopy
Tissue preparation
After perfusion with glutaraldehyde (0.1%), formaldehyde (4%), and picric acid (0.2%) in 0.12 M sodium phosphate buffer, pH 7.4, spinal cord blocks were cut in 200 µm transverse sections with a vibrating blade microtome, and slammed to a polished copper block cooled with liquid N2 in a MM80E cryofixation apparatus (Reichert, Vienna, Austria). Sections were then transferred to 0.5% uranyl acetate dissolved in anhydrous methanol (−90℃) in a freeze-substitution apparatus (CS Auto, Leica, Deerfield, IL). The temperature was raised stepwise to −50℃. Samples were finally infiltrated with Lowicryl HM20 resin (Chemische Werke Lowi, Waldkraiburg, Germany) and polymerized by ultraviolet light.25
Primary antibodies and controls
Primary antibodies were rabbit anti-GABAB2 (1:10; cat. number: ab 75838; Abcam, Cambridge, UK)26 and mouse anti-glutamate (1:10, antibodies-online.com, cat. number: 22523; Atlanta, GA). Routine immunocytochemical controls consisted in omission of primary antibodies or their substitution with normal serum.27
Post-embedding electron microscopy
Lowicryl ultrathin sections were cut with an ultramicrotome (EM UC6; Leica) and then immunostained on grids following a post-embedding protocol specifically developed for Lowicryl-embedded sections.28 All incubations were carried out at room temperature unless otherwise stated. Sections were first etched with a saturated solution of NaOH in absolute ethanol for 2–3 s, then rinsed with double-distilled water. They were then incubated sequentially in the following solutions: (1) 0.1% sodium borohydride and 50 mM glycine in Tris buffer (5 mM) containing 0.1% NaCl and 0.1% Triton X-100 (TBNT) (10 min); (2) 2% bovine serum albumin (BSA) in TBNT (10 min); (3) antibodies to glutamate (1:10) and/or GABAB2 (1:10) in TBNT containing 2% BSA (overnight); (4) TBNT (several rinses) and 2% BSA in TBNT (10 min); and (5) goat anti-mouse and/or goat anti-rabbit secondary antibodies coupled to 10 or 20 nm colloidal gold particles (GPs; British BioCell International, Cardiff, UK), diluted 1:20 in TBNT with 2% BSA and 0.05% polyethylene glycol (2 h). Grids were then rinsed several times in double-distilled water, counterstained with uranyl acetate and lead citrate, and examined with a JEM-1010 transmission electron microscope (Jeol, Tokyo, Japan) equipped with a side-mounted CCD camera (Mega View III, Olympus Soft Imaging System, Munster, Germany).
Quantitative immunogold analysis
To assess whether the GABAB2 subunit was localized at synapses and/or extrasynaptic sites, counts were performed on 90 randomly selected ultrathin sections cut across laminae III-IV of the spinal dorsal horn. The two laminae were first recognized in toluidine blue-stained semithin sections (Figure 1(a)) following the classical description of the spinal cord cytoarchitectonic organization.29 Then, the tissue block was trimmed accordingly, and random images (N = 153, n = 3 rats) were collected at a magnification of 30000×. The number of GPs/area (N/µ2) over the synaptic specializations, axon terminals, and dendrites were calculated using count particle function of the ImageJ software (NIH, Bethesda, MD). The density of GPs over mitochondria was chosen as an index of background staining, and subtracted from values obtained for the other cellular compartments. GPs were considered associated with the synaptic specializations when they were located over the synaptic cleft, the pre- or the postsynaptic membranes, or at a distance ≤30 nm from the membrane profiles engaged in the synapse. The distance between the center of each GP and the center of the synaptic cleft was measured to determine the distribution of GPs along the pre- or postsynaptic membrane.28 In this manner, GPs were considered at pre- or postsynaptic sites when their number on one side was three-fold higher than on the other side. A synapse was considered immunopositive when at least four GPs were associated to it according to the criteria described above.30
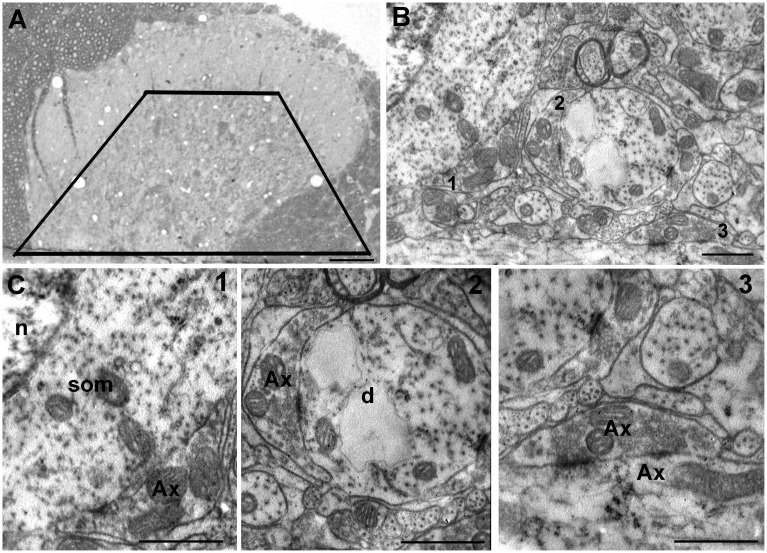
Figure 1.
Topographical identification and examples of synapses in laminae III-IV of the rat dorsal horn: (a) A toluidine blue-stained semithin section with a black trapezium indicating the area of the dorsal horn, comprising laminae III-IV, subsequently trimmed to cut ultrathin sections; (b) TEM low magnification of laminae III-IV immunolabeled for GABAB2; and (c) higher magnifications of axosomatic (1), axodendritic (2), and axo-axonic (3) profiles from the ultrathin section showed in (b). Scale bars: (a) 200 µm; (b) and (c) 250 nm.
Differences in GP densities among individual synaptic and extrasynaptic compartments at both axon terminals and dendrites were evaluated with two-way analysis of variance (ANOVA) test without repeated measures followed by Bonferroni multiple comparisons test. Data were expressed as mean ± SEM and differences were considered significant for p < 0.05.
To assess the degree of co-expression between glutamate and GABAB2 in 150 axon terminals, randomly selected profiles from three rats were directly counted at the electron microscope. Data were expressed as percentages of co-expression.
Electrophysiology
Spinal cord slice preparation
Animals were anesthetized with isoflurane and decapitated, the spinal cord and vertebrae were rapidly removed and placed in ice-cold dissecting Krebs’ solution (composition in mM:125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 25 glucose, 6 MgCl2, 1.5 CaCl2, and 1 kynurenic acid, pH 7.4, 320 mOsm), bubbled with 95% O2 and 5% CO2. The lumbar spinal cord was isolated, embedded in an agarose block (low-melting point agarose 3%, Thermo Fisher Scientific, Waltham, MA), and transverse slices (500 µm thick) were obtained using a vibrating microtome (WPI, Sarasota, FL). Slices were incubated in oxygenated Krebs’ solution (same as dissecting but without kynurenic acid) at 35℃ for 1 h and used for recording.
Patch-clamp recording and dorsal root stimulation
Patch-clamp recording in whole-cell configuration was performed on visually identified lamina III-IV neurons at room temperature. Neurons were visualized using an Axioskop microscope (Zeiss, Oberkochen, Germany), fitted with Nomarski optics and connected to a CCD camera (Dage-MTI, Michigan City, IN). Lamina III-IV neurons were identified as being ventral to the translucent lamina II layer and larger than the lamina II neurons. All recorded cells were located at a distance of 200–300 µm from the white matter, corresponding to the position of laminae III-IV in rat lumbar spinal cord.31,32 Slices were perfused at 2 ml/min with recording Krebs’ solution (in mM:125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 25 glucose, 1 MgCl2, and 2 CaCl2, pH 7.4, 320 mOsm). Recordings of EPSCs were performed in voltage-clamp using thick-walled borosilicate pipettes (3–5 MOhm resistance), filled with a solution having the following composition (in mM): 130 cesium methanesulfonate, 10 sodium methanesulfonate, 10 EGTA, 1 CaCl2, 10 HEPES, 5 lidocaine N-ethyl bromide quaternary salt-Cl, 2 ATP-Mg, pH adjusted to 7.2 with CsOH, osmolarity 300 mOsm. In order to isolate the contribution of postsynaptic GABABRs activated by baclofen, in a subset of experiments the recording pipettes were filled with a potassium-based solution having the following composition (in mM): 120 potassium methanesulfonate, 10 NaCl, 10 EGTA, 1 CaCl2, 10 HEPES, 5 ATP-Mg, pH adjusted to 7.2 with KOH, osmolarity 300 mOsm. Junction potential was corrected after recording. Data were recorded and acquired using a Multiclamp 700 A amplifier and the pClamp 10 software (Molecular Devices, Sunnyvale, CA). Sampling rate was 10 kHz, and data were filtered at 2 kHz.
