Abstract
The human primary motor cortex (M1) participates in motor learning and response selection, functions that rely on feedback on the success of behavior (i.e. reward). To investigate the possibility that behavioral contingencies alter M1 activity in humans, we tested intracortical inhibition with single and paired (subthreshold/suprathreshold) transcranial magnetic stimulation during a slot machine simulation that delivered variable money rewards for three-way matches and required no movement. A two-way match before the third barrel had stopped (increased reward expectation) was associated with more paired-pulse inhibition than no match. Receiving a large reward on the preceding trial augmented this effect. A control task that manipulated attention to the same stimuli produced no changes in excitability. The origin of this reward-related activity is not clear, although dopaminergic ventral tegmental area neurons project to M1, where they are thought to inhibit output neurons and could be the source of the finding. Transcranial magnetic stimulation of M1 may be useful as a quantitative measure of reward-related activity.
Keywords: motor cortex, reward, stimulation, VTA
Introduction
Rewarding and punishing events are processed by brain regions that decode their salience, predictability and comparability to prior events, thereby modifying emotional and motivational states, autonomic responses and behavior (Schultz & Dickinson, 2000; Schultz, 2000; Pessiglione et al., 2007). Studies in animals (Schultz, 2000, 2001, 2002, 2004; Fiorillo et al., 2003) and humans (Schultz, 2000; Pochon et al., 2002; Skolnick & Davidson, 2002; Knutson & Cooper, 2005; Oya et al., 2005; Hampton & O’Doherty, 2007; Van der Linden et al., 2007) have identified nodes comprising the ‘decoding arm’ of the network that analyses and stores the informational content of rewards: the orbitofrontal (O’Doherty et al., 2001; Gottfried et al., 2003) and medial pre-frontal (PFC)/anterior cingulate cortices (Williams et al., 2004; Oya et al., 2005), hippocampus (Chau et al., 2004; Wittmann et al., 2005), striatum (Haruno et al., 2004; Tanaka et al., 2004; Abler et al., 2006; Haruno & Kawato, 2006; O’Doherty et al., 2006; Rodriguez et al., 2006), amygdala (Gottfried et al., 2003) and ventral tegmental area (VTA) where the mesolimbic and mesocortical dopamine (DA) pathways arise (Schultz, 2000, 2004; Fiorillo et al., 2003; Chau et al., 2004). An ‘executive arm’ of the network links reward to future behavior and is thought to include the VTA (Schultz, 2004), striatum (Schultz & Dickinson, 2000; Morris et al., 2006), nucleus accumbens (Chau et al., 2004; Williams et al., 2004), PFC (Gaspar et al., 1992; Schultz & Dickinson, 2000; Frankle et al., 2006) and dorsal anterior cingulate cortex (Williams et al., 2004; Oya et al., 2005).
Generally, it is assumed that rewards influence behavior through their effects on PFC activity and that downstream motor areas are concerned only with execution (Carmichael & Price, 1996; Ongur & Price, 2000; Schultz & Dickinson, 2000; Haber, 2003; Frankle et al., 2006). However, the role of the primary motor cortex (M1) in learning, selecting and planning behavior may be broader than conventionally thought (Graziano, 2006).
Among the areas potentially influencing M1 through direct or indirect connections (Haber, 2003; Schmahmann & Pandya, 2006), many are involved in reward processing, e.g. the striatum (Schultz, 2000; Knutson et al., 2001; Haber, 2003), orbitofrontal cortex and amygdala (Schultz, 2000; Gottfried et al., 2003), anterior cingulate cortex (Williams et al., 2004), and supplementary motor area (Campos et al., 2005). However, VTA dopaminergic neurons also project directly to M1 in roughly equal numbers as to the ventral striatum and other frontal areas (Gaspar et al., 1992, 1995; Williams & Goldman-Rakic, 1993). Cortical DA projections synapse on both pyramidal cells and GABAergic interneurons (Sesack et al., 1998; Tseng et al., 2006) and, through these inputs, reward-related behavioral contingencies may modulate M1 activity, along with other frontal areas.
