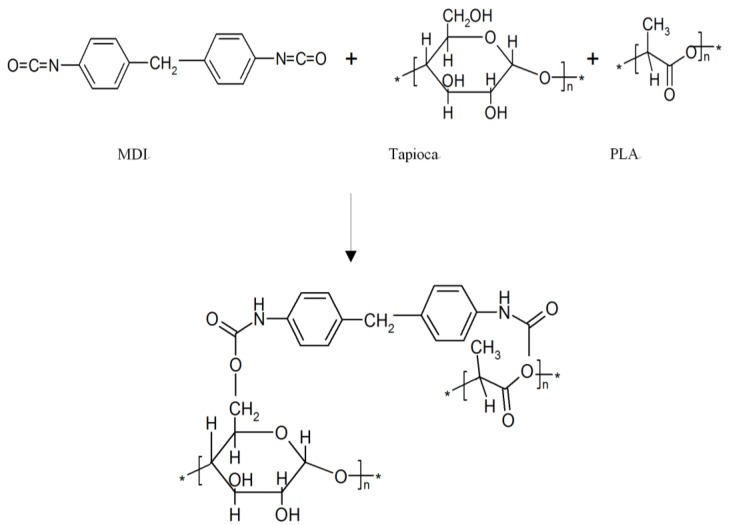
Abstract
Granular tapioca was thermally blended with poly(lactic acid) (PLA). All blends were prepared using a plasti-corder and characterized for tensile properties, thermal properties and morphology. Scanning electron micrographs showed that phase separation occurred, leading to poor tensile properties. Therefore, methylenediphenyl diisocyanate (MDI) was used as an interfacial compatibilizer to improve the mechanical properties of PLA/tapioca blends. The addition of MDI could improve the tensile strength of the blend with 60 wt% tapioca, from 19.8 to 42.6 MPa. In addition, because PLA lacked toughness, acetyl tributyl citrate (ATBC) was added as a plasticizer to improve the ductility of PLA. A significant decrease in the melting point and glass-transition temperature was observed on the basis of differential scanning calorimetry, which indicated that the PLA structure was not dense after ATBC was added. As such, the brittleness was improved, and the elongation at break was extended to several hundred percent. Therefore, mixing ATBC with PLA/tapioca/MDI blends did exhibit the effect of plasticization and biodegradation. The results also revealed that excessive plasticizer would cause the migration of ATBC and decrease the tensile properties.
Keywords: biodegradable, poly(lactic acid) (PLA), tapioca, methylenediphenyl diisocyanate (MDI), acetyl tributyl citrate (ATBC)
1. Introduction
Poly(lactic acid) (PLA) resins are well-known biodegradable, linear aliphatic thermoplastics, which can be produced from renewable resources [1,2]. They are one of the most promising polymers of their family [3] and are highly accepted as biomedical materials, because of their biocompatibility and good mechanical properties [4,5]. However, their brittleness, slow crystallization and being easily hydrolyzed limit their usage in many applications. In fact, it is difficult to use them for film blowing or extrusion, unless their moisture content and processing conditions are carefully controlled. Moreover, their price competitiveness in the biodegradable plastic market is an essential attribute that cannot be ignored. The most effective approach to reduce the capital cost of PLA is to use fillers. Cost-effective reinforcements are organic renewable resources [6], flax [7,8,9], sisal [10], lyocell [11], short abaca [12], jute [13], bamboo [14], paper pulp [15,16], pineapple [17], Cordenka [18], microcrystalline cellulose [19], and kenaf [20]. Starch is attractive because of its low cost, renewability, biodegradability, low density and non-abrasiveness. A lot of studies on the blending of PLA/starch [21,22,23,24,25,26,27,28,29,30], such as wheat starch [21,24,30], corn starch [26,27,29,31] and cassava starch [23], have been researched. Tapioca was used as a filler in [32], because it is cheap, and fewer reports compared it with other starches. However, the poor interfacial adhesion between the filler and the polymer generally leads to composites with worse mechanical properties. Surface and bulk modifications of the filler and/or matrix are necessary to increase the interfacial compatibility between the hydrophilic filler and the hydrophobic PLA matrix. Some studies used methylenediphenyl diisocyanate (MDI) as a compatibilizer to improve the compatibility between PLA and starch [21,30] or between PLA and rice husk [33]. These biopolymers are successfully prepared with starch or rice husk blends using MDI as a coupling agent. Copolymerization or blending PLA with other polymers [34,35,36,37,38,39,40,41,42,43] or compounds (e.g., plasticizers) [44,45,46,47,48,49] was proven to be a feasible way to improve its processability in film products for extrusion and/or film blowing. In this study, tapioca was used as a filler to reduce the cost of PLA products; MDI was used as a coupling agent to enhance the interfacial compatibility between PLA and tapioca; and ATBC was used as a plasticizer to improve the processability, flexibility and ductility of glassy PLA/tapioca composites. The result of this study could provide a database useful for the design and manufacture of biodegradable materials.
2. Results and Discussion
2.1. Tensile Property
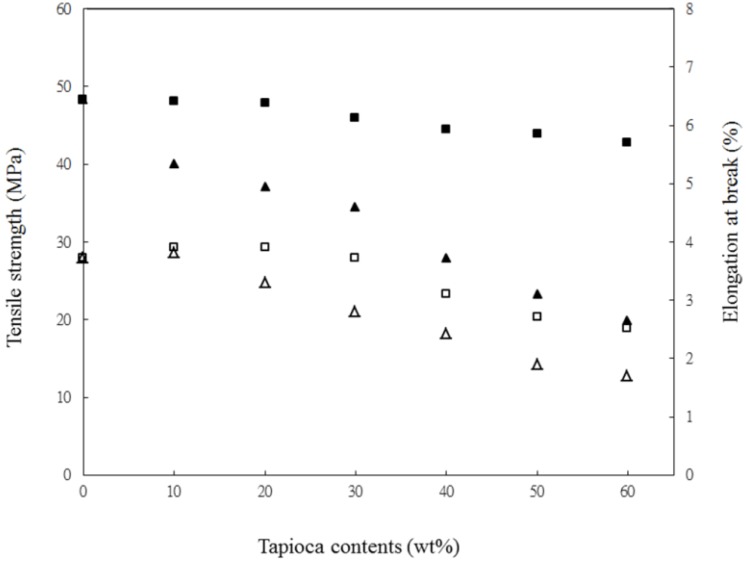
The tensile strength (σf) and elongation at break (εf) of PLAxTapiocay and PLA90Tapioca10MDI are plotted in Figure 1. The σf of PLA is 48.3 MPa. After blending PLA with tapioca, PLAxtapiocay specimens revealed a substantial reduction in σf and εf. For example, the σf of PLAxTapiocay specimens was reduced from 48.3 to 17.5 MPa as the tapioca content increased from 0 to 60 wt%. When MDI was added, the σf of the PLAxtapiocay specimens was improved; the σf of PLA40Tapioca60MDI was 42.6 MPa, whereas that of PLA40Tapioca60 was 25.1 MPa. This improvement in σf is due to the increase in the PLAxTapiocay interfacial adhesion, as a result of the formation of urethane linkages between MDI and PLA, as well as those between MDI and tapioca, because MDI acts as a coupling agent [21]. The εf for all samples are between 1% and 4%; thus, their toughness is similar.
