Abstract
We used preS2-S′-β-galactosidase, a three-domain fusion protein that aggregates extensively at 43°C in the cytoplasm of Escherichia coli, to search for multicopy suppressors of protein aggregation and inclusion body formation and took advantage of the known differential solubility of preS2-S′-β-galactosidase at 37 and 43°C to develop a selection procedure for the gene products that would prevent its aggregation in vivo at 43°C. First, we demonstrate that the differential solubility of preS2-S′-β-galactosidase results in a lactose-positive phenotype at 37°C as opposed to a lactose-negative phenotype at 43°C. We searched for multicopy suppressors of preS2-S′-β-galactosidase aggregation by selecting pink lactose-positive colonies on a background of white lactose-negative colonies at 43°C after transformation of bacteria with an E. coli gene bank. We discovered that protein isoaspartate methyltransferase (PIMT) is a multicopy suppressor of preS2-S′-β-galactosidase aggregation at 43°C. Overexpression of PIMT reduces the amount of preS2-S′-β-galactosidase found in inclusion bodies at 43°C and increases its amount in soluble fractions. It reduces the level of isoaspartate formation in preS2-S′-β-galactosidase and increases its thermal stability in E. coli crude extracts without increasing the thermostability of a control protein, citrate synthase, in the same extracts. We could not detect any induction of the heat shock response resulting from PIMT overexpression, as judged from amounts of DnaK and GroEL, which were similar in the PIMT-overproducing and control strains. These results suggest that PIMT might be overburdened in some physiological conditions and that its overproduction may be beneficial in conditions in which protein aggregation occurs, for example, during biotechnological protein overproduction or in protein aggregation diseases.
Protein misfolding and aggregation are major threats to living organisms. Protein aggregation can occur as a consequence of unfolding of native proteins during environmental stress or during de novo folding and is frequently observed upon synthesis of recombinant proteins in Escherichia coli (28).
Molecular chaperones are ubiquitous proteins that facilitate the folding of a fraction of newly synthesized polypeptides and participate in the reactivation or degradation of proteins damaged by heat shock and other types of stress. The major chaperone systems are the DnaK/DnaJ/GrpE and GroEL/GroES teams (12). Additional proteins may participate in cellular protein folding, the ribosome-associated peptidyl cis/trans isomerase trigger factor (6), the Clp ATPases (ClpA, ClpB, ClpX, and ClpY [37]), the Hsp90 homolog HtpG (30), the small heat shock proteins IbpA and IbpB (1), the redox-regulated chaperone Hsp33 (13), and the recently discovered chaperone YedU/Hsp31 (19, 26). Furthermore, the redox state of cytoplasmic proteins is controlled by the thioredoxin system (thioredoxin, thioredoxin reductase, and NADPH) and the glutathione/glutaredoxin system (5). Moreover, several protein repair processes have been identified, including protein proline isomerases that convert generally abnormal cis-proline residues to trans-proline residues (27), methionine sulfoxide reductases that convert oxidatively modified methionine sulfoxide residues to normal methionine residues (11), and protein isoaspartate methyltransferases that recognize l-isoaspartyl residues for repair (9). Finally, irreversibly damaged proteins are degraded by intracellular ATP-dependent proteases such as the proteasome in eukaryotes and Lon, ClpA/ClpP, ClpX/ClpP, or HslU/HslV in bacteria (29).
In this work, we used the preS2-S′-β-galactosidase fusion protein as a model to find novel multicopy suppressors (3, 35) of protein aggregation in vivo. Previous in vitro studies (17, 32) have shown that this fusion protein, which is soluble at 30 and 37°C, is highly prone to aggregation at 42°C. It has also been shown that increased levels of DnaK and DnaJ and of the heat shock sigma factor σ32 result in an increase in the recovery of active preS2-S′-β-galactosidase in crude bacterial extracts (17, 33). We took advantage of this differential solubility of preS2-S′-β-galactosidase observed in vitro to develop a selection procedure for gene products that would prevent preS2-S′-β-galactosidase aggregation in vivo. In this study, we show that the differential solubility of preS2-S′-β-galactosidase results in a lactose-positive phenotype at 37°C (pink colonies on MacConkey agar) and a lactose-negative phenotype at 43°C (white colonies on MacConkey agar). We searched for multicopy suppressors of preS2-S′-β-galactosidase aggregation by selecting pink lactose-positive colonies on a background of white lactose-negative colonies at 43°C after transformation of bacteria with an E. coli gene bank. Surprisingly, we discovered that protein isoaspartate methyltransferase (PIMT) is a multicopy suppressor of preS2-S′-β-galactosidase aggregation at 43°C.
Protein isoaspartate methyltransferase (the pcm gene product in E. coli) is an enzyme that recognizes l-isoaspartyl residues which arise mainly from the spontaneous rearrangement of aspartyl and asparaginyl residues in proteins (2). In the best-characterized pathway, a methyl group is transferred from S-adenosyl-l-methionine to form a methyl ester on the α-carboxyl group of an isoaspartyl residue. Subsequent nonenzymatic reactions result in the rapid formation of an unstable succinimide intermediate which can yield a normal l-aspartyl residue upon hydrolysis, thus ultimately catalyzing net repair of the damaged site (25). The formation of isoaspartyl residues can result in heterogeneity or loss of protein function. For example, isomerization of Asp-101 in lysozyme significantly reduces its affinity for its chitin substrate (38), and isomerization of Asp-11 in human epidermal growth factor reduces its mitogenic activity to 20% of normal levels (10). In E. coli, a few proteins, including ribosomal protein S11, are subjected to isoaspartate formation (4), and disruption of the l-isoaspartyl methyltransferase pcm gene results in a decreased rate of survival during stationary phase of bacteria subjected to environmental stress (36). In the present study, we isolated PIMT as a multicopy suppressor of preS2-S′-β-galactosidase aggregation at 43°C. We show that preS2-S′-β-galactosidase is a site of isoaspartate formation and that overexpression of PIMT reduces isoaspartate formation in preS2-S′-β-galactosidase, reduces the amount of preS2-S′-β-galactosidase found in inclusion bodies at 43°C, and increases its thermal stability in E. coli crude extracts.
