Abstract
BACKGROUND
Data reported during the past 5 years indicate that rates of survival have increased among infants born at the borderline of viability, but less is known about how increased rates of survival among these infants relate to early childhood neurodevelopmental outcomes.
METHODS
We compared survival and neurodevelopmental outcomes among infants born at 22 to 24 weeks of gestation, as assessed at 18 to 22 months of corrected age, across three consecutive birth-year epochs (2000–2003 [epoch 1], 2004–2007 [epoch 2], and 2008–2011 [epoch 3]). The infants were born at 11 centers that participated in the National Institute of Child Health and Human Development Neonatal Research Network. The primary outcome measure was a three-level outcome — survival without neurodevelopmental impairment, survival with neurodevelopmental impairment, or death. After accounting for differences in infant characteristics, including birth center, we used multinomial generalized logit models to compare the relative risk of survival without neurodevelopmental impairment, survival with neurodevelopmental impairment, and death.
RESULTS
Data on the primary outcome were available for 4274 of 4458 infants (96%) born at the 11 centers. The percentage of infants who survived increased from 30% (424 of 1391 infants) in epoch 1 to 36% (487 of 1348 infants) in epoch 3 (P<0.001). The percentage of infants who survived without neurodevelopmental impairment increased from 16% (217 of 1391) in epoch 1 to 20% (276 of 1348) in epoch 3 (P = 0.001), whereas the percentage of infants who survived with neurodevelopmental impairment did not change significantly (15% [207 of 1391] in epoch 1 and 16% [211 of 1348] in epoch 3, P = 0.29). After adjustment for changes in the baseline characteristics of the infants over time, both the rate of survival with neurodevelopmental impairment (as compared with death) and the rate of survival without neurodevelopmental impairment (as compared with death) increased over time (adjusted relative risks, 1.27 [95% confidence interval {CI}, 1.01 to 1.59] and 1.59 [95% CI, 1.28 to 1.99], respectively).
CONCLUSIONS
The rate of survival without neurodevelopmental impairment increased between 2000 and 2011 in this large cohort of periviable infants. (Funded by the National Institutes of Health and others; ClinicalTrials.gov numbers, NCT00063063 and NCT00009633.)
Care of periviable infants remains a great challenge in neonatal and perinatal medicine.1 Infants born between 22 and 24 weeks of gestation often die or survive with long-term neurodevelopmental impairment.2–4 The approach to resuscitation and management at these early gestational ages varies substantially.1,5
Data reported during the past 5 years indicate that mortality has declined among extremely premature infants.6–9 Investigators at the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) reported a decrease in mortality over the past two decades, with the greatest gains in survival seen among infants born at 23 and 24 weeks of gestation after 2008.8,9 Such studies raise questions about neurodevelopmental outcomes in surviving infants. NRN studies over previous periods have not shown significant improvement in neurodevelopmental outcomes over time among periviable infants,2,3 and there is concern that declining mortality in this population may lead to a greater number of infants surviving with neurodevelopmental impairment.10,11 The availability of data on both mortality and neurodevelopmental outcomes among survivors is important for families and clinicians making early care decisions for these high-risk infants.1
The objective of our study was to evaluate changes over time in survival and neurodevelopmental outcomes among infants born at 22 to 24 weeks of gestation, as assessed at 18 to 22 months of corrected age (with corrected age defined as the age the infant would be if born at term). We hypothesized that in addition to the rate of survival, the rate of survival without neurodevelopmental impairment also increased from 2000 through 2011.
METHODS
STUDY POPULATION AND DATA COLLECTION
We included infants born at 22 weeks 0 days to 24 weeks 6 days of gestation between January 1, 2000, and December 31, 2011, who were enrolled in the generic database registry of the NICHD NRN. Data from the 11 academic tertiary care centers that participated in the NRN for the entire duration of the study period were included in the analysis. A total of 427 infants who were not born at the centers were excluded. Data for mother–infant dyads were collected prospectively by trained research personnel for all live births, which included infants who died in the delivery room. Gestational age was defined as completed weeks of gestation, as determined according to the best obstetrical estimate that was based on the last menstrual period, obstetrical factors, or prenatal ultrasonogram (or a combination thereof), or — when obstetrical dating was unavailable — according to neonatal assessment that included the Ballard or Dubowitz examination.12,13 To assess whether there were changes in resuscitation practices over time, we evaluated the proportion of infants who did not receive active treatment after birth, which was defined by the use of surfactant, endotracheal intubation, ventilatory support (i.e., continuous positive airway pressure, bag-mask ventilation, or mechanical ventilation), chest compressions, epinephrine, or parenteral nutrition.5 Small for gestational age was defined as birth weight below the 10th percentile on Olsen growth curves.14 Severe intraventricular hemorrhage was defined as grade III to IV, according to the criteria of Papile et al.15 The presence of sepsis was determined by a positive blood culture and was classified as early onset (≤72 hours) or late onset (>72 hours). Retinopathy of prematurity was considered to be severe if the infant received surgical treatment, intravitreal bevacizumab, or both. Necrotizing enterocolitis was defined as Bell’s stage II to III, according to the modified Bell’s classification (with scores ranging from I to III and higher scores indicating greater severity of disease).16 Infants were considered to have bronchopulmonary dysplasia if they were receiving supplemental oxygen at 36 weeks of postmenstrual age (the infant’s gestational age at birth plus the time elapsed since birth [chronological age]).
STUDY OVERSIGHT
The institutional review boards at each of the 11 centers approved the protocol, available with the full text of this article at NEJM.org. Nine centers required written informed consent for the follow-up protocol (2 centers granted a waiver), and 1 center required oral informed consent for the in-hospital protocol (10 centers granted a waiver). The third author, a statistician at the data coordinating center, had full access to the data and performed the analysis. All the authors vouch for the integrity, accuracy, and completeness of the data and analyses and for the fidelity of the study to the protocol.
