Abstract
Obesity and diabetes is generally accompanied by a chronic state of oxidative stress, disequilibrium in the redox balance, implicated in the development and progression of complications such as micro- and macro-angiopathies. Disorders in the inner layer of blood vessels, the endothelium, play an early and critical role in the development of these complications. Blunted endothelium-dependent relaxation and/or contractions are quietly associated to oxidative stress. Thus, preserving endothelial function and oxidative stress seems to be an optimization strategy in the prevention of vascular complications associated with diabetes. Diet is a major lifestyle factor that can greatly influence the incidence and the progression of type 2 diabetes and cardiovascular complications. The notion that foods not only provide basic nutrition but can also prevent diseases and ensure good health and longevity is now attained greater prominence. Some dietary and lifestyle modifications associated to antioxidative supply could be an effective prophylactic means to fight against oxidative stress in diabesity and complications. A significant benefit of phytochemicals (polyphenols in wine, grape, teas), vitamins (ascorbate, tocopherol), minerals (selenium, magnesium), and fruits and vegetables in foods is thought to be capable of scavenging free radicals, lowering the incidence of chronic diseases. In this review, we discuss the role of oxidative stress in diabetes and complications, highlight the endothelial dysfunction, and examine the impact of antioxidant foods, plants, fruits, and vegetables, currently used medication with antioxidant properties, in relation to the development and progression of diabetes and cardiovascular complications.
Keywords: diabetes, complications, oxidative stress, antioxidants, plants, prevention
1. Introduction
Today, WHO and IDF (International Diabetes Federation) draws attention to the similarity of trends in obesity and diabetes in the World. The term “diabesity” is commonly used today to describe this epidemic or pandemic with exponential dramatic growth observed in all countries [1]. Our change of lifestyle to a sedentary attitude and massive industrialization with access from an early age to food and beverages rich in energy, fat, sugar, or a combination thereof is partly the cause of millions of obese and diabetic people [2]. Despite technical and technological progress accompanying therapeutic arsenal available and public health plans, we fail today to stop the progression of diabetes and its complications. In fact, diabetes is a silent and sneaky disease. Therefore, it is associated with many complications. Cardiovascular diseases are the major cause of death and disability among diabetic people [3], particularly for woman who have lost cardiovascular protection afforded by the classically female sex. Diabetic vascular complications are an important pathological issue in diabetes that leads to the further functional deterioration of several organs and caused micro- and macro-angiopathy [4]. Endothelial dysfunction, the loss of a balance between vasodilators and vasoconstrictors factors in the blood vessels, has largely been associated in several regions of the vasculature in T2D [5].
A common point of all these cardio-metabolic disorders is the appearance of oxidative stress. Oxidative stress is due to an imbalance between antioxidants (enzymes, vitamins, proteins, etc.) and pro-oxidants (UV radiations, alcohol, smoking, etc.) [6]. Oxidative stress along with chronic low-grade inflammation may initiate changes in cardiovascular structure and function such as endothelial dysfunction, cardiac hypertrophy, cardiac fibrosis, and ventricular contractile dysfunction [7]. Many studies have shown that diabetic patients undergo chronic oxidative stress, particularly due to hyperglycemia [8,9]. Thus, a strategy focus on both oxidative stress and endothelial function could help to prevent or delay the onset of vascular-related type 2 diabetes complications.
Much evidence shows that consumption of natural source substances confers chemopreventive and cytoprotectant activities. In fact, epidemiological studies suggest that consumption of fruits, vegetables [10,11,12,13,14], and plants [15] may be associated with a reduced risk of diabetes or a protective effect [16]. Some observations have also revealed an inverse relationship between the risk of cardiovascular mortality or morbidity and the consumption of polyphenol-rich products (red wine, cocoa and tea) [17,18,19,20]. Their consumption brings several exogenous antioxidants and vitamins, increasing the antioxidant status of the organism, in addition to their direct effect on blood vessels and in particular on the endothelium [21].
Many plants are also used for their benefits in traditional medicines. Some of them are at the origin of the development of drugs [16] such as biguanide, metformin, antidiabetic drugs, and Galega officinalis. In developed countries, traditional, complementary, and alternative medicines are becoming increasingly popular and are commonly used to treat or prevent chronic diseases and are improving quality of life [15].
Therefore, we will see through this review that many compounds surrounding us can be a real asset in the prevention of “diabesity” but also a valuable aid in addition to current treatments to prevent the occurrence of such complications. We will also discuss the appeal for the use of single molecules to the detriment of total extracts, thereby promoting molecular synergy.
2. Diabesity and Cardiovascular Complications
2.1. The Evolution of Obesity and Diabetes
Developed societies face two crucial health problems: overweight and obesity. Obesity is the most common metabolic disease, and the number of individuals who are overweight or obese is fast increasing worldwide [22]. Overweight and obesity are defined as abnormal or excessive fat accumulation that may impair health. Body mass index (BMI) is a simple index of weight-for-height that is commonly used to classify overweight and obesity in adults. It is defined as a person's weight in kilograms divided by the square of his height in meters (kg/m2). BMI is widely used as a measure of weight status and disease risk and is widely used for routine characterization of weight status in epidemiology, clinical nutrition, and research. Moreover, fat mass and fat-free mass as assessed by validated techniques (densitometry, dual impedance analysis, etc.) are also currently used. Thus, the term obesity in our review is used in a broad sense that includes BMI, fat mass, % body fat, etc.
Obesity has more than doubled since 1980. The prevalence of overweight and obese youth has increased dramatically over the past three decades [23]. In 2014, more than 1.9 billion adults, 18 years and older, were overweight. Of these over 600 million were obese. 39% of adults aged 18 years and over were overweight in 2014, and 13% were obese. Most of the world’s population lives in countries where overweight and obesity kills more people than does underweight.
Overweight and obesity have reached epidemic proportions globally along with an adoption of a Westernized lifestyle characterized by a combination of excessive food intake and inadequate physical activity. Raised BMI is a major risk factor for noncommunicable diseases such as cardiovascular diseases (mainly heart disease and stroke), which were the leading cause of death.
The dramatic rise in the prevalence of obesity and changes in lifestyle-related factors such as a reduction in physical activity have been accompanied by alarming increases in the incidence and prevalence of type 2 diabetes [24]. Diabetes is a chronic disease that occurs either when the pancreas does not produce enough insulin or when the body cannot effectively use the insulin it produces. Insulin is a hormone that regulates blood sugar [25]. Hyperglycemia, or raised blood sugar, is a common effect of uncontrolled diabetes and, over time, leads to serious damage to many of the body's systems, especially the nerves and blood vessels. In 2014, 9% of adults 18 years and older had diabetes. In 2012, diabetes was the direct cause of 1.5 million deaths. More than 80% of diabetes deaths occur in low- and middle-income countries.
Epidemiological studies have confirmed a strong positive association between excess adiposity and risk of developing type 2 diabetes. Based on the data from the Behavioral Risk Factors Surveillance System conducted by the United States, Mokdad et al. [26] estimated that, for every kilogram increment in self-reported body weight, the risk of diabetes increases by about 9%. The term “diabesity” has been coined to illustrate the close relationship between obesity and diabetes [27,28].
2.2. Lifestyle
Lifestyle habits have deteriorated over time with increases in obesity, central obesity, and diabetes and stagnating rates of persistent smoking. An increase in obesity and diabetes has paralleled the growth of urbanization and globalization in the region. For example, in China, the prevalence rates of diabetes in large provincial capital cities range from a high of 8% (in the Eastern region) to 4.6% (in the lowest in the Western region) [29]. Behavioral risk factors include tobacco use, alcohol consumption, unhealthy diet, and physical inactivity. Physical inactivity is the 4th mortality risk factor for mortality [30] with an increase of 20–30% of death compared with people who practice 30 min of exercise a day [31].
Finally, advances in agriculture and food systems, consequent increases in food availability, and a shift in dietary consumption patterns with economic development and urbanization of developing societies promotes overweight and obesity. This “new” diet favors consumption of fats, saturated fats largely from animal sources and sugars. The essence of these changes is captured by the term “nutrition transition” which accompanies the demographic and epidemiologic transition in these countries with economic development [32].
Epidemiological studies indicate that weight loss, even moderate, can improve insulin sensitivity, improve insulin action, and decrease the risk of developing type 2 diabetes. Improvements in insulin action after an average of 10% weight reduction were lost with weight regain but largely preserved with weight maintenance [33].
Physical activity is associated with a significant reduction in the risk of type 2 diabetes, whereas a sedentary lifestyle is associated with an increased risk [34,35]. There is a 20% increased risk of diabetes for each 2-h daily increment in watching television [36]. However, some studies have demonstrated the feasibility and efficiency of lifestyle intervention programs in the prevention of diabetes in individuals with impaired glucose tolerance [37,38]. The lifestyle intervention program permits a reduction in weight with moderate exercise and a controlled food intake (reduction of fat, increase in fiber, and frequent consumption of fruits, vegetables, etc.)
2.3. Diabetic Complications: Link with Oxidative Stress and Inflammation
Chronic hyperglycemia, disturbances of carbohydrate, and lipid and protein metabolism lead to metabolic derangements in diabetes and various complications including both macro- and microvascular dysfunctions [22]. Over time, diabetes can damage the heart, blood vessels, eyes, kidneys, and nerves. The incidence of cardiovascular diseases in people with diabetes, one of the major complications, is three to four times that in non-diabetic individuals. In a multinational study, 50% of people with diabetes die of cardiovascular disease (primarily heart disease and stroke) [39], with a twofold increase in risk of heart failure in male patients, and a fivefold increase in female patients [40]. Likewise, diabetes increased incidence of coronary artery disease and atherosclerotic lesions at a younger age, often associated with multivessel disease and involvement of distal coronary segments. Hypertension is also commonly found in both type 1 and type 2 diabetes [41]. Finally, diabetes can cause distinct pathologic alterations in the myocardium, independent of its effect on blood pressure and coronary atherosclerosis, termed “diabetic cardiomyopathy” (DMC) [42]. We will focus on cardiovascular complications below in this review.
Combined with reduced blood flow, neuropathy (nerve damage) in the feet increases the chance of foot ulcers, infection, and the eventual need for limb amputation and affects almost 30% to 50% of patients with diabetes. One percent of global blindness is attributed to diabetic retinopathy [43] due to a long-term accumulated damage to the small blood vessels in the retina, and the overall risk of dying among people with diabetes is at least double the risk of their peers without diabetes [44]. Another important microvascular complications is diabetic nephropathy, of which there is a ninefold higher risk in patients with diabetes, leading to end-stage renal disease requiring chronic dialysis and transplantation [23].
Oxidative stress has been suggested to be a common pathway for the pathogenesis of complications in diabetes [24,25]. For example, (1) the production of hydrogen peroxide by mesangial cells and lipid peroxidation, activation of protein kinase C (PKC), mitogen-activated protein (MAP) kinases, and cytokine production lead to renal injury [26]; (2) the redox-sensitive nuclear transcriptional factor, NFκB, accumulation of advanced-glycation end-products (AGEs) localized in sub-retinal membranes, and microvessels are activated earlier in the course of diabetic retinopathy [23,27] in addition to polyol accumulation and glycation associated to cataract [28]; (3) AGEs inhibit axonal regeneration [29], an increase in DNA damage and the stimulation of the PKC pathway, and NFκB and TGF-β increase deposition of the extracellular matrix [30], and all mechanisms have involved in neuropathy. Moreover, HbA1c, a biomarker of the overall glycemic exposure, is the most known diabetic parameter link to oxidative stress. In fact, it is due to the glycation of hemoglobin. The increase in Hb1Ac variability predicts the risk of microvascular complications in T1D [31,32,33] and the risk of nephropathy and cardiovascular diseases in T2D [34,35,36].
In addition to oxidative stress, inflammation stands out as a determinant process in the development of diabetic complications [37]. It is difficult, in fact, to understand the impact of these factors without each other, since numerous interplays exist between inflammation and oxidative stress and vice versa [38,39]. Hyperglycemia increases the levels of pro-inflammatory proteins [37], and infiltrated macrophages secrete inflammatory cytokines (correlate with a higher body mass index: IL-6, IL-8, MCP-1 [43]), thereby leading to a local and systemic inflammation. Increased production of TNF-α has also been widely associated with obesity-related insulin resistance and abnormal vascular reactivity, the vasculature being an important target of TNF-α [44] and closely linked to diabetic micro- and macro-complications [40,45].
3. Oxidative Stress and Cardiovascular Complications
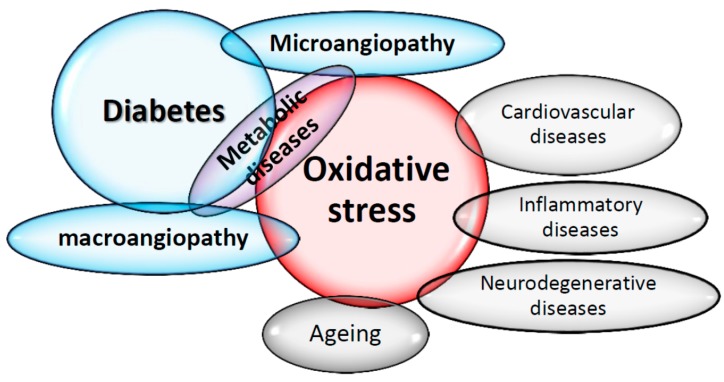
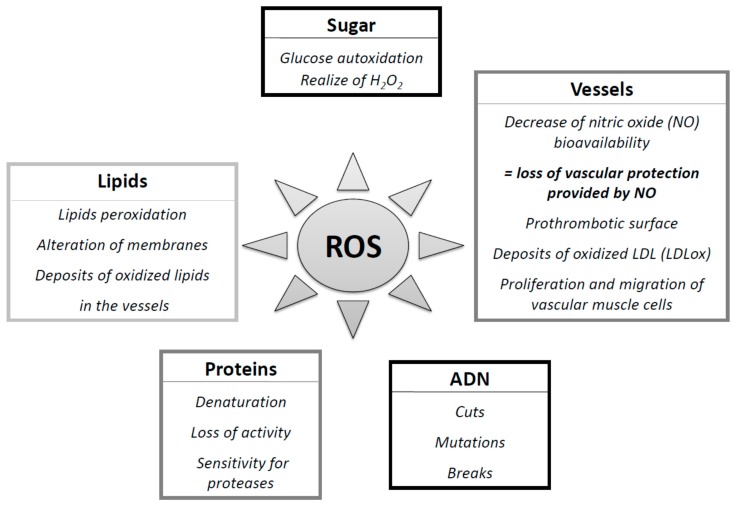
The concept that oxygen, which is essential to life, could be causing cell damage and involved in many diseases, was discovered in recent years. Today, many epidemiological and clinical studies strongly suggest the involvement of reactive oxygen species (ROS) in the genesis and evolution of chronic diseases, including diabetes and its complications [7] (Figure 1). Chronic hyperglycemia caused a major oxidative stress [22], and Yubero-Serrano et al. [46] recently proposed SOD activity as the most relevant oxidative stress biomarker in patients suffering from metabolic syndrome. It could be used as a predictive tool to determine the degree of the underlying oxidative stress in this pathology.
Figure 1.
Oxidative stress in the middle of diseases and complications, including diabetes.
3.1. Oxidative Stress: A Question of Balance
3.1.1. Oxygen Paradox and Anti-Oxygen
Oxygen, which first appeared three billion years ago in Earth's atmosphere, is an essential molecule for life. Through redox mechanisms, oxygen, the final electron acceptor, is transformed into water by the mitochondrial respiratory chain [41]. This reaction is a source of energy through ATP production and also the formation of 2% to 3% of reactive oxygen species (ROS), a free radical that is particularly unstable and reactive [42]. In 1954, Gerschman published the free radical theory of oxygen toxicity, due to partially reduced forms of oxygen [47], and, two years after, Harman proposed the concept of involving free radicals in the aging process [48]. Whereas McCord and Fridovich discovered the enzyme superoxide dismutase (SOD) in 1969 [49] and provided convincing evidence about the importance of free radicals in the living system [50], the concept of anti-oxidants has been reported for much longer by Dufraisse and Moureu in the 1920s, when they discovered that the polymerization of acrolein was inhibited by hydroquinone, an oxygen-dependent mechanism [51]. Originally named “anti-oxygen,” the Anglo-Saxon term “antioxidant” was quickly privileged and replaced. Since the properties as second messengers of ROS were discovered for the first time by Mittal and Murad in 1977 [52], many studies are now interested in this delicate balance between the beneficial and harmful effects of free radicals, which is the redox regulation for maintaining redox homeostasis and has provided protection to living organisms from various oxidative stresses.
3.1.2. Free Radicals, Oxidative Stress, and Diabetes
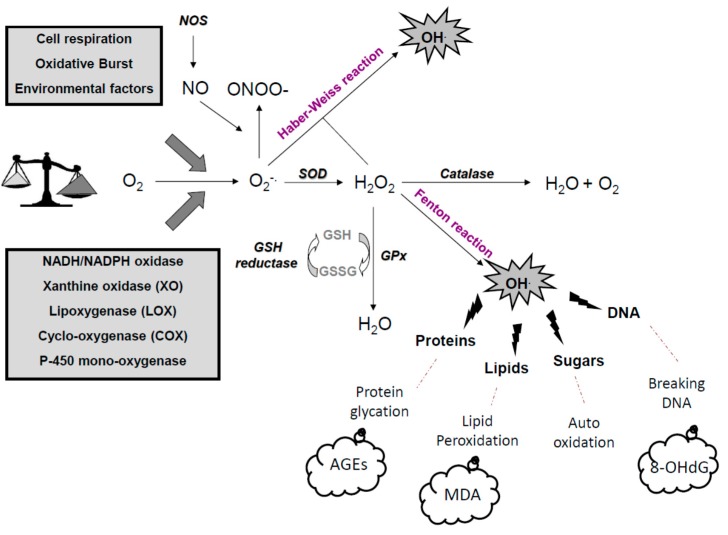
Beside physiological oxidations, many environmental processes have induced free radical formations: air pollutants [53], tobacco [54], UV radiation from sun [55], and industrialized lifestyle [56]. Different endogenous enzymes can also form free radicals at physiological concentrations: NADPH oxidase, xanthine oxidase, cyclo-oxygenases (COXs), and lipo-oxygenases (LPOs), nitric-oxide synthases (NOS), P450 cytochrome, and mitochondrial chain [57]. These free radical were reduced by the first line of antioxidant defense: the superoxide dismutase SOD [58]. Free radicals include reactive nitrogen species (RNS) and reactive oxygen species (ROS). The most important is superoxide anion (O2.−), which is rapidly dismutated into oxygen and hydrogen peroxide (H2O2) by superoxide dismutase (SOD). Then, catalase (CAT) dismutates H2O2 into water and oxygen, and glutathione peroxidase (GPx) reduces both H2O2 and organics hydroperoxides (ROOH). However, in the presence of transition metals such as iron or copper, O2.− and H2O2 form the strong oxidant hydroxyl radical (OH.) via Fenton reaction and the Haber–Weiss reaction. With chloride ions and H2O2, myeloperoxidase produce hypochlorous acid (HOCl). Nitric oxide (NO.) is produced from oxygen by various nitric oxide synthases (NOS) and produces the strong oxidant peroxynitrite (ONOO-) by reacting with O2.−. No enzymatic process can degrade ONOO–; however, with the presence of CO2, it form nitrate anion (NO3−) and nitrogen dioxide (NO2) (Figure 2).
Figure 2.
Oxidative defense and complications. AGEs: advanced glycated end-products; COX: cyclooxygenases; H2O2: hydrogen peroxide; LOX: lipoxygenases; NO: nitric oxide; NOS: NO synthase; NADPH oxydase: nicotinamide adenine dinucleotide oxidase; MDA: malondialdehyde (lipid peroxidation); SOD: superoxyde dismutases; GPx: glutathione peroxydase; GSH gluthathione; O2.−:superoxide anion; ONOO.−: peroxynitrite; OH. hydroxyl radical; 8-OHdG: 8-hydroxy-2’-deoxyguanosine (DNA damages).
In diabetes, the alteration of the first sites in the mitochondrial membrane lead to the activation of the complex II [59] and contribute to the formation of excessive O2.− by a leakage of electrons [60]. NADPH oxidases (Nox’s), a family of enzymes with the sole function of producing ROS, are implicated in the pathophysiology of many cardiovascular diseases [61,62,63,64] and are the major source of glucose-induced ROS production in the vasculature [65,66], kidney [65], liver [66,67], and β cells [68], confirming this enzyme as a mediator of diabetic complications. Recently, Brandes et al. [69] described molecular mechanisms of Nox activation and supported their implications in diabetes, hyperglycemia, and hyperinsulinemia through complex pathways involving NADPH oxidases. Xanthine oxidase is also implicated in diabetes and vascular complications [70], whereas treatment of T2D patients with Allopurinol, a XO inhibitor, reduces the level of oxidized lipids in plasma and improves blood flow [70]. Glucose itself, as well as its metabolites, is known to react with hydrogen peroxide in the presence of iron and copper ions to form hydroxyl radical during auto-oxidation, described in diabetes and complications by Wolff and Dean in 1987 (Figure 2).
3.1.3. Antioxidants Defenses
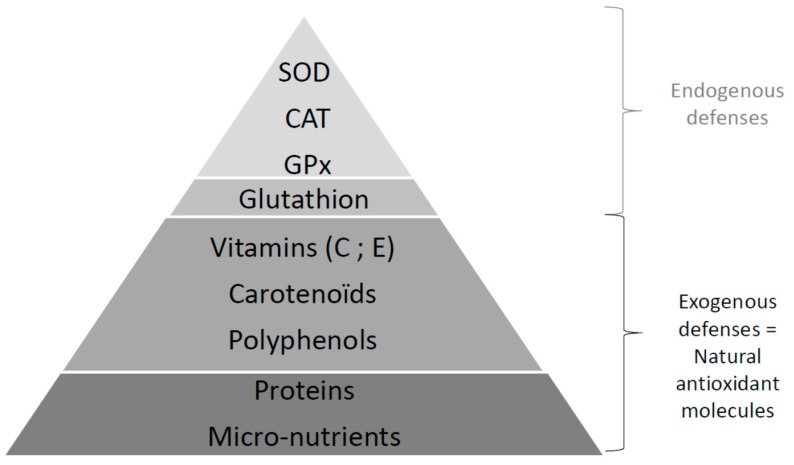
The body has a number of very effective antioxidant defense systems to lower the concentration of free radicals in the body. The term antioxidant refers to “any substance that, when present at low concentration compared with that of an oxidizable substrate, significantly delays or inhibits oxidation of the substrate” [71]. Nature of the antioxidant systems differs depending on the cell types, tissues, and localization in the intracellular or extracellular medium [72]. There are different types of molecules, natural or synthetic, with enzymatic or scavenging activities (Figure 3).
Figure 3.
Oxidative defense strategies. CAT: catalase; GPx: glutathione peroxydase; SOD: superoxyde dismutases.
The first line of defenses against free radicals groups these enzymatic systems (SOD, CAT, GPx) (Figure 2 and Figure 3) and are aided by micronutrients (copper, zinc, selenium) [73] as cofactors. There are three isoforms for the SOD described in mammals [74]: the manganese-SOD (MnSOD) in the mitochondria, copper (Cu), or zinc (Zn) in the cytoplasm and the mitochondria, and both Cu/Zn extracellular SOD (Cu/Zn SOD) in vessels. CAT is essentially present in peroxisomes and in erythrocytes [75]. GPx is present in the extracellular fluid (blood) and in the cytoplasm and membranes of cells [76] and forms a couple with glutathione reductase (GR) providing glutathione (GSH) bioavailability [7].
The second line of defenses involves non-enzymatic antioxidants, such as naturally nutrients provided by food, with a scavenging effect (capture of free electron and formation of more stable entities), a stimulatory effect on endogenous antioxidant enzymes, or both [77]. Main molecules are GSH, vitamin E (the most active form: α-tocopherol), vitamin C (L-ascorbic acid), vitamin A (carotenoids), but also polyunsaturated fatty acids or exogenous flavonoids (quercetin, rutin, resveratrol, etc.), which can strengthen the antioxidant defenses of the body [73]. For example, increasing concentration of GSH with these products can protect against cancer [78] and diabetic complications [79]. Vitamin E traps organic free radicals from the oxidation of lipids and helps reduce lipid peroxidation.
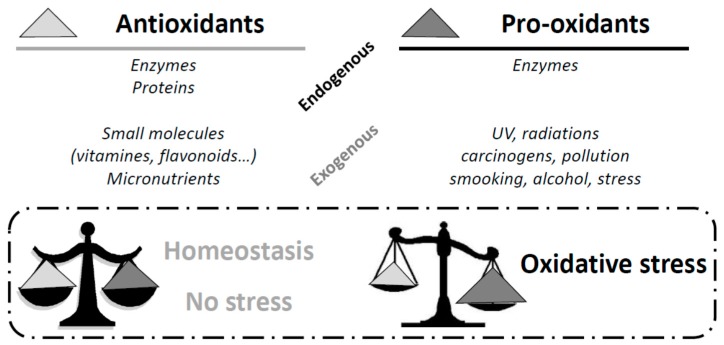
Β-cells are particularly sensitive to ROS because they are low in free radical quenching (antioxidant) enzymes such as CAT, SOD, and GPx [80,81,82] and have a lower level of GSH [82,83]. However, the balance between free radicals and antioxidant defense systems is crucial to maintaining homeostasis; if its equilibrium is broken in favor of the pro-oxidant entities, pathological oxidative stress appears [84] (Figure 4).
Figure 4.
Oxidative stress: A question of balance.
3.2. Free Radicals: Good and Bad Boys?
ROS and RNS are well recognized for playing a dual role as both deleterious and beneficial species, since they can be either harmful or beneficial to living systems [85], but it is a well-known feature that cells are capable of generating endogenously and constitutively ROS [6].
