Abstract
A polysaccharide deacetylase homologue, PdaA, was determined to act as an N-acetylmuramic acid deacetylase in vitro. Histidine-tagged truncated PdaA (with the putative signal sequence removed) was overexpressed in Escherichia coli cells and purified. Measurement of deacetylase activity showed that PdaA could deacetylate peptidoglycan treated with N-acetylmuramoyl-l-alanine amidase CwlH but could not deacetylate peptidoglycan treated with or without dl-endopeptidase LytF (CwlE). Reverse-phase high-performance liquid chromatography and mass spectrometry (MS) and MS-MS analyses indicated that PdaA could deacetylate the N-acetylmuramic acid residues of purified glycan strands derived from Bacillus subtilis peptidoglycan.
It is thought that the spore cell wall peptidoglycan of Bacillus subtilis plays a role in maintenance of heat resistance and dormancy. The B. subtilis spore cell wall is composed of two layers, the germ cell wall (inner layer) and the cortex (outer layer). In the outer layer (cortex) the muramic acid residues are replaced by single l-alanine residues and muramic δ-lactam (1, 13). The muramic δ-lactam structure is important for spore germination in B. subtilis because CwlD (l-alanine amidase homologue)- or PdaA (polysaccharide deacetylase homologue)-deficient spores have no muramic δ-lactam structure in the cortex and cannot germinate (6, 12, 15). Previously, we predicted that the first, second, and third steps of muramic δ-lactam formation are the cleavage of muropeptide side chains by CwlD, de-N-acetylation of muramic acid residues by PdaA, and transpeptidation of muramic acid residues, respectively (6).
In this study we found that PdaA of B. subtilis can individually deacetylate the N-acetylmuramic acid residues of purified glycan strands derived from B. subtilis peptidoglycan in vitro.
MATERIALS AND METHODS
Plasmid construction for h-ΔPdaA overexpression.
To construct a truncated PdaA expression plasmid, pUCΔSfjS (6) was digested with SacI and HindIII, and then a fragment containing the pdaA gene with a deletion of the putative signal sequence was prepared with a GeneCleanII kit (Funakoshi). The fragment was ligated to the corresponding site of pQE-30 (Apr; QIAGEN), resulting in pQEΔSfjS. The pQEΔSfjS plasmid was used for production of h-ΔPdaA, which is a truncated PdaA protein with a histidine tag at its N terminus.
Overexpression and purification of h-ΔPdaA.
To overexpress h-ΔPdaA, pQEΔSfjS was used for the transformation of Escherichia coli JM109 [recA1 Δ(lac-proAB) endA1 gyrA96 thi-1 hsdR17 relA1 supE44 (F′ traD36 proAB+ lacIq lacZ ΔM15)]. The resultant transformant, JM109(pQEΔSfjS), was cultured in Luria-Bertani medium (14) or 2× YT medium (16 g of Bacto Tryptone [Difco] per liter, 10 g of yeast extract per liter, 5 g of NaCl per liter; pH 7.3) at 37°C. When an optical density at 600 nm of 1.0 was reached, isopropyl-β-d-thiogalactopyranoside (IPTG) (final concentration, 1 mM) was added to the culture. After 1 h of incubation, the cells were harvested by centrifugation. Purification of h-ΔPdaA was performed basically as described previously (10) and was confirmed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (5).
Overexpression and purification of h-CwlH and H-LytF.
Overexpression and purification of h-CwlH (N-acetylmuramoyl-l-alanine amidase) and H-LytF (H-CwlE; dl-endopeptidase) were performed as described by Nugroho et al. (10) and Ohnishi et al. (11), respectively.
Preparation of cell wall and peptidoglycan of B. subtilis.
Cell wall was prepared from B. subtilis ATCC 6633 (Sigma) or 168 (trpC2; laboratory stock) essentially as described previously (4). In addition, peptidoglycan was prepared as described by DeHart et al. (3).