OUTCOME MEASURES
The primary outcome measure was a three-level outcome — survival without neurodevelopmental impairment, survival with neurodevelopmental impairment, or death, as assessed at 18 to 22 months of corrected age. Neurodevelopmental outcomes were assessed at 18 to 22 months of corrected age with the use of neurologic examinations and the Bayley Scales of Infant and Toddler Development, second edition (Bayley-II), for infants born between 2000 and 2005, and third edition (Bayley-III), for infants born between 2006 and 2011. Differences between the two editions are summarized in Table S1 in the Supplementary Appendix, available at NEJM.org. Infants were considered to have neurodevelopmental impairment if they had at least one of the following conditions: moderate or severe cerebral palsy, Gross Motor Function Classification System level of at least 2 (on a scale of 1 [mild impairment] to 5 [most severe impairment]), profound hearing loss requiring amplification in both ears, profound visual impairment with visual acuity of less than 20/200 in both eyes, or cognitive impairment, which was defined as a Mental Developmental Index score of less than 70 (two standard deviations below the mean ±SD score of 100±15 [scores range from 50 to 150, with the lower scores indicating a greater degree of developmental delay]) (Bayley-II) or a Cognitive Composite score of less than 85 (one standard deviation below the mean ±SD score of 100±15 [scores range from 55 to 145, with the lower scores indicating a greater degree of developmental delay]) (Bayley-III). We selected these cutoff points to adjust for the difference between Bayley-II and Bayley-III in estimating cognitive performance,17–20 on the basis of data showing 97% agreement between a Bayley-II Mental Developmental Index score lower than 70 and a Bayley-III Cognitive Composite score lower than 85.21 Bayley-II and Bayley-III motor scores and Bayley-III language scores were not included in the definition for neurodevelopmental impairment.
Given the potential discrepancy between Bayley-II and Bayley-III assessments, we chose neurosensory impairment as a secondary outcome to compare neurologic outcomes over time independent of Bayley scores. Neurosensory impairment was defined as moderate or severe cerebral palsy, Gross Motor Function Classification System level of at least 2, profound hearing loss, or profound visual impairment.
STATISTICAL ANALYSIS
We compared outcomes among infants in three birth-year epochs (2000–2003 [epoch 1], 2004–2007 [epoch 2], and 2008–2011 [epoch 3]). The study period and epoch definitions were chosen to be consistent with the definitions in a recent NRN report on changes in mortality over time8 and for consistency with previous NRN studies of neurodevelopmental outcomes in periviable infants over consecutive epochs.2,3 Demographics, perinatal characteristics, medical conditions, therapies, and outcomes were compared across epochs with the use of chi-square tests for categorical variables and median tests for continuous variables. Outcomes were stratified according to epoch for each of the participating centers. Multilevel generalized logit modeling was conducted to determine the effect of epoch on the three-level categorical outcome, with adjustment for gestational age, multiple gestation, maternal race, sex of the infant, and small-for-gestational-age status; birth center was included as a random effect. These covariates were selected to adjust for baseline factors that may influence outcomes.22,23 Variables related to care practices that may have changed over time, such as the use of antenatal glucocorticoids, were left out of the analysis so that the temporal effect would not be obscured. A similar modeling approach was used for the outcome of neurosensory impairment. Prespecified subgroup analyses were performed according to the gestational week in which the infants were born. Because changes in outcomes over time could reflect changes in resuscitation practices, we repeated the adjusted analyses with the sample limited to infants who received active treatment.
To further address the change from Bayley-II to Bayley-III, we performed a sensitivity analysis to evaluate changes in outcomes over the years since the implementation of Bayley-III. The analysis was restricted to infants born between 2006 and 2011. We used birth year, specified as a continuous variable, in place of epoch as the time variable in the model and adjusted for the same variables as in the regression model of the primary outcome.
All analyses were performed with the use of SAS software, version 9.3 (SAS Institute). Two-sided P values of less than 0.05 were considered to indicate statistical significance. The primary outcome analysis was considered to be confirmatory, and all other reported analyses were deemed exploratory and hypothesis generating, with P values presented for descriptive purposes. Thus, no adjustments were made for multiple testing.
RESULTS
INFANT AND MATERNAL CHARACTERISTICS
A total of 4458 infants were born at 11 participating centers during the study period. The analysis cohort included 4274 infants (96%) for whom data on the primary outcome were available. The cohort comprised 749 infants (18%) born at 22 weeks, 1435 infants (34%) born at 23 weeks, and 2090 infants (49%) born at 24 weeks. Birth weight, gestational age, and infant sex distributions did not differ significantly across epochs, although the proportion of infants who were small for their gestational age increased significantly over time (Table 1). The median maternal age increased over time, and the proportion of mothers with an education level less than high school decreased. The rates of multiple births, cesarean sections, and antenatal glucocorticoid use increased between epoch 1 and epoch 3, although the rate of antenatal antibiotic use decreased. Rates of active treatment did not change significantly across epochs. Active treatment was received by 22% of the infants (167 of 749) born at 22 weeks, by 71% (1015 of 1435) born at 23 weeks, and by 95% (1994 of 2090) born at 24 weeks.
Table 1.
Infant Characteristics, Medical Conditions, and Therapies.
