Abstract
Background: The heightened cardiovascular risk associated with metabolic syndrome (MetS) has been documented by several researchers. The Framingham risk score (FRS) provides a simple and efficient method for identifying individuals at cardiovascular risk. The objective was to describe the prevalence of MetS and its association with FRS in predicting cardiovascular disease among a cohort of semi-urban women; Method: Clinical and laboratory parameters were evaluated among 189 healthy women. The International Diabetes Federation definition was used to diagnose metabolic syndrome. FRS was calculated for each participant; Result: About two thirds of the participant make less than $US 90 per month. The mean systolic blood pressure was 131.80 ± 30. Eighty (42.3%) participants were overweight with a mean waist circumference of 91.64 ± 11.19 cm. MetS was present in 46 (24.3%). Individuals with MetS were more likely to have increased FRS, p = 0.012. One hundred and eighty seven (98.9%) were in the low risk category according to FRS. There was a significant difference in the mean FRS between participants with and without MetS (13.52 versus 10.29 p = 0.025); Conclusion: Prevalence of MetS in this study was comparable to the global rate, despite a low economic status. Individuals with MetS were more likely to have cardiovascular disease than persons without MetS, thus emphasizing the need for risk stratification and prompt management.
Keywords: metabolic syndrome, Framingham risk score, cardiovascular disease
1. Introduction
There is increased reported prevalence of obesity and metabolic syndrome in Sub-Saharan Africa. This may be attributed to the adoption of Western lifestyles, including diet and reduced physical activity. Several clinical conditions are specific to women. Stress associated with pregnancy, use of oral contraceptive medication, polycystic ovary syndrome, and the effects of menopause [1,2]. The aforementioned conditions, in association with traditional cardiovascular risk factors, put women at a greater disadvantage and, hence, are candidates for special considerations regarding risk factor identification, modification, and clinical management [2].
In the developed countries, cardiovascular mortality rates in women have declined due to modifications in risk behavior, such as increased physical activity, better management of hypertension and dyslipidaemia, and improved treatment of existing cardiovascular conditions [3].
These benefits are not apparent in developing countries, where only a fraction of women with cardiovascular disease (CVD) has access to optimal care [4,5].
Metabolic syndrome has been linked with cardiovascular disease. The heightened cardiovascular risk associated with metabolic syndrome has been documented by several researchers. Reports from developing countries showed that cardiovascular disease represents up to 75% of mortality from non-communicable diseases, and accounts for about 10% of the developing world’s burden of morbidity [5,6]. Cardiovascular disease is projected to be a principal cause of death and disability worldwide by 2030 [7,8,9].
Many reports also point out that the presence of metabolic syndrome (MetS) contributes to an increased risk for CVD and type-2 diabetes. A two- and five-fold increase risk has been associated with MetS in the development of CVD and diabetes, respectively [10,11].
The Framingham risk score (FRS) is a gender-based algorithm designed to estimate an individual’s 10-year chance of developing cardiovascular disease [12].
The scoring system is important as it gives an indication of the likely benefits of prevention and prompt management of cardiovascular risk factors. It also provides an economical and efficient method of identifying individuals at high cardiovascular risk who need preventive treatment, and persons with low risk who need not be unduly worried [13].
Many earlier studies in Nigeria described the prevalence of risk factors for CVD in specific populations, such as those with hypertension, diabetes, and the aged [14,15,16]. The aim of the study is to provide descriptive information on the prevalence of metabolic syndrome and its association with the Framingham risk score in predicting cardiovascular disease among a cohort of semi-urban African women.
2. Methods and Settings
A cross-sectional pilot study was conducted among Christian women during their annual congress meeting at Ado-Ekiti, Nigeria. The participants had an initial health-awareness talk a day before the screening exercise. One hundred and eighty-nine women voluntarily participated in the screening exercise. The International Diabetes Federation (IDF) definition was used to diagnose metabolic syndrome.
The anthropometry measurements, including the height, weight, and waist and hip circumferences, were taken. Weight was measured using a standardized bathroom scale, while the height was measured with a stadiometer. The waist circumference was taken at mid-way between the sub-costal margin and the iliac crest with an inelastic measuring tape, while the hip circumference was taken at the widest diameter. The body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters.