3.2.1. Physiological Roles: The Good Boy Side
Oxygen homeostasis at the tissue level is vital for development, growth, and survival, and cells hence have evolved a number of mechanisms to sense and respond to low oxygen levels. Under physiological conditions, beneficial effects of free radicals occur at low or moderate concentrations and involve physiological roles in the regulation of cellular signals implicated in proliferation and cell adhesion, apoptosis, inflammatory responses, and the regulation of transcription factors [6]. ROS, in low concentration, are generating when cells are stimulated by cytokines, growth factors, and hormones [86], and ROS can thus play a role as a secondary messengers [87,88] like the mitogen-activated protein kinase (MAPK) pathways [89], probably the most significant effect of metals and ROS. This involves the activation of nuclear transcription factors and control of the expression of protective genes that repair damaged DNA, power the immune system, arrest the proliferation of damaged cells, and induce apoptosis [89]. Cell adhesion plays an important role in embryogenesis, cell growth, differentiation, wound repair, and others, depending on redox regulation [90] and the involvement of NADPH oxidase [91]. In an inflammatory environment, activated neutrophils and macrophages produce a large quantity of ROS via NADPH oxidase and myeloperoxidase. This “oxidative burst” plays a key role in the defense against environmental pathogens [92]. Low and moderate levels of ROS also play important roles in regulating autophagy and apoptosis, therefore controlling cell death and survival [93,94], and ROS generated during ischemic preconditioning (alternation of short periods of ischemia and reperfusion) confer cardiac protection by reducing necrosis and the severity of arrhythmias, improving functional recovery when challenged with a longer period of ischemia [95]. This mechanism is very complex and involves triggers, mediators, and multiple second messengers’ pathways [96,97,98], but it is an innate physiologic adaptive process against potentially lethal ischemic injury. NO stimulates soluble guanylyl cyclase, leading to the relaxation of vascular smooth muscle [99] and the essential role of NO in endothelium-induced relaxation was discovered by Furchgott and Zawadzki in 1980 [100]. Nowadays, various studies report a pivotal role of NO on vascular homeostasis (anti-thrombotic, anti-aggregate, anti-migration, and relaxation) [101,102,103]. ROS play a crucial role in the activation of mechanotransduction signaling pathways and in cardiac contraction and relaxation [104]. In addition, in cardiovascular health, insulin sensitivity plays a vital role, and ROS intervene in the insulin signaling pathway. H2O2 induces typical metabolic actions of insulin, linking ROS to insulin [105], increases glucose uptake in adipocytes and muscles [106], is involved in the modulation of vascular endothelial function [107], and stimulates GLUT4 translocation and lipids synthesis in adipocytes [108]. However, ROS levels are the major determinants of impaired versus enhanced insulin sensitivity [109] through a ROS-induced increase in PI3K/Akt signaling [110].
3.2.2. Pathological Roles: The Bad Boy Side
Certainly necessary in many physiological pathways, their excessive production causes direct damage to biological molecules (DNA oxidation, proteins, lipids, and carbohydrates) as well as secondary damage due to cytotoxic and mutagenic character of metabolites released in particular during the lipid oxidation (Figure 2 and Figure 5). The body may also react against these abnormal compounds by producing antibodies, which unfortunately may be autoantibodies creating a third wave of attack.
Figure 5.
Reactive oxygen species (ROS) and complications. Impact of ROS on lipids, DNA, proteins, glucose, and vessels.
While DNA is the memory of all the biochemical live composition, it is very sensitive to free radical “attack.” At the very least, five main classes of oxidative damage mediated by OH• can be generated. Among them are oxidized bases, abasic sites, intra-catenary adducts, strand breaks, and DNA-protein bridges [111]. In addition to ROS, RNS such as peroxynitrites and nitric oxide have also been implicated in DNA damage [112]. The most extensively studied DNA lesion is the formation of 8-OH-G, and these changes are the first steps of carcinogenesis [85]; it is no coincidence that the carcinogenic agents are powerful free radical generators (UV and ionizing radiation, smoke, alcohol, asbestos fibers, carcinogenic metals, polycyclic hydrocarbons, etc.) (Figure 5).
The carbon reactive compounds (RCCs), such as malondialdehyde (MDA) and 4-hydroxynonenal (2-HNE), are formed endogenously during lipid peroxidation and glycoxidation of carbohydrates. They react with the tissue and cellular proteins to form AGEs (advanced glycation end-products) and ALEs (advanced lipid peroxidation end-products), inducing protein dysfunctions (loss of activity, increased sensitivity to proteases) [113,114] and damage in cellular responses—in particular, in inflammatory responses and apoptosis [114,115]. Lipids, mainly polyunsaturated fatty acids, are the main target of the attack by OH• and form conjugated diene radical [116]. These modifications concern circulating lipoproteins or membrane phospholipids. These derivatives are often hydrophobic and will therefore form in and around abnormal clusters of endothelial cells. These RCCs, MDA, 4-HNE, or oxidized-LDL were found in large quantities during mechanisms of carcinogenesis in various stages of cardiovascular diseases [117] such as atherosclerosis [118,119], metabolic syndrome [7], diabetes and complications [120], obesity, and insulin resistance [121], and in chronic inflammatory diseases such as lupus [122], asthma, chronic inflammation of the lungs, and respiratory allergies [123,124], and in degenerative diseases [120] (Figure 5).
3.3. Oxidative Stress, Diabetes, and Vascular Complications
Increased oxidative stress has been proposed to be one of the major causes of hyperglycemia-induced triggers of diabetic complications, implicates several mechanisms [125], and is a bipolar process. The first is the generation of ROS, and the second is a decrease in plasma antioxidants such as vitamin E, vitamin C, lipoic acid, and glutathione [126]. Both have been observed in diabetic patients [127,128] with micro- and macrovascular diabetic complications [3,129], linking metabolic-generated ROS to the development of diabetic complications [24]. This role of hyperglycemia has been established by large-scale prospective studies for both T1D and T2D, the DCCT/EDIC (Diabetes Control and Complications Trial) [130], the UKPDS (UK Prospective Diabetes Study) [131], and the Steno-2 study [132]. Diabetic cardiovascular complications appear to be multifactorial in origin [133,134], but, in particular, glycol-oxidative stress has been suggested to be the unifying link between the various molecular disorders in diabetes mellitus [59,135]. In fact, it is well established that hyperglycemia and acute glucose fluctuations have many side effects: modifying the redox balance, increasing circulating FFA, increasing NADPH oxidase activity and TNFα [126], and decreasing NADPH levels and glutathione, all of which generate by-products, activate oxidative, and inflammatory signaling. Hyperglycemia induces (1) an increase in glucose and other sugar fluxes through the polyol pathway, (2) an increase in advanced-glycation end-products (AGEs) formation through the hexosamine pathway, (3) expression of their receptor (RAGE) [136], and (4) the stimulation of protein kinase C (PKC) pathway. These mechanisms lead to increase production of glycative, glycoxidative, and carbonyl free radicals [22,137,138], which altered enzymatic and non-enzymatic antioxidant defenses. For example, oxidative stress increases mitochondrial DNA damages and causes axons cell death, leading to neuropathies [139]. Accumulation of sorbitol, due to an enzymatic conversion of excessive glucose, disrupts osmotic balance [140], a higher fructose production induces AGEs formation [141], and all participate in peripheral insulin resistance development [142,143] and β-cells injury [144]. Elevated AGEs may be a significant risk factor for T1D [145] and induce the progression of pre-diabetes to diabetes [146] and some complications such as diabetic retinopathy [147]. As shown before, oxidative stress is closely link to inflammation. Indeed, circulating TNF-α may impair vascular function by altering the balance between endothelial-derived vasodilator and vasoconstrictor substances because it downregulated the expression of eNOS and upregulated ET-1 production in endothelial cells [148]. Moreover, it may also directly activate NADPH oxidase and then increase the production of ROS in the vasculature [149].
Oxidative stress can be measured in vivo in multiple types including cells, solid tissues, urine, blood, and saliva. Several investigations correlated oxidative stress observed in serum and in saliva, and, today, saliva can be considerate as an oxidative stress diagnostic fluid [150,151]. Some human studies highlight reactive compounds in saliva in some pathologies, such as T1D [152,153,154,155] and T2D [153,156], with the detection of biomarkers such as 8-oxodG [152], MDA, and TBARS, proteins carbonyl [152], and total antioxidant capacity [153,154,155,156]. Recently, Wang et al. published a critical review of salivary biomarkers of oxidative stress [157], highlighting the problem of standardization in methods of saliva collection and measurements of composition. They proposed a guideline that could assist in discovery and validation of salivary oxidative stress biomarkers, allowing a diagnosis or even a simple predictive test of diabetes.
3.4. Endothelial Dysfunction, Diabetes, and Complications
As shown before, a large amount of evidence has demonstrated that hyperglycemia plays an important role in the pathogenesis of microvascular complications [158]. Dysfunction of the vascular endothelium is also regarded as an important factor [159,160], closely related to hyperglycemia and more recently to hypoglycemia [161], and has gained increasing attention in the study of vascular disease [162,163]. In fact, the endothelium is in constant interaction with the blood and subjected to mechanical stresses in the vessel, namely, intraluminal pressure, variations of flow including shear stress, and high glucose concentration. This strategical localization allows it a protective role as a detector toward theses stimuli. Endothelial cells respond to them through the production of messengers, addressed to cells by the blood. Thus, the endothelium plays a key role in vascular homeostasis by regulating the balance between relaxing and contracting factors. However, this protective role of the endothelium is also the first target of risk factors such as high cholesterol or high blood pressure [164], smoking [165], obesity and visceral fat distribution [166], impaired fasting glucose and hyperglycemia [167,168], insulin resistance [169,170,171,172] where this strategic balance is lost in favor to pro-mitogenic, pro-aggregation mediators [173,174], and inflammation [175]. Inflammation, in addition to oxidative stress, cause injury in cells (e.g., endothelial cells), leading to endothelial dysfunction [176] reported in numerous human and animal studies. In turn, this dysfunction promotes a pro-inflammatory environment as evidenced by increased endothelial expression of adhesion molecules, the imbalance of arachidonic acid metabolites, and chemoattractant molecules [176]. Forming a positive feedback loop, vascular inflammation leads to endothelial dysfunction (176). Lipopolysaccharide (LPS) from the bacterial cell wall [177] and C-reactive protein [178] are strong triggers for inflammation and endothelial vascular dysfunction in humans, as observed in T2D [179,180].
These disorders enable endothelial dysfunction as an early step in pathologies such as atherosclerosis and heart failure [181,182,183,184,185,186] and aging [187], as well as metabolic syndrome [188,189] and diabetes [190,191]. Endothelial dysfunction has been associated in several regions of the vasculature in animals and humans with T2D due to defects in NO-derived vasodilation [192,193], associated with diabetic complications such as nephropathy [194], retinopathy [195], and erectile function in animal models or human [190,196], and associated with cardiovascular and all-cause mortality in diabetic patients [191]. However, vascular complications may also be related to defects in endothelium-derived hyperpolarizing factor (EDHF) [193], which is thought to be an extremely important vasodilator substance, notably in resistance vasculature [64]. Unfortunately, the nature and, indeed, the very existence of EDHF remain obscure. Potentially, there are multiple EDHFs demonstrating vessel selectivity in their actions [197].
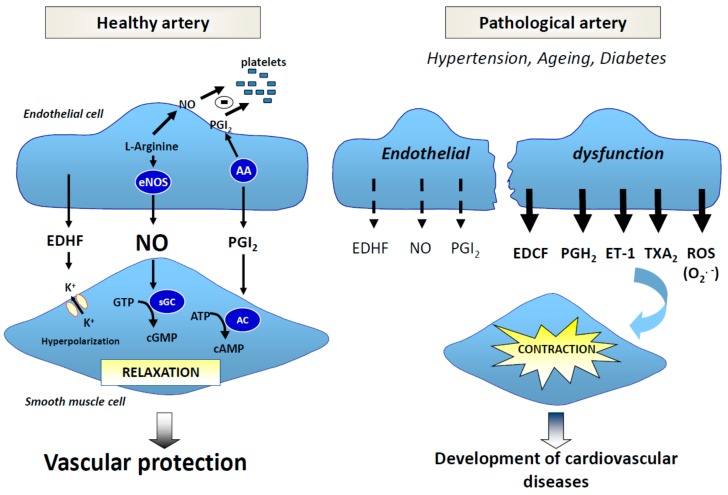
Mechanisms are complex and multiple, and etiologies are still at the heart of current research; however, oxidative stress are the common denominator [198] (Figure 6).
Figure 6.
Role of endothelium in vascular homeostasis. In a healthy artery, vasodilators factors such as nitric oxide (NO), endothelium-derived hyperpolarizing factor (EDHF), and prostacyclin (PGI2) play a key role in homeostasis. In a pathological artery, they decrease in favor of contractor factors such as endothelium-derived contracting factor (EDCF), prostaglandin (PGH2), endothelin-1 (ET-1), and thromboxane A2 (TXA2) in the presence of oxidative stress and superoxide anions (O2.−). AA: arachidonic acid, eNOS: endothelial nitric oxide synthase, sGC: soluble guanylate cyclase; AC: adenylate cyclase; K+: potassium.
3.4.1. Free radicals, NO and NO Synthases
Free radicals are able to modify relaxation or contraction balance in favor of contracting factor release, playing a primordial role in vascular pathologies [198]. O2.− decreases NO bioavailability, forms peroxinitrites [199,200], and inhibits activity and expression of soluble guanylate cyclase (sGC) [201,202,203]. Peroxinitrites themselves at a high concentration inhibits sGC, prostacyclin production through the nitration of the prostacyclin synthase, inhibits SOD [202], notably in diabetes [204], and uncouples NO synthase, leading to O2− synthesis. Peroxinitrite has a toxic effect on vasculature and contributes to the disease progression and myocardial damage [205]. This loss of NO availability induces disorders [57] such as the formation of a thrombogenic surface in the vessels, an increase in endothelium permeability and an accumulation of oxy-LDL, an attraction of monocytes and T lymphocytes, smooth muscle cell proliferation, and vascular wall growing, leading to vasculopathies. Deficiency of vascular NO is also associated with altered vasorelaxation in arterial pressure [206,207], atherosclerosis [208], hypercholesterolemia [209,210], vascular aging [62,211,212,213], metabolic syndrome [189], and diabetes [214,215]. Moreover, this blunted-NO availability is believed to be the primary defect that links insulin resistance and endothelial dysfunction [171], and is associated with oxidative stress, for example, in mesenteric arteries from established T2 models Otsuka Long–Evans Tokushima fatty (OLETF) rats [216].
In diabetes, the underlying mechanisms seem to be diverse, but include the effects of hyperglycemia [217], AGEs [211,214,218], uric acid [219], and oxidative stress [213] (Figure 6), and polymorphisms in eNOS lead to NO deficiency [220]. In fact, a high level of glucose induces an uncoupling of eNOS [221], and, although translocation to the membrane operates, this might be an inactivated form of the enzyme [222]. eNOS is not the only form to play a role in diabetes and its complications. In fact, NOS-opathies include three isoforms: neuronal (nNOS; NOS1), inducible (iNOS; NOS2), and the most well studied endothelial (eNOS; NOS3). Deletion of all three in mice results in spontaneous coronary artery diseases, myocardial infarction, and sudden cardiac death, [223,224] and results confirmed a protective role of eNOS and nNOS, whereas iNOS was found to exert an unfavorable role. Khanna et al. recently reviewed the implication of isoforms in diabetic cardiomyopathy and highlighted the important role of epigenetic modifications in the regulation of gene expression [225]. nNOS, originally expressed throughout the central and peripheral nervous system, is sympathoinhibitory in a range of diseases including chronic heart failure, chronic renal failure, and hypertension [226]. Moreover, nNOS, expressed also in macula densa cells and pylor, is involved in the pathogenesis of renal hemodynamic changes [227] and gastropyloric dysfunction [228] associated with diabetes. However, a characteristic feature of iNOS is its lack of expression in strictly resting cells. Instead, it is induced by immunological stimuli, which led to its original designation as inducible NO synthase [229]. The host cell localization of iNOS has been mainly investigated in macrophages, neutrophils, and smooth muscle cells, where the production of NO is more robust (µm vs. nM for eNOS and nNOS), continually (some days vs. min.) The authors of [230] initially intended to compensate the downregulation of eNOS by oxidative stress [231]. However, like a double-edged sword, the inflammatory cytokines, importantly, TNFα and C-reactive protein at the same time, will activate NADPH oxidase, which in turn produces O2.−. Excessive NO concentration produced reacts with O2.− forming peroxynitrite and contributes to an uncoupled iNOS due to the substrate limitation, and therefore the production of ROS [232]. Therefore, the link of oxidative stress and inflammatory response leads to decreased NO bioavailability causing endothelial dysfunction and contractile dysfunction [233], as shown in diabetic complications [234,235,236].
3.4.2. Free Radicals and EDHF
Alterations of EDHF signaling are also associated to animal and human pathologies [237], including hypercholesterolemia, arterial pressure [64], obesity [238], diabetes [239], and aging [62,213] and are characterized by O2− induce blunted EDHF-mediated relaxations through a decrease in potassium channels sensitive to calcium (SKCa and IKCa) [62,63,64,213] and myoendothelial gap junctions between endothelial cells and smooth muscle cells [64]. In mesenteric arteries from established T2D models such as OLETF-rats [240] and the insulin-resistant fatty Zucker rats (ZDF) [241,242], EDHF-mediated relaxation decreases due to alterations of both potassium channels, recently associated with oxidative stress [216,243,244] and probably involving renin-angiotensin-aldosterone systems (RAAS) [216] such as aging [62,213].
3.4.3. Free Radicals and Contractions
There is great heterogeneity in the formation of EDCF (endothelium-derived contracting factor)-dependent stimuli, vascular beds, age, and experimental animal models used. Among contractor factors produced by endothelial cells, we cite derivatives of arachidonic acid metabolism such as endoperoxides, thromboxane A2 (TXA2), prostaglandin H2 (PGH2), and prostacyclin (PGI2), but also superoxide anions (O2−), endothelin 1 (ET-1), and angiotensin II [245]. ET-1 is increased in metabolic syndrome [189] and obesity [238], and EDCF-mediated contraction is also exacerbated by obesity, hypertension and diabetes (e.g., OLETF-rats [216]) and thus are likely to contribute to the endothelial dysfunction [246].
3.4.4. Iron and Non-Transferrin-Bound Iron (NTBI)
Sometimes, the complex interactions between iron, oxidative stress, inflammation, and diabetic complications [247] have attracted considerable interest despite a poor understanding of the mechanisms involved. Numerous forms of body iron exist, and only forms not bound to transferrin or other iron-binding proteins named non-transferrin-bound iron (NTBI) seem to be implicated in oxidative damages due to their high reactivity [248]. NBTI could be considered a biomarker of the side effect of iron in diseases, greatly correlated with Hb1Ac [249]. Recently, Aljwaid et al. [249] confirmed association of NTBI with the risk of vascular complications in diabetes already highlighted 10 years earlier [250,251,252], because NBTI is easily accessible to plaque as well as endothelial cells, macrophages, and smooth muscle cells. Inflammation contributes to iron-mediated endothelial dysfunction, characterized by a high release of iron by infiltrated macrophages, an increase in E-selectin, and other adhesion molecules implicated in atherosclerotic plaque [247,253]. Iron can enter into the atherosclerotic lesion in the form of free hemoglobin, which is prone to oxidation, and can form methemoglobin, ferryhemoglobin, and release heme. All of these exert pro-oxidant and pro-inflammatory effects on the vascular wall [253]. Vinchi et al. [253] summarized current knowledge about the role of hemoglobin, heme, and iron through controversial epidemiological studies and concluded, given more evidence, their negative impact, compared with the innocent role of iron in atherosclerosis. The chronic increase in the release of hemoglobin and heme (hemolysis) is associated with endothelial dysfunction and reduced NO bioavailability [254] and with coagulopathy [255,256] and vasculopathy [256], as observed in diabetes [257], greatly reviewed by Vinchi et al. [258].
4. Nutritional Prevention: Antioxidants against Diabesity and Complications
Regarding the low level of antioxidant enzymes expression in the pancreas [80], combinations of conventional antidiabetic treatments with antioxidants were quickly privileged [259]. A Mediterranean diet (MedD) is characterized by abundant plant foods (fresh fruit, vegetables, breads, other forms of cereals, seeds, etc.), olive oil as the principal source of fat, and wine. The PREDIMED study examined the effect of a one-year MedD on oxidative and inflammatory parameters in subjects with a high risk for cardiovascular diseases. Results showing that the MedD increases plasma non-enzymatic antioxidant capacity, decrease the biomarkers of atherosclerosis,have anti-inflammatory effect in addition to the improvement of lipid profile, insulin sensitivity, blood pressure, and carotid atherosclerosis. Adherence to MedD reduces the incidence of T2D, metabolic syndrome, and diabetic retinopathy. However, the MedD have no effect on diabetic neuropathy, highlighting complexity to recommend an ideal model for diabetic complication prevention. In patients with newly diagnosed T2D, consumption of this diet resulted in a greater reduction of HbA1c levels, a higher rate of diabetes remission, and delayed need for diabetes medication [260]. Moreover, a Mediterranean diet enriched with extra-virgin olive oil but without energy restrictions reduced diabetes risk among persons with a high cardiovascular risk [261]. Antioxidants act synergistically or by trapping single electrons to free radicals or by reducing ROS enzymatically. Some antioxidants such as vitamins E (tocopherol), C (ascorbate), and Q (ubiquinone), and carotenoids or polyphenols come from food. Inhibition of hyperglycemia-induced ROS production using transgenic antioxidant enzyme expression or antioxidant compounds prevents the development of experimental diabetic retinopathy [262], nephropathy [263,264], neuropathy [265], and cardiomyopathy [266]. Additionally, the mechanisms behind the anti-inflammatory effect of carotenoids (β-carotene and lycopene) have been recently described: both decrease TNFα-mediated ROS generation and increase NO bioavailability at the endothelial level, linking oxidative stress inflammation and vascular beneficial impact [267]. In humans, some large epidemiological studies such as the Linxian study, the Clark study, the Qixia study, the NPC study, or the SU.VI.MAX study in France, the feasibility and efficacy to prevent cancer or mortality with moderate doses of antioxidants has been demonstrated in healthy subjects. Zatalia et al. [16] recently listed all the beneficial effects observed in animals and humans, from vitamins and supplements, plants but also drugs used for treating diabetes and its complications. These experimental and human studies led to a proposal for nutritional prevention to inhibit diabetic complications. Table 1 resumes some classical products that have potential cardiovascular protective effects.
Table 1.
Effects of functional foods and their bioactive compound on cardiovascular parameters [268].
| Functional Foods | Bioactive Compound | Mechanisms |
|---|---|---|
| Black tea |
|
|
| Citrus fruit |
|
|
| Dark chocolate |
|
|
| Extravirgin olive oil |
|
|
| Fish |
|
|
| Fruits and vegetables |
|
|
| Ginseng |
|
|
| Grapes and red wine |
|
|
| Green leafy vegetables |
|
|
| Green tea |
|
|
| Margarine |
|
|
| Nuts |
|
|
| Onion and garlic |
|
|
| Pomegranate |
|
|
| Soy proteins |
|
|
| Tomato |
|
|
| Vegetable oil |
|
|
| Whole grains |
|
|
Chol: cholesterol; ox-LDL: oxidation of LDL; TG: triglycerids.
We will now see different management strategies of diabetes and complications using non-exhaustive examples of the interest inspired by plants, fruits and vegetables, polyphenolic compounds, and even some drugs used today in the treatment of diabetes with an antioxidant activity (Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7).
Table 2.
Effects of plants on oxidative and metabolic parameters.
| Plants | Experimental studies | Efficacy |
|---|---|---|
|
Allium cepa Allium sativum |
Alloxan-induced diabetic rats [277] and STZ-induced diabetic rats [277] |
• ROS scavenger • ROS scavenger • ↓ oxidative stress (lipid peroxidation) • ↑ SOD, ↑ GST |
| Aralia elata | STZ-induced diabetic rats [277] | • Inhibition of aldose reductase • Inhibition of cataract (retinopathy) |
| Aloe verra | STZ-induced diabetic rats [277] | • ↑ antioxidant enzymes activities • ↓ oxidative stress (lipid peroxidation) |
| Anoectochilus formosanus | STZ-induced diabetic rats [277] | • ↑ antioxidant enzymes activities • ↓ oxidative stress (lipid peroxidation) |
| Cassia fistula | Alloxan-induced diabetic rats [277] | • ↑ antioxidant enzymes activities • ↓ oxidative stress (lipid peroxidation) |
| Coccinia indica | STZ-induced diabetic rats [278,279,280] | • hypoglycaemic/hypolipidaemic effects • ↑Vitamin C, antioxidant activity • ↑ antioxidant enzymes activities |
| Eugenia jambolana | STZ-induced diabetic rats [277] | • ROS scavenger |
| Ever green shrubs (Larrea divarita) | STZ-induced diabetic rats [277] | • ↓ XO activity, ion chelation, ROS scavenger, ↓ blood pressure, inhibition of nephropathy |
| Fomes fomentarius | STZ-induced diabetic rats [277] | • ↑ antioxidant enzymes activities • ↓ oxidative stress biomarkers |
| Juglans regia | T2D-mouse [277] | • ↓ oxidative stress biomarkers |
| Trigonella foenum-graecum (fenugreek) | T2D patients [281] | • Hypoglycemia effect |
| Lycium barbarum | Alloxan-induced diabetic rats [277] | • ↓ lipids |
| Panax ginseng | T2D rats [277] | • ROS scavenger • Erectile dysfunction protection |
| Potentilla chinesis | STZ-induced diabetic rats [282] | • ↑ antioxidant enzymes activities • ↓ oxidative stress (lipid peroxidation) • ↓ blood glucose • ↓ LDL, ↓TG, ↑HDL |
| Scoparia dulcis | STZ-induced diabetic rats [277] | • ↑ antioxidant enzymes activities • ↓ oxidative stress biomarkers • ↑ GSH |
| Stevia rebaudiana bertoni | STZ-induced diabetic rats [283] | • ↓ blood glucose, ↑glucose tolerance • ↑ insulin levels and ↑sensitivity • ↓ALT, ↓AST, ↑ filtration rate glomerular • Improve kidney damages (nephropathy) • ↓ oxidative stress (lipid peroxidation) • ↑ total antioxidant capacity • ↑ antioxidant enzymes activities |
| Trifolium alexandrium | STZ-induced diabetic rats [277] | • ↑ antioxidant enzymes activities • ↓ oxidative stress (lipid peroxidation) |
| Ulva lactuca polysaccharides (alga) | STZ-induced diabetic rats [284] | • ↓ blood glucose • ↓enzymes of lipid metabolism and absorption • ↓ LDL, ↓TG, ↑HDL • protection hepatic and renal functions |
| Vitis vinifera | Alloxan-induced diabetic rats [277] | • ROS scavenger • ↑ GSH • ↓ oxidative stress (lipid peroxidation) |
| Viburnim dilatatum | STZ-induced diabetic rats [277] | • ROS scavenger • ↓ oxidative stress (lipid peroxidation) |
| Viscum album | STZ-induced diabetic rats [277] | • ↑ antioxidant enzymes activities • ↓ oxidative stress biomarkers |
| Nopal (Opuntia streptacantha Lemaire) | Healthy people [285] T2D patients [286,287] |
• Hypoglycemia effect • ↓ blood glucose, ↓ insulin • ↑ insulin sensitivity |
| Pycnogenol® | Healthy people [288] Hypertensive patients [289] Metabolic syndrome patients [289] |
• ↑ NO-mediated forearm blood flow • ↓ blood pressure • Improve endothelial function |
| Zygophyllum album | Alloxan-induced diabetic rats [290] | • ↓ blood glucose, ↓obesity |
| Many plants | STZ-induced diabetic rats [291] | • ion chelation, ROS scavenger • ↓ oxidative stress (lipid peroxidation) |
| Plants like ferula assa-foetida | STZ-induced diabetic rats [277] KK-Ay mice [292] |
• ↑ antioxidant enzymes activities • ↓ oxidative stress biomarkers • ↓ blood glucose |
ALT and AST: hepatic transaminases; GSH: gluthatione; GST: glutathione S-transferase; ROS: reactive oxygen species; SOD: superoxide dismutase; STZ: streptozotocin; TG: triglycerides; XO: xanthine oxidase.