Transcranial magnetic stimulation (TMS) has been used extensively to study the physiology of M1. The amplitude of the motor evoked potential (MEP) to TMS reflects the integration of excitatory and inhibitory inputs to cortical and spinal motoneurons, also referred to as their ‘excitability’. However, the status of cortical circuits can be probed with little or no spinal outflow (Kujirai et al., 1993; Nakamura et al., 1997; Hanajima et al., 1998; Sanger et al., 2001; Matsunaga et al., 2002). Paired-pulse TMS can measure the inhibitory or facilitatory effect of a ‘conditioning’ pulse, too weak to evoke spinal cord activity, on the MEP from a suprathreshold ‘test’ pulse delivered a few milliseconds later. The effect is expressed as the ratio of the conditioned to the unconditioned (or ‘test’) MEP, evoked with no preceding pulse. The conditioning pulse inhibits the MEP when it is delivered about 1–4 ms before the test pulse and facilitates it at interstimulus intervals (ISIs) of about 6–20 ms (Ziemann et al., 1996a). Enhancers of GABAA receptor-mediated inhibition, such as benzodiazepines (Ziemann et al., 1996c) and neurosteroids (Smith et al., 2002b) as well as DA agonists (Ziemann et al., 1997), enhance intracortical inhibition during both the inhibitory and facilitatory phases (Di Lazzaro et al., 2006). This is consistent with the time course of the GABAA-mediated inhibitory post-synaptic potential (McCormick, 1989). The response to paired-pulse TMS is affected by a wide range of drugs (Ziemann et al., 1995, 1996d, 1997) as well as hormones (Smith et al., 1999, 2002b) and can serve as a marker for behavioral disorders (Greenberg et al., 2000; Gilbert et al., 2005) and individual differences (Wassermann et al., 2001).
In this study, we delivered single and paired TMS to M1 before and during a slot machine simulation that manipulated reward expectation, in order to look for evidence of reward-related input.
Materials and methods
Experimental design
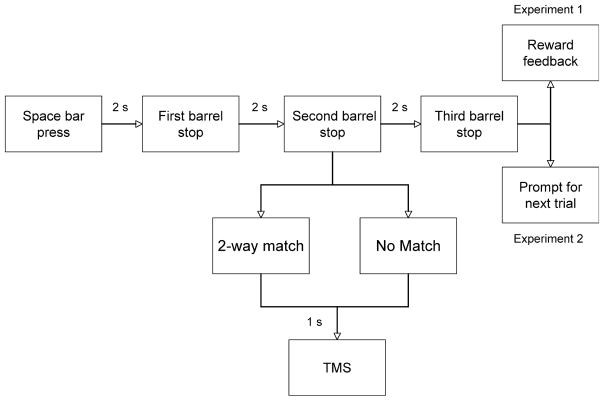
In order to manipulate behavioral states, we used a computer simulation of a three-barrel slot machine that delivered intermittent money rewards on trials resulting in a three-way match, i.e. when all three barrels showed matching items (cartoons of fruits). Subjects were familiarized with the task in a brief practice session with neither rewards nor TMS. Subjects started each trial by pressing the space bar with the non-dominant hand; the experiments required no other motor response. The dominant (tested) hand remained at rest throughout the experiment. At the beginning of each trial, the barrels began spinning and stopped one-by-one at 2-s intervals. We delivered TMS, time-locked to the phases of the game (Fig. 1), and recorded MEPs from the finger flexors or wrist extensor of the dominant hand.
Fig. 1.
Experimental design for Experiments 1 and 2.
Experiment 1 (N = 26)
This experiment contained 225 trials, randomized between behavioral conditions. A total of 180 trials resulted in a two-way match when two barrels had stopped and 90 of those in a three-way match after all three had stopped. Three-way matches were rewarded with $0.25 (45 trials), $1 (39 trials) or $5 (six trials). When two barrels had stopped, a two-way match indicated a 50% probability of a three-way match and produced an increased expectation of reward relative to the no-match condition. Feedback on the size of the reward was provided after all three barrels had stopped (Fig. 1). We delivered paired and single-pulse TMS at 1 s after the second barrel had stopped. We recorded MEPs from either the finger flexors (16 subjects) or the wrist extensors (10 subjects).
Experiment 2 (N = 15)
In naive subjects, we used the same slot machine simulation without rewards. In order to manipulate attention, we instructed subjects to keep track of the number of trials where a two-way match was followed by a three-way match. Thus, after a two-way match, they had to attend to the outcome of the trial, whereas, after no match, the outcome could be ignored. In this experiment, we recorded MEPs only from the finger flexors.
Study population
Forty-one healthy men (mean age 30.7 ± 9.3 years), drawn from the population in the NIH Clinical Research Volunteer Program, were enrolled. We included only men, as ovarian hormones affect paired-pulse TMS measures in women (Smith et al., 1999, 2002b,c, 2003). They underwent a neurological examination and were interviewed to exclude habitual gambling, alcoholism and illicit drug use. The experiments were undertaken with the written consent of each subject. The study conforms to the Code of Ethics of the World Medical Association (Declaration of Helsinki), printed in the British Medical Journal (18 July, 1964). Moreover, the study was approved by the Neuroscience Institutional Review Board of the National Institutes of Health.
Methods
The stimuli were programmed in Presentation® (Version 10.0, Neurobehavioral Systems, Albany, CA, USA) and presented on a computer that triggered and sent trial condition information to another computer. This computer, interfaced with a Micro 1401 processor (CED, Cambridge, UK), controlled the TMS, recorded the electromyogram and performed conditional averaging with Signal® software (Version 3.08, CED).