BMI status was categorized as follows: Underweight < 18.5; normal 18.5–24.9; overweight 25–29.9; obese ≥ 30.
Trained nurses and doctors took blood pressure measurements from the left upper arm with the participants sitting using Accusson’s mercury sphygmomanometer with appropriate cuff sizes. Fasting total and high-density lipoprotein cholesterol, triglycerides and blood glucose were estimated for all participants. The low-density cholesterol was calculated using the Friedewald formula [17]. All parameters were expressed as mmol/L.
Participants were diagnosed to have diabetes mellitus (DM) if they had a fasting blood glucose level (FBG) ≥ 7 mmol/L, or reported a history of diabetes, or use of glucose-lowering drugs.
Written inform consents were signed by all participants. Ethical clearance was obtained from the ethical review committee of the Ekiti State University Teaching Hospital, Ado Ekiti, project identification code EKSUTH/A67/2014/10/10, 10 October 2014.
The IDF criteria [18] were used to define the presence metabolic syndrome as follows: Central obesity (defined as waist circumference ≥ 80 cm) plus any two of the following four factors:
Raised TG level: ≥ 1.7 mmol/L, or specific treatment for this lipid abnormality
Reduced HDLchol cholesterol: <1.29 mmol/L in females, or specific treatment for this lipid abnormality
Raised blood pressure: Systolic BP ≥ 130 or diastolic BP ≥ 85 mmHg, or treatment of previously diagnosed hypertension
Raised fasting plasma glucose (FPG) ≥ 5.6 mmol/L, or previously diagnosed type 2 diabetes.
We use the Sub-Saharan threshold for waist circumference to define central obesity, i.e., ≥80 cm. Participants found to have diabetes mellitus or raised fasting plasma glucose were regarded to have dysglycaemia.
FRS for cardiovascular disease over 10 years was calculated for each participant based on age, gender, total cholesterol, HDL-cholesterol, and systolic blood pressure or currently on any medication to treat high blood pressure [12]. Individuals with a score of 10% or less had a low coronary disease risk at 10 years, while those with 10%–20% and 20% or more were categorized as having intermediate and high risk, respectively.
3. Statistical Analyses
Data for a continuous variable were presented as mean (SD), and as number and percentages for categorical variables. Chi-square and student’s T test were used for comparison of individuals with and without metabolic syndrome. A p value of <0.05 was taken as significant.
4. Results
One hundred and eighty-nine women participated in the screening exercise. Their mean age was 53.50 ± 9.64, with a range of 32–80 years, and about two thirds (58.2%) were between 46 and 60 years. About equal proportions were civil servants and petty trader 41.3% and 39.2%, respectively, while 16 (8.5%) practiced subsistence farming as an occupation (Table 1).
Table 1.
Socio-demography characteristic of the participants.
| Characteristic | All Participants N (%) | Individuals with MetS N (%) | Individuals without MetS N (%) |
|---|---|---|---|
| Occupation | |||
| House wife | 6 (3.2) | - | 6 (3.2) |
| Business | 74 (39.2) | 6 (3.2) | 68 (36) |
| Civil servant | 78 (41.3) | 13 (6.9) | 65 (34.4) |
| Farming | 16 (8.5) | - | 16 (8.5) |
| Clergy | 15 (7.9) | - | 15 (7.9) |
| Average monthly income | |||
| <90 Dollars | 114 (60.3) | 8 (4.2) | 106 (56.1) |
| 90–250 | 36 (19.0) | 3 (1.6) | 33 (17.5) |
| 251–500 | 35 (18.5) | 8 (4.2) | 27 (14.3) |
| >500 | 4 (2.1) | - | 4 (2.1) |
| Educational attainment | |||
| None | 10 (5.3) | 1 (0.5) | 9 (4.8) |
| Primary | 48 (25.4) | 5 (2.6) | 43 (22.8) |
| Secondary | 61 (32.3) | 6 (3.2) | 55 (29.1) |
| Tertiary | 59 (31.2) | 5 (2.9) | 54 (28.6) |
| Others | 11 (5.8) | 2 (1.1) | 9 (4.8) |
MetS = Metabolic syndrome.
