Abstract
Reservoir silting is an unavoidable issue. It is estimated that in Italy, the potential rate of silting-up in large reservoirs ranges from 0.1% to 1% in the presence of wooded river basins and intensive agricultural land use, respectively. In medium and small-sized reservoirs, these values vary between 0.3% and 2%. Considering both the types of reservoirs, the annual average loss of storage capacity would be of about 1.59%. In this paper, a management strategy aimed at sediment productive reuse is presented. Particularly, the main engineering outcomes of an extensive experimental program on geopolymer binder synthesis is reported. The case study deals with Occhito reservoir, located in Southern Italy. Clay sediments coming from this silted-up artificial lake were characterized, calcined and activated, by means of a wide set of alkaline activating solutions. The results showed the feasibility of this recovery process, optimizing a few chemical parameters. The possible reuse in building material production (binders, precast concrete, bricks, etc.) represents a relevant sustainable alternative to landfill and other more consolidated practices.
Keywords: sustainability, reservoir, silting-up, sediments, geopolymers, binders
1. Introduction
1.1. Italian Legislation Framework
In Italy, there are about 555 large dams, according to the definition of [1], where the “large dams” are the ones higher than 15 m with a total storage capacity greater than 1 × 106 m3. Among them, 494 are still in operation, together with a number of small dams that, according to different estimations, range from 8288 to 15,400. Large and small dams have an overall potential storage capacity of approximately 10,854 × 106 m3 and 300 × 106 m3, respectively.
The overall capacity of the reservoir is a poorly renewable resource, and the environmental problems resulting from the construction of new dams significantly reduces the number of areas suitable for the realization of new reservoirs. This is true for both large and small-sized reservoirs.
It is estimated that in Italy, the potential rate of silting-up in large reservoirs ranges from 0.1% to 1% in the presence of wooded river basins and intensive agricultural land use, respectively. In the reservoirs of medium and small size, these values vary between 0.3% and 2%. Considering both the types of reservoirs, the annual average loss of storage capacity would be about 1.59% [2].
An analysis performed on 13 large reservoirs located in Sicily showed a significant loss of storage capacity due to the deposit processes, with a consequent decreasing of the overall storage capacity from 489 to 432 × 106 m3 and an overall loss of about 12% of the initial storage volume [3,4].
The analysis carried out by [5] on a sample of 268 large dams built in Italy in the last 50 years has evidenced that already 20 years ago:
1.5% of the dams were almost completely filled by sediments;
17.5% showed a reservoir capacity reduction of more than 50%;
4.5% showed a reservoir capacity reduction of approximately 20%.
In addition to the high costs of desiltation measures, it is essential to point out that sediments removed from a reservoir bottom are essentially under anaerobic conditions, with high concentrations of soluble iron and manganese (reduced forms), S2−, S− and H2S [6,7]. Therefore, they are highly toxic to plants and can cause strong environmental impacts [5]; therefore, as a temporary remedy, they are often moved from the area close to the dam, where they can interfere with the hydraulic works, and deposited in a new area within the same reservoir.
The management of reservoir siltation aimed at the maintenance of useful storage capacity is a matter of Legislative Decree 152/2006 (Article 114) and subsequent amendments [8]. Article 114 requires the preparation of a Management Plan for each reservoir, prepared on the basis of the criteria established by decree of the Ministry of Infrastructures and Transport, and the Environmental and Territorial Protection with the advice of the Ministry of Productive Activities and the Ministry of Agriculture and Forestry. At present, the decree has not been issued yet and, according to [9], it will be needed therefore to refer to the Ministerial Decree of 30 June 2004 [10].
Currently, also, Article 43 of the Decree Law, 201/2011, and subsequent amendments provides for the obligation to draw up the Management Project of large dams on the basis of which the competent authorities (Ministry of Infrastructures and Transport, Autonomous Regions and Provinces) will identify the plants for which the removal of bottom sediment is necessary [11].
Article 1, Paragraph 2 of the [10], provides that the Italian Regions establish specific regulations for reservoirs, which are not subject to the provisions of the decree of the President of the Republic 1363/59 and for the pertinent water bodies (Small Reservoirs) [12].
At present, some Italian Regions have already enacted regional regulations for dams not subject to the requirements of [12], as provided by the Article 1, Paragraph 2 of [10]. These regulations are the only source of information and technical guidance for several specific issues.
In the current Italian legislative framework, sediments, once removed from the reservoir bottom, can be:
disposed of in a landfill as waste according to [13];
recovered under the normal or simplified procedure specific for dredging according to [14];
reused as a by-product, according to Article 184 of [8] and subsequent amendments (Legislative Decree 205/2010) [15].
Excavation soils should be regarded as being a by-product and not waste only when certain conditions are met, such as:
-
(a)
excavation soils result from the completing of a work and are part of a production process whose primary purpose is not the production of such material;
-
(b)
excavation soils are used in accordance to the Use Plan;
-
(c)
the excavation soils can be directly used without any further processing other than normal industrial practice (Annex 3 to the Ministerial Decree, 161/2012) [16];
-
(d)
for the specific use to which Point (c) is refers, the excavation soils fulfil the environmental protection requirements specified in Annex 4 to [16].
One of the critical issues in this decree concerns the lack of a simplified procedure for small yards where soil production does not exceed 6000 m3 (as specifically provided for by Article 266, Paragraph 7 of [8]).
