Abstract
In major depressive disorder, women exhibit higher lifetime prevalence and different antidepressant response rates than men, which illustrates the importance of examining individual differences in the pathophysiology of depression and therapeutic response. In recent years, the consideration of sex in related preclinical research has thus gained interest—particularly in light of novel evidence for rapid-acting antidepressants. Notably, the literature recently revealed a higher sensitivity of females to the antidepressant effects of the N-methyl-D-aspartate receptor antagonist ketamine, in both baseline and preclinical conditions. Combined with its fast-acting and relatively sustained properties, this evidence highlights ketamine as a particularly interesting therapeutic alternative for this sensitive population, and supports the value in considering sex as a critical factor for improved individualized therapeutic strategies.
Introduction
As the global burden of depression continues its rise as the leading cause of disability worldwide [1], the urgent need for more effective treatments is dire. A new wave of excitement, however, has been generated by recent discovery that the N-methyl d-aspartate receptor (NMDAR) antagonist, ketamine, rapidly relieves depressive symptoms and suicidal ideation, particularly amongst those with treatment-resistant depression [2]. Since then, a significant amount of effort has gone into understanding the underlying mechanisms by both preclinical and clinical researchers alike, with the hope of developing novel rapid-acting treatments effective in a broader range of patients [2].
In the era of personalized medicine, a greater focus on identifying biomarkers or predictors of rapid antidepressant response to ketamine has emerged [3], but despite the well-established female preponderance in depressive disorders [1] and sex differences in antidepressant efficacy [4], sex has yet to be investigated as a potential moderating variable. Much like genetic and environmental factors, sex is a naturally-occurring disease and treatment modifier [4,5], such that factors either protecting against disease or enhancing treatment response in one sex may indicate prevention or treatment strategies in the other sex [6]. This review will highlight recent preclinical evidence demonstrating sex differences in the rapid antidepressant-like response to acute low-dose ketamine, and discuss how a variety of factors including stress, hormonal state, context, and the presence of baseline sex differences, significantly contribute to behavioral or molecular readout following ketamine treatment. This new evidence encourages that sex be seen as an important factor influencing the individual’s response to antidepressant treatment rather than a phenotypic dichotomy.
Sex differences in effects of ketamine under baseline conditions
Sex differences in the rapid antidepressant-like effects of ketamine were first reported only a few years ago by work from our lab revealing the heightened sensitivity of female rats to these effects compared to males. These conclusions were demonstrated by the lower dose (2.5 mg/kg) required to rapidly reduce immobility in the forced swim test (FST) and latency to feed in the novelty-suppressed feeding test (NSFT) in naturally-cycling female rats compared to their male counterparts [7]. This finding, using FST measures as a behavioral readout, has since been replicated by our lab in rats [8], and corroborated in mice [9,10].
While studies conducted in mice to date have focused solely on intact females, our work in rats demonstrated that this heightened female sensitivity required cyclic fluctuations of both gonadal estradiol and progesterone in female rats [7,11]. This finding has significance, as we recently reported that cyclic progesterone administration to males was sufficient to significantly enhance their sucrose preference following the same acute low-dose of ketamine to which they have repeatedly been non-responsive [7,8,11]—providing an important example of how one facilitator of treatment response in females may enhance response in males. Conversely, testosterone does not influence male sensitivity to low-dose ketamine in rats in measures of hedonic behavior, but blocks hedonic response of naturally-cycling female rats to low-dose ketamine, likely via disruption of normal cyclic hormonal fluctuations [11].
The higher sensitivity of females to low-dose ketamine interestingly does not simply translate to greater activation of known molecular mediators mammalian target of rapamycin (mTOR) in the medial prefrontal cortex (mPFC), and eukaryotic elongation factor 2 in the hippocampus [7], suggesting that behavioral sex differences in response to ketamine extend beyond differential sensitivity at the molecular level, but rather involve distinct mechanisms in a dose-dependent manner.
Interestingly, daily injections of 10 mg/kg ketamine in mice for 21 days induce anti- or pro-depressant-like effects in males or females, respectively [12], which, while still potentially linked to the females’ higher sensitivity to ketamine, highlights the importance of administration paradigm in preclinical studies and warrants further investigations into the interaction between the treatment regimen and ketamine’s sex-dependent behavioral outcome.
Sex differences in antidepressant response under stress
Although substantial data on brain dysregulations in human depressed subjects are now available, preclinical studies have brought a detailed understanding of their underlying molecular mechanisms and response to therapeutic interventions. In this context, repeated exposures to stress triggers behavioral, molecular, and functional alterations resembling depressive symptoms observed in humans [13]. Notably, because several key mediators of the antidepressant response are sexually biased at baseline or following stress itself, it is critical to first investigate their regulation under chronic stress to better understand how males and females differ in response to antidepressants under pathological conditions.