Table 3.
Effects of fruits and vegetables on experimental diabetes models.
| Fruits or Vegetables | Experimental Studies | Efficacy |
|---|---|---|
| Apple | STZ-induced diabetic rats [300] |
|
| Asparagus | STZ-induced diabetic rats [301] |
|
| Black radish | STZ-induced diabetic ratsHigh Fat Diet rats [302] |
|
| Celery-root | Alloxane-induced diabetic mouse [303] |
|
| Cherry | Alloxane-induced diabetic rats [266] |
|
| Cucumber | Alloxane-induced diabetic mouse [304] |
|
| Garlic | STZ-induced diabetic rats [305,306] |
|
| Alloxane-induced diabetic rats [307] |
|
|
| High Fat Diet rats [308] |
|
|
| Resistant rats [280] |
|
|
| Green bean | STZ-induced diabetic rats [309] |
|
| Onion | STZ-induced diabetic rats [310,311,312] |
|
| High Fat High Sucrose rats [313] |
|
|
| Red cabbage | STZ-induced diabetic rats [314] |
|
| Shallot | Fructose-induced Insulin resistant rats [315] |
|
| Strawberry | High Fat Diet mouse [316] |
|
| Tomato | STZ-induced diabetic rats [317] |
|
| Zucchini | Alloxane-induced diabetic mouse [304] |
|
AGEs: advanced glycation end-products; CAT: catalase; SOD: superoxide dismutase; GPx: gluthatione peroxidase; TG: tryglicerides; TBARS: peroxided-lipids.
Table 4.
Effect of vitamins and supplements in diabetes and complications.
| Vitamins | Human or Experimental Studies | Efficacy |
|---|---|---|
| Vitamin C | T2D patients [343,344] |
|
| T1D patients [329] |
|
|
| Healthy patients [343,345] |
|
|
| Diabetic rats [346] |
|
|
| Vitamin D | Young predisposed child to T1D [347,348] |
|
| T2D-rats [349] |
|
|
| Vitamin E | Diabetic patients [350] |
|
| T2D patients [351] |
|
|
| T2D patients [352,353] |
|
|
| T2D patients [354] |
|
|
| T2D patients [355,356,357,358] |
|
|
| T2D patients [299,359,360] |
|
|
| T2D patients [341,360] |
|
|
| Diabetic patients [340,361] |
|
|
| T1D patients [330] |
|
|
| T1D patients [362] |
|
|
| Diabetic Balb/c mice [363] Diabetic rats [346] |
|
|
| Combined with nicotinamide | IMDIAB IX study T1D children [331,364] |
|
| Transitional metal chelating agent | STZ-induced diabetic rats [365,366] |
|
| Selenium | Alloxane-induced diabetic rats [367] |
|
| Zinc | STZ-induced diabetic rats [368] |
|
| Combined vitamin C, E, selenium, Zinc and Β-carotene | SU.VI.MAX Healthy patients [369] |
|
| B-carotene | Alloxane-induced diabetic rats [370] and T2D patients [371] |
|
CV: cardiovascular; FFA: free fatty acid; GSH: glutathione; ox-LDL: oxidized-LDL; OS: oxidative stress; ROS: reactive oxygen species; STZ: streptozotocin; T-Chol: total cholesterol; TG: triglycerides.
Table 5.
Beneficial effects of several polyphenol-rich natural sources on vessels in humans.
| Natural Sources | Human Studies | Efficacy |
|---|---|---|
| Plants | ||
| Soybean | Woman with CV risk factor [378] | ↑ FMD |
| Grape-derived products | ||
| Red wine + olive oil | Healthy people [379,380,381] Healthy people [382] |
↑ basal FMD ↑ basal FMD |
| Red wine | Atherogenic potential [383,384] Healthy people [385] |
↑ FMD, ↓ blood pressure |
| Hypercholesterolemic patients [386] | improved FMD, enhanced endothelium-independent vasodilation | |
| Coronary artery disease [387,388] | ↑ FMD | |
| Grape juice | Healthy people [389] | ↑ basal FMD |
| Hypercholesterolemic patients [386] | ↑ FMD protect against coronary artery disease | |
| Concord grape juice | Coronary artery disease [390] | ↑ FMD |
| Grape seed extract | Healthy people [391,392] Coronary artery disease [393,394] Hypertensive patients [395] |
↑ basal FMD ↑ FMD ↓ blood pressure |
| Dark chocolate | Atherogenic potential [396] | ↑ basal FMD, ↓ blood pressure |
| Hypertensive patients [397,398] | ↓ blood pressure | |
| Overweight adults [399] | ↑ FMD, ↓ blood pressure (sugar-free preparations) | |
| Healthy people [400] | ↓ blood pressure | |
| Cocoa | patients [401] Overweight adults [399] |
↑ basal FMD by 30% reverse vascular dysfunction no effect on glycaemia control ↑ FMD, ↓ blood pressure(may attenuate by sugar) |
| Hypertensive patients [402] | no effect on blood pressure | |
| Pomegranate juice | Severe carotid artery stenosis [403] Hypertensive patients [404] |
↓ blood pressure, ↓artery thickness ↓ blood pressure |
| Strawberry | Obese patients [405] | ↓ risk factors for CVD and stroke |
| Teas | ||
| Black tea | Coronary artery disease [406] | ↑ FMD |
| EGCG extract (Teavigo®) Green tea | Coronary artery disease [407] | ↑ FMD |
| Borderline diabetes or diabetes [408] | ↓ blood pressure | |
| Healthy prospective cohort [309] | ↓ CV mortality strongly vs. all cause↓ stroke | |
| Coronarien patients [407] | Endothelial cells protection (↑ NO) ↑ FMD |
|
| Maritime Pycnogenol® | Healthy people [288] | ↑ NO-mediated forearm blood flow |
| Hypertensive patients [289] | ↓ blood pressure | |
| Metabolic syndrome patients [289] | Improve endothelial function | |
| Oil | ||
| Krill oil (Ѡ3 and fatty acid) | T2D patients [409] | Improve endothelial function ↑ HDL |
CV: cardiovascular; FMD: flow-mediated dilatation (technic to measure endothelial function in humans).
Table 6.
Beneficial effects of several polyphenol-rich natural sources on Human cardio-metabolic diseases.
| Polyphenols | Human study | Efficacy |
|---|---|---|
| Single compounds | ||
| Quercetin Myricetin |
different national public health registers [418] | ↓ risk T2D an chronic disease |
| Quercetin Kaemferol Myricetin Apigenin Luteolin |
The Woman’s Health Study [419] | no effect |
| EGCG extract | Overweight or obese men [420] |
no effect on insulin sensitivity, no effect on glucose tolerance, modest ↓ in DBP |
| T2D patients [421] | no effect on insulin sensitivity, | |
| T2D patients [408,422] | no effect on HbA1c and glycaemia and Insulin resistance | |
| Lipoic acid | T2D patients [423] | ↑ insulin sensitivity |
| Ѡ-3 | DAISY (Diabetes Autoimmunity Study in the Young) = predisposed T1D-children [424] | ↓ risk of autoimmunity against islets, antioxidant effect |
| Pycnogenol® | Diabetes patients [289] | ↓ blood glucose |
| Hypertensive patients [289] | ↓ blood pressure | |
| Metabolic syndrome patients [289] | ↓ waist circumference, improve lipid profile, renal and endothelial functions | |
| Resveratrol | Diabetes patients [414] | Glucoregulation, ↑ insulin sensitivity, ↑ potency of hypoglycemic agents and antidiabetic therapies |
| Obeses patients [414] | ↑ or↓ insulin sensitivity ↓ adipocyte size ↓ or no effect on circulating inflammatory cytokines ↑ adiponectin |
|
| Overweight and obese adolescents [425] | ↓ insulin resistance ↓ non-alcoholic fatty liver disease (NAFLD) |
|
| NAFLD patients [426] |
no effect on anthropomorphic measurements, insulin markers, lipids profile, blood pressure ↓ NAFLD ↓ALT |
|
| Cardiovascular diseases [414] | ↓ or no effect on plasma lipid profile/Chol ↓ systolic blood pressure ↑ Flow-mediated dilatation ↓ pulse-wave velocity |
|
| Whole polyphenols diets/foods | ||
| Apple | Middle-age women [419] Men and women [418] |
↓ risk T2D ↓ risk T2D |
| Berry | Men and women [418] | ↓ risk T2D |
| Blueberry | T1D children [308] | ↓ HbA1c, ↑C-peptide, ↑ erythrocyte SOD |
| T2D patients [427] | ↓FBG, ↓ LDL, ↓ CRP ↓ AST, ↓AST, ↓GGT |
|
| Cinnamon | T2D patients [428] | ↓ CV risk, ↑ insulin sensitivity |
| Curcumin | Diabetic patients [308] | Improve microangiopathy |
| Healthy people [429] | ↑ HDL, ↓ cholesterol, ↓ lipids peroxidation |
|
| Coffee | Metabolic syndrome [430] | ↓ risk T2D |
| Cocoa drink | Hypertensive patients [402] |
no effect on insulin resistance no effect on blood pressure |
| Dark chocolate | Healthy people [400] and Hypertensive patients [398] Healthy people [400] |
↑ insulin sensitivity, ↓ blood pressure ↑ QUICKY (insulin sensitivity) ↓ HOMA-IR |
| Whole Grains rich diet | Obesity and T2D patients [431] | ↓ risk T2D |
| Grape seed extract | T2D patients [432] | ↓ glycaemia, ↓ inflammation no effect on HOMA-IR |
| Krill oil (rich inѠ-3) | T2D patients [409] | ↓ blood C-peptide levels, ↓ HOMA-IR, ↑ HDL |
| Purple grape juice | Coronaries patients [393] | ↓ ox LDL |
| Strawberry | Obese patients [405] | ↓ risk factors for CVD and stroke ↓ diabetes |
| Tea | Middle-age women [419] Meta-analysis [433] Non obese people [434] |
↓ risk T2D Prevention of T2D development ↓ risk of obesity, ↓ FBG |
| Green tea | T2D patients [435] Borderline diabetes or diabetes [408] |
↑ levels of insulin ↓ body weight and BMI ↓ blood pressure, ↓blood glucose ↓ HbA1c, ↓HOMA index |
| RWPs – french Corbières AOC | Healthy people [19,20] | ↓ weight, ↓ glycaemia Hypoglycemia effect |
AST, AST, GGT: transaminases; BMI: body mass index; CVD: cardiovascular disease; CRP: C-reactive protein; DBP: diastolic blood pressure; FBG: fasting blood glucose; HOMA-IR: insulin resistance index; NAFLD: non-alcoholic fatty liver disease.
Table 7.
Beneficial effects of several polyphenol-rich natural sources on in vitro and in vivo models of diabetes.
| Polyphenols | Experimental Models | Efficacy |
|---|---|---|
| Curcumin | T2D-rats [436] | ROS scavenger ↓ nephropathy |
| STZ-induced diabetic rats [437] | Protect endothelial dysfunction in the iris : ↓ retinopathy | |
| STZ-induced diabetic rats [438] | Improves mesenteric arteriolar function ↓ ROS artery, ↓ PKC-βII ↓ glycemia |
|
| db/db mice [439] | ↓ glycemia, ↓ weight | |
| Ob/ob mice [440] | ↑ glycemic control, ↑insulin sensitivity, ↑ leptin/adiponectin |
|
| Bovine aorta [441] | ↓ lipid peroxidation, ROS scavenger | |
| Tea Flavonoids | RINm5f (β-cells) [375] | ROS scavenger Fer and iron scavenger ↓ ROS production |
| Tea EGCG | RINm5f (β-cells) [442] | ↑ mitochondrial activityprotect against oxidative stress ↑ SOD activity ↓ ROS production, ↓ caspase 8 |
| ex vivo skin [443,444] | protection against UV ↑ GSH, ↑ GPx activity |
|
| in vitro [445] | prevention of hyperglycemia ↑ insulin activity protection of β cells |
|
| STZ-induced diabetes in rats [446] | ↓ β cells lost | |
| (OB/OB) mice [447] | ↓ hepatic steatosis ↓ injury in obese mice |
|
| (OB/OB) mice [448] | ↓ intestinal lipid absorption, ↓ body mass, ↓ lipid accumulation in liver and adipocyte, ↑ insulin sensitivity, ↑ TAOC | |
| α lipoic acid | STZ-induced diabetes in rats [449] | ↓ FBG, ↓ HbA1c improve dyslipidemia ↑ SOD activity, ↑endogenous Vit C ↓ MDA and 4-HNE in aorta ↓ DNA damages good vascular morphology |
| Procyanidin B2 (grape seed) | STZ-induced diabetes in rats [450] Β-cells |
↓ plasma glucose Insulin mimetic effect |
| Resveratrol | Zucker fatty (ZF) rats [451] (Obese and T2D) | ↓ T-Chol, ↓ TG |
| STZ-induced T2 diabetes in rats [452] | delay insulin resistance ↓ insulin secretion (hyperinsulinemia) |
|
| Endothelial cells of rats [453] | ↓ ROS, ↓NADPH oxidase, ↓inflammation ↓ LDL, antioxidant activity |
|
| RWPs extract ProvinolsTM | Zucker fatty (ZF) rats : Obese and T2D [242] | Improve glucose metabolism ↓ plasma glucose, ↓ fructosamine ↓ TG, ↓T-Chol, ↓ LDL Improve cardiac performance (↗ left ventricular and cardiac input) ↓ peripheral arteriole resistances Corrected endothelial dysfunction : in aorta : ↑ NO availability, ↑ NO, ↑ eNOS activity, ↓ O2, ↓ NADPH ox in mesenteric artery : ↑ EDHF |
| RWPs – french Corbières AOC | STZ-induced diabetes in rats and Fructose diet [19,20] | ↓ weight, ↓ glycemia ↓ plasma glucose ↓ plasma lipids |
| RINm5f (β-cells) [442] | ↑ mitochondrial activity protect against oxidative stress ↑ SOD activity ↓ ROS production, ↓ caspase 8 |
|
| SOD/CAT mimetics | animal models of diabetic neuropathy [263,264,265] | improve neuropathy |
| translocase of inner mitochondrial membrane | Mice [263] | improve nephropathy |
| tempol | Mice SOD-knockout [264] | improve nephropathy |
| overexpression of MnSOD | Mice [262] | improve retinopathy |
EDHF: endothelium derived hyperpolarizing factor; FBG: fasting blood glucose; MDA and 4-HNE: lipids peroxide; NO: nitric oxide; ROS: reactive oxygen species; SOD: superoxide dismutase; TG: triglycerids.
4.1. Plant Therapy
Plants have been used from a long time by Chinese, African, and South American peoples as traditional medicines and is used by about 60% of the world’s population. The first texts written about herbal medicine are etched in clay. It includes a series of tablets engraved in cuneiform, and its authors, the Sumerians, drafted them 3000 years before the common era. They used plants such as myrtle, hemp, thyme, and willow. From century to century, Theophrastus, Aristotle, Pliny the Elder, and Dioscorides deepened their knowledge of plants and their properties. Morphine, aspirin, quinine: What do they have in common? All come from nature and have led to major drugs. Morphine is extracted from opium (Papaver somniferum), aspirin is extracted from willow bark, and quinine is from a tree from the Cordilleras in the Andes called the cinchona. The world contains many molecules with interesting biological properties, but they must be highlighted. Recently, there has been considerable interest in finding natural antioxidants from plant materials to replace synthetic ones, and natural antioxidants occur in all higher plants and in all parts of the plant (wood, bark, stems, pods, leaves, fruit, roots, flowers, pollen, and seeds) [269]. There have been many investigations into the effects of these plants and their antioxidant ingredients on diabetes and its complications, and good results have been achieved. Dixit et al. focuses on Indian Herbal drugs and plants used in the treatment of diabetes, especially in India [270]. Dodda and Ciddi [15] reported on other plants used in the management of diabetic complications (nephropathy, neuropathy, cataract, and retinopathy) and, last year, Qiang et al. [271] demonstrated the protective effect of Sancaijiangtang on NO and ET-1 dysfunction observed in the vessels of T2D patients. Table 2 shows antioxidant properties of some of these plants, except from those treated by Dixit in his review.
If herbal medicine enjoys an extraordinary craze across the world, this is not just a matter of fashion. Of course, our era is deeply marked by the search for a healthier life, a return to nature and essential values. One recent example is the use of Stevia, with 200 species around the world growing primarily in the Amambay mountain range of Paraguay [272]. Stevia rebaudiana, the only species with the ability to sweeten with no caloric value, contain specific substances (glycosides) in leaves that are rich in vitamins and complements [273]. Research on diabetic rats has shown the antihyperglycemic, insulinotropic, and glucagonostatic actions of stevia [274] and its ability to reduce postprandial blood glucose levels in type 2 diabetic patients, indicating its beneficial effects on glucose metabolism [275]. Stevia offers an ideal alternative to sugar, well tolerated, with a zero glycemic index and no pharmacological effect in T1D and T2D patients [276].
4.2. Fruits and Vegetables
Scientific and medical interest in cardiovascular health benefits of fruit- and vegetable-rich diets has grown exponentially in recent years, due to compelling epidemiological evidence showing that the consumption of fruits and vegetables might reduce the risk of cardiovascular diseases [10,11,12,13,14]. Although studies demonstrate no significant beneficial effect against diabetes [293], others highlight a decrease in the risk to develop diabetes [294,295], which was confirmed by a recent meta-analysis on diets rich in green leafy vegetables [296]. Their antioxidant capacities in humans have also been demonstrated in many studies, namely, the effects of strawberries and tomato juice on metabolic syndrome, hyperlipidemia, and T2D [297,298,299]. Table 3 shows experimental studies that evaluate the effect of natural antioxidant products, fruits, and vegetables on diabetes and its related complications.
Recently, studies suggested that these beneficial effects could be due to nitrate content [318,319,320]. Machha and Schechter [321,322] reviewed and reported the beneficial effects of nitrite and nitrate on cardiovascular health, especially with respect to vascular function. Nitrites and nitrates, the content of the fruits and vegetables [323] and direct eNOS subtracts, can improve NO bioavailability in the vasculature and improve endothelial function and all the beneficial effects of NO, nitrites and nitrates as a substrate to eNOS. This evidence has been shown by several in vitro and in vivo animal models [324,325,326] and in humans [325,327,328] to increase the bioavailability of NO to reduce vascular tone, blood pressure, and micro- and macrovascular complications, and improving insulin sensitivity is certainly an attractive therapeutic target in T2D.
Even though antioxidant and anti-inflammatory mechanisms by which fruits and vegetables exert their protective effects are not entirely clear, some studies have identified several bioactive components such as carotenoids, vitamins, fiber, magnesium, and potassium as acting synergistically or antagonistically to promote a holistic beneficial effect. For example, vitamin C restores endothelial function in T1D patients, leading to decreased micro- and macrovascular complications [329]. Chronic vitamin E, with low (100 UI/d, 3 months) or high (250UI/d, 6 months) doses, decrease lipid peroxidation in T1D patients [330,331]. Vitamin E is the best example that shows the complexity of antioxidant studies. In fact, this antioxidant supplement has been investigated extensively. Since 1998, Heinonen et al. [332] has suspected an increase in prostate cancer, not confirmed later by Lippma et al. [333] and Gaziano et al. [334] in 2009. However, in 2005, Miller et al. [335] described an increase in all-cause mortality, and the SELECT study was stopped in 2008 due to an increase in prostate cancer with 400UI/d of vitamin E [336]. Moreover, a randomized clinical trial with vitamin E showed no cardiovascular benefits, mainly in non-diabetic subjects [337]; this was confirmed later by a HOPE clinical trial [338,339]. However, an analysis of all data in a sub-group of subjects with diabetes and haptoglobin 2-2 genotype in HOPE and ICARE studies revealed that, in fact, vitamin E (400UI/d, 18 months) reduced the rate of cardiovascular events in these high risk subjects [340,341], which was confirmed in a recent meta-analysis [342].
Table 4 shows antioxidant efficacy of vitamins and supplements focus in diabetes and its complications.
4.3. Polyphenols: Extract Versus Molecular Compound
Polyphenols are a large and heterogeneous group of phytochemicals of plant-based foods, including tea, coffee, wine, cereal grains, vegetables, legumes, fruits, and berries [372]. And the largest and best-studied polyphenols are flavonoids, which include several thousand compounds, among them flavonols, flavones, flavonones, flavan-3-ols, anthocyanins, and isoflavones [373]. The estimated intake of dietary polyphenols is approximately 1 g/day [374]. Increasingly, the dietary recommendations for individuals at risk of T2D emphasis the intake of plant food products, such as whole grains, berries, fruits, and vegetables, all known to be excellent sources of dietary fiber, but also good sources of variable polyphenolic compounds. In fact, epidemiological studies report an inverse association between dietary polyphenol consumption and both diabetes [17,18,19,20] and more generally in chronic diseases such as cardiovascular diseases, atherosclerosis, hypertension, and cancer [375].
As shown before, vascular protection may also be due to the direct action of polyphenols on the endothelial function. In fact, polyphenols are able to stimulate the endothelial formation of NO and EDHF in isolated blood vessels, and improve endothelial function in humans. Schini-Kerth et al. [21] described the vascular protection led by natural product-derived polyphenols in ex vivo and experimental models of cardiovascular disease, including metabolic syndrome and diabetes. Recently, Franzini et al. [376] indicated that diets that contain a high level of polyphenol-rich natural sources such as red wine, grapefruit, berries, and dark chocolate, improved endothelial function in a low cardiovascular risk population, and Khan et al. [377] discusses the effects of cocoa polyphenols on cardiovascular-related inflammation. Table 5 shows the effect of polyphenol-rich natural sources on human vascular function.
Besides their beneficial effects on endothelial function and vascular homeostasis, they also influence glucose metabolism by several mechanisms, such as the inhibition of carbohydrate digestion and glucose absorption in the intestine, the stimulation of insulin secretion from the pancreatic β-cells, the modulation of glucose release from liver, the activation of insulin receptors and glucose uptake in the insulin-sensitive tissues, and the modulation of hepatic glucose output [410]. Many polyphenols have been shown to inhibit mostly α-glucosidase activity in vitro (anthocyanins, catechins, flavanones, flavones, flavanols, isoflavones, phenolic acids, and proanthocyanidins), whereas α-amylase activity is inhibited only by phenolic acids and some flavonols such as quercetin, luteolin, and myricetin. As regards the various effects of polyphenols, very few of them are able to induce insulin secretion from cultured cells or islets isolated from pancreas (cyanidin and delphinidin, epicatechin and EGCG, rutin, quercetin, apigenin, etc.) and inhibit the sodium-dependent glucose transporter (SGLT1) and the glucose transporter GLUT2) (tea catechins and quercetins) [410]. Recently, Hanhineva et al. [410] listed the impacts of dietary polyphenols on glucose metabolism with in vitro and in vivo studies and highlight the protective role of dietary rich in polyphenols on carbohydrate metabolism in both animals and humans. For example, Rostami et al. [411] demonstrated that cocoa is effective in improving TG levels, decreasing blood pressure, and fasting blood sugar in T2D patients with hypertensive complications. A meta-analysis of eleven randomized controlled clinical trials showed that resveratrol significantly improves glucoregulation and insulin sensitivity in diabetic patients, but not control participants [412]. Similar results were obtained in a second meta-analysis that included only T2D patients [413]. One recent review [414] reported the latest advances regarding the timing, dosage, formulation, bioavailability, toxicity of resveratrol in human, focusing on cancer, neurogeneration and diabetes, obesity, and cardiovascular diseases. Curcumin has been reported as a potent scavenger of a variety of ROS [415], exhibiting anti-inflammatory activity as well as antioxidant properties [416]. The phenolic (OH) structure of curcumin was believed to be essential for curcumin’s anti-oxidant activity [417]. Novelle et al. [414] concluded about difficulties of establishing a specific range of safety/efficacy for particular doses of resveratrol for particular populations, and many discrepancies and conflicting information must be resolved before recommending the use of resveratrol. Table 6 and Table 7 show the effect of polyphenol-rich natural sources on human prevention of T2D on in vitro and in vivo models of diabetes and complications, respectively.
4.4. Current Medications
Some modern drugs are derived from traditional medicine: anti-malarials (artemisinin, quinine), anti-asthmatics (cromolyn), anti-cancer (etoposide, vinca alkaloids), anti-coagulants (huridine), anticholestérolémiants (Lavastatine), and analgesics (opiates) [454]. Moreover, drugs used to treat diabetes have an antioxidant activity [16]: a scavenger of ROS and a modulator of antioxidant enzymes activities by several mechanisms. Some of them have beneficial effects on diabetes complications such as nephropathy—angiotensin converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), and melatonin, and neuropathy and retinopathy—melatonin and α-lipoic acid. Many of them have beneficial effects against cardiovascular diseases: caffeic acid, phenethyl ester, carvedilol, and metformin [16]. In fact, metformin, the currently used biguanide antihyperglycemic agent, can decrease xanthine oxidase activity and TNFα production, chelates metal ions, and inhibits AGE formation [455] with an intracellular modulation of free radical production [72].
5. Discussion and General Conclusion
In the last few years, there has been an exponential growth in the field of herbal medicine, and these drugs are gaining popularity. Many traditional medicines in use are derived from medicinal plants, minerals, and organic matter, and many conventional drugs have been derived from prototypic molecules. The use of medicinal plants for therapeutic purposes is a practice as old as human history. Some studies and population observations highlight a real effect of plants on health and management of diabetes complications. Today, the WHO has listed 21,000 plants, which are used for medicinal purposes around the world, but the expert committee on diabetes has recommended that traditional medicinal herbs be further investigated.
