Abstract
Autobiographical memory, or memory for personal experiences, allows individuals to define themselves and construct a meaningful life story. Decline of this ability, as observed in Alzheimer’s disease (AD), results in an impaired sense of self and identity. In our model (AMAD: Autobiographical Memory in Alzheimer’s Disease), we present a critical review of theories and findings regarding cognitive and neuroanatomical underpinnings of autobiographical memory and its decline in AD and highlight studies on its clinical rehabilitation. We propose that autobiographical recall in AD is mainly characterized by loss of associated episodic information, which leads to de-contextualization of autobiographical memories and a shift from reliving past events to a general sense of familiarity. This decline refers to retrograde, but also anterograde amnesia that affects newly acquired memories besides remote ones. One consequence of autobiographical memory decline in AD is decreased access to memories that shape self-consciousness, self-knowledge, and self-images, leading to a diminished sense of self and identity. The link between autobiographical decline and compromised sense of self in AD can also manifest itself as low correspondence and coherence between past memories and current goals and beliefs. By linking cognitive, neuroanatomical, and clinical aspects of autobiographical decline in AD, our review provides a theoretical foundation, which may lead to better rehabilitation strategies.
Keywords: Alzheimer’ s disease, Autobiographical memory, Rehabilitation, Executive function, Hippocampus, Personal identity, Sense of self
1. Introduction
Autobiographical memory refers to memory for personal experiences and facts about the self. This ability allows individuals to define themselves, construct a life story, and attribute meaning to it. On the cognitive level, memory decline is a hallmark of Alzheimer’s Disease (AD) and this decline involves multiple memory systems, including those involved in autobiographical memory. Decline of autobiographical memory in AD leads to loss of knowledge about events and facts that defined the patients’ life, and consequently, degradation of their self-knowledge and sense of identity. Bearing in mind these clinical implications, our review aims at describing the current theoretical understanding about autobiographical memory decline in AD, with particular focus on clinical applicability and venues for future research. First, we provide an overview of sense of self and theoretical perspectives on the organization and construction of autobiographical memories. We then describe consequences of autobiographical decline in AD, such as over-generality of autobiographical recall, and discuss the impact of anterograde and retrograde amnesia, and impairment in the sense of identity. Next, we highlight recent findings on the cognitive and neuroanatomical underpinnings of the disorder, as well as studies on its clinical rehabilitation. Finally, we provide a theoretical framework for conceptualizing the empirical findings about autobiographical memory in AD.
This review was based on a literature survey that was performed by combining the keywords “Alzheimer’s disease”; “autobiographical memory”; “identity”; and “self”. The search was performed on the PubMed and PsycInfo databases from the first available year until 2015; and was limited to studies published in peer-reviewed journals. Additional exploration of the reference lists of these papers was carried out to identify additional papers.”
2. Sense of identity, sense of self
Since the present review emphasizes identity compromise in AD, it would be of interest to highlight some theoretical assumptions about identity and sense of self. Although several attempts have been made to categorize the different aspects of sense of self (e.g., Klein and Gangi, 2010; Neisser, 1988; Singer, 1995), we mainly follow the framework of framework by Prebble et al. (2013), according to which, sense of self is the mental processes that provide us with feelings of singularity, individuality, coherence, and unity that define our uniqueness (for a similar view, see Damasio, 2003). More precisely, the framework of Prebble et al. (2013) delineates sense of self along two dimensions: the subjective versus objective sense of self and the present versus temporally extended aspects of that sense. The first distinction refers to the difference between our phenomenological experience of identity (i.e., subjective sense of self) and our mental representation of identity, comprising all the things that we perceive and know about ourselves (i.e., objective sense of self). This division was posited by James (1892), who distinguished between the experience of knowing (the I-self) and the object of this awareness (the me-self); an assumption that still influences contemporary literature (Klein and Gangi, 2010). As for the second dimension of the framework of Prebble et al. (2013), these authors distinguish between sense of self that is related to the present moment (i.e., present self) and that that is extended over time (i.e., temporally extended self). This assumption fits with the idea that identity involves a temporally extended aspect, involving past, present, and future (Addis et al., 2009; Klein et al., 2002; Neisser, 1988; Schacter and Addis, 2007a,b; Schacter et al., 2007). The extension of identity over time is directly related to autobiographical memory, since the latter is fundamental to the formation and maintenance of identity in the present moment and to the continuity of the mental representation of identity over time (Conway, 2005). Hence, one consequence of autobiographical decline, as observed in AD, would be compromise in both the subjective experience of identity and its continuity over time.
3. Autobiographical memory: memory of the self
According to the autobiographical model of Conway (Conway, 2005; Conway and Pleydell-Pearce, 2000), autobiographical memories contain knowledge with different levels of specificity ranging from general knowledge about one’s past to highly contextual-specific knowledge [for the same view, see Kopelman, 1994]. More specifically, autobiographical memory is composed of two main components, a semantic component and an episodic one. The semantic component refers to generic representations rather than to particular events linked to particular times and places. These generic representations cover long lifetime periods (e.g., “when I was young”) and general events referring to thematic events that occur repeatedly (e.g., “I used to hike every weekend”). The episodic autobiographical component refers to memories for specific personal experiences that occurred at a particular time and place (e.g., “that day on that mountain when I lost my compass”). This distinction is essential in describing the re-experiencing aspect of autobiographical memory. Semantic autobiographical knowledge triggers a state of noetic consciousness by which awareness of the past is limited to feelings of knowing or familiarity, whereas episodic events trigger a state of autonoetic consciousness by which the subjective experience of past is relived thanks to mental time travel.
Besides the phenomenon of re-experiencing that characterizes autobiographical recall, another characteristic of autobiographical memory is its uneven temporal distribution. This distribution refers to three distinct features: childhood amnesia, reminiscence bump, and recency effect (Conway and Pleydell-Pearce, 2000; Janssen et al., 2012; Rubin and Wenzel, 1996). Childhood amnesia refers to a near complete extinction of memories from the earliest years of life, the reminiscence bump refers to a substantial rise of memories for events that occurred between the ages of 10 and 30 years, and the recency effect refers to preferential recall for recent (Conway and Pleydell-Pearce, 2000; Janssen et al., 2012; Rubin and Wenzel, 1996). Among these three features, the reminiscence bump is the most studied, as it covers the most important events of people’s lives (e.g., first day at high school, first meeting with a partner, or first driving lesson). Indeed, it has been argued that the reminiscence bump is the result of many first-time experiences and that these novel events are used later in life as milestones when people experience similar events (Pillemer, 2001). The reminiscence bump is the most self-defining memory component, as it covers self-defining memories and events that are vivid and emotion-laden with a large impact on the sense of identity (Conway et al., 2004). As we will emphasize later, the reminiscence bump can be utilized in rehabilitation efforts aiming to reinforce personal identity in AD.
Successful strategies for clinical rehabilitation of autobiographical memory require understanding of the two main principles that shape memory construction: coherence and correspondence (Conway, 2005). Coherence is a process that operates during the recall of autobiographical memories to make them consistent with our current beliefs and self-images, i.e., those mental models of the identity in relation to past, current, and future goals (Conway, 2005). In other words, memories are reconfigured during retrieval to support our beliefs for the world and ourselves. The principle of coherence may also be expressed in probabilistic terms: current beliefs may determine the prior probability that certain events could have happened and, from that, their likelihood of being remembered. The principle of correspondence dictates that autobiographical memories should correspond to the original experienced event. Hence, autobiographical retrieval depends on two forces, one aiming to represent the past as it was experienced (i.e., correspondence), and the other aiming to reconstruct it in such way as to support our current beliefs and goals (i.e., coherence) (Conway, 2005). In this theoretical framework, both principles are supervised by an executive center that was termed the “working-self” (Conway and Pleydell-Pearce, 2000). As we will later discuss, the severe impairment of executive function in AD may explain the weak coherence and correspondence in autobiographical memories and the impaired sense of self in the disease.