The dorsal root attached to each slice was stimulated using a suction electrode (stimulus intensities: 10–25 µA, duration: 0.1 ms). Monosynaptic EPSCs were identified by recording 20 consecutive traces at 20 Hz.32 Experiments on evoked synaptic responses were performed only on neurons where the EPSC was clearly visible at each trace and temporally linked to the stimulus artefact. Neurons exhibiting a large polysynaptic component shunting the subsequent EPSC were not included in this study.
Drugs, data analysis, and statistics
Drugs were bath applied for 3–5 min. Responses were recorded continuously in control and during drug application (Figures 3(b) and 4(b)). Baclofen and CGP 55845 were obtained from Sigma-Aldrich (St Louis, MO), gabazine (SR95531) was obtained from Abcam (Cambridge, UK). Data were analyzed off-line using pClamp10 software. Analysis of drug effects on synaptic responses was performed on EPSCs recorded 2–3 min after the start of drug application. EPSC peak amplitudes were determined in a constant 2 ms window. Mean peak amplitudes and paired-pulse ratios (PPRs) were calculated from 5–6 averaged traces in the different experimental conditions. The total charge transfer of EPSCs evoked by a stimulation train of four stimuli was determined by taking the area under the traces between the baseline time point immediately before the first EPSC and the time point at which the fourth EPSC recovered to baseline.
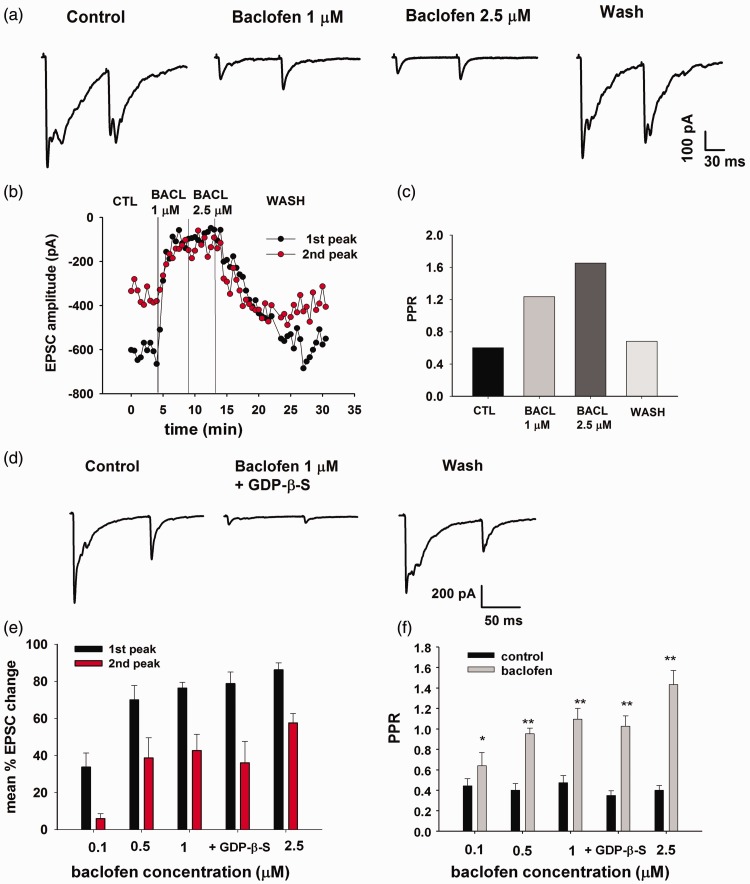
Figure 3.
The GABAB receptor agonist baclofen depresses the amplitude of evoked EPSCs and increases the PPR in laminae III/IV neurons. (a) Effect of 1 and 2.5 µM baclofen on EPSCs evoked by Aβ fiber stimulation and recorded from a laminae III/IV neuron held at −70 mV. The EPSCs were recorded applying the paired-pulse protocol (100 ms interval). At each stimulus, baclofen depresses the early, monosynaptic EPSC and completely suppresses the slower, polysynaptic response. (b) Time course of baclofen effect in the same neuron. (c) PPR determined from the EPSCs shown in (a), in the different experimental conditions. Both baclofen concentrations increase PPR, suggesting a presynaptic effect. (d) Intracellular perfusion of the G protein blocker GDP-β-S does not prevent the depressing effect of 1 µM baclofen on evoked EPSCs, recorded from a different laminae III/IV neuron. (e) Dose-dependent effect of baclofen on peak amplitudes of evoked EPSCs, recorded with the paired-pulse protocol from a total of 45 laminae III/IV neurons. (f) PPR values obtained from the same EPSCs analyzed in (e), determined in control and in the presence of different concentrations of baclofen. A significant increase of PPR was observed at each baclofen concentration (paired t-test, p < 0.05 or p < 0.01).
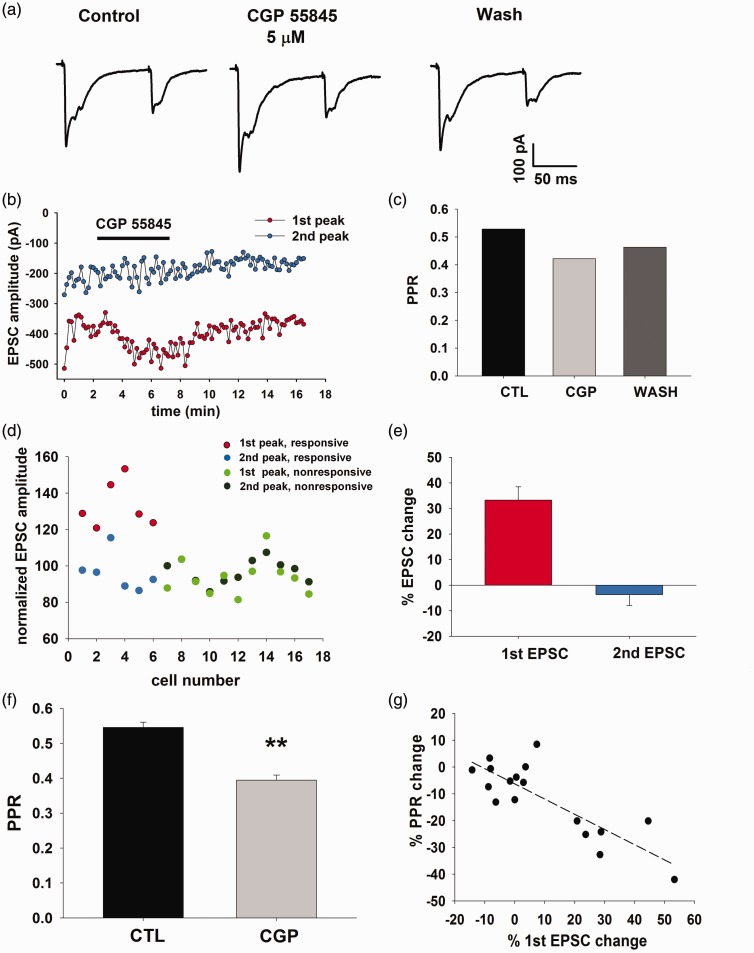
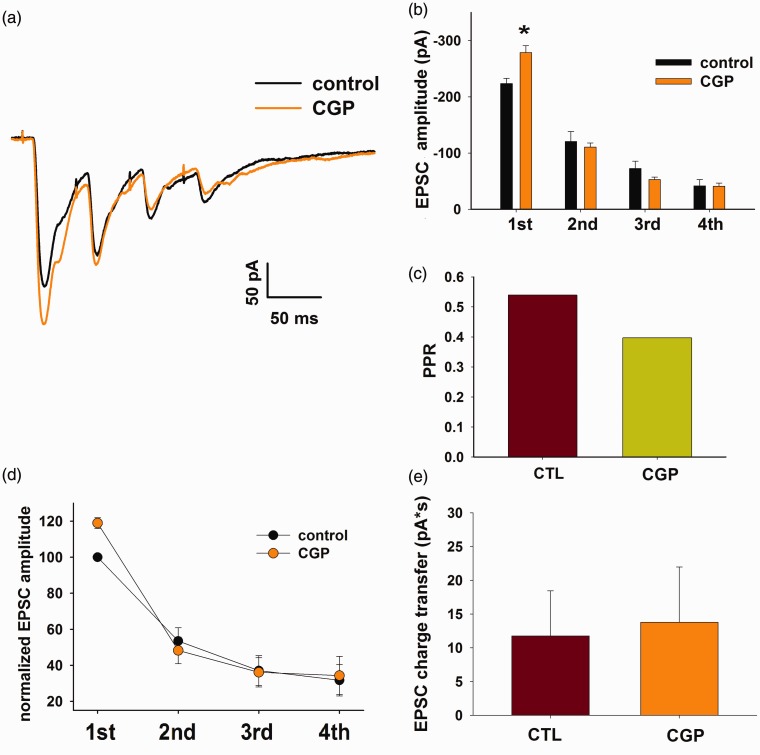
Figure 4.