In this sense, some Italian Regions have issued ad hoc regional laws, for instance:
Liguria Region, Regional Council Decree No. 3159 of 01 February 2013 [17];
Veneto Region, Regional Council Decree No. 179 of 11 February 2013 [18].
With reference to the sediments of a reservoir, based on the present state of scientific knowledge, the problem is to extend the life cycle of the dredged sediments by their sustainable reuse solutions [19].
In [20], the idea of the “economic defense” of a reservoir was presented, which combines reservoir rehabilitation and reservoir utilization for environmental, industrial and agricultural purposes. In this sense, the economic defense, which was introduced as a form of evolution of the reservoir sedimentation passive defense, involves also an economic benefit. In the next sections, this innovative perspective will be applied to the case study of Italian Occhito Reservoir, by considering geopolymers binder production as a management strategy oriented toward the “economic defense” paradigm.
1.2. Geopolymer Binders as an Innovative Management Strategy
The evolution of national and European legislations in the field of reservoir water and sediment management was a strong push factor for scientific research towards the determination of possible reuses of sediment [21,22].
Some interesting results were obtained in the following works concerning reservoir sediments:
use of harbor, river and reservoir sediments in brick production [23,24,25];
use of harbor and reservoir sediments in sintered lightweight aggregate manufacturing [26,27,28,29,30,31];
use of clay sediment for the production of Portland cement [32];
use of silt-clay sediment for agriculture amendment [33];
use of sandy sediments for beach nourishment and protection from erosion [34].
Concerning the beneficial reuse of sediments, two main research trends can be found: bricks [23,24,25] and sintered lightweight aggregates [26,27,28,29,30,31]. In both cases, high temperature industrial processes are required, implying a detrimental environmental effect. Lightweight aggregates could be obtained with alternative low temperature processes, such as cold bonding pelletization [35].
In this work, an extensive experimental program is presented in order to find the best management solution to employ reservoir sediments in the production of geopolymers. A remarkable growth of research in the field of geopolymers has been observed in the last fifteen years. This growth has been pushed first by the increasing pulse toward sustainability, which has involved also the construction sector. Furthermore, there have been highly promising results obtained by some pioneering research in terms of durability, fire resistance, etc. [36,37,38,39]. All of these factors have determined also new industrial applications, which have given a second development of geopolymers technology after those considered as forerunners in Belgium, Eastern Europe, China, etc. [40]. Even if many potential applications have been investigated in the literature [41,42], use in civil engineering works still remains the main market [43,44].
In a preliminary study [45], sediments coming from two reservoirs located in Southern Italy were characterized by means of several chemical tests, revealing the potential to be employed as raw materials for the synthesis of geopolymers after calcination at 650 °C and 750 °C. The effectiveness of this activation treatment increased with temperature. However, just one mix design was considered (5 M NaOH solution).
In this study, the abovementioned preliminary results were qualitatively and quantitatively compared with a broader set of binding mixtures, considering potassium and sodium aluminate solutions, with different concentrations. Furthermore, in this case, the experimental program relied on a more significant sample made by homogenizing ten cores randomly taken from the lake.
In the following sections, the optimization of binding systems synthesized with Occhito sediments and characterization procedures will be accurately described, and the main engineering outcomes and impacts on reservoir management will be discussed.
2. Results and Discussion
XRF analysis results are shown in Table 1. The results of the leaching test are not shown here, but they confirmed that the Occhito sediment can be classified as non-hazardous wastes. The code associated with this residue in the European Waste Catalogue is 17.05.06 (sludge dredging).
Table 1.
XRF analysis of Occhito sediment.
| Oxides | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | K2O | TiO2 | Na2O | MnO | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Occhito | 57.13 | 16.33 | 12.5 | 6.94 | 2.97 | 2.4 | 0.88 | 0.37 | 0.15 | 0.15 |
| MK1 | 51.2 | 43.98 | - | 1.12 | - | 0.54 | 1.67 | - | - | - |
| MK2 | 52.9 | 41.9 | 0.17 | 1.6 | 0.19 | 0.77 | 1.8 | - | - | - |
In Table 1 are also reported two commercial metakaolin samples, namely MK1 and MK2. As we can immediately observe, the clay coming from reservoir dredging is not a pure aluminosilicate solid precursor. Particularly, the alumina content is sensitively lower than in the cases of commercial metakaolin samples. Nevertheless, the high CaO content, if amorphous, can influence polycondensation, modifying the mineralogy of hardened samples by determining the presence of calcium silicate hydrate-based phases [46]. Iron, magnesium and potassium contents can also determine complex mineralogical systems in the matrix. However, the Occhito sample as received is unreactive and, so, unsuitable for geopolymer binder production. In the next lines, the effect of the calcination processes carried out at 650 and 750 °C will be discussed.
The results of semiquantitative analysis on Occhito sediments revealed that clay phases represent the main crystalline component (65%). Significantly lower contents of quartz (18%) and calcite (17%) were also detected, while a limited amount of feldspar (3%) was found [45].