| Variable | Epoch 1 (2000–2003) |
Epoch 2 (2004–2007) |
Epoch 3 (2008–2011) |
P Value* |
|---|---|---|---|---|
| All infants | ||||
| Total no. in cohort | 1391 | 1535 | 1348 | |
| Median birth weight (interquartile range) — g | 600 (533–670) | 590 (520–670) | 595 (511–680) | 0.11 |
| Gestational age at birth — no./total no. (%) | ||||
| 22 wk | 241/1391 (17) | 274/1535 (18) | 234/1348 (17) | 0.92 |
| 23 wk | 496/1391 (36) | 489/1535 (32) | 450/1348 (33) | 0.09 |
| 24 wk | 654/1391 (47) | 772/1535 (50) | 664/1348 (49) | 0.20 |
| Small for gestational age — no./total no. (%) | 39/1391 (3) | 65/1535 (4) | 105/1347 (8) | <0.001 |
| Male sex — no./total no. (%) | 759/1391 (55) | 834/1535 (54) | 702/1348 (52) | 0.35 |
| Race — no./total no. (%)† | ||||
| Black | 642/1385 (46) | 708/1521 (47) | 608/1322 (46) | 0.96 |
| White | 695/1385 (50) | 747/1521 (49) | 639/1322 (48) | 0.63 |
| Other | 48/1385 (3) | 66/1521 (4) | 75/1322 (6) | 0.02 |
| Multiple birth — no./total no. (%) | 330/1391 (24) | 422/1535 (27) | 362/1348 (27) | 0.049 |
| Antenatal glucocorticoids — no./total no. (%)‡ | ||||
| Any receipt | 799/1388 (58) | 881/1532 (58) | 860/1346 (64) | <0.001 |
| Full course | 398/1386 (29) | 511/1527 (33) | 593/1342 (44) | <0.001 |
| Antenatal antibiotics — no./total no. (%)§ | 987/1388 (71) | 991/1531 (65) | 884/1345 (66) | 0.001 |
| Cesarean delivery — no./total no. (%) | 428/1388 (31) | 587/1535 (38) | 505/1345 (38) | <0.01 |
| No active treatment, according to gestational age at birth — no./total no. (%)¶ | ||||
| 22 wk | 183/241 (76) | 213/274 (78) | 186/234 (79) | 0.65 |
| 23 wk | 134/496 (27) | 150/489 (31) | 136/450 (30) | 0.39 |
| 24 wk | 40/654 (6) | 32/772 (4) | 24/664 (4) | 0.07 |
| Total | 357/1391 (26) | 395/1535 (26) | 346/1348 (26) | >0.99 |
| Surfactant treatment — no./total no. (%) | 892/1391 (64) | 1009/1533 (66) | 889/1348 (66) | 0.53 |
| Median maternal age (interquartile range) — yr | 25 (21–31) | 26 (22–31) | 27 (22–32) | <0.001 |
| Maternal education less than high school — no./total no. (%) | 235/766 (31) | 305/1023 (30) | 189/792 (24) | 0.004 |
| Infants surviving >12 hr‖ | ||||
| Total no. in cohort | 855 | 968 | 865 | |
| Severe intraventricular hemorrhage — no./total no. (%) | 236/788 (30) | 260/889 (29) | 237/824 (29) | 0.87 |
| Periventricular leukomalacia — no./total no. (%) | 58/789 (7) | 53/889 (6) | 58/822 (7) | 0.48 |
| Posthemorrhagic hydrocephalus with shunt placement — no./total no. (%) | 26/854 (3) | 10/858 (1) | 5/704 (1) | <0.001 |
| Early-onset sepsis — no./total no. (%) | 30/855 (4) | 35/967 (4) | 19/863 (2) | 0.19 |
| Late-onset sepsis — no./total no. (%) | 370/734 (50) | 447/834 (54) | 331/790 (42) | <0.001 |
| Severe retinopathy of prematurity — no./total no. (%) | 84/495 (17) | 165/535 (31) | 126/547 (23) | <0.001 |
| Necrotizing enterocolitis — no./total no. (%) | ||||
| No surgery | 41/855 (5) | 57/968 (6) | 47/864 (5) | 0.59 |
| With surgery | 66/855 (8) | 87/968 (9) | 67/864 (8) | 0.52 |
| Surgery for patent ductus arteriosus — no./total no. (%) | 163/855 (19) | 187/968 (19) | 135/862 (16) | 0.08 |
| High-frequency ventilation — no./total no. (%) | 473/855 (55) | 611/966 (63) | 547/865 (63) | <0.001 |
| Bronchopulmonary dysplasia — no./total no. (%) | 372/470 (79) | 374/511 (73) | 386/536 (72) | 0.02 |
| Postnatal glucocorticoids — no./total no. (%) | 298/853 (35) | 154/953 (16) | 169/861 (20) | <0.001 |
P values were determined using chi-square tests for categorical variables and median tests for continuous variables.
Race was determined by investigators on the basis of chart abstraction using categories specified in the study manual of operation (black, white, Asian, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, or more than one race).
Any receipt of antenatal glucocorticoids was defined as a full or partial course during the current pregnancy for the purpose of accelerating fetal maturity. A full course was defined as two doses of betamethasone (12 or 24 hours apart) or four doses of dexamethasone (≥6 hours apart).
Receipt of antenatal antibiotics was defined as receipt of any antibiotic preceding birth during the hospital admission in which delivery occurred.
Active treatment was defined as the use of surfactant, endotracheal intubation, ventilatory support (i.e., continuous positive airway pressure, bag-mask ventilation, or mechanical ventilation), chest compressions, epinephrine, or parenteral nutrition.
Data on medical conditions and therapies were available for infants surviving more than 12 hours and undergoing the applicable examination (e.g., sonogram for intraventricular hemorrhage).
The incidence of posthemorrhagic hydrocephalus with shunt placement, late-onset sepsis, and bronchopulmonary dysplasia decreased between epoch 1 and epoch 3 (Table 1). The rate of postnatal glucocorticoid use also decreased between epoch 1 and epoch 3, although the rate of high-frequency ventilation increased. The percentages of infants with severe intraventricular hemorrhage, periventricular leukomalacia, early-onset sepsis, necrotizing enterocolitis, and ligation of a patent ductus arteriosus did not differ significantly across epochs. The incidence of severe retinopathy of prematurity was highest in epoch 2.
OUTCOMES
The percentage of infants who survived without neurodevelopmental impairment increased from 16% (217 of 1391) in epoch 1 to 20% (276 of 1348) in epoch 3 (P<0.001) (Table 2). The rate of death was lowest in epoch 3 (64% [861 of 1348 infants died]). The proportions of infants who survived with neurodevelopmental impairment did not differ significantly across epochs. Among infants born at 22 weeks, there was no significant change in outcomes (survival without neurodevelopmental impairment, survival with neurodevelopmental impairment, and death) over time. Among infants born at 23 and 24 weeks, the rate of survival without neurodevelopmental impairment increased between epoch 1 and epoch 3, although the rate of survival with neurodevelopmental impairment did not differ significantly.