As illustrated in Table 1, almost equal numbers of participants attained tertiary and secondary education; fifty-nine (31.2%) and 61 (32.3%), respectively. About two thirds of participants (60.3%) live on less than $US 90 per month, while only 4 (2.1%) earn more than $US 500 per month (Table 1).
The mean systolic and diastolic blood pressures were 131.80 ± 30.17 and 78.28 ± 13.58 mmHg, respectively. The mean body mass index (BMI) was 27.11 ± 6.84. The waist circumference, hip circumference and the waist-hip ratio were 91.64 ± 11.19 cm, 105.47 ± 13.64 cm and 0.87 ± 0.07, respectively.
The mean fasting blood sugar was 5.40 ± 1.76 mmol, while the values of fasting lipid profiles were as follows: Total cholesterol 5.43 ± 1.06 mmol/L and HDL-cholesterol 1.62 ± 0.47 mmol/L, TG 1.14 ± 0.46 mmol/L, LDL-cholesterol 3.28 ± 0.48 mmol/L (Table 2).
Table 2.
Clinical and laboratory parameters among the participants.
| Characteristic | All Participants | Individuals with MetS | Individuals without MetS | p Value |
|---|---|---|---|---|
| Age | 53.5 | 54.33 ± 10.55 | 53.24 ± 9.35 | |
| Weight (kg) | 70.12 ± 13.12 | 70.41 ± 12.47 | 70.02 ± 13.35 | |
| BMI (kg/m2) | 27.97 ± 4.79 | 26.98 ± 2.90 | 27.30 ± 6.83 | |
| Waist hip ratio | 0.87 ± 0.074 | 0.88 ± 0.059 | 0.86 ± 0.78 | |
| Tchol (mmol/L) | 5.43 ± 1.06 | 5.47 ± 0.78 | 5.41 ± 1.14 | |
| TG (mmol/L) | 1.14 ± 0.46 | 1.25 ± 0.43 | 1.11 ± 0.46 | |
| HDLchol (mmol/L) | 1.62 ± 0.47 | 1.50 ± 0.48 | 1.66 ± 0.47 | |
| LDLchol (mmol/L) | 3.28 ± 0.48 | 3.40 ± 0.70 | 3.23 ± 0.90 | |
| BP (mmHg) | ||||
| Systolic | 131.80 ± 30.17 | 152.39 ± 41.05 | 125.17 ± 22.13 | |
| Diastolic | 78.28 ± 13.58 | 85.54 ± 13.91 | 75.94 ± 12.66 | |
| FBS (mmol) | 5.40 ± 1.76 | 6.07 ± 2.82 | 5.18 ± 1.17 | |
| Categorical Data | ||||
| FRS ≤ 10% | 187 (98.9) | 44 (23.3) | 143 (75.7) | |
| FRS 10%–20% | 2 (1.1) | 2 (1.1) | - | 0.012 |
MetS = metabolic syndrome; BMI = body mass index; Tchol = total cholesterol; HDLchol = high density cholesterol; LDLchol = low density cholesterol; FRS = Framingham risk score; FBS = fasting blood sugar.
Fifty-three (28.0%) were either on antihypertensive medications or had systolic BP ≥ 130 or diastolic BP ≥ 85 mmHg, while 22 (11.6%) had fasting glucose level diagnostic of diabetes mellitus or on medication for diabetes mellitus. Eighty (42.3%) participants were overweight, while 55 (29.1%) and 6 (3.2%) had normal BMI and were underweight, respectively. The proportions of mild, moderate and severe obesity were 33 (17.5%), 10 (5.3%) and 5 (2.6%), respectively.
Metabolic syndrome was present in 46 (24.3%) of the participants. Individuals with metabolic syndrome were significantly more likely to have increased FRS, p = 0.012. Overall, 187 (98.9%) were in the low-risk category and only 2 (1.1%) were in moderate risk category group. Among those with metabolic syndrome, 44 (23.3%) had 10-year cardiovascular risk <10%, only 2 (1.1%) had 10%–20% risk, and none were in the high-risk category according to FRS.
