Abstract
Murraya paniculata (L.) Jack, a small tropical evergreen shrub growing in Nepal, has numerous uses in traditional medicine for treatment of abdominal pain, diarrhea, stomach ache, headache, edema, thrombosis, and blood stasis. The present study investigated the chemical composition and bioactivities of the leaf essential oil from M. paniculata from Nepal. The essential oil from leaves was obtained by hydrodistillation and a detailed chemical analysis was conducted by gas chromatography-mass spectrometry (GC-MS). The essential oil was screened for antimicrobial activity using the microbroth dilution test, for nematicidal activity against Caenorhabditis elegans, and for lethality against brine shrimp (Artemia salina). A total of 76 volatile components were identified from the essential oil. The major components were methyl palmitate (11.1%), isospathulenol (9.4%), (E,E)-geranyl linalool (5.3%), benzyl benzoate (4.2%), selin-6-en-4-ol (4.0%), β-caryophyllene (4.0%), germacrene B (3.6%), germacrene D (3.4%), and γ-elemene (3.2%). The essential oil showed no antibacterial activity, marginal antifungal activity against Aspergillus niger (MIC = 313 μg/mL), a moderate activity against A. salina (LC50 = 41 μg/mL), and a good nematicidal activity against C. elegans (LC50 = 37 μg/mL).
Keywords: Murraya paniculata, GC-MS, essential oil composition, hierarchical cluster analysis, antifungal, brine shrimp lethality, nematicidal
1. Introduction
The genus Murraya (Rutaceae) is made up of about 14 species. Murraya paniculata (L.) Jack is a small tropical evergreen shrub, native to the tropical and subtropical parts of the world, including southern China, Taiwan, India, Nepal, Northeastern Pakistan, Sri Lanka, Southeastern Asia (i.e., Cambodia, Laos, Myanmar, Thailand, Vietnam, Indonesia, Malaysia, and the Philippines), and Northern Australia. It is widely naturalized in the southern part of Australia, Southeastern USA and Central America. M. paniculata is also known as Chalcas exotica, Chalcas paniculata, and Camunium exoticum [1]. M. paniculata is commonly known as orange jasmine or mock orange. In Nepal, it is known as bajardante [2]. The average shrub can grow up to 7 m high. Morphologically, the plant can be distinguished by its alternate, glabrous, and glossy leaves that are once-compound, occurring in 3–7 oddly pinnate leaflets. Leaflets are elliptic to cuneate-obovate, 2–9 cm long × 1.5–6 cm wide [3,4]. M. paniculata blooms throughout the year. Inflorescences are terminal, corymbose, few-flowered, and dense. Flowers are pentamerous, bisexual, and sweetly fragrant. Petals are 12–18 mm long, narrowly elliptic to oblanceolate, curved backwards, and white to fading cream in color. The fruit is a fleshy berry, oblong-ovoid, red to orange, and grows up to 2.5 cm in length [3,4].
For many years, M. paniculata has been used as an ornamental and a medicinal plant [5]. Due to its hardiness and wide range of soil tolerance, orange jasmine is commonly used as a hedge. The leaves have been used as a food additive in many Indian and Malay dishes due to their strong fragrance [6]. M. paniculata is commonly used in traditional medicine for treatment of diarrhea, abdominal pain, stomach ache, dysentery, headache, edema, thrombosis, and stasis of blood. Moreover, it was used as a detoxication agent, anticonvulsant, local anesthetic, and expectorant. Previous reports have shown that the extracts from bark and leaf are stimulant and astringent, and had antinociceptive [7], anti-inflammatory, antidiarrheal [8], antitrypanocidal, antidiabetic, antimalarial, antibacterial, antifungal, and antioxidant activities [9,10]. The essential oil was reported to possess anti-amebic activity [11]. Pangnakorn and Poonpaiboonpipattana reported that the aqueous extract of M. paniculata leaves possesses phytotoxic effects on seed germination and seedling growth of Bidens pilosa, Amarathus spinosus, Echinochloa crusgalli, and Chloris barbata [12].
M. paniculata has been the subject of several phytochemical studies. The leaf extract was reported to contain coumarins [13,14] and flavonoids [15,16,17]. The components of leaf essential oils of M. paniculata from Bangladesh [18], China [19], Cuba [20], and Nigeria [5] have been previously reported. However, many factors, including provenance, weather, soil conditions, time of harvest, and the drying technique, can change the chemical composition and yield of essential oils [21]. M. paniculata has been described as synonymous with M. exotica [22], but this has been controversial and has recently been challenged [23]. The current study was conducted to investigate the composition of the leaf essential oil of M. paniculata from Nepal as well as its biological activities.
2. Materials and Methods
2.1. Plant Material
Leaves of Murraya paniculata, collected from city of Biratnagar (26°28′ N, 87°16′ E, and 1072 m above sea level), Morang district, Koshi Zone, Nepal in May 2011, were used in this study. The plant material was identified by Tilak P. Gautam and a voucher specimen has been deposited in the Botany Department, MMAMC Campus, Biratngar, Nepal. The essential oil was obtained from fresh leaf samples (100 g) that were crushed and hydrodistilled using a Clevenger-type apparatus for 4 h. The clear pale-yellow essential oil (1.0 g) produced was stored at 4 °C until analyzed (July 2011).
2.2. Gas Chromatographic–Mass Spectral Analysis
The essential oil of M. paniculata was analyzed by GC-MS using an Agilent 6890 GC with Agilent 5973 mass selective detector (Agilent Technologies, Santa Clara, CA, USA), an HP-5ms fused silica capillary column and an Agilent ChemStation data system [MSD, operated in the EI mode (electron energy = 70 eV), scan range = 40–400 amu, and scan rate = 3.99 scans/s], and an Agilent ChemStation data system as previously described [24]. The GC column was an HP-5ms fused silica capillary with a (5% phenyl)-polymethylsiloxane stationary phase, film thickness of 0.25 μm, a length of 30 m, and an internal diameter of 0.25 mm. The carrier gas was helium with a column head pressure of 48.7 kPa and a flow rate of 1.0 mL/min. Inlet temperature was 200 °C and interface temperature was 280 °C. The GC oven temperature program was used as follows: 40 °C initial temperature, hold for 10 min; increased at 3 °C/min to 200 °C; increased 2°/min to 220 °C. A 1% w/v solution of the sample in chloroform was prepared and 1 μL was injected using a 10:1 split ratio. Identification of the oil components was based on their retention indices (RI) and by comparison of their mass spectral fragmentation patterns with those reported in the literature [25].
