Abstract
The majority of Bordetella sp. virulence determinants are regulated by the BvgAS signal transduction system. BvgAS mediates the control of multiple phenotypic phases and a spectrum of gene expression profiles specific to each phase in response to incremental changes in the concentrations of environmental signals. Studies highlighting the critical role of this signaling circuitry in the Bordetella infectious cycle have focused on planktonically growing bacterial cells. It is becoming increasingly clear that the major mode of bacterial existence in the environment and within the body is a surface-attached state known as a biofilm. Biofilms are defined as consortia of sessile microorganisms that are embedded in a matrix. During routine growth of Bordetella under agitating conditions, we noticed the formation of a bacterial ring at the air-liquid interface of the culture tubes. We show here that this surface adherence property reflects the ability of these organisms to form biofilms. Our data demonstrate that the BvgAS locus regulates biofilm development in Bordetella. The results reported in this study suggest that the Bvg-mediated control in biofilm development is exerted at later time points after the initial attachment of bacteria to the different surfaces. Additionally, we show that these biofilms are highly tolerant of a number of antimicrobials, including the ones that are currently recommended for treatment of veterinary and human infections caused by Bordetella spp. Finally, we discuss the significance of the biofilm lifestyle mode as a potential contributor to persistent infections.
Bacteria belonging to the genus Bordetella are gram negative and colonize the respiratory tracts of humans and animals (37). Bordetella pertussis infects only humans and causes the acute respiratory disease known as whooping cough (66). Bordetella parapertussis strains can be divided into two genetically distinct types, those which infect humans (B. parapertussishu), causing a pertussis-like illness, and those which cause respiratory infections in sheep (B. parapertussisov). In contrast, Bordetella bronchiseptica has a broad host range, infecting a wide variety of animals (24). Although B. pertussis, B. bronchiseptica, and B. parapertussis vary in their host range, a number of studies have indicated that they are closely related phylogenetically and that they comprise a single bacterial species, which suggests that there was a very recent evolution of different subspecies (60).
The BvgAS signal transduction system controls a highly regulated program of gene expression in response to environmental stimuli. This regulatory cascade mediates the coordinated expression of almost all of the known or suspected colonization and virulence factors currently associated with the infectious cycle of Bordetella. BvgA and BvgS are members of a group of the two-component superfamily of regulatory signal transducing proteins that communicate by a four-step His-Asp-His-Asp phosphorelay. Phosphorylated BvgA can function as a transcriptional activator and repressor, depending on the cognate genes (reviewed in references 11 and 19 and in the references therein).
Although BvgAS activity can be inhibited by low temperature (<25°C), nicotinic acid, and sulfate anion in the laboratory, the true signals sensed in nature or in the mammalian hosts are unknown. In response to subtle changes in the intensities and the intervals of the different signals, BvgAS mediates a transition between multiple phenotypic phases (Bvg+, Bvgi, and Bvg−) that are characterized by distinct patterns of gene expression (20, 37). When BvgAS is active, bordetellae are in the Bvg+ phase, which is characterized by the expression of a number of Bvg-activated protein factors, including the adhesins and toxins encoded by the vag genes (Bvg-activated genes) and the repression of a group of genes designated vrg genes (Bvg-repressed genes). Inactivation of BvgAS by modulating signals or by mutation results in the transition to the Bvg− phase. In this phase, the vag genes are repressed and the vrg genes are expressed. For B. bronchiseptica, the prominent Bvg-repressed phenotype is flagellum-mediated motility (2), whereas in B. pertussis, the Bvg− phase is characterized by the expression of outer membrane proteins of unknown function (30). The Bvg-intermediate phase (Bvgi) is characterized principally by the maximal expression of a group of outer membrane proteins, of which BipA is the first known example (14, 18, 20, 55). For both B. pertussis and B. bronchiseptica, it has been demonstrated that the Bvg+ phase is necessary and sufficient for respiratory tract colonization (1, 35). Based on the increased survival of a Bvg− phase-locked mutant under conditions of nutrient limitation, it has been suggested that this phase may be responsible for the survival of B. bronchiseptica in the environment (14). The role of the Bvgi phase in the Bordetella infectious cycle is presently unclear. It has been hypothesized that the intermediate phase might be involved in aerosol transmission (14).
Although recent studies have clearly established that the ability of the BvgAS signal transduction system to regulate an entire spectrum of phase-specific gene expression states plays a central and essential role in determining different aspects of Bordetella pathogenesis, it is important to note that these studies were conducted with planktonically growing bacterial cells. It is becoming increasingly clear that in contrast to the free-floating planktonic mode, bacteria prefer a surface-bound community-based existence known as a biofilm. Biofilms are structured communities of sessile bacterial cells that are encased in a self-produced polymeric organic matrix (16, 61). The medical importance of the biofilm mode of existence is highlighted by its association with a number of chronic bacterial infections and its inherent resistance to antimicrobial agents (10, 21, 33).
During the growth of the wild-type (wt) B. bronchiseptica strain under agitating conditions, we noted the formation of a bacterial ring at the air-liquid interface of the culture tubes. Based on previous results from other bacterial systems, we hypothesized that this surface adherence property displayed by B. bronchiseptica is suggestive of its ability to form biofilms. Thus, we undertook this study with the goal of demonstrating a biofilm mode of existence for Bordetella. In this report, we provide evidence that bordetellae form biofilms and that the BvgAS-mediated signal transduction cascade regulates biofilm formation. While the present study was under preparation, Irie et al. reported that BvgAS regulates biofilm formation in B. bronchiseptica (28). Their study suggests that biofilm formation in B. bronchiseptica is primarily a Bvgi phase-specific phenotype. In contrast, by assaying the formation of biofilms at multiple time points and under both static and dynamic conditions, we show that B. bronchiseptica forms robust biofilms in both Bvg+ and Bvgi phase conditions. Additionally, we show that the ability of the BvgAS system to regulate biofilm formation is conserved in the three Bordetella species, B. bronchiseptica, B. pertussis, and B. parapertussis, which vary in their host specificity. Our results also suggest that the Bvg-mediated control of biofilm development is exerted at a step subsequent to the initial attachment. Finally, we show that biofilm development promotes tolerance to several clinically relevant antibiotics.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The wt B. bronchiseptica strain RB50 was isolated from a naturally infected New Zealand White rabbit (13). The Bvg+ phase-locked strain (RB53), the Bvgi phase-locked strain (RB53i), the Bvg− phase-locked strains (RB54 and RB55), and the chimeric strain (RB52) used in this study are isogenic derivatives of RB50 and have been described previously (13, 36). The wt B. pertussis strain Bp 536, its Bvg− phase-locked derivative Bp537 (47), and B. parapertussis strain 12822 (27) have also been previously described.