Table 2.
Survival and Neurodevelopmental Outcomes at 18 to 22 Months of Corrected Age.
| Outcome | Epoch 1 (2000–2003) |
Epoch 2 (2004–2007) |
Epoch 3 (2008–2011) |
P Value† | |||
|---|---|---|---|---|---|---|---|
| no./total no. | % (95% CI)* | no./total no. | % (95% CI)* | no./total no. | % (95%, CI)* | ||
| All infants‡ | |||||||
| Survival without neurodevelopmental impairment | 217/1391 | 16 (14–18) | 250/1535 | 16 (15–18) | 276/1348 | 20 (18–23) | 0.001 |
| Survival with neurodevelopmental impairment | 207/1391 | 15 (13–17) | 209/1535 | 14 (12–15) | 211/1348 | 16 (14–18) | 0.29 |
| Death | 967/1391 | 70 (67–72) | 1076/1535 | 70 (68–72) | 861/1348 | 64 (61–66) | <0.001 |
| Survival without neurosensory impairment | 340/1380 | 25 (22–27) | 391/1533 | 26 (23–28) | 395/1348 | 29 (27–32) | 0.01 |
| Survival with neurosensory impairment | 73/1380 | 5 (4–7) | 66/1533 | 4 (3–5) | 92/1348 | 7 (6–8) | 0.01 |
| Infants born at 22 wk | |||||||
| Survival without neurodevelopmental impairment§ | 2/241 | 1 (0–3) | 4/274 | 1 (1–4) | 3/234 | 1 (0–1) | 0.80 |
| Survival with neurodevelopmental impairment§ | 4/241 | 2 (1–1) | 9/274 | 3 (2–6) | 5/234 | 2 (1–5) | 0.46 |
| Death | 235/241 | 98 (95–99) | 261/274 | 95 (92–97) | 226/234 | 97 (93–98) | 0.39 |
| Infants born at 23 wk | |||||||
| Survival without neurodevelopmental impairment | 34/496 | 7 (5–9) | 55/489 | 11 (9–14) | 59/450 | 13 (10–17) | 0.005 |
| Survival with neurodevelopmental impairment | 63/496 | 13 (10–16) | 41/489 | 8 (6–11) | 51/450 | 11 (9–15) | 0.08 |
| Death | 399/496 | 80 (77–84) | 393/489 | 80 (77–84) | 340/450 | 76 (71–79) | 0.11 |
| Infants born at 24 wk | |||||||
| Survival without neurodevelopmental impairment | 181/654 | 28 (24–31) | 191/772 | 25 (22–28) | 214/664 | 32 (29–36) | 0.007 |
| Survival with neurodevelopmental impairment | 140/654 | 21 (18–25) | 159/772 | 21 (18–24) | 155/664 | 23 (20–27) | 0.44 |
| Death | 333/654 | 51 (47–55) | 422/772 | 55 (51–58) | 295/664 | 44 (41–18) | <0.001 |
Unadjusted binomial confidence intervals were determined with use of the Wilson method.
P values were determined using chi-square tests.
Included are 4274 infants who had data available on the primary outcome.
Among the 27 surviving infants born at 22 weeks, the median (interquartile range) gestational age was 22 weeks 5 days (22 weeks 4 days to 22 weeks 6 days) and birth weight was 570 g (510 to 620).
Among surviving infants, we found no significant difference in the incidence of neurodevelopmental impairment or neurosensory impairment across epochs (Table 3). The incidence of profound visual impairment declined significantly to 2 of 484 infants (<1%) in epoch 3, but the rates of other individual components of neurodevelopmental impairment were similar over time. Survival and neurodevelopmental outcomes varied across centers (Fig. 1).
Table 3.
Neurodevelopmental Outcomes among Infants Surviving to 18 to 22 Months of Corrected Age.
| Outcome | Epoch 1 (2000–2003) |
Epoch 2 (2004–2007) |
Epoch 3 (2008–2011) |
P Value† |
|---|---|---|---|---|
| no./total no. (%)* | ||||
| Neurodevelopmental impairment | 207/424 (49) | 209/459 (46) | 211/487 (43) | 0.25 |
| Neurosensory impairment | 73/413 (18) | 66/457 (14) | 92/487 (19) | 0.18 |
| Moderate or severe cerebral palsy | 62/423 (15) | 50/458 (11) | 56/487 (11) | 0.19 |
| Severe cerebral palsy | 34/424 (8) | 25/459 (5) | 26/487 (5) | 0.18 |
| Profound visual impairment | 10/424 (2) | 7/457 (2) | 2/484 (<1) | 0.04 |
| Profound hearing loss | 17/421 (4) | 16/457 (4) | 14/487 (3) | 0.63 |
| Cognitive impairment | 194/417 (47) | 204/457 (45) | 195/480 (41) | 0.19 |
| Cognitive impairment alone‡ | 123/417 (29) | 141/457 (31) | 119/480 (25) | 0.10 |
The number of children who underwent a Bayley Scales of Infant and Toddler Development examination was 421 in epoch 1, 458 in epoch 2, and 480 in epoch 3. The number of children who underwent a neurologic examination was 424 in epoch 1, 458 in epoch 2, and 487 in epoch 3.
P values were determined using chi-square tests.
Cognitive impairment alone indicates infants with cognitive impairment but without moderate or severe cerebral palsy, profound visual impairment, or profound hearing impairment.
Figure 1. Mortality and Neurodevelopmental Outcomes at 18 to 22 Months of Corrected Age by Birth Epoch and Center.
