Abstract
On the periplasmic side of LacY, two conserved Gly-Gly pairs in helices II and XI (Gly46 and Gly370, respectively) and helices V and VIII (Gly159 and Gly262, respectively) allow close packing of each helix pair in the outward (periplasmic)-closed conformation. Previous studies demonstrate that replacing one Gly residue in each Gly-Gly pair with Trp leads to opening of the periplasmic cavity with abrogation of transport activity, but an increased rate of galactoside binding. To further investigate the role of the Gly-Gly pairs, 11 double-replacement mutants were constructed for each pair at positions 46 (helix II) and 262 (helix VIII). Replacement with Ala or Ser results in decreased but significant transport activity, while replacements with Thr, Val, Leu, Asn, Gln, Tyr, Trp, Glu, or Lys exhibit very little or no transport. Remarkably, however, the double mutants bind galactoside with affinities 10–20-fold higher than that of the pseudo-WT or WT LacY. Moreover, site-directed alkylation of a periplasmic Cys replacement indicates that the periplasmic cavity becomes readily accessible in the double-replacement mutants. Molecular dynamics simulations with the WT and double-Leu mutant in the inward-open/outward-closed conformation provide support for this interpretation.
Graphical Abstract

The lactose permease of Escherichia coli (LacY) is a member of the major facilitator superfamily, the largest family of membrane transport proteins.1,2 As a sugar/H+ symporter, LacY utilizes the free energy released from downhill movement of H+ in response to a H+ electrochemical gradient (Δμ̃H+) to catalyze uphill translocation of galactopyranosides against a concentration gradient. Because coupling of sugar and H+ translocation is obligatory, in the absence of Δμ̃H+, LacY can utilize the energy released from downhill sugar movement to drive uphill H+ transport with the generation of Δμ̃H+, the polarity of which depends on the direction of the sugar gradient.3–5 A molecular mechanism for this chemiosmotically driven symporter has been proposed recently.5
The first X-ray crystal structure of LacY was obtained with the conformationally restricted mutant C154G,6,7 followed by structures of wild-type (WT) LacY8 and a single-Cys LacY mutant with a covalently bound affinity inactivator.9 Each of these structures exhibits the same inward-open/outward-closed conformation with two pseudosymmetrical six-helix bundles surrounding a large hydrophilic cavity. The cavity is open to the cytoplasmic side only, and the periplasmic side is closed (Figure 1A). However, a body of biochemical and biophysical studies10–21 demonstrates that sugar binding induces reciprocal opening and closing of hydrophilic cavities on the cytoplasmic and periplasmic sides of LacY. Thus, the sugar- and H+-binding sites are alternatively accessible from either side of the membrane (the alternating access model5,22).
Figure 1.
Structure of LacY. (A) Structure of LacY (PDB entry 2CFQ) viewed from the side. The Cα atoms of the Gly46-Gly370 pair (blue) and the Gly159-Gly262 pair (red) are shown as spheres. Helices V and VIII are labeled in red and helices II and XI in blue. The Cα atom of Asn245 is shown as a yellow sphere. (B) Cytoplasmic view. (C) Periplasmic view.
In the inward-open/outward-closed conformation, sealing of the periphery of the periplasmic cavity is due mainly to the proximity between helices II and XI and between helices V and VIII. The presence of two pairs of conserved Gly residues23,24 at the periplasmic ends of helices II and XI (Gly46 and Gly370, respectively) and helices V and VIII (Gly159 and Gly262, respectively) facilitates tight packing between these two pairs of helices (Figure 1A–C). Although Gly is a well-known “helix breaker”, when present at a similar level in neighboring transmembrane α-helices, Gly residues may also lead to tighter helix packing or oligomerization.25–27 Conversely, replacing Gly residues in Gly-Gly pairs may disrupt interactions between α-helices.
As evidenced by site-directed alkylation and stopped-flow binding measurements, simultaneously replacing a single Gly residue with a bulky Trp residue in both Gly-Gly pairs causes complete loss of transport activity, but an enhanced rate of sugar binding. These findings and the observation that a Cys replacement for Asn245, which is unreactive in WT LacY in the absence of sugar binding, alkylates readily in the double-Trp mutant indicate that the periplasmic cavity is patent.28 Surprisingly, however, X-ray structures obtained from the G46W/G262W mutant reveal an almost occluded conformation with a bound galactoside molecule, a narrowly open periplasmic pathway, and a sealed cytoplasmic surface.29,30 In all likelihood, in the absence of sugar, the periplasmic pathway in the mutant is sufficiently open to allow free access of sugar to the binding site, but when binding occurs and the mutant attempts to undergo the transition into an occluded state,5,29 it cannot do so completely because the bulky Trp residues at positions 46 and 262 block complete closure. Thus, the mutant is able to bind sugar and initiate transition into an occluded state, which it cannot complete. Therefore, although the mutant readily binds ligand, it is completely unable to catalyze transport of any type across the membrane.
To investigate further the role of the Gly pairs on the periplasmic side of LacY, 11 mutants were constructed with double replacements at positions 46 and 262. The mutants were assayed for transport activity, sugar affinity, and site-directed alkylation of a single-Cys replacement at position 245. In addition, the WT and a representative example of the mutants (the double-Leu mutant) were subjected to molecular dynamics (MD) simulations. The findings are uniformly consistent with the interpretation that disruption of the Gly-Gly pairs increases the probability of opening the periplasmic cavity.