B. bronchiseptica, B. pertussis, and B. parapertussis were maintained on Bordet-Gengou (BG) agar (Difco) containing 7.5% defibrinated sheep blood for the determination of colony morphology and hemolytic activity. For both planktonic and biofilm growth conditions, the bacteria were grown in Stainer-Scholte (SS) broth (52). For B. pertussis, SS broth was supplemented with heptakis(2,6-di-O-methyl)-β-cyclodextrin (Sigma). When needed for modulating BvgAS activity, the medium was supplemented with 40 mM MgSO4 or 20 mM nicotinic acid.
Microtiter dish assay of Bordetella biofilm formation.
Bordetella biofilm formation was monitored by the microtiter plate assay as described previously (42). Bordetella cells were grown in a total volume of 100 μl of SS broth at 37°C in 96-well polyvinylchloride (PVC) microtiter plates. The plates were inoculated at an optical density at 600 nm (OD600) of 0.05 for the respective B. bronchiseptica strains and an OD600 of 0.1 for B. parapertussis with an overnight culture grown with shaking in roller drums in SS broth. The microtiter plates were sealed with Scotch tape and were incubated under stationary conditions. The growth medium was exchanged with fresh SS broth every 24 h as described previously (26). At each time point, the nonattached and loosely adherent bacteria were removed by discarding the media from the wells, which were then washed thoroughly with water. Cells that remained adhered to the wells were stained with a 0.1% solution of crystal violet (CV) and were incubated at room temperature for 30 min. The washing process was repeated, and the CV staining the cells was solubilized with 200 μl of 95% ethanol. Biofilm formation was quantitated by measuring the OD540 for each well by transferring 125 μl of the solubilized CV stain to a fresh polystyrene microtiter dish.
Analysis of biofilm using the continuous flow model.
To compare the biofilm-forming ability of the different strains under dynamic conditions, we used a biofilm tubing assay as described previously (49). The silicone tubing was aseptically inoculated with a 500-μl suspension of mid-logarithmic phase cultures (A600 = 0.5) of the individual strains by using a 25 5/8-gauge needle. Bacteria were allowed to adhere to the inner surface of the tubing for either 2 or 14 h before the flow of medium (SS broth) was resumed at a rate of ≈1 ml/min. After 48 h, an 18.0-cm section of tubing was excised (6.0 cm before and 12.0 cm after the inoculation point). The excised tubing was sectioned longitudinally and biofilm-grown cells were harvested by scraping and resuspended into 1 ml of SS medium. Bacteria were enumerated by plating a series of dilutions on BG-blood agar plates.
Scanning electron microscopy.
Bordetella strains were cultured statically on glass coverslips partially submerged vertically in two-chambered polystyrene slides (Nunc) as described previously (59). The glass coverslips are placed such that an air-liquid interface was established. After the designated time points, the coverslip was removed and washed with sterile H2O, and the bacteria were fixed with 2.5% glutaraldehyde and processed for scanning electron microscopy (SEM) analysis as described previously (59).
Biofilm antibiotic resistance assay.
Antimicrobial reagents were purchased from the following manufacturers: ICN Biomedicals (erythromycin and ciprofloxacin), Cellgro (kanamycin monosulfate), and Research Products International Corp. (ampicillin, gentamicin sulfate, and streptomycin sulfate).
For determining the antibiotic resistance profile for planktonically growing bacterial cells, an overnight culture of the wt B. bronchiseptica strain was inoculated at an OD600 of 0.05 into 2 ml of the medium in 13-ml polystyrene tubes containing various concentrations of the different antibiotics. The culture tubes were incubated in a roller drum for 24 h at 37°C, and an aliquot of the growth medium containing bacteria (10 μl) was plated on BG-blood agar plates. MIC-P is defined as the minimal concentration of antibiotic at which there was no apparent growth on agar plates.
The microtiter dish assay for determining the relative resistance of Bordetella biofilms to antimicrobials was performed as described previously (64). Overnight culture of the wt B. bronchiseptica strain was subcultured at an OD600 of 0.05 into fresh medium and aliquoted (100 μl) into the wells of the microtiter dishes. The plates were incubated at 37°C to allow formation of the biofilms. After 24 h, the used medium was exchanged with fresh medium containing increasing concentrations of antibiotics and the plates were incubated for additional 24 h. The antibiotic-containing medium was then replaced with fresh antibiotic-free medium and incubated for 24 h. During this time, any viable cells in the biofilm grow and seed the growth medium. An aliquot of the growth medium (10 μl) containing bacteria was spotted on BG-blood agar plates and incubated at 37°C for 48 h. The minimum bactericidal concentration (MBC)-biofilm is defined as the minimal concentration of antibiotic at which no bacterial growth is apparent on the agar plates.
RESULTS
B. bronchiseptica forms a ring of cells under continuous agitating conditions.
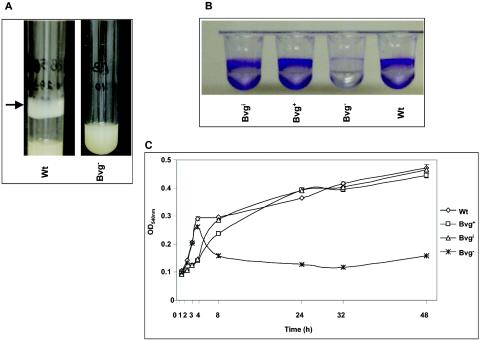
While growing the wt B. bronchiseptica strain RB50 in culture tubes in a roller drum at the maximum speed (≈60 rpm) at 37°C, we discovered the formation of a uniform bacterial ring at the surface of the culture tubes. During this experiment, the bacteria grew exponentially in the planktonic phase of the culture and the growth was monitored at regular intervals. Initially, we observed the formation of a thin film after 24 h of growth, which by 36 to 48 h developed into a thick opaque structure that is visible to the naked eye (Fig. 1A). Since the culture tubes were at an angle in the roller drum, the surface of the tube at which the film forms is the air-liquid interface. This film is formed on both polystyrene (Fig. 1A) and glass (data not shown) culture tubes. The cells of the growing film under agitating conditions were viable as determined by plating on BG agar plates (data not shown). Incubation of bacteria in standing cultures or cultures that were shaking at a high speed (≈300 rpm) also resulted in the formation of a film at the air-liquid interface. The microbial film formed under these conditions was thinner than that of the films formed in a roller drum (data not shown).
FIG. 1.