2.3. Antimicrobial Screening
The essential oil of M. paniculata was screened for antimicrobial activity against Bacillus cereus (ATCC No. 14579), Aspergillus niger (ATCC No. 16888), and Candida albicans (ATCC No. 10231). The minimum inhibitory concentration (MIC) was determined using the microbroth dilution technique as previously reported [26]. For B. cereus, a dilution of the essential oil were prepared in cation-adjusted Mueller Hinton broth (CAMBH) beginning with 50 μL of a 1% w/w solution of the sample in dimethylsulfoxide (DMSO) plus 50 μL CAMBH. The essential oil solution was serially diluted (1:1) in CAMBH in a 96-well plate. Organisms at a concentration of approximately 1.5 × 108 colony forming units (CFU)/mL were added to each well. Plates were incubated at 37 °C for 24 h; the final minimum inhibitory concentration (MIC) was determined as the lowest concentration without turbidity. Gentamicin was used as a positive antibiotic control. Antifungal activity against C. albicans was determined as above using yeast-nitrogen base growth medium with approximately 7.5 × 107 CFU/mL; amphotericin B was the positive control. Antifungal activity against A. niger was determined as above using potato dextrose broth inoculated with A. niger hyphal culture diluted to a McFarland turbidity of 1.0; amphotericin B was the positive control.
2.4. Nematicidal Assay
A nematicidal assay using Caenorhabditis elegans was done using a modification of the procedure of Park and co-workers [27]. Briefly, a 1% solution of M. paniculata leaf oil in dimethylsulfoxide (DMSO) was used to make dilutions for the sample solutions. The sample solutions were prepared in sterile water beginning with 50 µL of the 1% essential oil solution mixed in 50 µL sterile water. This sample solution was serially diluted (1:1) with sterile water in a 96-well plate. Into each well, 10–30 C. elegans (mixtures of juvenile and adult nematodes, male:female:juvenile ~1:1:2) per 50 µL of sample solution were added. Sterile water and serially diluted DMSO were used as controls. The dead and living nematodes were counted after 24 h using a microscope. Dead nematodes were identified by their immobility and straight body, even after transfer to clean water. Mean lethal concentration (LC50) values were determined using the method of Reed and Muench [28].
2.5. Brine Shrimp Lethality Assay
The brine shrimp (Artemia salina) lethality test was done using a modification of the procedure of McLaughlin [29]. A. salina eggs were hatched in a sea salt solution (Instant Ocean®, Spectrum Brands, Inc. Madison, WI, USA) (38 g/L) with an incandescent light bulb as the heat source. After 48 h, the newly hatched nauplii were counted using a micropipette and transferred to 20 mL vials. A total of nine vials, each containing 10 A. salina nauplii in 10 mL of sea salt solution (the same as the hatching solution) were prepared. Of these vials, three were labeled as controls with one vial containing no DMSO, a second vial containing 10 μL of DMSO, and the third vial containing 100 μL DMSO. A second set of three replicate vials contained 10 μL of 1% essential oil solution in DMSO, and the remaining three vials were prepared by adding 100 μL of 1% essential oil solution in DMSO. After 24 h, surviving A. salina nauplii were counted in each vial and LC50 values were determined using the Reed-Muench method [28].
2.6. Hierarchical Cluster Analysis
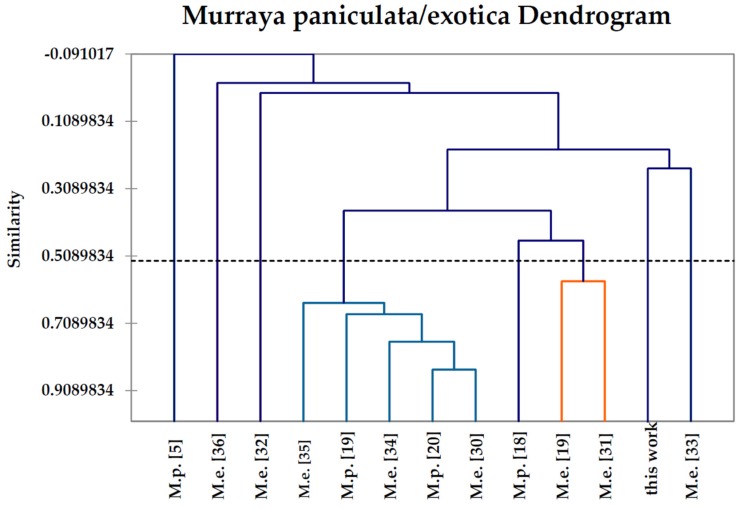
A total of 12 M. paniculata [5,18,19,20] and M. exotica [19,30,31,32,33,34,35,36] leaf essential oil compositions from the published literature, as well as the composition from this study, were treated as operational taxonomic units (OTUs). The percentage composition of 35 major essential oil components (α-pinene, methyl salicylate, β-cyclocitral, δ-elemene, α-cubebene, α-copaene, β-cubebene, β-elemene, β-caryophyllene, cedrene, (E)-α-bergamotene, β-humulene, (E)-β-farnesene, α-humulene, alloaromadendrene, germacrene D, germacrene B, bicyclogermacrene, α-zingiberene, trans-β-guaiene, γ-cadinene, cubebol, δ-cadinene, elemol, (E)-nerolidol, spathulenol, caryophyllene oxide, viridiflorol, 1,10-di-epi-cubenol, 1-epi-cubenol, τ-cadinol, β-eudesmol, α-cadinol, benzyl benzoate, and methyl palmitate) was used to determine the chemical relationship between the various Murraya essential oil samples by agglomerative hierarchical cluster (AHC) analysis using the XLSTAT software, version 2015.4.01 (Addinsoft SARL, Paris, France). Pearson correlation was selected as a measure of similarity, and the unweighted pair-group method with arithmetic average (UPGMA) was used for cluster definition. The resulting dendrogram is shown in Figure 1.