Surface electrodes were applied to the skin of the dominant hand over the long finger flexors (flexor digitorum profundus and superficialis; 16 subjects) or the wrist extensors (extensor carpi radialis longus and brevis; 10 subjects). The electromyogram was recorded and displayed online. Subjects were instructed to keep the recorded muscles relaxed at all times. TMS was delivered from two Magstim 200 stimulators connected via a Bistim® module, through a round coil placed in the optimal position and orientation for producing an MEP in the target muscle. Before each experiment, the resting motor threshold in the target muscle was determined, defined as the maximum stimulus intensity that did not produce an MEP > 50 μV in the targeted muscle (Ziemann et al., 1997). Mean resting motor threshold (both experiments) was 46.59 ± 6.2% of maximum stimulator output. For paired TMS, the intensity of the conditioning stimulus was set at 65% and the test stimulus intensity at 120% of resting motor threshold (Ziemann et al., 1996a; MacKinnon et al., 2005). The conditioning stimulus preceded the test stimulus by either 2 or 10 ms.
Before each experiment, we measured the subject’s baseline response to paired-pulse stimulation with 15 trials of the test stimulus alone, 15 pairs with a 2-ms ISI and 15 pairs with a 10-ms ISI. Subjects sat with eyes open, not conversing or performing any task. During each experiment, three different TMS conditions (the test stimulus given alone and pulse pairs at 2- and 10-ms ISI) were intermixed randomly. The interval between trials varied randomly by 0–20% around a mean of 6 s. In both experiments, each one of the three TMS conditions occurred in 15 no-match trials and 60 two-(or three-)way match trials. In Experiment 1, for each TMS condition, 30 of the ≥ two-way match trials were followed by no reward, 15 by $0.25 (small reward), 13 by $1 and 2 by $5 (large reward). In Experiment 2, for each TMS condition, 30 of the ≥ two-way match trials were followed by a three-way match and 30 by no three-way match.
Analysis
Trials contaminated by background electromyography (< 2% of all trials) were excluded from the analysis. Peak-to-peak MEP amplitudes were measured automatically.
The effect of the conditioning pulse was expressed as the ratio of conditioned MEP to unconditioned MEP. Ratios from individual trials were calculated by dividing the MEP from each pair by the mean MEP from the test pulse alone for the same experimental condition in each subject.
The conditioned MEP ratio was the dependent variable for all analyses. A decrease in the ratio signified an increase in inhibition. The independent variables in Experiment 1 were ISI (2 vs. 10 ms), match condition (two-way vs. no match), preceding trial reward (small or no reward vs. large reward), task condition (pre-task baseline vs. during task) and muscle (finger flexors vs. wrist extensors). The $5 rewards (two per TMS condition), which were included in order to increase the reward range, were grouped with the $1 reward trials (13 per condition) as ‘large’ rewards. In Experiment 2, the independent variables were ISI, match condition and task condition. Experiment was introduced as a variable in order to compare the results of Experiments 1 and 2.
All statistical analyses were performed with SPSS 15® software, based on a mixed linear model for fixed effects, a method of analysis similar to the general linear model but which makes fewer assumptions for the data. In all analyses, all models of Covariance Structure available in SPSS 15® were applied to the data and the best-fitting model was determined based on two criteria of fit, the Akaike’s Information Criterion and the Schwarz’s Bayesian Criterion. The best-fitting model proved to be the First Order Autoregressive Moving Average, which is typical for repeated measures analyses. This model does not assume constant variance for the different independent variable levels. Restricted maximum likelihood estimation was used to analyse incomplete data. Reported denominator degrees of freedom were calculated by SPSS 15® based on this estimation. The Bonferroni adjustment for multiple comparisons was applied by SPSS 15® in all post-hoc comparisons.
Results
Conditioned MEP ratios for the 2-ms ISI were significantly smaller than for the 10-ms ISI for both experiments (d.f. = 2179.825; F1,2179.8 = 429.6; P < 0.001). Conditioned MEP ratios for both ISIs were larger during the tasks compared with the pre-task baseline in Experiment 1 (both muscles pooled; F1,941.3 = 9.9; P = 0.002) and Experiment 2 (F1,198.9 = 7.9; P = 0.005; Figs 2 and 3). This decrease in inhibition was similar in the two experiments (interaction of experiment type and effect of task; F1,1366.6 = 1.0; P = 322).
Fig. 2.
In Experiment 1, the condition of two-way match associated with reward expectation significantly decreased the conditioned MEP ratio, both when the finger flexors (F = 8.8, P = 0.003) and the wrist extensor (F = 10.4, P = 0.002) were tested. Conditioned MEP ratios were larger during the experimental tasks compared with the pre-task baseline (for both muscles combined, F = 1.0, P = 0.002; for the flexors, F = 11.3, P = 0.001; for the extensor, F = 1.8, P = 0.178). Left panel: Conditioned MEP ratio by match condition and pre-task baseline for finger flexors. Right panel: Conditioned MEP ratio by match condition and pre-task baseline for wrist extensors. The columns represent means and the error bars their SEs.