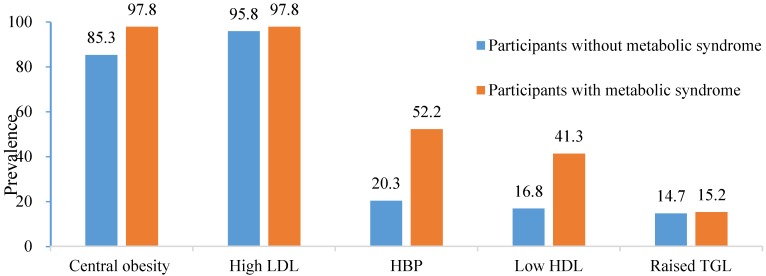
There was a significant difference in the mean FRS between participant with and without metabolic syndrome (13.52 versus 10.29 p = 0.025). Among the participants with metabolic syndrome, central obesity and high LDLchol were the prevalent disorders (Figure 1).
Figure 1.
Prevalence of individual components of metabolic syndrome in the entire study population and in the participants with metabolic syndrome.
Within the 45–60-year age group, 110 (58.8%) were in the low-risk category, while a lower prevalence (19.8% and 21.4%) was found in the 31–45 years and >60 years age groups, respectively.
5. Discussion
Women suffer from the pandemic of cardiovascular diseases (CVD). It has been documented that heart disease is the leading cause of death in women in resource-rich countries, with a worse outlook in developing countries [19]. CVD is often thought to be a disease of affluence, but mortality rates in women over the age of 60 are more than double in low- and middle-income countries than in high-income countries [20].
The first women-specific clinical recommendations for the prevention of CVD were published in 1999 [21], with a modification/reviewed in 2007. The guidelines emphasize the importance of pre-clinical detection of disease to identify asymptomatic individuals at high risk, with the sole aim of benefit from early intervention [22].
The prevalence of metabolic syndrome in this study was 24.3%. This is comparable to the 26% reported in a survey conducted among urban Asian Indians [23], and the report of Ogbu et al. from Nigeria [24].
A systematic review designed to estimate the overall distribution of Cardiometabolic syndrome in Nigeria by Oguoma et al. reported a prevalence of 28.1% using IDF criteria [25]. Similar prevalences were documented in reports from other studies [26,27,28,29].
The present study contrasts with the report of Adediran et al., who reported 7.7% and 14.9% prevalence rates of metabolic syndrome among rural and urban dwellers, respectively, in Abuja, Nigeria [30]. Olajire et al. reported the prevalence of metabolic syndrome to be 11.8% among the participants from three rural towns in Southwestern Nigeria [31].
In rural areas, the prevalence of the syndrome remains considerably lower, as reported by many studies. People with a traditional lifestyle in rural communities engage in daily physical activity and consume less energy-dense foods [30,32]. In addition, the observed discrepancy in the various studies might be due to differences in metabolic syndrome definitional criteria [33,34] and differences in genetic predisposition, various lifestyle patterns, nutritional behavior, as well as cultural differences [35,36,37,38].
It is also noteworthy that our study population was mainly women with a low socio-economic status, as more than one-third of them were petty traders, whose monthly incomes are less than $US 90.
However, there was a high prevalence of risk factors for metabolic syndrome among the participants, similar to those obtained in Western countries. This may explain the comparable rate of metabolic syndrome observed in our study.
Our observed prevalence falls within the global prevalence of this phenomenon, which varies from 14.2% to 24.0% [37]. Reports from Africa have shown that metabolic syndrome is more common in females and increases with age and urban dwelling [32,37,39,40].
Our present study, which involved asymptomatic subjects, has demonstrated that, among the subjects with metabolic syndrome, 44 (95.7%) had 10-year cardiovascular risk <10% and only 2 (4.3%) had >10% risk.