Autobiographical memory is essentially memory of the self and provides the foundation for self-consciousness, self-knowledge, and self-images. Hence, one deleterious consequence of the autobiographical memory decline in AD is the weakened sense of the self and identity. This issue will be next highlighted by illustrating how overgenerality of autobiographical recall and anterograde and retrograde amnesia contribute to a diminished sense of identity in AD.
4. Autobiographical memory decline in AD
4.1. Overgenerality of autobiographical memory in AD
With the passage of time and the repetition of similar events, there is a shift from specific to general memories. In addition, when people reminisce about their past, they often engage in episodic counterfactual thinking, or mental simulations of alternative ways in which autobiographic events could have occurred (De Brigard et al., 2013). In line with this assumption, studies in normal aging have showed lower production of episodic autobiographical memories (e.g., recall of locations, time, perceptions, and thoughts) than semantic autobiographical memories (e.g., general knowledge about one’s self) in healthy older adults (Levine et al., 2002; Piolino et al., 2010, 2006). This overgenerality is exaggerated in AD, since several studies have observed a substantial shift from episodic to semantic autobiographical recall in AD patients (Barnabe et al., 2012; El Haj et al., 2015a,b, 2011; Graham and Hodges, 1997; Greene et al., 1995; Hou et al., 2005; Irish et al., 2011; Ivanoiu et al., 2006; Leyhe et al., 2009; Meeter et al., 2006; Moses et al., 2004; Muller et al., 2013; Seidl et al., 2011). This shift was observed in these studies despite explicit instructions to AD participants to provide detailed descriptions of personal events that occurred at specific times and places, according to the norms for autobiographical memory assessment [see the Autobiographical Memory Test (Williams and Scott, 1988), the Autobiographical Memory Interview, the Autobiographical Interview, the Test Episodique de Mémoire du Passé autobiographique (Piolino et al., 2003)]. The autobiographical overgenerality in AD fits with research showing overdependence on gist when making memory decisions (Gallo et al., 2006). An interesting account for overgenerality in AD can be found in a model by Morris and Mograbi (2013), according to which semanticized self-judgments in the disease provide more rapid access compared to judgments made on the basis of episodic memory. Interestingly, this model assumes that semantic self-judgments in AD reflect lack of updating of personal knowledge, leading to an outdated sense of the self and inaccurate evaluation about current abilities leading to ignorance of the disease (i.e., anosognosia).
The overgenerality of autobiographical recall in AD may trigger a shift from autonoetic to noetic consciousness. This phenomenon was observed in a study by Piolino et al. (2003) who used the “Remember/Know” paradigm, according to which AD participants had to provide a “Remember” response, if they were able to recover a specific event with its encoding context, and a “Know” response, if they just knew this event had happened to them, but could not recall any contextual detail. Hence, “Remember” responses referred to autonoetic consciousness whereas “Know” responses referred to noetic consciousness. The study showed that patients with AD showed poor ability to retrieve specific autobiographical events, a distortion that was associated with a weakened ability to mentally relive those events (i.e., a decline in autonoetic consciousness). Disruption of autonoetic consciousness was also observed in subsequent replications that showed fewer “Remember” and more “Know” responses in AD participants than in healthy older adults (El Haj et al., 2014; Hudon et al., 2009; Rauchs et al., 2007). With the passage of time, the repetition of similar events, and the advancement of the disease, a substantial loss of episodic autobiographical details is observed in AD. This loss leads to the de-contextualisation or semantization of autobiographical memories and a shift from the ability to mentally relive past events to a general sense of familiarity that may be expressed by AD patients as a sense of “having experienced this before”.
4.2. Anterograde and retrograde amnesia
One of the earliest characteristics of autobiographical decline in AD is anterograde amnesia (i.e., inability to form new memories) followed by retrograde amnesia (i.e., inability to retrieve old memories). A body of empirical research has shown better retrieval for older memories than for recent memories in mild AD (Greene et al., 1995; Hou et al., 2005; Irish et al., 2011; Ivanoiu et al., 2006; Leyhe et al., 2009; Meeter et al., 2006). However, this pattern has been observed only when semantic and episodes autobiographical memories were grouped together to form one global autobiographical score. When considered alone, episodic memories tend to be impaired, whatever the time interval may be, whereas old semantic memories tend to be better preserved than recent ones (Irish et al., 2011; Ivanoiu et al., 2006; Piolino et al., 2003). It can be argued that representations of personally relevant knowledge that became part of semantic memories earlier in life become more strongly integrated in the brain and better consolidated and are, therefore, less easily degraded by AD. In a related vein, the Multiple Trace Theory (Nadel and Moscovitch, 1997; Nadel et al., 2007) proposes that, unlike episodic memory, personal semantic memory becomes independent of the medial temporal lobe over time. By this view, semantic memories are transferred over time to the neocortex [especially, the left anterior temporal lobe Budson and Price, 2005] and the mediotemporal structures are no longer necessary for their retrieval, whereas retrieval of episodic memories is always dependent on the interaction between hippocampus and neocortex. This model is of interest since the medial temporal lobes, and especially the hippocampus, are preferentially targeted by the neuropathological processes of AD [e.g., Pennanen et al., 2004]. Hence, the model predicts that semantic autobiographical memories are likely to be less affected by the pathological mechanisms of AD compared to episodic autobiographical memories, and consequently, less vulnerable to loss. However, the relative preservation of old semantic autobiographical memories holds only for mild AD, whereas with progression of the disease (perhaps, in association with the spread of AD pathology to the lateral temporal association cortices (Kim et al., 2011)) these memories also become prone to substantial loss.
Regardless of its neuroanatomical basis, the temporal gradient of autobiographical memory in AD can be attributed to differences in the methods used in assessing autobiographical recall. This issue was investigated by Barnabe et al. (2012) who assessed the Autobiographical Memory Interview (Kopelman, 1994) and the Autobiographical Interview (Levine et al., 2002). On the first task, AD participants were significantly impaired on both episodic and semantic autobiographical memory, with episodic memories showing a significant though modest temporal gradient sparing childhood memories. On the second task, the participants showed impaired recall of episodic but not of semantic memories, again with a modest temporal gradient. Differences between the two tasks (fewer epochs in the Autobiographical Memory Interview; fewer memories per epoch in the Autobiographical Interview) were found to have a significant impact on the pattern of findings; fewer epochs (in the first task) brought out the temporal gradient, whereas fewer memories per epoch (in the second task) diminished it. These findings show the importance of technical details when assessing autobiographical memory in AD.
4.3. Identity impairment
Due to anterograde amnesia, retrograde amnesia, and semantization of autobiographical memories, AD patients may have limited access to memories that shape their self-consciousness, self-knowledge, and self-image, resulting in a compromised sense of identity. This issue was assessed by a study (Addis and Tippett, 2004) in which mild AD patients were assessed with the Autobiographical Memory Interview and tests tapping into the cognitive structures underlying the sense of identity. In these tests, participants had to provide responses to the question “Who am I?” and were also assessed with a scale consisting of personal-self statements (e.g., I’m a cheerful person), family-self statements (e.g., “I am a member of a happy family”), social-self statements (e.g., “I’m a friendly person”), moral-self statements (e.g., “I am a decent person”), and physical-self statements (e.g., “I have a healthy body”). In these studies, poor autobiographical memory was significantly correlated with a weakened sense of identity.
Since older memories show better retrieval than recent ones, at least at the mild stage of AD, the reminiscence bump may provide AD patients with a significant portion of events that have defined their life-stories. Several studies have investigating the effect of AD on self-defining memories. In this area of research, a study reported that most autobiographical memories retrieved by AD participants originated from their reminisce bump (Fromholt et al., 2003). Another study assessed the impact of AD on self-defining memories by asking mild AD patients and healthy older adults to retrieve memories about lasting concerns or unresolved conflicts, that is, memories that could help them explain who they are (e.g., “memories from the day when I realized what mountain hiking meant to me”) (Martinelli et al., 2013). The latter study showed less specific self-defining memories in AD patients than in control participants. Hence, self-defining memories, or memories that are highly relevant for self-images, seem to be diminished even in mild stages of AD, which may explain the compromised sense of identity that occurs in the disease.