Figure 1.
Tensile strength vs. tapioca content for (▲) PLA/tapioca and (■) PLA/tapiocaMDI0.5; elongation at break vs. tapioca content for (△) PLA/tapioca and (□) PLA/tapiocayMDI0.5.
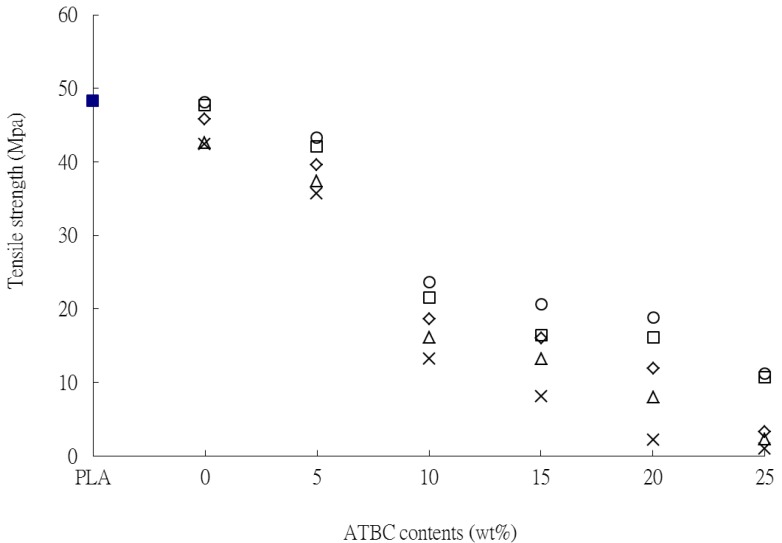
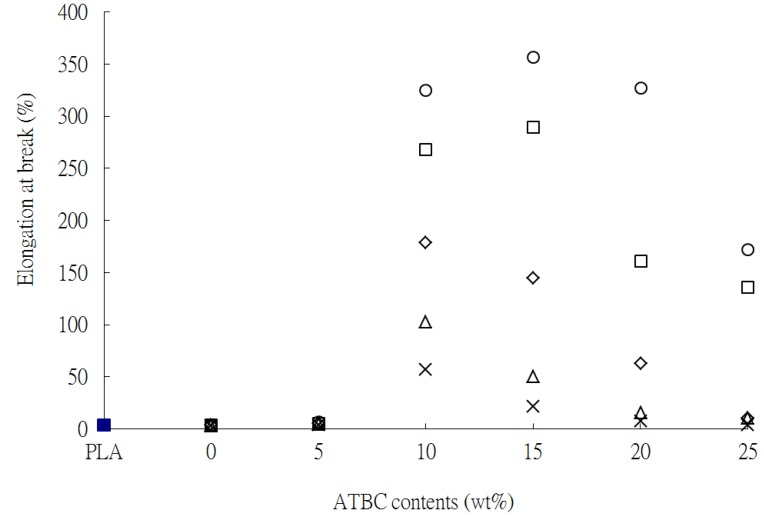
The σf and εf of PLA and PLAxtapiocayMDI specimens as a function of ATBC are shown in Figure 2 and Figure 3, respectively. With increasing plasticizer content, a common trend is shown by all series investigated: the σf decreases, whereas the εf increases. The σf of PLAxtapiocayMDI was reduced much more significantly after ATBC was added; the σf value of PLA50tapioca50MDI was reduced from 42.3 to 0.9 MPa, as the ATBC content was increased from 0 to 25 wt% (see Figure 2). This substantial reduction is due to the tapioca of PLAxtapiocayMDI that could not be plasticized by ATBC. In addition, the εf of PLA90tapioca10MDI, PLA80tapioca20MDI, PLA70tapioca30MDI, PLA60tapioca40MDI and PLA50tapioca50MDI approaches the maximum value at 357.3%, 289.7%, 178.8%, 102.5% and 56.3%, respectively, as the ATBC content reaches an optimum value of 10 or 15 wt%. The εf of the PLAxtapiocayMDI specimens was reduced as the ATBC content increased from 10 or 15 wt%. These results clearly suggest that the relatively poor ductility of PLAxtapiocayMDI was improved after blending proper amounts of ATBC with the PLAxTapiocayMDI composites.
Figure 2.
Tensile strength vs. ATBC content for (■) PLA; (○) PLA90tapioca10MDI0.5; (□) PLA80tapioca20MDI0.5; (◇) PLA70tapioca30MDI0.5; (△) PLA60tapioca40MDI0.5; and (×) PLA50tapioca50MDI0.5.
Figure 3.
Elongation at break vs. ATBC content for (■) PLA; (○) PLA90tapioca10MDI0.5; (□) PLA80tapioca20MDI0.5; (◇) PLA70tapioca30MDI0.5; (△) PLA60tapioca40MDI0.5; and (×) PLA50tapioca50MDI0.5.