Measurement of deacetylase activity with peptidoglycan by determination of acetic acid.
To determine the optimal pH of the deacetylase activity of h-ΔPdaA, Good's buffers (50 mM) containing 1 mg of B. subtilis peptidoglycan derived from B. subtilis ATCC 6633 cell wall per ml were used. Chitin oligomer (hexa-N-acetylchitohexaose; 1 mg/ml) was also used as a substrate. Purified h-ΔPdaA was added to the buffers to a final concentration of 2 μg/ml. After incubation at 37°C for 4 h, each solution was centrifuged, and then the supernatant was collected. The acetic acid released from the substrates by deacetylase activity was measured with an F-kit for the determination of acetic acid (Roche).
Measurement of deacetylase activity with peptidoglycan treated with CwlH or LytF.
To measure deacetylase activity, 5 mM HEPES buffer (pH 7.0) containing 2 mg of peptidoglycan per ml as a substrate was used. Purified h-CwlH and H-LytF (H-CwlE) were added to the buffer to final concentrations of 3.6 and 6.4 μg per ml, respectively. After incubation at 37°C for 4 h, the solution was boiled for 10 min. The hydrolyzed peptidoglycan became soluble in the buffer. After centrifugation, the supernatant was collected and then added to an equal volume of Good's buffer (100 mM). Purified h-ΔPdaA was added to the mixture to a final concentration of 2 μg/ml. After incubation at 37°C for 4 h, the solution was centrifuged, and then the supernatant was collected.
Preparation of glycan strands consisting of GlcNAc-MurNAc polymer.
Preparation of glycan strands consisting of GlcNAc-MurNAc polymer was performed basically as described by Vollmer and Tomasz (17). Ten milligrams of purified peptidoglycan derived from B. subtilis 168 cell wall and 5 ml of 5 mM sodium phosphate (pH 7.0) were mixed. Fifty micrograms of h-CwlH was added to the mixture, and then the mixture was incubated at 37°C for 12 h. To completely cleave peptide side chains from glycan strands, 50 μg of h-CwlH was added to the mixture, which was then incubated at 37°C for 12 h. After the mixture had been boiled for 5 min, it was centrifuged at 27,000 × g for 10 min, and then the supernatant was collected. To remove h-CwlH, Ni2+-chelated spherical beads derived from a Hi-Trap chelating column (Amersham Biosciences) were added to the supernatant, and then the mixture was allowed to stand for 15 min. After centrifugation of the mixture (27,000 × g, 10 min), the supernatant was collected. Glycan strands were separated from the peptides by size exclusion chromatography (TSKgelG2000SW column [TOSOH]; flow buffer, 5 mM sodium phosphate [pH 6.0] containing 100 mM NaCl; flow rate, 1 ml/min; monitoring wavelength, 202 nm; Shimadzu LC-6A high-performance liquid chromatography [HPLC] system). The collected sample containing glycan strands was freeze-dried, and then the sample was dissolved in ultrapure water. To remove NaCl and buffer constituents, gel filtration was performed (Hi-Trap desalting column [Amersham]; flow buffer, ultrapure water; flow rate, 1 ml/min; monitoring wavelength, 202 nm; Shimadzu LC-6A HPLC system). Glycan strands were collected and then freeze-dried.
Preparation of N-acetylated glycan strands.
Basically, N-acetylated glycan strands were prepared as previously described by Vollmer and Tomasz (17). Two milligrams of purified glycan strands was dissolved in 1 ml of ultrapure water, and then 0.25 ml of saturated NaHCO3 buffer and 0.25 ml of 5% anhydroacetic acid were added to the solution. After the mixture had been stirred at 0°C for 30 min, 375 μl of 5% anhydroacetic acid was added. After the mixture had been stirred at 0°C for 30 min, it was kept at room temperature for 1 h. To remove low-molecular-weight substances, gel filtration was performed (Hi-Trap desalting column; flow buffer, ultrapure water; flow rate, 1 ml/min; monitoring wavelength, 202 nm; Shimadzu LC-6A HPLC system). The N-acetylated glycan strands were collected and then freeze-dried.