Sex differences in response to chronic stress
Stress triggers a fast endocrine response characterized by the release of glucocorticoids, which, in the brain, directly control neurotransmission at multiple levels [14]. In addition to being highly dependent on the nature and intensity of the stressor, the consequences of this regulation are affected by both sex and hormonal fluctuations. In male rodents, prolonged exposure to stress or glucocorticoids impairs learning and memory, cognitive performances, and induces anxiety and depressive-like behaviors [13–15], whereas in females, the effects of chronic stress differ in a stress-specific manner [16,17]. For instance, chronic social defeat, restraint, isolation, or unpredictable stress, provoke learning and memory impairments as well as anxiety and depressive-like behaviors in male rats and mice [13], whereas chronic restraint stress induces memory deficits in male but not female rats [18,19]. Similarly, males generally appear more sensitive to the development of anhedonia following chronic mild or isolation stress [8,18,20], whereas females are more sensitive to induction of depressive-like symptoms by subchronic variable stress [21].
These behavioral sex differences are paralleled by coherent adaptations in neuronal activity underlined by dendritic and spine plasticity in key structures such as the hippocampus and mPFC. In these structures, chronic stress generally results in spine loss in both rats and mice [8,22], with concomitant down-regulation of synaptic proteins including synapsin I, PSD-95, and GluR1 [8,23,24], and reduced synaptic function and depressive-like behaviors, as observed in human depressed patients [25]. These effects are specific to males, however, as females show greater spine density in hippocampal CA1 [26,27] and infralimbic neurons projecting to the basolateral amygdala [28]. Notably, we recently found that chronic isolation stress down-regulates spine density and synaptic proteins in the mPFC of both male and female rats [8], indicating that sex differences in stress-induced spinogenesis in the mPFC are stress-specific. Furthermore, these sex differences are also species-specific as while observed in rats, both male and female mice exhibit hippocampal spine loss following chronic restraint stress [22], requiring further consideration when investigating sex-differences in the effects of ketamine on hippocampal spinogenesis.
Despite the lack of data in females, glutamatergic neurotransmission is critically involved in these events, as chronic stress-induced dendritic atrophy is prevented by NMDAR antagonists in males [29–31]. Importantly, stress-induced spine alterations can recover following interruption of the stress [32], in addition to illustrating the highly dynamic nature of neuronal and synaptic plasticity, opens the way for novel therapeutic intervention, and warrants targeting the glutamatergic neurotransmission for antidepressant treatment.
Sex differences in antidepressant response following chronic stress
Since the original detailed description of ketamine’s antidepressant effect in a preclinical model [23], the study of its underlying molecular mechanisms led to the development of a model whereby acute ketamine—through NMDAR inhibition on GABAergic interneurons—increases synaptic strength and subsequent activation of postsynaptic neuroplasticity-promoting pathways such as mTOR and brain-derived neurotrophic factor (BDNF), which, when coupled with extrasynaptic NMDAR inhibition, enhances synaptogenesis [2]. This model, however, originates from studies conducted solely in males. In light of the aforementioned sex differences in synaptic plasticity following chronic stress, and the higher sensitivity of stress-naive females to ketamine’s antidepressant-like effects when compared to males, it is difficult to directly extrapolate these findings to both sexes.
Accordingly, while acute ketamine can reverse chronic stress-induced depressive-like behaviors in male rats and mice, its effects differ in females (Table 1). For instance, although 10 mg/kg ketamine reduces chronic mild stress-induced behavioral despair in the FST 24 hours after acute treatment in both male and female mice, this effect is more pronounced in females but lasts longer in males [9], suggesting that the mechanisms underlying maintenance of ketamine’s lasting antidepressant-like effects in chronically stressed animals may be sexually biased. Alternatively, this sex discrepancy could result from the activation of sexually-distinct molecular mechanisms. Accordingly, we recently analyzed the dose-dependent effects of acute ketamine on spine density on apical dendrites of prelimbic pyramidal neurons of the socially-isolated male and female rat mPFC [8]. Similar to unstressed rats, a single dose of ketamine at 5 but not 2.5 mg/kg reversed isolation-induced behavioral despair in males, whereas both doses were effective in females [8]—findings coherent with the higher female sensitivity to ketamine’s antidepressant-like effects [7,11]. Consistent with the current model for ketamine’s effects on spinogenesis, ketamine reversed the stress-induced spine loss in the male mPFC only at the 5 mg/kg dose, associated with increases in synapsin I, PSD-95, and GluR1. In females, however, neither dose affected synaptic proteins expression or spine density within the mPFC, despite behavioral antidepressant-like effects [8]. Although pharmacokinetic differences or stress-specificity cannot be ruled out, these findings indicate that the molecular mechanisms underlying the reversal of stress-induced depressive-like behaviors differ between sexes.