MATERIALS AND METHODS
Materials
Oligonucleotides were synthesized by Integrated DNA Technologies (Coralville, CA). The QuikChange II mutagenesis kit was obtained from Agilent Technologies (Santa Clara, CA). [D-glucose-14C-(U)]Lactose was purchased from Moravek Biochemicals (Brea, CA). Tetramethylrhodamine 5-maleimide (TMRM) was purchased from Invitrogen (Carlsbad, CA). p-Nitrophenyl α-D-galactopyranoside (α-NPG) was obtained from Sigma-Aldrich (St. Louis, MO). α-D-Melibiose was obtained from CHEM-IMPEX (Wood Dale, IL), and immobilized monomeric avidin was purchased from Thermo Scientific (Rockford, IL). The penta-His antibody–horseradish peroxidase (HRP) conjugate was obtained from Qiagen (Hilden, Germany). All other materials were reagent grade and obtained from commercial sources.
Construction of Mutants
All mutants were constructed by site-directed mutagenesis using plasmid pT7-5 containing a cassette lacY gene encoding C-less LacY with a C-terminal biotin acceptor domain followed by a six-His tag. The double Lys replacement was also constructed in plasmid pT7-5 containing a cassette lacY gene encoding WT LacY with a six-His tag on the C-terminus. The entire lacY gene of each mutant was verified by DNA sequencing. The single-Cys245 mutant is termed “pseudo-WT”.
Lactose Transport
Transport of [14C]lactose was assayed as described previously.31 Briefly, E. coli T184 [lacI+ O+ Z−Y− (A) rpsL met− thr− recA hsdM hsdR/F’lacIq O+ ZD118 (Y+ A+)] cells32,33 transformed with plasmid pT7-5 encoding a given LacY mutant were induced for 2 h by addition of 1 mM IPTG. After 2 h, cells were harvested, washed with 100 mM KPi and MgSO4 (pH 7.0), and adjusted to an optical density of 10 at 420 nm (~0.7 mg of protein/mL). An aliquot of 50 μL was incubated with [14C]lactose (10 mCi/mmol, final concentration of 0.4 mM) for given times at room temperature. The cells were then rapidly diluted with 3 mL of 100 mM KPi and 10 mM LiCl (pH 5.5), immediately filtered, and washed once with the same solution. Radioactivity was determined by liquid scintillation spectrometry.
TMRM Labeling with Right-Side-Out (RSO) Membrane Vesicles
RSO membrane vesicles containing pseudo-WT or a given LacY mutant were prepared from a 0.5 L culture of E. coli T184 cells as described previously.34,35 For each mutant with a single Cys245, 50 μL of RSO membrane vesicles (0.1 mg of total protein) was incubated with 40 μM TMRM in the absence or presence of 2 mM α-NPG at 25 °C for 0, 5 10, 15, and 20 s. Labeling was terminated by addition of 10 mM (final concentration) dithiothreitol (DTT). The membranes were solubilized with 2% n-dodecyl β-D-maltopyranoside (DDM), and LacY was purified by avidin affinity chromatography as described previously.14 A purified LacY sample from each labeling reaction mixture was subjected to SDS–PAGE. The intensity of labeling and the amount of purified protein on the gel were determined by using an Amersham Typhoon 9410 Workstation and silver staining, respectively. The volumes of the labeled protein bands on the SDS–PAGE gel were quantified with ImageQuant 5.0 (GE Healthcare).14,15,17 Relative labeling was calculated as described previously.14 The rate of labeling was obtained by plotting relative labeling as a function of labeling time. Data were fitted by linear fit or a firstorder rate equation [At = A∞(1 – e−kt)] with GraFit 6 (Erithacus Software).
Purification of LacY Mutants
E. coli C41(DE3) cells transformed with plasmid pT7-5 encoding a given mutant were grown in 1 L of LB broth with ampicillin (100 mg/L) at 37 °C overnight. The overnight culture was diluted 10-fold in a fermenter and induced with 0.3 mM IPTG at an OD600 of 0.6. After a 3 h induction, cells were harvested, and the mutants were purified by metal affinity chromatography as described previously.19
α-NPG Binding
α-NPG binding with purified LacY mutants was measured as described previously.36 An SLM-Aminco 8100 spectrofluorometer was used to record the changes in fluorescence resulting from the displacement of α-NPG by addition of excess melibiose. KD values were also determined as described previously.36,37
Molecular Dynamics (MD) Simulations
Building the Model Systems
The WT simulations were based on the inward-open/outward-closed crystal structure of LacY (PDB entry 2V8N). The double-Leu replacements were introduced using the VMD psf plugin.38 The protein models were inserted by aligning the protein center of mass to a POPE lipid bilayer containing ~500 lipids generated by the CHARMM-GUI membrane builder.39 The system was subsequently solvated with ~18400 water molecules, and 59 Na+ and 67 Cl− ions were added to achieve electric neutrality and a 150 mM salt concentration. The simulation system was relaxed using a 10000-step conjugate-gradient energy minimization followed by gradual heating, from 0 to 310 K over 120 ps. The lipids and water molecules were then allowed to adjust to the restrained protein structure during a 10 ns equilibration step.