Deacetylation with h-ΔPdaA of N-acetylated or nontreated glycan strands.
One hundred micrograms of N-acetylated or nontreated glycan strands, 4 μg of purified h-ΔPdaA, and 100 μl of 25 mM sodium phosphate (pH 7.0) were mixed, and then the mixture was incubated at 37°C for 12 h. Twenty microliters of a 2-μg/μl lysozyme (hen egg white lysozyme [Sigma]) solution containing 5 mM sodium phosphate (pH 7.0) and 0.02% sodium azide was added to the mixture, and then the mixture was incubated at 37°C for 12 h. After 30 μl of sodium borate (pH 9.0; final concentration, 0.5 M) had been added, we confirmed that the pH of the mixture was about 9. One to two milligrams of NaBH4 was added to the mixture to reduce the reducing ends of amino sugars. Then one-third of the mixture was separated by reverse-phase HPLC (RP-HPLC) with a Symmetry Shield RP18 column (Waters) (flow rate, 1 ml/min; monitoring wavelength, 202 nm; Shimadzu LC-10AD HPLC system). Elution buffer A comprised 0.05% trifluoroacetic acid (TFA), and buffer B comprised 0.05% TFA and 40% CH3CN. Elution was performed for 10 min with buffer A at 40°C (with a column heater) with an isocratic gradient and then for 30 min with a linear gradient of buffer B (from 0 to 50%) at 40°C.
Identification of separated peaks on RP-HPLC by ESI-MS-MS.
Peaks separated by RP-HPLC were freeze-dried, and this was followed by addition of 50% CH3CN. Samples were identified by electrospray ionization (ESI)-mass spectrometry (MS) or ESI—MS-MS (Agilent 1100 series LC/MSD Trap VL).
Lysozyme treatment of the PdaA product.
To determine whether the PdaA product was deacetylated, the PdaA product and tetrasaccharide were treated with lysozyme. For this experiment, the PdaA product and tetrasaccharide were separated and freeze-dried. The dried samples were suspended in 50 mM sodium phosphate (pH 7.0). Fifty micrograms of lysozyme was added to each sample, and then the mixtures were incubated at 37°C for 12 h. The pH of each mixture was adjusted to 2 to 3 with TFA, and then the mixture was subjected to RP-HPLC with a Symmetry Shield RP18 column (Waters). The separation conditions were the same as those described above.
RESULTS
Purification of histidine-tagged truncated PdaA.
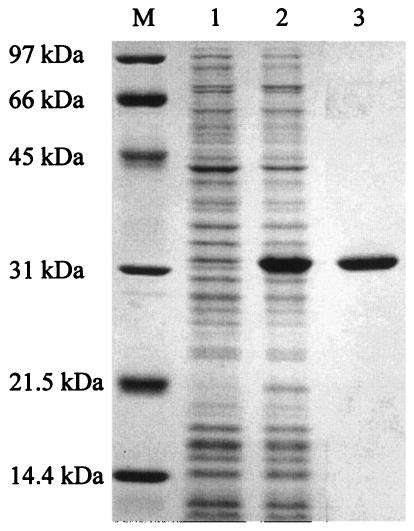
The histidine-tagged truncated PdaA protein (h-ΔPdaA [putative signal sequence of PdaA removed]) was overexpressed in E. coli JM109 cells and then purified on a Hi-Trap chelating column as described in Materials and Methods. Figure 1 shows the results of an SDS-PAGE analysis of the purified protein. The purified h-ΔPdaA (Mr, 29,636) produced one band at 32 kDa (Fig. 1, lane 3).
FIG. 1.
SDS-14% PAGE of h-ΔPdaA. h-ΔPdaA was expressed in E. coli JM109 cells in the presence of 1 mM IPTG. Lanes 1 and 2, proteins extracted from whole cells without and with IPTG added, respectively; lane 3, purified h-ΔPdaA; lane M, protein standards (Bio-Rad).