Figure 1.
Dendrogram obtained from the agglomerative hierarchical cluster analysis of 13 Murraya paniculata leaf essential oil samples.
3. Results and Discussion
The M. paniculata leaf essential oil composition is shown in Table 1. The leaf oil was mainly composed of methyl palmitate (11.05%), isospathulenol (9.44%), (E,E)-geranyl linalool (5.29%), benzyl benzoate (4.20%), selin-6-en-4-ol (4.01%), β-caryophyllene (3.97%), germacrene B (3.62%), germacrene D (3.39%), and γ-elemene (3.19%) ,with other minor constituents (<3%). The current study revealed that the essential oil composition and percentages are significantly different from the previously published reports from Bangladesh and China. The major constituents of the leaf oil of M. paniculata of Bangladeshi origin were caryophyllene oxide (16.6%), β-caryophyllene (11.8%), spathulenol (10.2%), β-elemene (8.9%), germacrene D (6.9%), and methylene-6-4-(1-propenylidene)cyclooctene (6.4%) [18], while the main components of Chinese M. paniculata essential oil were β-caryophyllene (23.3%), spathulenol (16.1%), (E)-α-bergamotene (9.3%), (E)-nerolidol (4.6%), and δ-elemene (3.3%) [19]. The leaf oil of M. paniculata from Cuba was also rich in β-caryophyllene (29.8%) and spathulenol (5.1%), but had significant quantities of caryophyllene oxide (6.3%), viridiflorol (5.7%), δ-cadinene (5.6%), bicyclogermacrene (5.6%), α-humulene (5.3%), and β-cubebene (5.3%) [20]. The leaf oil of Nigerian M. paniculata was mainly composed of β-cyclocitral (22.9%), methyl salicylate (22.4%), (E)-nerolidol (11.7%), α-cubebene (7.9%), cubenol (6.8%), β-cubebene (5.8%) and isogermacrene (5.7%) [5].
Table 1.
Chemical composition of the leaf essential oil of Murraya paniculata from Nepal.
| RI a | Compound | % |
|---|---|---|
| 1100 | Linalool | 1.20 |
| 1112 | Phenylethyl alcohol | 0.73 |
| 1138 | Benzeneacetonitrile | 0.14 |
| 1189 | α-Terpineol | 0.16 |
| 1254 | Geraniol | 0.19 |
| 1290 | Indole | 1.25 |
| 1325 | p-Vinylguaiacol | 0.69 |
| 1332 | Bicycloelemene | 0.12 |
| 1334 | δ-Elemene | 3.19 |
| 1336 | Methyl anthranilate | 2.05 |
| 1391 | β-Elemene | 0.38 |
| 1398 | (Z)-Jasmone | 0.59 |
| 1418 | β-Caryophyllene | 3.97 |
| 1451 | cis-Murrola-3,5-diene | 0.25 |
| 1453 | α-Humulene | 1.10 |
| 1476 | γ-Gurjunene | 1.07 |
| 1480 | Germacrene D | 3.39 |
| 1483 | α-Curcumene | 0.84 |
| 1485 | β-Selinene | 0.16 |
| 1491 | δ-Selinene | 0.52 |
| 1496 | α-Zingiberene | 2.16 |
| 1504 | Germacrene A | 0.42 |
| 1509 | (E,E)-α-Farnesene | 2.79 |
| 1514 | Cubebol | 0.39 |
| 1521 | α-Chamigrene | 0.91 |
| 1523 | β-Sesquiphellandrene | 0.71 |
| 1549 | α-Elemol | 0.45 |
| 1556 | Germacrene B | 3.62 |
| 1564 | (E)-Nerolidol | 0.43 |
| 1571 | (3Z)-Hexenyl benzoate | 0.27 |
| 1581 | trans-Sesquisabinene hydrate | 0.16 |
| 1583 | Caryophyllene oxide | 0.40 |
| 1586 | Phenylethyl tiglate | 0.27 |
| 1593 | Spathulenol isomer | 1.39 |
| 1606 | β-Nootkatol | 0.35 |
| 1614 | Zingiberenol | 0.39 |
| 1620 | Selin-6-en-4-ol | 4.01 |
| 1624 | 1,10-di-epi-Cubenol | 1.09 |
| 1628 | Isospathulenol | 9.44 |
| 1639 | τ-Cadinol | 2.02 |
| 1644 | epi-β-Muurolol | 0.34 |
| 1647 | β-Eudesmol | 0.17 |
| 1649 | τ-Muurolol | 1.01 |
| 1651 | α-Cadinol | 1.07 |
| 1663 | Intermedeol | 0.22 |
| 1680 | Germacra-4(15),5,10(14)-trien-1α-ol | 0.12 |
| 1689 | (2Z,6Z)-Farnesol | 0.22 |
| 1713 | Eudesma-4,11-dien-2-ol | 0.42 |
| 1716 | (2E,6Z)-Farnesol | 0.26 |
| 1720 | Nuciferol | 2.13 |
| 1733 | Oplopanone | 0.20 |
| 1745 | Curcumen-12-ol | 2.33 |
| 1761 | Benzyl benzoate | 4.20 |
| 1771 | Isospathulenol isomer | 0.93 |
| 1852 | Phenylethyl octanoate | 2.39 |
| 1865 | Benzyl salicylate | 0.29 |
| 1919 | Methyl palmitate | 11.05 |
| 1953 | Phenylethyl salicylate | 0.13 |
| 1957 | Palmitic acid | 0.80 |
| 1996 | Ethyl palmitate | 0.39 |
| 2031 | (E,E)-Geranyl linalool | 5.29 |
| 2094 | Methyl linoleate | 0.73 |
| 2100 | Methyl linolenate | 1.30 |
| 2110 | Phytol | 2.11 |
| 2123 | Methyl stearate | 0.60 |
| 2130 | Osthole | 2.58 |
| 2232 | Isogeigerin | 0.24 |
| 2238 | Suberosin epoxide | 0.82 |
| 2268 | 7-Methoxy-6-(3′-metylbuta-1′,3′-dienyl)coumarin | 0.47 |
| 2270 | Muurialongin | 0.59 |
| 2407 | Minimicrolin isovalerate | 0.45 |
| 2416 | Paniculol | tr b |
| 2504 | Octyl palmitate | 0.59 |
| 2705 | Octyl stearate | 0.21 |
| 2711 | Murpaniculol senecioate | tr |
| 2828 | Squalene | 0.25 |
| Compounds Identified | 76 (91.5%) | |
a RI determined with respect to a homologous series of n-alkanes on an HP-5ms column.b tr = “trace” (<0.05%).