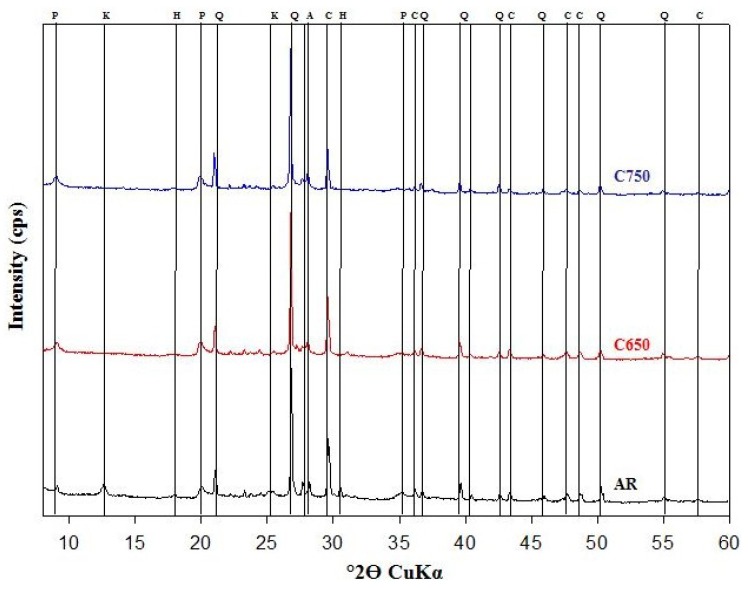
In Figure 1, XRD results are shown. The main detected mineralogical phases were: anorthite (A, CaO·Al2O3·2SiO2); calcite (C, CaCO3); hillebrandite (H, 2CaO·SiO2·H2O); kaolinite (K, Al2O3·2SiO2·2H2O); paragonite, (P, NaAl2 [Si3Al]O10(OH)2); quartz (Q, SiO2). The Occhito sample as received showed a mineralogical composition, which is mainly characterized by clay minerals, quartz and calcite. Iron, magnesium and potassium contents found by means of XRF did not find correspondent mineralogical phases in XRD, revealing that these elements could be present in amorphous phases. Thermal treatment determines the dehydration of clay minerals with the formation of reactive components.
Figure 1.
Mineralogical composition of Occhito sediments as received (indicated with “AR”), calcined at 650 °C (C650) and 750 °C (C750).
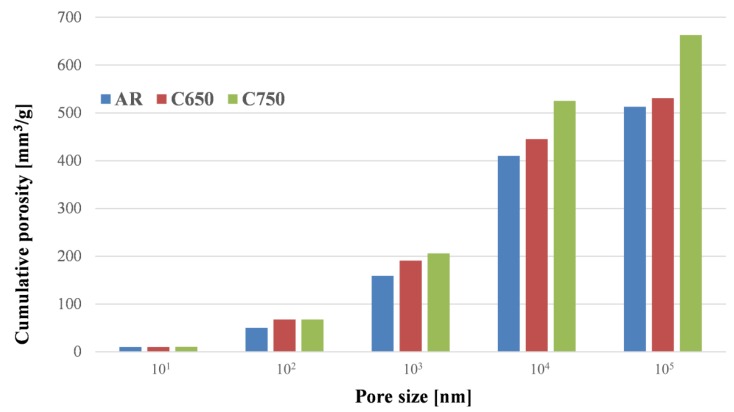
The physical effect of calcination was investigated by means of MIP (Figure 2). Five pore ranges were observed (101–105 nm), revealing an increase of porosity in calcined samples starting from 103 nm-sized pores. The total porosity values were 513, 531 and 664 mm3/g for as-received and calcined at 650 °C and 750 °C samples, respectively.
Figure 2.
Mercury intrusion porosimetry results.
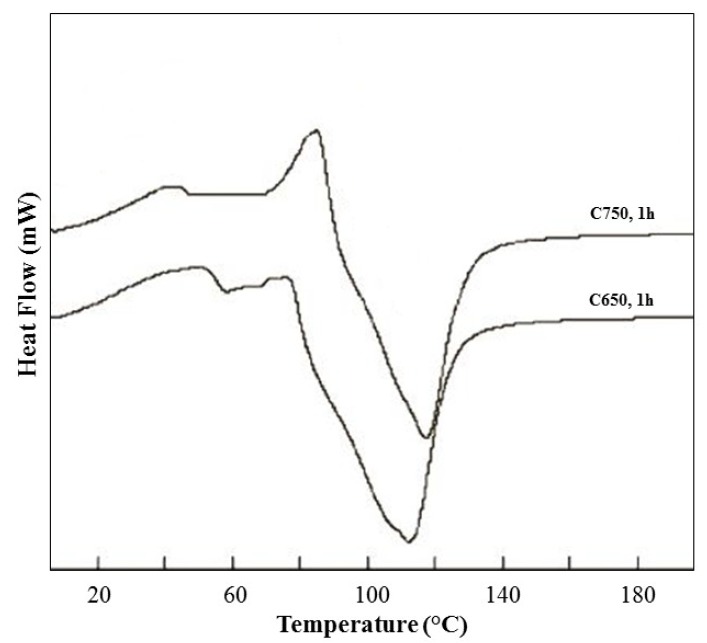
In Figure 3, the results of DSC analysis are shown. As outlined in previous sections, a dynamics test was carried out. The main outcomes of this experimental step were: (1) the exothermic peaks area was higher when calcination temperature increases; (2) the highest peaks were detected around 60 °C. The latter consideration is the reason for setting the curing temperature at 60 °C.
Figure 3.
DSC analysis results.