Deacetylation of peptidoglycan.
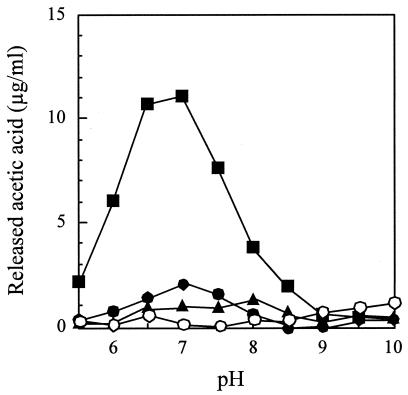
Since the amino acid sequence of PdaA exhibits high similarity to the sequences of several polysaccharide deacetylases, peptidoglycan was used as a substrate to determine deacetylase activity. The deacetylase activity of h-ΔPdaA was measured in Good's buffer at various pHs (from pH 5.5 to 10.0). As shown in Fig. 2, h-ΔPdaA exhibited little deacetylase activity with peptidoglycan. To further investigate the deacetylase activity, cell wall (containing anion polymers) was used as a substrate. However, h-ΔPdaA did not act on this substrate (data not shown).
FIG. 2.
Deacetylase activity of h-ΔPdaA with nontreated peptidoglycan (•), peptidoglycan treated with l-alanine amidase CwlH (▪), and peptidoglycan treated with dl-endopeptidase LytF (▴). One milligram of chitin oligomer (hexa-N-acetylchitohexaose) per ml was also used (○). Purified h-ΔPdaA (final concentration, 2 μg/ml) was added to the following buffers (final concentration, 50 mM) containing 1 mg of nontreated peptidoglycan per ml or peptidoglycan treated with CwlH or LytF as a substrate (see Materials and Methods): morpholineethanesulfonic acid (MES) buffer for pH 5.5, 6.0, and 6.5; HEPES buffer for pH 7.0, 7.5, 8.0, and 8.5; and CHES [2-(cyclohexylamino)-ethanesulfonic acid] buffer for pH 9.0, 9.5, and 10.0. The deacetylase reaction was carried out at 37°C for 4 h.
Deacetylation of peptidoglycan treated with CwlH (l-alanine amidase) or LytF (dl-endopeptidase).
Since several chitooligosaccharide deacetylases act on chitooligosaccharide and/or O-hydroxyethylated chitin (glycol chitin) (7, 9, 16) and glycan strands of Streptococcus pneumoniae peptidoglycan (17), we were curious to know whether h-ΔPdaA acts on chitin and glycan strands of B. subtilis peptidoglycan. Chitin oligomer (hexa-N-acetylchitohexaose) did not act as a substrate for h-ΔPdaA (Fig. 2). Then we used peptidoglycan treated with CwlH (l-alanine amidase) as a substrate for determination of deacetylase activity. As shown in Fig. 2, h-ΔPdaA exhibited deacetylase activity. The optimum pH of h-ΔPdaA was 7.0 (Fig. 2).
h-ΔPdaA was active with peptidoglycan treated with CwlH (soluble substrate) but was not active with peptidoglycan and cell wall (insoluble substrates). It is possible that h-ΔPdaA acts only on a soluble substrate. To rule out this possibility, we used peptidoglycan treated with LytF (dl-endopeptidase) as a substrate. h-ΔPdaA did not show any activity with this substrate (Fig. 2). These results indicate that h-ΔPdaA can deacetylate only peptidoglycan treated with CwlH.
Deacetylation of glycan strands.