Since M. paniculata has often been classified as a species, if it is synonymous with M. exotica [5], it is unclear about which essential oil composition may belong to which, or if they are, indeed, separate species. Lv and co-workers [19] have treated M. paniculata and M. exotica as separate species and have examined the essential oil compositions of both. These workers found M. exotica from Guangxi Province, China, to be qualitatively similar to M. paniculata (see above), and was dominated by spathulenol (25.6%), trans-β-guaiene (13.7%), β-caryophyllene (11.7%), and bicyclogermacrene (4.1%) [19]. There is much variation in the compositions of M. exotica essential oils, however. M. exotica oil from Hainan, China, was rich in β-caryophyllene (45.5%) and cedrene (15.1%) [30], while a sample from Guangdong, China, had spathulenol (17.7%), α-pinene (13.2%), caryophyllene oxide (8.6%), and bicyclogermacrene (7.1%) as major components [31]. In order to attempt to sort out the volatile phytochemistry of Murraya paniculata/exotica, a hierarchical cluster analysis was carried out on the essential oil compositions of M. paniculata and M. exotica reported in the literature (Figure 1) [5,18,19,20,30,31,32,33,34,35,36]. The components used in the cluster analysis are summarized in Table 2 and illustrate the chemical differences between these essential oil samples. Although there are only 13 essential oil samples, too few to provide a comprehensive chemotaxonomic representation of this species, this analysis does serve to place M. paniculata leaf oil from Nepal into context with previously-reported essential oils of M. paniculata and M. exotica.
Table 2.
Components used in the hierarchical cluster analysis of Murraya paniculata/Murraya exotica leaf essential oils.
| Compound | M.p. a | M.p. | M.p. | M.p. | M.p. | M.e. b | M.e. | M.e. | M.e. | M.e. | M.e. | M.e. | M.e. |
| This | [18] | [20] | [5] | [19] | [19] | [33] | [34] | [35] | [32] | [31] | [30] | [36] | |
| α-pinene | 0 | 0 | 0 | tr | 0 | 0 | 0 | tr | 0 | 62.5 | 13.2 | 0.3 | 0 |
| Methyl salicylate | 0 | 0 | 0 | 22.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| β-Cyclocitral | 0 | 0 | 0 | 22.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| δ-Elemene | 3.2 | 3.6 | 0.4 | 0 | 3.3 | 3.4 | 5.1 | 0.2 | 0 | 0.4 | 0 | 0 | 0 |
| α-Cubebene | 0 | 3.0 | 2.2 | 7.9 | 0.9 | 0.1 | 0 | 1.0 | 6.9 | 0 | 0 | 0.4 | 0 |
| α-Copaene | 0 | 2.3 | 3.8 | 0.2 | 0 | 0.1 | 0.5 | 4.4 | 1.4 | 0.4 | 1.7 | 0.2 | 0 |
| β-Cubebene | 0 | 0 | 5.3 | 5.8 | 1.6 | 0 | 1.6 | 10.5 | 0 | 1.6 | 0 | 0 | 0 |
| β-Elemene | 0.4 | 8.9 | 0 | 0 | 0.1 | 2.0 | 0 | 0 | 0 | 0 | 0.9 | 0.1 | 7.6 |
| β-Caryophyllene | 4.0 | 11.8 | 29.8 | 0 | 23.3 | 11.7 | 9.7 | 24.1 | 20.3 | 5.2 | 4.5 | 45.5 | 7.1 |
| Cedrene | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 15.1 | 0 |
| α-(E)-Bergamotene | 0 | 0 | 0 | 0 | 9.3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| β-Humulene | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40.6 |
| (E)-β-Farnesene | 0 | 0 | 0 | 0 | 2.6 | 0 | 1.5 | 0 | 2.4 | 0 | 0 | 0 | 0 |
| α-Humulene | 1.1 | 3.1 | 5.3 | tr | 0 | 3.0 | 0.6 | 5.8 | 0 | 0.8 | 7.3 | 0.3 | t |
| Alloaromadendrene | 0 | 0 | 0 | 0 | 0.1 | 1.9 | 0 | 0 | 5.9 | 0 | 0.2 | 0 | 0 |
| GermacreneD | 3.4 | 7.0 | 4.2 | 0 | 0.5 | 2.4 | 2.6 | 11.9 | 0 | 2.1 | 0.8 | 0 | 0 |
| GermacreneB | 0 | 0 | 0 | 0 | 0 | 0 | 3.5 | 0 | 0 | 1.9 | 0 | 0 | 0.9 |
| Bicyclogermacrene | 0 | 0 | 5.6 | 0 | 1.9 | 4.1 | 0 | 11.8 | 9.6 | 0 | 7.1 | 0 | 0 |
| α-Zingiberene | 2.2 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 12.7 | 0 | 0 | 0 | 0 |
| trans-β-Guaiene | 0 | 0 | 0 | 0 | 0 | 13.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| γ-Cadinene | 0 | 0 | 2.2 | 0 | 0 | 0 | 0 | tr | 2.1 | 1.1 | 0 | 0 | 0 |
| Cubebol | 0.4 | 0 | 0 | 6.8 | 0 | 0 | 0 | 3.1 | 0 | 0 | 0 | 0 | 0 |
| δ-Cadinene | 0 | 0 | 5.6 | tr | 2.2 | 1.2 | 0 | 4.4 | 8.0 | 0.5 | 4.4 | 0 | 0 |
| α-Elemol | 0.5 | 0 | 0 | 0 | 0.2 | 1.7 | 0.2 | 1.3 | 0 | 0 | 0 | 0 | 0.1 |
| (E)-Nerolidol | 0.4 | 0 | 0 | 11.7 | 4.6 | 0.4 | 27.8 | 1.5 | 2.7 | 0 | 0 | 0 | 0 |
| Spathulenol | 0 | 10.2 | 5.1 | 3.6 | 16.1 | 25.6 | 0.9 | 1.0 | 6.3 | 0.5 | 17.7 | 4.4 | 0.1 |
| Caryophyllene oxide | 0.4 | 16.6 | 6.3 | tr | 2.8 | tr | 0.1 | 0.8 | 4.0 | 0.5 | 8.6 | 1.3 | tr |
| Viridiflorol | 0 | 2.2 | 5.7 | 0 | 0 | 0 | 0.6 | 0.4 | 0 | 0 | 0 | 0 | 0 |