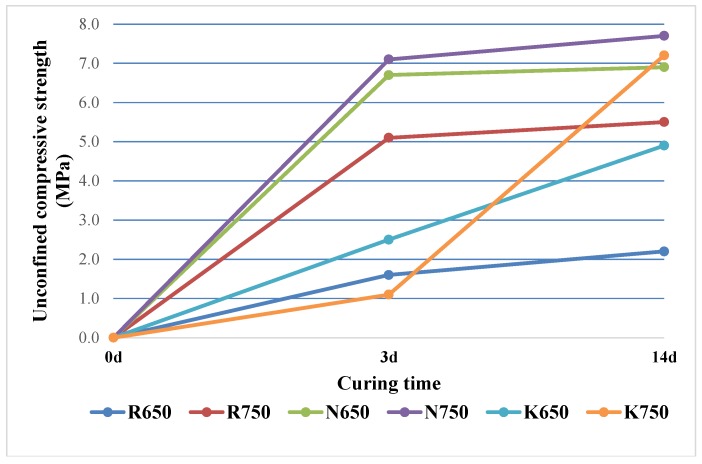
In Figure 4, the results of mechanical testing are reported. Strength development was monitored for 14 days. The curves indicated by R650 and R750 represent the reference systems, obtained by activating calcined samples by means of 5 M NaOH solution. These systems exhibited a strong dependence on calcination temperature. Passing from 650 °C to 750 °C, we observed an increase in the early strength by a factor of three. In both cases of R650 and R750, the curing phase carried out under room conditions did not determine a further strength increase.
Figure 4.
Unconfined compressive strength values after three and seven days of curing.
The qualitative analysis revealed that a proper mechanical performance development was achievable only in the cases of the 13 M sodium aluminate solution and 11 M potassium aluminate solution. Compressive strength development is reported here for brevity only for four aluminate-activated systems (namely N650, N750, K650, K750).
In Figure 4, the different influence of sodium and potassium aluminate solutions on hardened products strength is also observable. Samples obtained by activating calcined Occhito sediments with potassium aluminate, namely K650 and K750, exhibited a peculiar behavior. In the early age, calcination temperature seemed to bring no benefit to strength development, since the K650 compressive strength was higher than K750 by a factor of about 2.5. The situation is completely reversed after 14 days, when K750 samples show a tripled strength compared to K650 ones. Anyway, the geopolymers made with sediment calcined at 750 °C (K750) were able to develop relevantly the mechanical properties also at room conditions, while K650 showed a lower increase after the first three days at 60 °C. The systems activated with sodium aluminate showed the best mechanical performances. Furthermore, the kinetics were only slightly improved by increasing calcination temperature, as we can see from Figure 4. This is of course an interesting result, in terms of both performance development and sustainability indicators.
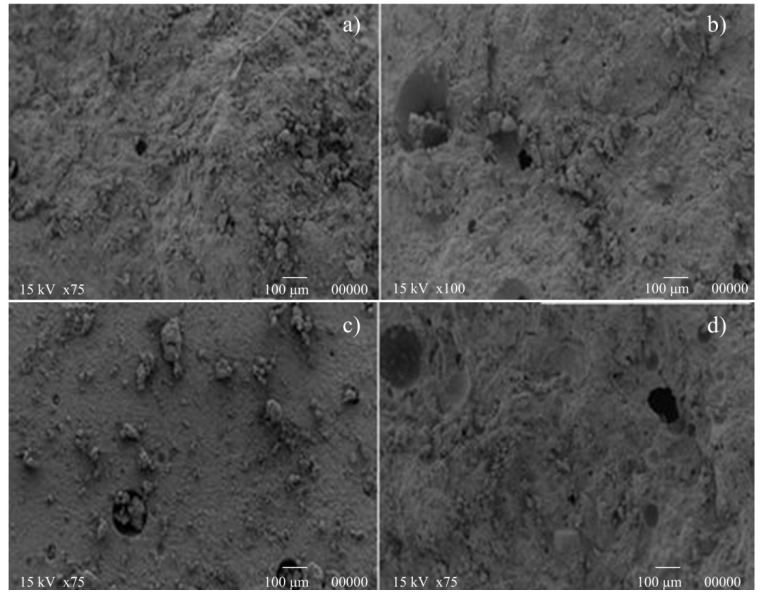
In Figure 5a,c, SEM images of N650 samples after three and 14 curing days are reported. As we can observe, no substantial microstructural changes occurred during the longer curing phase at room temperature. This result is in agreement with strength development, since no significant increase of compressive resistance was observed. A similar conclusion can be drawn considering Figure 5b,d, which is related to N750 samples after three and 14 curing days.
Figure 5.
Microstructural analysis of N650 (a,c) and N750 (b,d) samples.
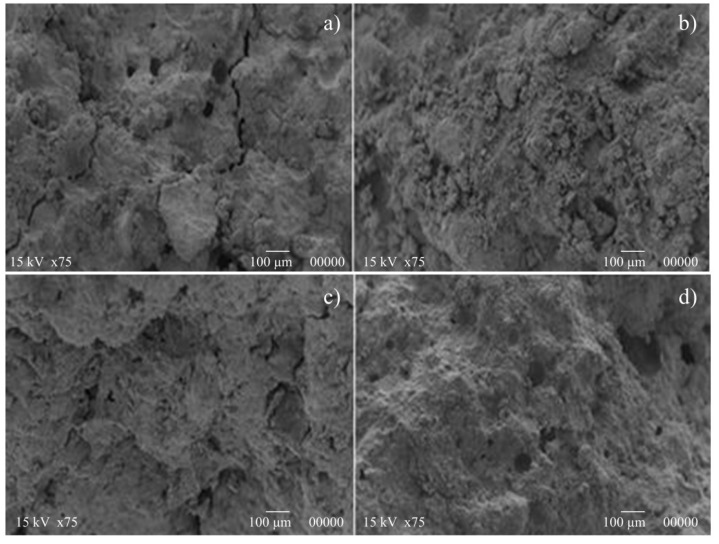
Figure 6 reports SEM images for K650 and K750 samples. The microstructure at the early age (after three days of curing) can be observed in Figure 6a (K650) and Figure 6b (K750). These observations explain the unsatisfactory mechanical performance obtained, since relevant microcracking patterns and poorly compacted microstructures were developed at the early age. Curing time definitely influences microstructure refinement only in the case of K750, with the development of a more compact microstructure (Figure 6d). Instead, in the case of K650, the presence of microcracks limits the strength gain, determining the premature brittle failure of the sample under testing. These results are in agreement with the outcomes of mechanical testing.