MATERIALS AND METHODS
Strains, plasmids, and media.
E. coli strain JC196 (parental strain MG1655) was used as a host strain in all experiments since it carries the Δ(lacZ)M15 deletion (absence of residual β-galactosidase activity). Plasmid pTBGH+, which encodes the fusion preS2-S′-β-galactosidase, has been described previously (17). We plated strain JC196 carrying plasmid pTBGH+ on MacConkey agar-1% lactose at 37 and 43°C in order to check the lactose-positive and -negative phenotypes.
We used an E. coli genomic library as starting material for the selection of multicopy suppressors. We constructed the library by partially digesting chromosomal DNA of strain JC196 with Sau3A and recovered 3- to 6-kb fragments after electrophoresis through a 1% agarose gel and ligating the fragments with BamHI-digested pACYC184 (low-copy-number plasmid carrying the origin of replication of plasmid p15A). We then used the E. coli bank to transform strain JC196 containing plasmid pTBGH+. Pink transformants at 43°C on a background of white colonies were picked, and plasmid DNA was extracted from these transformants and sequenced. We disrupted the pcm and nlpD genes by inserting a kanamycin cassette in each of the genes. Chloramphenicol, ampicillin, and kanamycin were added, as required, at 20, 50, and 50 μg/ml, respectively.
Shake flask cultures and cell fractionation.
We used cultures grown overnight at 37°C in Luria-Bertani (LB) medium to inoculate flasks containing 25 ml of LB medium. The cells were grown at 37°C to mid-exponential phase (optical density of 0.4), induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and returned to 37°C or transferred to 43°C. Cells were pelleted at 8,000 × g for 10 min, resuspended in 1 ml of 50 mM potassium phosphate (pH 6.5), and disrupted by sonication three times for 30 s. The crude extract was centrifuged for 15 min at 15,000 × g, leading to the soluble supernatant and inclusion bodies containing pellet. β-Galactosidase in fractionated extracts was quantified by enzymatic activity or immunodetection.
Enzymatic assays.
We performed β-galactosidase assays in triplicate on clarified soluble extract by using the chromogenic substrate o-nitrophenyl-β-d-galactoside as described previously by Miller (20). Citrate synthase assays were performed as described previously (14).
Methylesterification of proteins in intact cells.
E. coli was grown in exponential phase in 63 minimal medium (20) with 0.4% glycerol as the carbon source, 1 nmol of l-[methyl 3H]methionine (15 μCi) was added, and the mixture was incubated at 37°C for 30 min. Cells were lysed by sonication, and the proteins were immediately trichloroacetic acid precipitated for autoradiography after electrophoresis on polyacrylamide gels. Electrophoresis was carried at pH 2.4 as described previously by Fairbanks and Avruch (8). Gels were incubated for 30 min with the fluorographic reagent Amplify (Amersham Pharmacia Biotech) and dried before autoradiography.
Immunoblots, radiolabeling, and immunoprecipitation.
Immunoblots of DnaK, GroEL, and preS2-S′-β-galactosidase were done as described previously (7), and detection was made by using the chemiluminescent substrate SuperSignal West Pico from Pierce. Anti-DnaK and anti-GroEL antibodies were prepared as described previously (7). Anti-β-galactosidase antibodies were obtained from ICN. Radiolabeling and immunoprecipitation were done as described previously (7). Cells growing exponentially in glycerol medium at 30°C and then transferred to 43°C were pulse-labeled for 2 min with [35S]methionine (1,000 Ci/mmol; Amersham) at the indicated times, chased with nonradioactive methionine for 1 min, and kept on ice before lysis and immunoprecipitation of the crude extract with anti-DnaK antibodies. Proteins were resolved by electrophoresis, detected, and quantified by using a Molecular Dynamics PhosphorImager.
RESULTS
Bacteria producing the recombinant preS2-S′-β-galactosidase display a lactose-positive phenotype at 37°C and a lactose-negative phenotype at 43°C. Plasmid pTBGH+ encodes preS2-S′-β-galactosidase, a tripartite fusion protein consisting of the 55-residue preS2 domain and the 30-residue hydrophobic S′ domain of the hepatitis B surface antigen followed by E. coli β-galactosidase (17). In this construct, the β-galactosidase gene is under the control of the IPTG-inducible tac promoter. The β-galactosidase activity present in E. coli cells harboring pTBGH+ (measured in vitro after lysis of the cells) has been shown to decrease with increasing growth temperature due to the formation of preS2-S′-β-galactosidase inclusion bodies (17). This relationship between enzymatic activity and aggregation makes this fusion protein a useful model for the study of chaperone-assisted folding pathways in E. coli. As a consequence of preS2-S′-β-galactosidase inactivation at 43°C, the E. coli strain JC196 harboring pTBGH+ displays pink colonies at 37°C (lactose-positive phenotype) and white colonies at 43°C (lactose-negative phenotype) (data not shown). Thus, the increase in preS2-S′-β-galactosidase aggregation with increasing growth temperature observed in vitro by Thomas and Baneyx (32) corresponds to a lactose-positive phenotype at 37°C compared to a lactose-negative phenotype at 43°C.