(A) Formation of a bacterial ring (indicated by the arrow) at the air-liquid interface of polystyrene culture tubes rotating in a roller drum by the wt strain and the Bvg− phase-locked strain of B. bronchiseptica. (B) Microtiter assay of biofilm formation at 24 h by wt and mutant strains of B. bronchiseptica. (C) Kinetics of biofilm formation for B. bronchiseptica strains showing the OD540s of solubilized crystal violet from surface-attached cells grown in microtiter plates and assayed at specified time intervals. Each data point is the average for six wells, and error bars indicate the standard errors. Representative data from one of at least five independent experiments are shown. The wt and different mutant strains were grown in SS medium under Bvg+ phase conditions. (D) Complementation of the BvgAS locus restores biofilm formation. Microtiter assays were performed as described for panel C. (E) Conditions were the same as for panel C, except that the wt strain was grown in SS medium in the presence of modulator MgSO4 or nicotinic acid (Nic).
Biofilm formation by B. bronchiseptica.
Adherence to abiotic surfaces is the first step in the multistep process of biofilm development. The adherence of B. bronchiseptica to different surfaces under both static and shaking conditions resembles that in the formation of biofilm at the air-liquid interface of a standing liquid culture by Pseudomonas aeruginosa (also known as pellicle) and Vibrio cholerae (22, 25) and under shaking conditions by Salmonella enteritidis (51). Therefore, we became interested in determining whether bordetellae form biofilms. To demonstrate a biofilm mode of existence for Bordetella spp., we measured the ability of the wt B. bronchiseptica strain to adhere to PVC microtiter plates. Staining of the adhered cells with CV revealed the formation of a robust ring of attached cells at the air-liquid interface (Fig. 1B). Thus, the wt strain of B. bronchiseptica adheres to a variety of abiotic surfaces, including PVC, polystyrene, and glass.
The BvgAS signal transduction system controls biofilm development in B. bronchiseptica.
Biofilm formation in bacteria occurs in a highly coordinated stepwise manner (9, 15, 53, 62). The BvgAS signal transduction system is the principal regulatory locus of virulence gene expression in Bordetella spp. and plays an important role in their survival strategy. Therefore, we hypothesized that BvgAS would be important in mediating biofilm formation. To test this hypothesis, we compared the ability of the wt strain to adhere to microtiter plates with various isogenic signaling mutant strains (Bvg+, Bvgi, and Bvg−). The Bvg+ phase-locked strain (RB53) encodes a constitutively active BvgS protein (39) that is insensitive to modulators, whereas the Bvgi phase-locked strain (RB53i) is locked in the intermediate phase (14). The Bvg− phase-locked strain (RB54) contains a deletion of the gene encoding the BvgS sensor kinase, resulting in a strain that is locked in the Bvg− phase.
Both the Bvg+ and the Bvgi phase-locked strains formed a bacterial ring at the air-liquid interface (data not shown) and resulted in high levels of staining on PVC plates (Fig. 1B), indicating that similar to the wt strain, these strains form good biofilms on PVC surfaces. In contrast to any of these strains, the Bvg− phase-locked strain did not form a bacterial ring (Fig. 1A) and showed a very weakly staining band (Fig. 1B), suggesting that this strain is deficient in forming biofilms and that the BvgAS locus is required for efficient biofilm formation in B. bronchiseptica.
To confirm that there was no change in the phenotypic phase of the wt strain during biofilm growth, an aliquot of planktonic cells from the microtiter plates was streaked on a BG-blood agar plate. As apparent by colony morphology and zone of hemolysis, the wt strain remained in the Bvg+ phase during biofilm growth (data not shown).
Quantification of biofilm formation.
To correlate further the role of BvgAS-mediated signaling events in regulating the biofilm phenotype, we determined the time course of adherence to microtiter plates in a quantitative manner. CV associated with the adherent cells in a microtiter plates was solubilized and quantified by measuring absorbance at 540 nm. Figure 1C shows the kinetics of biofilm formation for the wt and the different B. bronchiseptica signaling mutant strains. Adherent cells were detected very early on (1 h) after inoculation for all of the strains. Interestingly, although the Bvg+ and Bvgi phase-locked strains were delayed in biofilm formation at early time points compared to the wt strain, they did not show significant differences compared to the wt strain at later time points (Fig. 1C). In agreement with high levels of biofilm formation by the wt bacteria under Bvg+ phase conditions and the Bvgi phase-locked strain, growth of wt bacteria under intermediate phase conditions (in the presence of 0.8 mM nicotinic acid) did not result in any significant differences in the levels of biofilm formation (data not shown).
In contrast, the Bvg− phase-locked strain was severely defective in its ability to form biofilms at both intermediate and later time points (Fig. 1C). Even after 100 h of incubation, the Bvg− phase-locked strain remained substantially defective in its ability to form biofilms compared to the wt strain (data not shown). It is important to note that there were no significant differences in the growth rates of the wt and various phase-locked mutant strains when grown planktonically (data not shown).
To confirm that the defect in biofilm formation for the Bvg− phase-locked strain was not due to any pleiotropic effects, we measured the adherence of a chimeric B. bronchiseptica strain RB52, which contains a precise chromosomal replacement of the B. bronchiseptica BvgAS locus with that of the corresponding locus from B. pertussis (36). It is clear from the results shown in Fig. 1D that RB52 showed high levels of biofilm formation. In contrast, the isogenic parental strain RB55, containing a deletion of the entire BvgAS locus, was defective in its ability to form biofilms.
In addition to the various phase-locked strains described above, we determined the amount of biofilm biomass formed by the wt strain under Bvg− phase conditions, i.e., in the presence of sulfate anion (MgSO4) or nicotinic acid. These chemical compounds serve as environmental modulators of BvgAS activity (18, 29, 40, 48). Quantitation of the biofilm-forming ability of the wt strain in the presence of modulators resulted in fewer numbers of adherent bacteria than for the wt strain grown in the absence of modulators (Fig. 1E). As observed earlier for the Bvg− phase-locked strain, the defect in biofilm formation in the presence of sulfate anion or nicotinic acid compared to the wt strain alone was more pronounced at later time points (compare Fig. 1C and E). Note that under planktonic conditions, there was no apparent defect in the growth of the wt and the Bvg− phase-locked strain in the presence of modulators.
Thus, these results taken together strongly suggest that a BvgAS-mediated signal transduction pathway regulates biofilm formation in B. bronchiseptica. Additionally, these results also suggest that the BvgAS-dependent regulation of biofilm formation is exerted at later time points (>6 h).
Quantitative analysis of the role of BvgAS in biofilm formation using continuous flow systems.