2.2. Fourier Transform Infra-Red Spectroscopy
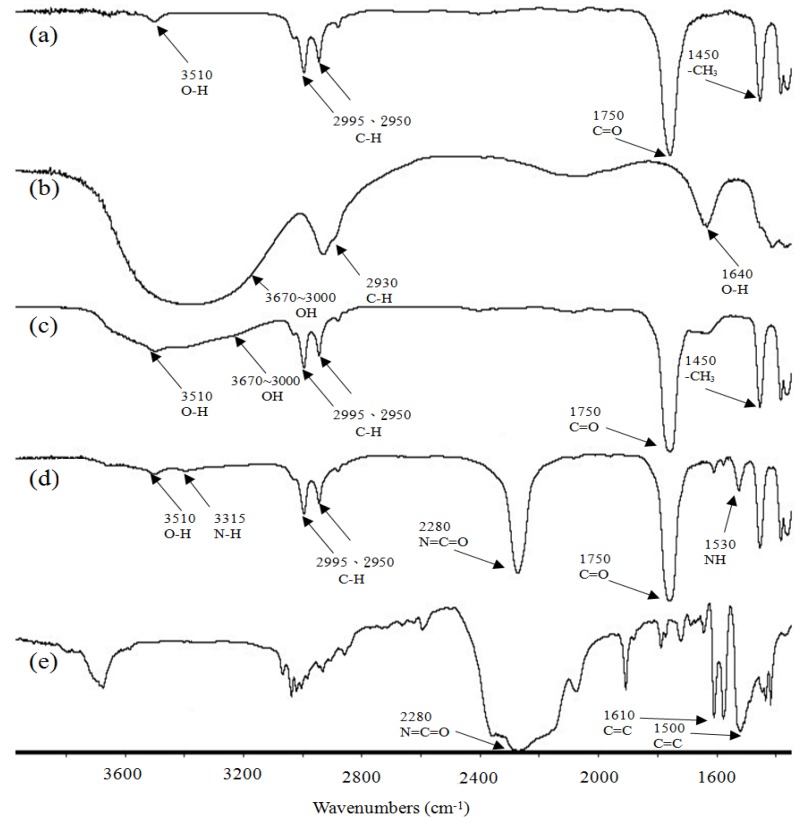
Figure 4 illustrates typical FTIR spectra of PLA, tapioca, MDI, PLAxtapiocay and PLAxtapiocayMDI specimens. Four characteristic absorption bands centered at 1750, 2950, 2995 and 3510 cm−1, corresponding to the motions of C=O bending, C-H aliphatic stretching, C-H aliphatic stretching (doublet) and C-O-O-H stretching vibrations, respectively, were found in the spectrum of PLA (see Figure 4a). The FTIR spectra of PLAxtapiocay specimens, indicated in Figure 4c, are very similar to those of PLA; the four main absorption bands centered at 1750, 2950, 2995 and 3510 cm−1 were also found in the spectra of PLAxtapiocay specimens. The absorption bands around 3000 to 3670 cm−1 were the O-H stretching vibration of tapioca. The FTIR spectra of PLAxtapiocay specimens are very similar to those of PLAxtapiocayMDI (see Figure 4d). However, the aforementioned 3000 to 3670 cm−1 absorption bands originally shown in the FTIR spectra of PLA were gradually replaced by a newly developed absorption band centered at 3315 cm−1, which corresponds to the motion of the amine (N-H) stretching vibration. The disappearance of the 3000 to 3670 cm−1 bending absorption bands and the appearance of 3315 and 1550 cm−1 (N-H) stretching absorption bands are attributed to the reaction of the hydroxyl (O-H) groups of tapioca molecules with the urethane (N=C=O) groups of MDI and/or to the reaction of the carboxylic acid (C-O-O-H) groups of PLA molecules with the urethane groups of MDI during the melt-blending of PLAxtapiocay specimens. The possible mechanism for PLA/tapioca/MDI composites is shown in Scheme 1.
Figure 4.
FTIR of: (a) PLA; (b) tapioca; (c) PLA/tapioca; (d) PLA/tapioca/MDI; and (e) MDI.
Scheme 1.
Possible reactions for PLA/tapioca/MDI composites.
2.3. Morphology Analysis
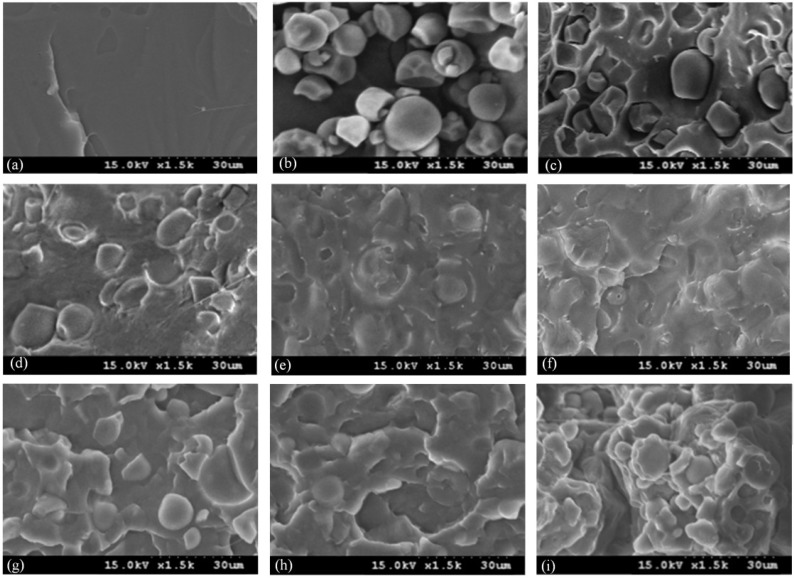
Typical SEM micrographs of (PLA70tapioca30MDI)aATBCb specimens are shown in Figure 5. As shown in Figure 5a, a relatively brittle and smooth surface morphology was found for PLA. Tapioca is shown in Figure 5b. After blending PLA with tapioca, intervals between PLA and tapioca granules appeared (see Figure 5c) These results are similar to some reports on PLA/wheat starch [21] and PLA/corn starch blends [27,29]. This morphology is typical of incompatible blends, resulting in a poor tensile property, which is consistent with the result in Figure 1. After MDI was added, the compatibility of the PLA70tapioca30 specimen was improved (see Figure 5d). It shows excellent compatible morphologies, without the interval and voids associated with poor interfacial adhesion. The better compatibility of PLA70tapioca30MDI was due to the reaction of the hydroxyl groups of tapioca with the urethane groups of MDI and the reaction of the carboxylic acid groups of PLA with the urethane groups of MDI. The SEM micrographs of PLA70tapioca30MDI as a function of the increasing ATBC content (from 5 to 25 wt%) are shown in Figure 5e–h. The surface of PLA70tapioca30MDI was still smooth and without intervals when the ATBC content was 5 wt%. Furthermore, two phases can be seen after the ATBC content reaches 10 wt%, which is the threshold limit value of high εf for (PLA70tapioca30MDI)aATBCb specimens (see Figure 3). With increasing ATBC content, more demarcated plastic-deformed PLA debris or fibrils were found on the surface of PLA70Tapioca30MDI (see Figure 5f–i). This was attributed to the increasing distance between PLA molecules, or the exudation of ATBC, and also to the deterioration of the interfacial adhesion between tapioca and PLA. Therefore, the σf of PLAxtapiocayMDI decreased significantly with increasing ATBC.
Figure 5.
SEM for: (a) PLA; (b) tapioca; (c) PLA70tapioca30; (d) PLA70Tapioca30MDI0.5; (e) (PLA70tapioca30MDI0.5)95ATBC5; (f) (PLA70tapioca30MDI0.5)90ATBC10; (g) (PLA70tapioca30MDI0.5)85ATBC15; (h) (PLA70tapioca30MDI0.5)80ATBC20; and (i) (PLA70tapioca30MDI0.5)75ATBC25.