Table 1.
Summary of sex differences in response to acute ketamine treatment.
| Reference | Species | Stress | Ketamine dose (i.p.) |
Readout | Time post injection |
Ketamine effects in Males vs Females |
|---|---|---|---|---|---|---|
| Behavioral correlates | ||||||
| Carrier and Kabbaj, 2013 | Rats (SD) | No stress | 2.5–10 mg/kg |
FST + NSFT | 30 min | Reduced immobility and latency to feed at 2.5, 5, and 10 mg/kg in females, but only at 5 and 10 mg/kg in males. |
| Carrier and Kabbaj, 2013 | Rats (SD) | No stress | 2.5–10 mg/kg |
Acute SPT | 48 hrs | Increased sucrose preference at 5 and 10 mg/kg in males only (ceiling effect in females). |
| Franceschelli et al., 2015 | Mice (C57BL/6J) |
No stress | 3, 5, 10 mg/kg |
FST | 30 min | Reduced immobility at 3, 5, and 10 mg/kg in females, but only at 5 and 10 mg/kg in males. |
| Franceschelli et al., 2015 | Mice (C57BL/6J) |
No stress | 3, 5, 10 mg/kg |
FST | 24 hrs | Reduced immobility at 5 and 10 mg/kg in females, but only at 10 mg/kg in males. |
| Saland et al., 2016 | Rats (SD) | No stress | 2.5 mg/kg | Continuous SPT monitoring |
Up to 7 days |
Hedonic effect sustained up to 7 days in females only, in an ovarian hormones-dependent manner. |
| Zanos et al., 2016 | Mice (CD-1) |
No stress | 1, 3, 10 mg/kg |
FST | 24 hrs | Reduced immobility at 3 and 10 mg/kg in females, but only at 10 mg/kg in males. |
| Franceschelli et al., 2015 | Mice (C57BL/6J) |
Stress (CMS) | 10 mg/kg | Acute SPT | 6 days | No effect in either sex. |
| Franceschelli et al., 2015 | Mice (C57BL/6J) |
Stress (CMS) | 10 mg/kg | FST | 1 or 7 days | Reduced immobility at day 1 and 7 in males, but only at day 1 in females. |
| Franceschelli et al., 2015 | Mice (C57BL/6J) |
Stress (CMS) | 10 mg/kg | Splash test | 5 days | Reversed CMS-induced reduction in grooming behavior in males, but not females. |
| Sarkar and Kabbaj, 2016 | Rats (SD) | No stress + Stress (IS) |
2.5, 5 mg/kg | FST | 3–5 days | Reduced immobility at 2.5, and 5 mg/kg in females, but only at 5 mg/kg in males. |
| Molecular correlates | ||||||
| Carrier and Kabbaj, 2013 | Rats (SD) | No stress | 2.5, 5 mg/kg | Protein expression in mPFC |
30 min | Increased mTOR phosphorylation in both males and females at 5, but not 2.5 mg/kg. Reduced eEF2 in the male HPC at 5 mg/kg; no effect in females. |
| Saland et al., 2016 | Rats (SD) | No stress | 2.5 mg/kg | Protein expression |
24 hrs | Up-regulation of BDNF in treatment-responsive females, but not males. |
| Sarkar and Kabbaj, 2016 | Rats (SD) | Stress (IS) | 2.5, 5 mg/kg | Dendritic spine density in mPFC |
3 hrs | Reversed stress-induced spine loss at 5 mg/kg in males but not in females. |
| Sarkar and Kabbaj, 2016 | Rats (SD) | Stress (IS) | 2.5, 5 mg/kg | Synaptic protein expression in mPFC |
3 days | Reversed stress-induced down-regulation of synapsin I, PSD95, and GluR1 at 5 mg/kg in males but not in females. |
| Pharmacokinetics | ||||||
| Zanos et al., 2016 | Mice (CD-1) | No stress | 10 mg/kg | Ketamine metabolites |
0–60 min | Higher HNK but not K or NK levels in the brain of females following ketamine administration. |
| Saland et al., unpublished |
Rats (SD) | No stress | 2.5 mg/kg | Ketamine metabolites |
0–180 min | Greater K and NK levels in the plasma and brain of females at early timepoints, but HPC and mPFC specificities in magnitude. |
| Zarate et al., 2012 | Humans | MDD/BP patients |
0.5 mg/kg (i.v.) |
Ketamine metabolites |
40–1,440 min | Higher DHNK and HNK4a/c plasma levels in female MDD and bipolar patients than males. |
| Sigtermans et al., 2009 | Humans | Healthy | 40 ng/ml/15 min (i.v.); 320 ng/ml max |
Ketamine, Norketamine |
2–300 min | Higher S(+)-K and -NK plasma levels in healthy females compared to males, associated with greater female clearance. |
Only experimental data displaying a sex difference are reported. BP: Bipolar Disorder, CMS: Chronic Mild Stress, DHNK: Dehydronorketamine, FST: Forced Swim Test, HNK: Hydroxynorketamine, HPC: Hippocampus, IS: Isolation Stress, K: Ketamine, mPFC: medial Prefrontal Cortex, MDD: Major Depressive Disorder, NSFT: Novelty-suppressed Feeding Test, NK: Norketamine, SD: Sprague-Dawley, SPT: Sucrose Preference Test.