Unlike mild AD, research suggests identity compromise in the advanced stages of the disease. Studies have demonstrated that patients with advanced AD are less likely to recognize themselves in the mirror compared to patients with mild AD (Biringer and Anderson, 1992; Biringer et al., 1988; Grewal, 1994). However, a study has found that participants with mild or moderate AD were able to correctly identify the photograph of themselves when faced with a selection of five photographs; probably because the distractor photographs featured complete strangers, rather than acquaintances or fellow residents, which might have made the task of self-recognition easier for the participants (Fazio and Mitchell, 2009). Caddell and Clare (2010) suggested that AD patients often do show difficulties with self-recognition, especially in advanced disease. Another aspect of identity compromise is the difficulty that AD patients face in updating their self-knowledge [(Eustache et al., 2013; Klein et al., 2003), for a similar view, see Morris and Mograbi, 2013]. This issue was addressed by Klein et al. (2003) who investigated the discrepancy between a patient’s ratings of current personality and the relative’s ratings of that person’s personality now and before the onset of dementia. Results showed that the patient’s ratings were accurate, but reflected her personality before the onset of disease. Hence, self-knowledge in AD may be intact, thanks to semantic knowledge (Klein and Lax, 2010), but outdated. In another study, Ruby et al. (2009) asked mild AD patients and relatives to rate themselves and each other on a list of 40 adjectives and found decreased self-judgment accuracy in AD. Interviews and conversations were also used in studies examining identity in AD. For instance, participants were asked to share their memories with the researcher (Usita et al., 1998), or were interviewed with reference to various components of their narrative or life story (Surr, 2006). This approach aimed to determine how well patients were able to share their memories, and whether they possessed a narrative identity and a sense of self. Studies in this field have shown that most patients in the moderate or severe stages of AD are able, at least to some degree, to construct a narrative, and that this construction enables them to preserve aspects of their self and identity (Surr, 2006; Usita et al., 1998). Interestingly, identity construction was found to be based on autobiographical memories, providing further support to the relationship between autobiographical memory and self in AD.
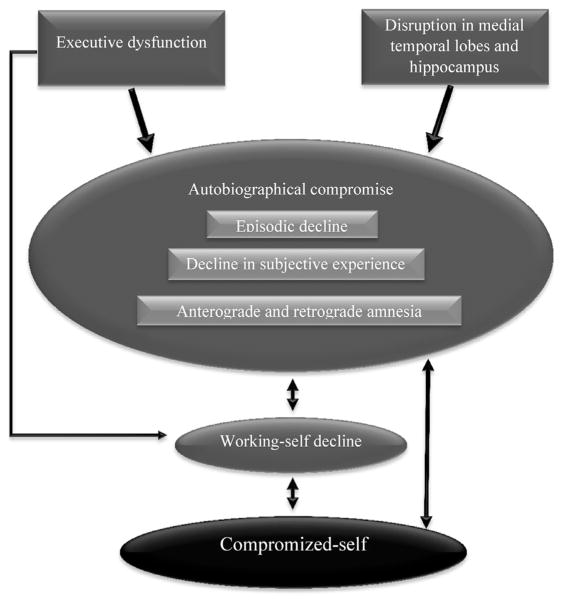
Together, and as illustrated in Fig. 1, the link between the compromised-self and autobiographical decline in AD may be attributed to poor correspondence and coherence between autobiographical memory and self-related goals and beliefs. This diminished correspondence and coherence is mainly mediated by disruption of the working-self that can be attributed to executive and frontal lobe dysfunction, as we will argue below.
Fig. 1.
According to our model (AMAD: Autobiographical Memory in Alzheimer’s Disease), the autobiographical compromise in Alzheimer’s disease (AD) is mainly characterized by a substantial loss of episodic information, weakened ability to mentally relive past events, and anterograde and retrograde amnesia. This autobiographical declineresults in a limited access to memories that shape self-consciousness, self-knowledge, and self-images, a limitation that leadsto a diminished sense of identity. The link between autobiographical decline and compromised-self in AD can also be attributed to disruption in the working-self, a disruption that can be connected to executive dysfunction. One consequence of the working-self disruption is low correspondence and coherence between autobiographical memory and current goals and believes of the self.
5. Cognitive and neuroanatomical underpinnings of autobiographical decline in AD
5.1. Executive dysfunction
Executive function refers to high-order control processes that are involved in regulation of thought and action (Friedman et al., 2006). Decline in executive function has been widely associated with impaired autobiographical memory in AD (Dall’Ora et al., 1989; Della Sala et al., 1993; Ivanoiu et al., 2006; Meeter et al., 2006). According to the executive account, autobiographical impairment in AD is the consequence of dysfunction affecting organization, elaboration, and memory search strategies rather than loss of stored memory representations. This view has been supported by empirical findings showing significant correlations between autobiographical performance in AD and tests of executive function, such as dual performance tasks (i.e., attentional tasks) and letter fluency (Della Sala et al., 1993). In a related vein, executive dysfunction has been found to be related with autobiographical overgenerality in AD (Moses et al., 2004). As executive function weakens, search for specific memories may stop at intermediate stages of more generic representations, which may result in semantic retrieval. Another implication of executive dysfunction is the decline of the hypothesized working-self, the executive center that shapes the correspondence and coherence of autobiographical memories (Conway and Pleydell-Pearce, 2000). As illustrated in Fig. 1, executive dysfunction may result in distortion of the working-self, and consequently, in low correspondence/coherence between autobiographical recall and current goals and beliefs. This may be reflected in incidents such as with a patient with AD that fails to explain why she/he is in the hospital claiming that she/he has come for a visit.
Executive function encompasses several processes, such as updating and shifting. These higher order executive functions are based on more fundamental frontal lobe functions, such as attention. Attention, is required for conscious recollection of autobiographic episodic memories (Dhanjal and Wise, 2014) and is diminished in AD (Kim et al., 2011). Therefore, attentional deficits in AD may contribute to autobiographical memory dysfunction. Cognitive control is a higher order function than attention that depends on the interplay of a cingulo-opercular salience network and a frontoparietal executive network. Both of these networks show dysfunction in AD, the level of which correlates with the level of memory impairment (Mormino et al., 2011). Another executive process that may be highlighted is inhibition since inhibitory deficits are an important feature of cognitive decline in AD (Amieva et al., 2004). Generally speaking, studies have associated the compromised inhibitory control in AD with performance on memory tasks (Collette et al., 2009; El Haj et al., 2015c), and, more specifically, a study has found that participants with AD have difficulties in inhibiting irrelevant autobiographical information (El Haj et al., 2011). The latter finding was produced with a directed forgetting task during which participants with AD were, first, instructed to dismiss certain autobiographical memories, but subsequently they were instructed to retrieve those memories. Taken together, there is evidence that executive dysfunction may hamper autobiographical memory retrieval in AD.
5.2. Neuroanatomical substrates of autobiographical decline in AD
As illustrated in this review, autobiographical recall involves many cognitive components and processes that contribute to the subjective experience of reliving. Not surprisingly, neuroimaging studies have shown that autobiographical recall involves a distributed network of brain regions that support a wide range of cognitive processes, such as memory storage, memory consolidation, memory search, autobiographical episodic counterfactual thinking, self-reference, and goal-related processes (Cabeza and St Jacques, 2007; Rubin, 2005; St Jacques et al., 2011; Svoboda et al., 2006). Neuroimaging studies have consistently implicated the default mode network (DMN) in autobiographical memory (Damoiseaux et al., 2012). The DMN is variably defined, but, in most accounts, it includes nodes at the medial and lateral prefrontal cortex (sometimes referred to as anterior DMN), the postero-medial parietal lobe (precuneus and retrosplenial cortices) and angular gyrus (sometimes referred to as posterior DMN and considered as the part of DMN specifically involved in episodic memory retrieval (Damoiseaux et al., 2012)), as well as medial temporal lobes (Cabeza and St Jacques, 2007; Spreng et al., 2010). With progressive beta-amyloid deposition and neurodegeneration in AD, functional connectivity within the DMN decreases, starting with the episodic memory-associated posterior DMN (Braak and Braak, 1991; Budson and Price, 2005; Damoiseaux et al., 2012). Generally speaking, the dorsomedial frontal and parietal DMN nodes are linked to autonoetic experience and mental time travel (Wheeler et al., 1997) and, more broadly, to self-referential processes [for a review, see Buckner et al., 2008]. In comparison, the ventral DMN nodes are linked to recovery of contextual information (Cabeza and St Jacques, 2007), whereas the lateral prefrontal cortex is linked to attention and executive processes (Seeley et al., 2007). Another functional dissociation within the DMN relevant to its autobiographical memory function may be attributable to emotional content, since dorsomedial regions (medial prefrontal cortex, posterior cingulate cortex) become more active during emotional autobiographical retrieval compared to ventral DMN regions that become more active during rest (Kim, 2010).