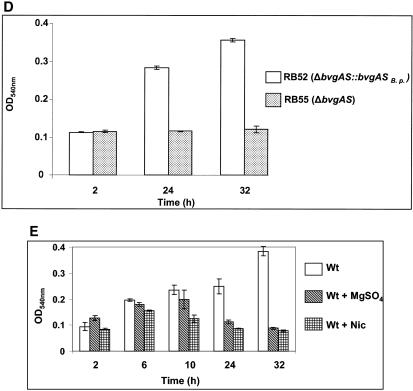
Although the microtiter assays described above provide a strong support for the involvement of the BvgAS locus in regulating biofilm formation, they represent a static system and do not take into account the possible nutrient deprivation during growth in microtiter plates or the role of shear forces generated by the fluids in a human or animal host. The growth and structures of biofilm biomass are strongly influenced by hydrodynamic conditions (46, 56). A quantitative assessment of biofilm formation under continuous flow systems will further strengthen our finding that the BvgAS locus plays a role in biofilm development. Therefore, we utilized a biofilm tube reactor system (49) to quantitate the population of biofilm-grown wt and various phase-locked cells under continuous laminar flow conditions with a constant supply of fresh Bordetella growth medium. The biofilms were formed by inoculating the growth medium-primed silicone tubes with an OD600 of 0.5 of the wt or the different phase-locked mutant strains. The cells were allowed to attach to the tubing for either 2 or 14 h and then the continuous flow of the growth medium was initiated. After 48 h of continuous passage of medium, a defined section of the tubes was longitudinally sectioned. The attached biofilm bacteria were harvested and enumerated. These results show that the Bvg− phase-locked strain was substantially impaired in its ability to form biofilms only at the later time point (14 h) (Fig. 2). At the earlier time point (2 h), there were no significant differences in the relative amounts of biofilm bacteria among the different bacterial strains (Fig. 2). Thus, these results further corroborate the observation that the Bvg-mediated effect on biofilm formation is exerted at later time points.
FIG. 2.
Quantitative analysis of biofilm populations of the wt and phase-locked mutant strains in silicone tubing continuous flow devices. Log phase cultures of each strain (OD600, ∼0.5) were inoculated into a section of silicone tubing that had first been primed with medium. The cells were allowed to attach to the tubing for the designated time points, followed by the continuous passage of growth medium through the tubing for 48 h. The tubings were then longitudinally sectioned, and the biofilm-grown cells were harvested, resuspended, and enumerated by plate counts (see Materials and Methods). The number of bacteria obtained for each strain was normalized to that recovered from the wt strain, which is represented as 100%.
Scanning electron microscopy of B. bronchiseptica cells adhered to glass coverslips.
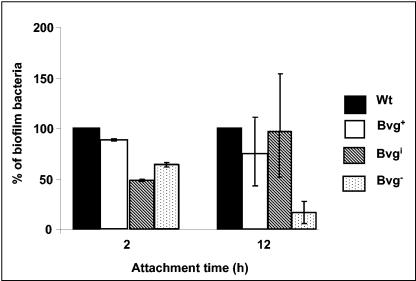
To further investigate the role of BvgAS in the biofilm development, SEM of biofilms formed at multiple time points by the wt and the Bvg− strains grown at the air-liquid interface in biphasic cultures on glass coverslips was performed. At the early time points (6 h), both the wt and the Bvg− phase-locked cells were spread around the disks and existed as a monolayer of cells (Fig. 3). At 24 and 72 h, while the wt strain formed a nearly uniform thick multilayered stack of cells resembling a bacterial community similar to those observed with other bacterial pathogens (Fig. 3), the Bvg− phase-locked cells were spread around the disks with large regions of the disks remaining uncolonized (Fig. 3). Similar to the wt strain, the Bvg+ phase-locked and Bvgi phase-locked strains were also aggregated in clusters when analyzed by SEM (data not shown).
FIG. 3.
Scanning electron micrographs of the B. bronchiseptica wt strain or the Bvg− phase-locked strain grown for 6, 24, and 72 h on glass coverslips as described in Materials and Methods. Bar = 1 μm.
Interestingly, SEM analyses at later time points revealed an unusual network of thread-like structures which radiate from the cell surface of both the wt and the Bvg− phase-locked strain (Fig. 3). For the wt bacteria, these thread-like structures result in a web-like architecture that appears to encase the bacterial microcolonies. While there was no significant difference between the relative numbers of attached bacteria for the wt and the Bvg− phase-locked strain at the early time point (6 h), substantially fewer numbers of adherent cells for the Bvg− phase-locked strain were detected at 24 and 72 h (Fig. 3). Thus, these results suggest that the Bvg-dependent effect on biofilm formation is exerted at a step subsequent to the initial attachment.
Bordetella biofilms and antibiotic tolerance.
Resistance to antimicrobials is one of the defining biofilm-specific properties that distinguish these surface-attached microbial populations from their planktonic counterparts (21, 33). We hypothesized that B. bronchiseptica cells in a biofilm would become more tolerant to antibiotics relative to their planktonic counterparts. Therefore, we compared the antimicrobial resistance properties of the biofilms produced by the wt B. bronchiseptica strain to that of the planktonically grown cultures. The antibiotic resistance assay was performed as reported previously (64). For this experiment, biofilms are formed in microtiter plates for 24 h, treated with antibiotics for 24 h, and grown in antibiotic-free medium for an additional 24 h. The survival of the bacteria with the different antibiotics was determined by spotting an aliquot onto BG agar plates. Depending on the antibiotics tested, the range of MBCs for biofilm-grown B. bronchiseptica was 160- to 1,000-fold higher than for that grown under planktonic conditions (Table 1). The data therefore suggest that the biofilm mode of growth promotes tolerance to a wide range of antibiotics.
TABLE 1.
Antibiotic sensitivity of biofilm-grown and planktonically grown wt B. bronchiseptica cellsa
| Drug | MBC-P (mg/ml) | MBC-B (mg/ml) | MBC-B/MBC-P |
|---|---|---|---|
| Erythromycin | 0.05 | >32b | >640 |
| Streptomycin | 0.75 | 300 | 400 |
| Kanamycin | 0.02 | 4 | 200 |
| Gentamicin | 0.005 | 0.8 | 160 |
| Ampicillin | 0.5 | 500 | 1,000 |
| Ciprofloxacin | 0.005 | 1 | 200 |
MBC-P, MBC of planktonically grown cells; MBC-B, MBC of biofilm-grown cells. Values are based on results of three different experiments.
The antibiotic was partially soluble above this concentration.
The BvgAS locus regulates biofilm formation in the human pathogens B. parapertussis and B. pertussis.
It has been estimated that between 50 and 80% of microbial infections in the human body involve biofilms (10, 57). Although B. bronchiseptica can occasionally infect humans, particularly immunocompromised individuals, it is primarily an animal pathogen. In contrast, B. pertussis and B. parapertussis infect humans. Thus, our goal was to demonstrate a biofilm mode of existence for B. parapertussis and B. pertussis and to determine whether BvgAS regulates biofilm formation in these organisms.