Simulations
The molecular dynamics simulations were run with the NAMD 2.9 software package.40 The CHARMM22, including CMAP correction, and CHARMM36 force fields41,42 were used for protein and lipids, respectively, and the TIP3P model was used for the water molecules.43 A time step of 1 fs was used to integrate the equations of motion, and a reversible multiple-time step algorithm44 of 4 fs was used for the electrostatic forces and 2 fs for short-range, nonbonded forces. The smooth particle mesh Ewald method45,46 was used to calculate electrostatic interactions. The short-range interactions were cut off at 12 Å. All bond lengths involving hydrogen atoms were held fixed using the SHAKE47 and SETTLE48 algorithms. A Langevin dynamics scheme was used for thermostating, and Nosé–Hoover–Langevin pistons were used for pressure control.49,50 Molecular graphics and simulation analyses were generated with VMD version 1.9.1.38
RESULTS
Lactose Transport
E. coli T184 transformed with plasmid pT7-5 encoding a given double replacement in a background with a single Cys at position 245 was used to assess the lactose transport activity of each mutant. Replacing Gly46 and Gly262 with Ala or Ser, which have similar masses and volumes,51 decreases activity to approximately 40 or 30%, respectively, of that of the Gly-Gly pseudo-WT (Figure 2A,B). However, increasing the mass of the double-Ser replacement by a single methylene group thereby making it into Thr abolishes transport activity, and similar losses of activity are observed with each of the other eight double replacements (Val, Leu, Asn, Gln, Tyr, Trp, Glu, and Lys).
Figure 2.
(A) Lactose transport of E. coli T184 cells expressing single-Cys245 LacY (●) or single-Cys245 LacY with double Ala (□) and Ser (△) replacements at positions 46 and 262, or no permease (○), was measured with [14C]lactose (10 mCi/mmol) at 0.4 mM for given times as described in Materials and Methods. The lactose transport by double-replacement mutants with Val, Leu, Thr, Asn, Gln, Tyr, Trp, Glu, or Lys is colored red, with bars indicating the activity spread of the different mutants. All mutants contain the given double replacement and a single Cys in place of Asn245. (B) Steady-state level of lactose accumulation of each mutant presented as the percentage of the Gly-Gly pseudo-WT. (C) Expression of the pseudo-WT and the double-replacement mutants as determined by Western blotting with the penta-His antibody–HRP conjugate. From left to right: Gly-Gly single Cys245 (1), empty vector (2), and double replacements with Ala (3), Glu (4), Lys (5), Leu (6), Asn (7), Gln (8), Ser (9), Thr (10), Val (11), or Tyr (12). Two micrograms of total membrane protein was analyzed in each lane by SDS–PAGE.
Western blots with an anti-His antibody show that all the double-replacement mutants are expressed at reasonable levels relative to that of pseudo-WT LacY, with the exception of the double-Lys mutant, which is expressed at <10% of that of the pseudo-WT LacY (Figure 2C). Although the level of expression of the double-Lys mutant increases significantly in the WT LacY background, no transport activity is observed (data not shown). Expression of the double-Trp mutant has been described previously.28
α-NPG Binding
As shown previously,37 binding of α-NPG undergoes Foörster resonance energy transfer (FRET) between Trp151 and the nitrophenyl moiety of α-NPG in the sugar-binding site of LacY. An increase in Trp fluorescence is observed by displacement of bound α-NPG with excess nonfluorescent galactopyranoside in a manner that is dependent on the α-NPG concentration. For each double-replacement mutant, the α-NPG affinity was determined by measuring the increase in Trp fluorescence after displacement of various concentrations of α-NPG by excess melibiose, and a KD was determined as described previously.36 As shown previously,28 the double-Trp replacement binds β-D-galactosyl 1-thio-β-D-galactopyranoside at a rate higher than that of WT LacY. Strikingly, the double-replacement mutants described here exhibit affinities for α-NPG 10–20-fold higher (i.e., lower KD) than that of the pseudo-WT LacY (Figure 3A and Table 1). Even the double-Lys replacement constructed in the WT background exhibits an affinity 10-fold higher than that of WT LacY (Figure 3B and Table 1). However, no direct correlation is observed between KD and the mass of the replacement, and the same effect is apparent regardless of charge (i.e., the double-Glu mutant exhibits the same 10-fold decrease in KD as observed with the double-Lys mutant).
Figure 3.
(A) Binding of α-NPG to purified single Cys245 (●) and single Cys245 with Ala (△), Ser (□), Thr (○), Leu (*), Glu (✩), Gln (◊) or Tyr (▽) double replacements at positions 46 and 262 measured by addition of 30 mM melibiose to samples preincubated with α-NPG at given concentrations. Binding curves were obtained by plotting the change in fluorescence as a function of α-NPG concentration. (B) Binding of α-NPG to purified WT LacY (●) and the double-Lys replacements at positions 46 and 262 in the WT background (○).
Table 1.
KD Values for NPG Binding
| replacement of Gly46 and Gly262 | KDapp (μM)a | standard error (±) |
|---|---|---|
| pseudo-WT background | ||
| pseudo-WT | 141.5 | 45.6 |
| Ala | 7.0 | 1.5 |
| Ser | 10.5 | 1.1 |
| Thr | 5.1 | 0.6 |
| Leu | 4.7 | 0.8 |
| Glu | 14.2 | 2.8 |
| Gin | 10.5 | 1.2 |
| Tyr | 5.3 | 1.2 |
| wild-type background | ||
| WT | 22.5 | 3.3 |
| Lys | 2.8 | 0.2 |
Data derived from Figure 3.