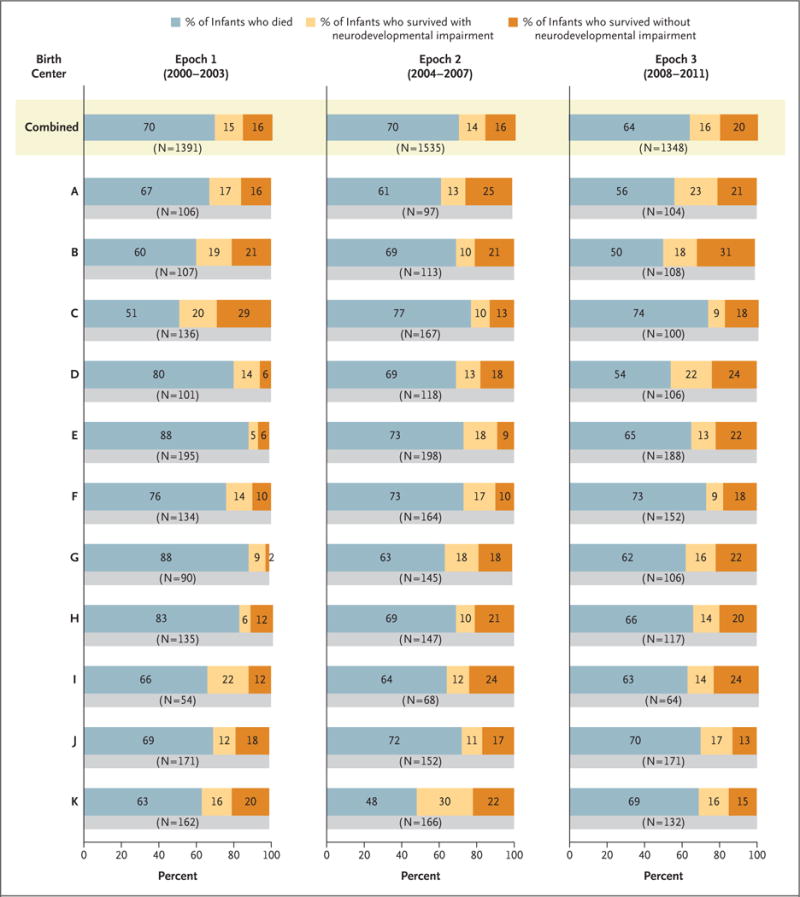
Shown are the rates of death, survival with neurodevelopmental impairment, and survival without neurodevelopmental impairment at the 11 centers that were included in the analysis. The rates were adjusted for gestational age at birth, multiple gestation, sex, race, and small-for-gestational-age status. Because of rounding, percentages may not total 100.
Of the 4274 infants with data on the primary outcome, 4227 infants had complete data on all of the variables included in the generalized logit regression model (46 infants were excluded because of missing data on race and 1 infant was excluded because of missing data on small-for-gestational-age status). After adjusting for baseline characteristics, we found that both the rate of survival with neurodevelopmental impairment versus death and the rate of survival without neurodevelopmental impairment versus death increased between epoch 1 and epoch 3 and between epoch 2 and epoch 3 (Table 4). The increase in the rate of survival without neurodevelopmental impairment was not significantly greater than that of survival with neurodevelopmental impairment. Our findings were similar when we limited the analysis to infants who received active treatment.
Table 4.
Survival and Neurodevelopmental Outcomes at 18 to 22 Months of Corrected Age.
| Outcome | Adjusted Relative Risk (95% CI)* | |
|---|---|---|
| Epoch 3 (2008–2011) vs. Epoch 2 (2004–2007) | Epoch 3 (2008–2011) vs. Epoch 1 (2000–2003) | |
| All infants† | ||
| Survived without neurodevelopmental impairment vs. died | 1.52 (1.22–1.88) | 1.59 (1.28–1.99) |
| Survived with neurodevelopmental impairment vs. died | 1.43 (1.14–1.79) | 1.27 (1.01–1.59) |
| Survived without neurodevelopmental impairment vs. survived with neurodevelopmental impairment | 1.08 (0.83–1.40) | 1.27 (0.99–1.65) |
| Survived without neurosensory impairment vs. died | 1.39 (1.15–1.68) | 1.44 (1.18–1.75) |
| Survived with neurosensory impairment vs. died | 1.93 (1.38–2.70) | 1.54 (1.11–2.15) |
| Survived without neurosensory impairment vs. survived with neurosensory impairment | 0.72 (0.51–1.02) | 0.93 (0.66–1.32) |
| Infants born at 22 wk‡ | ||
| Survived without neurodevelopmental impairment vs. died | 0.74 (0.16–3.47) | 1.30 (0.21–8.08) |
| Survived with neurodevelopmental impairment vs. died | 0.63 (0.20–1.97) | 1.30 (0.34–5.02) |
| Survived without neurodevelopmental impairment vs. survived with neurodevelopmental impairment | 1.13 (0.17–7.41) | 0.99 (0.11–9.34) |
| Infants born at 23 wk§ | ||
| Survived without neurodevelopmental impairment vs. died | 1.29 (0.86–1.94) | 2.31 (1.46–3.66) |
| Survived with neurodevelopmental impairment vs. died | 1.53 (0.98–2.40) | 1.07 (0.71–1.62) |
| Survived without neurodevelopmental impairment vs. survived with neurodevelopmental impairment | 0.86 (0.49–1.50) | 2.17 (1.23–3.83) |
| Infants born at 24 wk¶ | ||
| Survived without neurodevelopmental impairment vs. died | 1.63 (1.26–2.11) | 1.46 (1.12–1.90) |
| Survived with neurodevelopmental impairment vs. died | 1.46 (1.11–1.92) | 1.34 (1.01–1.78) |
| Survived without neurodevelopmental impairment vs. survived with neurodevelopmental impairment | 1.12 (0.83–1.51) | 1.08 (0.79–1.47) |
| Infants receiving active treatment‖ | ||
| Survived without neurodevelopmental impairment vs. died | 1.55 (1.24–1.93) | 1.64 (1.30–2.06) |
| Survived with neurodevelopmental impairment vs. died | 1.44 (1.14–1.81) | 1.30 (1.03–1.64) |
| Survived without neurodevelopmental impairment vs. survived with neurodevelopmental impairment | 1.08 (0.83–1.40) | 1.26 (0.97–1.65) |
Comparisons were adjusted for gestational age (defined as completed weeks of gestation), multiple gestation, sex, race, small-for-gestational-age status, and birth center (random effect). Gestational age was not included in 22-, 23-, and 24-week subgroup analyses.