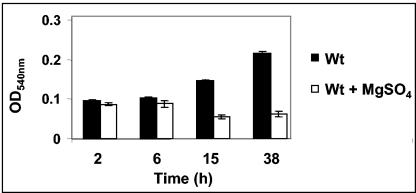
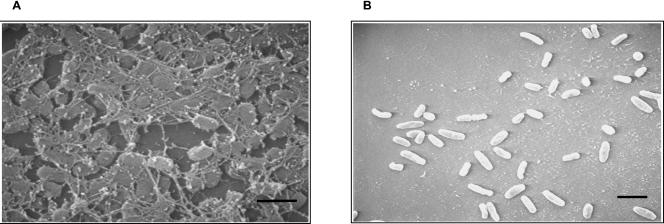
Quantification of biofilm formation by solubilizing the CV stain from the adherent bacteria suggests that similar to B. bronchiseptica, the BvgAS locus controls biofilm formation in B. parapertussis, since growth of the wt B. parapertussis strain 12822 in the presence of sulfate anion results in a defect in the formation of the relative amounts of biofilm biomass (Fig. 4). A SEM analysis of the adherent bacteria for B. parapertussis revealed a cluster of bacteria in a microcolony-like arrangement for the wt strain but isolated dispersed colonies when grown in the presence of sulfate anion (compare Fig. 5A and B). Similar results were obtained when the growth medium was supplemented with nicotinic acid (data not shown).
FIG. 4.
The kinetics of biofilm formation for B. parapertussis. The wt strain 12822 was grown in SS medium either in the absence (black bars) or presence (white bars) of the modulator MgSO4. The OD540 of solubilized crystal violet from surface-attached cells grown in microtiter plates and assayed at specified time-intervals is shown. Each data point is the average for six wells, and error bars indicate the standard errors. Representative data from one of at least three independent experiments are shown.
FIG. 5.
Scanning electron micrographs comparing biofilm formation for B. parapertussis wt strain 12822 grown in the absence (A) or presence (B) of the modulator MgSO4. The cultures were grown for 96 h on the surfaces of glass coverslips. Bars = 1 μm.
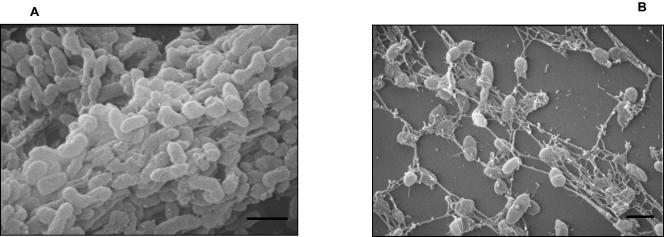
In contrast to B. parapertussis, neither the wt B. pertussis strain Bp 536 nor its isogenic Bvg− derivative Bp537 grew in microtiter plates. Although we were unable to grow B. pertussis in microtiter plates, we were successful in growing these bacteria on glass coverslips as described above for B. bronchiseptica. An inspection of the films at the liquid-air interface by SEM analysis revealed a cluster of bacteria in microcolonies for the wt B. pertussis strain (Fig. 6A). In contrast, the Bvg− phase-locked strain of B. pertussis showed isolated colonies (Fig. 6B). Thus, the above results collectively suggest that similar to B. bronchiseptica, the human pathogens B. pertussis and B. parapertussis also form BvgAS-regulated biofilms.
FIG. 6.
Scanning electron micrographs comparing biofilm formation for B. pertussis wt strain Bp 536 (A) and Bvg− phase-locked strain Bp537 (B) grown for 96 h on the surfaces of glass coverslips. Bars = 1 μm.
DISCUSSION
Biofilms are defined as communities of microorganisms that are adherent to an inert or living surface embedded in a self-produced organic polymeric matrix. It is now well established that bacteria exist primarily in sessile communities rather than as planktonic isolated cells. The experiments conducted in this study provide experimental evidence that Bordetella forms biofilms. Additionally, we have shown that the three species of Bordetella, B. bronchiseptica, B. parapertussis, and B. pertussis, which vary in their host range, attach and form biofilms on a number of abiotic surfaces under static, continuous agitating, and hydrodynamic conditions.
One of the major findings of this study is the demonstration that the BvgAS signal transduction system is involved in biofilm formation. Our studies clearly show the formation of high levels of biofilms by the wt strain grown under Bvg+ phase conditions (absence of modulators), the Bvg+ phase-locked strain, and the Bvgi phase-locked strain. The only mutant strain of Bordetella that consistently displayed a defect in biofilm formation was the Bvg− phase-locked strain. Biofilm formation was also inhibited in the presence of sulfate anion or nicotinic acid, known modulators of BvgS activity. Our finding that neither sulfate anion nor nicotinic acid significantly inhibit biofilm formation with the Bvg+ or the Bvgi phase-locked strain further supports the critical role of the BvgAS signaling circuitry in mediating this regulation. Although the exact mechanism by which these signaling compounds act is unknown, results from a number of studies provide indirect evidence that these chemical compounds lead to the inhibition of the transcription of Bvg-activated genes, presumably through a block in the signal transduction pathway (18, 29, 40, 48).
The development of a bacterial biofilm proceeds through a number of distinct programmed series of steps (9, 15, 62). Biofilms are initiated by bacteria attaching to a surface, followed by cells forming microcolonies that increase in size, resulting in mature biofilms. Mature biofilms are characterized by highly structured, distinct architectural and physicochemical properties. Each of the individual steps in biofilm formation is controlled by regulatory proteins and signaling processes (53). The results presented in this study suggest that the BvgAS signal transduction system regulates biofilm formation in Bordetella at a step following the adhesion of bacteria to the surface. Quantification of surface-adherent biofilm biomass indicates that there is a drastic difference in the abilities of the wt and the Bvg− phase-locked mutant to form biofilms only at later time points. These two strains do not differ significantly in surface adherence at early time points. The spatial distribution of the adherent bacteria, determined by SEM analyses, suggests that the BvgAS-mediated regulatory effect could be either at microcolony formation or at a later step leading to formation of a mature biofilm. Whereas wt Bordetella strains formed confluent clusters, the respective Bvg− phase-locked strains or the wt strain grown under Bvg− phase conditions (for B. parapertussis) existed in a monolayer of isolated attached cells.