We do possess several elements of interest for such alternative mechanisms, originating from the dependence of females’ higher sensitivity to ketamine on estrogen, progesterone, and hippocampal BDNF [11]. First, hippocampal spinogenesis is markedly influenced by ovarian hormones, and as such varies across the estrous cycle [33]. Second, BDNF is a critical regulator of the antidepressant and spinogenesis-enhancing effects of ketamine in the mPFC [34]. Interestingly, although controversial, multiple studies report differential stress-induced hippocampal BDNF regulation between male and female rodents [35–37], which places hippocampal BDNF as a potential critical mediator of sex differences in sensitivity to both the induction of a depressive-like state, as well as response to ketamine.
While behavioral responses to low-dose ketamine discussed thus far are generally consistent between rats and mice, they can vary between strains. In mice, for example, while ketamine’s antidepressant-like effects can be observed for up to 2 weeks [38], they dissipate by 7 days post-treatment in the male CD-1 strain [39]. Similarly, both male and female ICR mice exhibit an antidepressant-like response to acute 10 mg/kg ketamine, but not 5 mg/kg [40], a dose effective in C57BL6/J mice (Table 1). Moreover, ketamine dose-dependently reduces immobility in the FST in Wistar-Kyoto female rats but not in their relative control Wistar strain [41]. Although the non-response of Wistar rats could result from a flooring effect, these studies illustrate the need for an appropriate selection of strain based on study design. The Wistar-Kyoto strain, for instance, meets several criteria for modeling treatment-resistant depression [42], and given the interest in ketamine’s efficacy in treatment-resistant patients [43], represents an interesting choice for deciphering ketamine’s potential in this population.
Pharmacokinetic considerations and relevance to clinical populations
Once administered, ketamine is predominantly N-demethylated into norketamine (NK), which is further transformed into dehydronorketamine (DHNK) and six diastereomeric hydroxynorketamine (HNK) metabolites [44]. As a highly lipophilic, weak base, ketamine is rapidly distributed to the brain where it readily penetrates the blood-brain barrier via passive diffusion, along with its pharmacologically active metabolites—albeit to a slightly lesser extent as a result of greater hydrophilicity (SK Saland et al., unpublished; [10,45]). As a prerequisite, ketamine’s therapeutic efficacy in a given individual is limited by the availability of unbound drug (and/or metabolites) present at its relevant site(s) of action within the brain, making pharmacokinetic processes fundamental in understanding the heterogeneity in treatment response within and between sexes. Importantly, sex is a variable that influences nearly all pharmacokinetic processes—absorption, distribution, metabolism, and elimination—which may or may not ultimately influence treatment response [6].
Preclinical sex differences in ketamine pharmacokinetics
To this point, recent preclinical work has found that higher HNK, but not ketamine or NK, levels are observed in the brain of female mice following acute administration of 10 mg/kg ketamine (i.p.), in addition to greater female behavioral sensitivity to ketamine’s antidepressant-like effects when compared to males [10]. Excitingly, further experiments showed that systemically administered HNK is able to cross the blood-brain barrier and elicit antidepressant-like activity in mice without inducing ketamine-like side effects. However, sex differences were not examined in this case, so it is unclear whether behavioral sensitivity to HNK differs between males and females. Here, it should be noted that females, but not males, exhibited an antidepressant-like response to 3 mg/kg ketamine, whereas both sexes responded to the 10 mg/kg dose used for pharmacokinetic analysis. Therefore, a direct association between greater HNK levels and enhanced female antidepressant-like response to ketamine cannot be conclusively inferred. Interestingly, new findings from our lab demonstrated greater ketamine and NK exposure in the plasma and brain of cycling female versus male rats following 2.5 mg/kg ketamine—a dose behaviorally effective in females but not males—with regional differences observed when the mPFC and hippocampus were examined independently (SK Saland et al., unpublished). These findings further suggest species differences in not only behavioral, but also pharmacokinetic parameters following low-dose ketamine exposure, and highlight the need for pharmacokinetic analysis across multiple behaviorally-relevant doses across species in both sexes.