The endogenous activation of presynaptic GABAB receptors inhibits Aβ-fiber-mediated EPSCs. (a) Application of the GABAB receptor antagonist CGP 55845 (5 µM) causes the increase of the first EPSC, recorded with the paired-pulse protocol (averaged EPSCs). (b) Time course of the effect of 5 min application of CGP 55845. (c) The GABAB antagonist produces a decrease of the PPR of the EPSCs shown in A, indicating a presynaptic effect. (d) Normalized EPSCs amplitudes recorded in a sample of 17 neurons with the paired-pulse protocol. Red and blue circles represent the EPSC amplitudes (red: first peak and blue: second peak) recorded from the neurons significantly affected by CGP 55845 (n = 6). Light and dark green circles represent the amplitudes of the first and second EPSCs, respectively, not potentiated by CGP 55845 application (n = 11). (e) Mean percentage EPSC amplitude change in the six neurons responsive to CGP 55845. (f) PPR is significantly decreased by CGP 55845 in the responsive neurons (paired t-test, p < 0.01, n = 6). (g) Plot of the PPR change versus the first EPSC change, obtained from the whole sample of 17 neurons. The negative correlation between the two sets of data suggests that CGP 55845 causes an increase in release probability in the first EPSC (Pearson product moment correlation test: r = −0.82; p < 0.001, n = 17). EPSC: excitatory postsynaptic currents; PPR: paired-pulse ratio.
Graphs were obtained using Sigmaplot 11 (Systat software, San Jose, CA) and statistical analysis was performed using GraphPad (GraphPad software, San Diego, CA). Data were expressed as mean ± SEM and differences were considered significant for p < 0.05.
Results
Expression of GABAB2 subunit in laminae III-IV of the spinal dorsal horn
We focused our attention on the distribution of the GABAB2 subunit in laminae III-IV that are easily distinguished by the presence of a large number of myelinated axons and loosely packed neurons of heterogeneous size, with a prevailing incidence of larger cells than lamina II.29,33 Laminae III-IV are also characterized by the prevailing presence of axo-dendritic synapses and less numerous synapses of the axo-somatic, axo-axonic, and glomerular types (Figure 1(b) and (c)). Therefore, we evaluated the relative incidence of GABAB2 in these types of synapses in the two laminae (Figures 1 and 2). After observation of 100 randomly selected profiles, GABAB2 immunoreactivity was localized in 42% (30 of 72) of the axo-dendritic synapses, whereas no labeling was detected in axo-axonic (N = 10) or axo-somatic (N = 15) contacts and in glomeruli (N = 3).
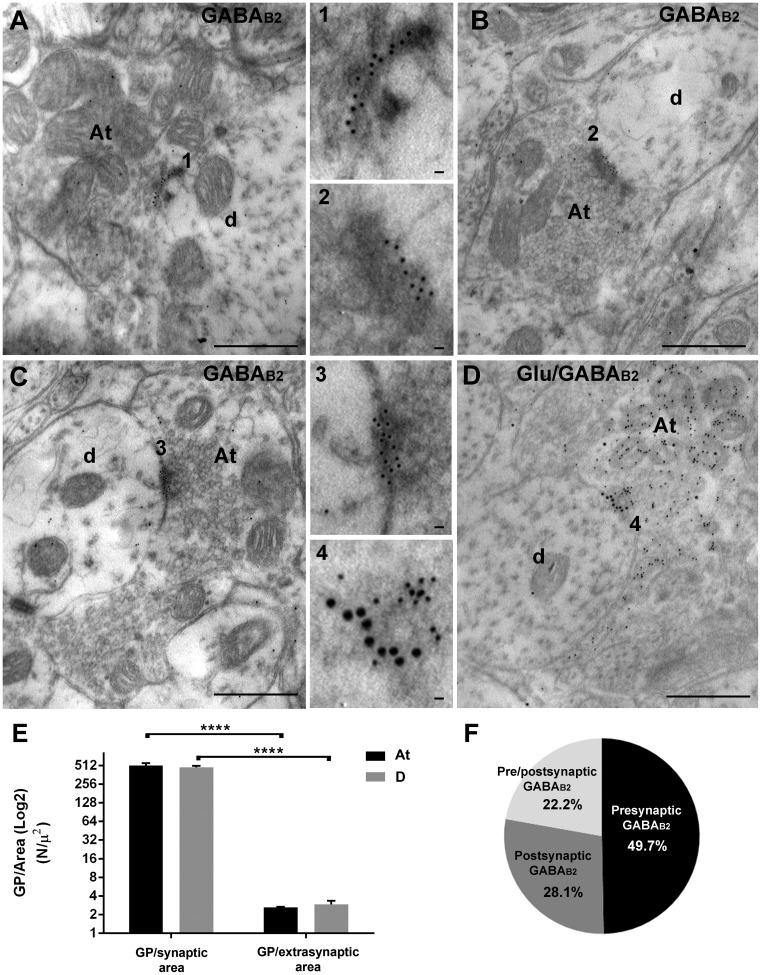
Figure 2.
Localization of GABAB2 at axo-dendritic synapses in laminae III-IV. (a) A GABAB2-immunoreactive At makes a synapse with an unlabeled dendrite (d). GPs are specifically distributed along the presynaptic membrane (insert number 1). (b) A unlabeled At makes a synapse with a GABAB2-immunoreactive dendrite (d). GPs are distributed along the postsynaptic density (insert number 2). (c): A GABAB2-immunoreactive At makes a synapse with a GABAB2-immunoreactive dendrite (d). GPs are scattered over both the pre- and postsynaptic densities (insert number 3). (d) A glutamate + GABAB2-immunoreactive At makes a synapse with an unlabeled dendrite (d). Glutamate-IR is characterized by 10 nm GPs, scattered all over the At, while GABAB2 is evidenced by 20 nm GPs specifically distributed along the presynaptic membrane (insert number 4). (e) Bar chart showing GPs densities (GP/Area, N/µ2) at synaptic and extrasynaptic sites of (At, black bars) and dendrites (D; gray bars).No statistical difference was shown between At and D for both GP/synaptic area (two-way ANOVA, not repeated measures, p = 0.5725) and GP/extrasynaptic area (two-way ANOVA, not repeated measures, p = 0.5725). GPs at the synapse were significantly different from GPs at extrasynaptic sites in both At and D (two-way ANOVA, not repeated measures followed by Bonferroni multiple comparisons test, ****p < 0.0001). (f) pie chart showing the results of quantitative analysis on the percentage of expression of GABAB2 at presynaptic sites (black; 49.7%), postsynaptic sites (dark gray; 28.1%), or both (light gray; 22.2%). Scale bar: 500 nm; inserts: 20 nm. GABA: γ-aminobutyric acid; At: axon terminal; D: dendrite.
Then, after observation of 153 labeled axodendritic profiles, 49.7% of GABAB2 subunit was distributed at the presynaptic axonal membrane of the synapse (Figure 2(a) and (f)), 28.1% at the postsynaptic dendritic membrane (Figure 2(b) and (f)), and 22.2% at both the pre- and postsynaptic membranes of the same axodendritic synapse (Figure 2(c) and (f)).
GPs indicative of GABAB2 localization were concentrated at synaptic specializations (two-way ANOVA, p < 0.0001; Bonferroni multiple comparisons test, p < 0.0001; Figure 2(e)). When both the axon and dendrite of the same synapse were immunoreactive, there was not a preferential axonal (presynaptic) or dendritic (postsynaptic) distribution (two-way ANOVA, p = 0.5725; Figure 2(e)). The few GPs at extrasynaptic sites were randomly distributed along the membrane of immunoreactive terminals or dendrites, without significant statistical differences between the two compartments (two-way ANOVA, p = 0.5752; Figure 2(e)).
Axon terminals expressing GABAB2 contained scattered small agranular vesicles and numerous mitochondria and made simple asymmetric synapses with dendrites. To better characterize the neurochemistry of these terminals, we performed double immunogold labeling experiments with a mixture of the glutamate and GABAB2 antibodies. In these experiments, a large fraction of GABAB2 immunoreactive axons were double labeled, with GPs indicative of GABAB2 localized at the synaptic specialization and those depicting glutamate immunoreactivity scattered all over the axoplasm and associated with the small agranular synaptic vesicles herein contained (Figure 2(d)). After observation of 150 GABAB2 (50/animal) randomly selected immunoreactive axon terminals, 47.3% were double labeled, 40% were GABAB2+ only, and 12.7% were glutamate+ only.