Fig. 3.
In Experiment 2, attention engagement to the two-way match condition without association with reward expectation does not change the conditioned MEP ratio (F = 1.157, P = 0.283). Conditioned MEP ratio by match condition and pre-task baseline. The columns represent means and the error bars their SEs.
In Experiment 1, two-way matches caused a significant decrease in the conditioned MEP ratio relative to no match in the finger flexors (F1,535.5 = 8.8; P = 0.003; Fig. 2) and wrist extensors (F1,159.2 = 10.4; P = 0.002; Fig. 2). This decrease effect was seen for both 2-ms (F1,820.8 = 5.2, P = 0.023) and 10-ms (F1,821.7 = 25.5, P < 0.001) ISI. Two-way matches produced significantly more inhibition when they followed large rewards than after small or no rewards (F1,273.0 = 6.6, P = 0.011; Fig. 4). When the no-reward condition was excluded, the effect of the preceding trial was still significant (F1,254.8 = 4.2, P = 0.017), suggesting possible scaling of the response with reward value. A comparison between the $1 and $5 rewards was not possible because of the small number of $5 rewards.
Fig. 4.
In Experiment 1 for finger flexors, the condition of two-way match associated with reward expectation decreased the conditioned MEP ratio for 2-and 10-ms ISI following large ($1 or $5) but not small ($0.25 or no reward) rewards. Two-way matches produced significantly more inhibition when they followed large ($1 or $5) compared with small ($0.25 or no reward) rewards (for large rewards, two-way matches vs. no matches, F = 8.0, P = 0.005; for small rewards, two-way matches vs. no matches, F = 2.0, P = 0.159; for two-way matches, large rewards vs. small rewards; F = 6.6, P = 0.011). The columns represent means and the error bars their SEs.
In Experiment 2, there was no significant difference in the conditioned MEP ratio between the two-way match and no-match conditions (F1,184.9 = 1, P = 0.283; Fig. 3). Notably, the estimated mean conditioned MEP ratio was higher for two-way match than for no match. Moreover, when we compared Experiment 2 with Experiment 1 directly, the interaction of experiment type with match condition was significant (F1,1403.5 = 6.8, P = 0.009). All subjects reported the correct number of three-way matches.
Discussion
In this study, we found an excitability change in M1 that was associated with the expectation of reward and modified by prior experience. Specifically, the inhibition produced by the conditioning pulse increased with reward expectation and this effect was greater when subjects had recently received a large reward. We regard this as evidence that M1 receives reward-related information similar to that reported for the PFC and striatum (Schultz et al., 1993; Schultz & Dickinson, 2000; Schultz, 2000; Haber, 2003; Oya et al., 2005).
Outcome uncertainty is coded by various brain areas, such as the VTA, following reward-predicting stimuli (Critchley et al., 2001; Fiorillo et al., 2003; Shizgal & Arvanitogiannis, 2003; Tobler et al., 2007) but also independent of reward (Critchley et al., 2001; Aron et al., 2004). In this study, both reward uncertainty and likelihood were maximal following a two-way match (i.e. 50% probability of any reward), posing a confound. The fact that large rewards preceding two-way matches resulted in more inhibition than equally probable small rewards indicates that reward value modulated the response, at least on successive trials. However, the present study was not designed to distinguish between reward value and probability.
The possibility that aspects of the stimuli, other than their reward significance but specific to the two-way match condition, caused the increase in inhibition was addressed in Experiment 2 where the subject’s attention was drawn to the two-way match condition but the stimuli had no reward-related significance. This was not a perfect control for attention to the two-way match condition, which may have been greater during Experiment 1. However, we do not believe that it would have been possible to match the degree of attention in Experiment 1 without involving the reward system in some way (Maunsell, 2004; Schultz, 2006). Although disentangling the effects of attention and reward can be challenging, we doubt that the increase in ICI observed in Experiment 1 was due to attention alone. Attention/arousal appears to decrease rather than increase ICI (Rosenkranz & Rothwell, 2004; Thomson et al., 2008), which is what we observed in the comparison between pre-task baseline and task in both experiments.
To address the focality of the increase in ICI associated with two-way matches and the possibility that it was related to the covert preparation of a latent motor response, we tested a flexor and an extensor muscle acting across two different joints. Outputs to both muscles changed similarly with reward likelihood. Although this could still represent stereotyped activity related to movement preparation, we note that movement preparation, per se, is associated with increased, rather than decreased, M1 excitability (Alexander & Crutcher, 1990). Moreover, paired-pulse inhibition in M1 decreases during response preparation (Leocani et al., 2000).
Reward-related activity has been observed in M1 in functional neuroimaging studies, when (unlike our paradigm) the task included a motor response (Pessiglione et al., 2007) or when ventral pre-motor areas were also activated (Ponseti et al., 2006). In tasks not requiring motor responses, net inhibitory inputs, such as we measured, would probably not produce regional blood flow changes large enough to be captured by functional neuroimaging (Waldvogel et al., 2000; Smith et al., 2002a).