Human sex differences in ketamine pharmacokinetics and relationship to clinical response
Surprisingly, very little evidence exists regarding sex differences in ketamine pharmacokinetics in humans, primarily owing to a lack of studies investigating such effects. However, Zarate and colleagues (2012) recently identified modest sex differences in metabolism of low-dose ketamine in MDD and bipolar patients, where females displayed greater plasma levels of DHNK and HNK4a/c metabolites compared to males—however, these differences had no association with treatment response, and no sex differences in antidepressant response were observed. In fact, HNK was negatively associated with treatment response in bipolar depression patients (independent of sex) [46], suggesting that pharmacokinetic sex differences may not actually impact treatment response in clinical depression. Of note, hormone levels were not controlled for in these studies, which may have obscured potential differences in clinical response between sexes. These observations are likely dose-dependent, however, as 20% greater ketamine and NK clearance and lower drug/metabolite concentrations are observed in women than men following ketamine infusion at a higher dose [47]. These sex differences were also reflected at the behavioral level in healthy men and women, with greater effects on cardiac output and heat pain-related indices in men compared to women [48]. Together, the limited clinical data available do not currently support sex differences in rapid antidepressant-response to acute low-dose ketamine; however, preclinical evidence discussed herein may warrant further investigation through the use of proper experimental design and controls for within- and between-sex differences in hormone levels.
Possible explanations for sex- and species-specific pharmacokinetic differences
While underlying factors responsible for these varying differences in ketamine metabolism observed between males and females remain unknown, sex differences in hepatic expression and activity of ketamine-metabolizing cytochrome P450 enzymes are well-known [49]—and subject to hormonal regulation by estrogen, progesterone and testosterone, which also happen to be substrates of several P450 enzymes responsible for ketamine metabolism [6,49]. As well, physiological differences influencing xenobiotic distribution, metabolism and clearance (i.e., body weight, adipose tissue levels and distribution) are present between males and females of a variety of species [6]. Ultimately, whether sex-dependent pharmacokinetic processes contribute to differences between males and females in ketamine’s antidepressant response is unclear, but the evidence strongly supports their consideration both preclinically and clinically. Likewise, non-negligible pharmacokinetic-related species differences have been highlighted herein, encouraging further examination to better translate findings between rodents and humans—an appreciable barrier currently hindering translational neuroscience.
Conclusions
In this review, we discussed the recent advances on sex differences in antidepressant response, while focusing on the novel fast-acting drug ketamine. The increasing inclusion of females in animal models of depression and antidepressant response in the recent years revealed sex-specificities reflected in the case of ketamine by an overall higher sensitivity of females to its antidepressant properties. These observations, however, aren’t consistent, but rather depend on a variety of additional factors including hormonal state, context, and most importantly the presence of baseline sex differences likely to interfere with the behavioral or molecular readout following ketamine treatment. It becomes important to investigate the multiple factors influencing one’s behavior together, revealing the advantage of an individualized approach over a group-based strategy (Figure 1). Indeed, the integration of sex as covariate among other factors would bring preclinical experimental designs closer to the reality of the clinical population’s heterogeneity. Leveraging this heterogeneity in preclinical studies would thus help improve the translatability from preclinical to clinical, and ultimately promote our understanding of sex and individual differences in antidepressant response.