We observed that individuals with metabolic syndrome were significantly more likely to have increased FRS. None of the participants were in the high-risk category according to FRS. While many have reported FRS as a better predictor of coronary heart disease and stroke than metabolic syndrome [41], previous studies [41,42,43,44,45,46] have shown inconsistencies in the utility of metabolic syndrome and FRS for prediction of cardiovascular risk. It has yet to be determined whether one predicting tool is superior to the other in assessing cardiovascular risk among patients. However, both metabolic syndrome and FRS can be effectively used for predicting the long-term appearance of cardiovascular events, bearing in mind some of the potential limitations of each tool.
The findings from this study are consistent with other research, with respect to the prevalence of FRS. It is noteworthy that the majority of our study population was apparently normal individuals, with no history of smoking, although about 29% and 5.8% of them had family or personal history of hypertension and stroke, respectively.
Zarich et al. [47], in their assessment of the prevalence of metabolic syndrome and estimates of global risk using the Framingham Risk Score in young subjects with acute myocardial infarction, documented that two thirds of their subjects had metabolic syndrome. Their study also showed that 62% of the patients had the lowest Framingham risk category, and only 28% of subjects with MS had a Framingham Risk Score greater than 20%.
Yousefzadeh et al. [48] showed that 74.3% of their subjects with MetS were low-risk, 18.1% were intermediate-risk, and 7.6% were high risk for 10-year CVD. It was concluded in their report that there was a significant association between the presence of metabolic syndrome and a high risk for cardiovascular disease, based on FRS. In the same study, women with metabolic syndrome were found to have a higher risk of development of cardiovascular events compared to men. Similarly, a meta-analysis examining the association between the metabolic syndrome and risk of cardiovascular disease, conducted by Galassi et al. [49], showed that women were at higher risk of cardiovascular disease associated with the metabolic syndrome.
6. Conclusions
The prevalence of metabolic syndrome in these cohorts of semi-urban women was comparable to the global rate, despite their low economic status and lifestyles. Individuals with metabolic syndrome were significantly more likely to have cardiovascular disease than persons without metabolic syndrome, thus emphasizing the need for risk stratification and prompt management. As Nigeria is witnessing tremendous socio-economic changes and rural-urban migration, a call for urgent action to combat the growing epidemic of metabolic syndrome is advocated.
7. Limitations
The use of cross-sectional data could not help to examine the causal pathways and the generalization of the result obtained. Our data analysis was restricted to providing a snapshot of the prevalence of metabolic syndrome and evaluating cardiovascular risk among semi-urban women. The sample was not randomly selected, which could affect the actual prevalence of metabolic syndrome in the study population, albeit, the volunteers were asymptomatic. Selection bias might have accounted for the observed high prevalence of metabolic syndrome among these apparently healthy volunteers, as those who perceived they could be at risk were more likely to present themselves, having listened to the health-awareness talk earlier.
Abbreviations
The following abbreviations are used in this manuscript:
| FRS | Framingham risk score |
| MetS | Metabolic syndrome |
| BMI | body mass index |
| Tchol | total cholesterol |
| HDLchol | high density cholesterol |
| LDLchol | low density cholesterol |
| CVD | cardiovascular disease |
Author Contributions