Nutrition and diet quality are key elements in the acquisition, control, and potential treatment of many chronic diseases and adverse health conditions. Higher consumption of fruits and vegetables has been associated with a lower risk of several diseases, including cardiovascular disease [11,12]. Increased physical activity and dietary management implemented by health-care professionals is fundamental to initial treatment of T2D and has been recommended for a long time by international consensus [456]. Meta-analyses of exercise and diet studies have concluded that concentrations of HbA1c can be lowered by aerobic and resistance exercise and by dietary intervention [457,458], more precisely, intensified, targeted, multifactorial interventions compared to conventional intervention [459]. However, few studies have determined whether treatments affect endothelial dysfunction and oxidative stress. If multifactorial treatment does have an effect, then markers of endothelial dysfunction and oxidative stress would be expected to be less associated with cardiovascular death and all-cause mortality in these patients. In fact, most of the studies have reported the beneficial effects of natural products-rich in antioxidant activities, leading to protect vessels against oxidative stress, loss of vascular homeostasis, and diabetic complications. Recent data have supported that the hyperglycemic environment may be remembered in the vasculature, a metabolic or “hyperglycemic memory” explaining the progression of diabetic vascular complications despite normoglycemia restoration [460]. Moreover, endothelial progenitor cells as a biological marker of peripheral artery disease [390] have highlighted a real interest in protecting the vascular arch [461]. Thus, taking all of this literature together, blood vessels could be a good marker and strategy to monitor complications, especially in diabetes.
Observational cohort studies support that consumption of sugar-sweetened beverages, including artificially beverages and fruit juice, are associated with incident T2D, independently of obesity. Both were unlikely to be healthy alternatives to sugar-sweetened beverages for the prevention of type 2 diabetes, and, under assumption of causality, there consumptions may be related to a substantial number of cases of new onset diabetes [462]. The local food environment may influence individual (including food choices) and community health [463]. Today, the objective is to promote the consumption of non-industrial and natural products instead of concentrated fruit juice intake. In fact, the association between fruits and vegetables consumption and weight development has been summarized in the ISA-FRUIT Project of the EU from 2008, and 7/16 studies [464] and several prospective cohort studies [296,465] and the EPIC-Norfolk Study [466] have highlighted an inverse association between “unworked” fruits and vegetables consumption and health outcomes including obesity, cardiovascular, and diabetes. However, others studies have not demonstrated the effectiveness of fruits and vegetables to have health effects or to prevent chronic diseases. These results suggest that there are sub-types within larger categories of food environments that are differentially associated with adverse health outcomes [467]. Differences in the nutrient contents by group could explain differences and raise difficulties of interpreting the results of different human studies. There is a need to conduct clinical research, developing simple bioassays for biological standardization, pharmacological, and toxicological evaluation, to study the effects of natural food on health.
It would actually be profitable to propose tables’ effects of antioxidants, with corresponding doses and diseases treated; still, however, the study of antioxidants is very difficult and complex. Many parameters can influence the results of clinical studies: a different design in terms of types and origins of antioxidants, doses, formulation, absorption, biodisponibility, and times of treatments; the studied population, genotype sub-type of patients, types of medication, and progression of the disease, with a time course of diabetes and complications; and the methods of assessment and their limitations [468,469,470]. The international society of antioxidants in nutrition and health (ISANH) work today to propose guidelines with all these objectives. Although the results of clinical studies about the therapeutic use of antioxidants are quite controversial, all data reported in this review and in others provide real hope for their use, especially in the prevention of diabetic complications. Food is the first pillar of patient care before the introduction of medications. Wealth, nutritious additions, and a contribution of bioactive molecules (vitamins, polyphenols, etc.) with antioxidant properties is actually a real asset in the prevention of chronic diseases, while the importance of prevention should not be underestimated. All publications by Dal et al. demonstrated the interest of the use of natural antioxidants (red wine polyphenols) to prevent and treat diseases with endothelial dysfunction related to oxidative stress [62,64,213,471,472]. Moreover, our recent works carried out on in vitro and in vivo models of metabolic disorders have allowed us not only to involve oxidative stress in the pathophysiology of disorders but also to demonstrate that natural antioxidant compounds help to prevent or reduce complications (polyphenols from green tea and red wine [442,473], red cabbage, Dal S, under publication). One study in process in the lab focusing on antioxidants in T1D seems be a new target for the diabetic optimization of management [474].
All of our studies, and studies mentioned in this review, demonstrate the ability of antioxidants to prevent or counteract excessive ROS production by increasing endogenous antioxidant defenses. We think now that a new strategy might be to prevent the overproduction of ROS instead of only scavenging the already formed ones, because of the “cardio-metabolic memory.” Today, an optimal understanding of the beneficial mechanisms of functional products or functional foods [475] will not allow for more personalized care, depending on the status of cardiovascular and metabolic patients.
Author Contributions
SD and SS design the paper, SS wrote part 2 and reviewed it, and SD wrote the other parts and reviewed the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.IDF . IDF Diabetes Atlas. 7th ed. IDF; Brussels, Belgium: 2015. [Google Scholar]
- 2.Nolan C.J., Damm P., Prentki M. Type 2 diabetes across generations: From pathophysiology to prevention and management. Lancet. 2011;378:169–181. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- 3.Orasanu G., Plutzky J. The pathologic continuum of diabetic vascular disease. J. Am. Coll. Cardiol. 2009;53(Suppl. S5):S35–S42. doi: 10.1016/j.jacc.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosolova H., Petrlova B., Simon J., Sifalda P., Sipova I., Sefrna F. Macrovascular and microvascular complications in type 2 diabetes patients. Vnitrni lekarstvi. 2008;54:229–237. [PubMed] [Google Scholar]
- 5.Sena C.M., Pereira A.M., Seica R. Endothelial dysfunction—A major mediator of diabetic vascular disease. Biochimica et Biophysica Acta. 2013;1832:2216–2231. doi: 10.1016/j.bbadis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 7.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Chong Z.Z., Maiese K., Targeting W.N.T. protein kinase B, and mitochondrial membrane integrity to foster cellular survival in the nervous system. Histol. Histopathol. 2004;19:495–504. doi: 10.14670/hh-19.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.You Z., Saims D., Chen S., Zhang Z., Guttridge D.C., Guan K.L., MacDougald O.A., Brown A.M., Evan G., Kitajewski J., et al. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J. Cell Biol. 2002;157:429–440. doi: 10.1083/jcb.200201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ness A.R., Powles J.W. Fruit and vegetables, and cardiovascular disease: A review. Int. J. Epidemiol. 1997;26:1–13. doi: 10.1093/ije/26.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Van’T Veer P., Jansen M.C., Klerk M., Kok F.J. Fruits and vegetables in the prevention of cancer and cardiovascular disease. Public Health Nutr. 2000;3:103–107. doi: 10.1017/S1368980000000136. [DOI] [PubMed] [Google Scholar]
- 12.Bazzano L.A., Serdula M.K., Liu S. Dietary intake of fruits and vegetables and risk of cardiovascular disease. Curr. Atheroscler. Rep. 2003;5:492–499. doi: 10.1007/s11883-003-0040-z. [DOI] [PubMed] [Google Scholar]
- 13.Joshipura K.J., Hu F.B., Manson J.E., Stampfer M.J., Rimm E.B., Speizer F.E., Colditz G., Ascherio A., Rosner B., Spiegelman D., et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann. Intern. Med. 2001;134:1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 14.Joshipura K.J., Ascherio A., Manson J.E., Stampfer M.J., Rimm E.B., Speizer F.E., Hennekens C.H., Spiegelman D., Willett W.C. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA. 1999;282:1233–1239. doi: 10.1001/jama.282.13.1233. [DOI] [PubMed] [Google Scholar]
- 15.Dodda D., Ciddi V. Plants used in the management of diabetic complications. Indian J. Pharm. Sci. 2014;76:97–106. [PMC free article] [PubMed] [Google Scholar]
- 16.Zatalia S.R., Sanusi H. The role of antioxidants in the pathophysiology, complications, and management of diabetes mellitus. Acta Med. Indones. 2013;45:141–147. [PubMed] [Google Scholar]
- 17.Maritim A.C., Sanders R.A., Watkins J.B., 3rd Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 18.Vincent H.K., Innes K.E., Vincent K.R. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes. Metab. 2007;9:813–839. doi: 10.1111/j.1463-1326.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 19.Al-Awwadi N., Azay J., Poucheret P., Cassanas G., Krosniak M., Auger C., Gasc F., Rouanet J.M., Cros G., Teissedre P.L. Antidiabetic activity of red wine polyphenolic extract, ethanol, or both in streptozotocin-treated rats. J. Agric. Food Chem. 2004;52:1008–1016. doi: 10.1021/jf030417z. [DOI] [PubMed] [Google Scholar]
- 20.Al-Awwadi N.A., Bornet A., Azay J., Araiz C., Delbosc S., Cristol J.P., Linck N., Cros G., Teissedre P.L. Red wine polyphenols alone or in association with ethanol prevent hypertension, cardiac hypertrophy, and production of reactive oxygen species in the insulin-resistant fructose-fed rat. J. Agric. Food Chem. 2004;52:5593–5597. doi: 10.1021/jf049295g. [DOI] [PubMed] [Google Scholar]
- 21.Schini-Kerth V.B., Etienne-Selloum N., Chataigneau T., Auger C. Vascular protection by natural product-derived polyphenols: In vitro and in vivo evidence. Planta Med. 2011;77:1161–1167. doi: 10.1055/s-0030-1250737. [DOI] [PubMed] [Google Scholar]
- 22.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 23.Yamagishi S., Maeda S., Matsui T., Ueda S., Fukami K., Okuda S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochimica et Biophysica Acta. 2012;1820:663–671. doi: 10.1016/j.bbagen.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ. Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitocco D., Tesauro M., Alessandro R., Ghirlanda G., Cardillo C. Oxidative stress in diabetes: Implications for vascular and other complications. Int. J. Mol. Sci. 2013;14:21525–21550. doi: 10.3390/ijms141121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anjaneyulu M., Chopra K. Nordihydroguairetic acid, a lignin, prevents oxidative stress and the development of diabetic nephropathy in rats. Pharmacology. 2004;72:42–50. doi: 10.1159/000078631. [DOI] [PubMed] [Google Scholar]
- 27.Kowluru R.A., Koppolu P., Chakrabarti S., Chen S. Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic. Res. 2003;37:1169–1180. doi: 10.1080/10715760310001604189. [DOI] [PubMed] [Google Scholar]
- 28.Chung Y.S., Choi Y.H., Lee S.J., Choi S.A., Lee J.H., Kim H., Hong E.K. Water extract of Aralia elata prevents cataractogenesis in vitro and in vivo. J. Ethnopharmacol. 2005;101:49–54. doi: 10.1016/j.jep.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Sytze Van Dam P., Cotter M.A., Bravenboer B., Cameron N.E. Pathogenesis of diabetic neuropathy: Focus on neurovascular mechanisms. Eur. J. Pharmacol. 2013;719:180–186. doi: 10.1016/j.ejphar.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 30.Hosseini A., Abdollahi M. Diabetic neuropathy and oxidative stress: Therapeutic perspectives. Oxid. Med. Cell. Longev. 2013;2013:168039. doi: 10.1155/2013/168039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kilpatrick E.S., Rigby A.S., Atkin S.L. A1C variability and the risk of microvascular complications in type 1 diabetes: Data from the Diabetes Control and Complications Trial. Diabetes Care. 2008;31:2198–2202. doi: 10.2337/dc08-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcovecchio M.L., Dalton R.N., Chiarelli F., Dunger D.B. A1C variability as an independent risk factor for microalbuminuria in young people with type 1 diabetes. Diabetes Care. 2011;34:1011–1013. doi: 10.2337/dc10-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waden J., Forsblom C., Thorn L.M., Gordin D., Saraheimo M., Groop P.H. A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes. 2009;58:2649–2655. doi: 10.2337/db09-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugawara A., Kawai K., Motohashi S., Saito K., Kodama S., Yachi Y., Hirasawa R., Shimano H., Yamazaki K., Sone H. HbA(1c) variability and the development of microalbuminuria in type 2 diabetes: Tsukuba Kawai Diabetes Registry 2. Diabetologia. 2012;55:2128–2131. doi: 10.1007/s00125-012-2572-7. [DOI] [PubMed] [Google Scholar]
- 35.Penno G., Solini A., Bonora E., Fondelli C., Orsi E., Zerbini G., Morano S., Cavalot F., Lamacchia O., Laviola L., et al. HbA1c variability as an independent correlate of nephropathy, but not retinopathy, in patients with type 2 diabetes: The Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study. Diabetes Care. 2013;36:2301–2310. doi: 10.2337/dc12-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Group A.C., Patel A., MacMahon S., Chalmers J., Neal B., Billot L., Woodward M., Marre M., Cooper M., Glasziou P., et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 37.Wellen K.E., Hotamisligil G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005;115:1111–1119. doi: 10.1172/JCI200525102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Touyz R.M. Molecular and cellular mechanisms in vascular injury in hypertension: Role of angiotensin II. Curr. Opin. Nephrol. Hypertens. 2005;14:125–131. doi: 10.1097/00041552-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Ishibashi T. Molecular hydrogen: New antioxidant and anti-inflammatory therapy for rheumatoid arthritis and related diseases. Curr. Pharm. Des. 2013;19:6375–6381. doi: 10.2174/13816128113199990507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westermann D., Van Linthout S., Dhayat S., Dhayat N., Schmidt A., Noutsias M., Song X.-Y., Spillmann F., Riad A., Schultheiss H.-P., et al. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res. Cardiol. 2007;102:500–507. doi: 10.1007/s00395-007-0673-0. [DOI] [PubMed] [Google Scholar]
- 41.Rolfe D.F., Brown G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 42.Koppenol W.H. The Haber-Weiss cycle--70 years later. Redox Rep. 2001;6:229–234. doi: 10.1179/135100001101536373. [DOI] [PubMed] [Google Scholar]
- 43.Derosa G., Fogari E., D'Angelo A., Bianchi L., Bonaventura A., Romano D., Maffioli P. Adipocytokine levels in obese and non-obese subjects: An observational study. Inflammation. 2013;36:914–920. doi: 10.1007/s10753-013-9620-4. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L., Wheatley C.M., Richards S.M., Barrett E.J., Clark M.G., Rattigan S. TNF-alpha acutely inhibits vascular effects of physiological but not high insulin or contraction. Am. J. Physiol. Endocrinol. Metab. 2003;285:E654–E660. doi: 10.1152/ajpendo.00119.2003. [DOI] [PubMed] [Google Scholar]
- 45.Moriwaki Y., Inokuchi T., Yamamoto A., Ka T., Tsutsumi Z., Takahashi S., Yamamoto T. Effect of TNF-alpha inhibition on urinary albumin excretion in experimental diabetic rats. Acta Diabetol. 2007;44:215–218. doi: 10.1007/s00592-007-0007-6. [DOI] [PubMed] [Google Scholar]
- 46.Yubero-Serrano E.M., Delgado-Lista J., Pena-Orihuela P., Perez-Martinez P., Fuentes F., Marin C., Tunez I., Tinahones F.J., Perez-Jimenez F., Roche H.M., et al. Oxidative stress is associated with the number of components of metabolic syndrome: LIPGENE study. Exp. Mol. Med. 2013;45:e28. doi: 10.1038/emm.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerschman R., Gilbert D.L., Nye S.W., Dwyer P., Fenn W.O. Oxygen poisoning and x-irradiation: A mechanism in common. Science. 1954;119:623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- 48.Harman D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 49.McCord J.M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 50.McCord J.M., Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J. Biol. Chem. 1969;244:6056–6063. [PubMed] [Google Scholar]
- 51.Moureu C.D.C. Sur l'autoxydation: Essai sur le mécanisme de l'action antioxygène. CR Acad. Sci. (Paris) 1923;176:624–629. [Google Scholar]
- 52.Mittal C.K., Murad F. Activation of guanylate cyclase by superoxide dismutase and hydroxyl radical: A physiological regulator of guanosine 3′,5′-monophosphate formation. Proc. Natl. Acad. Sci. USA. 1977;74:4360–4364. doi: 10.1073/pnas.74.10.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boffetta P., Nyberg F. Contribution of environmental factors to cancer risk. Br. Med. Bull. 2003;68:71–94. doi: 10.1093/bmp/ldg023. [DOI] [PubMed] [Google Scholar]
- 54.Youn Y.K., Lalonde C., Demling R. Oxidants and the pathophysiology of burn and smoke inhalation injury. Free Rad. Biol. Med. 1992;12:409–415. doi: 10.1016/0891-5849(92)90090-4. [DOI] [PubMed] [Google Scholar]
- 55.Black H.S. Potential involvement of free radical reactions in ultraviolet light-mediated cutaneous damage. Photochem. Photobiol. 1987;46:213–221. doi: 10.1111/j.1751-1097.1987.tb04759.x. [DOI] [PubMed] [Google Scholar]
- 56.Grattagliano I., Palmieri V.O., Portincasa P., Moschetta A., Palasciano G. Oxidative stress-induced risk factors associated with the metabolic syndrome: A unifying hypothesis. J. Nutr. Biochem. 2008;19:491–504. doi: 10.1016/j.jnutbio.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 57.Cai H., Harrison D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000;87:840–844. doi: 10.1161/01.RES.87.10.840. [DOI] [PubMed] [Google Scholar]
- 58.Ducrocq C., Servy C., Cudic M., Blanchard E.B. Intervention by nitric oxide, NO, and its oxide derivatives particularly in mammals. Can. J. Physiol. Pharmacol. 2001;79:95–102. doi: 10.1139/y00-077. [DOI] [PubMed] [Google Scholar]
- 59.Nishikawa T., Edelstein D., Du X.L., Yamagishi S., Matsumura T., Kaneda Y., Yorek M.A., Beebe D., Oates P.J., Hammes H.P., et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 60.Al Ghouleh I., Khoo N.K., Knaus U.G., Griendling K.K., Touyz R.M., Thannickal V.J., Barchowsky A., Nauseef W.M., Kelley E.E., Bauer P.M., et al. Oxidases and peroxidases in cardiovascular and lung disease: New concepts in reactive oxygen species signaling. Free Rad. Biol. Med. 2011;51:1271–1288. doi: 10.1016/j.freeradbiomed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Selemidis S., Sobey C.G., Wingler K., Schmidt H.H., Drummond G.R. NADPH oxidases in the vasculature: Molecular features, roles in disease and pharmacological inhibition. Pharmacol. Ther. 2008;120:254–291. doi: 10.1016/j.pharmthera.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 62.Dal-Ros S., Zoll J., Lang A.L., Auger C., Keller N., Bronner C., Geny B., Schini-Kerth V.B. Chronic intake of red wine polyphenols by young rats prevents aging-induced endothelial dysfunction and decline in physical performance: Role of NADPH oxidase. Biochem. Biophys. Res. Commun. 2011;404:743–749. doi: 10.1016/j.bbrc.2010.12.060. [DOI] [PubMed] [Google Scholar]
- 63.Dal-Ros S., Oswald-Mammosser M., Pestrikova T., Schott C., Boehm N., Bronner C., Chataigneau T., Geny B., Schini-Kerth V.B. Losartan prevents portal hypertension-induced, redox-mediated endothelial dysfunction in the mesenteric artery in rats. Gastroenterology. 2010;138:1574–1584. doi: 10.1053/j.gastro.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 64.Dal-Ros S., Bronner C., Schott C., Kane M.O., Chataigneau M., Schini-Kerth V.B., Chataigneau T. Angiotensin II-induced hypertension is associated with a selective inhibition of endothelium-derived hyperpolarizing factor-mediated responses in the rat mesenteric artery. J. Pharmacol. Exp. Ther. 2009;328:478–486. doi: 10.1124/jpet.108.145326. [DOI] [PubMed] [Google Scholar]
- 65.Li J.M., Shah A.M. ROS generation by nonphagocytic NADPH oxidase: Potential relevance in diabetic nephropathy. J. Am. Soc. Nephrol. 2003;14(8 Suppl. S3):S221–S226. doi: 10.1097/01.ASN.0000077406.67663.E7. [DOI] [PubMed] [Google Scholar]
- 66.Dal S., Jeandidier N., Seyfritz E., Bietiger W., Peronet C., Moreau F., Pinget M., Maillard E., Sigrist S. Oxidative stress status and liver tissue defenses in diabetic rats during intensive subcutaneous insulin therapy. Exp. Biol. Med. 2016;241:184–192. doi: 10.1177/1535370215603837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dal S., Jeandidier N., Schaschkow A., Spizzo A.H., Seyfritz E., Sookhareea C., Bietiger W., Peronet C., Moreau F., Pinget M., et al. Portal or subcutaneous insulin infusion: Efficacy and impact on liver inflammation. Fundam. Clin. Pharmacol. 2015;29:488–498. doi: 10.1111/fcp.12129. [DOI] [PubMed] [Google Scholar]
- 68.Newsholme P., Morgan D., Rebelato E., Oliveira-Emilio H.C., Procopio J., Curi R., Carpinelli A. Insights into the critical role of NADPH oxidase(s) in the normal and dysregulated pancreatic beta cell. Diabetologia. 2009;52:2489–2498. doi: 10.1007/s00125-009-1536-z. [DOI] [PubMed] [Google Scholar]
- 69.Brandes R.P., Weissmann N., Schroder K. Nox family NADPH oxidases in mechano-transduction: Mechanisms and consequences. Antioxid. Redox Signal. 2014;20:887–898. doi: 10.1089/ars.2013.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Butler R., Morris A.D., Belch J.J., Hill A., Struthers A.D. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35:746–751. doi: 10.1161/01.HYP.35.3.746. [DOI] [PubMed] [Google Scholar]
- 71.Halliwell B., Gutteridge J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 72.Bonnefont-Rousselot D., Raji B., Walrand S., Gardes-Albert M., Jore D., Legrand A., Peynet J., Vasson M.P. An intracellular modulation of free radical production could contribute to the beneficial effects of metformin towards oxidative stress. Metabolism. 2003;52:586–589. doi: 10.1053/meta.2003.50093. [DOI] [PubMed] [Google Scholar]
- 73.Evans P., Halliwell B. Micronutrients: Oxidant/antioxidant status. Br. J. Nutr. 2001;85(Suppl. 2):S67–S74. doi: 10.1079/BJN2000296. [DOI] [PubMed] [Google Scholar]
- 74.Fukai T., Ushio-Fukai M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alfonso-Prieto M., Biarnes X., Vidossich P., Rovira C. The molecular mechanism of the catalase reaction. J. Am. Chem. Soc. 2009;131:11751–11761. doi: 10.1021/ja9018572. [DOI] [PubMed] [Google Scholar]
- 76.Prabhakar R., Vreven T., Morokuma K., Musaev D.G. Elucidation of the mechanism of selenoprotein glutathione peroxidase (GPx)-catalyzed hydrogen peroxide reduction by two glutathione molecules: A density functional study. Biochemistry. 2005;44:11864–11871. doi: 10.1021/bi050815q. [DOI] [PubMed] [Google Scholar]
- 77.Pietta P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000;63:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 78.Bounous G. Whey protein concentrate (WPC) and glutathione modulation in cancer treatment. Anticancer Res. 2000;20:4785–4792. [PubMed] [Google Scholar]
- 79.Thornalley P.J., McLellan A.C., Lo T.W., Benn J., Sonksen P.H. Negative association between erythrocyte reduced glutathione concentration and diabetic complications. Clin. Sci. 1996;91:575–582. doi: 10.1042/cs0910575. [DOI] [PubMed] [Google Scholar]
- 80.Grankvist K., Marklund S.L., Taljedal I.B. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem. J. 1981;199:393–398. doi: 10.1042/bj1990393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lenzen S., Drinkgern J., Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Rad. Biol. Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 82.Acharya J.D., Ghaskadbi S.S. Islets and their antioxidant defense. Islets. 2010;2:225–235. doi: 10.4161/isl.2.4.12219. [DOI] [PubMed] [Google Scholar]
- 83.Ammon H.P., Hagele R., Youssif N., Eujen R., El-Amri N. A possible role of intracellular and membrane thiols of rat pancreatic islets in calcium uptake and insulin release. Endocrinology. 1983;112:720–726. doi: 10.1210/endo-112-2-720. [DOI] [PubMed] [Google Scholar]