2.4. Differential Scanning Calorimetry
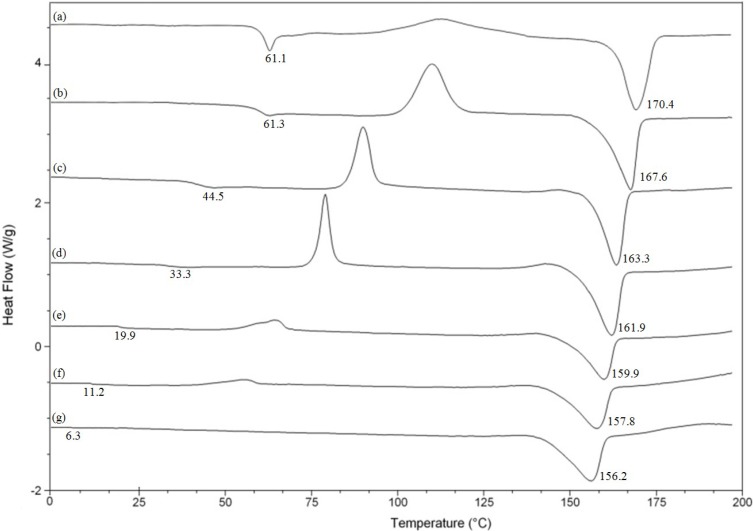
The thermal behavior of PLA and (PLA70tapioca30MDI)aATBCb specimens was investigated. Figure 6 shows the DSC curves of PLA, the (PLA70tapioca30MDI)aATBCb specimens and the plasticizer. The crystallization and melting enthalpies are identical, showing that PLA is totally amorphous after melt quenching. The DSC curves of PLA70tapioca30MDI show a single glass transition that decreases with increasing ATBC concentration (from 0 to 25 wt%). This result agrees with the data by Yeh et al. [50] and Baiardo et al. [51], who analyzed PLA/triacetin (TAc) and PLA/ATBC composites, respectively, over a more limited composition range (0%–30% TAc and ATBC). A decreasing Tg trend with increasing ATBC content is also shown in Figure 6, which is related to the cold crystallization and melting phenomena.
Figure 6.
DSC for: (a) PLA; (b) PLA70tapioca30MDI; (c) (PLA70tapioca30MD)95ATBC5; (d) (PLA70tapioca30MDI)90ATBC10; (e) (PLA70tapioca30)85ATBC15; (f) (PLA70tapioca30MDI)aATBCb;and (g) (PLA70tapioca30MDI)75ATBC25.
2.5. Water Absorption
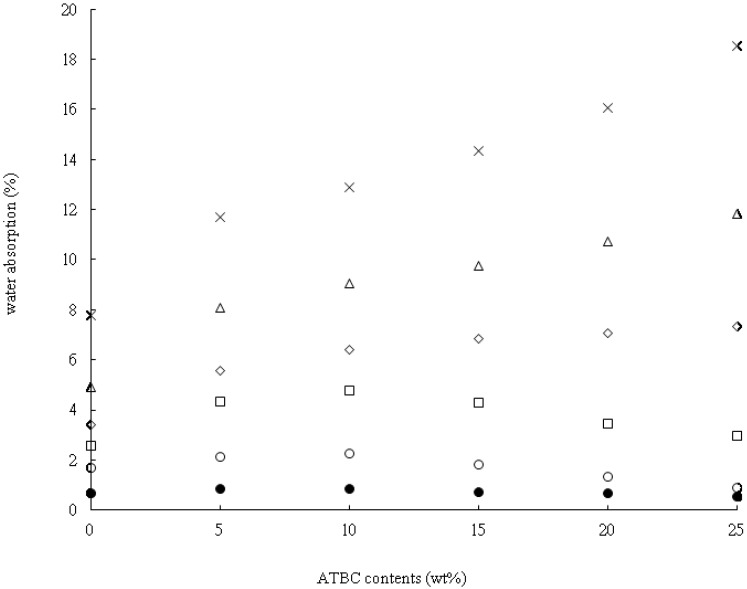
Figure 7 presents data on the water absorption of the PLAxATBCy specimens as a function of varying tapioca content. It shows a rising water absorption rate with increasing tapioca content at the same ATBC content. Water absorption rates were 0.58% to 18.57% when the tapioca content was from 0% to 50% at 25% ATBC content. This is due to the hydrophilic property of tapioca, causing the percentage of water absorption to increase. Furthermore, water absorption rates increased when ATBC was added; the water absorption rate of PLA60tapioca40 was 4.91% to 11.85% when the ATBC content was from 0% to 25%. ATBC is hydrophobic, but shows an interesting phenomenon of increasing moisture content. The trend of increasing water absorption is attributed to ATBC, which could enhance the free volume in PLA. This is evidenced by DSC analysis (see Figure 6): Tg decreased with increasing ATBC content. Therefore, the water molecule could be absorbed by PLAxtapiocay easily when the ATBC content increased the mobility of PLA molecules. The water adsorption for the lower tapioca content (10% to 30%) was first increasing and then decreasing. This might be due to slight exudation when the ATBC content is from 10% to 25%. It could be evidenced from the morphology analysis. The phase separation may be because of the exudation of ATBC; the phase separation worsens when the amount of ATBC was higher than 10% (See Figure 5f–i). However, when the tapioca content approached 40% and 50%, the ATBC might be absorbed by tapioca. Therefore, the exudation effect might not be apparent. On the basis of the results from the tensile property, morphology analysis and water absorption, the miscibility limits of ATBC content are suggested to be 10% for PLAxtapiocayMDI0.5 composites.
Figure 7.
Water absorption vs. ATBC content for (●) PLA; (○) PLA90tapioca10MDI0.5; (□) PLA80tapioca20MDI0.5; (◇) PLA70tapioca30MDI0.5; (△) PLA60tapioca40MDI0.5; and (×) PLA50tapioca50MDI0.5.