Multicopy suppressors of the lactose-negative phenotype at 43°C.
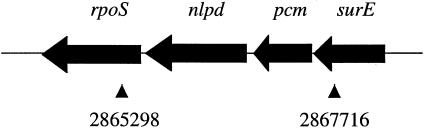
A mixture of recombinant plasmids randomly carrying chromosomal E. coli fragments (cloned in the low-copy-number plasmid pACYC184) was introduced into strain JC196 harboring pTBGH+. Lactose-positive transformants (pink) were selected on MacConkey plates at 43°C (on a background of white colonies). Plasmid DNAs were extracted from these transformants and reintroduced into JC196 harboring pTBGH+ in order to confirm the lactose-positive phenotype. We obtained 24 clones from 80,000 transformants. We extracted and then sequenced plasmid DNA from the lactose-positive clones. Sixteen clones contained the chbB and chbC genes, two genes that belong to the N,N′-diacetylchitobiose phosphotransferase transport system (unpublished data) (see Discussion), and six clones contained a 2.5-kb DNA fragment (Fig. 1) harboring both the pcm gene (which codes for protein isoaspartate methyltransferase) and the nlpD gene (which codes for an outer membrane protein possibly involved in peptidoglycan remodeling) (16).
FIG. 1.
Gene organization around the pcm gene. The genes are rpoS (RNA polymerase, σS subunit, stationary phase), nlpD (may function in cell wall formation), pcm (l-isoaspartyl protein carboxylmethyltransferase; repair of isoaspartyl residues), and surE (required for stationary-phase survival). The multicopy suppressor fragment is delimited by the two arrows, and numbers indicate the location (bases) of its extremities on the E. coli chromosome.
PIMT is a multicopy suppressor of the lactose-negative phenotype at 43°C.
In order to determine whether pcm or nlpD overproduction leads to the lactose-positive phenotype, we interrupted the pcm gene of the pACYC184 plasmid with a kanamycin cassette and determined the lactose phenotype of the JC196(pTBGH+) strain transformed with the pcm-interrupted plasmid. Bacteria transformed with the pcm-interrupted plasmid displayed white colonies at 43°C, suggesting that it is the pcm gene product that suppresses the lactose-negative phenotype at 43°C (data not shown). We interrupted the nlpD gene in parallel, which resulted in pink colonies on MacConkey agar at 43°C, suggesting that it is not involved in the suppression activity. These results strongly suggest that it is the overproduction of the pcm gene which leads to the lactose-positive phenotype at 43°C. The results also suggest that neither the small 5′ fragment of the rpoS gene (276 nucleotides) nor the small 3′ fragment of the surE gene (180 nucleotides) is responsible for the lactose-positive phenotype (moreover, neither of these two genes was selected as a multicopy suppressor of the lactose-negative phenotype at 43°C).
Effect of PIMT overexpression on the cellular localization of preS2-S′-β-galactosidase.
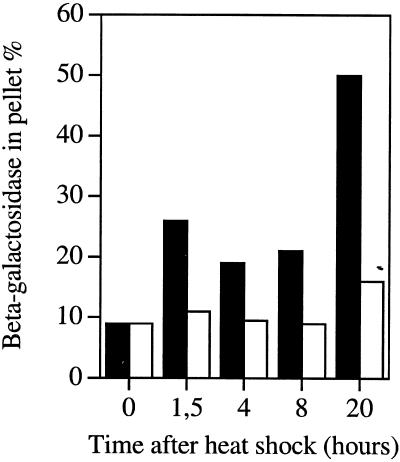
We investigated the effect of protein isoaspartate methyltransferase overproduction on the cellular localization of preS2-S′-β-galactosidase by using enzymatic assays after fractionation of extracts. Control cells and bacteria overproducing protein isoaspartate methyltransferase were grown in LB medium at 37°C in the presence of 1 mM IPTG and shifted to 43°C. Extracts were prepared from cells before and several hours after heat shock. Soluble protein and inclusion bodies were separated by centrifugation at 15,000 × g after bacterial lysis, and β-galactosidase was measured in soluble and aggregated fractions. As shown in Fig. 2, 10% of β-galactosidase precipitates as inclusion bodies in both strains at 37°C. In control cells at 43°C, this amount increased to 25% 1.5 to 8 h after heat shock and up to 50% 16 h after heat shock. In contrast, in the protein isoaspartate methyltransferase-overproducing strain, this amount increased only slightly, to 10 to 13% 1.5 to 8 h after heat shock and 17% 16 h after heat shock. Thus, overexpression of protein isoaspartate methyltransferase efficiently reduces (two- to threefold) the amount of overproduced preS2-S′-β-galactosidase in inclusion bodies. Conversely, the amount of β-galactosidase found in soluble fraction decreased as a function of time at 43°C in the control strain (down to 19% of its value at 37°C), whereas it remained more or less constant (around 80% of its value at 37°C) in the protein isoaspartate methyltransferase-overproducing strain (data not shown). These results concerning preS2-S′-β-galactosidase localization in control and PIMT-overproducing cells at 43°C are consistent with the ability of protein isoaspartate methyltransferase overproduction to reverse the lactose-negative phenotype at 43°C.
FIG. 2.
Effect of overproduction of PIMT on the recovery of soluble preS2-S′-β-galactosidase. Insoluble fractions corresponding to identical amounts of PIMT-overproducing (open bars) or control (filled bars) bacteria were collected at indicated times after transfer from 37 to 43°C, and β-galactosidase was quantified by immunoblot (the results are expressed as a percentage of total β-galactosidase); 100% represents 1,100 and 960 Miller units (20) for the control and PIMT-overproducing strains, respectively.