Autobiographical recall may be impaired to a degree in normal aging as it depends on an interaction between retrieval of contextual details mediated by the hippocampus and cognitive control processes. These processes, and their neuroanatomical substrates, are known to be sensitive to aging (St Jacques et al., 2012). For instance, hippocampal activity related to episodic recollection is attenuated by aging (Cabeza et al., 2004). Reduced frontal activation during controlled memory retrieval that lacks environmental support is also observed in aging (Paxton et al., 2008). In a related vein, a study has found an age-related decrease in contextual richness of autobiographical memory, a decline that was attributed to impaired strategic retrieval processes and poor recruitment of the hippocampus and ventrolateral prefrontal cortex (St Jacques et al., 2012). Taken together, these findings suggest that aging may impair to a degree the retrieval of episodic autobiographical details due to neuroanatomical changes in the hippocampus and ventrolateral prefrontal cortex.
Regarding AD, decreased episodic autobiographical performance has been associated with anterior lateral temporal cortex and medial temporal lobes atrophy (Gilboa et al., 2005); this is not surprising, since the medial temporal lobes, and especially the hippocampus, are preferentially targeted by the neuropathological processes underlying AD (Pennanen et al., 2004). Interestingly, a study has found associations between hippocampal volume and episodic autobiographical memory in AD for both recent and remote memories (Philippi et al., 2012). In contrast, a functional MRI study has demonstrated that retrieval of recent memories in AD participants was more associated with right prefrontal activation whereas retrieval of remote memories was more associated with left prefrontal activation, regardless of hippocampal activation (Eustache et al., 2004). This finding fits well with the hypothesis that the right prefrontal cortex is more involved in episodic memory whereas the left prefrontal cortex is more involved in semantic memory (Tulving et al., 1994). In this respect, the overgenerality of autobiographical memories in AD may be attributed to over-activation of the left prefrontal cortex during episodic retrieval. This hypothesis is further supported by an fMRI study that found that, relatively to episodic autobiographical retrieval, semantic retrieval in AD is associated with activation in the left prefrontal cortex (Meulenbroek et al., 2010). A recent lesion study directly addressing the dichotomy of semantic versus episodic autobiographical memory found that semantic deficits were associated with left mPFC and MTL damage, whereas episodic deficits were associated with right mPFC and MTL damage (De Brigard et al., 2015). In sum, the AD-related autobiographical decline can be linked to functional and structural impairment within the DMN and the hippocampus, but the exaggerated shift from episodic to semantic content may be due to compensatory overactivation of the left prefrontal cortex.
As for neuroanatomical support for the relationship between self and memory in AD, Genon et al. (2014) asked AD participants to indicate whether certain traits described them or not. Findings showed activation in the medial prefrontal cortex during encoding of self-relevance information. In addition, better memory for self-related information was associated with higher gray matter density in the lateral prefrontal cortex. According to the authors (Genon et al., 2014), the lateral prefrontal cortex supports high-order processes linking self and memory.
After we highlighted the cognitive and neuroanatomical underpinnings of autobiographical decline in AD, we will turn our attention to studies on clinical rehabilitation of autobiographical recall in the disease.
6. Clinical rehabilitation of autobiographical recall in AD
The aim of clinical rehabilitation of autobiographical memory in AD is to restore, as far as possible, inaccessible memories or to, at least, maintain the available pool of autobiographical memories of AD patients. Three main therapeutic strategies can be highlighted: reminisce therapy, auditory stimulation, and use of technological tools. We should note that various other therapeutic strategies are also being used toward this aim, but they are not being included in our review due to lack of empirical studies supporting their validity.
6.1. Reminiscence therapy
Reminiscence therapy has been the most widely studied clinical rehabilitation strategy for autobiographical recall in AD. This therapy focuses on conscious recall of personal memories from one’s life in order to inform thinking, telling, or teaching about past experiences. Reminiscence therapy was first proposed by Butler (Butler, 1963) who promoted it as a tool for enhancing wellbeing and reducing depression in older adults. Since then, the use of reminiscence has been widely applied in the cognitive rehabilitation of older adults, including those with AD, following two main directions: “life review” and “life review therapy”. Life review promotes evaluation and integration of positive and negative memories to help older adults understand how they have developed throughout their lives to become the person they are now; they are encouraged to evaluate what they have learned from their positive and negative past experiences. This clinical intervention is widely applied to older adults who are struggling to find meaning in their lives. In contrast with this “life review”, “life review therapy” addresses older adults with depression or anxiety since this therapy aims at reducing boredom and bitterness revival by focusing on life-stories that promote positive self-identities.
Life review therapy has been applied in patients with AD, mainly through the use of life books depicting important personal events (e.g., familial events, professional events) in different life periods (e.g., childhood, adolescence) [for comprehensive reviews, see Cotelli et al., 2012]. Although there is some evidence for beneficial effects of this therapy in AD, many studies show no effect, probably because studied participants were often at advanced stages of the disease [for the same view, see Bohlmeijer et al., 2007; Moos and Björn, 2006]. In some studies, life review therapy has also been applied without any structured methodology, and many studies have not included pre/post autobiographical evaluations to demonstrate any effects of the life review therapy [for the same view, see Moos and Björn, 2006]. These shortcomings were taken into account by a clinical intervention assessing the impact of an autobiographical training program on both the episodic and semantic components of autobiographical performance across lifetime periods (Lalanne et al., 2015). This training program was applied to patients with early to moderate AD, and effects were compared with a control program that focused on collective memory (e.g., memory for celebrities). The autobiographical training program included six sessions. On the first two sessions, participants had to retrieve semantic autobiographical knowledge of five life periods: 0–17 years, 18–30 years, age 30, the last 5 years, and the last 12 months. This semantic knowledge referred to names of family members, friends, teachers, or coworkers; participants had also to describe information about their residences, schools, workplaces, leisure activities, and holidays. Besides these two sessions, four sessions were dedicated to retrieval of episodic autobiographical memories, during which, participants had to remember unique personal events that occurred at specific times and locations, triggering specific emotions, perception and thoughts. After the program of Lalanne et al. (2015) was applied, the authors found better autobiographical recall in AD participants who took part in this program compared to those who took part in the control program, an enhancement that was specifically pronounced for autobiographical semantic memory.
The semantic aspect of autobiographical recall in AD is worth considering in designing rehabilitation studies, since research has shown that personal semantic memory contributes to numerous cognitive processes that involve self-referential thinking, such as reflecting on one’s past life, imagining a personal future, and maintaining self-identity [for a review, see Grilli and Verfaellie, 2014]. Indeed, autobiographical semantic memory supports knowledge of one’s traits, roles, thoughts, and beliefs, which may support self-referential thinking that is highly conceptual and independent of the medial temporal lobe (Grilli and Verfaellie, 2014). Hence, autobiographical semantic memory, which is relatively preserved in mild to moderate AD, can be utilized to help improve self-identity and self-continuity, as well as in developing self-referential cognitive strategies.