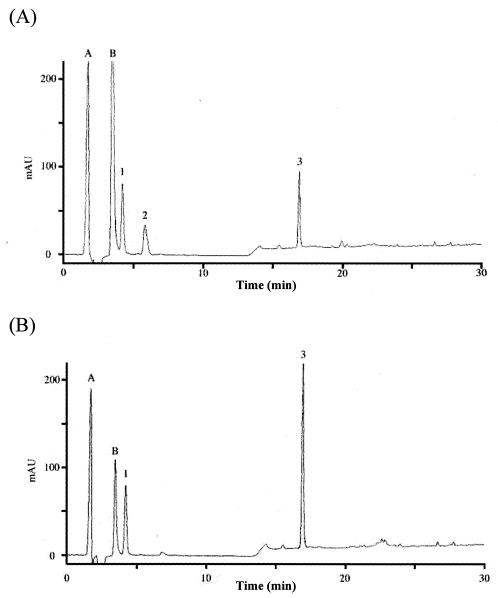
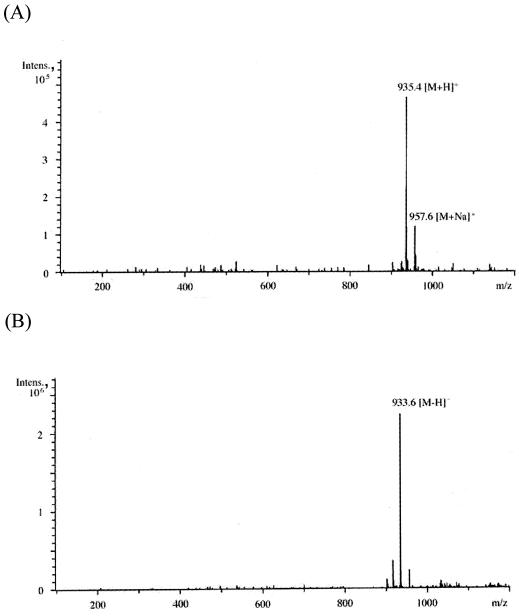
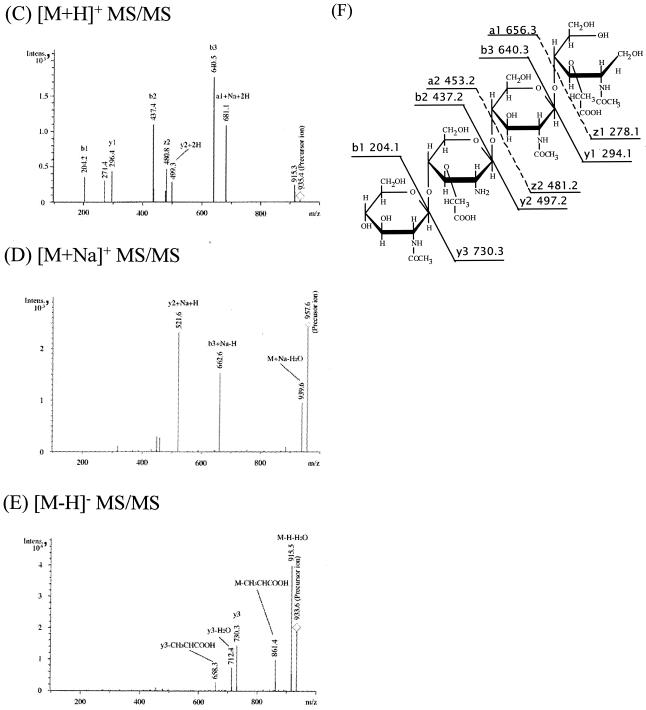
To confirm that h-ΔPdaA deacetylates only glycan strands, glycan strands were N acetylated, purified, and used as a substrate for h-ΔPdaA, as follows. After digestion of peptidoglycan with or without h-ΔPdaA, the reaction products were cleaved with hen egg white lysozyme (muramidase), and then the reducing ends of amino sugars were reduced with sodium borohydride. Figure 3 shows that the samples treated with and without h-ΔPdaA were separated by RP-HPLC. As shown in Fig. 3, peak 2 (retention time, 6 min) appeared and the level of peak 3 (retention time, 17 min) decreased when h-ΔPdaA was present. Peak A (retention time, 1 min) and peak B (retention time, 3 min) were background artifacts (reaction buffer and NaN3, respectively), because both of these peaks also appeared when only the lysozyme solution was separated by RP-HPLC (data not shown). MS analysis of peak 1 in the positive and negative modes gave fragment ions at m/z 521.5 and 497.3, respectively (data not shown). These values correspond to [M+Na]+ and [M-H]−, respectively. MS-MS analysis of peak 1 indicated that this peak represented a disaccharide (N-acetylglucosamine [GlcNAc]-N-acetylmuramic acid reduced at its reducing end [MurNAcr]) (data not shown). MS analysis of peak 3 in the positive and negative modes gave fragment ions at m/z 999.6 and 975.6, respectively (data not shown). These values correspond to [M+Na]+ and [M-H]−, respectively. MS-MS analysis of peak 3 indicated that this peak represented a tetrasaccharide (GlcNAc-N-acetylmuramic acid [MurNAc]-GlcNAc-MurNAcr) (data not shown). MS analysis of peak 2 in the positive and negative modes gave fragment ions at m/z 935.4 and 957.6 and at m/z 933.6, respectively (Fig. 4A and B).
FIG. 3.