Site-Directed Alkylation
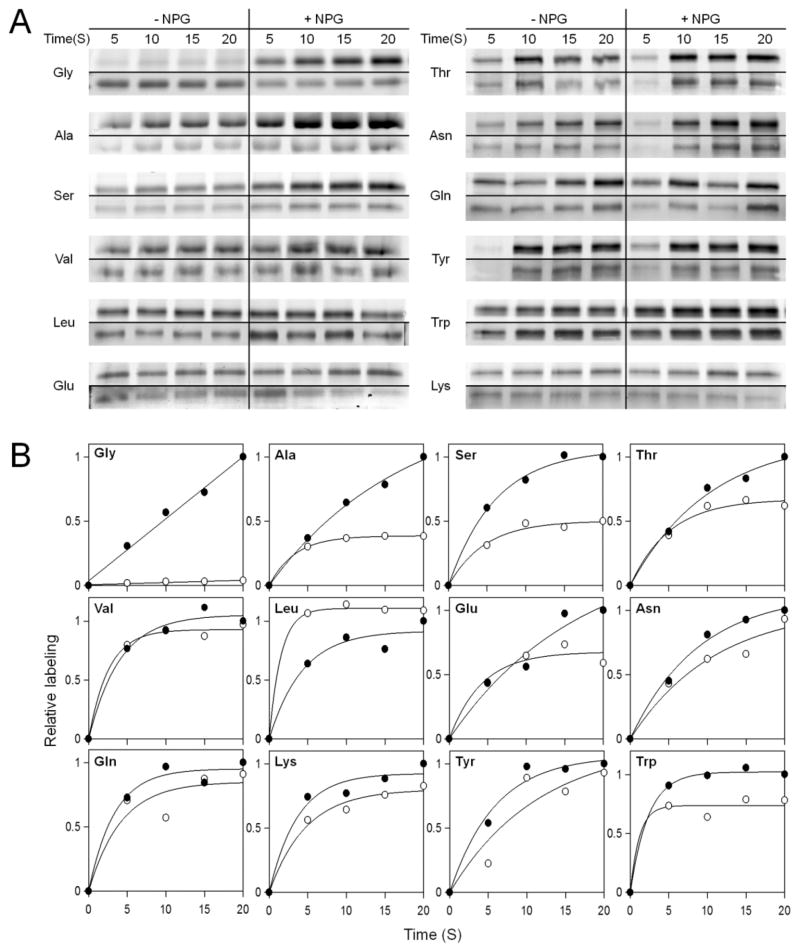
Asn245 is near the end of helix VII on the periplasmic side of LacY (Figure 1), and sitedirected alkylation studies with N-ethylmaleimide10,52 or TMRM11,12,14,17 demonstrate that the accessibility or activity of a single-Cys replacement at position 245 in an otherwise Cys-less background is very sensitive to conformational change. More specifically, a marked increase in the level of labeling is observed upon binding of sugar. Thus, with pseudo-WT LacY, almost no TMRM labeling is observed during a 20 s incubation in the absence of α-NPG. In contrast, in the presence of the galactoside, labeling of Cys245 is markedly stimulated (Figure 4A), and the level increases linearly throughout the time course (Figure 4B). Double replacement of Gly46 and Gly262 with Ala, Ser, or Thr increases the level of labeling in the absence of sugar in a manner that appears to correlate with the mass of the side chain, as the stimulatory effect of α-NPG becomes smaller from Ala to Ser to Thr (Figure 4A,B). For the remainder of the double-replacement mutants, there is little or no systematic difference observed in the absence or presence of α-NPG. The findings are consistent with the interpretation that simultaneous replacement of Gly46 and Gly262, neighboring positions in helices II and VIII that allow tighter helix packing, with side chains with a mass greater than that of Ser markedly increases the probability of periplasmic cavity opening.
Figure 4.
(A) RSO membrane vesicles containing single Cys245 or single Cys245 with Ala, Val, Leu, Ser, Thr, Glu, Lys, Asn, Gln, Tyr, or Trp double replacements at positions 46 and 262 labeled with 40 μM TMRM for given times in the absence or presence of 2 mM α-NPG. For each mutant, the TMRM labeling intensity (top panel) was obtained by scanning the SDS–PAGE gel with a phosphorimager, and protein (bottom panel) was visualized by silver staining. (B) TMRM labeling rates of Cys245 in single-Cys245 and double-Ala, -Ser, -Thr, -Val, -Leu, -Glu, -Asn, -Gln, -Lys, -Tyr, or -Trp replacements at positions 46 and 262 obtained by plotting the relative labeling in the presence (●) or absence (○) of 2 mM α-NPG as a function of labeling time. For each mutant, the relative labeling of 20 s in the presence of sugar point was set to 1.