6.2. Auditory stimulation
Due to the beneficial effect of music on the mood of patients with AD, a body of research has assessed the effects of music exposure on autobiographical recall in AD. In a pioneering study, Foster and Valentine (2001) asked patients with mild to moderate AD to complete an autobiographical assessment while listening to Vivaldi’s Four Seasons, unfamiliar music, cafeteria noise, or in silence. The participants’ autobiographical performance was significantly better in the sound conditions than in silence, and while listening to music than in the cafeteria noise. These findings were replicated by another study that found better autobiographical recall in AD patients during exposure to Vivaldi’s Four Seasons music than in silence, a pattern that was related to a significant reduction in anxiety (Irish et al., 2006). The effect of auditory stimulation on autobiographical recall in AD was also assessed in a study that found more positive emotional words being retrieved during music exposure than in silence (El Haj et al., 2013). This emotional account is worth consideration since AD patients tend to show better memory for emotional than for neutral information, a trend that was found to be associated with integrity of the right orbitofrontal and sub-callosal cortices (Kumfor et al., 2013). Another study on emotional effect of auditory stimulation in AD found that music-evoked autobiographical memories in AD patients triggered more emotional content, had a greater impact on mood, were retrieved faster and engaged less executive processes than memories evoked in silence (El Haj et al., 2012a); these features are characteristic of involuntary memories, or those autobiographical memories that appear in consciousness spontaneously (Berntsen et al., 2013). Another account of music-evoked autobiographical memories was given by a study demonstrating that these memories were characterized by fewer empty words, higher grammatical complexity and propositional density than memories evoked in silence (El Haj et al., 2013). Hence, the negative effects of AD on autobiographical memory may to some extent be alleviated by exposure to music. However, we should note that most of these studies assessed only a single autobiographical event (El Haj et al., 2013, 2012a,b) or only semantic autobiographical knowledge (Foster and Valentine, 2001). Also, participants in these studies were in mild to moderate stages of the disease, whereas those in advanced stages seem to not benefit from music exposure. Regardless of its autobiographical implications, it would be of interest to assess whether music stimulation may influence the trajectory of disease progression in mild to moderate AD.
6.3. Use of technological tools
Clinical rehabilitation of autobiographical memory can benefit from technological tools that deliver more contextually rich and reliable memory cues and promote better realization of existing autobiographical deficits. In this area of research, a study has used SenseCam to promote autobiographical memory in AD (De Leo et al., 2011). SenceCam is a small wearable device that includes a digital camera and multiple sensors that detect changes in motion, light levels, and ambient temperature. This device also incorporates a passive infrared sensor to detect the presence of any person around the patient. SenceCam can record up to 3000 images per day, as well as data from all sensors. In the study by De Leo et al. (2011), SenseCam was used to record the daily lives of six patients with mild to moderate AD. Every two days for two weeks participants had to retrieve recent memories, and recall was followed by a structured review of the SenseCam images. A written diary control condition was also applied. Long-term recall of all events was tested one and three months later. During this post-intervention assessment, better autobiographical recall was observed for events reviewed on the SenseCam than on those reviewed on the dairy. In a similar vein, another study programmed a smartphone to take pictures of one patient’s life at five-minute intervals for 12 h each day for four weeks (Woodberry et al., 2014). The pictures were combined into a video slide show and were reviewed by the participant and caregiver. The participant was asked to retrieve memories before and after watching the slide show, with results showing better autobiographical recall after watching the slide show. Moreover, carrying the smart phone was not intrusive to the patient’s daily routines.
Taken together, there is a slim body of research showing beneficial effects of technological tools on autobiographical memory in mild to moderate AD. However, one impediment to the use of technological tools by patients with AD is their anosognosia, which prevents them from seeking external aids.
7. Summary and suggestions
Autobiographical memory decline in AD results in loss of knowledge about events that shaped patients’ lives, and consequently, degradation in their sense of identity. Autobiographical recall in AD is mainly characterized by a substantial loss of episodic information leading to a decontextualization of autobiographical memories and a shift from mentally reliving past events to a general sense of familiarity. This decline is exacerbated by anterograde and retrograde amnesia, leaving AD patients with a limited access to memories that shape self-consciousness, self-knowledge, and self-images. The ultimate outcome is a compromised sense of identity in the disease.
AD-related autobiographical impairment can be also the consequence of impaired attention and executive dysfunction affecting cognitive control, organization, elaboration, and memory search strategies. The decline of these working-self-mediated processes also results in poor correspondence and coherence between autobiographical memory and self-goals and beliefs. From neuroanatomical standpoint, the autobiographical decline in AD is attributed to structural and functional disruption of the default mode network and its medial temporal lobes and hippocampus connections.
AD-related autobiographical memory impairment can be alleviated, especially in the mild stages of the disease, thanks to therapeutic interventions such as reminisce therapy, auditory stimulation, and the use of technological tools. These clinical therapies can benefit from future research that can build on the below-mentioned venues.
7.1. Future venues
AD-related autobiographical decline has been widely associated with a weakened ability to mentally reliving past events, and this decline has been demonstrated using the Remember/Know paradigm (El Haj et al., 2014; Hudon et al., 2009; Rauchs et al., 2007). The study of autonoetic decline in AD requires specific rather than general assessment of subjective reliving. A prominent element of subjective reliving that should be studies in AD is visual imagery. According to Conway (Conway, 2005, 2009), autobiographical memories are predominantly reconstructed in the form of visual images. Visual imagery is also believed to trigger mental time travel (Tulving, 2002). Assessment of visual imagery in AD can be implemented through the “in-field/observer paradigm”. In this paradigm, participants are typically instructed to provide (1) an observer response if they see themselves in the remembered episode as a spectator or (2) a field response if they see the episode through their own eyes, as if they were reliving the episode from an actor perspective. Another component of subjective reliving worth considering is emotion. Although emotion is a key factor in autobiographical reconstruction (Conway, 2005), and may lead to the assembly of a specialized DMN sub-network during autobiographical memory recall (Bado et al., 2014), little is known about its role in AD-related autobiographical decline.
Besides targeting specific components of subjective reliving, clinical interventions targeting AD-related autobiographical decline, especially those employing reminiscence, should adopt a more rigorous methodology. Homogeneity of participants in terms of residual cognitive function and blinded pre-/post-intervention autobiographical evaluations should be performed. Also, long-term benefits of reminiscence should be assessed. Employment of rigorous study methodologies may explain controversial reports about reminiscence effects in AD. Another venue for future research is cuing autobiographical memory with non-auditory sensory stimuli, especially odors. Because odors are present in most everyday life contexts, associations between olfactory information and episodic autobiographical memories are highly probable, and odors may serve as a potential cue for episodic autobiographical recall. Olfactory stimulation may activate autobiographical memory without any effortful retrieval. According to Conway and Pleydell-Pearce (2000), autobiographical memory can be recovered via either direct or generative (strategic) retrieval. In direct retrieval, the cue alone is sufficient for retrieval, whereas in the strategic retrieval the cue only provides the starting point for a memory search. Therefore, and unlike generative retrieval, direct retrieval is an automatic mechanism that bypasses strategic processes (linked to executive function and the frontal lobes) by directly mapping cues onto stored information. Following this distinction, studies using odor cues to trigger autobiographical memories in healthy participants attributed their effects to direct retrieval (Larsson and Willander, 2009). These studies also found that these memories were more emotional and associated with stronger reliving as compared to memories evoked by other sensory cues (Larsson and Willander, 2009). Although AD patients tend to show impaired sense of smell, especially on odor identification and recognition tasks, olfactory abilities relying on low-level perceptual processing may be relatively preserved in the disease (Rahayel et al., 2012), enabling the potential use of odors for evoking autobiographical memories in AD.
Curiously for our age of technological advancement, few studies have investigated the benefits of electronic tools on autobiographical memory in AD, and there is a crucial need to fill this paucity.
7.2. Conclusion
Autobiographical memory is fundamental for the sense of identity and its impairment in AD results in an impaired sense of self and identity. Our review is an attempt to provide a comprehensive picture of the current status of empirical knowledge about autobiographical decline in AD encompassing cognitive, neuroanatomical, and clinical data. The deleterious consequences of autobiographical decline in AD are fairly well characterized, but there is much to learn about its potential rehabilitation. We hope that our review will motivate the study of different strategies for rehabilitation with the ultimate goal of maintaining the sense of self and identity of AD patients.