Lysozyme digestion of isolated N-acetylated peptidoglycan glycan strands treated with (A) or without (B) PdaA. Peptidoglycan from B. subtilis 168 was digested with l-alanine amidase CwlH, and then N-acetylated glycan strands were purified mainly by size exclusion chromatography (see Materials and Methods). After the N-acetylated glycan strands had been treated with (A) or without (B) PdaA, samples were treated with lysozyme, and this was followed by addition of NaBH4. The samples were separated by RP-HPLC. Peak 1, disaccharide (GlcNAc-MurNAcr); peak 2, N-deacetylated tetrasaccharide (GlcNAc-Mur-GlcNAc-MurNAcr); peak 3, tetrasaccharide (GlcNAc-MurNAc-GlcNAc-MurNAcr); peak A, reaction buffer; peak B, NaN3.
FIG. 4.
(A to E) ESI-MS analyses (A and B) and ESI-MS-MS analyses (C to E) of peak 2 (GlcNAc-Mur-GlcNAc-MurNAcr) in Fig. 3A. Panels A and B show ESI-MS analysis in the positive and negative modes, respectively. Panels C to E show ESI-MS-MS analyses of the [M+H]+ precursor ion (m/z 935.4), [M+Na]+ precursor ion (m/z 957.6), and [M-H]− precursor ion (m/z 933.6), respectively. (F) Putative structure and calculated molecular weight of each fragment of peak 2 in Fig. 3A. Ion series b, y, a, and z correspond to the fragment peaks in panels C to E. Intens., intensity.
The results of the MS and MS-MS analyses suggested that PdaA deacetylates MurNAc. To confirm that PdaA is an N-acetylmuramic acid deacetylase, peak 2 was treated with hen egg white lysozyme. If peak 2 was an N-deacetylated tetrasaccharide (GlcNAc-Mur-GlcNAc-MurNAcr), lysozyme could not cleave the Mur-GlcNAc bond (lysozyme can cleave only MurNAc-GlcNAc bonds). After lysozyme treatment, peak 2 was separated by RP-HPLC. We found that the retention time of peak 2 with lysozyme treatment was the same as that without treatment (data not shown). After lysozyme treatment, peak 3 was separated by RP-HPLC as a control. The profile of peak 3 with lysozyme treatment was different from the profile of peak 3 without the treatment, and three new peaks (a disaccharide [GlcNAc-MurNAcr] and α- and β-anomers of a disaccharide [GlcNAc-MurNAc]) were found (data not shown). These results strongly suggest that PdaA acts as an N-acetylmuramic acid deacetylase in vitro.