Molecular Dynamics (MD) Simulations
To characterize the underlying molecular details associated with double replacements at positions 46 and 262, simulated molecular dynamics of the WT and double-Leu mutant were contrasted. Simulations starting from the inward-open/outward-closed conformation (PDB entry 2V8N) show significant differences in structural dynamics in the vicinity of both positions 46 and 262 (see arrows in Figure 5A). When Cα–Cα interatomic distances are tracked in the WT simulation, the helices at positions 46 and 262 remain in the crystallographic positions, while the corresponding helices in the double-Leu mutant simulations undergo extensive structural shifts (Figure 5B). Upon visual inspection of snapshots corresponding to the last frames of each simulation, it is clear that the observed differences in Cα–Cα interatomic distances are associated with a significant separation of the II–XI and V–VIII helix pairs (Figure 5C). Therefore, the sugar-binding site of the double-Leu mutant structure becomes more accessible from the periplasmic side, which is consistent with the experimental observations.
Figure 5.
Dynamics and structural rearrangements in wild-type and double-Leu mutant LacY. (A) Root-mean-square fluctuations (rmsf) extracted from the full simulations of wild-type (black) and double-Leu (red) LacY. The arrows mark sequence regions of significant differences. (B) Cα–Cα interatomic distances tracked between positions 46–370 and 159–262 in the wild-type and mutant proteins. (C) Superimposed frames corresponding to the ends of both the wild-type (blue) and mutant (cyan) simulations. The protein is viewed from the periplasmic side, and the mutated leucine residues are shown in licorice representation. The dashed black lines highlight the separation between the II–XI and V–VIII helix pairs.
DISCUSSION
Gly residues play unique structural roles in both soluble and membrane proteins because of the absence of a side chain. Although considered a major helix breaker in soluble proteins,53–55 Gly is commonly found in transmembrane α-helices in membrane proteins.56,57 In the low-dielectric, hydrophobic environment of the membrane, strong intrahelical hydrogen bonds stabilize transmembrane helices, thereby allowing tolerance to Gly residues,58 but it has also been shown that Gly residues can facilitate tight helix packing in membrane proteins.25,27 For example, an LIxxGVxxGVxxT motif is necessary for dimerization of the single-transmembrane α-helical protein human glycophorin A,59 and replacing either Gly in the motif eliminates dimerization.60
Among the 36 Gly residues in LacY, several are highly conserved in the members of the major facilitator super-family. 24,61 Two pairs of conserved Gly residues on helices II and XI (Gly46 and Gly370, respectively) and helices V and VIII (Gly159 and Gly262, respectively) facilitate tight interaction of each pair of helices to seal the periplasmic side of LacY in the inward-open/outward-closed conformation (Figure 1). Replacing one Gly residue in each Gly-Gly pair with a bulky Trp residue inactivates transport and increases the open probability on the periplasmic side, leading to an increase in the rate of α-NPG binding.28
All double replacements for Gly46 and Gly262 described here reduce transport activity (Figure 2), indicating that a tight seal between helices II and XI and helices V and VIII on the periplasmic side is essential for the alternating access mechanism. Progressing from Gly to Ala to Ser to Thr, we find there is a correlation between the mass of the side chain and decreased transport activity, as well as an increased probability of opening the periplasmic cavity (Figure 4). However, no further correlation with mass or charge is observed with the remaining replacements, each of which exhibits little or no transport activity and little or no effect of galactoside binding on the accessibility of the periplasmic cavity. Furthermore, all of the double-replacement mutants bind α-NPG with affinities 10–20-fold higher than that of the pseudo-WT, which is likely caused by increased access to the binding site with an increase in kon (Figure 3). It is also notable that the transport data obtained here are in agreement with a previous Cys-scanning study of the Gly residues in LacY,62 where Ala replacements for the important Gly residues Gly115 and Gly147 exhibit the best transport activity.
The MD simulations are consistent with the experimental findings. The observed differences in Cα–Cα interatomic distances are a direct result of increased patency of the periplasmic cavity in the double-Leu replacement mutant due to greater interhelical separation (Figure 5). Thus, in the inward-open/outward-closed state, significant helical rearrangements are observed in the mutant relative to the WT at both position 46 and position 262 (Figure 5B,C) that result in opening of the periplasmic cavity.
It is interesting to compare these observations to the conformationally restricted mutant C154G, which also has very little transport activity but binds galactoside with a high affinity.63 Although the X-ray structure of mutant C154G exhibits an inward-open/outward-closed conformation like WT LacY,6,8 site-directed alkylation indicates that the periplasmic cavity in mutant C154G is patent in the absence of sugar.13,17 A likely explanation for the lack of transport activity in the C154G mutant is that position 154 in helix V is in direct opposition to Gly24 in helix I where the two helices cross in the middle of the membrane. Therefore, when Cys154 is mutated to Gly, helices I and V become more tightly packed and may prevent a “scissors-like” interhelical movement required for transport.64 Experimental support for this notion is provided by the finding that replacing Gly24 with Cys effectively rescues transport activity in the C154G mutant.65
Acknowledgments
Funding
This work was supported by the National Science Foundation (Eager Grant MCB-1547801 to H.R.K.) and by the National Institutes of Health (Grant R01 DK51131 to H.R.K.), as well as a grant from Ruth and Bucky Stein. This work was also supported by grants from Marie Curie Career Integration Grant (FP7-MC-CIG-618558), Magnus Bergvalls Stiftelse (2014-00170), Åke Wibergs Stiftelse (M15-0148), and Stiftelsen Olle Engkvist Byggmaästare (2015/768) to M.A. Computational resources were provided by the Swedish National Infrastructure for Computing (2014/11-33).