2.6. Enzymatic Hydrolysis
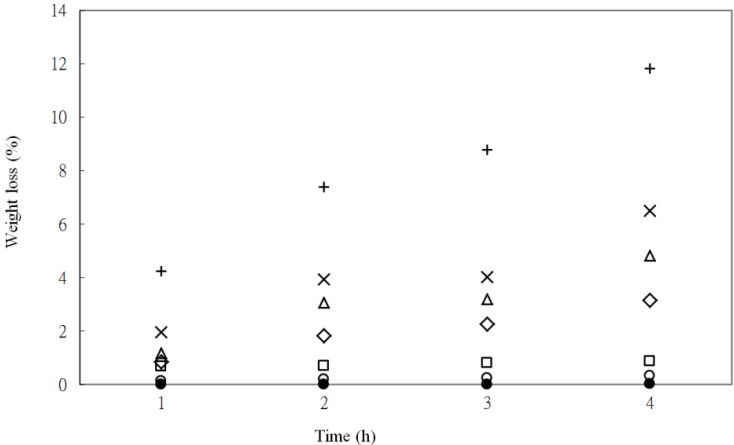
The weight loss of PLA70tapioca30MDI and (PLA70tapioca30MDI)aATBCb at varying enzymatic hydrolysis time is indicated in Figure 8. It shows a common result: the increasing percentage of weight loss for all series, as the hydrolysis time increases. The weight loss of PLA70tapioca30MDI increased significantly from 0.33% to 11.82%, as the ATBC content increased from 0 to 25 wt% after 120 h of hydrolysis time. Enzymes could attack the molecules of PLA easily after ATBC was blended with PLA70tapioca30MDI. Furthermore, the interfacial adhesion between PLA and tapioca might deteriorate when ATBC was added. The weight loss increased significantly when the ATBC content was higher than 5%. The migration of ATBC occurred from 10% content. This conjecture of exudation was evidenced by the morphological analysis and water absorption.
Figure 8.
Weight loss for (●) PLA; (○) PLA70tapioca30MDI0.5; (□) (PLA70tapioca30MDI0.5)95ATBC5; (◇) (PLA70tapioca30MDI0.5)90ATBC10;(△) (PLA70tapioca30MDI0.5)85ATBC15; (×) (PLA70tapioca30MDI0.5)80ATBC20; and (+) (PLA70tapioca30MDI0.5)75ATBC25.
3. Experimental Section
3.1. Materials and Preparation
PLA resin, with a trade name of Nature Green 4032D, was obtained from Cargill-Dow. Tapioca was purchased from United Global Agencies (Bangkok, Thailand). Before melt-blending, PLA and tapioca were dried in a vacuum oven at 80 °C for 8 h to remove the residual water. Acetyl tributyl citrate (ATBC-food grade) was supplied by Chou Feng Enterprise Co., Ltd. (Shulin, Taiwan). Dried components of PLA/starch at varying weight ratios were melt-blended using a Brabender. Three compounds were evaluated: PLAxTapiocay, PLAxTapiocayMDI and (PLAxTapiocayMDI)aATBCb. During each compounding process, the Brabender was operated at a temperature of 190 °C and a screw speed of 120 rpm for 3 min for all samples without ATBC and for an additional 2 min after adding ATBC. All prepared series were then hot-pressed at 190 °C and 10 MPa for 2 min and then cooled in air at about 25 °C. The compositions of all specimens are summarized in Table 1. Before hot-pressing, the specimens were dried in a vacuum oven at 80 °C for 12 h.
Table 1.
Compositions of PLA, PLAxtapiocay, PLAxtapiocayMDI and (PLAxtapiocayMDI)aATBCb specimens.
| Sample | PLA (%) | Tapioca (%) | ATBC (%) | MDI (phr) |
|---|---|---|---|---|
| PLA | 100 | 0 | 0 | 0 |
| PLA90tapioca10 | 90 | 10 | 0 | 0 |
| PLA80tapioca20 | 80 | 20 | 0 | 0 |
| PLA70tapioca30 | 70 | 30 | 0 | 0 |
| PLA60tapioca40 | 60 | 40 | 0 | 0 |
| PLA50tapioca50 | 50 | 50 | 0 | 0 |
| PLA40tapioca60 | 0 | 60 | 0 | 0 |
| PLA90tapioca10MDI | 90 | 10 | 0 | 0.5 |
| PLA80tapioca20MDI | 80 | 20 | 0 | 0.5 |
| PLA70tapioca30MDI | 70 | 30 | 0 | 0.5 |
| PLA60tapioca40MDI | 60 | 40 | 0 | 0.5 |
| PLA50tapioca50MDI | 50 | 50 | 0 | 0.5 |
| PLA60tapioca40MDI | 40 | 60 | 0 | 0.5 |
| (PLA90tapioca10MDI)95ATBC5 | 85.5 | 9.5 | 5 | 0.5 |
| (PLA80tapioca20MDI)95ATBC5 | 76 | 19 | 5 | 0.5 |
| (PLA70tapioca30MDI)95ATBC5 | 66.5 | 28.5 | 5 | 0.5 |
| (PLA60tapioca40MDI)95ATBC5 | 57 | 38 | 5 | 0.5 |
| (PLA50tapioca50MDI)95ATBC5 | 47.5 | 47.5 | 5 | 0.5 |
| (PLA90tapioca10MDI)90ATBC10 | 81 | 9 | 10 | 0.5 |
| (PLA80tapioca20MDI)90ATBC10 | 72 | 18 | 10 | 0.5 |
| (PLA70tapioca30MDI)90ATBC10 | 63 | 27 | 10 | 0.5 |
| (PLA60tapioca40MDI)90ATBC10 | 54 | 36 | 10 | 0.5 |
| (PLA50tapioca50MDI)90ATBC10 | 45 | 45 | 10 | 0.5 |
| (PLA90tapioca10MDI)85ATBC15 | 76.5 | 8.5 | 15 | 0.5 |
| (PLA80tapioca20MDI)85ATBC15 | 68 | 17 | 15 | 0.5 |
| (PLA70tapioca30MDI)85ATBC15 | 59.5 | 25.5 | 15 | 0.5 |
| (PLA60tapioca40MDI)85ATBC15 | 51 | 34 | 15 | 0.5 |
| (PLA50tapioca50MDI)85ATBC15 | 42.5 | 42.5 | 15 | 0.5 |
| (PLA90tapioca10MDI)80ATBC20 | 72 | 8 | 20 | 0.5 |
| (PLA80tapioca20MDI)80ATBC20 | 64 | 16 | 20 | 0.5 |
| (PLA70tapioca30MDI)80ATBC20 | 56 | 24 | 20 | 0.5 |
| (PLA60tapioca40MDI)80ATBC20 | 48 | 32 | 20 | 0.5 |
| (PLA50tapioca50MDI)80ATBC20 | 40 | 40 | 20 | 0.5 |
| (PLA90tapioca10MDI)75ATBC25 | 67.5 | 7.5 | 25 | 0.5 |
| (PLA80tapioca20MDI)75ATBC25 | 60 | 15 | 25 | 0.5 |
| (PLA70tapioca30MDI)75ATBC25 | 52.2 | 22.5 | 25 | 0.5 |
| (PLA60tapioca40MDI)75ATBC25 | 45 | 30 | 25 | 0.5 |
| (PLA50tapioca50MDI)75ATBC25 | 37.5 | 37.5 | 25 | 0.5 |
3.2. Tensile Property
The tensile properties of the hot-pressed PLA, PLAxTapiocay and (PLAxTapiocay)/ATBC specimens at 25 °C were determined using a tensile testing machine (model AG-10KNA, Shimadzu Corporation, Kyoto, Japan) with a crosshead speed of 50 mm/min. A 35-mm gauge length was used for each tensile experiment. Dog-bone-shaped specimens were prepared according to the ASTM D638 Type IV standard [52]. On the basis of the average tensile results of at least five tensile specimens, the values of tensile strength and elongation at break were obtained.