| 1,10-di-epi-Cubenol | 1.1 | 2.4 | 1.9 | 0 | 0 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1-epi-Cubenol | 0 | 0 | 2.5 | 1.2 | 0.4 | 0.1 | 0 | 1.1 | 0 | 0 | 0 | 0 | 0 |
| τ-Cadinol | 2.0 | 0 | 4.3 | 3.2 | 0 | 0 | 2.2 | 0 | 0 | 0.6 | 3.6 | 0 | 0 |
| β-Eudesmol | 0.2 | 0 | 0 | 0 | 2.9 | 2.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| α-Cadinol | 1.1 | 0 | 1.7 | 0 | 0 | 0 | 0.2 | 1.4 | 0.6 | 0.3 | 0 | 0 | 0 |
| Benzyl benzoate | 4.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 24.0 |
| Methyl palmitate | 11.1 | 0 | 0 | 0 | tr | tr | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
a M.p. = Murraya paniculata; b M.e. = Murraya exotica.
The cluster analysis reveals at least eight chemotypes for the Murraya paniculata/exotica complex based on volatiles: (1) a methyl salicylate/β-cyclocitral chemotype represented by the M. paniculata sample from Nigeria [5]; (2) a β-humulene chemotype represented by the M. exotica sample from India [36]; (3) a chemotype dominated by α-pinene represented by the M. exotica sample from Egypt [32]; (4) a cluster rich in β-caryophyllene with M. paniculata samples from China [19] and Cuba [20] and M. exotica samples from China [30,35] and Cuba [34]; (5) a caryophyllene oxide/β-caryophyllene/spathulenol chemotype represented by the M. paniculata sample from Bangladesh [18]; (6) a spathulenol-rich cluster with M. exotica samples from China [19,31]; (7) a β-caryophyllene/α-zingiberene chemotype represented by the M. exotica sample from India [33]; and (8) the sample from Nepal (this work), rich in methyl palmitate. In addition to genetic variation [23], age [37], vegetative cycle stage [38], climate [39], season [40], soil composition [41], and edaphic factors [42] are among several factors responsible for the considerable variation in essential oil compositions [43,44]. Based on the observed composition, the Nepalese M. paniculata leaf oil is chemically distinct from previously-reported analyses and may represent a distinct chemotype.
The essential oil of M. paniculata was screened for potential antimicrobial activity. Based on our experience [26,45,46], we consider samples to have good antimicrobial activity with MIC < 156 μg/mL, moderate activity with MIC between 156 and 313 μg/mL, and weak activity between 313 and 625 μg/mL. Samples with MIC > 625 we consider to be inactive. Based on these criteria, M. paniculata essential oil was inactive against Bacillus cereus and Candida albicans (MIC = 2500 μg/mL) and marginally antifungal against Aspergillus niger (MIC = 313 μg/mL). Nevertheless, the antifungal activity of M. paniculata leaf oil was better than many of the essential oils we have tested [47,48], comparable to Mitracarpus scaber leaf essential oil (MIC = 313 μg/mL) [49] and Betula nigra buds essential oil (MIC = 313 μg/mL) [50], but not as effective as Pinus roxburghii cone essential oil (MIC = 39 μg/mL) [51], Cinnamomum camphora leaf essential oil (MIC = 19.5 μg/mL) [52], Curcuma longa leaf essential oil (MIC = 19.5 μg/mL) [53], or Canthium subcordatum fruit essential oil (MIC = 39 μg/mL) [54]. Although in low concentrations, α-humulene (1.1%) and germacrene D (3.4%) may contribute to the antifungal activity of M. paniculata leaf oil, both have shown activity against A. niger (MIC = 78 and 39 μg/mL, respectively, for α-humulene and germacrene D [47]). Methyl palmitate (11.1% in M. paniculata oil) has shown antifungal activity (MIC = 333 μg/mL) against Blumeria graminis [55].
M. paniculata oil showed moderate activity in the brine shrimp (Artemia salina) lethality test with LC50 value of 41 μg/mL. Essential oils showing A. salina toxicity with LC50 < 10 μg/mL are considered very active [56], 10 μg/mL < LC50 < 50 μg/mL are moderately active [51,57], and 50 μg/mL < LC50 < 100 μg/mL, weakly active. In our nematicidal activity screening against C. elegans, we have found LC50 values ranging from 18 to 1100 μg/mL (unpublished), and we consider nematicidal activities LC50 < 100 μg/mL to be very active, LC50 values between 100 and 200 μg/mL to be moderately active [52,58], between 200 and 300 μg/mL to be weakly active, and > 300 μg/mL to be inactive. Thus, M. paniculata oil was highly nematicidal to Caenorhabditis elegans (LC50 = 37 μg/mL). It is difficult to speculate as to which compound(s) in the essential oil may be responsible for the brine shrimp lethality or nematicidal activity; there are many components in the leaf oil and none are especially dominant.