- 84.Sies H. Role of reactive oxygen species in biological processes. Klini. Wochenschr. 1991;69:965–968. doi: 10.1007/BF01645140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Valko M., Rhodes C.J., Moncol J., Izakovic M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biol. Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 86.Thannickal V.J., Fanburg B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 87.Lowenstein C.J., Dinerman J.L., Snyder S.H. Nitric oxide: A physiologic messenger. Ann. Intern. Med. 1994;120:227–237. doi: 10.7326/0003-4819-120-3-199402010-00009. [DOI] [PubMed] [Google Scholar]
- 88.Storz P. Reactive oxygen species in tumor progression. Front. Biosci. 2005;10:1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 89.Sun Y., Oberley L.W. Redox regulation of transcriptional activators. Free Rad. Biol. Med. 1996;21:335–348. doi: 10.1016/0891-5849(96)00109-8. [DOI] [PubMed] [Google Scholar]
- 90.Frenette P.S., Wagner D.D. Adhesion molecules--Part 1. N. Engl. J. Med. 1996;334:1526–1529. doi: 10.1056/NEJM199606063342308. [DOI] [PubMed] [Google Scholar]
- 91.Schroder K. NADPH oxidases in redox regulation of cell adhesion and migration. Antioxid. Redox Signal. 2014;20:2043–2058. doi: 10.1089/ars.2013.5633. [DOI] [PubMed] [Google Scholar]
- 92.Keisari Y., Braun L., Flescher E. The oxidative burst and related phenomena in mouse macrophages elicited by different sterile inflammatory stimuli. Immunobiology. 1983;165:78–89. doi: 10.1016/S0171-2985(83)80048-5. [DOI] [PubMed] [Google Scholar]
- 93.Scherz-Shouval R., Elazar Z. Regulation of autophagy by ROS: Physiology and pathology. Trends Biochem. Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 94.Zhang L., Wang K., Lei Y., Li Q., Nice E.C., Huang C. Redox signaling: Potential arbitrator of autophagy and apoptosis in therapeutic response. Free Rad. Biol. Med. 2015;89:452–465. doi: 10.1016/j.freeradbiomed.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 95.Murry C.E., Richard V.J., Reimer K.A., Jennings R.B. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ. Res. 1990;66:913–931. doi: 10.1161/01.RES.66.4.913. [DOI] [PubMed] [Google Scholar]
- 96.Kalikiri P.C., Sachan R.S.G.S. Ischemic and anesthetic preconditioning of the heart: An insith into concepts and mechanisms. Internet J. Anesthesiol. 2004;8:2. [Google Scholar]
- 97.Zhao T.C., Hines D.S., Kukreja R.C. Adenosine-induced late preconditioning in mouse hearts: Role of p38 MAP kinase and mitochondrial K(ATP) channels. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H1278–H1285. doi: 10.1152/ajpheart.2001.280.3.H1278. [DOI] [PubMed] [Google Scholar]
- 98.Laude K., Favre J., Thuillez C., Richard V. NO produced by endothelial NO synthase is a mediator of delayed preconditioning-induced endothelial protection. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H2053–H2060. doi: 10.1152/ajpheart.00627.2002. [DOI] [PubMed] [Google Scholar]
- 99.Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J. Cycl. Nucleotide Res. 1977;3:23–35. [PubMed] [Google Scholar]
- 100.Furchgott R.F., Zawadzki J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 101.Vanhoutte P.M. Endothelium and control of vascular function. State of the Art lecture. Hypertension. 1989;13(6 Pt 2):658–667. doi: 10.1161/01.HYP.13.6.658. [DOI] [PubMed] [Google Scholar]
- 102.Vanhoutte P.M. Role of the endothelium in control of vascular smooth muscle function. Verhandelingen—Koninklijke Academie voor Geneeskunde van Belgie. 1982;44:411–418. [PubMed] [Google Scholar]
- 103.Schini-Kerth V.B. Vascular biosynthesis of nitric oxide: Effect on hemostasis and fibrinolysis. Transfusion clinique et biologique. 1999;6:355–363. doi: 10.1016/S1246-7820(00)88980-6. [DOI] [PubMed] [Google Scholar]
- 104.Prosser B.L., Ward C.W., Lederer W.J. X-ROS signaling: Rapid mechano-chemo transduction in heart. Science. 2011;333:1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 105.Czech M.P., Lawrence J.C., Jr., Lynn W.S. Evidence for the involvement of sulfhydryl oxidation in the regulation of fat cell hexose transport by insulin. Proc. Natl. Acad. Sci. USA. 1974;71:4173–4177. doi: 10.1073/pnas.71.10.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Higaki Y., Mikami T., Fujii N., Hirshman M.F., Koyama K., Seino T., Tanaka K., Goodyear L.J. Oxidative stress stimulates skeletal muscle glucose uptake through a phosphatidylinositol 3-kinase-dependent pathway. Am. J. Physiol. Endocrinol. Metab. 2008;294:E889–E897. doi: 10.1152/ajpendo.00150.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Christon R., Drouin O., Marette A. Redox modulation of insulin signaling and endothelial function. Antioxid. Redox Signal. 2005;7:1062–1070. doi: 10.1089/ars.2005.7.1062. [DOI] [PubMed] [Google Scholar]
- 108.May J.M., de Haen C. The insulin-like effect of hydrogen peroxide on pathways of lipid synthesis in rat adipocytes. J. Biol. Chem. 1979;254:9017–9021. [PubMed] [Google Scholar]
- 109.Tiganis T. Reactive oxygen species and insulin resistance: The good, the bad and the ugly. Trends Pharmacol. Sci. 2011;32:82–89. doi: 10.1016/j.tips.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 110.Loh K., Deng H., Fukushima A., Cai X., Boivin B., Galic S., Bruce C., Shields B.J., Skiba B., Ooms L.M., et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10:260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cadet J., Bellon S., Berger M., Bourdat A.G., Douki T., Duarte V., Frelon S., Gasparutto D., Muller E., Ravanat J.L., et al. Recent aspects of oxidative DNA damage: Guanine lesions, measurement and substrate specificity of DNA repair glycosylases. Biol. Chem. 2002;383:933–943. doi: 10.1515/BC.2002.100. [DOI] [PubMed] [Google Scholar]
- 112.Hehner S.P., Breitkreutz R., Shubinsky G., Unsoeld H., Schulze-Osthoff K., Schmitz M.L., Droge W. Enhancement of T cell receptor signaling by a mild oxidative shift in the intracellular thiol pool. J. Immunol. 2000;165:4319–4328. doi: 10.4049/jimmunol.165.8.4319. [DOI] [PubMed] [Google Scholar]
- 113.Zarkovic N. 4-hydroxynonenal as a bioactive marker of pathophysiological processes. Mol. Asp. Med. 2003;24:281–291. doi: 10.1016/S0098-2997(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 114.Petersen D.R., Doorn J.A. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Rad. Biol. Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 115.Dalle-Donne I., Giustarini D., Colombo R., Rossi R., Milzani A. Protein carbonylation in human diseases. Trends Mol. Med. 2003;9:169–176. doi: 10.1016/S1471-4914(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 116.Esterbauer H., Gebicki J., Puhl H., Jurgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Rad. Biol. Med. 1992;13:341–390. doi: 10.1016/0891-5849(92)90181-F. [DOI] [PubMed] [Google Scholar]
- 117.Fraley A.E., Tsimikas S. Clinical applications of circulating oxidized low-density lipoprotein biomarkers in cardiovascular disease. Curr. Opin. Lipidol. 2006;17:502–509. doi: 10.1097/01.mol.0000245255.40634.b5. [DOI] [PubMed] [Google Scholar]
- 118.Torzewski M., Klouche M., Hock J., Messner M., Dorweiler B., Torzewski J., Gabbert H.E., Bhakdi S. Immunohistochemical demonstration of enzymatically modified human LDL and its colocalization with the terminal complement complex in the early atherosclerotic lesion. Arterioscler. Thromb. Vasc. Biol. 1998;18:369–378. doi: 10.1161/01.ATV.18.3.369. [DOI] [PubMed] [Google Scholar]
- 119.Makita Z., Yanagisawa K., Kuwajima S., Bucala R., Vlassara H., Koike T. The role of advanced glycosylation end-products in the pathogenesis of atherosclerosis. Nephrol. Dial. Transplant. 1996;11(Suppl. S5):31–33. doi: 10.1093/ndt/11.supp5.31. [DOI] [PubMed] [Google Scholar]
- 120.Ramasamy R., Vannucci S.J., Yan S.S., Herold K., Yan S.F., Schmidt A.M. Advanced glycation end products and RAGE: A common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15:16R–28R. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- 121.Grimsrud P.A., Picklo M.J., Sr., Griffin T.J., Bernlohr D.A. Carbonylation of adipose proteins in obesity and insulin resistance: Identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Mol. Cell. Proteom. MCP. 2007;6:624–637. doi: 10.1074/mcp.M600120-MCP200. [DOI] [PubMed] [Google Scholar]
- 122.Morgan P.E., Sturgess A.D., Davies M.J. Increased levels of serum protein oxidation and correlation with disease activity in systemic lupus erythematosus. Arthritis Rheum. 2005;52:2069–2079. doi: 10.1002/art.21130. [DOI] [PubMed] [Google Scholar]
- 123.Boldogh I., Bacsi A., Choudhury B.K., Dharajiya N., Alam R., Hazra T.K., Mitra S., Goldblum R.M., Sur S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J. Clin. Investig. 2005;115:2169–2179. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Castro S.M., Guerrero-Plata A., Suarez-Real G., Adegboyega P.A., Colasurdo G.N., Khan A.M., Garofalo R.P., Casola A. Antioxidant treatment ameliorates respiratory syncytial virus-induced disease and lung inflammation. Am. J. Respir. Crit. Care Med. 2006;174:1361–1369. doi: 10.1164/rccm.200603-319OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Madamanchi N.R., Runge M.S. Redox signaling in cardiovascular health and disease. Free Rad. Biol. Med. 2013;61:473–501. doi: 10.1016/j.freeradbiomed.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bloch-Damti A., Bashan N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid. Redox Signal. 2005;7:1553–1567. doi: 10.1089/ars.2005.7.1553. [DOI] [PubMed] [Google Scholar]
- 127.Maxwell S.R., Thomason H., Sandler D., Leguen C., Baxter M.A., Thorpe G.H., Jones A.F., Barnett A.H. Antioxidant status in patients with uncomplicated insulin-dependent and non-insulin-dependent diabetes mellitus. Eur. J. Clin. Investig. 1997;27:484–490. doi: 10.1046/j.1365-2362.1997.1390687.x. [DOI] [PubMed] [Google Scholar]
- 128.Rocic B., Vucic M., Knezevic-Cuca J., Radica A., Pavlic-Renar I., Profozic V., Metelko Z. Total plasma antioxidants in first-degree relatives of patients with insulin-dependent diabetes. Exp. Clin. Endocrinol. Diabetes. 1997;105:213–217. doi: 10.1055/s-0029-1211754. [DOI] [PubMed] [Google Scholar]
- 129.Santini S.A., Marra G., Giardina B., Cotroneo P., Mordente A., Martorana G.E., Manto A., Ghirlanda G. Defective plasma antioxidant defenses and enhanced susceptibility to lipid peroxidation in uncomplicated IDDM. Diabetes. 1997;46:1853–1858. doi: 10.2337/diab.46.11.1853. [DOI] [PubMed] [Google Scholar]
- 130.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 131.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 132.Gaede P., Lund-Andersen H., Parving H.H., Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 133.Luscher T.F., Creager M.A., Beckman J.A., Cosentino F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part II. Circulation. 2003;108:1655–1661. doi: 10.1161/01.CIR.0000089189.70578.E2. [DOI] [PubMed] [Google Scholar]
- 134.Creager M.A., Luscher T.F., Cosentino F., Beckman J.A. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 135.Du X.L., Edelstein D., Rossetti L., Fantus I.G., Goldberg H., Ziyadeh F., Wu J., Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc. Natl. Acad. Sci. USA. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yao D., Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010;59:249–255. doi: 10.2337/db09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr. Opin. Clin. Nutr. Metab. Care. 2002;5:561–568. doi: 10.1097/00075197-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 138.Spitaler M.M., Graier W.F. Vascular targets of redox signalling in diabetes mellitus. Diabetologia. 2002;45:476–494. doi: 10.1007/s00125-002-0782-0. [DOI] [PubMed] [Google Scholar]
- 139.Figueroa-Romero C., Sadidi M., Feldman E.L. Mechanisms of disease: The oxidative stress theory of diabetic neuropathy. Rev. Endocr. Metab. Disord. 2008;9:301–314. doi: 10.1007/s11154-008-9104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Defraigne J.O. A central pathological mechanism explaining diabetic complications? Revue medicale de Liege. 2005;60:472–478. [PubMed] [Google Scholar]
- 141.Suarez G., Rajaram R., Bhuyan K.C., Oronsky A.L., Goidl J.A. Administration of an aldose reductase inhibitor induces a decrease of collagen fluorescence in diabetic rats. J. Clin. Investig. 1988;82:624–627. doi: 10.1172/JCI113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cai W., He J.C., Zhu L., Chen X., Zheng F., Striker G.E., Vlassara H. Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan. Am. J. Pathol. 2008;173:327–336. doi: 10.2353/ajpath.2008.080152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cai W., He J.C., Zhu L., Chen X., Wallenstein S., Striker G.E., et al. Reduced oxidant stress and extended lifespan in mice exposed to a low glycotoxin diet: Association with increased AGER1 expression. Am. J. Pathol. 2007;170:1893–1902. doi: 10.2353/ajpath.2007.061281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhao Z., Zhao C., Zhang X.H., Zheng F., Cai W., Vlassara H., Ma Z.A. Advanced glycation end products inhibit glucose-stimulated insulin secretion through nitric oxide-dependent inhibition of cytochrome c oxidase and adenosine triphosphate synthesis. Endocrinology. 2009;150:2569–2576. doi: 10.1210/en.2008-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Beyan H., Riese H., Hawa M.I., Beretta G., Davidson H.W., Hutton J.C., Burger H., Schlosser M., Snieder H., Boehm B.O., et al. Glycotoxin and autoantibodies are additive environmentally determined predictors of type 1 diabetes: A twin and population study. Diabetes. 2012;61:1192–1198. doi: 10.2337/db11-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Uribarri J., Cai W., Peppa M., Goodman S., Ferrucci L., Striker G., Vlassara H. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:427–433. doi: 10.1093/gerona/62.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chung S.S., Ho E.C., Lam K.S., Chung S.K. Contribution of polyol pathway to diabetes-induced oxidative stress. J. Am. Soc. Nephrol. 2003;14(Suppl. S3):S233–S236. doi: 10.1097/01.ASN.0000077408.15865.06. [DOI] [PubMed] [Google Scholar]
- 148.Mohamed F., Monge J.C., Gordon A., Cernacek P., Blais D., Stewart D.J. Lack of role for nitric oxide (NO) in the selective destabilization of endothelial NO synthase mRNA by tumor necrosis factor-alpha. Arterioscler. Thromb. Vasc. Biol. 1995;15:52–57. doi: 10.1161/01.ATV.15.1.52. [DOI] [PubMed] [Google Scholar]
- 149.De Keulenaer G.W., Alexander R.W., Ushio-Fukai M., Ishizaka N., Griendling K.K. Tumour necrosis factor alpha activates a p22phox-based NADH oxidase in vascular smooth muscle. Biochem. J. 1998;329(Pt 3):653–657. doi: 10.1042/bj3290653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pfaffe T., Cooper-White J., Beyerlein P., Kostner K., Punyadeera C. Diagnostic potential of saliva: Current state and future applications. Clin. Chem. 2011;57:675–687. doi: 10.1373/clinchem.2010.153767. [DOI] [PubMed] [Google Scholar]
- 151.Lee Y.H., Wong D.T. Saliva: An emerging biofluid for early detection of diseases. Am. J. Dent. 2009;22:241–248. [PMC free article] [PubMed] [Google Scholar]
- 152.Su H., Velly A.M., Salah M.H., Benarroch M., Trifiro M., Schipper H.M., Gornitsky M. Altered redox homeostasis in human diabetes saliva. J. Oral Pathol. Med. 2012;41:235–241. doi: 10.1111/j.1600-0714.2011.01092.x. [DOI] [PubMed] [Google Scholar]
- 153.Gumus P., Buduneli N., Cetinkalp S., Hawkins S.I., Renaud D., Kinane D.F., Scott D.A. Salivary antioxidants in patients with type 1 or 2 diabetes and inflammatory periodontal disease: A case-control study. J. Periodontol. 2009;80:1440–1446. doi: 10.1902/jop.2009.090159. [DOI] [PubMed] [Google Scholar]
- 154.Reznick A.Z., Shehadeh N., Shafir Y., Nagler R.M. Free radicals related effects and antioxidants in saliva and serum of adolescents with Type 1 diabetes mellitus. Arch. Oral Biol. 2006;51:640–648. doi: 10.1016/j.archoralbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 155.Astaneie F., Afshari M., Mojtahedi A., Mostafalou S., Zamani M.J., Larijani B., Abdollahi M. Total antioxidant capacity and levels of epidermal growth factor and nitric oxide in blood and saliva of insulin-dependent diabetic patients. Arch. Med. Res. 2005;36:376–381. doi: 10.1016/j.arcmed.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 156.Pendyala G., Thomas B., Joshi S.R. Evaluation of Total Antioxidant Capacity of Saliva in Type 2 Diabetic Patients with and without Periodontal Disease: A Case-Control Study. N. Am. J. Med. Sci. 2013;5:51–57. doi: 10.4103/1947-2714.106208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang J., Schipper H.M., Velly A.M., Mohit S., Gornitsky M. Salivary biomarkers of oxidative stress: A critical review. Free Rad. Biol. Med. 2015;85:95–104. doi: 10.1016/j.freeradbiomed.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 158.United Kingdom Prospective Diabetes Study (UKPDS) 13: Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ. 1995;310:83–88. [PMC free article] [PubMed] [Google Scholar]
- 159.De Caterina R. Endothelial dysfunctions: Common denominators in vascular disease. Curr. Opin. Lipidol. 2000;11:9–23. doi: 10.1097/00041433-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 160.Stehouwer C.D., Lambert J., Donker A.J., van Hinsbergh V.W. Endothelial dysfunction and pathogenesis of diabetic angiopathy. Cardiovasc. Res. 1997;34:55–68. doi: 10.1016/S0008-6363(96)00272-6. [DOI] [PubMed] [Google Scholar]
- 161.Desouza C.V., Bolli G.B., Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010;33:1389–1394. doi: 10.2337/dc09-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Paneni F., Beckman J.A., Creager M.A., Cosentino F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013;34:2436–2443. doi: 10.1093/eurheartj/eht149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Beckman J.A., Paneni F., Cosentino F., Creager M.A. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part II. Eur. Heart J. 2013;34:2444–2452. doi: 10.1093/eurheartj/eht142. [DOI] [PubMed] [Google Scholar]
- 164.John S., Schmieder R.E. Impaired endothelial function in arterial hypertension and hypercholesterolemia: Potential mechanisms and differences. J. Hypertens. 2000;18:363–374. doi: 10.1097/00004872-200018040-00002. [DOI] [PubMed] [Google Scholar]
- 165.Hutchison S. Smoking as a risk factor for endothelial dysfunction. Can. J. Cardiol. 1998;14:20D–22D. [PubMed] [Google Scholar]
- 166.Arcaro G., Zamboni M., Rossi L., Turcato E., Covi G., Armellini F., Bosello O., Lechi A. Body fat distribution predicts the degree of endothelial dysfunction in uncomplicated obesity. Int. J. Obes. Relat. Metab. Disord. 1999;23:936–942. doi: 10.1038/sj.ijo.0801022. [DOI] [PubMed] [Google Scholar]
- 167.Kawano H., Motoyama T., Hirashima O., Hirai N., Miyao Y., Sakamoto T., Kugiyama K., Ogawa H., Yasue H. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J. Am. Coll. Cardiol. 1999;34:146–154. doi: 10.1016/S0735-1097(99)00168-0. [DOI] [PubMed] [Google Scholar]
- 168.Vehkavaara S., Seppala-Lindroos A., Westerbacka J., Groop P.H. Yki-Jarvinen H.in vivo endothelial dysfunction characterizes patients with impaired fasting glucose. Diabetes Care. 1999;22:2055–2060. doi: 10.2337/diacare.22.12.2055. [DOI] [PubMed] [Google Scholar]
- 169.Steinberg H.O., Chaker H., Leaming R., Johnson A., Brechtel G., Baron A.D. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J. Clin. Investig. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Defronzo R.A. Is insulin resistance atherogenic? Possible mechanisms. Atheroscler. Suppl. 2006;7:11–15. doi: 10.1016/j.atherosclerosissup.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 171.Cersosimo E., DeFronzo R.A. Insulin resistance and endothelial dysfunction: The road map to cardiovascular diseases. Diabetes/Metab. Res. Rev. 2006;22:423–436. doi: 10.1002/dmrr.634. [DOI] [PubMed] [Google Scholar]
- 172.Patel T.P., Rawal K., Bagchi A.K., Akolkar G., Bernardes N., Dias D.D., Gupta S., Singal P.K. Insulin resistance: An additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Fail. Rev. 2016;21:11–23. doi: 10.1007/s10741-015-9515-6. [DOI] [PubMed] [Google Scholar]
- 173.Vanhoutte P.M. Hypercholesterolaemia, atherosclerosis and release of endothelium-derived relaxing factor by aggregating platelets. Eur. Heart J. 1991;12(Suppl.):E:25–E:32. doi: 10.1093/eurheartj/12.suppl_E.25. [DOI] [PubMed] [Google Scholar]
- 174.Vanhoutte P.M., Boulanger C.M. Endothelium-dependent responses in hypertension. Hypertens. Res. 1995;18:87–98. doi: 10.1291/hypres.18.87. [DOI] [PubMed] [Google Scholar]
- 175.Cleland S.J., Sattar N., Petrie J.R., Forouhi N.G., Elliott H.L., Connell J.M. Endothelial dysfunction as a possible link between C-reactive protein levels and cardiovascular disease. Clin. Sci. 2000;98:531–535. doi: 10.1042/cs0980531. [DOI] [PubMed] [Google Scholar]
- 176.Ng C.Y., Kamisah Y., Faizah O., Jaarin K. The role of repeatedly heated soybean oil in the development of hypertension in rats: Association with vascular inflammation. Int. J. Exp. Pathol. 2012;93:377–387. doi: 10.1111/j.1365-2613.2012.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Becker L., Prado K., Foppa M., Martinelli N., Aguiar C., Furian T., Clausell N., Rohde L.E. Endothelial dysfunction assessed by brachial artery ultrasound in severe sepsis and septic shock. J. Crit. Care. 2012;27:316 e9–316 e14. doi: 10.1016/j.jcrc.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 178.Ridker P.M., Cannon C.P., Morrow D., Rifai N., Rose L.M., McCabe C.H., Pfeffer M.A., Braunwald E. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 179.Henry R.M., Ferreira I., Kostense P.J., Dekker J.M., Nijpels G., Heine R.J., Kamp O., Bouter L.M., Stehouwer C.D. Type 2 diabetes is associated with impaired endothelium-dependent, flow-mediated dilation, but impaired glucose metabolism is not; The Hoorn Study. Atherosclerosis. 2004;174:49–56. doi: 10.1016/j.atherosclerosis.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 180.Esser N., Paquot N., Scheen A.J. Inflammatory markers and cardiometabolic diseases. Acta Clin. Belg. 2015;70:193–199. doi: 10.1179/2295333715Y.0000000004. [DOI] [PubMed] [Google Scholar]
- 181.Busse R., Fleming I. Endothelial dysfunction in atherosclerosis. J. Vasc. Res. 1996;33:181–194. doi: 10.1159/000159147. [DOI] [PubMed] [Google Scholar]
- 182.Barton M. Endothelial dysfunction and atherosclerosis: Endothelin receptor antagonists as novel therapeutics. Curr. Hypertens. Rep. 2000;2:84–91. doi: 10.1007/s11906-000-0064-5. [DOI] [PubMed] [Google Scholar]
- 183.Bousette N., Giaid A. Endothelin-1 in atherosclerosis and other vasculopathies. Can. J. Physiol. Pharmacol. 2003;81:578–587. doi: 10.1139/y03-010. [DOI] [PubMed] [Google Scholar]
- 184.Tabas I., Garcia-Cardena G., Owens G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015;209:13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Celermajer D.S., Sorensen K.E., Gooch V.M., Spiegelhalter D.J., Miller O.I., Sullivan I.D., Lloyd J.K., Deanfield J.E. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-F. [DOI] [PubMed] [Google Scholar]
- 186.Bonetti P.O., Lerman L.O., Lerman A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003;23:168–175. doi: 10.1161/01.ATV.0000051384.43104.FC. [DOI] [PubMed] [Google Scholar]
- 187.Andrawis N., Jones D.S., Abernethy D.R. Aging is associated with endothelial dysfunction in the human forearm vasculature. J. Am. Geriatr. Soc. 2000;48:193–198. [PubMed] [Google Scholar]
- 188.Fornoni A., Raij L. Metabolic syndrome and endothelial dysfunction. Curr. Hypertens. Rep. 2005;7:88–95. doi: 10.1007/s11906-005-0080-6. [DOI] [PubMed] [Google Scholar]
- 189.Ahirwar A.K., Jain A., Singh A., Goswami B., Bhatnagar M.K., Bhatacharjee J. The study of markers of endothelial dysfunction in metabolic syndrome. Horm. Mol. Biol. Clin. Investig. 2015;24:131–136. doi: 10.1515/hmbci-2015-0039. [DOI] [PubMed] [Google Scholar]
- 190.Angulo J., Cuevas P., Fernandez A., Gabancho S., Allona A., Martin-Morales A., Moncada I., Videla S., de Tejada I.S. Diabetes impairs endothelium-dependent relaxation of human penile vascular tissues mediated by NO and EDHF. Biochem. Biophys. Res. Commun. 2003;312:1202–1208. doi: 10.1016/j.bbrc.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 191.Von Scholten B.J., Reinhard H., Hansen T.W., Schalkwijk C.G., Stehouwer C., Parving H.H., Jacobsen P.K., Rossing P. Markers of inflammation and endothelial dysfunction are associated with incident cardiovascular disease, all-cause mortality, and progression of coronary calcification in type 2 diabetic patients with microalbuminuria. J. Diabetes Complicat. 2016;30:248–255. doi: 10.1016/j.jdiacomp.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 192.Pieper G.M. Review of alterations in endothelial nitric oxide production in diabetes: Protective role of arginine on endothelial dysfunction. Hypertension. 1998;31:1047–1060. doi: 10.1161/01.HYP.31.5.1047. [DOI] [PubMed] [Google Scholar]