3.3. Fourier Transform Infra-Red Spectroscopy
FTIR measurements were performed on a PerkinElmer spectrometer (model Spectrum One, PerkinElmer Inc., Waltham, MA, USA). The spectra of the samples were obtained by averaging 15 scans, with a wavenumber range of 4000 to 650 cm−1 and a resolution of 2 cm−1.
3.4. Morphology Analysis
The morphology of specimens, before and after the hydrolytic degradation, was observed by using a scanning electron microscope (model SU1510, Hitachi High-Technologies Corporation, Tokyo, Japan). Specimens of a 2 × 2 cm2 area were fixed on a sample holder using a conductive adhesive tape. They were coated with a thin layer of gold at 15 keV for 15 s to improve the image resolution and were then photographed at 3.00 K magnification and a low voltage of 2.1 kV.
3.5. Differential Scanning Calorimetry
The thermal properties of PLA composite resins were determined using a TA Q100 differential scanning calorimetry (DSC). All DSC scans were performed at a heating rate of 10 °C/min and under flowing nitrogen with a flow rate of 50 mL/min. The DSC was calibrated using pure indium. For Tg and Tm determination, samples weighing approximately 0.5 mg were placed in standard aluminum-sample pans.
3.6. Water Absorption
Five specimens (10 mm × 10 mm × 0.5 mm) were used for the water absorption test. After conditioning in desiccators for three weeks, specimens were weighed. They were immersed in distilled water at room temperature for 24 h. Then, they were dabbed with tissue paper to remove the water from the surface. Water absorption was calculated using the following Equation (1):
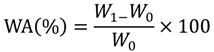 |
(1) |
where W0 is the weight of the dry sample and W1 is the weight of the sample immersed in distilled water for 24 h.
3.7. Enzymatic Hydrolysis
The degradation of the enzymatic hydrolysis of specimens was evaluated at 27 °C using 50 mg starch enzyme in (0.025 mol Na2HPO4 + 0.025 mol KH2PO4) aqueous solution. Specimens with a dimension of 5 × 5 cm2 were tested for various days, washed with distilled water and dried completely in a vacuum oven at 70 °C for 8 h. On the basis of weight loss, the degree of degradation was determined using Equation (2):
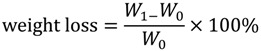 |
(2) |
where W0 is the dry weight before degradation and Wt is the dry weight at time t.
4. Conclusions
The σf of PLAxtapiocayMDI specimens was significantly higher than that of PLAxtapiocay specimens. The εf of PLA and PLAxtapiocayMDI specimens approached the maximum value, as the ATBC content reached an optimum value of 10 and 15 wt%, respectively. The threshold limits of the εf were high when the ATBC content was 10 wt%. FTIR demonstrated the disappearance of the 3000 to 3670 cm−1 bending absorption band and the appearance of the 3315 and 1550 cm−1 NH stretching absorption band, which were attributed to the reaction of the OH groups of tapioca molecules with the N=C=O groups of MDI and/or to the reaction of the C-O-O-H groups of PLA molecules with the urethane groups of MDI during the melt-blending of PLAxtapiocay specimens. SEM micrographs revealed the intervals between PLA and tapioca. Voids from the matrix of PLAxtapiocay were significantly improved after MDI was added. Furthermore, two phases can be seen after the ATBC content reached 10 wt%. This is due to the exudation of ATBC; with increasing ATBC content, more demarcated plastic deformation was found on the surface of PLA70Tapioca30MDI. DSC curves of the PLA70tapioca30MDI specimen showed a single glass transition and cold crystallization that decreased as the ATBC content increased from 0 to 25 wt%. The increasing trend of water absorption with increasing ATBC content was attributed to the increasing free volume in PLA, causing the water molecules to be easily absorbed in the PLAxtapiocay specimens. Enzymatic hydrolysis tests indicated that the weight loss of PLA70tapioca30MDI increased significantly as the ATBC content increased.
Acknowledgments
The authors express their appreciation to Grabio Greentech Corporation, Ching-Yung Trading Co., Ltd., Fabric King Textile Co., Ltd., Ministry of Economic Affairs (100-EC-17-A-10-S1-186) and the National Science Council (NSC 102-2221-E-161-011, NSC 100-3113-E-033-001 and NSC 102-2221-E-003-065) for support of this work.
Author Contributions
All authors contributed to this study. Chi-Hui Tsou and Wei-Song Hung designed the research and wrote this paper. Chi-Hui Tsou, Maw-Cherng Suen, and Chih-Yuan Tsou edited the paper and gave final approval of the version to be submitted. Chih-Yuan Tsou and Ruo Yao Wang did the analysis. Manuel De Guzman, Chien-Chieh Hu, and Kueir-Rarn Lee also contributed in analyzing data and in rewriting the revised manuscript. Jen-Taut Yeh, Wei-Hua Yao, Chin-San Wu, Shih-Hsuan Chiu, Jui-Chin Chen, Shang-Ming Lin, and Manuel De Guzman supervised the conduct of experiments and performed the theoretical analysis of the data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Handra R., Rustgi R. Biodegradable polymers. Prog. Polym. Sci. 1998;23:1273–1335. doi: 10.1016/S0079-6700(97)00039-7. [DOI] [Google Scholar]
- 2.Warwel S., Brüse F., Demes C., Kunz M., Rüsch gen Klaas M. Polymers and surfactants on the basis of renewable resources. Chemosphere. 2001;43:39–48. doi: 10.1016/S0045-6535(00)00322-2. [DOI] [PubMed] [Google Scholar]
- 3.Tsutsumi N., Kono Y., Oya M., Sakai W., Nagata M. Recent development of biodegradable network polyesters obtained from renewable natureal resources. Clean Soil Air Water. 2008;36:682–686. doi: 10.1002/clen.200800052. [DOI] [Google Scholar]