In contrast with the overproduced recombinant preS2-S′-β-galactosidase, other proteins (found in the pellet at 15,000 × g at 43°C) were not significantly solubilized upon PIMT overexpression (data not shown), suggesting that PIMT overexpression did not exert a global effect on protein localization and aggregation in E. coli.
Methylesterification of E. coli proteins in the protein isosaspartate methyltransferase-overproducing strain and in the control strain.
Protein methylation can be used as a marker of aspartate damage in cells (4). We determined whether overproduced preS2-S′-β-galactosidase contained isoaspartate in control and PIMT-overproducing cells. Bacteria at the end of exponential phase (grown at 37°C) were incubated for 30 min with methyl-labeled methionine, the in vivo precursor of S-adenosylmethionine. After sonication, whole-cell extracts were subjected to electrophoresis on sodium dodecyl sulfate-polyacrylamide gel (at acidic pH to preserve the stability of the methyl ester group), followed by autoradiography. Interestingly, control cells displayed methyl incorporation, as a broad band (possibly a result of partial proteolytic degradation), in a protein whose molecular weight corresponds to that of the β-galactosidase fusion protein (Fig. 3, lane 1), whereas PIMT-overproducing cells do not display such a methyl incorporation (Fig. 3, lane 2). Methyl incorporation in the band around 125 kDa was absent in extracts from bacteria which do not overproduce the β-galactosidase fusion protein (Fig. 3, lane 3). This result suggests that the overproduced β-galactosidase fusion protein is subjected to isoaspartate formation and that isoaspartate formation is prevented by protein isoaspartate methyltransferase overproduction.
FIG. 3.
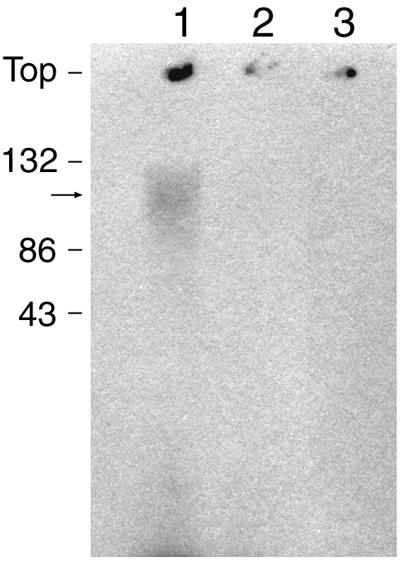
Methylesterification of E. coli proteins in PIMT-overproducing and control strains. The methyl-accepting activity of E. coli extracts was determined after incubation of intact bacteria (lane 1, strain JC196(pTBGH+); lane 2, strain JC196(pTBGH+), overproducing PIMT; lane 3, strain JC196) at the end of exponential phase with 3H-labeled methionine for 30 min, ultrasonic lysis of bacteria, and autoradiography of polyacrylamide gels after electrophoresis at pH 2.4, as described in Materials and Methods. The arrow indicates the position of preS2-S′-β-galactosidase.
Increased thermal stability of preS2-S′-β-galactosidase in extracts from the protein isoaspartate methyltransferase-overproducing strain.
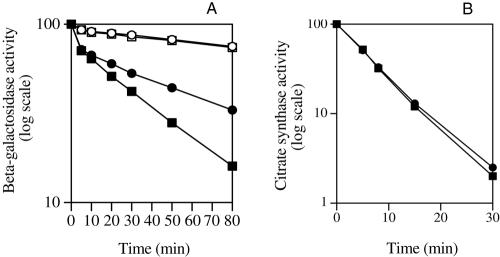
In order to understand the increased solubility and activity of preS2-S′-β-galactosidase in the PIMT-overproducing strain at 43°C, we measured its thermal stability in extracts from control and PIMT-overproducing cells. The velocity of preS2-S′-β-galactosidase inactivation at 56°C followed biphasic kinetics, as observed previously by others (23) (Fig. 4A). The first phase of thermodenaturation was similar for extracts from control and PIMT-overproducing strains, but the second phase was much slower for extracts from the PIMT-overproducing strain than for those from the control strain (half-life of 55 min instead of 30 min) (Fig. 4A). Similar results were obtained with extracts from bacteria grown at 37°C or after transfer at 43°C (data not shown). Thus, the stability of preS2-S′-β-galactosidase in crude extracts from PIMT-overproducing bacteria is increased. The thermal stability of wild-type β-galactosidase in crude extracts from control and PIMT-overproducing strains was also measured (these extracts were prepared from wild-type strain MG1655 transformed with either pACYC184 or the PIMT-overproducing plasmid). The velocity of wild-type β-galactosidase inactivation at 56°C was similar in both extracts, and it was much slower than the inactivation of preS2-S′-β-galactosidase. This results suggests that there is no enhanced chaperone activity in the crude extract from the PIMT-overproducing strain, and the lower thermal stability of preS2-S′-β-galactosidase, compared with that of normal β-galactosidase, is consistent with the lactose-negative phenotype at 43°C of the preS2-S′-β-galactosidase producing strain.
FIG. 4.