Acknowledgments
Drs. El Haj and Dr. Antoine were supported by the LABEX (excellence laboratory, program investment for the future) DIS-TALZ (Development of Innovative Strategies for a Transdisciplinary approach to Alzheimer disease). Dr. Kapogiannis was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health (NIA/NIH).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Addis DR, Sacchetti DC, Ally BA, Budson AE, Schacter DL. Episodic simulation of future events is impaired in mild Alzheimer’s disease. Neuropsychologia. 2009;47:2660–2671. doi: 10.1016/j.neuropsychologia.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Tippett LJ. Memory of myself: autobiographical memory and identity in Alzheimer’s disease. Memory. 2004;12:56–74. doi: 10.1080/09658210244000423. [DOI] [PubMed] [Google Scholar]
- Amieva H, Phillips LH, Della Sala S, Henry JD. Inhibitory functioning in Alzheimer’s disease. Brain. 2004;127:949–964. doi: 10.1093/brain/awh045. [DOI] [PubMed] [Google Scholar]
- Bado P, Engel A, de Oliveira-Souza R, Bramati IE, Paiva FF, Basilio R, Sato JR, Tovar-Moll F, Moll J. Functional dissociation of ventral frontal and dorsomedial default mode network components during resting state and emotional autobiographical recall. Hum Brain Mapp. 2014;35:3302–3313. doi: 10.1002/hbm.22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabe A, Whitehead V, Pilon R, Arsenault-Lapierre G, Chertkow H. Autobiographical memory in mild cognitive impairment and Alzheimer’s disease: a comparison between the Levine and Kopelman interview methodologies. Hippocampus. 2012;22:1809–1825. doi: 10.1002/hipo.22015. [DOI] [PubMed] [Google Scholar]
- Berntsen D, Staugaard SR, Sørensen LMT. Why am I remembering this now? Predicting the occurrence of involuntary (spontaneous) episodic memories. J Exp Psychol Gen. 2013;142:426. doi: 10.1037/a0029128. [DOI] [PubMed] [Google Scholar]
- Biringer F, Anderson JR. Self-recognition in Alzheimer’s disease: a mirror and video study. J Gerontol. 1992;47:P385–388. doi: 10.1093/geronj/47.6.p385. [DOI] [PubMed] [Google Scholar]
- Biringer F, Anderson JR, Strubel D. Self-recognition in senile dementia. Exp Aging Res. 1988;14:177–180. doi: 10.1080/03610738808259745. [DOI] [PubMed] [Google Scholar]
- Bohlmeijer E, Roemer M, Cuijpers P, Smit F. The effects of reminiscence on psychological well-being in older adults: a meta-analysis. Aging Mental Health. 2007;11:291–300. doi: 10.1080/13607860600963547. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Budson AE, Price BH. Memory dysfunction. N Engl J Med. 2005;352:692–699. doi: 10.1056/NEJMra041071. [DOI] [PubMed] [Google Scholar]
- Butler RN. The life review: an interpretation of reminiscence in the aged. Psychiatry. 1963;26:65–76. doi: 10.1080/00332747.1963.11023339. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cabeza R, St Jacques P. Functional neuroimaging of autobiographical memory. Trends Cogn Sci. 2007;11:219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Caddell LS, Clare L. The impact of dementia on self and identity: a systematic review. Clin Psychol Rev. 2010;30:113–126. doi: 10.1016/j.cpr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Collette F, Schmidt C, Scherrer C, Adam S, Salmon E. Specificity of inhibitory deficits in normal aging and Alzheimer’s disease. Neurobiol Aging. 2009;30:875–889. doi: 10.1016/j.neurobiolaging.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Conway MA. Memory and the self. J Mem Lang. 2005;53:594–628. [Google Scholar]
- Conway MA. Episodic memories. Neuropsychologia. 2009;47:2305–2313. doi: 10.1016/j.neuropsychologia.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychol Rev. 2000;107:261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Conway MA, Singer JA, Tagini A. The self and autobiographical memory: correspondence and coherence. Soc Cognit. 2004;22:491–529. [Google Scholar]
- Cotelli M, Manenti R, Zanetti O. Reminiscence therapy in dementia: a review. Maturitas. 2012;72:203–205. doi: 10.1016/j.maturitas.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Dall’Ora P, Della Sala S, Spinnler H. Autobiographical memory. Its impairment in amnesic syndromes. Cortex. 1989;25:197–217. doi: 10.1016/s0010-9452(89)80037-1. [DOI] [PubMed] [Google Scholar]
- Damasio A. Feelings of emotion and the self. Ann N Y Acad Sci. 2003;1001:253–261. doi: 10.1196/annals.1279.014. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Prater KE, Miller BL, Greicius MD. Functional connectivity tracks clinical deterioration in Alzheimer’s disease. Neurobiol Aging. 2012;33:828e819–830. doi: 10.1016/j.neurobiolaging.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brigard F, Nathan Spreng R, Mitchell JP, Schacter DL. Neural activity associated with self, other, and object-based counterfactual thinking. Neuroimage. 2015;109C:12–26. doi: 10.1016/j.neuroimage.2014.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brigard F, Szpunar KK, Schacter DL. Coming to grips with the past: effect of repeated simulation on the perceived plausibility of episodic counterfactual thoughts. Psychol Sci. 2013;24:1329–1334. doi: 10.1177/0956797612468163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leo G, Brivio E, Sautter SW. Supporting autobiographical memory in patients with Alzheimer’s disease using smart phones. Appl Neuropsychol. 2011;18:69–76. doi: 10.1080/09084282.2011.545730. [DOI] [PubMed] [Google Scholar]
- Della Sala S, Laiacona M, Spinnler H, Trivelli C. Autobiographical recollection and frontal damage. Neuropsychologia. 1993;31:823–839. doi: 10.1016/0028-3932(93)90131-i. [DOI] [PubMed] [Google Scholar]
- Dhanjal NS, Wise RJ. Frontoparietal cognitive control of verbal memory recall in Alzheimer’s disease. Ann Neurol. 2014;76:241–251. doi: 10.1002/ana.24199. [DOI] [PubMed] [Google Scholar]
- El Haj M, Antoine P, Kapogiannis D. Flexibility decline contributes to similarity of past and future thinking in Alzheimer’s disease. Hippocampus. 2015a doi: 10.1002/hipo.22465. http://dx.doi.org/10.1002/hipo.22465. [DOI] [PMC free article] [PubMed]
- El Haj M, Antoine P, Kapogiannis D. Similarity between remembering the past and imagining the future in Alzheimer’s disease: implication of episodic memory. Neuropsychologia. 2015b;66:119–125. doi: 10.1016/j.neuropsychologia.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Haj M, Clément S, Fasotti L, Allain P. Effects of music on autobiographical verbal narration in Alzheimer’s disease. J Neurolinguist. 2013;26:691–700. [Google Scholar]
- El Haj M, Fasotti L, Allain P. The involuntary nature of music-evoked autobiographical memories in Alzheimer’s disease. Conscious Cogn. 2012a;21:238–246. doi: 10.1016/j.concog.2011.12.005. [DOI] [PubMed] [Google Scholar]
- El Haj M, Fasotti L, Allain P. Directed forgetting of source memory in normal aging and Alzheimer’s disease. Aging Clin Exp Res. 2015c;27:329–336. doi: 10.1007/s40520-014-0276-1. [DOI] [PubMed] [Google Scholar]
- El Haj M, Moroni C, Luyat M, Omigie D, Allain P. To what extent does destination recall induce episodic reliving? Evidence from Alzheimer’s disease. J Clin Exp Neuropsychol. 2014;36:127–136. doi: 10.1080/13803395.2013.869309. [DOI] [PubMed] [Google Scholar]
- El Haj M, Postal V, Allain P. Music enhances autobiographical memory in mild Alzheimer’s disease. Educ Gerontol. 2012b;38:30–41. [Google Scholar]