DISCUSSION
h-ΔPdaA is active with peptidoglycan treated with CwlH (l-alanine amidase) but is not active with peptidoglycan treated with LytF (dl-endopeptidase) (Fig. 2). Moreover, h-ΔPdaA can also deacetylate purified glycan strands (GlcNAc-MurNAc polymer) (Fig. 3B). However, this enzyme is not active with purified glycan strands carrying an l-Ala-d-Glu dipeptide, GlcNAc-MurNAc(-l-Ala-d-Glu) polymer (data not shown). The data indicate that PdaA can deacetylate glycan strands and strictly recognizes this substrate for catalysis. Peptide side chains in peptidoglycan may prevent the deacetylase activity of PdaA because PdaA deacetylates glycan strands.
In a previous study, PdaA was found to be required for the production of muramic δ-lactam residues in the spore cortex of B. subtilis, and the spore cortex of a pdaA-deficient mutant had muramic acid residues without peptide side chains (6). In vitro, PdaA is an N-acetylmuramic acid deacetylase (Fig. 4) (unpublished results) and can deacetylate glycan strands (Fig. 3). These results support the hypothesis that PdaA deacetylates N-acetylmuramic acid residues without peptide side chains.
We previously proposed the following pathway for δ-lactam biosynthesis (6). The first step of δ-lactam formation appears to be cleavage of peptide side chains bound to N-acetylmuramic acid residues by CwlD, which is an l-alanine amidase homologue and is required for muramic δ-lactam synthesis (12). The second step is the de-N-acetylation of muramic acid residues by PdaA. The last step in δ-lactam formation involves transpeptidase activity. Recently, Gilmore et al. reported that introduction of the pdaA and cwlD genes into E. coli cells led to lactam formation in peptidoglycan (8). This finding suggests that PdaA acts in both the de-N-acetylation of muramic acid residues (second step) and the transpeptidase reaction (lactam cyclization; third step) for E. coli peptidoglycan. However, we could not detect any transpeptidase activity in vitro. This may reflect the difference between the in vitro and in vivo conditions. At least, PdaA can deacetylate N-acetylmuramic acid residues without peptide side chains in vitro.
Recently, Forouhar et al. determined the structure of PdaA by X-ray diffraction (Protein Data Bank [PDB] identification no. 1NY1). PdaA has a beta/alpha barrel structure, and this structure is conserved in several oligosaccharide-related proteins (PDB identification no. 1TML, 1QJW, 1OCN, 1K1X, 1HTY, and 1O7D). Moreover, Blair and van Aalten determined the complex structures of PdaA and N-acetylglucosamine (2). Thus, these results supported the hypothesis that PdaA is associated with GlcNAc-MurNAc glycan chain modification.
PdaA is similar to several polysaccharide deacetylases. Rhizobium leguminosarum NodB, Streptococcus pneumoniae PgdA (peptidoglycan N-acetylglucosamine deacetylase A), Saccharomyces cerevisiae CDA1 and CDA2 (chitin deacetylases 1 and 2), and Mucor rouxii CHDE (chitin deacetylase) are highly homologous to PdaA (40.1, 36.4, 31.7, 26.8, and 25.5% identity with the catalytic domain of PdaA, respectively). However, these enzymes mainly deacetylate N-acetylglucosamine, and PdaA deacetylates N-acetylmuramic acid. Thus, PdaA is a unique and new member of the polysaccharide deacetylase family.
This is the first report that a polysaccharide deacetylase homologue mainly exhibits N-acetylmuramic acid deacetylase activity in vitro.
Acknowledgments
We thank H. Karasawa, Food Technology Research Institute of Nagano Prefecture, for kindly helping with the MS and MS-MS analyses.