Figure 6.
Microstructural analysis of K650 (a,c) and K750 (b,d) samples.
3. Experimental Section
The samples of clay sediments were collected from the Italian reservoir of Occhito (Carlantino, Foggia, Italy). Many samples were obtained by coring, obtaining ten cores from Occhito Lake. The cores were 10 cm in diameter and 1 m in height. The cores were first crushed in a jaw crusher; then, they were milled in a ring mill and carefully mixed to get a homogeneous sample.
The sample was dried in an oven at 105 °C and then submitted to quantitative chemical analysis. The composition in terms of main oxides was obtained by means of X-ray fluorescence, making use of a Bruker Explorer S4 apparatus. Calcination was carried out at 650 °C and 750 °C for 1 h. The mineralogical analysis was performed by means of a Philips PW 1730 diffractometer (Cu Kα radiation, 5°–60° 2ϴ range, step width of 0·02° 2ϴ and 1 s data collection per step).
Semiquantitative evaluation of the mineralogical phases was performed according to the following conditions: powders with a grain size <10 µm have been obtained by means of a McCrone micronizing mill (agate cylinders and wet grinding time = 15 min). An α-Al2O3 internal standard (1 µm, Buehler Micropolish) has been added to each sample in the amount of 20 wt%. Powder data sets were obtained with a PANalytical X’Pert Pro modular diffractometer equipped with an RTMS detector (Cu Kα radiation, 40 kV, 40 mA, 2θ range from 3° to 80°, equivalent step size of 0·0179° 2θ, equivalent counting time of 120 s per step). X’Pert High Score Plus 2·2d software and the inorganic crystal structure database (ICSD) were used for mineral identification and semiquantitative evaluation.
In order to assess the physical effect of calcination, mercury intrusion porosimetry (MIP) testing was carried out on both as-received and calcined samples. The pore structure of powder is strictly related to the specific surface, and any variation can influence reactivity. The experimental equipment consisted of two porosimeters apparatus, namely Thermo Pascal 140 and Thermo Pascal 440, which reach a maximum pressure of 400 kPa and 400 MPa, respectively. The pore shapes are assumed to be cylindrical, and their diameters can be calculated by the Washburn equation:
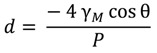 |
(1) |
In this formula, d is the pore diameter (m); γM is the mercury surface tension (N/m); P is the applied pressure (MPa); and ʘ is the contact angle between the mercury and the solid surface (°).
Calcined clay samples dredged from Occhito Lake were employed in geopolymer binder preparation. The case study presented here was concerned with the optimization of the binding mixture by means of several alkaline activating solutions. Particularly, considering the low content of alumina in the raw sediments, it was suggested to design binding systems with alumina-containing activating solutions. Most of the literature study cases consider aluminosilicate precursors activated by means of alkaline hydroxide/silicate solutions for geopolymers synthesis. In the case of this experimental work, where sediments showed a low alumina content, the use of alkaline aluminate solutions appeared to be a very interesting solution. In the literature, only a small amount of data about aluminate-based activation of geopolymers can be found. Nevertheless, the results obtained with the aluminate option were considerably promising. In [47], a separate mixing procedure was applied to a silica fume-based geopolymer system activated by means of: (1) sodium hydroxide solution; (2) sodium aluminate solution. The hardened binder samples exhibited good agreement with the geopolymer science criteria, e.g., they were X-ray amorphous, set and hardened in mild conditions, achieving fine mechanical performance (about 26 MPa). The only crystalline phase detected was gibbsite, mainly attributable to the carbonation of 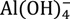 species [47]. Hajimohammadi et al. [48] studied a one-part geopolymer composition obtained by adding water to a solid geothermal silica and sodium aluminate blend, but engineering characterization was not carried out. In [49], many binding mixtures were studied and, among those, sodium aluminate activated kaolinite, slag, K-feldspar and fly ash mixtures. The alumina content obtained by means of XRF analysis was equal to 28.6, 17.6, 14.1 and 27.1 wt% for kaolinite, k-feldspar, slag and fly ash, respectively. Compressive strength values after 7 days of curing ranged from 3.3 to 26.6 MPa when only sodium aluminate was used and from 23.0 to 86.5 MPa when a binary aluminosilicate alkaline solution was used [49]. Recently, other works have demonstrated the interest of research in aluminate activation. Rickard et al. investigated the thermal properties of fly ash-based geopolymers activated by means of sodium silicate and sodium aluminate solutions [50]. Van Riessen et al. obtained average performance geopolymers (compressive strength in the range 33–43 MPa) activating fly ash by means of industrial liquors coming from the Bayer process [51]. All of these considerations reasonably push research toward the further development of aluminate activation, which could bring not just average performance composites for the construction sector, but also high performance ones.