- El Haj M, Postal V, Le Gall D, Allain P. Directed forgetting of autobiographical memory in mild Alzheimer’s disease. Memory. 2011;19:993–1003. doi: 10.1080/09658211.2011.626428. [DOI] [PubMed] [Google Scholar]
- Eustache F, Piolino P, Giffard B, Viader F, De La Sayette V, Baron JC, Desgranges B. ‘In the course of time’: a PET study of the cerebral substrates of autobiographical amnesia in Alzheimer’s disease. Brain. 2004;127:1549–1560. doi: 10.1093/brain/awh166. [DOI] [PubMed] [Google Scholar]
- Eustache ML, Laisney M, Juskenaite A, Letortu O, Platel H, Eustache F, Desgranges B. Sense of identity in advanced Alzheimer’s dementia: a cognitive dissociation between sameness and selfhood? Conscious Cogn. 2013;22:1456–1467. doi: 10.1016/j.concog.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Fazio S, Mitchell DB. Persistence of self in individuals with Alzheimer’s disease: evidence from language and visual recognition. Dementia. 2009;8:39–59. [Google Scholar]
- Foster NA, Valentine ER. The effect of auditory stimulation on autobiographical recall in dementia. Exp Aging Res. 2001;27:215–228. doi: 10.1080/036107301300208664. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Corley RP, Young SE, DeFries JC, Hewitt JK. Not all executive functions are related to intelligence. Psychol Sci. 2006;17:172–179. doi: 10.1111/j.1467-9280.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Fromholt P, Mortensen DB, Torpdahl P, Bender L, Larsen P, Rubin DC. Life-narrative and word-cued autobiographical memories in centenarians: comparisons with 80-year-old control, depressed, and dementia groups. Memory. 2003;11:81–88. doi: 10.1080/741938171. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Shahid KR, Olson MA, Solomon TM, Schacter DL, Budson AE. Overdependence on degraded gist memory in Alzheimer’s disease. Neuropsychology. 2006;20:625–632. doi: 10.1037/0894-4105.20.6.625. [DOI] [PubMed] [Google Scholar]
- Genon S, Bahri MA, Collette F, Angel L, d’Argembeau A, Clarys D, Kalenzaga S, Salmon E, Bastin C. Cognitive and neuroimaging evidence of impaired interaction between self and memory in Alzheimer’s disease. Cortex. 2014;51:11–24. doi: 10.1016/j.cortex.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Ramirez J, Kohler S, Westmacott R, Black SE, Moscovitch M. Retrieval of autobiographical memory in Alzheimer’s disease: relation to volumes of medial temporal lobe and other structures. Hippocampus. 2005;15:535–550. doi: 10.1002/hipo.20090. [DOI] [PubMed] [Google Scholar]
- Graham KS, Hodges JR. Differentiating the roles of the hippocampal complex and the neocortex in long-term memory storage: evidence from the study of semantic dementia and Alzheimer’s disease. Neuropsychology. 1997;11:77–89. doi: 10.1037//0894-4105.11.1.77. [DOI] [PubMed] [Google Scholar]
- Greene JD, Hodges JR, Baddeley AD. Autobiographical memory and executive function in early dementia of Alzheimer type. Neuropsychologia. 1995;33:1647–1670. doi: 10.1016/0028-3932(95)00046-1. [DOI] [PubMed] [Google Scholar]
- Grewal RP. Self-recognition in dementia of the Alzheimer type. Percept Mot Skills. 1994;79:1009–1010. doi: 10.2466/pms.1994.79.2.1009. [DOI] [PubMed] [Google Scholar]
- Grilli MD, Verfaellie M. Personal semantic memory: insights from neuropsychological research on amnesia. Neuropsychologia. 2014;61:56–64. doi: 10.1016/j.neuropsychologia.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Hou CE, Miller BL, Kramer JH. Patterns of autobiographical memory loss in dementia. Int J Geriatr Psychiatry. 2005;20:809–815. doi: 10.1002/gps.1361. [DOI] [PubMed] [Google Scholar]
- Hudon C, Belleville S, Gauthier S. The assessment of recognition memory using the Remember/Know procedure in amnestic mild cognitive impairment and probable Alzheimer’s disease. Brain Cogn. 2009;70:171–179. doi: 10.1016/j.bandc.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Irish M, Cunningham CJ, Walsh JB, Coakley D, Lawlor BA, Robertson IH, Coen RF. Investigating the enhancing effect of music on autobiographical memory in mild Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;22:108–120. doi: 10.1159/000093487. [DOI] [PubMed] [Google Scholar]
- Irish M, Hornberger M, Lah S, Miller L, Pengas G, Nestor PJ, Hodges JR, Piguet O. Profiles of recent autobiographical memory retrieval in semantic dementia, behavioural-variant frontotemporal dementia, and Alzheimer’s disease. Neuropsychologia. 2011;49:2694–2702. doi: 10.1016/j.neuropsychologia.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Ivanoiu A, Cooper JM, Shanks MF, Venneri A. Patterns of impairment in autobiographical memory in the degenerative dementias constrain models of memory. Neuropsychologia. 2006;44:1936–1955. doi: 10.1016/j.neuropsychologia.2006.01.030. [DOI] [PubMed] [Google Scholar]
- James W. Psychology: The Briefer Course. Holt; New York, NY: 1892. [Google Scholar]
- Janssen SM, Rubin DC, Conway MA. The reminiscence bump in the temporal distribution of the best football players of all time: Pele, Cruijff or Maradona? Q J Exp Psychol (Hove) 2012;65:165–178. doi: 10.1080/17470218.2011.606372. [DOI] [PubMed] [Google Scholar]
- Kim SH. The global security perspective on the effects of executive cognitive function on complex behavioral screening intervention and HIV/AIDS. Soc Work Public Health. 2010;25:591–608. doi: 10.1080/19371910903127083. [DOI] [PubMed] [Google Scholar]
- Kim SY, Park MH, Han SH, Na HR, Cho S, Choi MS, Lee JH, Na DL, Kim JE, Park KW. Validation analysis of the Attention Questionnaire Scale. J Alzheimers Dis. 2011;24:393–402. doi: 10.3233/JAD-2011-100660. [DOI] [PubMed] [Google Scholar]
- Klein SB, Cosmides L, Costabile KA. Preserved knowledge of self in a case of Alzheimer’s dementia. Soc Cogn. 2003;21:157–165. [Google Scholar]
- Klein SB, Gangi CE. The multiplicity of self: neuropsychological evidence and its implications for the self as a construct in psychological research. Ann N Y Acad Sci. 2010;1191:1–15. doi: 10.1111/j.1749-6632.2010.05441.x. [DOI] [PubMed] [Google Scholar]
- Klein SB, Lax ML. The unanticipated resilience of trait self-knowledge in the face of neural damage. Memory. 2010;18:918–948. doi: 10.1080/09658211.2010.524651. [DOI] [PubMed] [Google Scholar]
- Klein SB, Loftus J, Kihlstrom JF. Memory and temporal experience: the effects of episodic memory loss on an amnesic patient’s ability to remember the past and imagine the future. Soc Cogn. 2002;20:353–379. [Google Scholar]
- Kopelman MD. The Autobiographical Memory Interview (AMI) in organic and psychogenic amnesia. Memory. 1994;2:211–235. doi: 10.1080/09658219408258945. [DOI] [PubMed] [Google Scholar]
- Kumfor F, Irish M, Hodges JR, Piguet O. The orbitofrontal cortex is involved in emotional enhancement of memory: evidence from the dementias. Brain. 2013:awt185. doi: 10.1093/brain/awt185. [DOI] [PubMed] [Google Scholar]
- Lalanne J, Gallarda T, Piolino P. The castle of Remembrance: new insights from a cognitive training programme for autobiographical memory in Alzheimer’s disease. Neuropsychol Rehabil. 2015;25:254–282. doi: 10.1080/09602011.2014.949276. [DOI] [PubMed] [Google Scholar]
- Larsson M, Willander J. Autobiographical odor memory. Ann N Y Acad Sci. 2009;1170:318–323. doi: 10.1111/j.1749-6632.2009.03934.x. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychol Aging. 2002;17:677–689. [PubMed] [Google Scholar]