Presynaptic GABABRs activated by baclofen modulate glutamate release from Aβ fibers
In most-recorded laminae III/IV neurons, low-intensity (10–25 µA) dorsal root stimulation evoked monosynaptic glutamatergic EPSCs that did not show failures during high-frequency stimulation (20 Hz) and exhibited a stable latency (latency variability <1 ms). These electrophysiological features were compatible with EPSCs evoked by Aβ fiber stimulation, as shown in previous studies performed in juvenile rats.32,34 We tested the effect of the GABAB receptor agonist baclofen on monosynaptic EPSCs, recorded with the paired-pulse protocol (100 ms interval). Both 1 and 2.5 µM baclofen rapidly caused the depression of the two EPSCs (Figure 3(a) and (b)). As shown in Figure 3(a), baclofen also caused a marked change in shape of the EPSCs, due to the complete block of the polysynaptic response. This effect was observed in all neurons exhibiting polysynaptic activity before baclofen application.
The PPR was determined from the average peak amplitudes of the monosynaptic EPSCs, in control and in presence of baclofen. Since in all tested neurons the first EPSC was depressed more than the second was, the value of PPR increased, as expected for a presynaptic effect (Figure 3(c)).
We tested a total of 35 neurons for different concentrations of baclofen, ranging from 0.1 to 2.5 µM: at each dose, we observed significant depressions of the first EPSC in all tested neurons (t-test on individual neurons, p < 0.05, Figure 3(e)) and significant increases of the PPR value (Figure 3(f)).
Polysynaptic EPSCs evoked by the first stimulus and overlapping with the second EPSC could cause postsynaptic shunting. Thus, the loss of polysynaptic EPSCs in baclofen could affect the measurement of the second EPSC and the determination of PPR. However, we did not find any significant correlation between the amplitude of the first polysynaptic response in control (measured just before the second stimulus and normalized by the second peak) and the effect of baclofen on PPR (baclofen 1 µM, Pearson correlation test, p = 0.94, n = 10). This would suggest that changes in postsynaptic shunting did not significantly contribute to the increase of PPR by baclofen.
To exclude a contribution of postsynaptic GABABRs and further confirm the presynaptic nature of the baclofen effect, we performed a separate set of experiments in which the neurons were intracellularly perfused for at least 20 min with the G protein blocker Guanosine 5'-[beta-thio]diphosphate (GDP-β-S). In these conditions, we tested 1 µM baclofen on the two EPSCs recorded with the paired-pulse protocol (Figure 3(d)), obtaining values of percentage depression not significantly different from those observed in absence of GDP-β-S (Figure 3(e); t-test, first peak: p = 0.85 and second peak: p = 0.76; n = 10).
Data represented in Figure 3 were obtained by perfusing the neurons with a cesium-based intracellular solution. In these conditions, we did not observe a significant change in the holding current, excluding a substantial activation of postsynaptic GABABRs. Since postsynaptic GABABRs are supposed to act mainly through the opening of inwardly rectifying potassium channels,35 the presence of intracellular cesium could have prevented their activation. Therefore, we tested for baclofen (1 and 2.5 µM), a group of neurons intracellularly perfused with a potassium-based solution. At the holding potential of −60 mV, we recorded an outward current in 11 out of 12 cells tested (mean current amplitude: baclofen 1 µM, 24.9 ± 5.9 pA; baclofen 2.5 µM, 38.9 ± 10.1 pA), confirming the presence of postsynaptic GABABRs on laminae III/IV neurons.
Endogenously activated GABABRs regulate synaptic transmission in laminae III/IV
We then investigated whether GABABRs expressed on Aβ fibers could be endogenously activated, by applying the GABAB antagonist CGP 55845 (5 µM) during recording of evoked EPSCs, and using the paired-pulse protocol. As shown in Figure 4(a) and (b), CGP 55845 significantly increased the first EPSC, while the second EPSC was unchanged (or, in some cases, depressed) and the PPR value decreased (Figure 4(c)). Since these recordings were performed using a cesium-based intracellular solution, we were not able to determine whether CGP 55845 blocked a tonic current mediated by postsynaptic GABABRs, which usually involves the activation of potassium channels.
We observed a significant potentiation of the first EPSC in the presence of CGP 55845 in 6 out of 17 neurons tested (Figure 4(d) and (e); t-test on individual neurons, p < 0.05), accompanied by a significant decrease of PPR in the responsive neurons (Figure 4(f)). In addition, the percentage change of PPR was significantly correlated with the variation of the first EPSC (Figure 4(g)), suggesting that the GABAB antagonist causes an increase in release probability at the first response.36
The effect of CGP 55845 on the first EPSC suggested that GABA tonically activates GABABRs expressed on Aβ fibers, causing a significant depression of glutamate release at the first stimulus. This could imply that GABAB presynaptic action is particularly effective only at the beginning of a stimulation train, as observed at other central synapses.36 We tested this hypothesis by repetitively stimulating the dorsal root with a four pulses train at the frequency of 20 Hz. The responses evoked at the second, third, and fourth stimulus in control were usually strongly depressed compared to the first EPSC, as previously reported at this synapse.21 Application of CGP 55845 (5 µM) induced a significant potentiation only of the first EPSC and the decrease of the PPR value (Figure 5(a) to (c)). The subsequent three EPSCs were not significantly affected by CGP 55845. In a sample of 13 neurons, we observed a significant effect of CGP 55845 on the first EPSCs in five neurons, with an average increase of 18.9 ± 2.9% (Figure 5(d); t-test on individual cells, p < 0.05). In all five responsive neurons, the subsequent peaks were not significantly changed. High-frequency stimulation trains produce the temporal summation of the synaptic responses, that can be monitored through the total charge transferred along the trains.36 As shown in Figure 5(e), CGP 55845 failed to induce a significant change in the total EPSC charge transfer during repetitive stimulation, confirming that GABABRs specifically affect the initial release events triggered by a stimulation train.
Figure 5.
GABABR-mediated presynaptic modulation specifically affects the first EPSC during repetitive stimulation. (a) to (c): Example of EPSCs recorded from a lamina III/IV neuron, during dorsal root repetitive stimulation (20 Hz), in control and in the presence of CGP 55845. Only the first peak is significantly affected by CGP 55845 (b) (t-test performed on samples of eight traces for each condition, p < 0.05), and the PPR is decreased (c). (d) Normalized EPSC amplitude during the four-pulses train, in control and CGP 55845, obtained from the five neurons (of 13) where CGP 55845 had a significant effect on the first EPSC. (e) Despite different short-term dynamics, no relevant difference was observed in EPSC charge transfer between control and CGP 55845 in the same neurons (paired t-test, p = 0.26, n = 5).
We have previously shown that A fibers synapsing onto laminae III/IV neurons express functional GABAA receptors, whose activation depresses glutamate release.21 As observed here for GABABRs, GABAA receptors were also activated by endogenously released GABA. Application of gabazine, a GABAA receptor antagonist, during paired-pulse or repetitive stimulation of Aβ fibers, caused the increase of the second EPSC recorded from laminae III/IV neurons, leaving the first EPSC unaffected. This would suggest that although presynaptic GABAARs and GABABRs are both expressed on Aβ fibers, they require different patterns of activation and exert a different impact on the excitatory synaptic transmission in deep dorsal horn neurons. To confirm this hypothesis, we tested the effect of both CGP 55845 (5 µM) and gabazine (10 µM) on Aβ-mediated EPSCs, recorded with the paired-pulse protocol. Experiments performed on 10 laminae III/IV neurons showed that CGP 55845 potentiates the first EPSC (5/10 neurons), while gabazine (added to CGP) increases the second EPSC (6/10 neurons) (Figure 6(a) and (b)). In two neurons, we observed the effects of both CGP 55845 and gabazine, while in the remaining cells only one of the two antagonists was effective.