Ayokunle S. Dada and Daisi D. Ajayi, Taiwo H. Raimi conceived and designed the study; Daisi D. Ajayi performed the laboratory analysis; Olusegun A. Aremu, Eyitayo E. Emmanuel enter the data and contributed to manuscript writing. Ayokunle S. Dada and Peter O. Areo analyzed the data and wrote the paper. Olusola O. Odu contributed to literature search and writing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Banerjee M., Cruickshank J. Pregnancy as the prodrome to vascular dysfunction and cardiovascular risk. Nat. Clin. Pract. Cardiovasc. Med. 2006;3:596–603. doi: 10.1038/ncpcardio0683. [DOI] [PubMed] [Google Scholar]
- 2.Rapeport N. Cardiovascular disease prevention in women: Are we up to date? Cardiovasc. J. Afr. 2011;22:119–120. [PMC free article] [PubMed] [Google Scholar]
- 3.Ford E.S., Ajani U.A., Croft J.B., Critchley J.A., Labarthe D.R., Kottke T.E., Giles W.H., Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N. Engl. J. Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . World Health Statistics 2010. World Health Organization; Geneva, Switzerland: 2010. [Google Scholar]
- 5.Gaziano T.A. Cardiovascular disease in the developing world and its cost-effective management. Circulation. 2005;112:3547–3553. doi: 10.1161/CIRCULATIONAHA.105.591792. [DOI] [PubMed] [Google Scholar]
- 6.Alwan A. Global Status Report on Noncommunicable Diseases 2010. World Health Organization; Geneva, Switzerland: 2011. [Google Scholar]
- 7.Kelly B.B., Fuster V. Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health. National Academies Press; Washington, DC, USA: 2010. [PubMed] [Google Scholar]
- 8.Mathers C.D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray C.J., Lopez A.D. Alternative projections of mortality and disability by cause 1990–2020: Global burden of disease study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 10.Kaur J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Wenger N.K. Women and coronary heart disease: A century after Herrick understudied, underdiagnosed, and undertreated. Circulation. 2012;126:604–611. doi: 10.1161/CIRCULATIONAHA.111.086892. [DOI] [PubMed] [Google Scholar]
- 12.Wilson P.W., D’Agostino R.B., Levy D., Belanger A.M., Silbershatz H., Kannel W.B. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.CIR.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 13.Grundy S.M., Bazzarre T., Cleeman J., D’Agostino R.B., Hill M., Houston-Miller N., Kannel W.B., Krauss R., Krumholz H.M., Lauer R.M. Prevention conference V beyond secondary prevention: Identifying the high-risk patient for primary prevention: Medical office assessment: Writing group I. Circulation. 2000;101:e3–e11. doi: 10.1161/01.CIR.101.1.e3. [DOI] [PubMed] [Google Scholar]
- 14.Akintunde A., Salawu A., Opadijo O. Prevalence of traditional cardiovascular risk factors among staff of Ladoke Akintola University of Technology, Ogbomoso, Nigeria. Niger. J. Clin. Pract. 2015;17:750–755. doi: 10.4103/1119-3077.144390. [DOI] [PubMed] [Google Scholar]
- 15.Ejim E., Okafor C., Emehel A., Mbah A., Onyia U., Egwuonwu T., Akabueze J., Onwubere B. Prevalence of cardiovascular risk factors in the middle-aged and elderly population of a Nigerian rural community. J. Trop. Med. 2011;2011:308687. doi: 10.1155/2011/308687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oguoma V.M., Nwose E.U., Skinner T.C., Digban K.A., Onyia I.C., Richards R.S. Prevalence of cardiovascular disease risk factors among a Nigerian adult population: Relationship with income level and accessibility to CVD risks screening. BMC Public Health. 2015;15:397. doi: 10.1186/s12889-015-1709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warnick G.R., Knopp R.H., Fitzpatrick V., Branson L. Estimating low-density lipoprotein cholesterol by the friedewald equation is adequate for classifying patients on the basis of nationally recommended cutpoints. Clin. Chem. 1990;36:15–19. [PubMed] [Google Scholar]