Effect of overproduction of PIMT on the thermal stability of preS2-S′-β-galactosidase in vitro. A) Crude extracts from strain JC196(pTBGH+) (producing preS2-S′-β-galactosidase and overproducing PIMT) (filled circles), strain JC196(pTBGH+) (producing the preS2-S′-β-galactosidase strain but not overproducing PIMT) (filled squares), and parental strain MG1655 (producing wild-type β-galactosidase and overproducing PIMT [open circles)] and producing wild-type β-galactosidase but not overproducing PIMT [open squares]) were incubated at 56°C. Samples were removed at intervals and cooled, and β-galactosidase activity was subsequently determined at 25°C as described in Materials and Methods. B) Crude extracts from strain JC196(pTBGH+) overproducing PIMT (circles) or strain JC196(pTBGH+) not overproducing PIMT (squares) were incubated at 56°C. Samples were removed at intervals and cooled, and citrate synthase activity was subsequently determined at 25°C as described in Materials and Methods.
The thermal stability of a control protein, citrate synthase, was not increased in PIMT-overproducing extracts compared with control extracts (Fig. 4B). This result suggests that the improved thermal stability of preS2-S′-β-galactosidase in the PIMT-overproducing strain probably results from a specific action of protein isoaspartate methyltransferase on the recombinant protein.
Absence of induction of DnaK and GroEL in the PIMT-overproducing strain.
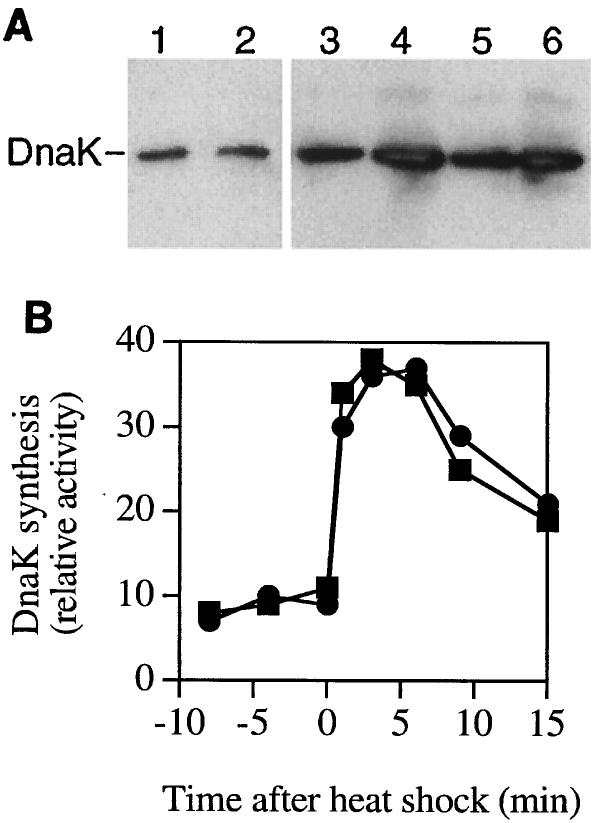
DnaK has been shown to be the main chaperone involved in protection against thermal stress and protein aggregation (21), and we suspected that the effects of PIMT overproduction might be mediated by a consequent overproduction of DnaK. Recently, Kindrachuk et al. (15) found that massive PIMT overproduction (under the control of the highly efficient T7 promoter) induces overexpression of DnaK and triggers the heat shock response. In our study, we took care to use the low-copy-number pACYC184 plasmid in order to avoid important physiological perturbations resulting from a massive expression of cloned genes. We measured steady-state DnaK levels in the PIMT-overproducing and control strains. As shown in Fig. 5A, there was no significant increase in steady-state DnaK levels in the PIMT-overproducing strain at 37°C (lane 1, control; lane 2, PIMT overproducer) or 43°C (lanes 3 and 4, 2 and 4 μg, respectively, of control extract; lanes 5 and 6, 2 and 4 μg, respectively, of PIMT overproducer extract). In similar conditions, Kindrachuk et al. found a two- to threefold increase in the steady-state level of DnaK upon massive PIMT overproduction. The steady-state GroEL level was also tested and found to be similar in both strains (data not shown). Since the determination of steady-state levels is not very sensitive, we measured DnaK synthesis in the PIMT-overproducing and control strains at 30 and 43°C. DnaK synthesis is similar for both strains at 30°C, and a fivefold stimulation of DnaK synthesis is observed for both strains during their transfer from 30 to 43°C (Fig. 5B). Similarly, the synthesis of GroEL at 30 and 43°C is not affected by PIMT overexpression (data not shown). This result suggests that DnaK (the main chaperone involved in thermoprotection [21]) and GroEL are not responsible for the solubilizing effects of PIMT overproduction described in this work. The increased stability and solubility of preS2-S′-β-galactosidase in the PIMT-overproducing strain therefore does not appear to result from an indirect induction of the heat shock system. This result is in accordance with the similar thermal stability of citrate synthase in extracts from the PIMT-overproducing strain and from the control strain; since citrate synthase is a client protein of DnaK, overproduction of the chaperone should have increased the thermal protection of citrate synthase.
FIG. 5.
Absence of effect of PIMT overexpression on synthesis and levels of DnaK. A) Steady-state DnaK levels. The amount of DnaK in crude extracts from PIMT-overproducing and control strains was measured after electrophoresis on 11% polyacrylamide gels and immunoblotting with anti-DnaK antibodies. Lanes 1 and 2 were loaded with 2 μg of crude extracts from control and PIMT-overproducing cells grown at 37°C; lanes 3 and 4 were loaded with 2 and 4 μg, respectively, of crude extracts from control cells grown at 37°C and transferred to 43°C for 2 h; lanes 5 and 6 were loaded with 2 and 4 μg, respectively, of crude extracts from PIMT-overproducing cells grown at 37°C and transferred to 43°C for 2 h. B) DnaK synthesis. Cells (PIMT overproducing [squares] or control [circles]) growing exponentially in glycerol medium at 30°C and then transferred to 43°C were pulse-labeled for 2 min with [35S]methionine at the indicated times, chased with nonradioactive methionine for 1 min, and kept on ice before lysis and immunoprecipitation of the crude extract with anti-DnaK antibodies. Proteins were resolved by electrophoresis, detected, and quantified by using a Molecular Dynamics PhosphorImager.