species [47]. Hajimohammadi et al. [48] studied a one-part geopolymer composition obtained by adding water to a solid geothermal silica and sodium aluminate blend, but engineering characterization was not carried out. In [49], many binding mixtures were studied and, among those, sodium aluminate activated kaolinite, slag, K-feldspar and fly ash mixtures. The alumina content obtained by means of XRF analysis was equal to 28.6, 17.6, 14.1 and 27.1 wt% for kaolinite, k-feldspar, slag and fly ash, respectively. Compressive strength values after 7 days of curing ranged from 3.3 to 26.6 MPa when only sodium aluminate was used and from 23.0 to 86.5 MPa when a binary aluminosilicate alkaline solution was used [49]. Recently, other works have demonstrated the interest of research in aluminate activation. Rickard et al. investigated the thermal properties of fly ash-based geopolymers activated by means of sodium silicate and sodium aluminate solutions [50]. Van Riessen et al. obtained average performance geopolymers (compressive strength in the range 33–43 MPa) activating fly ash by means of industrial liquors coming from the Bayer process [51]. All of these considerations reasonably push research toward the further development of aluminate activation, which could bring not just average performance composites for the construction sector, but also high performance ones.
In this work, three different series of alkaline solutions were employed:
-
(1)
5 M NaOH in an amount so as to have a Na/Al ratio at most equal to 1:1; this is possible by assuming the hypothesis that all of the alumina is available for polycondensation reactions;
-
(2)
sodium aluminate solution obtained by dissolving pure Al(OH)3 in NaOH solutions in an amount so as to have a molar Al/Na ratio equal to 0.65 (maximum solubility);
-
(3)
potassium aluminate solution obtained by dissolving pure Al(OH)3 in KOH solutions in an amount so as to have a molar Al/K ratio equal to 0.53 (maximum solubility).
In both of the two latter cases, several alkaline solution concentrations were evaluated by means of a qualitative inspection of hardened samples. Particularly, sodium aluminate solutions with concentration of 8.5, 11, 13, 15 and 17 and potassium aluminate solutions with a concentration of 8.5, 11, 13 and 17 were considered.
The reactivity of samples was tested by measuring polycondensation occurrence through a differential scanning calorimetry (DSC) apparatus, TA Instruments model Q20, according to the following experimental details: the reactants (calcined sediments and 5 M NaOH solution) were separately kept at 4 °C and intimately mixed for 3 min before testing. Particularly, a dynamics test was carried out on a sample of 10 mg by heating up to 200 °C at 5 °C min−1. The reactivity was evaluated by measuring the heat evolved in each experimental condition, assessing in this way the effect of calcination temperature on polycondensation.
In order to verify the actual occurrence of geopolymerization for Occhito sediment in the whole set of alkaline environments investigated here, cylindrical samples (diameter, 3 cm; height, 6 cm) were prepared by pouring the mixtures into polyethylene molds. Three samples were tested for each binding mixture. These samples were cured for 3 days at 60 °C in an oven, keeping the lids closed to ensure 100% relative humidity conditions. Afterwards, the specimens were extracted from the molds and kept in air for 11 days, until they were subjected to unconfined compressive strength (UCS) determination using a 100-kN capacity Controls MCC8 testing machine. Finally, in order to relate mechanical testing results with microstructural changes, SEM analysis was carried out by means of an FEI Quanta 200 FEG microscope.
4. Conclusions and Further Developments
In this paper, we reported a case study on the beneficial reuse of clay sediments coming from the dredging of Occhito Lake, located in Southern Italy. The problem of extending the life cycle of the dredged sediments by their sustainable reuse was approached here by considering geopolymer binder production. This management solution is favored by the push toward sustainability, which has involved also the construction industry and the promising results obtained in terms of mechanical performance, durability, fire resistance, etc. Furthermore, building material production in both precast and in situ applications represents a wide market, which could imply a positive trend, considering the huge amount of raw materials usually employed.
Sodium aluminate solution revealed to be the best activator, among those tested, in terms of mechanical property development (three days of compressive of about 7 MPa). In the case of this activator, calcination temperature can be beneficially reduced from 750 °C to 650 °C, implying further improvement of sustainability indicators. Other literature applications [23,24,25,26,27,28,29,30,31], as already pointed out, deal with a high temperature industrial process (about 1000–1150 °C). Calcination temperature reduction by means of binding system optimization represents a good way to improve sustainability indicators for the recycling process proposed here.
In a forthcoming experimental phase, starting from this initial, but wide research on binder kinetics and hardening properties, civil engineering composites (concrete, mortars and bricks) will be designed and characterized in terms of rheology, shrinkage and durability (freeze-thaw, sulfate attack, alkali silica reaction, chloride attack, etc.).