Where does the M1 reward expectation signal come from? Among the possible direct and indirect sources [striatum, orbitofrontal cortex and amygdala, anterior cingulate cortex and supplementary motor area, VTA (Schmahmann & Pandya, 2006)], circumstantial evidence suggests direct or indirect input from the VTA as a likely source. In Experiment 1, the task parameters and reward schedule (intermittent, variable reward and 0.5 reward probability after a two-way match) paralleled the values shown to maximize tonic VTA DA neuron activity in primates (Fiorillo et al., 2003) and TMS delivery was timed to coincide with our estimate of peak VTA pre-reward activity (Fiorillo et al., 2003). In addition, the increment in the response to a two-way match following large rewards is consistent with the observation that large rewards seem to ‘reset’ the tonic VTA activity at a higher level (Fiorillo et al., 2003).
The data are also consistent with a direct effect of DA in M1 via the known VTA–M1 pathway (Gaspar et al., 1992, 1995; Williams & Goldman-Rakic, 1993). Increased M1 inhibition is physiologically similar to the action of exogenous DA agonists (Ziemann et al., 1996b, 1997). VTA cells are thought to fire en masse in response to reward-related stimuli (Schultz, 2001; Fiorillo et al., 2003) and VTA projections to M1 and PFC are thought to act in parallel (Berger et al., 1988; Gaspar et al., 1992). VTA DA projections terminate on inhibitory cortical interneurons (Gao et al., 2003; Tseng et al., 2006) and pyramidal (including corticospinal) cells (Sesack et al., 1998; Huda et al., 2001; Awenowicz & Porter, 2002), modulating their excitability, spatial tuning and temporal dynamics (Fellous et al., 2001; Gao et al., 2003; Markram et al., 2004). Tonic DA release (as in reward expectation) acts mostly on D2 and D4 receptors on interneurons (Huda et al., 2001; Awenowicz & Porter, 2002; Gao et al., 2003; Onn et al., 2006; Tseng et al., 2006), suppressing recurrent pyramidal cell excitation (Onn et al., 2006). Recurrent excitation within the motor cortex is thought to be the basis of the repeated spinal volleys, or ‘I waves’ (Amassian et al., 1987), that summate at the spinal segment to produce the MEP. Human spinal recordings show that I wave suppression is the mechanism by which the conditioning pulse inhibits the MEP (Nakamura et al., 1997; Di Lazzaro et al., 1998).
What is the role of a reward-related signal to M1 in shaping behavior? The current concept of M1 is no longer that of a low-level output stage (Penfield & Boldrey, 1937; Penfield & Jasper, 1954; Graziano, 2006) but a highly plastic processor, able to resolve response conflicts (Praamstra & Seiss, 2005) and learn. M1 maps are continually reshaped by information from multiple sources (Graziano, 2006). Reward signals are thought to drive the plastic changes that underlie learning (Waelti et al., 2001; Schultz, 2004) in cortical (Ueki et al., 2006) and striatal (Haruno et al., 2004; Tanaka et al., 2004; Rodriguez et al., 2006) neurons. Learning in M1 alters movement representations (Karni et al., 1995, 1998; Cohen et al., 2005; Robertson et al., 2005; Graziano, 2006; Richardson et al., 2006) and probably depends, in part, on DA signals (Ueki et al., 2006) to indicate behavioral salience, guide plasticity and optimize motor/pre-motor interactions (Mir et al., 2005). Other cortical areas in the executive chain, medial PFC (response selection) and pre-motor areas (motor preparation) also receive VTA input (Berger et al., 1988; Gaspar et al., 1992). Therefore, it is consistent that M1 (motor learning and sequence execution) should receive analogous information. Simultaneous input to all of these structures could enhance coordination between the stages of the executive arm of the reward network.
In addition to implicating M1 in reward processing, TMS may open a physiological window into the human reward system and provide a new level of ease and precision for measuring the neural coding of reward value.
Acknowledgments
Statistical advice was provided by Drs James Dambrosia and David Luckenbaugh. Devee Schoenberg edited the manuscript. This research was supported by the Intramural Research Program of the NIH, NINDS.