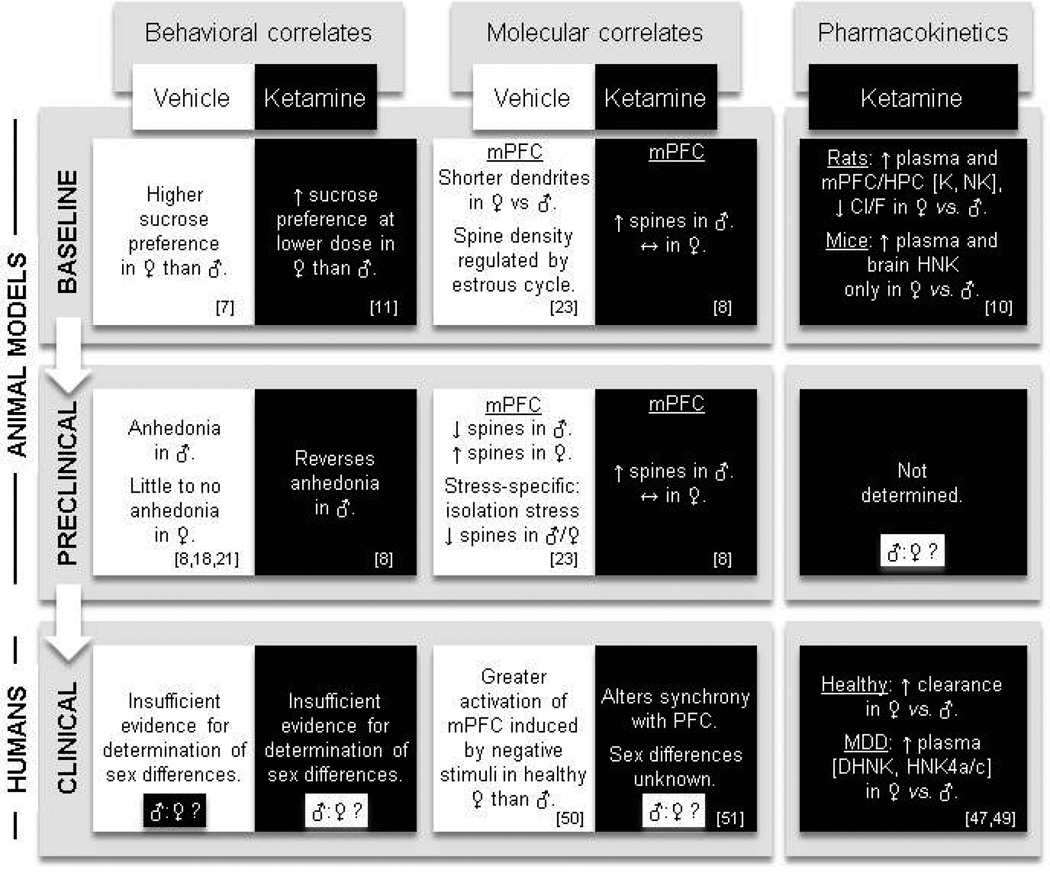
Figure 1.
Conceptual framework for an individualized multi-domain analysis of antidepressant response, applied to sex differences in anti-anhedonic effects of ketamine (as discussed throughout this review). In this diagram, key findings on the current state of knowledge of sex differences in ketamine’s effects on hedonic behaviors across three primary domains of investigation (“Behavioral correlates”, “Molecular correlates”, and “Pharmacokinetics”) are depicted at three main study levels (“Baseline”, “Preclinical”, and “Clinical”). Furthermore, each level × domain combination is summarized with (black background) and without (white background) ketamine treatment, thereby accounting for baseline sex differences when analyzing the drug’s effect(s). Note that all changes in the “Ketamine” category refer to comparisons with vehicle-treated controls (“Vehicle” category of the same “level”). Despite a previous sparsity in experimental evidence, growing preclinical research further characterizes the similarities and inconsistencies in sex differences in the effects of ketamine between rodents and humans across the three study levels defined (baseline, preclinical, and clinical). In this example, for instance, a parallel comparison reveals a consistent sex difference in ketamine-induced spinogenesis in rats between baseline and a preclinical model, whereas such a comparison can be limited either by the presence of a sex difference in the preclinical model itself (as seen for the development of anhedonia in males but not females, “Behavioral correlates” domain), or by the absence of data on eventual sex differences (as seen in the “Pharmacokinetics” domain). Moreover, this example also illustrates the missing consideration of sex differences in clinical populations—both in vehicle- and drug-treated groups—that further limits the translation from the preclinical to clinical level and thus represents a significant barrier to progress in individualized treatment approaches. For each description, corresponding references are listed in the lower right corner. Cl/F: oral clearance, DHNK: dehydronorketamine, HNK: hydroxynorketamine, HPC: hippocampus, K: ketamine, MDD: major depressive disorder, mPFC: medial prefrontal cortex, NK: norketamine.
Highlights.