We propose that biofilm formation in Bordetella requires an initial Bvg- independent step and a subsequent Bvg-dependent step. The exact mechanism or the step at which BvgAS influences biofilm formation is not clear at this point. If BvgAS is required for microcolony formation, it might lead to stable cell-cell interactions by controlling the production of surface structures, such as pili, and/or an extracellular matrix, as shown for other gram-negative bacteria (45, 58). A later step in biofilm formation, maturation in P. aeruginosa and V. cholerae, has been shown to be regulated by quorum sensing, a control mechanism switched on at high cell density (17, 25, 68). In V. cholerae, this regulation occurs through HapR, a protein that is homologous to the LuxR family of regulatory proteins (25, 41, 68). Interestingly, the activity of HapR in turn is regulated by a phosphorylation cascade that involves multiple proteins that include the sensor kinase LuxN and the response regulator LuxO. Quorum sensing has not yet been reported for Bordetella. Annotation of the Bordetella genome database has revealed the presence of a number of open reading frames that display homology to the LuxR family of transcriptional regulators (43). Note that the BvgAS-mediated difference in biofilm formation is most prominent at later time points. Thus, it is possible that when Bordetella spp. reach a critical cell density, the accumulation of a yet-unknown Bvg-regulated signal, possibly similar to N-acylhomoserine lactones in other gram-negative bacteria, promotes efficient biofilm formation (17, 25, 44, 68). We are in the process of establishing genetic screens to identify genes that are responsible for biofilm development in Bordetella.
After the submission of the present study for publication, Irie et al. reported that B. bronchiseptica forms biofilms primarily in the intermediate phase (28). Their studies also suggested a potential interacting role for adenylate cyclase-hemolysin and filamentous hemagglutinin in biofilm formation in the Bvgi phase. Although our study and that by Irie et al. reached different conclusions about the Bordetella phenotypic phase that is important for biofilm formation, both studies demonstrated the importance of the BvgAS locus in regulating Bordetella biofilm formation. Although it appears that identical strains were used in both studies, subtle variations in the experiments might account for the discrepancies.
It is becoming increasingly clear that biofilm-borne bacteria possess physicochemical properties distinct from those of the planktonic cells. Biofilms confer on bacterial cells protection from host defenses and resistance to antimicrobial agents, e.g., antibiotics, reactive oxygen species, and detergents (33, 34, 54). In this study, we chose a number of different antibiotics for several reasons. Our wt B. bronchiseptica strain RB50 was isolated from rabbits and is naturally resistant to streptomycin and ampicillin (12). Erythromycin is widely used for both prophylaxis and treatment of pertussis. Ciprofloxacin, a representative of the fluoroquinolones, was chosen because of its increasing use in both veterinary medicine and human infections to treat bacterial infections, including Bordetella (6, 7). Our results demonstrate that biofilms formed by B. bronchiseptica are 160- to 1,000-fold more resistant to these antibiotics than are their planktonic counterparts. In particular, our finding of a very high level of tolerance of Bordetella biofilms to erythromycin and ciprofloxacin is quite a concern and becomes highly important with the recent isolation of multiple erythromycin-resistant strains in the United States (4, 32, 63). For a number of bacterial diseases, including pertussis, current therapeutic agents are selected based on their efficacy against planktonic cells. Since increasing evidence indicates that many bacteria form biofilms in vivo (3, 31, 50), the current antibacterial agents might not be sufficient in the future to treat recalcitrant antibiotic resistant infections.
Although vaccination has decreased mortality considerably, B. pertussis continues to circulate and persists even in populations that have traditionally achieved high vaccination coverage. A number of studies have indicated that pertussis is a common cause of persistent cough in adults (5, 8, 23, 65). It is estimated that 20 to 30% of adolescents and adults who have chronic cough lasting for more than 1 week appear to be persistently colonized with B. pertussis in the nasopharynx (8). It is hypothesized that adults carrying B. pertussis may serve as a reservoir for familial spread of infections in young children, in whom the disease can be severe and sometimes lethal (66). In a recent study, it was found that 37% of infections in children were secondary infections acquired from the parents (6). Additionally, B. bronchiseptica remains persistently colonized in the nose of rabbits and rats. By using phase-locked mutants, it has been shown previously that for both B. pertussis and B. bronchiseptica, the Bvg+ phase is necessary and sufficient for respiratory tract colonization (1, 35). It is noteworthy that although some of the Bvg-activated factors in B. bronchiseptica are required for efficient or persistent colonization of the trachea, none of those elicit a defect in the colonization of the nose (38, 67). The only mutant strain of Bordetella that does not colonize any of the upper respiratory tract sites is the Bvg− phase-locked strain (12, 13). In this study, we have shown that the Bvg− phase-locked strain of B. bronchiseptica is substantially defective in biofilm formation compared to the wt and the Bvg+ phase-locked mutant. Thus, we suggest that the biofilm mode of existence in a particular niche, especially in the nasopharynx or at a specific stage during respiratory infection, allows Bordetella to escape immune defenses and lead to persistent infections.
In conclusion, a great deal of information about the complexities of gene expression profiles mediated by the BvgAS signal transduction system has recently been obtained by studying Bordetella in a planktonic growth state. Instead of the previous emphasis on studying the ability of the BvgAS phosphorelay system in coordinating environmental responses in free-living cells, it should be now possible to study these events in a more appropriate multicellular biological system. Given the increasing awareness of the contribution of biofilms in nature and diseased individuals, the identification and characterization of the BvgAS-regulated determinants and their mechanism of action in Bordetella biofilm formation should prove helpful to realistically assess the role of this locus in Bordetella pathogenesis. Future investigation of the biochemical and genetic events associated with biofilm formation in Bordetella may provide for the development of new chemotherapeutic or immunological strategies for the eradication of infections caused by this important group of bacterial pathogens.
Acknowledgments
We thank Amy Foreman-Wykert, Jeff F. Miller, and Eric Harvill for sending strains. We are grateful to Ken Grant for help with the scanning electron microscopy. We thank W. E. Swords and Yolanda Sanchez for critical reading of the manuscript.
This work was supported by faculty development funds from Wake Forest University Health Sciences and from the North Carolina Chapter of the American Lung Association to R.D. D.J.W. is supported by Public Health Service grants AI-35177 and HL-58334. K.J. is supported by the David and Lucile Packard Foundation.