Abbreviations
- DA
dopamine
- ICI
intracortical inhibition
- ISI
interstimulus interval
- M1
primary motor cortex
- MEP
motor evoked potential
- PFC
pre-frontal cortex
- TMS
transcranial magnetic stimulation
- VTA
ventral tegmental area
References
- Abler B, Walter H, Erk S, Kammerer H, Spitzer M. Prediction error as a linear function of reward probability is coded in human nucleus accumbens. Neuroimage. 2006;31:790–795. doi: 10.1016/j.neuroimage.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Neural representations of the target (goal) of visually guided arm movements in three motor areas of the monkey. J Neurophysiol. 1990;64:164–178. doi: 10.1152/jn.1990.64.1.164. [DOI] [PubMed] [Google Scholar]
- Amassian VE, Stewart M, Quirk GJ, Rosenthal JL. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery. 1987;20:74–93. [PubMed] [Google Scholar]
- Aron AR, Shohamy D, Clark J, Myers C, Gluck MA, Poldrack RA. Human midbrain sensitivity to cognitive feedback and uncertainty during classification learning. J Neurophysiol. 2004;92:1144–1152. doi: 10.1152/jn.01209.2003. [DOI] [PubMed] [Google Scholar]
- Awenowicz PW, Porter LL. Local application of dopamine inhibits pyramidal tract neuron activity in the rodent motor cortex. J Neurophysiol. 2002;88:3439–3451. doi: 10.1152/jn.00078.2002. [DOI] [PubMed] [Google Scholar]
- Berger B, Trottier S, Verney C, Gaspar P, Alvarez C. Regional and laminar distribution of the dopamine and serotonin innervation in the macaque cerebral cortex: a radioautographic study. J Comp Neurol. 1988;273:99–119. doi: 10.1002/cne.902730109. [DOI] [PubMed] [Google Scholar]
- Campos M, Breznen B, Bernheim K, Andersen RA. Supplementary motor area encodes reward expectancy in eye-movement tasks. J Neurophysiol. 2005;94:1325–1335. doi: 10.1152/jn.00022.2005. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Chau DT, Roth RM, Green AI. The neural circuitry of reward and its relevance to psychiatric disorders. Curr Psychiat Rep. 2004;6:391–399. doi: 10.1007/s11920-004-0026-8. [DOI] [PubMed] [Google Scholar]
- Cohen DA, Pascual-Leone A, Press DZ, Robertson EM. Offline learning of motor skill memory: a double dissociation of goal and movement. Proc Natl Acad Sci USA. 2005;102:18 237–18 241. doi: 10.1073/pnas.0506072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29:537–545. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P, Insola A, Profice P, Ranieri F, Capone F, Tonali PA, Rothwell JC. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol. 2006;96:1765–1771. doi: 10.1152/jn.00360.2006. [DOI] [PubMed] [Google Scholar]
- Fellous JM, Houweling AR, Modi RH, Rao RP, Tiesinga PH, Sejnowski TJ. Frequency dependence of spike timing reliability in cortical pyramidal cells and interneurons. J Neurophysiol. 2001;85:1782–1787. doi: 10.1152/jn.2001.85.4.1782. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Laruelle M, Haber SN. Prefrontal cortical projections to the midbrain in primates: evidence for a sparse connection. Neuropsychopharmacology. 2006;31:1627–1636. doi: 10.1038/sj.npp.1300990. [DOI] [PubMed] [Google Scholar]
- Gao WJ, Wang Y, Goldman-Rakic PS. Dopamine modulation of perisomatic and peridendritic inhibition in prefrontal cortex. J Neurosci. 2003;23:1622–1630. doi: 10.1523/JNEUROSCI.23-05-01622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Stepniewska I, Kaas JH. Topography and collateralization of the dopaminergic projections to motor and lateral prefrontal cortex in owl monkeys. J Comp Neurol. 1992;325:1–21. doi: 10.1002/cne.903250102. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Bloch B, Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur J Neurosci. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Sallee FR, Zhang J, Lipps TD, Wassermann EM. Transcranial magnetic stimulation-evoked cortical inhibition: a consistent marker of attention-deficit/hyperactivity disorder scores in tourette syndrome. Biol Psychiat. 2005;57:1597–1600. doi: 10.1016/j.biopsych.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Graziano M. The organization of behavioral repertoire in motor cortex. Annu Rev Neurosci. 2006;29:105–134. doi: 10.1146/annurev.neuro.29.051605.112924. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Ziemann U, Corá-Locatelli G, Harmon A, Murphy DL, Keel JC, Wassermann EM. Altered cortical excitability in obsessive-compulsive disorder. Neurology. 2000;54:142–147. doi: 10.1212/wnl.54.1.142. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hampton AN, O’Doherty JP. Decoding the neural substrates of reward-related decision making with functional MRI. Proc Natl Acad Sci USA. 2007;104:1377–1382. doi: 10.1073/pnas.0606297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol. 1998;509(2):607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruno M, Kawato M. Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus–action–reward association learning. J Neurophysiol. 2006;95:948–959. doi: 10.1152/jn.00382.2005. [DOI] [PubMed] [Google Scholar]