Author Contributions
Bruno Molino and Raffaele Cioffi shared the innovative idea of sediments reuse in geopolymer synthesis process and planned the project activities. Annamaria De Vincenzo studied Italian legislative framework for this topic, in order to find viable management solutions. Claudio Ferone and Francesco Colangelo coordinated materials design and characterization aspects, while Francesco Messina focused on literature survey, experimental activities and data analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Law No. 584. Italy: Oct 21, 1994. [Google Scholar]
- 2.Bazzoffi P., Vanino S. Rapporto. Istituto Nazionale di Economia Agraria; Rome, Italy: 2009. L’interrimento degli invasi ad uso irriguo nelle regioni meridionali: Rilievi diretti metodologie e modellistica. (In Italian) [Google Scholar]
- 3.Barbagallo S., Tamburino V. Valutazione dei deflussi in alcuni bacini siciliani mediante il bilancio idrico dei serbatoi. Idrotecnica. 1989;1:5–12. (In Italian) [Google Scholar]
- 4.Tamburino V., Barbagallo S., Vella P. Indagine sull’interrimento dei serbatoi artificiali siciliani. Ing. Agrar. 1989;3:156–164. (In Italian) [Google Scholar]
- 5.Tomasi L. Operation and maintenance problems due to sedimentation in reservoir; Proceedings of the International Conference on Reservoir Sedimentation; Fort Collins, CO, USA. 9–13 September 1996; Fort Collins, CO, USA: Colorado State University Press; 1996. pp. 15–28. [Google Scholar]
- 6.Barko J.W., Adams M.S., Clesceri N.L. Environmental factors and their considerations in the managements of submersed aquatic vegetations: A review. J. Aquat. Plant Manag. 1986;24:1–10. [Google Scholar]
- 7.Nichols S.A. Depth, substrate, and turbidity relationships of some Wisconsin lake plants. Trans. Wis. Acad. Sci. Arts Lett. 1992;80:97–118. [Google Scholar]
- 8.Article 114, Legislative Decree No. 152. Italy: 2006. [Google Scholar]
- 9.Legislative Decree No. 152. Italy: 1999. [Google Scholar]
- 10.Ministerial Decree. Italy: Jun 30, 2004. [Google Scholar]
- 11.Article 43, Decree Law No. 201. Italy: 2011. [Google Scholar]
- 12.Decree of the President of the Italian Republic No. 1363. Italy: 1959. [Google Scholar]
- 13.Ministerial Decree. Italy: Sep 27, 2011. [Google Scholar]
- 14.Ministerial Decree. Italy: Feb 5, 1998. [Google Scholar]
- 15.Legislative Decree No. 205. Italy: 2010. [Google Scholar]
- 16.Ministerial Decree No. 161. Italy: 2012. [Google Scholar]
- 17.Decree No. 3159. Italy: Feb 1, 2013. [Google Scholar]
- 18.Decree No. 179. Italy: Feb 11, 2013. [Google Scholar]
- 19.Magri M. Profili giuridici del riuso dei materiali. Quarry Constr. 2009;9:13–20. (In Italian) [Google Scholar]
- 20.Molino B. Combined reservoir rehabilitation and sediment utilization: Economic defence; Proceedings of the International Conference on Trends in Water and Environmental Engineering for Safety and Life; Capri, Italy. 3–7 July 2000. [Google Scholar]
- 21.Molino B., Sole A., Zuccaro G. Rehabilitation of artificial reservoir and environmental reuse of sediment; Proceedings of the 3rd International Conference on Management Information System; Halkidiki, Greece. 24–26 April 2002. [Google Scholar]
- 22.Molino B. Environmental protection in processes of reservoir restoration and use of sediments; Proceedings of the International Workshop from Watersheds Slopes to Coastal Areas: Sedimentation Processes at Different Scales; Venice, Italy. 3–6 December 2003. [Google Scholar]
- 23.Chiang K.Y., Chien K.L., Hwang S.J. Study on the characteristics of building bricks produced from reservoir sediment. J. Hazard. Mater. 2008;159:499–504. doi: 10.1016/j.jhazmat.2008.02.046. [DOI] [PubMed] [Google Scholar]
- 24.Hamer K., Karius V. Brick production with dredged harbour sediments. An industrial-scale experiment. Waste Manag. 2002;22:521–530. doi: 10.1016/s0956-053x(01)00048-4. [DOI] [PubMed] [Google Scholar]
- 25.Samara M., Lafhaj Z., Chapiseau C. Valorization of stabilized river sediments in fired clay bricks: Factory scale experiment. J. Hazard. Mater. 2009;163:701–710. doi: 10.1016/j.jhazmat.2008.07.153. [DOI] [PubMed] [Google Scholar]
- 26.Liao Y.C., Huang C.Y. Effects of heat treatment on the physical properties of lightweight aggregate from water reservoir sediment. Ceram. Int. 2011;37:3723–3730. doi: 10.1016/j.ceramint.2011.04.122. [DOI] [Google Scholar]
- 27.Liao Y.C., Huang C.Y. Effects of CaO addition on lightweight aggregates produced from water reservoir sediment. Constr. Build. Mater. 2011;25:2997–3002. doi: 10.1016/j.conbuildmat.2010.12.034. [DOI] [Google Scholar]
- 28.Tang C.W., Chen H.J., Wang S.Y., Spaulding J. Production of synthetic lightweight aggregate using reservoir sediments for concrete and masonry. Cem. Concr. Compos. 2011;33:292–300. doi: 10.1016/j.cemconcomp.2010.10.008. [DOI] [Google Scholar]