- 18.Alberti G., Zimmet P., Shaw J., Grundy S. IDF Worldwide Definition of the Metabolic Syndrome. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 19.Gholizadeh L., Davidson P. More similarities than differences: An international comparison of CVD mortality and risk factors in women. Health Care Women Int. 2007;29:3–22. doi: 10.1080/07399330701723756. [DOI] [PubMed] [Google Scholar]
- 20.Mathers C., Fat D.M., Boerma J.T. The Global Burden of Disease: 2004 Update. World Health Organization; Geneva, Switzerland: 2008. [Google Scholar]
- 21.Mosca L., Grundy S.M., Judelson D., King K., Limacher M., Oparil S., Pasternak R., Pearson T.A., Redberg R.F., Smith S.C., Jr., et al. Guide to preventive cardiology for women. AHA/ACC scientific statement consensus panel statement. Circulation. 1999;99:2480–2484. doi: 10.1161/01.CIR.99.18.2480. [DOI] [PubMed] [Google Scholar]
- 22.Mosca L., Banka C.L., Benjamin E.J., Berra K., Bushnell C., Dolor R.J., Ganiats T.G., Gomes A.S., Gornik H.L., Gracia C., et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115:1481–1501. doi: 10.1161/CIRCULATIONAHA.107.181546. [DOI] [PubMed] [Google Scholar]
- 23.Deepa M., Farooq S., Datta M., Deepa R., Mohan V. Prevalence of metabolic syndrome using WHO, ATPIII and IDF definitions in Asian Indians: The chennai urban rural epidemiology study (cures-34) Diabetes Metab. Res. Rev. 2007;23:127–134. doi: 10.1002/dmrr.658. [DOI] [PubMed] [Google Scholar]
- 24.Ogbu I., Chukwukelu E. Prevalence of metabolic syndrome using weight and weight indices in an apparently healthy Nigerian population. Int. J. Med. Biomed. Res. 2013;1:141–146. doi: 10.14194/ijmbr.129. [DOI] [Google Scholar]
- 25.Oguoma V., Nwose E., Richards R. Prevalence of cardio-metabolic syndrome in Nigeria: A systematic review. Public Health. 2015;129:413–423. doi: 10.1016/j.puhe.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Park Y., Zhu S., Palaniappan L., Heshka S., Carnethon M.R., Heymsfield S.B. The metabolic syndrome: Prevalence and associated risk factor findings in the U.S. population from the third national health and nutrition examination survey, 1988–1994. Arch. Intern. Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramos R.G., Olden K. The prevalence of metabolic syndrome among U.S. women of childbearing age. Am. J. Public Health. 2008;98:1122–1127. doi: 10.2105/AJPH.2007.120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran A., Gelaye B., Girma B., Lemma S., Berhane Y., Bekele T., Khali A., Williams M.A. Prevalence of metabolic syndrome among working adults in Ethiopia. Int. Hypertens. 2011;2011:193719. doi: 10.4061/2011/193719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh J.-Y., Hong Y.S., Sung Y.-A., Barrett-Connor E. Prevalence and factor analysis of metabolic syndrome in an urban Korean population. Diabetes Care. 2004;27:2027–2032. doi: 10.2337/diacare.27.8.2027. [DOI] [PubMed] [Google Scholar]
- 30.Adediran O., Akintunde A.A., Edo A.E., Opadijo O.G., Araoye A.M. Impact of urbanization and gender on frequency of metabolic syndrome among native Abuja settlers in Nigeria. J. Cardiovasc. Dis. Res. 2012;3:191–196. doi: 10.4103/0975-3583.98890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adegoke O.A., Adedoyin R.A., Balogun M.O., Adebayo R.A., Bisiriyu L.A., Salawu A.A. Prevalence of metabolic syndrome in a rural community in Nigeria. Metab. Syndr. Relat. Disord. 2010;8:59–62. doi: 10.1089/met.2009.0037. [DOI] [PubMed] [Google Scholar]
- 32.Ulasi I.I., Ijoma C.K., Onodugo O.D. A community-based study of hypertension and cardio-metabolic syndrome in semi-urban and rural communities in Nigeria. BMC Health Serv. Res. 2010;10:1–6. doi: 10.1186/1472-6963-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonora E., Kiechl S., Willeit J., Bonadonna R., Muggeo M. The Metabolic Syndrome in Subjects Aged 40–79 from the Bruneck Study. Epidemiology and Cardiovascular Risk. American Diabetes Association; Alexandria, VA, USA: 2001. p. A203. [Google Scholar]