4. Conclusion
The leaf essential oil of Murraya paniculata growing in Nepal has been analyzed by GC-MS and revealed this to be a distinct chemotype, rich in methyl palmitate. Biological screening of the leaf oil showed it to have good nematicidal activity, marginal activity against brine shrimp and Aspergillus niger, and inactive against bacteria. The particular chemotype of this plant could have important implication on its biological activity and traditional medicinal uses.
Acknowledgments
Prabodh Satyal is grateful to Ajaya Bhattarai, and Sanjaya Neupane for assistance in hydrodistillation of essential oil.
Abbreviations
The following abbreviations are used in this manuscript:
- GC-MS
Gas chromatography–mass spectrometry
- RI
Retention indices
- ATCC
American type culture collection
- DMSO
Dimethylsulfoxide
- LC50
Median lethal concentration
Author Contributions
P.S. conceived and designed the experiments; P.S. and N.S.D. performed the experiments; P.S. and W.N.S. analyzed the data; T.P.S. identified the plant specimen; W.N.S. contributed reagents/materials/analysis tools; N.S.D., P.S. and W.N.S. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Seidemann J. World Spice Plants: Economic Usage, Botany, Taxonomy. Springer; Berlin, Germany: 2005. [Google Scholar]
- 2.Quattrocchi U. CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology. CRC Press; Boca Raton, FL, USA: 2012. p. 1747. [Google Scholar]
- 3.Sambamurty A.V.S.S. Taxonomy of Angiosperms. I.K. International Pvt. Ltd.; New Delhi, India: 2005. [Google Scholar]
- 4.Schmelzer G.H., Gurib-Fakim A. Plant Resources of Tropical Africa 11(2). Medicinal Plants 2. PROTA Foundation; Wageningen, The Netherlands: 2013. [Google Scholar]
- 5.Olawore N.O., Ogunwande I.A., Ekundayo O., Adeleke K.A. Chemical composition of the leaf and fruit essential oils of Murraya paniculata (L.) Jack. (Syn. Murraya exotica Linn.) Flavour Fragr. J. 2005;20:54–56. doi: 10.1002/ffj.1365. [DOI] [Google Scholar]
- 6.Ng M.K., Abdulhadi-Noaman Y., Cheah Y.K., Yeap S.K., Alitheen N.B. Bioactivity studies and chemical constituents of Murraya paniculata (Linn) Jack. Int. Food Res. J. 2012;19:1307–1312. [Google Scholar]
- 7.Sharker S.M., Shahid I.J., Hasanuzzaman M. Antinociceptive and bioactivity of leaves of Murraya paniculata (L.) Jack, Rutaceae. Braz. J. Pharmacog. 2009;19:746–748. [Google Scholar]
- 8.Rahman M.A., Hasanuzzaman M., Uddin N., Shahid I.Z. Antidiarrhoeal and anti-inflammatory activities of Murraya paniculata (L.) Jack. Pharmacologyonline. 2010;3:768–776. [Google Scholar]
- 9.Sundaram M., Sivakumar, Karthikeyan, Bhuvaneshwar, Aishwarya, Thirumalai, Pennarasi Studies on in vitro antibacterial, antifungal property and antioxidant potency of Murraya paniculata. Pak. J. Nutr. 2011;10:925–928. [Google Scholar]
- 10.Narkhede M.B., Ajmire P.V., Wagh A.E. Evaluation of antinociceptive and anti-inflammatory activity of ethanol extract of Murraya paniculata leaves in experimental rodents. Int. J. Pharm. Pharmaceut. Sci. 2012;4:247–251. [Google Scholar]
- 11.Sawangjaroen N., Phongpaichit S., Subhadhirasakul S., Visutthi M., Srisuwan N., Thammapalerd N. The anti-amoebic activity of some medicinal plants used by AIDS patients in southern Thailand. Parasitol. Res. 2006;98:588–592. doi: 10.1007/s00436-005-0119-2. [DOI] [PubMed] [Google Scholar]
- 12.Pangnakorn U., Poonpaiboonpipattana T. Allelopathic potential of orange jessamine (Murraya paniculata L.) against weeds. J. Agric. Sci. Technol. 2013;3:790–796. [Google Scholar]
- 13.Aziz S.S.S.A., Sukari M.A., Rahmani M., Kitajima M., Aimi N., Ahpandi N.J. Coumarins from Murraya paniculata (Rutaceae) Malay. J. Anal. Sci. 2010;14:1–5. [Google Scholar]
- 14.Saeed S., Shah S., Mehmood R., Malik A. Paniculacin, a new coumarin derivative from Murraya paniculata. J. Asian Nat. Prod. Res. 2011;13:724–727. doi: 10.1080/10286020.2011.586343. [DOI] [PubMed] [Google Scholar]
- 15.Kinoshita T., Firman K. Highly oxygenated flavonoids from Murraya paniculata. Phytochemistry. 1996;42:1207–1210. doi: 10.1016/0031-9422(96)00058-1. [DOI] [Google Scholar]
- 16.Sukari M.A., Azziz S.S.S., Rahmani M., Ali A.M., Aimi N., Kitajima M. Polysubstituted flavonoids from the leaves of Murraya paniculata (Rutaceae) Nat. Prod. Sci. 2003;9:56–59. [Google Scholar]
- 17.Zhang Y., Li J., Zhou S.X., Tu P.F. Polymethoxylated flavonoids from the leaves of Murraya paniculata. Chin. Pharmaceut. J. 2010;45:1139–1141. [Google Scholar]
- 18.Chowdhury J.U., Bhuiyan M.N.I., Yusuf M. Chemical composition of the leaf essential oils of Murraya koenigii (L.) Spreng and Murraya paniculata (L.) Jack. Bangladesh J. Pharmacol. 2008;3:59–63. doi: 10.3329/bjp.v3i2.841. [DOI] [Google Scholar]
- 19.Lv H.N., Guo X.Y., Tu P.F., Jiang Y. Comparative analysis of the essential oil composition of Murraya paniculata and M. exotica. Nat. Prod. Commun. 2013;8:1473–1475. [PubMed] [Google Scholar]
- 20.Rodriguez E.J., Ramis-Ramos G., Vander Heyden Y., Simó-Alfonso E.F., Lerma-García M.J., Saucedo-Hernández Y., Monteagudo U., Morales Y., Holgado B., Herrero-Martínez J.M. Chemical composition, antioxidant properties and antimicrobial activity of the essential oil of Murraya paniculata leaves from the mountains of central Cuba. Nat. Prod. Commun. 2012;7:1527–1530. [PubMed] [Google Scholar]
- 21.Boira H., Blanquer A. Environmental factors affecting chemical variability of essential oils in Thymus piperella L. Biochem. Systemat. Ecol. 1997;26:811–822. doi: 10.1016/S0305-1978(98)00047-7. [DOI] [Google Scholar]
- 22.Tropicos.org Missouri Botanical Garden. [(accessed on 8 January 2016)]. Available online: http://www.tropicos.org/Name/28100030.