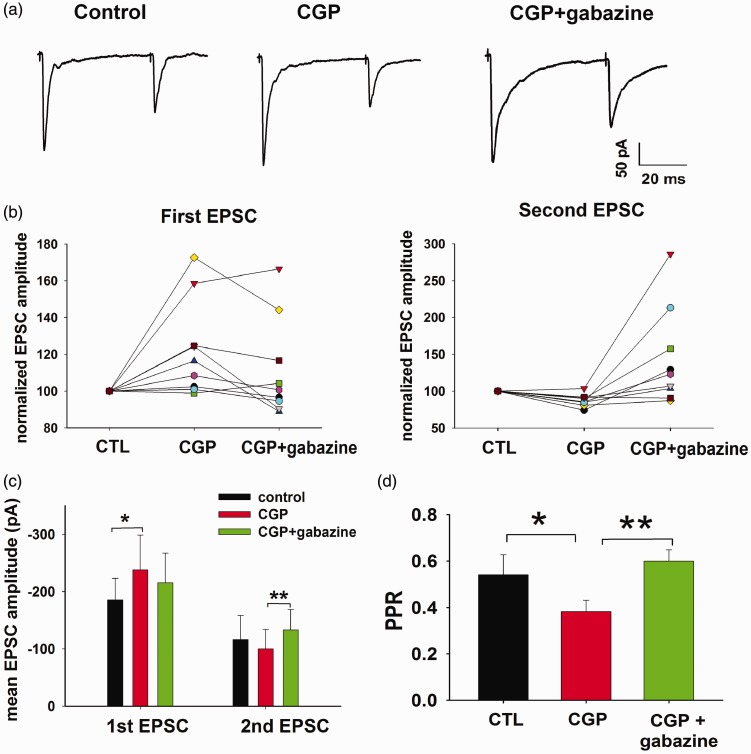
Figure 6.
Comparison between the presynaptic modulations exerted by GABAB and GABAA receptors on the EPSCs evoked by Aβ fiber stimulation. (a) Averaged EPSCs recorded with the paired-pulse protocol, in presence of CGP 55845 (5 µM) and CGP 55845 + gabazine (10 µM). In this example, CGP 55845 causes the increase in the first EPSC, while gabazine potentiates the second peak. (b) Normalized EPSC amplitudes obtained from a sample of 10 laminae III/IV neurons, in CGP 55845 and CGP 55845 + gabazine. (c) CGP 55845 causes a significant change in the first EPSC amplitude (ANOVA, repeated measures, p = 0.02, n = 10), while gabazine significantly increases the second peak (ANOVA, repeated measures, p < 0.01, n = 10). (d) PPR decreases significantly in CGP 55845, while it increases by adding gabazine (ANOVA, repeated measures, p = 0.02 and 0.002 respectively, n = 10).
In the majority of neurons responsive to gabazine (4 of 6), we observed a significant increase of the polysynaptic responses, revealed by the slower decay of the EPSCs (Figure 6(a)). Polysynaptic EPSCs evoked by the first stimulus could potentially cause the shunting of the second EPSC, affecting its amplitude and the determination of PPR. However, the percentage increase of the first polysynaptic response in gabazine was not significantly correlated with the percentage change of the second EPSC (Pearson correlation test, p = 0.9, n = 6). This is consistent with our previous observation that the effect of gabazine on the second EPSC is not significantly affected by postsynaptic shunting.21
Analysis performed on the whole neuron sample confirmed that the first EPSC was significantly increased by CGP 55845 and the second EPSC was significantly potentiated by gabazine (Figure 6(c)). Both receptor antagonists act at the presynaptic site, since the PPR was significantly decreased in CGP 55845 and increased by adding gabazine (Figure 6(d)).
Discussion
We investigated the GABABRs-mediated inhibition of Aβ fibers synapsing onto laminae III/IV neurons of the rat spinal cord. First, we demonstrated that presynaptic GABAB2 is highly expressed at synaptic specializations of glutamatergic terminals engaged in axo-dendritic synapses in laminae III-IV (47.3%) Then, we functionally showed that the presynaptic GABABRs expressed on Aβ fibers are tonically activated by endogenous GABA and involved in the modulation of the synaptic response of laminae III/IV neurons to primary afferent stimulation. These data suggest that presynaptic GABABRs could play a relevant role in the processing of tactile information and the generation of mechanical allodynia.
GABAB2 immunocytochemical localization as a mean to label GABABRs in laminae III-IV of spinal cord
The GABABR, as any other member of the class C of the G protein-coupled receptors, consists of two subunits referred to as GABAB1 and GABAB2. Both GABAB1 and GABAB2 are essential for the normal function of GABABRs, following the assembly of heterodimers made by combinations of the two.37–39 The GABAB1, in turn, exists in 14 isoforms named from GABAB1a to GABAB1n40 GABAB1a and GABAB1b are the most abundant isoforms expressed in the nervous tissue.
By light microscopy immunocytochemistry and in situ hybridization, it was previously demonstrated that GABAB1a, GABAB1b, and GABAB2 are all present in DRG neurons and the spinal cord, where highest expression occurs in laminae I-III.23,41 In DRGs, GABAB1b was restricted to the large diameter neurons, in contrast to GABAB1a (the predominant isoform) and GABAB2, which were detected in both large and small diameter neurons.23,41 In the spinal cord, GABAB1a and GABAB2 were localized to the neuropil, whereas GABAB1b was associated with the neuronal cell bodies.23 Electron microscopy using an affinity-purified antibody that recognized the GABAB1a and the GABAB1b subunits showed that, in laminae I-II, GABAB1 was localized to myelinated fibers, the central boutons deriving from unmyelinated primary afferent fibers in glomeruli, as well as the somato-dendritic domains of dorsal horn neurons.5
Collectively, these results indicate that: (i) GABAB1b has a specific somatic localization in spinal cord and large DRG neurons and is thus not suitable for localization studies at synapses; (ii) GABAB1a and GABAB2 are expressed in DRG neurons irrespectively of size and in the spinal cord neuropil, and are thus expressed at synapses. As none of the two appears to have an origin-specific distribution, this leaves open the possibility that the GABAB2 subunit, which we have here localized, is expressed in primary afferent terminals as well as the terminals of descending fibers42 and the axons/dendrites of the spinal cord neurons. However, expression of GABAB2 can be considered highly suggestive of a primary afferent origin of the immunoreactive nerve terminals in the spinal cord neuropil, and staining for this subunit would, in any case, allow labeling all synaptic components of the neuropil irrespective of their origin.
Localization of functional GABABRs in laminae III/IV
With high-resolution electron microscopy, we here showed that, in laminae III-IV of the dorsal horn, the GABAB2 subunit is only detected at axo-dendritic synapses but not at axo-axonic synapses, axo-somatic synapses, or glomeruli. On the light of the discussion above, lack of GABAB2 immunoreactivity in the primary afferent central boutons of glomeruli was quite unexpected. However, one has to keep in mind that glomeruli only represent a minimal fraction of total synapses in the dorsal horn-about 5% in lamina II, which displays the highest concentration33 and 3% in our sample.
In GABAB2 immunoreactive axo-dendritic synapses, the receptor subunit had a prevalent presynaptic (axonal) localization (about 70%), with a small fraction of these synapses (about 22%) where GABAB2 was concurrently detected on the membrane of the postsynaptic dendrite. On purely theoretical grounds, the spatial resolution of an indirect immunogold labeling procedure using 20 nm GPs is around 26 nm.43 Therefore, considering the size of the synaptic cleft (12–20 nm) and the plane of section at which the synaptic cleft is cut, these data must be considered with extreme caution, as it is well possible that we have underestimated the concurrent presence of pre- or postsynaptic receptors at the same synapse.
About half of the presynaptic axons expressing GABAB2 were excitatory, as they contained a variable number of round, small, clear agranular vesicles, numerous mitochondria, made asymmetric axo-dendritic synapses,29 and were immunolabeled with the anti-glutamate antiserum. Expression of GABABRs in glutamatergic terminals is not a new finding, and it was shown that the cell surface expression of the receptor is independent of agonist stimulation but controlled by glutamate.44 As these excitatory GABAB2 immunoreactive axons are engaged in simple axo-dendritic synapses, they may have a heterogeneous origin from primary afferent fibers, or glutamatergic dorsal horn interneurons. Nonetheless, the electrophysiological experiments, where the primary afferent Aβ fiber release of glutamate was depressed by the GABABR agonist baclofen with a presynaptic mechanism, confirmed the primary afferent origin of at least a fraction of immunoreactive axons after ultrastructural examination.
In about 28% of GABAB2 immunoreactive synapses, the receptor subunit was instead (or concurrently) expressed at the postsynaptic dendrite. This demonstrates that some spinal cord neurons express GABAB2 at their dendritic domain and suggests that at least some of these neurons have their cell bodies located in laminae III-IV. In accordance with ultrastructural data, our electrophysiological recordings, obtained from neurons intracellularly perfused with a potassium-based solution in the presence of baclofen, have confirmed the presence of postsynaptic GABABRs in lamina III/IV neurons.
Finally, it is worth mentioning that it is the GABAB1 subunit that binds baclofen and CGP 55845, which we have used to activate/inhibit the receptor in electrophysiological studies, whereas the GABAB2 subunit only binds positive allosteric modulators.45 Therefore, our combined structural and functional observations provide evidence that fully functional receptor heterodimers have pre- and postsynaptic localizations at axo-dendritic synapses made by Aβ fibers and laminae III-IV neurons.