REFERENCES
- 1.Akerley, B. J., P. A. Cotter, and J. F. Miller. 1995. Ectopic expression of the flagellar regulon alters development of the Bordetella host interaction. Cell 80:611-620. [DOI] [PubMed] [Google Scholar]
- 2.Akerley, B. J., and J. F. Miller. 1993. Flagellin gene transcription in Bordetella bronchiseptica is regulated by the BvgAS virulence control system. J. Bacteriol. 175:3468-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. [DOI] [PubMed] [Google Scholar]
- 4.Bartkus, J. M., B. A. Juni, K. Ehresmann, C. A. Miller, G. N. Sanden, P. K. Cassiday, M. Saubolle, B. Lee, J. Long, A. R. Harrison, Jr., and J. M. Besser. 2003. Identification of a mutation associated with erythromycin resistance in Bordetella pertussis: implications for surveillance of antimicrobial resistance J. Clin. Microbiol. 41:1167-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birkebaek, N. H., M. Kristiansen, T. Seefeldt, J. Degn, A. Moller, I. Heron, P. L. Andersen, J. K. Moller, and L. Ostergard. 1999. Bordetella pertussis and chronic cough in adults. Clin. Infect. Dis. 29:1239-1242. [DOI] [PubMed] [Google Scholar]
- 6.Bourgeois, N., J. C. Ghnassia, and F. Doucet-Populaire. 2003. In vitro activity of fluoroquinolones against erythromycin-susceptible and -resistant Bordetella pertussis. J. Antimicrob. Chemother. 51:742-743. [DOI] [PubMed] [Google Scholar]
- 7.Carbone, M., M. T. Fera, M. G. Pennisi, M. Masucci, A. De Sarro, and C. Macri. 1999. Activity of nine fluoroquinolones against strains of Bordetella bronchiseptica. Int. J. Antimicrob. Agents 12:355-358. [DOI] [PubMed] [Google Scholar]
- 8.Cherry, J. D. 1999. Epidemiological, clinical, and laboratory aspects of pertussis in adults. Clin. Infect. Dis. 28(Suppl. 2):S112-S117. [DOI] [PubMed] [Google Scholar]
- 9.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappinscott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 10.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 11.Cotter, P. A., and A. M. Jones. 2003. Phosphorelay control of virulence gene expression in Bordetella. Trends Microbiol. 11:367-373. [DOI] [PubMed] [Google Scholar]
- 12.Cotter, P. A., and J. F. Miller. 1994. A rabbit model for the analysis of virulence gene-regulation in Bordetella-Bronchiseptica. J. Cell. Biochem. Suppl. 18A:65.
- 13.Cotter, P. A., and J. F. Miller. 1994. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect. Immun. 62:3381-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotter, P. A., and J. F. Miller. 1997. A mutation in the Bordetella bronchiseptica bvgS gene results in reduced virulence and increased resistance to starvation, and identifies a new class of Bvg-regulated antigens. Mol. Microbiol. 24:671-685. [DOI] [PubMed] [Google Scholar]
- 15.Danese, P. N., L. A. Pratt, and R. Kolter. 2001. Biofilm formation as a developmental process. Methods Enzymol. 336:19-26. [DOI] [PubMed] [Google Scholar]
- 16.Davey, M. E., and G. A. O'Toole. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64:847-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 18.Deora, R. 2002. Differential regulation of the Bordetella bipA gene: Distinct roles for different BvgA binding sites. J. Bacteriol. 184:6942-6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deora, R. 2004. Multiple mechanisms of bipA gene regulation by the Bordetella BvgAS phosphorelay system. Trends Microbiol. 12:63-65. [DOI] [PubMed] [Google Scholar]
- 20.Deora, R., H. J. Bootsma, J. F. Miller, and P. A. Cotter. 2001. Diversity in the Bordetella virulence regulon: transcriptional control of a Bvg-intermediate phase gene. Mol. Microbiol. 40:669-683. [DOI] [PubMed] [Google Scholar]
- 21.Drenkard, E., and F. M. Ausubel. 2002. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416:740-743. [DOI] [PubMed] [Google Scholar]
- 22.Friedman, L., and R. Kolter. 2004. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol. Microbiol. 51:675-690. [DOI] [PubMed] [Google Scholar]
- 23.Gilberg, S., E. Njamkepo, I. P. du Chatelet, H. Partouche, P. Gueirard, C. Ghasarossian, M. Schlumberger, and N. Guiso. 2002. Evidence of Bordetella pertussis infection in adults presenting with persistent cough in a French area with very high whole-cell vaccine coverage. J. Infect. Dis. 186:415-418. [DOI] [PubMed] [Google Scholar]
- 24.Goodnow, R. A. 1980. Biology of Bordetella bronchiseptica. Microbiol. Rev. 44:722-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammer, B. K., and B. L. Bassler. 2003. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 50:101-114. [DOI] [PubMed] [Google Scholar]
- 26.Hamon, M. A., and B. A. Lazazzera. 2001. The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol. Microbiol. 42:1199-1209. [DOI] [PubMed] [Google Scholar]
- 27.Heininger, U., P. A. Cotter, H. W. Fescemyer, G. M. de Tejada, M. H. Yuk, J. F. Miller, and E. T. Harvill. 2002. Comparative phenotypic analysis of the Bordetella parapertussis isolate chosen for genomic sequencing. Infect. Immun. 70:3777-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irie, Y., S. Mattoo, and M. H. Yuk. 2004. The Bvg virulence control system regulates biofilm formation in Bordetella bronchiseptica. J. Bacteriol. 186:5692-5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinnear, S. M., P. E. Boucher, S. Stibitz, and N. H. Carbonetti. 1999. Analysis of BvgA activation of the pertactin gene promoter in Bordetella pertussis. J. Bacteriol. 181:5234-5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knapp, S., and J. J. Mekalanos. 1988. Two trans-acting regulatory genes (vir and mod) control antigenic modulation in Bordetella pertussis. J. Bacteriol. 170:5059-5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lam, J., R. Chan, K. Lam, and J. W. Costerton. 1980. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect. Immun. 28:546-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis, K., M. A. Saubolle, F. C. Tenover, M. F. Rudinsky, S. D. Barbour, and J. D. Cherry. 1995. Pertussis caused by an erythromycin-resistant strain of Bordetella pertussis. Pediatr. Infect. Dis. J. 14:388-391. [DOI] [PubMed] [Google Scholar]
- 33.Mah, T. F., B. Pitts, B. Pellock, G. C. Walker, P. S. Stewart, and G. A. O'Toole. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306-310. [DOI] [PubMed] [Google Scholar]
- 34.Mah, T. F. C., and G. A. O'Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34-39. [DOI] [PubMed] [Google Scholar]
- 35.Martinez de Tejada, G., P. A. Cotter, U. Heininger, A. Camilli, B. J. Akerley, J. J. Mekalanos, and J. F. Miller. 1998. Neither the Bvg− phase nor the vrg6 locus of Bordetella pertussis is required for respiratory infection in mice. Infect. Immun. 66:2762-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez de Tejada, G., J. F. Miller, and P. A. Cotter. 1996. Comparative analysis of the virulence control systems of Bordetella pertussis and Bordetella bronchiseptica. Mol. Microbiol. 22:895-908. [DOI] [PubMed] [Google Scholar]