Data on survival and neurodevelopmental outcomes were available for 4227 infants (46 infants were excluded because of missing data on race, and 1 infant was excluded because of missing data on small-for-gestational-age status).
Data on survival and neurodevelopmental outcomes were available for 737 infants.
Data on survival and neurodevelopmental outcomes were available for 1417 infants.
Data on survival and neurodevelopmental outcomes were available for 2073 infants.
Data on survival and neurodevelopmental outcomes were available for 3158 infants.
After we excluded the Bayley score from our outcomes, we found that both the rate of survival with neurosensory impairment versus death and the rate of survival without neurosensory impairment versus death increased over time, but the increase in survival rate with neurosensory impairment did not differ significantly from the increase in survival rate without neurosensory impairment (Table 4).
Among the infants born at 23 weeks, both the rate of survival without neurodevelopmental impairment versus death and the rate of survival without neurodevelopmental impairment versus survival with neurodevelopmental impairment increased between epoch 1 and epoch 3 (Table 4). Among the infants born at 24 weeks, both the rate of survival with neurodevelopmental impairment versus death and the rate of survival without neurodevelopmental impairment versus death increased over time, but the rates of increase did not differ significantly. There was no significant change in outcomes among the infants born at 22 weeks. However, the 95% confidence intervals for these estimates were wide, which reflects the small sample size in this gestational age group.
In the sensitivity analyses to evaluate changes in outcomes among the infants eligible for the Bayley-III examination (i.e., infants who were born between 2006 and 2011), in which birth year was used in place of epoch in the regression model, we found that the rate of survival without neurodevelopmental impairment versus death increased from 2006 to 2011 (adjusted relative risk, 1.08 per 1-year increase; 95% confidence interval [CI], 1.04 to 1.13). The rate of survival with neurodevelopmental impairment versus death also increased (adjusted relative risk, 1.08; 95% CI, 1.03 to 1.13). The rate of survival without neurodevelopmental impairment versus survival with neurodevelopmental impairment did not change significantly over time (adjusted relative risk, 1.00; 95% CI, 0.96 to 1.05).
DISCUSSION
Our study showed an increase in the rate of survival without neurodevelopmental impairment from 2000 through 2011 in a large cohort of periviable infants born at a consortium of U.S. academic tertiary care centers. A significant decline in mortality over the study period was accompanied by relative increases in both the rate of survival with neurodevelopmental impairment and the rate of survival without neurodevelopmental impairment. The increase in the rate of survival was not associated with a disproportionate increase in the rate of survival with neurodevelopmental impairment; rather, the rate of survival without neurodevelopmental impairment and the rate of survival with neurodevelopmental impairment increased similarly (adjusted relative risk, 1.27; 95% CI, 0.99 to 1.65). These findings are important for guiding counseling and decision making with respect to periviable birth. Prognosis continues to be guarded; in the most recent epoch, mortality was 64%, and 43% of surviving infants had neurodevelopmental impairment.
The improvements in survival and neurodevelopmental outcomes that we observed may reflect advances in obstetrical and neonatal care. We observed declines in the rates of postnatal glucocorticoid use, late-onset sepsis, posthemorrhagic hydrocephalus with shunt placement, and bronchopulmonary dysplasia over time, each of which has been independently associated with adverse neurodevelopmental outcomes.24–27 Proactive perinatal management has been associated with better outcomes among extremely premature infants, including an increased rate of survival and unchanged or reduced rates of disability among survivors.5,20,28–30 In our study, the rates of cesarean delivery and antenatal glucocorticoid use increased over time. The improvement in outcomes was unlikely to be due to changes in the use of active treatment for infants, because the rates of active treatment were similar across epochs and the study findings did not change significantly when we restricted the analysis to infants who received active treatment. Changes in maternal characteristics may have contributed to the improvement in outcomes, because maternal age and level of education increased over time.
Previous studies of survival and neurodevelopmental outcomes among extremely premature infants have shown mixed results, with reports of increased,10,11 unchanged,2,3,31 or decreased rates of neurodevelopmental impairment over time.32,33 Many of these studies involved primarily more mature infants, and it is unclear whether the results can be extrapolated to the periviable population. Comparing neurodevelopmental outcomes across studies in this population would be complicated by sparse data and differences in sample selection, criteria used to define impairment, and age at follow-up. Studies from the United Kingdom, Sweden, and Japan, published in 2012 and 2013, showed rates of neurodevelopmental impairment of 34% (46 of 136 infants), 41% (56 of 138), and 47% (130 of 279), respectively, among surviving infants born at 22 to 24 weeks, as evaluated at 2.5 to 3 years of corrected age, as compared with a rate of 46% (627 of 1370) in our study.20,31,34 Among infants born at 24 weeks, rates of neurodevelopmental impairment were higher in our study (44%) than in these three studies (30 to 37%). It is unclear how much of this variation is due to differences in sample ascertainment, study design, infant characteristics, or care practices. We found variation in outcomes by center; this observation is consistent with the findings from other studies published in 2015 and 2004 that assessed extremely premature infants.5,22
In the subgroup analysis performed according to the gestational week in which the infants were born, we found that the rate of survival without neurodevelopmental impairment increased over time among infants born at 23 weeks and 24 weeks. However, only 1% of infants born at 22 weeks survived without neurodevelopmental impairment in each epoch. Among the 167 infants born at 22 weeks who received active treatment after birth, 9 (5%) survived without neurodevelopmental impairment.