We thank Vladimir Kasho, Irina Smirnova, and Junichi Sugihara for useful discussions and technical help.
ABBREVIATIONS
- C-less LacY
functional LacY devoid of native Cys residues
- RSO
right-side-out
- TMRM
tetramethylrhodamine 5-maleimide
- α-NPG
p-nitrophenyl α-D-galactopyranoside
- IPTG
isopropyl β-D-1-thiogalactopyranoside
- DDM
n-dodecyl β-D-maltopyranoside
- DTT
1,4-dithiothreitol
- SDS
sodium dodecyl sulfate
- SDS–PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- PDB
Protein Data Bank
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Saier MH, Jr, Beatty JT, Goffeau A, Harley KT, Heijne WH, Huang SC, Jack DL, Jahn PS, Lew K, Liu J, Pao SS, Paulsen IT, Tseng TT, Virk PS. The major facilitator superfamily. J Mol Microbiol Biotechnol. 1999;1:257–279. [PubMed] [Google Scholar]
- 2.Saier MH., Jr Families of transmembrane sugar transport proteins. Mol Microbiol. 2000;35:699–710. doi: 10.1046/j.1365-2958.2000.01759.x. [DOI] [PubMed] [Google Scholar]
- 3.Kaback HR, Sahin-Toth M, Weinglass AB. The kamikaze approach to membrane transport. Nat Rev Mol Cell Biol. 2001;2:610–620. doi: 10.1038/35085077. [DOI] [PubMed] [Google Scholar]
- 4.Guan L, Kaback HR. Lessons from lactose permease. Annu Rev Biophys Biomol Struct. 2006;35:67–91. doi: 10.1146/annurev.biophys.35.040405.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaback HR. A chemiosmotic mechanism of symport. Proc Natl Acad Sci U S A. 2015;112:1259–1264. doi: 10.1073/pnas.1419325112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 7.Mirza O, Guan L, Verner G, Iwata S, Kaback HR. Structural evidence for induced fit and a mechanism for sugar/ H(+) symport in LacY. EMBO J. 2006;25:1177–1183. doi: 10.1038/sj.emboj.7601028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan L, Mirza O, Verner G, Iwata S, Kaback HR. Structural determination of wild-type lactose permease. Proc Natl Acad Sci U S A. 2007;104:15294–15298. doi: 10.1073/pnas.0707688104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaptal V, Kwon S, Sawaya MR, Guan L, Kaback HR, Abramson J. Crystal structure of lactose permease in complex with an affinity inactivator yields unique insight into sugar recognition. Proc Natl Acad Sci U S A. 2011;108:9361–9366. doi: 10.1073/pnas.1105687108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaback HR, Dunten R, Frillingos S, Venkatesan P, Kwaw I, Zhang W, Ermolova N. Site-directed alkylation and the alternating access model for LacY. Proc Natl Acad Sci U S A. 2007;104:491–494. doi: 10.1073/pnas.0609968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nie Y, Ermolova N, Kaback HR. Site-directed Alkylation of LacY: Effect of the Proton Electrochemical Gradient. J Mol Biol. 2007;374:356–364. doi: 10.1016/j.jmb.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nie Y, Kaback HR. Sugar binding induces the same global conformational change in purified LacY as in the native bacterial membrane. Proc Natl Acad Sci U S A. 2010;107:9903–9908. doi: 10.1073/pnas.1004515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nie Y, Sabetfard FE, Kaback HR. The Cys154– > Gly mutation in LacY causes constitutive opening of the hydrophilic periplasmic pathway. J Mol Biol. 2008;379:695–703. doi: 10.1016/j.jmb.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang X, Nie Y, Kaback HR. Site-Directed Alkylation Studies with LacY Provide Evidence for the Alternating Access Model of Transport. Biochemistry. 2011;50:1634–1640. doi: 10.1021/bi101988s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang X, Driessen AJ, Feringa BL, Kaback HR. The Periplasmic Cavity of LacY Mutant Cys154– > Gly: How Open Is Open? Biochemistry. 2013;52:6568–6574. doi: 10.1021/bi401026d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Y, Guan L, Freites JA, Kaback HR. Opening and closing of the periplasmic gate in lactose permease. Proc Natl Acad Sci U S A. 2008;105:3774–3778. doi: 10.1073/pnas.0800825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang X, Guan L, Zhou Y, Hong WX, Zhang Q, Kaback HR. Evidence for an intermediate conformational state of LacY. Proc Natl Acad Sci U S A. 2012;109:E698–704. doi: 10.1073/pnas.1201107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majumdar DS, Smirnova I, Kasho V, Nir E, Kong X, Weiss S, Kaback HR. Single-molecule FRET reveals sugar-induced conformational dynamics in LacY. Proc Natl Acad Sci U S A. 2007;104:12640–12645. doi: 10.1073/pnas.0700969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smirnova I, Kasho V, Choe JY, Altenbach C, Hubbell WL, Kaback HR. Sugar binding induces an outward facing conformation of LacY. Proc Natl Acad Sci U S A. 2007;104:16504–16509. doi: 10.1073/pnas.0708258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smirnova I, Kasho V, Sugihara J, Kaback HR. Probing of the rates of alternating access in LacY with Trp fluorescence. Proc Natl Acad Sci U S A. 2009;106:21561–21566. doi: 10.1073/pnas.0911434106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smirnova I, Kasho V, Sugihara J, Kaback HR. Opening the periplasmic cavity in lactose permease is the limiting step for sugar binding. Proc Natl Acad Sci U S A. 2011;108:15147–15151. doi: 10.1073/pnas.1112157108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smirnova I, Kasho V, Kaback HR. Lactose permease and the alternating access mechanism. Biochemistry. 