- 193.De Vriese A.S., Verbeuren T.J., Van de Voorde J., Lameire N.H., Vanhoutte P.M. Endothelial dysfunction in diabetes. Br. J. Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nakagawa T., Tanabe K., Croker B.P., Johnson R.J., Grant M.B., Kosugi T., Li Q. Endothelial dysfunction as a potential contributor in diabetic nephropathy. Nat. Rev. Nephrol. 2011;7:36–44. doi: 10.1038/nrneph.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bianchi E., Ripandelli G., Taurone S., Feher J., Plateroti R., Kovacs I., Magliulo G., Orlando M.P., Micera A., Battaglione E., et al. Age and diabetes related changes of the retinal capillaries: An ultrastructural and immunohistochemical study. Int. J. Immunopathol. Pharmacol. 2016;29:40–53. doi: 10.1177/0394632015615592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Angulo J., Cuevas P., Gabancho S., Gonzalez-Corrochano R., Videla S., Saenz de Tejada I. Enhancement of both EDHF and NO/cGMP pathways is necessary to reverse erectile dysfunction in diabetic rats. J. Sex. Med. 2005;2:341–346. doi: 10.1111/j.1743-6109.2005.20348.x. [DOI] [PubMed] [Google Scholar]
- 197.Triggle C.R., Ding H., Anderson T.J., Pannirselvam M. The endothelium in health and disease: A discussion of the contribution of non-nitric oxide endothelium-derived vasoactive mediators to vascular homeostasis in normal vessels and in type II diabetes. Mol. Cell. Biochem. 2004;263:21–27. doi: 10.1023/B:MCBI.0000041845.62061.c9. [DOI] [PubMed] [Google Scholar]
- 198.Griendling K.K., FitzGerald G.A. Oxidative stress and cardiovascular injury: Part II: Animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- 199.Rajagopalan S., Kurz S., Munzel T., Tarpey M., Freeman B.A., Griendling K.K., Harrison D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Investig. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Miller F.J., Jr., Gutterman D.D., Rios C.D., Heistad D.D., Davidson B.L. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ. Res. 1998;82:1298–1305. doi: 10.1161/01.RES.82.12.1298. [DOI] [PubMed] [Google Scholar]
- 201.Rubanyi G.M., Vanhoutte P.M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am. J. Physiol. 1986;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- 202.Price D.T., Vita J.A., Keaney J.F., Jr. Redox control of vascular nitric oxide bioavailability. Antioxid. Redox Signal. 2000;2:919–935. doi: 10.1089/ars.2000.2.4-919. [DOI] [PubMed] [Google Scholar]
- 203.Munzel T., Daiber A., Ullrich V., Mulsch A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler. Thromb. Vasc. Biol. 2005;25:1551–1557. doi: 10.1161/01.ATV.0000168896.64927.bb. [DOI] [PubMed] [Google Scholar]
- 204.Zou M.H., Cohen R., Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium. 2004;11:89–97. doi: 10.1080/10623320490482619. [DOI] [PubMed] [Google Scholar]
- 205.Ceriello A., Quagliaro L., D’Amico M., Di Filippo C., Marfella R., Nappo F., Berrino L., Rossi F., Giugliano D. Acute hyperglycemia induces nitrotyrosine formation and apoptosis in perfused heart from rat. Diabetes. 2002;51:1076–1082. doi: 10.2337/diabetes.51.4.1076. [DOI] [PubMed] [Google Scholar]
- 206.Tang E.H., Vanhoutte P.M. Endothelial dysfunction: A strategic target in the treatment of hypertension? Pflugers Archiv. 2010;459:995–1004. doi: 10.1007/s00424-010-0786-4. [DOI] [PubMed] [Google Scholar]
- 207.Vanhoutte P.M. Endothelial dysfunction in hypertension. J. hypertens. Suppl. 1996;14:S83–S93. [PubMed] [Google Scholar]
- 208.Vanhoutte P.M. Endothelial dysfunction and atherosclerosis. Eur. Heart J. 1997;18(Suppl. SE):E19–E29. doi: 10.1016/S0195-668X(97)90005-1. [DOI] [PubMed] [Google Scholar]
- 209.Seppo L., Karjala K., Nevala R., Korpela R., Lahteenmaki T., Solatunturi E., Tikkanen M.J., Vapaatalo H. A long-term fish diet modifies the toxic properties of human partially oxidized LDL on vascular preparations in vitro. J. Physiol. Pharmacol. 2000;51:251–265. [PubMed] [Google Scholar]
- 210.Vidal F., Colome C., Martinez-Gonzalez J., Badimon L. Atherogenic concentrations of native low-density lipoproteins down-regulate nitric-oxide-synthase mRNA and protein levels in endothelial cells. Eur. J. Biochem./FEBS. 1998;252:378–384. doi: 10.1046/j.1432-1327.1998.2520378.x. [DOI] [PubMed] [Google Scholar]
- 211.Matz R.L., de Sotomayor M.A., Schott C., Stoclet J.C., Andriantsitohaina R. Vascular bed heterogeneity in age-related endothelial dysfunction with respect to NO and eicosanoids. Br. J. Pharmacol. 2000;131:303–311. doi: 10.1038/sj.bjp.0703568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Matz R.L., Schott C., Stoclet J.C., Andriantsitohaina R. Age-related endothelial dysfunction with respect to nitric oxide, endothelium-derived hyperpolarizing factor and cyclooxygenase products. Physiol. Res./Acad. Scientiarum Bohemoslov. 2000;49:11–18. [PubMed] [Google Scholar]
- 213.Dal-Ros S., Bronner C., Auger C., Schini-Kerth V.B. Red wine polyphenols improve an established aging-related endothelial dysfunction in the mesenteric artery of middle-aged rats: Role of oxidative stress. Biochem. Biophys. Res. Commun. 2012;419:381–387. doi: 10.1016/j.bbrc.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 214.Akbari C.M., LoGerfo F.W. Diabetes and peripheral vascular disease. J. Vasc. Surg. 1999;30:373–384. doi: 10.1016/S0741-5214(99)70154-0. [DOI] [PubMed] [Google Scholar]
- 215.Wolff S.P., Dean R.T. Glucose autoxidation and protein modification. The potential role of 'autoxidative glycosylation' in diabetes. Biochem. J. 1987;245:243–250. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Matsumoto T., Ishida K., Nakayama N., Taguchi K., Kobayashi T., Kamata K. Mechanisms underlying the losartan treatment-induced improvement in the endothelial dysfunction seen in mesenteric arteries from type 2 diabetic rats. Pharmacol. Res. 2010;62:271–281. doi: 10.1016/j.phrs.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 217.Brodsky S.V., Morrishow A.M., Dharia N., Gross S.S., Goligorsky M.S. Glucose scavenging of nitric oxide. Am. J. Physiol. Renal Physiol. 2001;280:F480–F486. doi: 10.1152/ajprenal.2001.280.3.F480. [DOI] [PubMed] [Google Scholar]
- 218.Vlassara H., Uribarri J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. diabetes Rep. 2014;14:453. doi: 10.1007/s11892-013-0453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Khosla U.M., Zharikov S., Finch J.L., Nakagawa T., Roncal C., Mu W., Krotova K., Block E.R., Prabhakar S., Johnson R.J. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 220.Noiri E., Satoh H., Taguchi J., Brodsky S.V., Nakao A., Ogawa Y., Nishijima S., Yokomizo T., Tokunaga K., Fujita T. Association of eNOS Glu298Asp polymorphism with end-stage renal disease. Hypertension. 2002;40:535–540. doi: 10.1161/01.HYP.0000033974.57407.82. [DOI] [PubMed] [Google Scholar]
- 221.Brodsky S.V., Gao S., Li H., Goligorsky M.S. Hyperglycemic switch from mitochondrial nitric oxide to superoxide production in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H2130–H2139. doi: 10.1152/ajpheart.00196.2002. [DOI] [PubMed] [Google Scholar]
- 222.Komers R., Schutzer W.E., Reed J.F., Lindsley J.N., Oyama T.T., Buck D.C., Mader S.L., Anderson S. Altered endothelial nitric oxide synthase targeting and conformation and caveolin-1 expression in the diabetic kidney. Diabetes. 2006;55:1651–1659. doi: 10.2337/db05-1595. [DOI] [PubMed] [Google Scholar]
- 223.Nakata S., Tsutsui M., Shimokawa H., Suda O., Morishita T., Shibata K., Tanimoto A., Nagasaki M., Tasaki H., Sasaguri Y.N., et al. Spontaneous myocardial infarction in mice lacking all nitric oxide synthase isoforms. Circulation. 2008;117:2211–2223. doi: 10.1161/CIRCULATIONAHA.107.742692. [DOI] [PubMed] [Google Scholar]
- 224.Shibata K., Shimokawa H., Yanagihara N., Otsuji Y., Tsutsui M. Nitric oxide synthases and heart failure—Lessons from genetically manipulated mice. J. UOEH. 2013;35:147–158. doi: 10.7888/juoeh.35.147. [DOI] [PubMed] [Google Scholar]
- 225.Khanna S., Singh G.B., Khullar M. Nitric oxide synthases and diabetic cardiomyopathy. Nitric Oxide. 2014;43:29–34. doi: 10.1016/j.niox.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 226.Carnicer R., Crabtree M.J., Sivakumaran V., Casadei B., Kass D.A. Nitric oxide synthases in heart failure. Antioxid. Redox Signal. 2013;18:1078–1099. doi: 10.1089/ars.2012.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Komers R., Lindsley J.N., Oyama T.T., Allison K.M., Anderson S. Role of neuronal nitric oxide synthase (NOS1) in the pathogenesis of renal hemodynamic changes in diabetes. Am. J. Physiol. Renal Physiol. 2000;279:F573–F583. doi: 10.1152/ajprenal.2000.279.3.F573. [DOI] [PubMed] [Google Scholar]
- 228.Watkins C.C., Sawa A., Jaffrey S., Blackshaw S., Barrow R.K., Snyder S.H., Ferris C.D. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J. Clin. Investig. 2000;106:803. doi: 10.1172/JCI8273C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 230.Nathan C., Xie Q.W. Nitric oxide synthases: Roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 231.Weldy C.S., Wilkerson H.W., Larson T.V., Stewart J.A., Kavanagh T.J. DIESEL particulate exposed macrophages alter endothelial cell expression of eNOS, iNOS, MCP1, and glutathione synthesis genes. Toxicol. in Vitro. 2011;25:2064–2073. doi: 10.1016/j.tiv.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jo H., Otani H., Jo F., Shimazu T., Okazaki T., Yoshioka K., Fujita M., Kosaki A., Iwasaka T. Inhibition of nitric oxide synthase uncoupling by sepiapterin improves left ventricular function in streptozotocin-induced diabetic mice. Clin. Exp. Pharmacol. Physiol. 2011;38:485–493. doi: 10.1111/j.1440-1681.2011.05535.x. [DOI] [PubMed] [Google Scholar]
- 233.Pautz A., Art J., Hahn S., Nowag S., Voss C., Kleinert H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide. 2010;23:75–93. doi: 10.1016/j.niox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 234.Nagareddy P.R., Soliman H., Lin G., Rajput P.S., Kumar U., McNeill J.H., MacLeod K.M. Selective inhibition of protein kinase C beta(2) attenuates inducible nitric oxide synthase-mediated cardiovascular abnormalities in streptozotocin-induced diabetic rats. Diabetes. 2009;58:2355–2364. doi: 10.2337/db09-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Soliman H., Gador A., Lu Y.H., Lin G., Bankar G., MacLeod K.M. Diabetes-induced increased oxidative stress in cardiomyocytes is sustained by a positive feedback loop involving Rho kinase and PKCbeta2. Am. J. Physiol. Heart Circ. Physiol. 2012;303:H989–H1000. doi: 10.1152/ajpheart.00416.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lei S., Li H., Xu J., Liu Y., Gao X., Wang J., Ng K.F., Lau W.B., Ma X.L., Rodrigues B., et al. Hyperglycemia-induced protein kinase C beta2 activation induces diastolic cardiac dysfunction in diabetic rats by impairing caveolin-3 expression and Akt/eNOS signaling. Diabetes. 2013;62:2318–2328. doi: 10.2337/db12-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Feletou M., Vanhoutte P.M. EDHF: New therapeutic targets? Pharmacol. Res. 2004;49:565–580. doi: 10.1016/j.phrs.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 238.Mauricio M.D., Aldasoro M., Ortega J., Vila J.M. Endothelial dysfunction in morbid obesity. Curr. Pharm. Des. 2013;19:5718–5729. doi: 10.2174/1381612811319320007. [DOI] [PubMed] [Google Scholar]
- 239.Rvdw I.L., Bietiger W., Seyfritz E., Peronet C., Pinget M., Jeandidier N., Maillard E., Marchioni E., Sigrist S., Dal S. High-fructose and high-fat diet-induced disorders in rats: Impact on diabetes risk, hepatic and vascular complications. Nutr. Metab. 2015 doi: 10.1186/s12986-016-0074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Matsumoto T., Kobayashi T., Kamata K. Mechanisms underlying the impaired EDHF-type relaxation response in mesenteric arteries from Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Eur. J. Pharmacol. 2006;538:132–140. doi: 10.1016/j.ejphar.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 241.Burnham M.P., Johnson I.T., Weston A.H. Impaired small-conductance Ca2+-activated K+ channel-dependent EDHF responses in Type II diabetic ZDF rats. Br. J. Pharmacol. 2006;148:434–441. doi: 10.1038/sj.bjp.0706748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Agouni A., Lagrue-Lak-Hal A.H., Mostefai H.A., Tesse A., Mulder P., Rouet P., Desmoulin F., Heymes C., Martinez M.C., Andriantsitohaina R. Red wine polyphenols prevent metabolic and cardiovascular alterations associated with obesity in Zucker fatty rats (Fa/Fa) PLoS ONE. 2009;4:e5557. doi: 10.1371/journal.pone.0005557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Brondum E., Kold-Petersen H., Simonsen U., Aalkjaer C. NS309 restores EDHF-type relaxation in mesenteric small arteries from type 2 diabetic ZDF rats. Br. J. Pharmacol. 2010;159:154–165. doi: 10.1111/j.1476-5381.2009.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Matsumoto T., Noguchi E., Ishida K., Nakayama N., Kobayashi T., Kamata K. Cilostazol improves endothelial dysfunction by increasing endothelium-derived hyperpolarizing factor response in mesenteric arteries from Type 2 diabetic rats. Eur. J. Pharmacol. 2008;599:102–109. doi: 10.1016/j.ejphar.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 245.Tang E.H., Vanhoutte P.M. Prostanoids and reactive oxygen species: Team players in endothelium-dependent contractions. Pharmacol. Ther. 2009;122:140–149. doi: 10.1016/j.pharmthera.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 246.Vanhoutte P.M., Tang E.H. Endothelium-dependent contractions: when a good guy turns bad! J. Physiol. 2008;586(Pt 22):5295–5304. doi: 10.1113/jphysiol.2008.161430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Basuli D., Stevens R.G., Torti F.M., Torti S.V. Epidemiological associations between iron and cardiovascular disease and diabetes. Front. Pharmacol. 2014;5:117. doi: 10.3389/fphar.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Brissot P., Ropert M., Le Lan C., Loreal O. Non-transferrin bound iron: A key role in iron overload and iron toxicity. Biochimica et Biophysica Acta. 2012;1820:403–410. doi: 10.1016/j.bbagen.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 249.Aljwaid H., White D.L., Collard K.J., Moody A.J., Pinkney J.H. Non-transferrin-bound iron is associated with biomarkers of oxidative stress, inflammation and endothelial dysfunction in type 2 diabetes. J. Diabetes Complicat. 2015;29:943–949. doi: 10.1016/j.jdiacomp.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 250.Sulieman M., Asleh R., Cabantchik Z.I., Breuer W., Aronson D., Suleiman A., Miller-Lotan R., Hammerman H., Levy A.P. Serum chelatable redox-active iron is an independent predictor of mortality after myocardial infarction in individuals with diabetes. Diabetes Care. 2004;27:2730–2732. doi: 10.2337/diacare.27.11.2730. [DOI] [PubMed] [Google Scholar]
- 251.Lee D.H., Liu D.Y., Jacobs D.R., Jr., Shin H.R., Song K., Lee I.K., Kim B., Hider R.C. Common presence of non-transferrin-bound iron among patients with type 2 diabetes. Diabetes Care. 2006;29:1090–1095. doi: 10.2337/dc05-2471. [DOI] [PubMed] [Google Scholar]
- 252.Leoncini S., Rossi V., Signorini C., Tanganelli I., Comporti M., Ciccoli L. Oxidative stress, erythrocyte ageing and plasma non-protein-bound iron in diabetic patients. Free Radic. Res. 2008;42:716–724. doi: 10.1080/10715760802317655. [DOI] [PubMed] [Google Scholar]
- 253.Vinchi F., Muckenthaler M.U., Da Silva M.C., Balla G., Balla J., Jeney V. Atherogenesis and iron: From epidemiology to cellular level. Front. Pharmacol. 2014;5:94. doi: 10.3389/fphar.2014.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Balla J., Vercellotti G.M., Jeney V., Yachie A., Varga Z., Eaton J.W., Balla G. Heme, heme oxygenase and ferritin in vascular endothelial cell injury. Mol. Nutr. Food Res. 2005;49:1030–1043. doi: 10.1002/mnfr.200500076. [DOI] [PubMed] [Google Scholar]
- 255.Kumar S., Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 2005;157:175–188. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 256.Morris C.R. Mechanisms of vasculopathy in sickle cell disease and thalassemia. Hematol. Am. Soc. Hematol. Educ. Program. 2008:177–185. doi: 10.1182/asheducation-2008.1.177. [DOI] [PubMed] [Google Scholar]
- 257.Hess K. The vulnerable blood. Coagulation and clot structure in diabetes mellitus. Hamostaseologie. 2015;35:25–33. doi: 10.5482/HAMO-14-09-0039. [DOI] [PubMed] [Google Scholar]
- 258.Vinchi F., Tolosano E. Therapeutic approaches to limit hemolysis-driven endothelial dysfunction: Scavenging free heme to preserve vasculature homeostasis. Oxid. Med. Cell. Longev. 2013;2013:396527. doi: 10.1155/2013/396527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Bonnefont-Rousselot D.T.P., Delattre J. Free radical and antioxidants (article in French) In: Jardillier J.C., editor. Biochimie Pathologique Aspects moléculaires et cellulaires JDelattre, GDurand. Médecine-Sciences; Flammarion, France: 2003. p. 317. [Google Scholar]
- 260.Esposito K., Maiorino M.I., Petrizzo M., Bellastella G., Giugliano D. The effects of a Mediterranean diet on the need for diabetes drugs and remission of newly diagnosed type 2 diabetes: Follow-up of a randomized trial. Diabetes Care. 2014;37:1824–1830. doi: 10.2337/dc13-2899. [DOI] [PubMed] [Google Scholar]
- 261.Salas-Salvado J., Bullo M., Estruch R., Ros E., Covas M.I., Ibarrola-Jurado N., Corella D., Aros F., Gomez-Gracia E., Ruiz-Gutierrez V.R., et al. Prevention of diabetes with Mediterranean diets: A subgroup analysis of a randomized trial. Ann. Intern. Med. 2014;160:1–10. doi: 10.7326/M13-1725. [DOI] [PubMed] [Google Scholar]
- 262.Kowluru R.A., Kowluru V., Xiong Y., Ho Y.S. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Rad. Biol. Med. 2006;41:1191–1196. doi: 10.1016/j.freeradbiomed.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 263.Zhang Y., Wada J., Hashimoto I., Eguchi J., Yasuhara A., Kanwar Y.S., Shikata K., Makino H. Therapeutic approach for diabetic nephropathy using gene delivery of translocase of inner mitochondrial membrane 44 by reducing mitochondrial superoxide production. J. Am. Soc. Nephrol. JASN. 2006;17:1090–1101. doi: 10.1681/ASN.2005111148. [DOI] [PubMed] [Google Scholar]
- 264.DeRubertis F.R., Craven P.A., Melhem M.F. Acceleration of diabetic renal injury in the superoxide dismutase knockout mouse: Effects of tempol. Metabolism. 2007;56:1256–1264. doi: 10.1016/j.metabol.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 265.Vincent A.M., Russell J.W., Sullivan K.A., Backus C., Hayes J.M., McLean L.L., Feldman E.L. SOD2 protects neurons from injury in cell culture and animal models of diabetic neuropathy. Exp. Neurol. 2007;208:216–227. doi: 10.1016/j.expneurol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Shen X., Zheng S., Metreveli N.S., Epstein P.N. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes. 2006;55:798–805. doi: 10.2337/diabetes.55.03.06.db05-1039. [DOI] [PubMed] [Google Scholar]
- 267.Di Tomo P., Canali R., Ciavardelli D., di Silvestre S., de Marco A., Giardinelli A., Pipino C., di Pietro N., Virgili F., Pandolfi A. Beta-Carotene and lycopene affect endothelial response to TNF-alpha reducing nitro-oxidative stress and interaction with monocytes. Mol. Nutr. Food Res. 2012;56:217–227. doi: 10.1002/mnfr.201100500. [DOI] [PubMed] [Google Scholar]
- 268.Alissa E.M., Ferns G.A. Functional foods and nutraceuticals in the primary prevention of cardiovascular diseases. J. Nutr. Metab. 2012;2012:569486. doi: 10.1155/2012/569486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Chanwitheesuk A.T.A., Rakariyatham N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005;92:491–497. doi: 10.1016/j.foodchem.2004.07.035. [DOI] [Google Scholar]
- 270.Dixit P.P., Devasagayam T.P., Ghaskadbi S. Formulated antidiabetic preparation Syndrex has a strong antioxidant activity. Eur. J. Pharmacol. 2008;581:216–225. doi: 10.1016/j.ejphar.2007.11.052. [DOI] [PubMed] [Google Scholar]
- 271.Qiang G., Wenzhai C., Huan Z., Yuxia Z., Dongdong Y., Sen Z., Qiu C. Effect of Sancaijiangtang on plasma nitric oxide and endothelin-1 levels in patients with type 2 diabetes mellitus and vascular dementia: A single-blind randomized controlled trial. J. Tradit. Chin. Med. 2015;35:375–380. doi: 10.1016/S0254-6272(15)30112-6. [DOI] [PubMed] [Google Scholar]
- 272.Mizutani K., Tanaka O. Stevia, the genus Stevia. Medicinal and aromatic plants—industrial profiles. In: Kinghorn A.D., editor. Use of Stevia Rebaudiana Sweeteners in Japan. Volume 19. Taylor and Francis; London, UK: 2002. pp. 178–195. [Google Scholar]
- 273.Kim J., Choi C.H. Stevia, the genus Stevia. Medicinal and aromatic plants—Industrial profiles. In: Kinghorn A.D., editor. Use of Stevioside and Cultivation. Departement of medicinal chemistry and pharmacognosy, University of Illinois at Chicago; Chicago, IL, USA: 2002. [Google Scholar]
- 274.Jeppesen P.B., Gregersen S., Alstrup K.K., Hermansen K. Stevioside induces antihyperglycaemic, insulinotropic and glucagonostatic effectsin vivo: Studies in the diabetic Goto-Kakizaki (GK) rats. Phytomedicine. 2002;9:9–14. doi: 10.1078/0944-7113-00081. [DOI] [PubMed] [Google Scholar]
- 275.Gregersen S., Jeppesen P.B., Holst J.J., Hermansen K. Antihyperglycemic effects of stevioside in type 2 diabetic subjects. Metabolism. 2004;53:73–76. doi: 10.1016/j.metabol.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 276.Barriocanal L.A., Palacios M., Benitez G., Benitez S., Jimenez J.T., Jimenez N., Rojas V. Apparent lack of pharmacological effect of steviol glycosides used as sweeteners in humans. A pilot study of repeated exposures in some normotensive and hypotensive individuals and in Type 1 and Type 2 diabetics. Regul. Toxicol. Pharmacol. RTP. 2008;51:37–41. doi: 10.1016/j.yrtph.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 277.Rahimi R., Nikfar S., Larijani B., Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005;59:365–373. doi: 10.1016/j.biopha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 278.Pari L., Venkateswaran S. Protective effect of Coccinia indica on changes in the fatty acid composition in streptozotocin induced diabetic rats. Die Pharm. 2003;58:409–412. [PubMed] [Google Scholar]
- 279.Venkateswaran S., Pari L. Effect of Coccinia indica leaf extract on plasma antioxidants in streptozotocin- induced experimental diabetes in rats. Phytother. Res. PTR. 2003;17:605–608. doi: 10.1002/ptr.1195. [DOI] [PubMed] [Google Scholar]
- 280.Venkateswaran S., Pari L. Effect of Coccinia indica leaves on antioxidant status in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2003;84:163–168. doi: 10.1016/S0378-8741(02)00294-5. [DOI] [PubMed] [Google Scholar]
- 281.Modak M., Dixit P., Londhe J., Ghaskadbi S., Devasagayam T.P. Indian herbs and herbal drugs used for the treatment of diabetes. J. Clin. Biochem. Nutr. 2007;40:163–173. doi: 10.3164/jcbn.40.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Qiao W., Zhao C., Qin N., Zhai H.Y., Duan H.Q. Identification of trans-tiliroside as active principle with anti-hyperglycemic, anti-hyperlipidemic and antioxidant effects from Potentilla chinesis. J. Ethnopharmacol. 2011;135:515–521. doi: 10.1016/j.jep.2011.03.062. [DOI] [PubMed] [Google Scholar]
- 283.Shivanna N., Naika M., Khanum F., Kaul V.K. Antioxidant, anti-diabetic and renal protective properties of Stevia rebaudiana. J. Diabetes Complicat. 2013;27:103–113. doi: 10.1016/j.jdiacomp.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 284.BelHadj S., Hentati O., Elfeki A., Hamden K. Inhibitory activities of Ulva lactuca polysaccharides on digestive enzymes related to diabetes and obesity. Arch. Physiol. Biochem. 2013;119:81–87. doi: 10.3109/13813455.2013.775159. [DOI] [PubMed] [Google Scholar]
- 285.Frati Munari A.C., Quiroz Lazaro J.L., Altamirano Bustamante P., Banales Ham M., Islas Andrade S., Ariza Andraca C.R. The effect of various doses of nopal (Opuntia streptacantha Lemaire) on the glucose tolerance test in healthy individuals. Archivos de Investigacion Medica. 1988;19:143–148. [PubMed] [Google Scholar]
- 286.Frati A.C., Gordillo B.E., Altamirano P., Ariza C.R., Cortes-Franco R., Chavez-Negrete A. Acute hypoglycemic effect of Opuntia streptacantha Lemaire in NIDDM. Diabetes Care. 1990;13:455–456. doi: 10.2337/diacare.13.4.455. [DOI] [PubMed] [Google Scholar]
- 287.Frati-Munari A.C., Gordillo B.E., Altamirano P., Ariza C.R. Hypoglycemic effect of Opuntia streptacantha Lemaire in NIDDM. Diabetes Care. 1988;11:63–66. doi: 10.2337/diacare.11.1.63. [DOI] [PubMed] [Google Scholar]
- 288.Nishioka K., Hidaka T., Nakamura S., Umemura T., Jitsuiki D., Soga J., Goto C., Chayama K., Yoshizumi M., Higashi Y. Pycnogenol, French maritime pine bark extract, augments endothelium-dependent vasodilation in humans. Hypertens. Res. 2007;30:775–780. doi: 10.1291/hypres.30.775. [DOI] [PubMed] [Google Scholar]