Ketamine’s antidepressant effects in preclinical models are sexually different
The higher sensitivity of females to ketamine is modulated by hormonal fluctuations
Ketamine response is controlled by interactions between sex, hormones, and context
Sparse clinical consideration of sex differences limits translation from preclinical
Greater consideration of sex would help improve individualized therapeutic approaches
Acknowledgments
This work was supported by the National Institute of Mental Health [grants number MHR01 MH87583 and MH099085 to M.K]. The funding source had no involvement in the study design, collection, analysis and interpretation of data, writing of the manuscript, or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patel V, Vikram P, Dan C, Rachana P, Charlson FJ, Louisa D, Tarun D, Ferrari AJ, Steve H, Ramanan L, et al. Addressing the burden of mental, neurological, and substance use disorders: key messages from Disease Control Priorities, 3rd edition. Lancet. 2016;387:1672–1685. doi: 10.1016/S0140-6736(15)00390-6. [DOI] [PubMed] [Google Scholar]
- 2.Abdallah CG, Adams TG, Kelmendi B, Esterlis I, Sanacora G, Krystal JH. Ketamine’s mechanisms of action: a path to rapid-acting antidepressants. Depress. Anxiety. 2016 doi: 10.1002/da.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarate CA, Mathews DC, Furey ML. Human Biomarkers of Rapid Antidepressant Effects. Biol. Psychiatry. 2013;73:1142–1155. doi: 10.1016/j.biopsych.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keers R, Aitchison KJ. Gender differences in antidepressant drug response. Int. Rev. Psychiatry. 2010;22:485–500. doi: 10.3109/09540261.2010.496448. [DOI] [PubMed] [Google Scholar]
- 5.Becker JB, Prendergast BJ, Liang JW. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol. Sex Differ. 2016;7 doi: 10.1186/s13293-016-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vries GJ, Forger NG. Sex differences in the brain: a whole body perspective [Internet] Biol. Sex Differ. 2015;6 doi: 10.1186/s13293-015-0032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrier N, Kabbaj M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 2013;70:27–34. doi: 10.1016/j.neuropharm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 8. Sarkar A, Kabbaj M. Sex Differences in Effects of Ketamine on Behavior, Spine Density, and Synaptic Proteins in Socially Isolated Rats. Biol. Psychiatry. 2016;80:448–456. doi: 10.1016/j.biopsych.2015.12.025. * Using the chronic social isolation model, the authors report that ketamine dose-dependently reverses stress-induced depressive-like behaviors in both male and female rats, but induces spinogenesis in the medial prefrontal cortex of males but not females. This is the first report of sex-differences in ketamine’s effects on spines density in rodents, suggesting sex-specific mechanisms in the molecular underpinnings of ketamine’s antidepressant effects.
- 9. Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and “depressed” mice exposed to chronic mild stress. Neuroscience. 2015;290:49–60. doi: 10.1016/j.neuroscience.2015.01.008. * In this study, the authors report a higher sensitivity of female C57BL/6J mice to the antidepressant effects of acute ketamine when compared to males, at baseline and following the induction of a depressive-like state by chronic mild-stress. This represents the first report of sex-differences in sensitivity to ketamine in mice.
- 10. Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. * Through the use of pharmacological, behavioral and electrophysiological techniques, this study revealed that the higher sensitivity of female mice to ketamine’s antidepressant effects were associated with greater levels of ketamine’s HNK metabolite in an NMDAR-independent manner--via early and sustained AMPAR activation. Further, through this novel mechanism, HNK produced rapid antidepressant effects without ketamine-related side effects.
- 11. Saland SK, Schoepfer KJ, Mohamed K. Hedonic sensitivity to low-dose ketamine is modulated by gonadal hormones in a sex-dependent manner. Sci. Rep. 2016;6:21322. doi: 10.1038/srep21322. * Through the combination of cyclic hormonal treatments and continuous monitoring of hedonic response to low-dose ketamine in rats, this study reveals the dependence of ketamine’s hedonic properties in female rats on estradiol and progesterone—but not testosterone—associated with greater BDNF hippocampal levels. This study represents the first systematic characterization of the influence of cyclic ovarian but not testicular hormones on the sex-specific effects of ketamine.
- 12.Thelen C, Sens J, Mauch J, Pandit R, Pitychoutis PM. Repeated ketamine treatment induces sex-specific behavioral and neurochemical effects in mice. Behav. Brain Res. 2016;312:305–312. doi: 10.1016/j.bbr.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 13.Czéh B, Fuchs E, Wiborg O, Simon M. Animal models of major depression and their clinical implications. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;64:293–310. doi: 10.1016/j.pnpbp.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 14.McEwen BS, Nasca C, Gray JD. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darcet F, Gardier AM, Gaillard R, David DJ, Guilloux J-P. Cognitive Dysfunction in Major Depressive Disorder. A Translational Review in Animal Models of the Disease [Internet] Pharmaceuticals. 2016;9 doi: 10.3390/ph9010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luine V. Estradiol: Mediator of memories, spine density and cognitive resilience to stress in female rodents. J. Steroid Biochem. Mol. Biol. 2016;160:189–195. doi: 10.1016/j.jsbmb.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franceschelli A, Herchick S, Thelen C, Papadopoulou-Daifoti Z, Pitychoutis PM. Sex differences in the chronic mild stress model of depression. Behav. Pharmacol. 2014;25:372–383. doi: 10.1097/FBP.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 18.Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z. Sex differences in response to stress and expression of depressive-like behaviours in the rat. Curr. Top. Behav. Neurosci. 2011;8:97–118. doi: 10.1007/7854_2010_94. [DOI] [PubMed] [Google Scholar]