2011;50:9684–9693. doi: 10.1021/bi2014294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bockmann J, Heuel H, Lengeler JW. Characterization of a chromosomally encoded, non-PTS metabolic pathway for sucrose utilization in E. coli EC3132. Mol Gen Genet. 1992;235:22–32. doi: 10.1007/BF00286177. [DOI] [PubMed] [Google Scholar]
- 24.Kasho VN, Smirnova IN, Kaback HR. Sequence alignment and homology threading reveals prokaryotic and eukaryotic proteins similar to lactose permease. J Mol Biol. 2006;358:1060–1070. doi: 10.1016/j.jmb.2006.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Javadpour MM, Eilers M, Groesbeek M, Smith SO. Helix packing in polytopic membrane proteins: role of glycine in transmembrane helix association. Biophys J. 1999;77:1609–1618. doi: 10.1016/S0006-3495(99)77009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemmon MA, Flanagan JM, Hunt JF, Adair BD, Bormann BJ, Dempsey CE, Engelman DM. Glycophorin A dimerization is driven by specific interactions between transmembrane alpha-helices. J Biol Chem. 1992;267:7683–7689. [PubMed] [Google Scholar]
- 27.Dong H, Sharma M, Zhou HX, Cross TA. Glycines: role in alpha-helical membrane protein structures and a potential indicator of native conformation. Biochemistry. 2012;51:4779–4789. doi: 10.1021/bi300090x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smirnova I, Kasho V, Sugihara J, Kaback HR. Trp replacements for tightly interacting Gly-Gly pairs in LacY stabilize an outward-facing conformation. Proc Natl Acad Sci U S A. 2013;110:8876–8881. doi: 10.1073/pnas.1306849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar H, Kasho V, Smirnova I, Finer-Moore JS, Kaback HR, Stroud RM. Structure of sugar-bound LacY. Proc Natl Acad Sci U S A. 2014;111:1784–1788. doi: 10.1073/pnas.1324141111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar H, Finer-Moore JS, Kaback HR, Stroud RM. Structure of LacY with an alpha-substituted galactoside: Connecting the binding site to the protonation site. Proc Natl Acad Sci U S A. 2015;112:9004–9009. doi: 10.1073/pnas.1509854112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaback HR. Transport in isolated bacterial membrane vesicles. Methods Enzymol. 1974;31:698–709. doi: 10.1016/0076-6879(74)31075-0. [DOI] [PubMed] [Google Scholar]
- 32.Teather RM, Müller-Hill B, Abrutsch U, Aichele G, Overath P. Amplification of the lactose carrier protein in Escherichia coli using a plasmid vector. Mol Gen Genet. 1978;159:239–248. doi: 10.1007/BF00268260. [DOI] [PubMed] [Google Scholar]
- 33.Teather RM, Bramhall J, Riede I, Wright JK, Furst M, Aichele G, Wilhelm U, Overath P. Lactose carrier protein of Escherichia coliStructure and expression of plasmids carrying the Y-gene of the lac operon. Eur J Biochem. 1980;108:223–231. doi: 10.1111/j.1432-1033.1980.tb04715.x. [DOI] [PubMed] [Google Scholar]
- 34.Short SA, Kaback HR, Kohn LD. Localization of D-lactate dehydrogenase in native and reconstituted Escherichia coli membrane vesicles. J Biol Chem. 1975;250:4291–4296. [PubMed] [Google Scholar]
- 35.Kaback HR. Bacterial Membranes. In: Kaplan NP, Jakoby WB, Colowick NP, editors. Methods in Enzymology. Elsevier; New York: 1971. pp. 99–120. [Google Scholar]
- 36.Jiang X, Villafuerte MK, Andersson M, White SH, Kaback HR. Galactoside-binding site in LacY. Biochemistry. 2014;53:1536–1543. doi: 10.1021/bi401716z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smirnova IN, Kasho VN, Kaback HR. Direct Sugar Binding to LacY Measured by Resonance Energy Transfer. Biochemistry. 2006;45:15279–15287. doi: 10.1021/bi061632m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graphics. 1996;14:27–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 39.Jo S, Kim T, Im W. Automated builder and database of protein/membrane complexes for molecular dynamics simulations. PLoS One. 2007;2:e880. doi: 10.1371/journal.pone.0000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klauda JB, Venable RM, Freites JA, O’Connor JW, Tobias DJ, Mondragon-Ramirez C, Vorobyov I, MacKerell AD, Pastor RW. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J Phys Chem B. 2010;114:7830–7843. doi: 10.1021/jp101759q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mackerell AD, Jr, Feig M, Brooks CL., 3rd Extending the treatment of backbone energetics in protein force fields: limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J Comput Chem. 2004;25:1400–1415. doi: 10.1002/jcc.20065. [DOI] [PubMed] [Google Scholar]