- 289.Gulati O.P. Pycnogenol(R) in Metabolic Syndrome and Related Disorders. Phytother. Res. PTR. 2015;29:949–968. doi: 10.1002/ptr.5341. [DOI] [PubMed] [Google Scholar]
- 290.Mnafgui K., Kchaou M., Hamden K., Derbali F., Slama S., Nasri M., Salah H.B., Allouche N., Elfeki A. Inhibition of carbohydrate and lipid digestive enzymes activities by Zygophyllum album extracts: Effect on blood and pancreas inflammatory biomarkers in alloxan-induced diabetic rats. J. Physiol. Biochem. 2014;70:93–106. doi: 10.1007/s13105-013-0284-1. [DOI] [PubMed] [Google Scholar]
- 291.Coskun O., Kanter M., Korkmaz A., Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol. Res. 2005;51:117–123. doi: 10.1016/j.phrs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 292.Ohnishi M., Matuo T., Tsuno T., Hosoda A., Nomura E., Taniguchi H., Sasaki H., Morishita H. Antioxidant activity and hypoglycemic effect of ferulic acid in STZ-induced diabetic mice and KK-Ay mice. BioFactors. 2004;21:315–319. doi: 10.1002/biof.552210161. [DOI] [PubMed] [Google Scholar]
- 293.Liu S., Serdula M., Janket S.J., Cook N.R., Sesso H.D., Willett W.C., Manson J.E., Buring J.E. A prospective study of fruit and vegetable intake and the risk of type 2 diabetes in women. Diabetes Care. 2004;27:2993–2996. doi: 10.2337/diacare.27.12.2993. [DOI] [PubMed] [Google Scholar]
- 294.Ford E.S., Mokdad A.H. Fruit and vegetable consumption and diabetes mellitus incidence among U.S. adults. Prev. Med. 2001;32:33–39. doi: 10.1006/pmed.2000.0772. [DOI] [PubMed] [Google Scholar]
- 295.Martinez-Gonzalez M.A., de la Fuente-Arrillaga C., Nunez-Cordoba J.M., Basterra-Gortari F.J., Beunza J.J., Vazquez Z., Benito S., Tortosa A., Bes-Rastrollo M. Adherence to Mediterranean diet and risk of developing diabetes: Prospective cohort study. BMJ. 2008;336:1348–1351. doi: 10.1136/bmj.39561.501007.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Carter P., Gray L.J., Troughton J., Khunti K., Davies M.J. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: Systematic review and meta-analysis. BMJ. 2010;341:c4229. doi: 10.1136/bmj.c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Basu A., Wilkinson M., Penugonda K., Simmons B., Betts N.M., Lyons T.J. Freeze-dried strawberry powder improves lipid profile and lipid peroxidation in women with metabolic syndrome: Baseline and post intervention effects. Nutr. J. 2009;8:43. doi: 10.1186/1475-2891-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Burton W.N., Chen C.Y., Schultz A.B., Edington D.W. The association between achieving low-density lipoprotein cholesterol (LDL-C) goal and statin treatment in an employee population. Popul. Health Manag. 2010;13:1–8. doi: 10.1089/pop.2009.0020. [DOI] [PubMed] [Google Scholar]
- 299.Upritchard J.E., Sutherland W.H., Mann J.I. Effect of supplementation with tomato juice, vitamin E, and vitamin C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diabetes Care. 2000;23:733–738. doi: 10.2337/diacare.23.6.733. [DOI] [PubMed] [Google Scholar]
- 300.Najafian M., Jahromi M.Z., Nowroznejhad M.J., Khajeaian P., Kargar M.M., Sadeghi M., Arasteh A. Phloridzin reduces blood glucose levels and improves lipids metabolism in streptozotocin-induced diabetic rats. Mol. Biol. Rep. 2012;39:5299–5306. doi: 10.1007/s11033-011-1328-7. [DOI] [PubMed] [Google Scholar]
- 301.Hafizur R.M., Kabir N., Chishti S. Asparagus officinalis extract controls blood glucose by improving insulin secretion and beta-cell function in streptozotocin-induced type 2 diabetic rats. Br. J. Nutr. 2012;108:1586–1595. doi: 10.1017/S0007114511007148. [DOI] [PubMed] [Google Scholar]
- 302.Lugasi A., Blazovics A., Hagymasi K., Kocsis I., Kery A. Antioxidant effect of squeezed juice from black radish (Raphanus sativus L. var niger) in alimentary hyperlipidaemia in rats. Phytother. Res. PTR. 2005;19:587–591. doi: 10.1002/ptr.1655. [DOI] [PubMed] [Google Scholar]
- 303.Panda S., Kar A. Antidiabetic and antioxidative effects of Annona squamosa leaves are possibly mediated through quercetin-3-O-glucoside. BioFactors. 2007;31:201–210. doi: 10.1002/biof.5520310307. [DOI] [PubMed] [Google Scholar]
- 304.Dixit Y., Kar A. Protective role of three vegetable peels in alloxan induced diabetes mellitus in male mice. Plant Foods Hum. Nutr. 2010;65:284–289. doi: 10.1007/s11130-010-0175-3. [DOI] [PubMed] [Google Scholar]
- 305.Eidi A., Eidi M., Esmaeili E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2006;13:624–629. doi: 10.1016/j.phymed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 306.Baluchnejadmojarad T., Roghani M. Garlic extract attenuates time-dependent changes in the reactivity of isolated aorta in streptozotocin-diabetic rats. Life Sci. 2003;73:2281–2289. doi: 10.1016/S0024-3205(03)00604-0. [DOI] [PubMed] [Google Scholar]
- 307.El-Demerdash F.M., Yousef M.I., Zoheir M.A. Stannous chloride induces alterations in enzyme activities, lipid peroxidation and histopathology in male rabbit: Antioxidant role of vitamin C. Food Chem. Toxicol. 2005;43:1743–1752. doi: 10.1016/j.fct.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 308.Kempaiah R.K., Srinivasan K. Influence of dietary curcumin, capsaicin and garlic on the antioxidant status of red blood cells and the liver in high-fat-fed rats. Ann. Nutr. Metab. 2004;48:314–320. doi: 10.1159/000081198. [DOI] [PubMed] [Google Scholar]
- 309.Pari L., Venkateswaran S. Effect of an aqueous extract of Phaseolus vulgaris on the properties of tail tendon collagen of rats with streptozotocin-induced diabetes. Braz. J. Med. Biol. Res. 2003;36:861–870. doi: 10.1590/S0100-879X2003000700006. [DOI] [PubMed] [Google Scholar]
- 310.Babu P.S., Srinivasan K. Influence of dietary capsaicin and onion on the metabolic abnormalities associated with streptozotocin induced diabetes mellitus. Mol. Cell. Biochem. 1997;175:49–57. doi: 10.1023/A:1006881027166. [DOI] [PubMed] [Google Scholar]
- 311.Kang M.J., Kim J.H., Choi H.N., Kim M.J., Han J.H., Lee J.H., Kim J.I. Hypoglycemic effects of Welsh onion in an animal model of diabetes mellitus. Nutr. Res. Practice. 2010;4:486–491. doi: 10.4162/nrp.2010.4.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Azuma K., Minami Y., Ippoushi K., Terao J. Lowering effects of onion intake on oxidative stress biomarkers in streptozotocin-induced diabetic rats. J. Clin. Biochem. Nutr. 2007;40:131–140. doi: 10.3164/jcbn.40.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Yamamoto Y., Aoyama S., Hamaguchi N., Rhi G.S. Antioxidative and antihypertensive effects of Welsh onion on rats fed with a high-fat high-sucrose diet. Biosci. Biotechnol. Biochem. 2005;69:1311–1317. doi: 10.1271/bbb.69.1311. [DOI] [PubMed] [Google Scholar]
- 314.Kataya H.A., Hamza A.A. Red Cabbage (Brassica oleracea) Ameliorates Diabetic Nephropathy in Rats. Evid.-based Complement. Altern. Med. eCAM. 2008;5:281–287. doi: 10.1093/ecam/nem029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Jalal R., Bagheri S.M., Moghimi A., Rasuli M.B. Hypoglycemic effect of aqueous shallot and garlic extracts in rats with fructose-induced insulin resistance. J. Clin. Biochem. Nutr. 2007;41:218–223. doi: 10.3164/jcbn.2007031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Parelman M.A., Storms D.H., Kirschke C.P., Huang L., Zunino S.J. Dietary strawberry powder reduces blood glucose concentrations in obese and lean C57BL/6 mice, and selectively lowers plasma C-reactive protein in lean mice. Br. J. Nutr. 2012;108:1789–1799. doi: 10.1017/S0007114512000037. [DOI] [PubMed] [Google Scholar]
- 317.Ali M.M., Agha F.G. Amelioration of streptozotocin-induced diabetes mellitus, oxidative stress and dyslipidemia in rats by tomato extract lycopene. Scand. J. Clin. Lab. Investig. 2009;69:371–379. doi: 10.1080/00365510802658473. [DOI] [PubMed] [Google Scholar]
- 318.Lundberg J.O., Feelisch M., Bjorne H., Jansson E.A., Weitzberg E. Cardioprotective effects of vegetables: Is nitrate the answer? Nitric Oxide. 2006;15:359–362. doi: 10.1016/j.niox.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 319.Carlstrom M., Larsen F.J., Nystrom T., Hezel M., Borniquel S., Weitzberg E., Lundberg J.O. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc. Natl. Acad. Sci. USA. 2010;107:17716–17720. doi: 10.1073/pnas.1008872107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Gilchrist M., Benjamin N. Vegetables and diabetes. Is nitrate the answer? BMJ. 2010;341:c5306. doi: 10.1136/bmj.c5306. [DOI] [PubMed] [Google Scholar]
- 321.Machha A., Schechter A.N. Dietary nitrite and nitrate: A review of potential mechanisms of cardiovascular benefits. Eur. J. Nutr. 2011;50:293–303. doi: 10.1007/s00394-011-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Machha A., Schechter A.N. Inorganic nitrate: A major player in the cardiovascular health benefits of vegetables? Nutr. Rev. 2012;70:367–372. doi: 10.1111/j.1753-4887.2012.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Bryan N.S. Cardioprotective actions of nitrite therapy and dietary considerations. Front. Biosci. 2009;14:4793–4808. doi: 10.2741/3568. [DOI] [PubMed] [Google Scholar]
- 324.Stokes K.Y., Dugas T.R., Tang Y., Garg H., Guidry E., Bryan N.S. Dietary nitrite prevents hypercholesterolemic microvascular inflammation and reverses endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H1281–H1288. doi: 10.1152/ajpheart.01291.2008. [DOI] [PubMed] [Google Scholar]
- 325.Dejam A., Hunter C.J., Tremonti C., Pluta R.M., Hon Y.Y., Grimes G., Partovi K., Pelletier M.M., Oldfield E.H., Cannon R.O., 3rd, et al. Nitrite infusion in humans and nonhuman primates: Endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 326.Bryan N.S., Calvert J.W., Gundewar S., Lefer D.J. Dietary nitrite restores NO homeostasis and is cardioprotective in endothelial nitric oxide synthase-deficient mice. Free Rad. Biol. Med. 2008;45:468–474. doi: 10.1016/j.freeradbiomed.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Webb A.J., Patel N., Loukogeorgakis S., Okorie M., Aboud Z., Misra S., Rashid R., Miall P., Deanfield J., Benjamin N., et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Larsen F.J., Ekblom B., Sahlin K., Lundberg J.O., Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N. Engl. J. Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 329.Timimi F.K., Ting H.H., Haley E.A., Roddy M.A., Ganz P., Creager M.A. Vitamin C improves endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. J. Am. Coll. Cardiol. 1998;31:552–557. doi: 10.1016/S0735-1097(97)00536-6. [DOI] [PubMed] [Google Scholar]
- 330.Jain S.K., McVie R., Jaramillo J.J., Palmer M., Smith T., Meachum Z.D., Little R.L. The effect of modest vitamin E supplementation on lipid peroxidation products and other cardiovascular risk factors in diabetic patients. Lipids. 1996;31(Suppl):S87–S90. doi: 10.1007/BF02637057. [DOI] [PubMed] [Google Scholar]
- 331.Engelen W., Keenoy B.M., Vertommen J., De Leeuw I. Effects of long-term supplementation with moderate pharmacologic doses of vitamin E are saturable and reversible in patients with type 1 diabetes. Am. J. Clin. Nutr. 2000;72:1142–1149. doi: 10.1093/ajcn/72.5.1142. [DOI] [PubMed] [Google Scholar]
- 332.Heinonen O.P., Albanes D., Virtamo J., Taylor P.R., Huttunen J.K., Hartman A.M., Haapakoski J., Malila N., Rautalahti M., Ripatti S., et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: Incidence and mortality in a controlled trial. J. Natl. Cancer Inst. 1998;90:440–446. doi: 10.1093/jnci/90.6.440. [DOI] [PubMed] [Google Scholar]
- 333.Lippman S.M., Klein E.A., Goodman P.J., Lucia M.S., Thompson I.M., Ford L.G., Parnes H.L., Minasian L.M., Gaziano J.M., Hartline J.A.P., et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Gaziano J.M., Glynn R.J., Christen W.G., Kurth T., Belanger C., MacFadyen J., Bubes V., Manson J.E., Sesso H.D., Buring J.E. Vitamins E and C in the prevention of prostate and total cancer in men: The Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Miller E.R., 3rd, Pastor-Barriuso R., Dalal D., Riemersma R.A., Appel L.J., Guallar E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 336.Klein E.A., Thompson I.M., Jr., Tangen C.M., Crowley J.J., Lucia M.S., Goodman P.J., Minasian L.M., Ford L.G., Parnes H.L., Gaziano J.M., et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Sacco M., Pellegrini F., Roncaglioni M.C., Avanzini F., Tognoni G., Nicolucci A., Group PPPC Primary prevention of cardiovascular events with low-dose aspirin and vitamin E in type 2 diabetic patients: Results of the Primary Prevention Project (PPP) trial. Diabetes Care. 2003;26:3264–3272. doi: 10.2337/diacare.26.12.3264. [DOI] [PubMed] [Google Scholar]
- 338.Yusuf S., Dagenais G., Pogue J., Bosch J., Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N. Engl. J. Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 339.Gerstein H.C., Bosch J., Pogue J., Taylor D.W., Zinman B., Yusuf S. Rationale and design of a large study to evaluate the renal and cardiovascular effects of an ACE inhibitor and vitamin E in high-risk patients with diabetes. The MICRO-HOPE Study. Microalbuminuria, cardiovascular, and renal outcomes. Heart Outcomes Prevention Evaluation. Diabetes Care. 1996;19:1225–1228. doi: 10.2337/diacare.19.11.1225. [DOI] [PubMed] [Google Scholar]
- 340.Blum S., Vardi M., Brown J.B., Russell A., Milman U., Shapira C., Levy N.S., Miller-Lotan R., Asleh R., Levy A.P. Vitamin E reduces cardiovascular disease in individuals with diabetes mellitus and the haptoglobin 2–2 genotype. Pharmacogenomics. 2010;11:675–684. doi: 10.2217/pgs.10.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Milman U., Blum S., Shapira C., Aronson D., Miller-Lotan R., Anbinder Y., Alshiek J., Bennett L., Kostenko M., Landau M., et al. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2–2 genotype: A prospective double-blinded clinical trial. Arterioscler. Thromb. Vasc. Biol. 2008;28:341–347. doi: 10.1161/ATVBAHA.107.153965. [DOI] [PubMed] [Google Scholar]
- 342.Vardi M., Blum S., Levy A.P. Haptoglobin genotype and cardiovascular outcomes in diabetes mellitus—Natural history of the disease and the effect of vitamin E treatment. Meta-analysis of the medical literature. Eur. J. Intern. Med. 2012;23:628–632. doi: 10.1016/j.ejim.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Paolisso G., D’Amore A., Balbi V., Volpe C., Galzerano D., Giugliano D., Sgambato S., Varricchio M., D'Onofrio F. Plasma vitamin C affects glucose homeostasis in healthy subjects and in non-insulin-dependent diabetics. Am. J. Physiol. 1994;266(2 Pt 1):E261–E268. doi: 10.1152/ajpendo.1994.266.2.E261. [DOI] [PubMed] [Google Scholar]
- 344.Chen H., Karne R.J., Hall G., Campia U., Panza J.A., Cannon R.O., 3rd, Wang Y., Katz A., Levine M., Quon M.J. High-dose oral vitamin C partially replenishes vitamin C levels in patients with Type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistance. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H137–H145. doi: 10.1152/ajpheart.00768.2005. [DOI] [PubMed] [Google Scholar]
- 345.Hirai N., Kawano H., Hirashima O., Motoyama T., Moriyama Y., Sakamoto T., Kugiyama K., Ogawa H., Nakao K., Yasue H. Insulin resistance and endothelial dysfunction in smokers: Effects of vitamin C. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H1172–H1178. doi: 10.1152/ajpheart.2000.279.3.H1172. [DOI] [PubMed] [Google Scholar]
- 346.Kern T.S., Engerman R.L. Comparison of retinal lesions in alloxan-diabetic rats and galactose-fed rats. Curr. Eye Res. 1994;13:863–867. doi: 10.3109/02713689409015087. [DOI] [PubMed] [Google Scholar]
- 347.Hypponen E., Laara E., Reunanen A., Jarvelin M.R., Virtanen S.M. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 348.Stene L.C., Joner G., Norwegian Childhood Diabetes Study G Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: A large, population-based, case-control study. Am. J. Clin. Nutr. 2003;78:1128–1134. doi: 10.1093/ajcn/78.6.1128. [DOI] [PubMed] [Google Scholar]
- 349.Kono K., Fujii H., Nakai K., Goto S., Kitazawa R., Kitazawa S., Shinohara M., Hirata M., Fukagawa M., Nishi S. Anti-oxidative effect of vitamin D analog on incipient vascular lesion in non-obese type 2 diabetic rats. Am. J. Nephrol. 2013;37:167–174. doi: 10.1159/000346808. [DOI] [PubMed] [Google Scholar]
- 350.Reunanen A., Knekt P., Aaran R.K., Aromaa A. Serum antioxidants and risk of non-insulin dependent diabetes mellitus. Eur. J. Clin. Nutr. 1998;52:89–93. doi: 10.1038/sj.ejcn.1600519. [DOI] [PubMed] [Google Scholar]
- 351.Jialal I., Devaraj S., Venugopal S.K. Oxidative stress, inflammation, and diabetic vasculopathies: The role of alpha tocopherol therapy. Free Rad. Res. 2002;36:1331–1336. doi: 10.1080/1071576021000038531. [DOI] [PubMed] [Google Scholar]
- 352.Davie S.J., Gould B.J., Yudkin J.S. Effect of vitamin C on glycosylation of proteins. Diabetes. 1992;41:167–173. doi: 10.2337/diab.41.2.167. [DOI] [PubMed] [Google Scholar]
- 353.Ceriello A., Giugliano D., Quatraro A., Donzella C., Dipalo G., Lefebvre P.J. Vitamin E reduction of protein glycosylation in diabetes. New prospect for prevention of diabetic complications? Diabetes Care. 1991;14:68–72. doi: 10.2337/diacare.14.1.68. [DOI] [PubMed] [Google Scholar]
- 354.Sinclair A.J., Girling A.J., Gray L., Lunec J., Barnett A.H. An investigation of the relationship between free radical activity and vitamin C metabolism in elderly diabetic subjects with retinopathy. Gerontology. 1992;38:268–274. doi: 10.1159/000213339. [DOI] [PubMed] [Google Scholar]
- 355.Caballero B. Vitamin E improves the action of insulin. Nutr. Rev. 1993;51:339–340. doi: 10.1111/j.1753-4887.1993.tb03761.x. [DOI] [PubMed] [Google Scholar]
- 356.Paolisso G., D'Amore A., Galzerano D., Balbi V., Giugliano D., Varricchio M., D'Onofrio F. Daily vitamin E supplements improve metabolic control but not insulin secretion in elderly type II diabetic patients. Diabetes Care. 1993;16:1433–1437. doi: 10.2337/diacare.16.11.1433. [DOI] [PubMed] [Google Scholar]
- 357.Paolisso G., D'Amore A., Giugliano D., Ceriello A., Varricchio M., D’Onofrio F. Pharmacologic doses of vitamin E improve insulin action in healthy subjects and non-insulin-dependent diabetic patients. Am. J. Clin. Nutr. 1993;57:650–656. doi: 10.1093/ajcn/57.5.650. [DOI] [PubMed] [Google Scholar]
- 358.Penckofer S., Schwertz D., Florczak K. Oxidative stress and cardiovascular disease in type 2 diabetes: The role of antioxidants and pro-oxidants. J. Cardiovasc. Nurs. 2002;16:68–85. doi: 10.1097/00005082-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 359.Devaraj S., Jialal I. Alpha tocopherol supplementation decreases serum C-reactive protein and monocyte interleukin-6 levels in normal volunteers and type 2 diabetic patients. Free Rad. Biol. Med. 2000;29:790–792. doi: 10.1016/S0891-5849(00)00420-2. [DOI] [PubMed] [Google Scholar]
- 360.Neri S., Signorelli S.S., Torrisi B., Pulvirenti D., Mauceri B., Abate G., Ignaccolo L., Bordonaro F., Cilio D., Calvagno S., et al. Effects of antioxidant supplementation on postprandial oxidative stress and endothelial dysfunction: A single-blind, 15-day clinical trial in patients with untreated type 2 diabetes, subjects with impaired glucose tolerance, and healthy controls. Clin. Therapeutics. 2005;27:1764–1773. doi: 10.1016/j.clinthera.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 361.Blum S., Vardi M., Levy N.S., Miller-Lotan R., Levy A.P. The effect of vitamin E supplementation on cardiovascular risk in diabetic individuals with different haptoglobin phenotypes. Atherosclerosis. 2010;211:25–27. doi: 10.1016/j.atherosclerosis.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Bursell S.E., Clermont A.C., Aiello L.P., Aiello L.M., Schlossman D.K., Feener E.P., Laffel L., King G.L. High-dose vitamin E supplementation normalizes retinal blood flow and creatinine clearance in patients with type 1 diabetes. Diabetes Care. 1999;22:1245–1251. doi: 10.2337/diacare.22.8.1245. [DOI] [PubMed] [Google Scholar]
- 363.Otero P., Bonet B., Herrera E., Rabano A. Development of atherosclerosis in the diabetic BALB/c mice. Prevention with Vitamin E administration. Atherosclerosis. 2005;182:259–265. doi: 10.1016/j.atherosclerosis.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 364.Crino A., Schiaffini R., Manfrini S., Mesturino C., Visalli N., Anguissola G.B., Suraci C., Pitocco D., Spera S., Corbi S., et al. A randomized trial of nicotinamide and vitamin E in children with recent onset type 1 diabetes (IMDIAB IX) Eur. J. Endocrinol./Eur. Fed. Endocr. Soc. 2004;150:719–724. doi: 10.1530/eje.0.1500719. [DOI] [PubMed] [Google Scholar]
- 365.Cameron N.E., Cotter M.A. The relationship of vascular changes to metabolic factors in diabetes mellitus and their role in the development of peripheral nerve complications. Diabetes/Metab. Rev. 1994;10:189–224. doi: 10.1002/dmr.5610100302. [DOI] [PubMed] [Google Scholar]
- 366.Cameron N.E., Cotter M.A. Neurovascular dysfunction in diabetic rats. Potential contribution of autoxidation and free radicals examined using transition metal chelating agents. J. Clin. Investig. 1995;96:1159–1163. doi: 10.1172/JCI118104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Sheng X.Q., Huang K.X., Xu H.B. Influence of alloxan-induced diabetes and selenite treatment on blood glucose and glutathione levels in mice. J. Trace elem. Med. Biol. 2005;18:261–267. doi: 10.1016/j.jtemb.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 368.Moustafa S.A. Zinc might protect oxidative changes in the retina and pancreas at the early stage of diabetic rats. Toxicol. Appl. Pharmacol. 2004;201:149–155. doi: 10.1016/j.taap.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 369.Czernichow S., Couthouis A., Bertrais S., Vergnaud A.C., Dauchet L., Galan P., Hercberg S. Antioxidant supplementation does not affect fasting plasma glucose in the Supplementation with Antioxidant Vitamins and Minerals (SU.VI.MAX) study in France: Association with dietary intake and plasma concentrations. Am. J. Clin. Nutr. 2006;84:395–359. doi: 10.1093/ajcn/84.1.394. [DOI] [PubMed] [Google Scholar]
- 370.Maritim A., Dene B.A., Sanders R.A., Watkins J.B., 3rd Effects of beta-carotene on oxidative stress in normal and diabetic rats. J. Biochem. Mol. Toxicol. 2002;16:203–208. doi: 10.1002/jbt.10038. [DOI] [PubMed] [Google Scholar]
- 371.Levy Y., Zaltsberg H., Ben-Amotz A., Kanter Y., Aviram M. Dietary supplementation of a natural isomer mixture of beta-carotene inhibits oxidation of LDL derived from patients with diabetes mellitus. Ann. Nutr. Metab. 2000;44:54–60. doi: 10.1159/000012821. [DOI] [PubMed] [Google Scholar]
- 372.Bravo L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 373.Crozier A., Jaganath I.B., Clifford M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009;26:1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 374.Ovaskainen M.L., Torronen R., Koponen J.M., Sinkko H., Hellstrom J., Reinivuo H., Mattila P. Dietary intake and major food sources of polyphenols in Finnish adults. J. Nutr. 2008;138:562–566. doi: 10.1093/jn/138.3.562. [DOI] [PubMed] [Google Scholar]
- 375.Middleton E., Jr., Kandaswami C., Theoharides T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 376.Franzini L., Ardigo D., Valtuena S., Pellegrini N., Del Rio D., Bianchi M.A., Scazzina F., Piatti P.M., Brighenti F., Zavaroni I. Food selection based on high total antioxidant capacity improves endothelial function in a low cardiovascular risk population. Nutr. Metab. Cardiovasc. Dis. NMCD. 2012;22:50–57. doi: 10.1016/j.numecd.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 377.Khan N., Khymenets O., Urpi-Sarda M., Tulipani S., Garcia-Aloy M., Monagas M., Mora-Cubillos X., Llorach R., Andres-Lacueva C. Cocoa polyphenols and inflammatory markers of cardiovascular disease. Nutrients. 2014;6:844–880. doi: 10.3390/nu6020844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Hall W.L., Formanuik N.L., Harnpanich D., Cheung M., Talbot D., Chowienczyk P.J., Sanders T.A. A meal enriched with soy isoflavones increases nitric oxide-mediated vasodilation in healthy postmenopausal women. J. Nutr. 2008;138:1288–1292. doi: 10.1093/jn/138.7.1288. [DOI] [PubMed] [Google Scholar]
- 379.Boban M., Modun D., Music I., Vukovic J., Brizic I., Salamunic I., Obad A., Palada I., Dujic Z. Red wine induced modulation of vascular function: Separating the role of polyphenols, ethanol, and urates. J. Cardiovasc. Pharmacol. 2006;47:695–701. doi: 10.1097/01.fjc.0000211762.06271.ce. [DOI] [PubMed] [Google Scholar]
- 380.Agewall S., Wright S., Doughty R.N., Whalley G.A., Duxbury M., Sharpe N. Does a glass of red wine improve endothelial function? Eur. Heart J. 2000;21:74–78. doi: 10.1053/euhj.1999.1759. [DOI] [PubMed] [Google Scholar]
- 381.Hashimoto M., Kim S., Eto M., Iijima K., Ako J., Yoshizumi M., Akishita M., Kondo K., Itakura H., Hosoda K., et al. Effect of acute intake of red wine on flow-mediated vasodilatation of the brachial artery. Am. J. Cardiol. 2001;88:1457–1460. doi: 10.1016/S0002-9149(01)02137-3. [DOI] [PubMed] [Google Scholar]