- 29.Wei Y.L., Yang J.C., Lin Y.Y., Chuang S.Y., Wang H.P. Recycling of harbor sediment as lightweight aggregate. Mar. Pollut. Bull. 2008;57:867–872. doi: 10.1016/j.marpolbul.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 30.Wei Y.L., Lin Y.Y. Role of Fe compounds in light aggregate formation from a reservoir sediment. J. Hazard. Mater. 2009;171:111–115. doi: 10.1016/j.jhazmat.2009.05.122. [DOI] [PubMed] [Google Scholar]
- 31.Wei Y.L., Lin C.Y., Ko K.W., Wang H.P. Preparation of low water-sorption lightweight aggregates from harbor sediment added with waste glass. Mar. Pollut. Bull. 2011;63:135–140. doi: 10.1016/j.marpolbul.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 32.Valenti G., Bernardo G., Marroccoli M., Molino B. Beneficial reuse of reservoir sediment in the cement industry; Proceedings of the International Conference on Remediation of Contaminated Sediments; Venice, Italy. 30 September–3 October 2003. [Google Scholar]
- 33.De Vincenzo A., Molino B., Viparelli R., Albergo G. Silting-up in reservoir: A risk and a resource; Proceedings of the 32nd IAHR World Congress; Venice, Italy. 1–6 July 2007; pp. 2–10. [Google Scholar]
- 34.Molino B. L’interrimento degli invasi e utilizzo dei sedimenti per la tutela dell’ambiente costiero. Geol. Ambient. 2008;2:77–86. (In Italian) [Google Scholar]
- 35.Ferone C., Colangelo F., Messina F., Iucolano F., Liguori B., Cioffi R. Coal combustion wastes reuse in low energy artificial aggregates manufacturing. Materials. 2013;6:5000–5015. doi: 10.3390/ma6115000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bakharev T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005;35:658–670. doi: 10.1016/j.cemconres.2004.06.005. [DOI] [Google Scholar]
- 37.Bakharev T. Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 2005;35:1233–1246. doi: 10.1016/j.cemconres.2004.09.002. [DOI] [Google Scholar]
- 38.Bakharev T. Thermal behaviour of geopolymers prepared using class F fly ash and elevated temperature curing. Cem. Concr. Res. 2006;36:1134–1147. doi: 10.1016/j.cemconres.2006.03.022. [DOI] [Google Scholar]
- 39.Davidovits J. Geopolymers and geopolymeric materials. J. Therm. Anal. Calorim. 1989;35:429–441. doi: 10.1007/BF01904446. [DOI] [Google Scholar]
- 40.Provis J.L., Brice D.G., Buchwald A., Duxson P., Kavalerova E., Krivenko P.V., Shi C., van Deventer J.S.J., Wiercx J.A.L.M. Alkali-Activated Materials: State-of-the-Art Report. Springer; Berlin, Germany: 2014. Demonstration projects and applications in building and civil infrastructure; pp. 309–338. [Google Scholar]
- 41.Migliaccio M., Ferrara G., Gifuni A., Sorrentino A., Colangelo F., Ferone C., Cioffi R., Messina F. Shielding effectiveness tests of low-cost civil engineering materials in a reverberating chamber. Prog. Electromagn. Res. B. 2013;54:227–243. doi: 10.2528/PIERB13071703. [DOI] [Google Scholar]
- 42.Ferone C., Colangelo F., Messina F., Santoro L., Cioffi R. Recycling of pre-washed municipal solid waste incinerator fly ash in the manufacturing of low temperature setting geopolymer materials. Materials. 2013;6:3420–3437. doi: 10.3390/ma6083420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menna C., Asprone D., Ferone C., Colangelo F., Balsamo A., Prota A., Cioffi R., Manfredi G. Use of geopolymers for composite external reinforcement of RC members. Compos. Part B Eng. 2013;45:1667–1676. doi: 10.1016/j.compositesb.2012.09.019. [DOI] [Google Scholar]
- 44.Ferone C., Colangelo F., Cioffi R., Montagnaro F., Santoro L. Mechanical performances of weathered coal fly ash based geopolymer bricks. Proc. Eng. 2011;21:745–752. doi: 10.1016/j.proeng.2011.11.2073. [DOI] [Google Scholar]
- 45.Ferone C., Colangelo F., Cioffi R., Montagnaro F., Santoro L. Use of reservoir clay sediments as raw materials for geopolymer binders. Adv. Appl. Ceram. 2013;112:184–189. doi: 10.1179/1743676112Y.0000000064. [DOI] [Google Scholar]
- 46.Brew D.R.M., MacKenzie K.J.D. Geopolymer synthesis using silica fume and sodium aluminate. J. Mater. Sci. 2007;42:3990–3993. doi: 10.1007/s10853-006-0376-1. [DOI] [Google Scholar]
- 47.Hajimohammadi A., Provis J.L., van Deventer J.S. One-part geopolymer mixes from geothermal silica and sodium aluminate. Ind. Eng. Chem. Res. 2008;47:9396–9405. doi: 10.1021/ie8006825. [DOI] [Google Scholar]
- 48.Phair J.W., van Deventer J.S.J. Characterization of fly-ash-based geopolymeric binders activated with sodium aluminate. Ind. Eng. Chem. Res. 2002;41:4242–4251. doi: 10.1021/ie010937o. [DOI] [Google Scholar]
- 49.Garcia-Lodeiro I., Palomo A., Fernández-Jiménez A., Macphee D.E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011;41:923–931. [Google Scholar]
- 50.Rickard W.D., Temuujin J., van Riessen A. Thermal analysis of geopolymer pastes synthesised from five fly ashes of variable composition. J. Non Cryst. Solids. 2012;358:1830–1839. doi: 10.1016/j.jnoncrysol.2012.05.032. [DOI] [Google Scholar]
- 51.Van Riessen A., Jamieson E., Kealley C.S., Hart R.D., Williams R.P. Bayer-Geopolymers: An exploration of synergy between the alumina and geopolymer industries. Cem. Conc. Compos. 2013;41:29–33. doi: 10.1016/j.cemconcomp.2013.04.010. [DOI] [Google Scholar]