The best measures of neurodevelopmental outcomes in premature infants continue to be debated. Our definition of neurodevelopmental impairment included measures of motor function, sensory impairment, and cognitive delay, which is consistent with the definition used in other studies. Clinicians and families should note that there is likely to be substantial variation in the long-term functioning of children classified as having neurodevelopmental impairment in early childhood. Although early neurodevelopmental assessment is important for the timely identification of children at risk for long-term neurologic impairment or developmental delay, its capacity to predict later functioning is limited.35–37 Many children will catch up to their peers by school age, whereas other children will have persistent impairment. Conversely, some children without signs of neurodevelopmental impairment in early childhood will have impairments that manifest at school age.37,38 Bayley-II Mental Developmental Index and Bayley-III Cognitive Composite scores have been shown to correlate with later cognitive outcomes but account for only a minority of the variance in later cognitive functioning.35,36,39 Reported rates of impairment at school age among children who were born extremely premature are generally lower than those reported in early childhood, but studies have been limited by small sample sizes and heterogeneous results.35,37,40 Additional research is needed to better understand long-term outcomes among periviable infants.
Our analysis was complicated by the transition from Bayley-II to Bayley-III during the study period. Studies involving extremely premature infants have shown that Bayley-III Cognitive Composite scores are, on average, 10 to 11 points higher than the Bayley-II Mental Developmental Index scores.17,18 To address this limitation, we defined cognitive impairment using a conservative Bayley-III Cognitive Composite score lower than 85, which is one standard deviation below the mean score of 100. Johnson et al.21 observed 97% agreement between a Bayley-II Mental Developmental Index score lower than 70 and a Bayley-III Cognitive Composite score lower than 85. Furthermore, we showed an increase in the rate of survival without neurosensory impairment over time, with neurosensory impairment defined by the same components as neurodevelopmental impairment with the exclusion of the Bayley scores. Finally, when we evaluated changes in outcomes in the years since the implementation of Bayley-III, the results were consistent.
Our study has additional limitations. The data represent a select group of infants born in a subset of academic centers and may not be generalizable to other populations. Furthermore, we did not correct for multiple testing, which increases the probability that some of the significant differences that we observed in our secondary outcomes analyses may have occurred by chance. There is a small chance that the changes in outcomes over time reflect random variation alone.
In conclusion, our study showed a small but significant increase in the rate of survival without neurodevelopmental impairment at 18 to 22 months of corrected age among periviable infants. Despite improvements over time, the incidence of death, neurodevelopmental impairment, and other adverse outcomes remains high in this population.
Supplementary Material
Acknowledgments
Although staff members at the Eunice Kennedy Shriver National Institute of Child Health and Human Development contributed to the design and conduct of the study, the analysis of the data, and the drafting of the manuscript, the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supported by grants from the National Institutes of Health (including grants 5T32HD043728-10, HD060558-05, and 4K12HD043494-14 to Dr. Younge) and from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, grants from the National Center for Research Resources, and cooperative agreements from the National Center for Advancing Translational Sciences for the Neonatal Research Network’s Generic Database and Follow-up Studies (U10 HD27904, U10 HD21364, M01 RR80, U10 HD68284, U10 HD27853, M01 RR8084, U10 HD40492, M01 RR30, U10 HD27851, M01 RR39, U10 HD27856, M01 RR750, U10 HD68278, U10 HD36790, U10 HD27880, M01 RR70, UL1 TR93, U10 HD53119, M01 RR54, U10 HD34216, M01 RR32, U10 HD68270, U10 HD40461, U10 HD53109, M01 RR59, U10 HD21397, M01 RR16587, U10 HD27881, U10 HD53089, M01 RR997, U10 HD68244, U10 HD68263, U10 HD40521, UL1 RR24160, M01 RR44, UL1 TR42, U10 HD21415, U10 HD21373, U10 HD40689, M01 RR633, U10 HD53124, M01 RR64, UL1 TR105, U10 HD40498, M01 RR7122, U10 HD21385, U10 HD27871, UL1 RR24139, M01 RR125, and UL1 TR142).
Dr. Patel reports receiving an honorarium from Pediatrix Medical Group, a unit of MEDNAX; and Dr. Cotten, receiving fees for serving on a data and safety monitoring board from rEVO Biologics.
We thank our medical and nursing colleagues and the parents who agreed to have their infants take part in this study.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Raju TN, Mercer BM, Burchfield DJ, Joseph GF. Periviable birth: executive summary of a Joint Workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. J Perinatol. 2014;34:333–42. doi: 10.1038/jp.2014.70. [DOI] [PubMed] [Google Scholar]
- 2.Hintz SR, Kendrick DE, Vohr BR, Poole WK, Higgins RD. Changes in neurodevelopmental outcomes at 18 to 22 months’ corrected age among infants of less than 25 weeks’ gestational age born in 1993–1999. Pediatrics. 2005;115:1645–51. doi: 10.1542/peds.2004-2215. [DOI] [PubMed] [Google Scholar]
- 3.Hintz SR, Kendrick DE, Wilson-Costello DE, et al. Early-childhood neurodevelopmental outcomes are not improving for infants born at <25 weeks’ gestational age. Pediatrics. 2011;127:62–70. doi: 10.1542/peds.2010-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mercier CE, Dunn MS, Ferrelli KR, Howard DB, Soll RF. Neurodevelopmental outcome of extremely low birth weight infants from the Vermont Oxford network: 1998–2003. Neonatology. 2010;97:329–38. doi: 10.1159/000260136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rysavy MA, Li L, Bell EF, et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. 2015;372:1801–11. doi: 10.1056/NEJMoa1410689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kusuda S, Fujimura M, Uchiyama A, Totsu S, Matsunami K. Trends in morbidity and mortality among very-low-birth-weight infants from 2003 to 2008 in Japan. Pediatr Res. 2012;72:531–8. doi: 10.1038/pr.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horbar JD, Carpenter JH, Badger GJ, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012;129:1019–26. doi: 10.1542/peds.2011-3028. [DOI] [PubMed] [Google Scholar]