- 43.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of Simple Potential Functions for Simulating Liquid Water. J Chem Phys. 1983;79:926–935. [Google Scholar]
- 44.Grubmuller H, Heller H, Windemuth A, Schulten K. Generalized Verlet Algorithm for Efficient Molecular Dynamics Simulations with Long-Range Interactions. Mol Simul. 1991;6:121–142. [Google Scholar]
- 45.Darden T, York D, Pedersen L. Particle Mesh Ewald - an N.Log(N) Method for Ewald Sums in Large Systems. J Chem Phys. 1993;98:10089–10092. [Google Scholar]
- 46.Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG. A Smooth Particle Mesh Ewald Method. J Chem Phys. 1995;103:8577–8593. [Google Scholar]
- 47.Ryckaert JP, Ciccotti G, Berendsen H. Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J Comput Phys. 1977;23:327–341. [Google Scholar]
- 48.Miyamoto S, Kollman PA. Settle - an Analytical Version of the Shake and Rattle Algorithm for Rigid Water Models. J Comput Chem. 1992;13:952–962. [Google Scholar]
- 49.Feller SE, Zhang YH, Pastor RW, Brooks BR. Constant-Pressure Molecular-Dynamics Simulation - the Langevin Piston Method. J Chem Phys. 1995;103:4613–4621. [Google Scholar]
- 50.Martyna GJ, Tobias DJ, Klein ML. Constant-Pressure Molecular-Dynamics Algorithms. J Chem Phys. 1994;101:4177–4189. [Google Scholar]
- 51.Zamyatnin AA. Protein volume in solution. Prog Biophys Mol Biol. 1972;24:107–123. doi: 10.1016/0079-6107(72)90005-3. [DOI] [PubMed] [Google Scholar]
- 52.Venkatesan P, Kwaw I, Hu Y, Kaback HR. Site-directed sulfhydryl labeling of the lactose permease of Escherichia coli: helix VII. Biochemistry. 2000;39:10641–10648. doi: 10.1021/bi000438b. [DOI] [PubMed] [Google Scholar]
- 53.O’Neil KT, DeGrado WF. A thermodynamic scale for the helix-forming tendencies of the commonly occurring amino acids. Science. 1990;250:646–651. doi: 10.1126/science.2237415. [DOI] [PubMed] [Google Scholar]
- 54.Lyu PC, Liff MI, Marky LA, Kallenbach NR. Side chain contributions to the stability of alpha-helical structure in peptides. Science. 1990;250:669–673. doi: 10.1126/science.2237416. [DOI] [PubMed] [Google Scholar]
- 55.Chou PY, Fasman GD. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974;13:211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- 56.Eilers M, Patel AB, Liu W, Smith SO. Comparison of helix interactions in membrane and soluble alpha-bundle proteins. Biophys J. 2002;82:2720–2736. doi: 10.1016/S0006-3495(02)75613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Landolt-Marticorena C, Williams KA, Deber CM, Reithmeier RA. Non-random distribution of amino acids in the transmembrane segments of human type I single span membrane proteins. J Mol Biol. 1993;229:602–608. doi: 10.1006/jmbi.1993.1066. [DOI] [PubMed] [Google Scholar]
- 58.Kim S, Cross TA. Uniformity, ideality, and hydrogen bonds in transmembrane alpha-helices. Biophys J. 2002;83:2084–2095. doi: 10.1016/S0006-3495(02)73969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lemmon MA, Treutlein HR, Adams PD, Brunger AT, Engelman DM. A dimerization motif for transmembrane alpha-helices. Nat Struct Biol. 1994;1:157–163. doi: 10.1038/nsb0394-157. [DOI] [PubMed] [Google Scholar]
- 60.Lemmon MA, Flanagan JM, Treutlein HR, Zhang J, Engelman DM. Sequence specificity in the dimerization of transmembrane alpha-helices. Biochemistry. 1992;31:12719–12725. doi: 10.1021/bi00166a002. [DOI] [PubMed] [Google Scholar]
- 61.Bockmann J, Heuel H, Lengeler JW. Characterization of a chromosomally encoded, non-PTS metabolic pathway for sucrose utilization in Escherichia coli EC3132. Mol Gen Genet. 1992;235:22–32. doi: 10.1007/BF00286177. [DOI] [PubMed] [Google Scholar]
- 62.Jung K, Jung H, Colacurcio P, Kaback HR. Role of glycine residues in the structure and function of lactose permease, an Escherichia coli membrane transport protein. Biochemistry. 1995;34:1030–1039. doi: 10.1021/bi00003a038. [DOI] [PubMed] [Google Scholar]
- 63.Smirnova IN, Kaback HR. A mutation in the lactose permease of Escherichia coli that decreases conformational flexibility and increases protein stability. Biochemistry. 2003;42:3025–3031. doi: 10.1021/bi027329c. [DOI] [PubMed] [Google Scholar]
- 64.Zhou Y, Madej MG, Guan L, Nie Y, Kaback HR. An early event in the transport mechanism of LacY: Interaction between helices V and I. J Biol Chem. 2011;286:30415–30422. doi: 10.1074/jbc.M111.268433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ermolova NV, Smirnova IN, Kasho VN, Kaback HR. Interhelical packing modulates conformational flexibility in the lactose permease of Escherichia coli. Biochemistry. 2005;44:7669–7677. doi: 10.1021/bi0502801. [DOI] [PubMed] [Google Scholar]