- 382.Karatzi K., Papamichael C., Karatzis E., Papaioannou T.G., Voidonikola P.T., Vamvakou G.D., Lekakis J., Zampelas A. Postprandial improvement of endothelial function by red wine and olive oil antioxidants: A synergistic effect of components of the Mediterranean diet. J. Am. Coll. Nutr. 2008;27:448–453. doi: 10.1080/07315724.2008.10719724. [DOI] [PubMed] [Google Scholar]
- 383.Papamichael C., Karatzi K., Karatzis E., Papaioannou T.G., Katsichti P., Zampelas A., Lekakis J. Combined acute effects of red wine consumption and cigarette smoking on haemodynamics of young smokers. J. Hypertens. 2006;24:1287–1292. doi: 10.1097/01.hjh.0000234108.08368.01. [DOI] [PubMed] [Google Scholar]
- 384.Papamichael C., Karatzis E., Karatzi K., Aznaouridis K., Papaioannou T., Protogerou A., Lekakis J., Mavrikakis M. Red wine's antioxidants counteract acute endothelial dysfunction caused by cigarette smoking in healthy nonsmokers. Am. Heart J. 2004;147:E5. doi: 10.1016/S0002-8703(03)00468-X. [DOI] [PubMed] [Google Scholar]
- 385.Hampton S.M., Isherwood C., Kirkpatrick V.J., Lynne-Smith A.C., Griffin B.A. The influence of alcohol consumed with a meal on endothelial function in healthy individuals. J. Hum. Nutr. Diet. 2010;23:120–125. doi: 10.1111/j.1365-277X.2009.01021.x. [DOI] [PubMed] [Google Scholar]
- 386.Coimbra S.R., Lage S.H., Brandizzi L., Yoshida V., da Luz P.L. The action of red wine and purple grape juice on vascular reactivity is independent of plasma lipids in hypercholesterolemic patients. Braz. J. Med. Biol. Res. 2005;38:1339–1347. doi: 10.1590/S0100-879X2005000900008. [DOI] [PubMed] [Google Scholar]
- 387.Whelan A.P., Sutherland W.H., McCormick M.P., Yeoman D.J., de Jong S.A., Williams M.J. Effects of white and red wine on endothelial function in subjects with coronary artery disease. Intern. Med. J. 2004;34:224–228. doi: 10.1111/j.1444-0903.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- 388.Karatzi K., Papamichael C., Aznaouridis K., Karatzis E., Lekakis J., Matsouka C., Boskou G., Chiou A., Sitara M., Feliou G., et al. Constituents of red wine other than alcohol improve endothelial function in patients with coronary artery disease. Coron. Artery Dis. 2004;15:485–490. doi: 10.1097/00019501-200412000-00005. [DOI] [PubMed] [Google Scholar]
- 389.Clifton P.M. Effect of Grape Seed Extract and Quercetin on Cardiovascular and Endothelial Parameters in High-Risk Subjects. J. Biomed. Biotechnol. 2004;2004:272–278. doi: 10.1155/S1110724304403088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Lekakis J., Rallidis L.S., Andreadou I., Vamvakou G., Kazantzoglou G., Magiatis P., Skaltsounis A.L., Kremastinos D.T. Polyphenolic compounds from red grapes acutely improve endothelial function in patients with coronary heart disease. Eur. J. Cardiovasc. Prev. Rehabil. 2005;12:596–600. doi: 10.1097/00149831-200512000-00013. [DOI] [PubMed] [Google Scholar]
- 391.Engler M.B., Engler M.M., Chen C.Y., Malloy M.J., Browne A., Chiu E.Y., Kwak H.K., Milbury P., Paul S.M., Blumberg J., et al. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J. Am. Coll. Nutr. 2004;23:197–204. doi: 10.1080/07315724.2004.10719361. [DOI] [PubMed] [Google Scholar]
- 392.Schroeter H., Heiss C., Balzer J., Kleinbongard P., Keen C.L., Hollenberg N.K., Sies H., Kwik-Uribe C., Schmitz H.H., Kelm M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Stein J.H., Keevil J.G., Wiebe D.A., Aeschlimann S., Folts J.D. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation. 1999;100:1050–1055. doi: 10.1161/01.CIR.100.10.1050. [DOI] [PubMed] [Google Scholar]
- 394.Chou E.J., Keevil J.G., Aeschlimann S., Wiebe D.A., Folts J.D., Stein J.H. Effect of ingestion of purple grape juice on endothelial function in patients with coronary heart disease. Am. J. Cardiol. 2001;88:553–555. doi: 10.1016/S0002-9149(01)01738-6. [DOI] [PubMed] [Google Scholar]
- 395.Park Y.K., Kim J.S., Kang M.H. Concord grape juice supplementation reduces blood pressure in Korean hypertensive men: Double-blind, placebo controlled intervention trial. BioFactors. 2004;22:145–147. doi: 10.1002/biof.5520220128. [DOI] [PubMed] [Google Scholar]
- 396.Heiss C., Kleinbongard P., Dejam A., Perre S., Schroeter H., Sies H., Kelm M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J. Am. Coll. Cardiol. 2005;46:1276–1283. doi: 10.1016/j.jacc.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 397.Taubert D., Berkels R., Roesen R., Klaus W. Chocolate and blood pressure in elderly individuals with isolated systolic hypertension. JAMA. 2003;290:1029–1030. doi: 10.1001/jama.290.8.1029. [DOI] [PubMed] [Google Scholar]
- 398.Grassi D., Desideri G., Necozione S., Lippi C., Casale R., Properzi G., Blumberg J.B., Ferri C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J. Nutr. 2008;138:1671–1676. doi: 10.1093/jn/138.9.1671. [DOI] [PubMed] [Google Scholar]
- 399.Faridi Z., Njike V.Y., Dutta S., Ali A., Katz D.L. Acute dark chocolate and cocoa ingestion and endothelial function: A randomized controlled crossover trial. Am. J. Clin. Nutr. 2008;88:58–63. doi: 10.1093/ajcn/88.1.58. [DOI] [PubMed] [Google Scholar]
- 400.Grassi D., Lippi C., Necozione S., Desideri G., Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am. J. Clin. Nutr. 2005;81:611–614. doi: 10.1093/ajcn/81.3.611. [DOI] [PubMed] [Google Scholar]
- 401.Balzer J., Rassaf T., Heiss C., Kleinbongard P., Lauer T., Merx M., Heussen N., Gross H.B., Keen C.L., Schroeter H., et al. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. J. Am. Coll. Cardiol. 2008;51:2141–2149. doi: 10.1016/j.jacc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 402.Muniyappa R., Hall G., Kolodziej T.L., Karne R.J., Crandon S.K., Quon M.J. Cocoa consumption for 2 wk enhances insulin-mediated vasodilatation without improving blood pressure or insulin resistance in essential hypertension. Am. J. Clin. Nutr. 2008;88:1685–1696. doi: 10.3945/ajcn.2008.26457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Aviram M., Rosenblat M., Gaitini D., Nitecki S., Hoffman A., Dornfeld L., Volkova N., Presser D., Attias J., et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004;23:423–433. doi: 10.1016/j.clnu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 404.Aviram M., Dornfeld L. Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis. 2001;158:195–198. doi: 10.1016/S0021-9150(01)00412-9. [DOI] [PubMed] [Google Scholar]
- 405.Zunino S.J., Parelman M.A., Freytag T.L., Stephensen C.B., Kelley D.S., Mackey B.E., Woodhouse L.R., Bonnel E.L. Effects of dietary strawberry powder on blood lipids and inflammatory markers in obese human subjects. Br. J. Nutr. 2012;108:900–909. doi: 10.1017/S0007114511006027. [DOI] [PubMed] [Google Scholar]
- 406.Duffy S.J., Keaney J.F., Jr., Holbrook M., Gokce N., Swerdloff P.L., Frei B., Vita J.A. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation. 2001;104:151–156. doi: 10.1161/01.CIR.104.2.151. [DOI] [PubMed] [Google Scholar]
- 407.Widlansky M.E., Hamburg N.M., Anter E., Holbrook M., Kahn D.F., Elliott J.G., Keaney J.F., Jr., Vita J.A. Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. J. Am. Coll. Nutr. 2007;26:95–102. doi: 10.1080/07315724.2007.10719590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Fukino Y., Shimbo M., Aoki N., Okubo T., Iso H. Randomized controlled trial for an effect of green tea consumption on insulin resistance and inflammation markers. J. Nutr. Sci. Vitaminol. 2005;51:335–342. doi: 10.3177/jnsv.51.335. [DOI] [PubMed] [Google Scholar]
- 409.Lobraico J.M., DiLello L.C., Butler A.D., Cordisco M.E., Petrini J.R., Ahmadi R. Effects of krill oil on endothelial function and other cardiovascular risk factors in participants with type 2 diabetes, a randomized controlled trial. BMJ Open Diabetes Res. Care. 2015;3:e000107. doi: 10.1136/bmjdrc-2015-000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Hanhineva K., Torronen R., Bondia-Pons I., Pekkinen J., Kolehmainen M., Mykkanen H., Poutanen K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010;11:1365–1402. doi: 10.3390/ijms11041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Rostami A., Khalili M., Haghighat N., Eghtesadi S., Shidfar F., Heidari I., Ebrahimpour-Koujan S., Eghtesadi M. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscler. 2015;11:21–29. [PMC free article] [PubMed] [Google Scholar]
- 412.Liu K., Zhou R., Wang B., Mi M.T. Effect of resveratrol on glucose control and insulin sensitivity: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2014;99:1510–1519. doi: 10.3945/ajcn.113.082024. [DOI] [PubMed] [Google Scholar]
- 413.Hausenblas H.A., Schoulda J.A., Smoliga J.M. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus--systematic review and meta-analysis. Mol. Nutr. Food Res. 2015;59:147–159. doi: 10.1002/mnfr.201400173. [DOI] [PubMed] [Google Scholar]
- 414.Novelle M.G., Wahl D., Dieguez C., Bernier M., de Cabo R. Resveratrol supplementation: Where are we now and where should we go? Ageing Res. Rev. 2015;21:1–15. doi: 10.1016/j.arr.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Reddy A.C., Lokesh B.R. Studies on the inhibitory effects of curcumin and eugenol on the formation of reactive oxygen species and the oxidation of ferrous iron. Mol. Cell. Biochem. 1994;137:1–8. doi: 10.1007/BF00926033. [DOI] [PubMed] [Google Scholar]
- 416.Ali Hussain H.E. Hypoglycemic, hypolipidemic and antioxidant properties of combination ofCurcumin fromCurcuma longa, Linn, and partially purified product fromAbroma augusta, Linn. in streptozotocin induced diabetes. Indian J. Clin. Biochem. IJCB. 2002;17:33–43. doi: 10.1007/BF02867969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Priyadarsini K.I., Maity D.K., Naik G.H., Kumar M.S., Unnikrishnan M.K., Satav J.G., Mohan H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Rad. Biol. Med. 2003;35:475–484. doi: 10.1016/S0891-5849(03)00325-3. [DOI] [PubMed] [Google Scholar]
- 418.Knekt P., Kumpulainen J., Jarvinen R., Rissanen H., Heliovaara M., Reunanen A., Hakulinen T., Aromaa A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002;76:560–568. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 419.Song Y., Manson J.E., Buring J.E., Sesso H.D., Liu S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: A prospective study and cross-sectional analysis. J. Am. Coll. Nutr. 2005;24:376–384. doi: 10.1080/07315724.2005.10719488. [DOI] [PubMed] [Google Scholar]
- 420.Brown A.L., Lane J., Coverly J., Stocks J., Jackson S., Stephen A., Bluck L., Coward A., Hendrickx H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: Randomized controlled trial. Br. J. Nutr. 2009;101:886–894. doi: 10.1017/S0007114508047727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Ryu O.H., Lee J., Lee K.W., Kim H.Y., Seo J.A., Kim S.G., Baik S.H., Choi D.S., Choi K.M. Effects of green tea consumption on inflammation, insulin resistance and pulse wave velocity in type 2 diabetes patients. Diabetes Res. Clin. Pract. 2006;71:356–358. doi: 10.1016/j.diabres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 422.Mackenzie T., Leary L., Brooks W.B. The effect of an extract of green and black tea on glucose control in adults with type 2 diabetes mellitus: Double-blind randomized study. Metabolism. 2007;56:1340–1344. doi: 10.1016/j.metabol.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 423.Evans J.L., Goldfine I.D. Alpha-lipoic acid: A multifunctional antioxidant that improves insulin sensitivity in patients with type 2 diabetes. Diabetes Technol. Therapeutics. 2000;2:401–413. doi: 10.1089/15209150050194279. [DOI] [PubMed] [Google Scholar]
- 424.Norris J.M., Yin X., Lamb M.M., Barriga K., Seifert J., Hoffman M., Orton H.D., Baron A.E., Clare-Salzler M., Chase H.P.S., et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 2007;298:1420–1428. doi: 10.1001/jama.298.12.1420. [DOI] [PubMed] [Google Scholar]
- 425.Wicklow B., Wittmeier K., t’Jong G.W., McGavock J., Robert M., Duhamel T., Dolinsky V.W. Proposed trial: Safety and efficacy of resveratrol for the treatment of non-alcoholic fatty liver disease (NAFLD) and associated insulin resistance in adolescents who are overweight or obese adolescents—Rationale and protocol. Biochem. Cell Biol. 2015;10:1–9. doi: 10.1139/bcb-2014-0136. [DOI] [PubMed] [Google Scholar]
- 426.Faghihzadeh F., Adibi P., Hekmatdoost A. The effects of resveratrol supplementation on cardiovascular risk factors in patients with non-alcoholic fatty liver disease: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2015;114:796–803. doi: 10.1017/S0007114515002433. [DOI] [PubMed] [Google Scholar]
- 427.Abidov M., Ramazanov A., Jimenez Del Rio M., Chkhikvishvili I. Effect of Blueberin on fasting glucose, C-reactive protein and plasma aminotransferases, in female volunteers with diabetes type 2: Double-blind, placebo controlled clinical study. Georgian Med. News. 2006;141:66–72. [PubMed] [Google Scholar]
- 428.Khan A., Safdar M., Ali Khan M.M., Khattak K.N., Anderson R.A. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26:3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 429.Soni K.B., Kuttan R. Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian J. Physiol. Pharmacol. 1992;36:273–275. [PubMed] [Google Scholar]
- 430.van Dam R.M., Hu F.B. Coffee consumption and risk of type 2 diabetes: A systematic review. JAMA. 2005;294:97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]
- 431.de Munter J.S., Hu F.B., Spiegelman D., Franz M., van Dam R.M. Whole grain, bran, and germ intake and risk of type 2 diabetes: A prospective cohort study and systematic review. PLoS Med. 2007;4:e261. doi: 10.1371/journal.pmed.0040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Kar P., Laight D., Rooprai H.K., Shaw K.M., Cummings M. Effects of grape seed extract in Type 2 diabetic subjects at high cardiovascular risk: A double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabet. Med. 2009;26:526–531. doi: 10.1111/j.1464-5491.2009.02727.x. [DOI] [PubMed] [Google Scholar]
- 433.Jing Y., Han G., Hu Y., Bi Y., Li L., Zhu D. Tea consumption and risk of type 2 diabetes: A meta-analysis of cohort studies. J. Gen. Intern. Med. 2009;24:557–562. doi: 10.1007/s11606-009-0929-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Polychronopoulos E., Zeimbekis A., Kastorini C.M., Papairakleous N., Vlachou I., Bountziouka V., Panagiotakos D.B. Effects of black and green tea consumption on blood glucose levels in non-obese elderly men and women from Mediterranean Islands (MEDIS epidemiological study) Eur. J. Nutr. 2008;47:10–16. doi: 10.1007/s00394-007-0690-7. [DOI] [PubMed] [Google Scholar]
- 435.Nagao T., Meguro S., Hase T., Otsuka K., Komikado M., Tokimitsu I., Yamamoto T., Yamamoto K. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity. 2009;17:310–317. doi: 10.1038/oby.2008.505. [DOI] [PubMed] [Google Scholar]
- 436.Sharma S., Kulkarni S.K., Chopra K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin. Exp. Pharmacol. Physiol. 2006;33:940–945. doi: 10.1111/j.1440-1681.2006.04468.x. [DOI] [PubMed] [Google Scholar]
- 437.Patumraj S., Wongeakin N., Sridulyakul P., Jariyapongskul A., Futrakul N., Bunnag S. Combined effects of curcumin and vitamin C to protect endothelial dysfunction in the iris tissue of STZ-induced diabetic rats. Clin. Hemorheol. Microcirc. 2006;35:481–489. [PubMed] [Google Scholar]
- 438.Rungseesantivanon S., Thenchaisri N., Ruangvejvorachai P., Patumraj S. Curcumin supplementation could improve diabetes-induced endothelial dysfunction associated with decreased vascular superoxide production and PKC inhibition. BMC Complement. Altern. Med. 2010;10 doi: 10.1186/1472-6882-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Seo K.I., Choi M.S., Jung U.J., Kim H.J., Yeo J., Jeon S.M., Lee M.K. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol. Nutr. Food Res. 2008;52:995–1004. doi: 10.1002/mnfr.200700184. [DOI] [PubMed] [Google Scholar]
- 440.Weisberg S.P., Leibel R., Tortoriello D.V. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149:3549–3558. doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Motterlini R., Foresti R., Bassi R., Green C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Rad. Biol. Med. 2000;28:1303–1312. doi: 10.1016/S0891-5849(00)00294-X. [DOI] [PubMed] [Google Scholar]
- 442.Auberval N., Dal S., Bietiger W., Seyfritz E., Peluso J., Muller C., Zhao M., Marchioni E., Pinget M., Jeandidier N., et al. Oxidative Stress Type Influences the Properties of Antioxidants Containing Polyphenols in RINm5F Beta Cells. Evid.-based Complement. Altern. Med. 2015;2015 doi: 10.1155/2015/859048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Katiyar S.K., Afaq F., Perez A., Mukhtar H. Green tea polyphenol (-)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis. 2001;22:287–294. doi: 10.1093/carcin/22.2.287. [DOI] [PubMed] [Google Scholar]
- 444.Katiyar S.K. Elmets ca. Green tea polyphenolic antioxidants and skin photoprotection (Review) Int. J. Oncol. 2001;18:1307–1313. doi: 10.3892/ijo.18.6.1307. [DOI] [PubMed] [Google Scholar]
- 445.Bolling B.W., Blumberg J.B., Chen C.Y. Extraction methods determine the antioxidant capacity and induction of quinone reductase by soy products in vitro. Food Chem. 2009;116:351–355. doi: 10.1016/j.foodchem.2009.01.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Song E.K., Hur H., Han M.K. Epigallocatechin gallate prevents autoimmune diabetes induced by multiple low doses of streptozotocin in mice. Arch. Pharm. Res. 2003;26:559–563. doi: 10.1007/BF02976881. [DOI] [PubMed] [Google Scholar]
- 447.Bruno R.S., Dugan C.E., Smyth J.A., DiNatale D.A., Koo S.I. Green tea extract protects leptin-deficient, spontaneously obese mice from hepatic steatosis and injury. J. Nutr. 2008;138:323–331. doi: 10.1093/jn/138.2.323. [DOI] [PubMed] [Google Scholar]
- 448.Koo S.I., Noh S.K. Green tea as inhibitor of the intestinal absorption of lipids: Potential mechanism for its lipid-lowering effect. J. Nutr. Biochem. 2007;18:179–183. doi: 10.1016/j.jnutbio.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Budin S.B., Othman F., Louis S.R., Bakar M.A., Das S., Mohamed J. The effects of palm oil tocotrienol-rich fraction supplementation on biochemical parameters, oxidative stress and the vascular wall of streptozotocin-induced diabetic rats. Clinics. 2009;64:235–244. doi: 10.1590/S1807-59322009000300015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Pinent M., Blay M., Blade M.C., Salvado M.J., Arola L., Ardevol A. Grape seed-derived procyanidins have an antihyperglycemic effect in streptozotocin-induced diabetic rats and insulinomimetic activity in insulin-sensitive cell lines. Endocrinology. 2004;145:4985–4990. doi: 10.1210/en.2004-0764. [DOI] [PubMed] [Google Scholar]
- 451.Rivera L., Moron R., Zarzuelo A., Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem. Pharmacol. 2009;77:1053–1063. doi: 10.1016/j.bcp.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 452.Su H.C., Hung L.M., Chen J.K. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am. J. Physiol. Endocrinol. Metab. 2006;290:E1339–E1346. doi: 10.1152/ajpendo.00487.2005. [DOI] [PubMed] [Google Scholar]
- 453.Orallo F., Alzueta A.F. Preliminary study of the vasorelaxant effects of (+)-nantenine, an alkaloid isolated from Platycapnos spicata, in rat aorta. Planta Med. 2001;67:800–806. doi: 10.1055/s-2001-18848. [DOI] [PubMed] [Google Scholar]
- 454.Ghalib H., Modabber F. Consultation meeting on the development of therapeutic vaccines for post kala azar dermal leishmaniasis. Kinetoplastid Biol. Dis. 2007;6:7. doi: 10.1186/1475-9292-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Ouslimani N., Peynet J., Bonnefont-Rousselot D., Therond P., Legrand A., Beaudeux J.L. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism. 2005;54:829–834. doi: 10.1016/j.metabol.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 456.Nathan D.M., Buse J.B., Davidson M.B., Ferrannini E., Holman R.R., Sherwin R., Zinman B. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Thomas D., Elliott E.J. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst. Rev. 2009:CD006296. doi: 10.1002/14651858.CD006296.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Nield L., Moore H.J., Hooper L., Cruickshank J.K., Vyas A., Whittaker V., Summerbell C.D. Dietary advice for treatment of type 2 diabetes mellitus in adults. Cochrane Database Syst. Rev. 2007:CD004097. doi: 10.1002/14651858.CD004097.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Gaede P.H., Jepsen P.V., Larsen J.N., Jensen G.V., Parving H.H., Pedersen O.B. The Steno-2 study. Intensive multifactorial intervention reduces the occurrence of cardiovascular disease in patients with type 2 diabetes. Ugeskrift Laeger. 2003;165:2658–2661. [PubMed] [Google Scholar]
- 460.Costantino S., Paneni F., Cosentino F. Hyperglycemia: A bad signature on the vascular system. Cardiovasc. Diagn. Ther. 2015;5:403–406. doi: 10.3978/j.issn.2223-3652.2015.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Kuschnerus K., Landmesser U., Krankel N. Vascular repair strategies in type 2 diabetes: Novel insights. Cardiovasc. Diagn. Ther. 2015;5:374–386. doi: 10.3978/j.issn.2223-3652.2015.05.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Imamura F., O'Connor L., Ye Z., Mursu J., Hayashino Y., Bhupathiraju S.N., Forouhi N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576. doi: 10.1136/bmj.h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Larson N.I., Story M.T., Nelson M.C. Neighborhood environments: Disparities in access to healthy foods in the U.S. Am. J. Prev. Med. 2009;36:74–81. doi: 10.1016/j.amepre.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 464.Alinia S., Hels O., Tetens I. The potential association between fruit intake and body weight—A review. Obes. Rev. 2009;10:639–647. doi: 10.1111/j.1467-789X.2009.00582.x. [DOI] [PubMed] [Google Scholar]
- 465.Hamer M., Chida Y. Intake of fruit, vegetables, and antioxidants and risk of type 2 diabetes: Systematic review and meta-analysis. J. Hypertens. 2007;25:2361–2369. doi: 10.1097/HJH.0b013e3282efc214. [DOI] [PubMed] [Google Scholar]
- 466.Harding A.H., Wareham N.J., Bingham S.A., Khaw K., Luben R., Welch A., Forouhi N.G. Plasma vitamin C level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: The European prospective investigation of cancer—Norfolk prospective study. Arch. Intern. Med. 2008;168:1493–1499. doi: 10.1001/archinte.168.14.1493. [DOI] [PubMed] [Google Scholar]
- 467.Frankenfeld C.L., Leslie T.F., Makara M.A. Diabetes, obesity, and recommended fruit and vegetable consumption in relation to food environment sub-types: A cross-sectional analysis of Behavioral Risk Factor Surveillance System, United States Census, and food establishment data. BMC Public Health. 2015;15:491. doi: 10.1186/s12889-015-1819-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Sies H. Total antioxidant capacity: Appraisal of a concept. J. Nutr. 2007;137:1493–1495. doi: 10.1093/jn/137.6.1493. [DOI] [PubMed] [Google Scholar]
- 469.Litescu S.C., Eremia S., Radu G.L. Methods for the determination of antioxidant capacity in food and raw materials. Adv. Exp. Med. Biol. 2010;698:241–249. doi: 10.1007/978-1-4419-7347-4_18. [DOI] [PubMed] [Google Scholar]
- 470.Young I.S. Measurement of total antioxidant capacity. J. Clin. Pathol. 2001;54:339. doi: 10.1136/jcp.54.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Kane M.O., Etienne-Selloum N., Dal-Ros S., Demougeot C., Berthelot A., Schini-Kerth V.B., Auger C. Role of arginases in the angiotensin II-induced hypertension-associated endothelial dysfunction in the rat aorta: Preventive effect of red wine polyphenols. J. Phys. Pharm. Adv. 2015;5:667–678. doi: 10.5455/jppa.20150713061620. [DOI] [Google Scholar]
- 472.Kane M.O., Etienne-Selloum N., Madeira S.V., Sarr M., Walter A., Dal-Ros S., Schott C., Chataigneau T., Schini-Kerth V.B. Endothelium-derived contracting factors mediate the Ang II-induced endothelial dysfunction in the rat aorta: Preventive effect of red wine polyphenols. Pflugers Arch. 2010;459:671–679. doi: 10.1007/s00424-009-0759-7. [DOI] [PubMed] [Google Scholar]
- 473.Auberval N., Dal S., Maillard E., Bietiger W., Peronet C., Pinget M., Schini-Kerth V., Sigrist S. Beneficial effects of a red wine polyphenol extract on high-fat diet-induced metabolic syndrome in rats. Eur. J. Nutr. 2016 doi: 10.1007/s00394-016-1192-2. [DOI] [PubMed] [Google Scholar]
- 474.Dal S S.A., Maillard-Pedracini E., Pinget M., Jeandidier N., Sigrist N. Le stress oxydant: Nouvelle cible pour optimiser la prise en charge du patient diabétique de type 1. Infusystems Fr. 2014;31:25–30. [Google Scholar]
- 475.Sikand G., Kris-Etherton P., Boulos N.M. Impact of functional foods on prevention of cardiovascular disease and diabetes. Curr. Cardiol. Rep. 2015;17:39. doi: 10.1007/s11886-015-0593-9. [DOI] [PubMed] [Google Scholar]