DISCUSSION
In this study, we report that PIMT is a multicopy suppressor of preS2-S′-β-galactosidase aggregation in E. coli at 43°C. PIMT overexpression increases the amount of soluble preS2-S′-β-galactosidase, decreases its aggregation at 43°C, decreases the amount of isoaspartate found in this recombinant protein, and increases its thermal stability in crude extracts, without affecting the synthesis and levels of the DnaK and GroEL chaperones.
It is noteworthy that only two DNA fragments from our E. coli gene bank, one of which codes for protein isoaspartate methyltransferase, can reverse the lactose-negative phenotype of the preS2-S′-β-galactosidase-overproducing strain at 43°C. The other multicopy suppressor found codes for the chbB and chbC genes, two membrane components of the phosphotransferase transport system for N,N′-diacetylchitobiose (this suppressor activity appears to result from a specific interaction between preS2-S′-β-galactosidase and the membrane in the chbBC-overproducing strain, leading to preS2-S′-β-galactosidase solubilization [unpublished data]). We did not find rpoH (whose gene product, σ32, increases the solubility of preS2-S′-β-galactosidase in vitro by two- to threefold [33]) as a multicopy suppressor of preS2-S′-β-galactosidase aggregation, although our bank contains rpoH (unpublished results). Nor did we find dnaK/dnaJ (whose products increase the solubility of preS2-S′-β-galactosidase in vitro by four- to sixfold [33]) as a multicopy suppressor of preS2-S′-β-galactosidase aggregation. These results are possibly due to an insufficient effect of these gene products on preS2-S′-β-galactosidase solubility in vivo, and the negative result concerning dnaK/dnaJ might also be explained by the necessity for concomitant overexpression of the grpE gene product (located in another part of the chromosome) for optimal efficiency of the DnaK/DnaJ/GrpE chaperone machine. This requirement for all three dnaK/dnaJ/grpE gene products for protein solubilization has already been reported (33).
Overexpression of the pcm gene results in a threefold decrease in the amount of preS2-S′-β-galactosidase found in inclusion bodies (from 25 to 50% [depending on the length of the incubation time at 43°C] to 10 to 17%) and a two- to fourfold increase in the amount of soluble preS2-S′-β-galactosidase. The amounts of soluble preS2-S′-β-galactosidase in the control and PIMT-overproducing strains 20 h after transfer to 43°C are 220 and 700 Miller units, respectively (these two values are lower and higher than the white-pink colony limit, in the range of 200 to 300 Miller units [20]), and it is possible that the amount of soluble preS2-S′-β-galactosidase at 43°C in the control strain is even lower in solid medium than in liquid medium (resulting in white lactose-negative colonies on MacConkey agar plates). Furthermore, it cannot be excluded that preS2-S′-β-galactosidase aggregation exerts an inhibitory action on a metabolic pathway required for lactose metabolism (lactose transport, for instance); in several protein aggregation diseases, an inhibitory interaction between protein aggregates (the polyglutamine aggregates of Huntington disease, for instance) and cellular factors (such as transcription factors) has been observed (24), leading to the concept of toxic protein aggregates.
PIMT overexpression reduces the degree of isoaspartate formation in preS2-S′-β-galactosidase. The control strain incorporates radioactive methionine in the recombinant protein, whereas the PIMT-overproducing strain does not. This finding suggests that some degree of isoaspartate formation occurs in the overproduced preS2-S′-β-galactosidase, which can be prevented by PIMT overproduction. The stoichiometry of isoaspartate formation in preS2-S′-β-galactosidase could not be determined from our in vivo labeling experiment. Our results suggest that overproducing a protein triggers a risk of isoaspartate formation in the overexpressed protein. This is possibly due to either increased molecular aging of the recombinant protein resulting from folding defects or an increase in the number of translational errors leading to isoaspartate misincorporation during protein synthesis.
PIMT overexpression increases the thermal stability of preS2-S′-β-galactosidase in crude extracts, whereas the thermal stabilities of wild-type β-galactosidase and of the control protein citrate synthase remain unchanged. This result suggests that PIMT overexpression specifically affects the overproduced protein. Furthermore, we did not detect a global effect of PIMT overexpression on protein localization and aggregation.
Neither preS2-S′-β-galactosidase solubilization nor thermoprotection appears to result from an induction of the heat shock response triggered by PIMT overexpression. If they did, one would expect a thermoprotection of citrate synthase (which is a good substrate of several chaperones, including DnaK [14, 19]) and an increase in the amount of DnaK and GroEL in PIMT-overproducing strains. Recently, Kindrachuk et al. (15) found that massive PIMT overproduction (under the control of the highly efficient T7 promoter) induces overexpression of DnaK and triggers the heat shock response. In our study, we took care to use the low-copy-number pACYC184 plasmid in order to avoid important physiological perturbations resulting from a massive expression of cloned genes. Although we did not observe any detectable increase in the steady-state level or in the synthesis of DnaK or GroEL in the PIMT-overproducing strain, we cannot exclude the possibility of an interaction between preS2-S′-β-galactosidase and a chaperone or chaperone-like protein, perhaps PIMT itself. No chaperone activity has ever been reported for PIMT, and the active isoaspartate-binding site does not display any preferential affinity for hydrophobic peptides (18). One cannot, however, exclude the presence of a distinct binding site which might display chaperone properties, such as those found in protein oxidoreductases (14, 34) and protein prolyl isomerases (a hypothetical chaperone activity of PIMT has not been tested in the present work) (31). However, our results strongly favor the hypothesis that the increased solubility and stability of the recombinant protein in the PIMT-overproducing strain results from a decrease in its isoaspartate content.