- 23.Verma S., Rana T.S., Ranade S.A. Genetic variation and clustering in Murraya paniculata complex as revealed by single primer amplification reaction methods. Curr. Sci. 2009;96:1210–1216. [Google Scholar]
- 24.Monzote L., Nance M.R., Garcia M., Scull R., Setzer W.N. Comparative chemical, cytotoxicity and antileishmanial properties of essential oils from Chenopodium ambrosioides. Nat. Prod. Commun. 2011;6:281–286. [PubMed] [Google Scholar]
- 25.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing; Carol Stream, IL, USA: 2007. [Google Scholar]
- 26.Setzer M.C., Setzer W.N., Jackes B.R., Gentry G.A., Moriarity D.M. The medicinal value of tropical rainforest plants from Paluma, North Queensland, Australia. Pharmaceut. Biol. 2001;39:67–78. doi: 10.1076/phbi.39.1.67.5944. [DOI] [Google Scholar]
- 27.Park I.K., Kim J., Lee S.G., Shin S.C. Nematicidal activity of plant essential oils and components from ajowan (Trachyspermum ammi), allspice (Pimenta dioica) and litsea (Litsea cubeba) essential oils against pine wood nematode (Bursaphelenchus xylophilus) J. Nematol. 2007;39:257–279. [PMC free article] [PubMed] [Google Scholar]
- 28.Reed L.J., Muench H. A simple method of estimating fifty percent end points. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- 29.McLaughlin J.L. Bench-top bioassays for the discovery of bioactive compounds in higher plants. Brenesia. 1990;34:1–14. [Google Scholar]
- 30.Huang Y.S., Wang Y., Luo X.P., Yuan K. Composition, antimicrobial and antioxidant activities of the essential oil of Murraya exotica from Hainan of China. Asian J. Chem. 2013;25:5055–5058. [Google Scholar]
- 31.Li W.Q., Jiang C.H., Chu S.S., Zuo M.X., Liu Z.L. Chemical composition and toxicity against Sitophilus zeamais and Tribolium castaneum of the essential oil of Murraya exotica aerial parts. Molecules. 2010;15:5831–5839. doi: 10.3390/molecules15085831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Sakhawy F.S., El-Tantawy M.E., Ross S.A., El-Sohly M.A. Composition and antimicrobial activity of the essential oil of Murraya exotica L. Flavour Fragr. J. 1998;13:59–62. doi: 10.1002/(SICI)1099-1026(199801/02)13:1<59::AID-FFJ693>3.0.CO;2-L. [DOI] [Google Scholar]
- 33.Raina V.K., Verma S.C., Dhawan S., Khan M., Ramesh S., Singh S.C., Yadav A., Srivastava S.K. Essential oil composition of Murraya exotica from the plains of northern India. Flavour Fragr. J. 2006;21:140–142. doi: 10.1002/ffj.1547. [DOI] [Google Scholar]
- 34.Pino J.A., Marbot R., Fuentes V. Aromatic plants from western Cuba. VI. Composition of the leaf oils of Murraya exotica L., Amyris balsamifera L., Severinia buxifolia (Poir.) Ten. and Triphasia trifolia (Burm. f.) P. Wilson. J. Essent. Oil Res. 2006;18:24–28. doi: 10.1080/10412905.2006.9699376. [DOI] [Google Scholar]
- 35.You C., Zhang W., Guo S., Wang C., Yang K., Liang J., Wang Y., Geng Z., Du S., Deng Z. Chemical composition of essential oils extracted from six Murraya species and their repellent activity against Tribolium castaneum. Ind. Crops Prod. 2015;76:681–687. doi: 10.1016/j.indcrop.2015.07.044. [DOI] [Google Scholar]
- 36.Krishnamoorthy S., Chandrasekaran M., Raj G.A., Jayaraman M., Venkatesalu V. Identification of chemical constituents and larvicidal activity of essential oil from Murraya exotica L. (Rutaceae) against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus (Diptera: Culicidae) Parasitol. Res. 2015;114:1839–1845. doi: 10.1007/s00436-015-4370-x. [DOI] [PubMed] [Google Scholar]
- 37.Miller S.L., Villanueva H.E., Palazzo M.C., Wright B.S., Setzer W.N. Seasonal variation and bioactivity in the leaf oil of Liriodendron tulipifera growing in Huntsville, Alabama. Nat. Prod. Commun. 2009;4:839–843. [PubMed] [Google Scholar]
- 38.Hudaib M., Speroni E., Di Pietra A.M., Cavrini V. GC/MS evaluation of thyme (Thymus vulgaris L.) oil composition and variations during the vegetative cycle. J. Pharmaceut. Biomed. Anal. 2002;29:691–700. doi: 10.1016/S0731-7085(02)00119-X. [DOI] [PubMed] [Google Scholar]
- 39.Telci I., Devirtas I., Bayram E., Arabaci O., Kacar O. Environmental variation on aroma components of pulegone/piperitone rich spearmint (Mentha spicata L.) Ind. Crops Prod. 2010;32:588–592. doi: 10.1016/j.indcrop.2010.07.009. [DOI] [Google Scholar]
- 40.Angioni A., Barra A., Coroneo V., Dessi S., Cabras P. Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J. Agric. Food Chem. 2006;54:4364–4370. doi: 10.1021/jf0603329. [DOI] [PubMed] [Google Scholar]