Functional properties of GABABRs expressed at axo-dendritic synapses between Aβ fibers and laminae III/IV neurons
Electrophysiological data and localization studies converged to demonstrate that both pre- and postsynaptic GABABRs at synapses between Aβ fibers and laminae III/IV neurons were fully functional. The activation of presynaptic GABABRs by baclofen, in fact, caused a significant depression of evoked EPSCs in all tested neurons, accompanied by a change in PPR. Baclofen also induced an outward current, indicating that postsynaptic GABABRs were effective in activating a potassium conductance in most recorded neurons.
GABABR-mediated modulation of glutamate release from primary afferents was described by several electrophysiological studies, whose findings, however, were often contradictory. Specifically, while some studies reported that unmyelinated C fibers were more sensitive to baclofen than myelinated Aβ fibers and that touch-evoked EPSCs were less inhibited by baclofen than pinch-evoked EPSCs,13,24 other authors did not observe any significant difference in baclofen sensitivity between the fibers mediating different sensory modalities.5,46 In addition, a recent report showed that the EPSCs, evoked by optogenetic activation of low threshold, VGluT3 + C fibers and recorded from laminae I-II neurons, were strongly depressed by low (1 µM) baclofen concentrations.47 Our present data show that Aβ fibers synapsing onto laminae III/IV neurons were indeed highly sensitive to GABABR activation, since even low concentrations of baclofen (0.1–0.5 µM) produced substantial depressions of the evoked EPSCs. Therefore, our results suggest that GABABRs control a subpopulation of these synapses under physiological conditions.
The modulation of Aβ fibers is mediated by endogenously activated presynaptic GABABRs
At all primary afferent synapses, tested with the paired-pulse protocol, there was a strong paired-pulse depression in the absence of any drugs. Blockade of GABABRs by CGP 55845 induced, in a subpopulation of laminae III/IV neurons, the facilitation of the first EPSC, accompanied by a decrease of PPR. This indicates that the glutamate release from Aβ fibers is endogenously regulated by presynaptic GABABRs at a subpopulation of synapses in the deep dorsal horn. The fact that the facilitating effect of CGP 55845 was visible already at the first stimulus implies that presynaptic GABABRs were tonically active. Our results expand what previously observed in several different areas of the CNS, where GABABRs are tonically activated by endogenous GABA and regulate glutamate release through voltage-gated calcium channels.14,36,48,49
Since GABABRs have higher affinity for GABA than GABAARs and their activation requires lower concentrations of neurotransmitter,50 a spillover of GABA from the synaptic cleft or from the surrounding glial cells might be sufficient to tonically activate these receptors. Variable local levels of GABA could explain why only a subpopulation of neurons responded to CGP 55845, despite the fact that all neurons responded to baclofen. Alternatively, the different response of laminae III/IV neurons to baclofen and CGP 55845 suggests that baclofen is able to activate all GABABRs, whereas CGP 55845 only blocks the synaptic GABABRs activated by endogenously released GABA.
A number of studies have described the fine structure of individual axons in laminae III-IV according to specific sensory modalities. In general, these excitatory axons are contacted by inhibitory terminals containing flat or pleiomorphic synaptic vescicles and forming symmetric GABAergic/glycinergic synapses.33 Therefore, one might expect that the GABABRs responsible for the presynaptic inhibition of glutamate release from Aβ fibers would be located either at the axo-axonic synapses that these terminals receive from GABAergic/glycinergic boutons or at extrasynaptic sites in the primary afferent axonal membrane. However, our ultrastructural data show that GABAB2 is not expressed at axo-axonic synapses and poorly represented at extrasynaptic sites. As we have not tried to localize the GABAB1 subunit in our material, it cannot be excluded that GABAB1 has additional localizations in the neuropil of laminae III-IV. If so, axo-axonic synapses and/or the extrasynaptic membrane of the primary afferent axons would only express GABAB1. It is still unclear if these receptors may be functional in the brain, as a series of observations suggested the possibility that GABAB1 couples to G proteins also in the absence of GABAB245 Thus, an intervention of GABABRs expressed at axo-axonic synapses or at extrasynaptic sites cannot be totally ruled out in explaining the effects of baclofen, only if one accepts that dimers, tetramers, or higher order oligomers only consisting of GABAB1 are fully functional at laminae III-IV synapses. Figure 7(a) depicts in graphic form a presumptive circuit that is compatible with both our ultrastructural and functional observations.
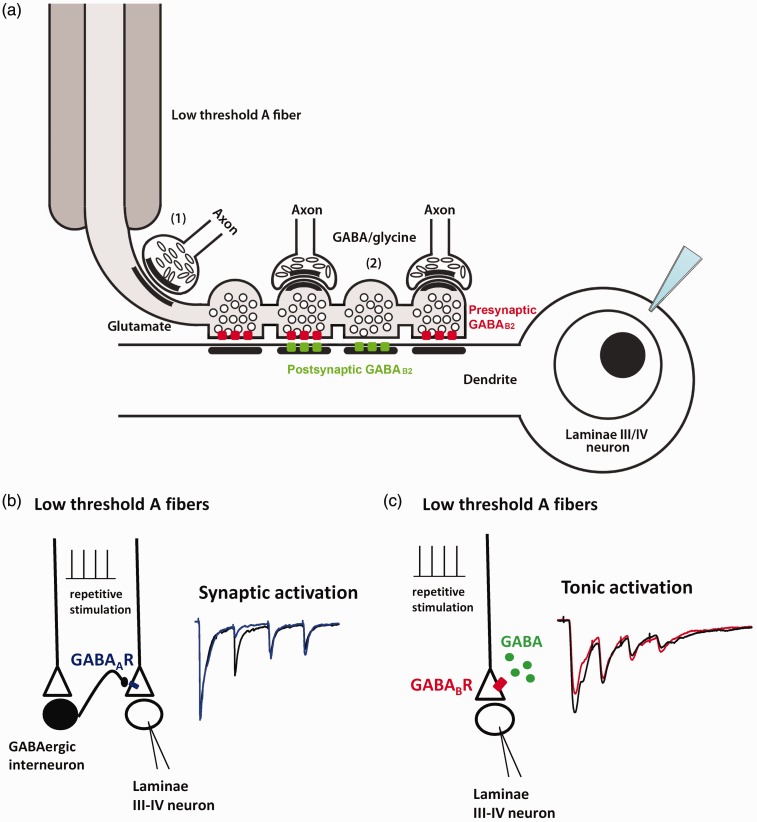
Figure 7.
Schematic summary of the ultrastructural and electrophysiological results obtained in this study. (a) Representation of a simplified hypothetical circuit compatible with the ultrastructural findings and electrophysiological recordings from rat laminae III/V neurons. The synaptic organization of the low-threshold A fiber is based on a drawing from Maxwell et al.60 showing that functionally identified hair follicle afferents receive axo-axonic synapses forming en passant (1) or terminal (2) boutons. These inhibitory terminals can be recognized as they contain flat or pleomorphic vesicles, form symmetric contacts and are immunoreactive for GABA/glycine. The GABAB2 subunit is not expressed at these synapses but at the axo-dendritic glutamatergic synapses made by the A fiber onto the laminae III-IV neurons. These synapses are asymmetric and contain round vesicles labeled with the anti-glutamate antiserum. For simplicity, we have represented four synapses made by the same fiber impinging onto a patch-clamped laminae III-IV neuron. This recalls the results of quantitative analysis of immunogold labeling (see text). The scheme also takes in account the quantitative TEM data on GABAB2 subunit-specific localization at the synaptic specializations. The pre and/or postsynaptic localization of GABAB2 at these synapses is consistent with the results of patch-clamp experiments. For simplicity, we have not considered the possibility that presynaptic GABAB2 is also expressed by glutamatergic excitatory interneurons and by glutamate-unreactive axons of (presumptive) descending origin. Both possibilities are considered in Discussion section.
(b) to (c): Schematic illustration of the presynaptic modulation mediated by GABAA and GABAB receptors expressed on low-threshold A fiber terminals in dorsal horn laminae III/IV. Synaptic activation of GABAARs causes the depression of the second EPSC during repetitive stimulation of A fibers (b), while tonic activation of GABABRs by local GABA depresses the first response (c). On the right of each panel, example traces of evoked EPSCs showing the effect of presynaptic inhibition exerted by GABAARs (blue trace) or GABABRs (red trace). GABA: γ-aminobutyric acid; GABAB2: subunit B2 of the GABA receptor; GABAAR: GABAA receptor; GABABR: GABAB receptor.