For the first time, to our knowledge, PIMT is described as a suppressor of protein aggregation, without any apparent implication of the heat shock chaperones. This effect could be used to improve the efficiency of protein overproduction in biotechnology. It is possible that certain folding problems encountered by overproduced proteins increase their rate of spontaneous isoaspartyl formation or that stalling at rare codons during translation of recombinant proteins increases the mischarging of an aspartyl-tRNA with l-aspartate as its β-carboxyl group (22).
Acknowledgments
We thank E. d'Alençon for her help in the early course of this work, M. Kohiyama for critical reading of the manuscript, and A. Kropfinger for correction of the English language.
This work was supported by grant CR 521090 from the DGA to G.R.
REFERENCES
- 1.Allen, S. P., J. O. Polazzi, J. K. Gierse, and A. M. Easton. 1992. Two novel heat shock genes encoding proteins produced in response to heterologous protein expression in Escherichia coli. J. Bacteriol. 174:6938-6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke, S. 2003. Aging as war between chemical and biochemical processes: protein methylation and the recognition of age-damaged proteins for repair. Ageing Res. Rev. 2:263-285. [DOI] [PubMed] [Google Scholar]
- 3.Danese, P. N., C. K. Murphy, and T. J. Silhavy. 1995. Multicopy suppression of cold-sensitive sec mutations in Escherichia coli. J. Bacteriol. 177:4969-4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.David, C. L., J. Keener, and D. W. Aswad. 1999. Isoaspartate in ribosomal protein S11 of Escherichia coli. J. Bacteriol. 181:2872-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debarbieux, L., and J. Beckwith. 1999. Electron avenue: pathways of disulfide bond formation and isomerization. Cell 99:117-119. [DOI] [PubMed] [Google Scholar]
- 6.Deuerling, E., H. Patzelt, S. Vorderwulbecke, T. Rauch, G. Kramer, A. Mogk, A. Schulze-Specking, H. Langen, and B. Bukau. 2003. Trigger factor and DnaK possess overlapping substrate pools and binding specificities. Mol. Microbiol. 47:1317-1328. [DOI] [PubMed] [Google Scholar]
- 7.el Yaagoubi, A., M. Kohiyama, and G. Richarme. 1996. Defect in export and synthesis of the periplasmic galactose receptor MglB in dnaK mutants of Escherichia coli, and decreased stability of the mglB mRNA. Microbiology 142:2595-2602. [DOI] [PubMed] [Google Scholar]
- 8.Fairbanks, G., and J. Avruch. 1972. Four gel systems for electrophoretic fractionation of membrane proteins using ionic detergents. J. Supramol. Struct. 1:66-75. [DOI] [PubMed] [Google Scholar]
- 9.Fu, J. C., L. Ding, and S. Clarke. 1991. Purification, gene cloning, and sequence analysis of an l-isoaspartyl protein carboxyl methyltransferase from Escherichia coli. J. Biol. Chem. 266:14562-14572. [PubMed] [Google Scholar]
- 10.George-Nascimento, C., J. Lowenson, M. Borissenko, M. Calderon, A. Medina-Selby, J. Kuo, S. Clarke, and A. Randolph. 1990. Replacement of a labile aspartyl residue increases the stability of human epidermal growth factor. Biochemistry 29:9584-9591. [DOI] [PubMed] [Google Scholar]
- 11.Grimaud, R., B. Ezraty, J. K. Mitchell, D. Lafitte, C. Briand, P. J. Derrick, and F. Barras. 2001. Repair of oxidized proteins. Identification of a new methionine sulfoxide reductase. J. Biol. Chem. 276:48915-48920. [DOI] [PubMed] [Google Scholar]
- 12.Hartl, F. U., and M. Hayer-Hartl. 2002. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295:1852-1858. [DOI] [PubMed] [Google Scholar]
- 13.Jakob, U., W. Muse, M. Eser, and J. C. Bardwell. 1999. Chaperone activity with a redox switch. Cell 96:341-352. [DOI] [PubMed] [Google Scholar]
- 14.Kern, R., A. Malki, A. Holmgren, and G. Richarme. 2003. Chaperone properties of Escherichia coli thioredoxin and thioredoxin reductase. Biochem. J. 371:965-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kindrachuk, J., J. Parent, G. F. Davies, M. Dinsmore, S. Attah-Poku, and S. Napper. 2003. Overexpression of l-isoaspartate O-methyltransferase in Escherichia coli increases heat shock survival by a mechanism independent of methyltransferase activity. J. Biol. Chem. 278:50880-50886. [DOI] [PubMed] [Google Scholar]
- 16.Lange, R., and R. Hengge-Aronis. 1994. The nlpD gene is located in an operon with rpoS on the Escherichia coli chromosome and encodes a novel lipoprotein with a potential function in cell wall formation. Mol. Microbiol. 13:733-743. [DOI] [PubMed] [Google Scholar]
- 17.Lee, S. C., Y. C. Choi, and M. H. Yu. 1990. Effect of the N-terminal hydrophobic sequence of hepatitis B virus surface antigen on the folding and assembly of hybrid beta-galactosidase in Escherichia coli. Eur. J. Biochem. 187:417-424. [DOI] [PubMed] [Google Scholar]