- 37.Mattoo, S., A. K. Foreman-Wykert, P. A. Cotter, and J. F. Miller. 2001. Mechanisms of Bordetella pathogenesis. Front. Biosci. 6:E168-E186. [DOI] [PubMed] [Google Scholar]
- 38.Mattoo, S., J. F. Miller, and P. A. Cotter. 2000. Role of Bordetella bronchiseptica fimbriae in tracheal colonization and development of a humoral immune response. Infect. Immun. 68:2024-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller, J. F., S. A. Johnson, W. J. Black, D. T. Beattie, J. J. Mekalanos, and S. Falkow. 1992. Constitutive sensory transduction mutations in the Bordetella pertussis bvgS gene. J. Bacteriol. 174:970-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller, J. F., C. R. Roy, and S. Falkow. 1989. Analysis of Bordetella pertussis virulence gene regulation by use of transcriptional fusions in Escherichia coli. J. Bacteriol. 171:6345-6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller, M. B., K. Skorupski, D. H. Lenz, R. K. Taylor, and B. L. Bassler. 2002. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110:303-314. [DOI] [PubMed] [Google Scholar]
- 42.O'Toole, G. A., L. A. Pratt, P. I. Watnick, D. K. Newman, V. B. Weaver, and R. Kolter. 1999. Genetic approaches to study of biofilms. Methods Enzymol. 310:91-109. [DOI] [PubMed] [Google Scholar]
- 43.Parkhill, J., M. Sebaihia, A. Preston, L. D. Murphy, N. Thomson, D. E. Harris, M. T. G. Holden, C. M. Churcher, S. D. Bentley, K. L. Mungall, A. M. Cerdeno-Tarraga, L. Temple, K. James, B. Harris, M. A. Quail, M. Achtman, R. Atkin, S. Baker, D. Basham, N. Bason, I. Cherevach, T. Chillingworth, M. Collins, A. Cronin, P. Davis, J. Doggett, T. Feltwell, A. Goble, N. Hamlin, H. Hauser, S. Holroyd, K. Jagels, S. Leather, S. Moule, H. Norberczak, S. O'Neil, D. Ormond, C. Price, E. Rabbinowitsch, S. Rutter, M. Sanders, D. Saunders, K. Seeger, S. Sharp, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, L. Unwin, S. Whitehead, B. G. Barrell, and D. J. Maskell. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 35:32-40. [DOI] [PubMed] [Google Scholar]
- 44.Parsek, M. R., and E. P. Greenberg. 2000. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. USA 97:8789-8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pratt, L. A., and R. Kolter. 1998. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 30:285-293. [DOI] [PubMed] [Google Scholar]
- 46.Purevdorj, B., J. W. Costerton, and P. Stoodley. 2002. Influence of hydrodynamics and cell signaling on the structure and behavior of Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 68:4457-4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Relman, D., E. Tuomanen, S. Falkow, D. T. Golenbock, K. Saukkonen, and S. D. Wright. 1990. Recognition of a bacterial adhesin by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell 61:1375-1382. [DOI] [PubMed] [Google Scholar]
- 48.Scarlato, V., and R. Rappuoli. 1991. Differential response of the bvg virulence regulon of Bordetella pertussis to MgSO4 modulation. J. Bacteriol. 173:7401-7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schaefer, A. L., E. P. Greenberg, and M. R. Parsek. 2001. Acylated homoserine lactone detection in Pseudomonas aeruginosa biofilms by radiolabel assay. Methods Enzymol. 336:41-47. [DOI] [PubMed] [Google Scholar]
- 50.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 51.Solano, C., B. Garcia, J. Valle, C. Berasain, J. M. Ghigo, C. Gamazo, and I. Lasa. 2002. Genetic analysis of Salmonella enteritidis biofilm formation: critical role of cellulose. Mol. Microbiol. 43:793-808. [DOI] [PubMed] [Google Scholar]
- 52.Stainer, D. W., and M. J. Scholte. 1970. A simple chemically defined medium for the production of phase I Bordetella pertussis. J. Gen. Microbiol. 63:211-220. [DOI] [PubMed] [Google Scholar]
- 53.Stanley, N. R., and B. A. Lazazzera. 2004. Environmental signals and regulatory pathways that influence biofilm formation. Mol. Microbiol. 52:917-924. [DOI] [PubMed] [Google Scholar]
- 54.Stewart, P. S., and J. W. Costerton. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135-138. [DOI] [PubMed] [Google Scholar]
- 55.Stockbauer, K. E., B. Fuchslocher, J. F. Miller, and P. A. Cotter. 2001. Identification and characterization of BipA, a Bordetella Bvg-intermediate phase protein. Mol. Microbiol. 39:65-78. [DOI] [PubMed] [Google Scholar]
- 56.Stoodley, P., I. Dodds, J. D. Boyle, and H. M. Lappin-Scott. 1999. Influence of hydrodynamics and nutrients on biofilm structure. J. Appl. Microbiol. 85:S19-S28. [DOI] [PubMed] [Google Scholar]
- 57.Stoodley, P., K. Sauer, D. G. Davies, and J. W. Costerton. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187-209. [DOI] [PubMed] [Google Scholar]
- 58.Sutherland, I. W. 2001. The biofilm matrix—an immobilized but dynamic microbial environment. Trends Microbiol. 9:222-227. [DOI] [PubMed] [Google Scholar]
- 59.Swords, W. E., M. L. Moore, L. Godzicki, G. Bukofzer, M. J. Mitten, and J. VonCannon. 2004. Sialylation of lipooligosaccharides promotes biofilm formation by nontypeable Haemophilus influenzae. Infect. Immun. 72:106-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Zee, A., F. Mooi, J. Van Embden, and J. Musser. 1997. Molecular evolution and host adaptation of Bordetella spp.: phylogenetic analysis using multilocus enzyme electrophoresis and typing with three insertion sequences. J. Bacteriol. 179:6609-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watnick, P., and R. Kolter. 2000. Biofilm, city of microbes. J. Bacteriol. 182:2675-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watnick, P. I., and R. Kolter. 1999. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol. Microbiol. 34:586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson, K. E., P. K. Cassiday, T. Popovic, and G. N. Sanden. 2002. Bordetella pertussis isolates with a heterogeneous phenotype for erythromycin resistance. J. Clin. Microbiol. 40:2942-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wozniak, D. J., T. J. O. Wyckoff, M. Starkey, R. Keyser, P. Azadi, G. A. O'Toole, and M. R. Parsek. 2003. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. USA 100:7907-7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wright, S. W. 1998. Pertussis infection in adults. South. Med. J. 91:702-708. [DOI] [PubMed] [Google Scholar]
- 66.Yeh, S. H. 2003. Pertussis: persistent pathogen, imperfect vaccines. Expert Rev. Vaccines 2:113-127. [DOI] [PubMed] [Google Scholar]
- 67.Yuk, M. H., E. T. Harvill, P. A. Cotter, and J. F. Miller. 2000. Modulation of host immune responses, induction of apoptosis and inhibition of NF-kappa B activation by the Bordetella type III secretion system. Mol. Microbiol. 35:991-1004. [DOI] [PubMed] [Google Scholar]
- 68.Zhu, J., M. B. Miller, R. E. Vance, M. Dziejman, B. L. Bassler, and J. J. Mekalanos. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 99:3129-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]