Recordings performed during repetitive high-frequency (20 Hz) stimulation of Aβ fibers showed that only the first response was increased by CGP 55845, while the subsequent responses were not significantly affected. Therefore, presynaptic GABABRs on Aβ fibers seem to act specifically on the initial part of the synaptic response, selectively regulating the onset of excitation in laminae III/IV neurons. Our data are consistent with studies performed at other central synapses, showing that the GABABR inhibition of evoked glutamate or GABA release is frequency dependent: during low-frequency trains (0.1–10 Hz), GABABRs depressed each evoked EPSCs or IPSCs, while, at higher frequencies, they modulated only the first response.36,49 Decrease in release probability (due to vesicular depletion or other activity-dependent mechanisms) could prevent an effective modulation by GABABRs at each stimulus during high-frequency stimulation. Frequency dependence of GABABR modulation could also be due to the voltage-dependent relief of G protein inhibition on calcium channels during high-frequency firing of presynaptic neurons.51,52
Functional significance of GABABR-mediated presynaptic modulation in deep dorsal horn
The data here obtained in the presence of gabazine confirmed previous results that, during repetitive stimulation, Aβ fibers synapsing onto laminae III/IV neurons were endogenously modulated by GABAARs.21 In that case, only the response to the second stimulus was depressed, indicating that GABAARs were not tonically active and that the first stimulus was necessary to induce GABA release onto Aβ fiber terminals. Figure 7(b) and (c) summarize the different modalities of activation and the effects of presynaptic GABAARs and GABABRs receptors expressed on low-threshold A fibers in the deep dorsal horn.
Aβ fibers convey the tactile information to laminae III/IV by firing action potentials at a high frequency that gradually decreases during the mechanical stimulus, with different adaptation rates. Our data show that both presynaptic GABAARs and GABABRs transiently inhibit glutamate release from low-threshold afferents, affecting only the beginning of a train of afferent action potentials. Therefore, this mechanism could selectively affect the early phase of the tactile response, when afferent fibers fire at the highest frequency, by regulating synaptic excitation and action potential firing.
Besides the role in touch sensation, GABABRs expressed on Aβ fibers could also be involved in the genesis of tactile allodynia, the painful reaction to innocuous mechanical stimuli observed in pathological pain. Indeed, in animal models of neuropathic pain, pharmacological antagonism of spinal GABABRs produces mechanical allodynia,53 while intrathecal injection of baclofen produces anti-allodynic effects.54,55 Moreover, baclofen decreases the hypersensitivity of dorsal horn wide dynamic range neurons to low-intensity mechanical stimulation induced by spinal cord ischemia.56 A decrease in the number, affinity, or coupling of GABABRs in the deep dorsal horn may underlie the allodynia occurring in neuropathic pain. As reported by recent studies, peripheral nerve injury leads to the decrease of GABABRs expression, both in DRGs8 and in the spinal cord dorsal horn,57,58 and to impairment of the GABAB1/GABAB2 heterodimer assembly.39,59 Downregulation of GABABRs could decrease the tonic inhibitory control exerted on low-threshold afferent fibers, resulting in the increase in the excitatory drive on laminae III/IV neurons and, ultimately, in allodynia.
In conclusion, our data provide the first ultrastructural localization of GABABRs in the rat deep dorsal horn, demonstrate that these receptors are functional and can be activated by endogenous GABA, and suggest that, besides mediating some touch-related sensory modalities, they may be involved in the genesis of allodynia under chronic pain conditions.
Acknowledgment
We thank professor Amy MacDermott for helpful suggestions.
Author Contributions
All authors have read and approved the final manuscript. CS and RB performed the experiments. CS, AM, and RB designed the study, interpreted the results, and wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Fondazione Cassa di Risparmio di Vignola to RB. and by local grants from the University of Turin to CS. and AM.
References
- 1.Price GW, Wilkin GP, Turnbull MJ, et al. Are baclofen-sensitive GABAB receptors present on primary afferent terminals of the spinal cord? Nature 1984; 307: 71–74. [DOI] [PubMed] [Google Scholar]
- 2.Price GW, Kelly JS, Bowery NG. The location of GABAB receptor binding sites in mammalian spinal cord. Synapse 1987; 1: 530–538. [DOI] [PubMed] [Google Scholar]
- 3.Margeta-Mitrovic M, Mitrovic I, Riley RC, et al. Immunohistochemical localization of GABA(B) receptors in the rat central nervous system. J Comp Neurol 1999; 405: 299–321. [DOI] [PubMed] [Google Scholar]
- 4.Calver AR, Medhurst AD, Robbins MJ, et al. The expression of GABA(B1) and GABA(B2) receptor subunits in the CNS differs from that in peripheral tissues. Neuroscience 2000; 100: 155–170. [DOI] [PubMed] [Google Scholar]
- 5.Yang K, Wang D, Li YQ. Distribution and depression of the GABA(B) receptor in the spinal dorsal horn of adult rat. Brain Res Bull 2001; 55: 479–485. [DOI] [PubMed] [Google Scholar]
- 6.Yang K, Ma WL, Feng YP, et al. Origins of GABA(B) receptor-like immunoreactive terminals in the rat spinal dorsal horn. Brain Res Bull 2002; 58: 499–507. [DOI] [PubMed] [Google Scholar]
- 7.Castro AR, Pinto M, Lima D, et al. Nociceptive spinal neurons expressing NK1 and GABAB receptors are located in lamina I. Brain Res 2004; 1003: 77–85. [DOI] [PubMed] [Google Scholar]
- 8.Engle MP, Merrill MA, Marquez De Prado B, et al. Spinal nerve ligation decreases γ-aminobutyric acidB receptors on specific populations of immunohistochemically identified neurons in L5 dorsal root ganglion of the rat. J Comp Neurol 2012; 520: 1663–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goudet C, Magnaghi V, Landry M, et al. Metabotropic receptors for glutamate and GABA in pain. Brain Res Rev 2009; 60: 43–56. [DOI] [PubMed] [Google Scholar]
- 10.Kangrga I, Jiang M, Randić M. Actions of (−)-baclofen on rat dorsal horn neurons. Brain Res 1991; 562: 265–275. [DOI] [PubMed] [Google Scholar]
- 11.Malcangio M, Bowery N. (−)Baclofen inhibition of electrically-evoked release of substance P from rat spinal cord. Neuropeptide 1993; 24: 211–212. [Google Scholar]
- 12.Iyadomi M, Iyadomi I, Kumamoto E, et al. Presynaptic inhibition by baclofen of miniature EPSCs and IPSCs in substantia gelatinosa neurons of the adult rat spinal dorsal horn. Pain 2000; 85: 385–393. [DOI] [PubMed] [Google Scholar]
- 13.Ataka T, Kumamoto E, Shimoji K, et al. Baclofen inhibits more effectively C-afferent than Aδ-afferent glutamatergic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. Pain 2000; 86: 273–282. [DOI] [PubMed] [Google Scholar]
- 14.Yang K, Ma H. Blockade of GABAB receptors facilitates evoked neurotransmitter release at spinal dorsal horn synapse. Neuroscience 2011; 193: 411–420. [DOI] [PubMed] [Google Scholar]
- 15.Yang K, Ma R, Wang Q, et al. Optoactivation of parvalbumin neurons in the spinal dorsal horn evokes GABA release that is regulated by presynaptic GABAB receptors. Neurosci Lett 2015; 594: 55–59. [DOI] [PubMed] [Google Scholar]
- 16.Hugel S, Schlichter R. Convergent control of synaptic GABA release from rat dorsal horn neurones by adenosine and GABA autoreceptors. J Physiol 2003; 551: 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Désarmenien M, Feltz P, Occhipinti G, et al. Coexistence of GABAA and GABAB receptors on Aδ and C primary afferents. Br J Pharmacol 1984; 81: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott RH, Dolphin A. Activation of a G protein promotes agonist responses to calcium channel ligands. Nature 1987; 330: 760–762. [DOI] [PubMed] [Google Scholar]
- 19.Huang D, Huang S, Peers C, et al. GABAB receptors inhibit low-voltage activated and high-voltage activated Ca2+ channels in sensory neurons via distinct mechanisms. Biochem Biophys Res Commun 2015; 465: 188–193. [DOI] [PubMed] [Google Scholar]
- 20.Dittman JS, Regehr WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci 1996; 16: 1623–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Betelli C, MacDermott AB, Bardoni R. Transient, activity dependent inhibition of transmitter release from low threshold afferents mediated by GABAA receptors in spinal cord lamina III/IV. Mol Pain 2015; 11: 64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bohlhalter S, Weinmann O, Mohler H, et al. Laminar compartmentalization of GABAA-receptor subtypes in the spinal cord: an immunohistochemical study. J Neurosci 1996; 16: 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Towers S, Princivalle A, Billinton A, et al. GABAB receptor protein and mRNA distribution in rat spinal cord and dorsal root ganglia. Eur J Neurosci 2000; 12: 3201–3210. [DOI] [PubMed] [Google Scholar]
- 24.Fukuhara K, Katafuchi T, Yoshimura M. Effects of baclofen on mechanical noxious and innocuous transmission in the spinal dorsal horn of the adult rat: in vivo patch-clamp analysis. Eur J Neurosci 2013; 38: 3398–3407. [DOI] [PubMed] [Google Scholar]
- 25.Cesa R, Morando L, Strata P. Glutamate receptor delta2 subunit in activity-dependent heterologous synaptic competition. J Neurosci 2003; 23: 2363–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuny H, de Faoite A, Huynh TG, et al. γ-Aminobutyric acid type B (GABAB) receptor expression is needed for inhibition of N-type (Cav2.2) calcium channels by analgesic α-conotoxins. J Biol Chem 2012; 287: 23948–23957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salio C, Lossi L, Ferrini F, et al. Ultrastructural evidence for a pre- and postsynaptic localization of full-length trkB receptors in substantia gelatinosa (lamina II) of rat and mouse spinal cord. Eur J Neurosci 2005; 22: 1951–1966. [DOI] [PubMed] [Google Scholar]
- 28.Sassoè-Pognetto M, Ottersen OP. Organization of ionotropic glutamate receptors at dendrodendritic synapses in the rat olfactory bulb. J Neurosci 2000; 20: 2192–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown AG. Organization in the Spinal Cord: The Anatomy and Physiology of Identified Neurones. Illustrated Edition. Berlin: Springer-Verlag, 1981.