- Leyhe T, Muller S, Milian M, Eschweiler GW, Saur R. Impairment of episodic and semantic autobiographical memory in patients with mild cognitive impairment and early Alzheimer’s disease. Neuropsychologia. 2009;47:2464–2469. doi: 10.1016/j.neuropsychologia.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Martinelli P, Anssens A, Sperduti M, Piolino P. The influence of normal aging and Alzheimer’s disease in autobiographical memory highly related to the self. Neuropsychology. 2013;27:69–78. doi: 10.1037/a0030453. [DOI] [PubMed] [Google Scholar]
- Meeter M, Eijsackers EV, Mulder JL. Retrograde amnesia for autobiographical memories and public events in mild and moderate Alzheimer’s disease. J Clin Exp Neuropsychol. 2006;28:914–927. doi: 10.1080/13803390591001043. [DOI] [PubMed] [Google Scholar]
- Meulenbroek O, Rijpkema M, Kessels RP, Rikkert MG, Fernandez G. Autobiographical memory retrieval in patients with Alzheimer’s disease. Neuroimage. 2010;53:331–340. doi: 10.1016/j.neuroimage.2010.05.082. [DOI] [PubMed] [Google Scholar]
- Moos I, Björn A. Use of the life story in the institutional care of people with dementia: a review of intervention studies. Ageing Soc. 2006;26:431–454. [Google Scholar]
- Mormino EC, Smiljic A, Hayenga AO, Onami SH, Greicius MD, Rabinovici GD, Janabi M, Baker SL, Yen IV, Madison CM, Miller BL, Jagust WJ. Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cereb Cortex. 2011;21:2399–2407. doi: 10.1093/cercor/bhr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Mograbi DC. Anosognosia, autobiographical memory and self knowledge in Alzheimer’s disease. Cortex. 2013;49:1553–1565. doi: 10.1016/j.cortex.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Moses A, Culpin V, Lowe C, McWilliam C. Overgenerality of autobiographical memory in Alzheimer’s disease. Br J Clin Psychol. 2004;43:377–386. doi: 10.1348/0144665042388964. [DOI] [PubMed] [Google Scholar]
- Muller S, Saur R, Greve B, Melms A, Hautzinger M, Fallgatter AJ, Leyhe T. Similar autobiographical memory impairment in long-term secondary progressive multiple sclerosis and Alzheimer’s disease. Mult Scler. 2013;19:225–232. doi: 10.1177/1352458512450352. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Nadel L, Winocur G, Ryan L, Moscovitch M. Systems consolidation and hippocampus: two views. Debates Neurosci. 2007;1:55–66. [Google Scholar]
- Neisser U. Five kinds of self-knowledge. Philos Psychol. 1988;1:35–59. [Google Scholar]
- Paxton JL, Barch DM, Racine CA, Braver TS. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cereb Cortex. 2008;18:1010–1028. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hanninen T, Laakso MP, Hallikainen M, Vanhanen M, Nissinen A, Helkala EL, Vainio P, Vanninen R, Partanen K, Soininen H. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol Aging. 2004;25:303–310. doi: 10.1016/S0197-4580(03)00084-8. [DOI] [PubMed] [Google Scholar]
- Philippi N, Noblet V, Botzung A, Despres O, Renard F, Sfikas G, Cretin B, Kremer S, Manning L, Blanc F. MRI-based volumetry correlates of autobiographical memory in Alzheimer’s disease. PLoS One. 2012;7:e46200. doi: 10.1371/journal.pone.0046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillemer DB. Momentous events and the life story. Rev Gen Psychol. 2001;5:123. [Google Scholar]
- Piolino P, Coste C, Martinelli P, Mace AL, Quinette P, Guillery-Girard B, Belleville S. Reduced specificity of autobiographical memory and aging: do the executive and feature binding functions of working memory have a role. Neuropsychologia. 2010;48:429–440. doi: 10.1016/j.neuropsychologia.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Belliard S, Matuszewski V, Lalevee C, De la Sayette V, Eustache F. Autobiographical memory and autonoetic consciousness: triple dissociation in neurodegenerative diseases. Brain. 2003;126:2203–2219. doi: 10.1093/brain/awg222. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Clarys D, Guillery-Girard B, Taconnat L, Isingrini M, Eustache F. Autobiographical memory, autonoetic consciousness, and self-perspective in aging. Psychol Aging. 2006;21:510–525. doi: 10.1037/0882-7974.21.3.510. [DOI] [PubMed] [Google Scholar]
- Prebble SC, Addis DR, Tippett LJ. Autobiographical memory and sense of self. Psychol Bull. 2013;139:815–840. doi: 10.1037/a0030146. [DOI] [PubMed] [Google Scholar]
- Rahayel S, Frasnelli J, Joubert S. The effect of Alzheimer’s disease and Parkinson’s disease on olfaction: a meta-analysis. Behav Brain Res. 2012;231:60–74. doi: 10.1016/j.bbr.2012.02.047. [DOI] [PubMed] [Google Scholar]
- Rauchs G, Piolino P, Mézenge F, Landeau B, Lalevée C, Pélerin A, Viader F, De La Sayette V, Eustache F, Desgranges B. Autonoetic consciousness in Alzheimer’s disease: neuropsychological and PET findings using an episodic learning and recognition task. Neurobiol Aging. 2007;28:1410–1420. doi: 10.1016/j.neurobiolaging.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Rubin DC. A basic-systems approach to autobiographical memory. Curr Dir Psychol Sci. 2005;14:79–83. [Google Scholar]
- Rubin DC, Wenzel AE. One hundred years of forgetting: a quantitative description of retention. Psychol Rev. 1996;103:734. [Google Scholar]
- Ruby P, Collette F, D’Argembeau A, Peters F, Degueldre C, Balteau E, Luxen A, Maquet P, Salmon E. Perspective taking to assess self-personality: what’s modified in Alzheimer’s disease. Neurobiol Aging. 2009;30:1637–1651. doi: 10.1016/j.neurobiolaging.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philos Trans R Soc Lond B Biol Sci. 2007a;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. Constructive memory: the ghosts of past and future. Nature. 2007b;445:27. doi: 10.1038/445027a. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl U, Lueken U, Thomann PA, Geider J, Schroder J. Autobiographical memory deficits in Alzheimer’s disease. J Alzheimers Dis. 2011;27:567–574. doi: 10.3233/JAD-2011-110014. [DOI] [PubMed] [Google Scholar]
- Singer JA. Seeing one’s self: locating narrative memory in a framework of personality. J Pers. 1995;63:429–457. doi: 10.1111/j.1467-6494.1995.tb00502.x. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Kragel PA, Rubin DC. Dynamic neural networks supporting memory retrieval. Neuroimage. 2011;57:608–616. doi: 10.1016/j.neuroimage.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Rubin DC, Cabeza R. Age-related effects on the neural correlates of autobiographical memory retrieval. Neurobiol Aging. 2012;33:1298–1310. doi: 10.1016/j.neurobiolaging.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surr CA. Preservation of self in people with dementia living in residential care: a socio-biographical approach. Soc Sci Med. 2006;62:1720–1730. doi: 10.1016/j.socscimed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci U S A. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usita PM, Hyman IE, Jr, Herman KC. Narrative intentions: listening to life stories in Alzheimer’s disease. J Aging Stud. 1998;12:185–197. [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: the frontal lobes and autonoetic consciousness. Psychol Bull. 1997;121:331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- Williams JM, Scott J. Autobiographical memory in depression. Psychol Med. 1988;18:689–695. doi: 10.1017/s0033291700008370. [DOI] [PubMed] [Google Scholar]
- Woodberry E, Browne G, Hodges S, Watson P, Kapur N, Woodberry K. The use of a wearable camera improves autobiographical memory in patients with Alzheimer’s disease. Memory. 2014:1–10. doi: 10.1080/09658211.2014.886703. [DOI] [PubMed] [Google Scholar]