- 41.Amzallag G.N., Larkov O., Ben Hur M., Dudai N. Soil microvariations as a source of variability in the wild: The case of secondary metabolism in Origanum dayi Post. J. Chem. Ecol. 2005;31:1235–1254. doi: 10.1007/s10886-005-5283-4. [DOI] [PubMed] [Google Scholar]
- 42.Sáez F. Volatile oil variability in Thymus serpylloides ssp. gadorensis growing wild in southeastern Spain. Biochem. System. Ecol. 2001;29:189–198. doi: 10.1016/s0305-1978(00)00040-5. [DOI] [PubMed] [Google Scholar]
- 43.Satyal P., Crouch R.A., Monzote L., Cos P., Ali N.A.A., Alhaj M.A., Setzer W.N. The chemical diversity of Lantana camara: Analyses of essential oil samples from Cuba, Nepal, and Yemen. Chem. Biodivers. 2016 doi: 10.1002/cbdv.00005. in press. [DOI] [PubMed] [Google Scholar]
- 44.Satyal P., Paudel P., Lamichhane B., Setzer W.N. Leaf essential oil composition and bioactivity of Psidium guajava from Kathmandu, Nepal. Am. J. Essent. Oils Nat. Prod. 2016 in press. [Google Scholar]
- 45.Setzer M.C., Moriarity D.M., Lawton R.O., Setzer W.N., Gentry G.A., Haber W.A. Phytomedicinal potential of tropical cloudforest plants from Monteverde, Costa Rica. Rev. Biol. Trop. 2003;51:647–674. [PubMed] [Google Scholar]
- 46.Setzer M.C., Werka J.S., Irvine A.K., Jackes B.R., Setzer W.N. Biological activity of rainforest plant extracts from far north Queensland, Australia. In: Williams L.A.D., editor. Biologically Active Natural Products for the 21st Century. Research Signpost; Trivandrum, India: 2006. pp. 21–46. [Google Scholar]
- 47.Schmidt J.M., Noletto J.A., Vogler B., Setzer W.N. Abaco bush medicine: Chemical composition of the essential oils of four aromatic medicinal plants from Abaco Island, Bahamas. J. Herbs Spices Med. Plants. 2007;12:43–65. doi: 10.1300/J044v12n03_04. [DOI] [Google Scholar]
- 48.Sharopov F.S., Wink M., Khalifaev D.R., Zhang H., Dosoky N.S., Setzer W.N. Composition and bioactivity of the essential oil of Melissa officinalis L. growing wild in Tajikistan. Int. J. Trad. Nat. Med. 2013;2:86–96. [Google Scholar]
- 49.Owolabi M.S., Lawal O.A., Dosoky N.S., Satyal P., Setzer W.N. Chemical composition, antimicrobial, and cytotoxic assessment of Mitracarpus scaber Zucc. (Rubiaceae) essential oil from southwestern Nigeria. Am. J. Essent. Oil Nat. Prod. 2013;1:4–6. [Google Scholar]
- 50.Woods K.E., Chhetri B.K., Jones C.D., Goel N. Bioactivities and compositions of Betula nigra essential oils. J. Med. Active Plants. 2013;2:1–9. [Google Scholar]
- 51.Satyal P., Paudel P., Raut J., Deo A., Dosoky N.S., Setzer W.N. Volatile constituents of Pinus roxburghii from Nepal. Phcog. Res. 2013;5:43–48. doi: 10.4103/0974-8490.105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Satyal P., Paudel P., Poudel A., Dosoky N.S., Pokharel K.K., Setzer W.N. Bioactivities and compositional analyses of Cinnamomum essential oils from Nepal: C. camphora, C. tamala, and C. glaucescens. Nat. Prod. Commun. 2013;8:1777–1784. [PubMed] [Google Scholar]
- 53.Essien E.E., Newby J.S., Walker T.M., Setzer W.N., Ekundayo O. Chemotaxonomic characterization and in-vitro antimicrobial and cytotoxic activities of Curcuma longa grown in southern Nigeria. Medicines. 2015;2:340–349. doi: 10.3390/medicines2040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Essien E.E., Newby J.S., Walker T.M., Setzer W.N., Ekundayo O. Characterization and antimicrobial activity of volatile constituents from fresh fruits of Alchornea cordifolia and Canthium subcordatum. Medicines. 2016;3:1. doi: 10.3390/medicines3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi G.J., Jang K.S., Choi Y.H., Yu J.H., Kim J.C. Antifungal activity of lower alkyl fatty acid esters against powdery mildews. Plant Pathol. J. 2010;26:360–366. doi: 10.5423/PPJ.2010.26.4.360. [DOI] [Google Scholar]
- 56.Setzer W.N., Park G., Agius B.R., Stokes S.L., Walker T.M., Haber W.A. Chemical compositions and biological activities of leaf essential oils of twelve species of Piper from Monteverde, Costa Rica. Nat. Prod. Commun. 2008;3:1367–1374. [Google Scholar]
- 57.Werka J.S., Boehme A.K., Setzer W.N. Biological activities of essential oils from Monteverde, Costa Rica. Nat. Prod. Commun. 2007;2:1215–1219. [Google Scholar]
- 58.Satyal P., Woods K.E., Dosoky N.S., Neupane S., Setzer W.N. Biological activities and volatile constituents of Aegle marmelos (L.) Corrêa from Nepal. J. Med. Active Plants. 2012;1:114–122. [Google Scholar]