- 30.Baude A, Nusser Z, Molnar E, et al. High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neuroscience 1995; 69: 1031–1055. [DOI] [PubMed] [Google Scholar]
- 31.Naim M, Spike RC, Watt C, et al. Cells in laminae III and IV of the rat spinal cord that possess the neurokinin-1 receptor and have dorsally directed dendrites receive a major synaptic input from tachykinin-containing primary afferents. J Neurosci 1997; 17: 5536–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci 2006; 26: 1833–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willis WD, Coggeshall RE. Structure of the dorsal horn. Sensory mechanisms of the spinal cord - Primary afferent neurons and the spinal dorsal horn, New York: Kluver Academic/Plenum Publisher, 2004, pp. 155–186. [Google Scholar]
- 34.Park J, Nakatsuka T, Nagata K, et al. Reorganization of the primary afferent termination in the rat spinal dorsal horn during post-natal development. Brain Res Dev Brain Res 1999; 113: 29–36. [DOI] [PubMed] [Google Scholar]
- 35.Lüscher C, Jan L, Stoffel M, et al. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 1997; 19: 687–695. [DOI] [PubMed] [Google Scholar]
- 36.Mapelli L, Rossi P, Nieus T, et al. Tonic activation of GABAB receptors reduces release probability at inhibitory connections in the cerebellar glomerulus. J Neurophysiol 2009; 101: 3089–3099. [DOI] [PubMed] [Google Scholar]
- 37.White JH, Wise A, Main MJ, et al. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature 1998; 396: 679–682. [DOI] [PubMed] [Google Scholar]
- 38.Kuner R, Köhr G, Grünewald S, et al. Role of heteromer formation in GABAB receptor function. Science 1999; 283: 74–77. [DOI] [PubMed] [Google Scholar]
- 39.Laffray S, Bouali-Benazzouz R, Papon MA, et al. Impairment of GABAB receptor dimer by endogenous 14-3-3ζ in chronic pain conditions. EMBO J 2012; 31: 3239–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bettler B, Kaupmann K, Mosbacher J, et al. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev 2004; 84: 835–867. [DOI] [PubMed] [Google Scholar]
- 41.Charles KJ, Evans ML, Robbins MJ, et al. Comparative immunohistochemical localization of GABA(B1a), GABA(B1b) and GABA(B2) subunits in rat brain, spinal cord and dorsal root ganglion. Neuroscience 2001; 106: 447–467. [DOI] [PubMed] [Google Scholar]
- 42.Jiménez I, Rudomin P, Enriquez M. Differential effects of (-)-baclofen on Ia and descending monosynaptic EPSPs. Exp Brain Res 1991; 85: 103–113. [DOI] [PubMed] [Google Scholar]
- 43.Merighi A, Polak JM. Post-embedding immunogold staining. In: Cuello AC. (ed). Immunohistochemistry II, London: John Wiley & Sons, 1993, pp. 229–264. [Google Scholar]
- 44.Guetg N, Abdel Aziz S, Holbro N, et al. NMDA receptor-dependent GABAB receptor internalization via CaMKII phosphorylation of serine 867 in GABAB1. PNAS 2010; 107: 13924–13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu C, Zhang W, Rondard P, et al. Complex GABAB receptor complexes: how to generate multiple functionally distinct units from a single receptor. Front Pharmacol 2014; 5: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blake JF, Cao CQ, Headley PM, et al. Antagonism of baclofen-induced depression of whole-cell synaptic currents in spinal dorsal horn neurones by the potent GABAB antagonist CGP55845. Neuropharmacology 1993; 32: 1437–1440. [DOI] [PubMed] [Google Scholar]
- 47.Honsek SD, Seal RP, Sandkühler J. Presynaptic inhibition of optogenetically identified VGluT3+ sensory fibres by opioids and baclofen. Pain 2015; 156: 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kulik A, Vida I, Lujan R, et al. Subcellular localization of metabotropic GABAB receptor subunits GABAB1a/b and GABAB2 in the rat hippocampus. J Neurosci 2003; 23: 11026–11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitchell SJ, Silver RA. GABA spillover from single inhibitory axons suppresses low-frequency excitatory transmission at the cerebellar glomerulus. J Neurosci 2000; 20: 8651–8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chéry N, De Koninck Y. GABA(B) receptors are the first target of released GABA at lamina I inhibitory synapses in the adult rat spinal cord. J Neurophysiol 2000; 84: 1006–1011. [DOI] [PubMed] [Google Scholar]
- 51.Brody DL, Patil PG, Mulle JG, et al. Bursts of action potential waveforms relieve G-protein inhibition of recombinant P/Q-type Ca2+ channels in HEK 293 cells. J Physiol 1997; 499: 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dolphin AC. Mechanisms of modulation of voltage-dependent calcium channels by G proteins. J Physiol 1998; 506: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hao JX, Xu XJ, Wiesenfeld-Hallin Z. Intrathecal gamma-aminobutyric acidB (GABAB) receptor antagonist CGP 35348 induces hypersensitivity to mechanical stimuli in the rat. Neurosci Lett 1994; 182: 299–302. [DOI] [PubMed] [Google Scholar]
- 54.Hwang JH, Yaksh TL. The effect of spinal GABA receptor agonists on tactile allodynia in a surgically-induced neuropathic pain model in the rat. Pain 1997; 70: 15–22. [DOI] [PubMed] [Google Scholar]
- 55.Cui JG, Linderoth B, Meyerson BA. Effects of spinal cord stimulation on touch-evoked allodynia involve GABAergic mechanisms. An experimental study in the mononeuropathic rat. Pain 1996; 66: 287–295. [DOI] [PubMed] [Google Scholar]
- 56.Hao JX, Xu XJ, Yu YX, et al. Baclofen reverses the hypersensitivity of dorsal horn wide dynamic range neurons to mechanical stimulation after transient spinal cord ischemia; implications for a tonic GABAergic inhibitory control of myelinated fiber input. J Neurophysiol 1992; 68: 392–396. [DOI] [PubMed] [Google Scholar]
- 57.Wang XL, Zhang Q, Zhang YZ, et al. Downregulation of GABAB receptors in the spinal cord dorsal horn in diabetic neuropathy. Neurosci Lett 2011; 490: 112–115. [DOI] [PubMed] [Google Scholar]
- 58.Wu J, Xu Y, Pu S, et al. p38/MAPK inhibitor modulates the expression of dorsal horn GABA(B) receptors in the spinal nerve ligation model of neuropathic pain. Neuroimmunomodulation 2011; 18: 150–155. [DOI] [PubMed] [Google Scholar]
- 59.Benke D. GABAB receptor trafficking and interacting proteins: targets for the development of highly specific therapeutic strategies to treat neurological disorders? Biochem Pharmacol 2013; 86: 1525–1530. [DOI] [PubMed] [Google Scholar]
- 60.Maxwell DJ, Bannatyne BA, Fyffe RE, et al. Ultrastructure of hair follicle afferent fibre terminations in the spinal cord of the cat. J Neurocytol 1982; 11: 571–582. [DOI] [PubMed] [Google Scholar]