- 4.Sinclair R.G.J. The case for polylactic acid as a commodity packaging plastic. Macromol. Sci. Pure Appl. Chem. 1996;33:585–597. doi: 10.1080/10601329608010880. [DOI] [Google Scholar]
- 5.Kricheldorf H.R., Kreiser-Saunders I. Polylactides—Synthesis, characterization and medical application. Macromol. Symp. 1996;103:85–102. [Google Scholar]
- 6.Jang W.Y., Shin B.Y., Lee T.J., Narayan R. Thermal properties and morphology of biodegradable PLA/starch compatibilized blends. J. Ind. Eng. Chem. 2007;1:457–464. [Google Scholar]
- 7.Oksman K., Skrifvars M., Selin J.F. Natural fibres as reinforcement in polylactic acid (PLA) composites. Compos. Sci. 2003;63:1317–1324. doi: 10.1016/S0266-3538(03)00103-9. [DOI] [Google Scholar]
- 8.Shanks R.A., Hodzic A., Ridderhof D. Composites of poly(lactic acid) with flax fibers modified by interstitial polymerization. J. Appl. Polym. Sci. 2006;101:3620–3629. doi: 10.1002/app.22715. [DOI] [Google Scholar]
- 9.Bodros E., Pillin I., Montrelay N., Baley C. Could biopolymers reinforced by randomly scattered flax fiber be used in structural applications. Compos. Sci. Technol. 2007;67:462–470. doi: 10.1016/j.compscitech.2006.08.024. [DOI] [Google Scholar]
- 10.Alvarez V.A., Ruscekaite R.A., Vázquez A. Mechanical properties and water absorption behavior of composites made from a biodegradable matrix and alkaline-treated sisal fibers. J. Compos. Mater. 2003;37:1575–1588. doi: 10.1177/0021998303035180. [DOI] [Google Scholar]
- 11.Lee S.Y., Kang I.A., Doh G.H., Yoon H.G., Park B.D., Wu Q. Thermal and mechanical properties of wood flour/talc-filled polylactic acid composites: Effect of filler content and coupling treatment. J. Thermoplast. Compos. Mater. 2008;21:209–223. doi: 10.1177/0892705708089473. [DOI] [Google Scholar]
- 12.Shibata M., Ozawa K., Teramoto N., Yosomiya R., Takeishi H. Biocomposites made from short abaca fiber and biodegradable polyesters. Macromol. Mater. Eng. 2003;288:35–43. doi: 10.1002/mame.200290031. [DOI] [Google Scholar]
- 13.Plackett D., Andersen T.L., Pedersen W.B., Nielsen L. Biodegradable composites based on l-polylactide and jute fibres. Compos. Sci. Technol. 2003;63:1287–1296. doi: 10.1016/S0266-3538(03)00100-3. [DOI] [Google Scholar]
- 14.Shibata M., Oyamada S., Kobayashi S.I., Yaginuma D. Mechanical properties and biodegradability of green composites based on biodegradable polyesters and lyocell fabric. J. Appl. Polym. Sci. 2004;92:3857–3863. doi: 10.1002/app.20405. [DOI] [Google Scholar]
- 15.Huda M.S., Drzal L.T., Misra M., Mohanty A.K. Wood-fiber-reinforced poly(lactic acid) composites: Evaluation of the physicomechanical and morphological properties. J. Appl. Polym. Sci. 2006;102:4856–4869. doi: 10.1002/app.24829. [DOI] [Google Scholar]
- 16.Huda M.S., Drzal L.T., Misra M., Mohanty A.K., Williams K., Mielewski D.F. A study onbiocomposites from recycled newspaper fiber and poly(lactic acid) Ind. Eng. Chem. Res. 2005;44:5593–5601. doi: 10.1021/ie0488849. [DOI] [Google Scholar]
- 17.Liu W., Misra M., Askeland P., Drzal L., Mohanty A.K. Green composites from soy based plastic and pineapple leaf fiber: Fabrication and properties evaluation. Polymer. 2005;46:2710–2721. doi: 10.1016/j.polymer.2005.01.027. [DOI] [Google Scholar]
- 18.Ardente F., Beccali M., Cellura M., Mistretta M. Building energy performance: A LCA case study of kenaf-fibres insulation board. Energy Build. 2008;40:1–10. doi: 10.1016/j.enbuild.2006.12.009. [DOI] [Google Scholar]
- 19.Mathew A.P., Oksman K., Sain M. Mechanical properties of biodegradable composites from poly lactic acid (PLA) and microcrystalline cellulose (MCC) J. Appl. Polym. Sci. 2005;97:10–20. [Google Scholar]
- 20.Avella M., Buzarovska A., Errico M.E. Gennaro gentile and grozdanov aeco-challenges of bio-based polymer composites. Materials. 2009;2:911–925. doi: 10.3390/ma2030911. [DOI] [Google Scholar]
- 21.Wang H., Sun X.Z., Seib P. Strengthening blends of poly(lactic acid) and starch with methylenediphenyl diisocyanate. J. Appl. Polym. Sci. 2001;82:1761–1767. doi: 10.1002/app.2018. [DOI] [Google Scholar]
- 22.Ohkita T., Lee S.H. Effect of aliphatic isocyanates (HDI and LDI) as a coupling agent on the properties of eco-composite from biodegradable polymers and corn starch. J. Adhes. Sci. Technol. 2004;18:905–924. doi: 10.1163/156856104840516. [DOI] [Google Scholar]
- 23.Teixeira E.M., Da Roz A.L., Carvalho A.J.F., Curvelo A.A.S. The effect of glycerol/sugar/water and sugar/water mixtures on the plasticization of thermoplastic cassava starch. Carbohydr. Polym. 2007;69:619–624. doi: 10.1016/j.carbpol.2007.01.022. [DOI] [Google Scholar]
- 24.Rodriguez-Gonzalez F.J., Ramsay B.A., Favis B.D. Rheological and thermal properties of thermoplastic starch with high glycerol content. Carbohydr. Polym. 2004;58:139–147. doi: 10.1016/j.carbpol.2004.06.002. [DOI] [Google Scholar]
- 25.Ma X.F., Yu J.G., Wan J.J. Urea and ethanolamine as a mixed plasticizer for thermoplastic starch. Carbohydr. Polym. 2006;64:267–273. doi: 10.1016/j.carbpol.2005.11.042. [DOI] [Google Scholar]
- 26.Shi R., Zhang Z.Z., Liu Q.Y., Han Y.M., Zhang L.Q., Chen D.F. Characterization of citric acid/glycerol co-plasticized thermoplastic starch prepared by melt blending. Carbohydr. Polym. 2007;69:748–755. doi: 10.1016/j.carbpol.2007.02.010. [DOI] [Google Scholar]
- 27.Jang J.W.Y., Shin B.Y. Thermal properties and morphology of biodegradable PLA/starch Compatibilized Blends. Eng. Chem. 2007;13:457–464. [Google Scholar]