This research was supported by Grants-in-Aid for Scientific Research on Priority Areas, Genome Biology (grant 12206005), the 21st Century COE Program, and Scientific Research (B) (grant 16380059 to J.S.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
REFERENCES
- 1.Atrih, A., P. Zöllner, G. Allmaier, and S. J. Foster. 1996. Structural analysis of Bacillus subtilis 168 endospore peptidoglycan and its role during differentiation. J. Bacteriol. 178:6173-6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blair, D. E., and D. M. van Aalten. 2004. Structures of Bacillus subtilis PdaA, a family 4 carbohydrate esterase, and a complex with N-acetyl-glucosamine. FEBS Lett. 570:13-19. [DOI] [PubMed] [Google Scholar]
- 3.DeHart, H. P., H. E. Heath, L. S. Heath, P. A. LeBlanc, and G. L. Sloan. 1995. The lysostaphin endopeptidase resistance gene (epr) specifies modification of peptidoglycan cross bridges in Staphylococcus simulans and Staphylococcus aureus. Appl. Environ. Microbiol. 61:1475-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fein, J. E., and H. J. Rogers. 1976. Autolytic enzyme-deficient mutants of Bacillus subtilis 168. J. Bacteriol. 127:1427-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukushima, T., S. Ishikawa, H. Yamamoto, N. Ogasawara, and J. Sekiguchi. 2003. Transcriptional, functional and cytochemical analyses of the veg gene in Bacillus subtilis. J. Biochem. 133:475-483. [DOI] [PubMed] [Google Scholar]
- 6.Fukushima, T., H. Yamamoto, A. Atrih, S. J. Foster, and J. Sekiguchi. 2002. A polysaccharide deacetylase gene (pdaA) is required for germination and for production of muramic δ-lactam residues in the spore cortex of Bacillus subtilis. J. Bacteriol. 184:6007-6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao, X. D., T. Katsumoto, and K. Onodera. 1995. Purification and characterization of chitin deacetylase from Absidia coerulea. J. Biochem. 117:257-263. [DOI] [PubMed] [Google Scholar]
- 8.Gilmore, M. E., D. Bandyopadhyay, A. M. Dean, S. D. Linnstaedt, and D. L. Popham. 2004. Production of muramic δ-lactam in Bacillus subtilis spore peptidoglycan. J. Bacteriol. 186:80-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kafetzopoulos, D., G. Thireos, J. N. Vournakis, and V. Bouriotis. 1993. The primary structure of a fungal chitin deacetylase reveals the function for two bacterial gene products. Proc. Natl. Acad. Sci. USA 90:8005-8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nugroho, F. A., H. Yamamoto, Y. Kobayashi, and J. Sekiguchi. 1999. Characterization of a new sigma-K-dependent peptidoglycan hydrolase gene that plays a role in Bacillus subtilis mother cell lysis. J. Bacteriol. 181:6230-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohnishi, R., S. Ishikawa, and J. Sekiguchi. 1999. Peptidoglycan hydrolase LytF plays a role in cell separation with CwlF during vegetative growth of Bacillus subtilis. J. Bacteriol. 181:3178-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popham, D. L., J. Helin, C. E. Costello, and P. Setlow. 1996. Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc. Natl. Acad. Sci. USA 93:15405-15410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Popham, D. L., J. Helin, C. E. Costello, and P. Setlow. 1996. Analysis of the peptidoglycan structure of Bacillus subtilis endospores. J. Bacteriol. 178:6451-6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 15.Sekiguchi, J., K. Akeo, H. Yamamoto, F. K. Khasanov, J. C. Alonso, and A. Kuroda. 1995. Nucleotide sequence and regulation of a new putative cell wall hydrolase gene, cwlD, which affects germination in Bacillus subtilis. J. Bacteriol. 177:5582-5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsigos, I., and V. Bouriotis. 1995. Purification and characterization of chitin deacetylase from Colletotrichum lindemuthianum. J. Biol. Chem. 270:26286-26291. [DOI] [PubMed] [Google Scholar]
- 17.Vollmer, W., and A. Tomasz. 2000. The pgdA gene encodes for a peptidoglycan N-acetylglucosamine deacetylase in Streptococcus pneumoniae. J. Biol. Chem. 275:20496-20501. [DOI] [PubMed] [Google Scholar]