- 34.Rantala A., Kauma H., Lilja M., Savolainen M., Reunanen A., Kesäniemi Y. Prevalence of the metabolic syndrome in drug-treated hypertensive patients and control subjects. J. Intern. Med. 1999;245:163–174. doi: 10.1046/j.1365-2796.1999.00429.x. [DOI] [PubMed] [Google Scholar]
- 35.Oldroyd J., Banerjee M., Heald A., Cruickshank K. Diabetes and ethnic minorities. Postgrad. Med. J. 2005;81:486–490. doi: 10.1136/pgmj.2004.029124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruno G., Merletti F., Biggeri A., Bargero G., Ferrero S., Runzo C., Cerai S.P., Pagano G., Cavallo-Perin P. Metabolic syndrome as a predictor of all-cause and cardiovascular mortality in type 2 diabetes the casale monferrato study. Diabetes Care. 2004;27:2689–2694. doi: 10.2337/diacare.27.11.2689. [DOI] [PubMed] [Google Scholar]
- 37.Ford E.S., Giles W.H., Dietz W.H. Prevalence of the metabolic syndrome among U.S. adults: Findings from the third national health and nutrition examination survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 38.Bender R., Jöckel K.-H., Richter B., Spraul M., Berger M. Body weight, blood pressure, and mortality in a cohort of obese patients. Am. J. Epidemiol. 2002;156:239–245. doi: 10.1093/aje/kwf015. [DOI] [PubMed] [Google Scholar]
- 39.Vorster H.H. The emergence of cardiovascular disease during urbanisation of Africans. Public Health Nutr. 2002;5:239–243. doi: 10.1079/PHN2001299. [DOI] [PubMed] [Google Scholar]
- 40.Njelekela M.A., Mpembeni R., Muhihi A., Mligiliche N.L., Spiegelman D., Hertzmark E., Liu E., Finkelstein J.L., Fawzi W.W., Willett W.C., et al. Gender-related differences in the prevalence of cardiovascular disease risk factors and their correlates in urban Tanzania. BMC Cardiovasc. Disord. 2009;9:30. doi: 10.1186/1471-2261-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wannamethee S.G., Shaper A.G., Lennon L., Morris R.W. Metabolic syndrome vs. Framingham risk score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch. Intern. Med. 2005;165:2644–2650. doi: 10.1001/archinte.165.22.2644. [DOI] [PubMed] [Google Scholar]
- 42.Sattar N., Gaw A., Scherbakova O., Ford I., O’Reilly D.S.J., Haffner S.M., Isles C., Macfarlane P.W., Packard C.J., Cobbe S.M. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the west of Scotland coronary prevention study. Circulation. 2003;108:414–419. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 43.Gami A.S., Witt B.J., Howard D.E., Erwin P.J., Gami L.A., Somers V.K., Montori V.M. Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J. Am. Coll. Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 44.Najarian R.M., Sullivan L.M., Kannel W.B., Wilson P.W., D’Agostino R.B., Wolf P.A. Metabolic syndrome compared with type 2 diabetes mellitus as a risk factor for stroke: The Framingham offspring study. Arch. Intern. Med. 2006;166:106–111. doi: 10.1001/archinte.166.1.106. [DOI] [PubMed] [Google Scholar]
- 45.Grundy S.M., Brewer H.B., Cleeman J.I., Smith S.C., Lenfant C. Definition of metabolic syndrome report of the national heart, lung, and blood institute/american heart association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 46.Stern M.P., Williams K., González-Villalpando C., Hunt K.J., Haffner S.M. Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care. 2004;27:2676–2681. doi: 10.2337/diacare.27.11.2676. [DOI] [PubMed] [Google Scholar]
- 47.Zarich S., Luciano C., Hulford J., Abdullah A. Prevalence of metabolic syndrome in young patients with acute mi: Does the Framingham risk score underestimate cardiovascular risk in this population? Diabetes Vasc. Dis. Res. 2006;3:103–107. doi: 10.3132/dvdr.2006.012. [DOI] [PubMed] [Google Scholar]
- 48.Yousefzadeh G., Shokoohi M., Najafipour H., Shadkamfarokhi M. Applying the Framingham risk score for prediction of metabolic syndrome: The kerman coronary artery disease risk study, Iran. ARYA Atheroscler. 2015;11:179–185. [PMC free article] [PubMed] [Google Scholar]
- 49.Galassi A., Reynolds K., He J. Metabolic syndrome and risk of cardiovascular disease: A meta-analysis. Am. J. Med. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]