- Haruno M, Kuroda T, Doya K, Toyama K, Kimura M, Samejima K, Imamizu H, Kawato M. A neural correlate of reward-based behavioral learning in caudate nucleus: a functional magnetic resonance imaging study of a stochastic decision task. J Neurosci. 2004;24:1660–1665. doi: 10.1523/JNEUROSCI.3417-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huda K, Salunga TL, Matsunami K. Dopaminergic inhibition of excitatory inputs onto pyramidal tract neurons in cat motor cortex. Neurosci Lett. 2001;307:175–178. doi: 10.1016/s0304-3940(01)01960-7. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci USA. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123:1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Gilley EA, Weis-McNulty A, Simuni T. Pathways mediating abnormal intracortical inhibition in Parkinson’s disease. Ann Neurol. 2005;58:516–524. doi: 10.1002/ana.20599. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Matsunaga K, Akamatsu N, Uozumi T, Urasaki E, Tsuji S. Early and late inhibition in the human motor cortex studied by paired stimulation through subdural electrodes. Clin Neurophysiol. 2002;113:1099–1109. doi: 10.1016/s1388-2457(02)00079-2. [DOI] [PubMed] [Google Scholar]
- Maunsell JH. Neuronal representations of cognitive state: reward or attention? Trends Cogn Sci. 2004;8:261–265. doi: 10.1016/j.tics.2004.04.003. [DOI] [PubMed] [Google Scholar]
- McCormick DA. GABA as an inhibitory neurotransmitter in human cerebral cortex. J Neurophysiol. 1989;62:1018–1027. doi: 10.1152/jn.1989.62.5.1018. [DOI] [PubMed] [Google Scholar]
- Mir P, Matsunaga K, Gilio F, Quinn NP, Siebner HR, Rothwell JC. Dopaminergic drugs restore facilitatory premotor–motor interactions in Parkinson disease. Neurology. 2005;64:1906–1912. doi: 10.1212/01.WNL.0000163772.56128.A8. [DOI] [PubMed] [Google Scholar]
- Morris G, Nevet A, Arkadir D, Vaadia E, Bergman H. Midbrain dopamine neurons encode decisions for future action. Nat Neurosci. 2006;9:1057–1063. doi: 10.1038/nn1743. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498(3):817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Buchanan TW, Seymour B, Dolan RJ. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 2006;49:157–166. doi: 10.1016/j.neuron.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Onn SP, Wang XB, Lin M, Grace AA. Dopamine D1 and D4 receptor subtypes differentially modulate recurrent excitatory synapses in prefrontal cortical pyramidal neurons. Neuropsychopharmacology. 2006;31:318–338. doi: 10.1038/sj.npp.1300829. [DOI] [PubMed] [Google Scholar]
- Oya H, Adolphs R, Kawasaki H, Bechara A, Damasio A, Howard MA., 3rd Electrophysiological correlates of reward prediction error recorded in the human prefrontal cortex. Proc Natl Acad Sci USA. 2005;102:8351–8356. doi: 10.1073/pnas.0500899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- Penfield W, Jasper H. Epilepsy and the Functional Anatomy of the Human Brain. Little, Brown; Boston: 1954. [Google Scholar]
- Pessiglione M, Schmidt L, Draganski B, Kalisch R, Lau H, Dolan RJ, Frith CD. How the brain translates money into force: a neuroimaging study of subliminal motivation. Science. 2007;316:904–906. doi: 10.1126/science.1140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, Le Bihan D, Dubois B. The neural system that bridges reward and cognition in humans: an fMRI study. Proc Natl Acad Sci USA. 2002;99:5669–5674. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponseti J, Bosinski HA, Wolff S, Peller M, Jansen O, Mehdorn HM, Buchel C, Siebner HR. A functional endophenotype for sexual orientation in humans. Neuroimage. 2006;33:825–833. doi: 10.1016/j.neuroimage.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Seiss E. The neurophysiology of response competition: motor cortex activation and inhibition following subliminal response priming. J Cogn Neurosci. 2005;17:483–493. doi: 10.1162/0898929053279513. [DOI] [PubMed] [Google Scholar]
- Richardson AG, Overduin SA, Valero-Cabre A, Padoa-Schioppa C, Pascual-Leone A, Bizzi E, Press DZ. Disruption of primary motor cortex before learning impairs memory of movement dynamics. J Neurosci. 2006;26:12 466–12 470. doi: 10.1523/JNEUROSCI.1139-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM, Press DZ, Pascual-Leone A. Off-line learning and the primary motor cortex. J Neurosci. 2005;25:6372–6378. doi: 10.1523/JNEUROSCI.1851-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PF, Aron AR, Poldrack RA. Ventral-striatal/nucleus-accumbens sensitivity to prediction errors during classification learning. Hum Brain Mapp. 2006;27:306–313. doi: 10.1002/hbm.20186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Rothwell JC. The effect of sensory input and attention on the sensorimotor organization of the hand area of the human motor cortex. J Physiol. 2004;561:307–320. doi: 10.1113/jphysiol.2004.069328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. Oxford University Press; Oxford: 2006. [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Schultz W. Reward signaling by dopamine neurons. Neuroscientist. 2001;7:293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Neural coding of basic reward terms of animal learning theory, game theory, microeconomics and behavioural ecology. Curr Opin Neurobiol. 2004;14:139–147. doi: 10.1016/j.conb.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T, Romo R, Scarnati E. Reward-related activity in the monkey striatum and substantia nigra. Prog Brain Res. 1993;99:227–235. doi: 10.1016/s0079-6123(08)61349-7. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Melchitzky DS, Lewis DA. Dopamine innervation of a subclass of local circuit neurons in monkey prefrontal cortex: ultrastructural analysis of tyrosine hydroxylase and parvalbumin immunoreactive structures. Cereb Cortex. 1998;8:614–622. doi: 10.1093/cercor/8.7.614. [DOI] [PubMed] [Google Scholar]