- 8.Patel RM, Kandefer S, Walsh MC, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372:331–40. doi: 10.1056/NEJMoa1403489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314:1039–51. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115:997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- 11.Claas MJ, Bruinse HW, Koopman C, van Haastert IC, Peelen LM, de Vries LS. Two-year neurodevelopmental outcome of preterm born children ≤750 g at birth. Arch Dis Child Fetal Neonatal Ed. 2011;96:F169–F177. doi: 10.1136/adc.2009.174433. [DOI] [PubMed] [Google Scholar]
- 12.Ballard JL, Novak KK, Driver M. A simplified score for assessment of fetal maturation of newly born infants. J Pediatr. 1979;95:769–74. doi: 10.1016/s0022-3476(79)80734-9. [DOI] [PubMed] [Google Scholar]
- 13.Dubowitz LM, Dubowitz V, Goldberg C. Clinical assessment of gestational age in the newborn infant. J Pediatr. 1970;77:1–10. doi: 10.1016/s0022-3476(70)80038-5. [DOI] [PubMed] [Google Scholar]
- 14.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125(2):e214–e224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 15.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of sub-ependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 16.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vohr BR, Stephens BE, Higgins RD, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J Pediatr. 2012;161(2):222–8.e3. doi: 10.1016/j.jpeds.2012.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore T, Johnson S, Haider S, Hennessy E, Marlow N. Relationship between test scores using the second and third editions of the Bayley Scales in extremely preterm children. J Pediatr. 2012;160:553–8. doi: 10.1016/j.jpeds.2011.09.047. [DOI] [PubMed] [Google Scholar]
- 19.Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW. Underestimation of developmental delay by the new Bayley-III Scale. Arch Pediatr Adolesc Med. 2010;164:352–6. doi: 10.1001/archpediatrics.2010.20. [DOI] [PubMed] [Google Scholar]
- 20.Serenius F, Källén K, Blennow M, et al. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA. 2013;309:1810–20. doi: 10.1001/jama.2013.3786. [DOI] [PubMed] [Google Scholar]
- 21.Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr Res. 2014;75:670–4. doi: 10.1038/pr.2014.10. [DOI] [PubMed] [Google Scholar]
- 22.Vohr BR, Wright LL, Dusick AM, et al. Center differences and outcomes of extremely low birth weight infants. Pediatrics. 2004;113:781–9. doi: 10.1542/peds.113.4.781. [DOI] [PubMed] [Google Scholar]
- 23.Tyson JE, Parikh NA, Langer J, Green C, Higgins RD. Intensive care for extreme prematurity — moving beyond gestational age. N Engl J Med. 2008;358:1672–81. doi: 10.1056/NEJMoa073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–65. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 25.Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R. Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics. 2008;121(5):e1167–e1177. doi: 10.1542/peds.2007-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vohr BR, Wright LL, Dusick AM, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics. 2000;105:1216–26. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 27.Yeh TF, Lin YJ, Lin HC, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. 2004;350:1304–13. doi: 10.1056/NEJMoa032089. [DOI] [PubMed] [Google Scholar]
- 28.Serenius F, Blennow M, Maršál K, Sjörs G, Källen K. Intensity of perinatal care for extremely preterm infants: outcomes at 2.5 years. Pediatrics. 2015;135(5):e1163–e1172. doi: 10.1542/peds.2014-2988. [DOI] [PubMed] [Google Scholar]
- 29.Håkansson S, Farooqi A, Holmgren PA, Serenius F, Högberg U. Proactive management promotes outcome in extremely preterm infants: a population-based comparison of two perinatal management strategies. Pediatrics. 2004;114:58–64. doi: 10.1542/peds.114.1.58. [DOI] [PubMed] [Google Scholar]
- 30.Carlo WA, McDonald SA, Fanaroff AA, et al. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks’ gestation. JAMA. 2011;306:2348–58. doi: 10.1001/jama.2011.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore T, Hennessy EM, Myles J, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. 2012;345:e7961. doi: 10.1136/bmj.e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlapbach LJ, Adams M, Proietti E, et al. Outcome at two years of age in a Swiss national cohort of extremely preterm infants born between 2000 and 2008. BMC Pediatr. 2012;12:198. doi: 10.1186/1471-2431-12-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doyle LW, Roberts G, Anderson PJ. Outcomes at age 2 years of infants < 28 weeks’ gestational age born in Victoria in 2005. J Pediatr. 2010;156(1):49–53.e1. doi: 10.1016/j.jpeds.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Ishii N, Kono Y, Yonemoto N, Kusuda S, Fujimura M. Outcomes of infants born at 22 and 23 weeks’ gestation. Pediatrics. 2013;132:62–71. doi: 10.1542/peds.2012-2857. [DOI] [PubMed] [Google Scholar]
- 35.Hack M, Taylor HG, Drotar D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005;116:333–41. doi: 10.1542/peds.2005-0173. [DOI] [PubMed] [Google Scholar]
- 36.Spencer-Smith MM, Spittle AJ, Lee KJ, Doyle LW, Anderson PJ. Bayley-III Cognitive and Language Scales in preterm children. Pediatrics. 2015;135(5):e1258–e1265. doi: 10.1542/peds.2014-3039. [DOI] [PubMed] [Google Scholar]
- 37.Serenius F, Ewald U, Farooqi A, et al. Neurodevelopmental outcomes among extremely preterm infants 6.5 years after active perinatal care in Sweden. JAMA Pediatr. 2016;170:954–63. doi: 10.1001/jamapediatrics.2016.1210. [DOI] [PubMed] [Google Scholar]
- 38.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 39.Luttikhuizen dos Santos ES, de Kieviet JF, Königs M, van Elburg RM, Oosterlaan J. Predictive value of the Bayley Scales of Infant Development on development of very preterm/very low birth weight children: a meta-analysis. Early Hum Dev. 2013;89:487–96. doi: 10.1016/j.earlhumdev.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Moore GP, Lemyre B, Barrowman N, Daboval T. Neurodevelopmental outcomes at 4 to 8 years of children born at 22 to 25 weeks’ gestational age: a meta-analysis. JAMA Pediatr. 2013;167:967–74. doi: 10.1001/jamapediatrics.2013.2395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