- 28.Wang J.W., Zhai W.T., Zheng W.G. Poly(ethylene glycol) grafted starch introducing a novel interphase in poly(lactic acid)/poly(ethylene glycol)/starch ternary composite. J. Polym. Environ. 2012;2:528–539. doi: 10.1007/s10924-012-0416-7. [DOI] [Google Scholar]
- 29.Xiong Z., Yang Y., Feng J.X., Zhang X., Zhang C.Z., Tang Z.B. Preparation and characterization of poly(lactic acid)/starch composites toughened with epoxidized soybean oil. Carbohydr. Polym. 2013;92:810–816. doi: 10.1016/j.carbpol.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Acioli-Moura R., Sun X.S. Thermal degradation and physical aging of poly(lactic acid) and its blends with starch ricardo. Polym. Eng. Sci. 2008;48:829–836. doi: 10.1002/pen.21019. [DOI] [Google Scholar]
- 31.Xiong Z., Zhang L.S., Ma S.Q., Yang Y., Zhang C.Z., Tang Z.B., Zhu J. Effect of castor oil enrichment layer produced by reaction on the properties of PLA/HDI-g-starch blends. Carbohydr. Polym. 2013;94:235–243. doi: 10.1016/j.carbpol.2013.01.038. [DOI] [PubMed] [Google Scholar]
- 32.Poramacom N., Ungsuratana A.O., Ungsuratana P., Supavititpattana P. Cassava production, prices and related policy in Thailand. Am. Int. J. Contemp. Res. 2013;3:43–51. [Google Scholar]
- 33.Tsou C.H., Hung W.S., Chen J.C., Huang C.Y., Wu C.S., Chiu S.H., Tsou C.Y., Chen J.C., Chu C.K., Hu C.C., et al. New composition of maleic-anhydride-grafted poly(lactic acid)/rice husk with methylenediphenyl diisocyanate. Mater.Sci. 2014 in press. [Google Scholar]
- 34.Zhao Y.L., Cai Q., Shuai X.T., Bei J.Z., Chen C.F., Xi F. Synthesis and thermal properties of novel star-shaped poly(l-lactide)s with starburst PAMAM–OH dendrimer macroinitiator. Polymer. 2002;43:5819–5825. doi: 10.1016/S0032-3861(02)00529-3. [DOI] [Google Scholar]
- 35.Kim E.S., Kim B.C., Kim S.H. Structural effect of linear and star-shaped poly(l-lactic acid) on physical properties. J. Polym. Sci. Part BPolym. Phys. 2004;42:939–946. doi: 10.1002/polb.10685. [DOI] [Google Scholar]
- 36.Liu H., Chen F., Liu B., Estep G., Zhang J. Super toughened poly(lactic acid) ternary blends by simultaneous dynamic vulcanization and interfacial compatibilization. Macromolecules. 2010;43:6058–6066. doi: 10.1021/ma101108g. [DOI] [Google Scholar]
- 37.Jiang L., Wolcott M.P., Zhang J. Study of biodegradable polylactide/poly(butylene adipate-co-terephthalate) blends. Biomacromolecules. 2006;7:199–207. doi: 10.1021/bm050581q. [DOI] [PubMed] [Google Scholar]
- 38.Williams G.I., Wool R.P. Composites from natural fibers and soy oil resins. Wool. Appl. Compos. Mater. 2000;7:421–432. doi: 10.1023/A:1026583404899. [DOI] [Google Scholar]
- 39.Wallenberger F.T., Weston N.E., editors. Natural Fibre: Plastics and Composites. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2004. [Google Scholar]
- 40.Martin P., Maquet C., Legras R., Bailly C., Leemans L., van Gurp M. Conjugated effects of the compatibilization and the dynamic vulcanization on the phase inversion behavior in poly(butylene terephthalate)/epoxide-containing rubber reactive polymer blends. Polymer. 2004;45:5111–5125. doi: 10.1016/j.polymer.2004.03.093. [DOI] [Google Scholar]
- 41.Martin P., Maquet R.C., Legras C., Bailly L., van Gurp Leemans M. Particle-in-particle morphology in reactively compatibilized poly(butylene terephthalate)/epoxide-containing rubber blends. Polymer. 2004;45:3277–3284. doi: 10.1016/j.polymer.2004.03.031. [DOI] [Google Scholar]
- 42.Lim J.S., Park K.I., Chung G.S., Kim J.H. Effect of composition ratio on the thermal and physical properties of semicrystalline PLA/PHB-HHx composites. Mater. Sci. Eng. 2013;33:2131–2137. doi: 10.1016/j.msec.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 43.Gerard T., Budtova T. Morphology and molten-state rheology of polylactide and polyhydroxyalkanoate blends. Eur. Polym. J. 2002;48:1110–1117. doi: 10.1016/j.eurpolymj.2012.03.015. [DOI] [Google Scholar]
- 44.Labrecque L.V., Dave R.A., Gross R.A. Citrate esters as plasticizers for poly(lactic acid) J. Appl. Polym. Sci. 1997;66:1507–1513. doi: 10.1002/(SICI)1097-4628(19971121)66:8<1507::AID-APP11>3.0.CO;2-0. [DOI] [Google Scholar]
- 45.Kulinski Z., Piorkowska E., Gadzinowska K. Plasticization of poly(l-lactide) with poly(propylene glycol) Biomacromolecules. 2006;7:2128–2135. doi: 10.1021/bm060089m. [DOI] [PubMed] [Google Scholar]
- 46.Ljungberg N., Wesslen B. Preparation and properties of plasticized poly(lactic acid) films. Biomacromolecules. 2005;6:1789–1796. doi: 10.1021/bm050098f. [DOI] [PubMed] [Google Scholar]
- 47.Ljungberg N., Andersson T., Wesslen B. Film extrusion and film weldability of poly(lactic acid) plasticized with triacetine and tributyl citrate. J. Appl. Polym. Sci. 2003;88:3239–3247. doi: 10.1002/app.12106. [DOI] [Google Scholar]
- 48.Martin O., Averous L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer. 2001;42:6209–6219. doi: 10.1016/S0032-3861(01)00086-6. [DOI] [Google Scholar]
- 49.Jacobsen S., Fritz H.G. Plasticizing polylactide-the effect of different plasticizers on the mechanical properties. Polym. Eng. Sci. 1999;39:1303–1310. doi: 10.1002/pen.11517. [DOI] [Google Scholar]
- 50.Yeh J.T., Huang C.Y., Chai W.L., Chen K.N. Plasticized properties of poly (lactic acid) and triacetine blends. J. Appl. Polym. Sci. 2009;112:2757–2763. doi: 10.1002/app.29761. [DOI] [Google Scholar]
- 51.Baiardo M., Frisoni G., Scandola M., Michel R., Lips D., Ruffieux K., Wintermantel E. Thermal and mechanical properties of plasticized poly(l-lactic acid) J. Appl. Polym. Sci. 2003;90:1731–1738. doi: 10.1002/app.12549. [DOI] [Google Scholar]
- 52.Standard Test Method for Tensile Properties of Plastics. [(accessed on 31 July 2014)]. Available online: http://www.astm.org/Standards/D638.htm.