- Shizgal P, Arvanitogiannis A. Neuroscience. Gambling on dopamine Science. 2003;299:1856–1858. doi: 10.1126/science.1083627. [DOI] [PubMed] [Google Scholar]
- Skolnick AI, Davidson RI. Affective modulation of eyeblink startle with reward and threat. Psychophysiology. 2002;39:835–850. doi: 10.1111/1469-8986.3960835. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Keel JC, Greenberg BD, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Menstrual cycle effects on cortical excitability. Neurology. 1999;53:2069–2072. doi: 10.1212/wnl.53.9.2069. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shulman RG, Hyder F. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. Proc Natl Acad Sci USA. 2002a;99:10 765–10 770. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Ovarian hormone effects on human cortical excitability. Ann Neurol. 2002b;51:599–603. doi: 10.1002/ana.10180. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Effects of ovarian hormones on human cortical excitability. Ann Neurol. 2002c;51:599–603. doi: 10.1002/ana.10180. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Adams LF, Schmidt PJ, Rubinow DA, Wassermann EM. Abnormal luteal phase excitability of the motor cortex in women with PMS. Biol Psychiat. 2003;54:757–762. doi: 10.1016/s0006-3223(02)01924-8. [DOI] [PubMed] [Google Scholar]
- Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat Neurosci. 2004;7:887–893. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- Thomson RH, Garry MI, Summers JJ. Attentional influences on short-interval intracortical inhibition. Clin Neurophysiol. 2008;119:52–62. doi: 10.1016/j.clinph.2007.09.060. [DOI] [PubMed] [Google Scholar]
- Tobler PN, O’Doherty JP, Dolan RJ, Schultz W. Reward value coding distinct from risk attitude-related uncertainty coding in human reward systems. J Neurophysiol. 2007;97:1621–1632. doi: 10.1152/jn.00745.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Mallet N, Toreson KL, Le Moine C, Gonon F, O’Donnell P. Excitatory response of prefrontal cortical fast-spiking interneurons to ventral tegmental area stimulation in vivo. Synapse. 2006;59:412–417. doi: 10.1002/syn.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki Y, Mima T, Kotb MA, Sawada H, Saiki H, Ikeda A, Begum T, Reza F, Nagamine T, Fukuyama H. Altered plasticity of the human motor cortex in Parkinson’s disease. Ann Neurol. 2006;59:60–71. doi: 10.1002/ana.20692. [DOI] [PubMed] [Google Scholar]
- Van der Linden A, Van Camp N, Ramos-Cabrer P, Hoehn M. Current status of functional MRI on small animals: application to physiology, pathophysiology, and cognition. NMR Biomed. 2007;20:522–545. doi: 10.1002/nbm.1131. [DOI] [PubMed] [Google Scholar]
- Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412:43–48. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- Waldvogel D, van Gelderen P, Muellbacher W, Ziemann U, Immisch I, Hallett M. The relative metabolic demand of inhibition and excitation. Nature. 2000;406:995–998. doi: 10.1038/35023171. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Greenberg BD, Nguyen MB, Murphy DL. Motor cortex excitability correlates with an anxiety-related personality trait. Biol Psychiat. 2001;50:377–382. doi: 10.1016/s0006-3223(01)01210-0. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Characterization of the dopaminergic innervation of the primate frontal cortex using a dopamine-specific antibody. Cereb Cortex. 1993;3:199–222. doi: 10.1093/cercor/3.3.199. [DOI] [PubMed] [Google Scholar]
- Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci. 2004;7:1370–1375. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze HJ, Duzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Paulus W. Inhibition of human motor cortex by ethanol. A transcranial magnetic stimulation study. Brain. 1995;118:1437–1446. doi: 10.1093/brain/118.6.1437. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol (Lond) 1996a;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Bruns D, Paulus W. Enhancement of human motor cortex inhibition by the dopamine receptor agonist pergolide: evidence from transcranial magnetic stimulation. Neurosci Lett. 1996b;208:187–190. doi: 10.1016/0304-3940(96)12575-1. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996c;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996d;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Bruns D, Baudewig J, Paulus W. Changes in human motor cortex excitability induced by dopaminergic and anti-dopaminergic drugs. Electroencephalogr Clin Neurophysiol. 1997;105:430–437. doi: 10.1016/s0924-980x(97)00050-7. [DOI] [PubMed] [Google Scholar]