- 18.Lowenson, J. D., and S. Clarke. 1991. Structural elements affecting the recognition of l-isoaspartyl residues by the -isoaspartyl/d-aspartyl protein methyltransferase. Implications for the repair hypothesis. J. Biol. Chem. 266:19396-19406. [PubMed] [Google Scholar]
- 19.Malki, A., R. Kern, J. Abdallah, and G. Richarme. 2003. Characterization of the Escherichia coli YedU protein as a molecular chaperone. Biochem. Biophys. Res. Commun. 301:430-436. [DOI] [PubMed] [Google Scholar]
- 20.Miller, J. H. 1972. Experiments in molecular genetics, p. 431. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 21.Mogk, A., T. Tomoyasu, P. Goloubinoff, S. Rudiger, D. Roder, H. Langen, and B. Bukau. 1999. Identification of thermolabile Escherichia coli proteins: prevention and reversion of aggregation by DnaK and ClpB. EMBO J. 18:6934-6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Momand, J. A., and S. Clarke. 1990. The fidelity of protein synthesis: can mischarging by aspartyl-tRNA(Asp) synthetase lead to the formation of isoaspartyl residues in proteins? Biochim. Biophys. Acta 1040:153-158. [DOI] [PubMed] [Google Scholar]
- 23.Moses, V., and P. B. Sharp. 1970. Adenosine 3′:5′-cyclic monophosphate and catabolite repression in Escherichia coli. Biochem. J. 118:491-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross, C. A. 2002. Polyglutamine pathogenesis: emergence of unifying mechanisms for Huntington's disease and related disorders. Neuron 35:819-822. [DOI] [PubMed] [Google Scholar]
- 25.Ryttersgaard, C., S. C. Griffith, M. R. Sawaya, D. C. MacLaren, S. Clarke, and T. O. Yeates. 2002. Crystal structure of human l-isoaspartyl methyltransferase. J. Biol. Chem. 277:10642-10646. [DOI] [PubMed] [Google Scholar]
- 26.Sastry, M. S., K. Korotkov, Y. Brodsky, and F. Baneyx. 2002. Hsp31, the Escherichia coli yedU gene product, is a molecular chaperone whose activity is inhibited by ATP at high temperatures. J. Biol. Chem. 277:46026-46034. [DOI] [PubMed] [Google Scholar]
- 27.Schiene, C., and G. Fischer. 2000. Enzymes that catalyse the restructuring of proteins. Curr. Opin. Struct. Biol. 10:40-45. [DOI] [PubMed] [Google Scholar]
- 28.Schlieker, C., B. Bukau, and K. Mogk. 2002. Prevention and reversion of protein aggregation by molecular chaperones in the E. coli cytosol: implications for their applicability in biotechnology. J. Biotechnol. 96:13-21. [DOI] [PubMed] [Google Scholar]
- 29.Sherman, M. Y., and A. L. Goldberg. 1996. Involvement of molecular chaperones in intracellular protein breakdown. EXS 77:57-78. [DOI] [PubMed] [Google Scholar]
- 30.Spence, J., and C. Georgopoulos. 1989. Purification and properties of the Escherichia coli heat shock protein, HtpG. J. Biol. Chem. 264:4398-4403. [PubMed] [Google Scholar]
- 31.Suzuki, R., K. Nagata, F. Yumoto, M. Kawakami, N. Nemoto, M. Furutani, K. Adachi, T. Maruyama, and M. Tanokura. 2003. Three-dimensional solution structure of an archaeal FKBP with a dual function of peptidyl prolyl cis-trans isomerase and chaperone-like activities. J. Mol. Biol. 328:1149-1160. [DOI] [PubMed] [Google Scholar]
- 32.Thomas, J. G., and F. Baneyx. 1996. Protein misfolding and inclusion body formation in recombinant Escherichia coli cells overexpressing heat-shock proteins. J. Biol. Chem. 271:11141-11147. [DOI] [PubMed] [Google Scholar]
- 33.Thomas, J. G., and F. Baneyx. 1996. Protein folding in the cytoplasm of Escherichia coli: requirements for the DnaK-DnaJ-GrpE and GroEL-GroES molecular chaperone machines. Mol. Microbiol. 21:1185-1196. [DOI] [PubMed] [Google Scholar]
- 34.Tsai, B., C. Rodighiero, W. I. Lencer, and T. A. Rapoport. 2001. Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin. Cell 104:937-948. [DOI] [PubMed] [Google Scholar]
- 35.Ueguchi, C., and K. Ito. 1992. Multicopy suppression: an approach to understanding intracellular functioning of the protein export system. J. Bacteriol. 174:1454-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Visick, J. E., H. Cai, and S. Clarke. 1998. The l-isoaspartyl protein repair methyltransferase enhances survival of aging Escherichia coli subjected to secondary environmental stresses. J. Bacteriol. 180:2623-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wickner, S., M. R. Maurizi, and S. Gottesman. 1999. Post-translational quality control: folding, refolding, and degrading proteins. Science 286:1888-1893. [DOI] [PubMed] [Google Scholar]
- 38.Yamada, H., T. Ueda, R. Kuroki, T. Fukumura, T. Yasukochi, T. Hirabayashi, K. Fujita, and T. Imoto. 1985. Isolation and characterization of 101-beta-lysozyme that possesses the beta-aspartyl sequence at aspartic acid-101. Biochemistry 24:7953-7959. [DOI] [PubMed] [Google Scholar]