- 19.Kitraki E, Kremmyda O, Youlatos D, Alexis MN, Kittas C. Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience. 2004;125:47–55. doi: 10.1016/j.neuroscience.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Mileva GR, Bielajew C. Environmental manipulation affects depressive-like behaviours in female Wistar-Kyoto rats. Behav. Brain Res. 2015;293:208–216. doi: 10.1016/j.bbr.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, Magida J, Brancato A, Takahashi A, Flanigan ME, et al. Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J. Neurosci. 2015;35:16362–16376. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiao H, Li M-X, Xu C, Chen H-B, An S-C, Ma X-M. Dendritic Spines in Depression: What We Learned from Animal Models. Neural Plast. 2016;2016:8056370. doi: 10.1155/2016/8056370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, Li X-Y, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li N, Liu R-J, Dwyer JM, Banasr M, Lee B, Son H, Li X-Y, Aghajanian G, Duman RS. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duman CH, Duman RS. Spine synapse remodeling in the pathophysiology and treatment of depression. Neurosci. Lett. 2015;601:20–29. doi: 10.1016/j.neulet.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLaughlin KJ, Wilson JO, Harman J, Wright RL, Wieczorek L, Gomez J, Korol DL, Conrad CD. Chronic 17beta-estradiol or cholesterol prevents stress-induced hippocampal CA3 dendritic retraction in ovariectomized female rats: possible correspondence between CA1 spine properties and spatial acquisition. Hippocampus. 2010;20:768–786. doi: 10.1002/hipo.20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conrad CD, McLaughlin KJ, Huynh TN, El-Ashmawy M, Sparks M. Chronic stress and a cyclic regimen of estradiol administration separately facilitate spatial memory: relationship with hippocampal CA1 spine density and dendritic complexity. Behav. Neurosci. 2012;126:142–156. doi: 10.1037/a0025770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb. Cortex. 2010;20:2560–2567. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 30.Rubio-Casillas A, Fernández-Guasti A. The dose makes the poison: from glutamatemediated neurogenesis to neuronal atrophy and depression [Internet] Rev. Neurosci. 2016 doi: 10.1515/revneuro-2015-0066. [DOI] [PubMed] [Google Scholar]
- 31.Shors TJ, Falduto J, Leuner B. The opposite effects of stress on dendritic spines in male vs. female rats are NMDA receptor-dependent. Eur. J. Neurosci. 2004;19:145–150. doi: 10.1046/j.1460-9568.2003.03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radley JJ, Rocher AB, Janssen WGM, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp. Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Kato A, Hojo Y, Higo S, Komatsuzaki Y, Murakami G, Yoshino H, Uebayashi M, Kawato S. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front. Neural Circuits. 2013;7:149. doi: 10.3389/fncir.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu R-J, Lee FS, Li X-Y, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol. Psychiatry. 2012;71:996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Y, Ter Horst GJ, Wichmann R, Bakker P, Liu A, Li X, Westenbroek C. Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cereb. Cortex. 2009;19:1978–1989. doi: 10.1093/cercor/bhn225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niknazar S, Nahavandi A, Peyvandi AA, Peyvandi H, Akhtari AS, Karimi M. Comparison of the Adulthood Chronic Stress Effect on Hippocampal BDNF Signaling in Male and Female Rats. Mol. Neurobiol. 2016;53:4026–4033. doi: 10.1007/s12035-015-9345-5. [DOI] [PubMed] [Google Scholar]
- 37.Autry AE, Adachi M, Cheng P, Monteggia LM. Gender-specific impact of brainderived neurotrophic factor signaling on stress-induced depression-like behavior. Biol. Psychiatry. 2009;66:84–90. doi: 10.1016/j.biopsych.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alphaamino- 3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol. Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 39.Bechtholt-Gompf AJ, Smith KL, John CS, Kang HH, Carlezon WA, Jr, Cohen BM, Ongür D. CD-1 and Balb/cJ mice do not show enduring antidepressant-like effects of ketamine in tests of acute antidepressant efficacy. Psychopharmacology. 2011;215:689–695. doi: 10.1007/s00213-011-2169-8. [DOI] [PubMed] [Google Scholar]
- 40.Kara NZ, Agam G, Anderson GW, Zitron N, Einat H. Lack of effect of chronic ketamine administration on depression-like behavior or frontal cortex autophagy in female and male ICR mice [Internet] Behav. Brain Res. 2016 doi: 10.1016/j.bbr.2016.09.056. [DOI] [PubMed] [Google Scholar]
- 41.Tizabi Y, Bhatti BH, Manaye KF, Das JR, Akinfiresoye L. Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar-Kyoto rats. Neuroscience. 2012;213:72–80. doi: 10.1016/j.neuroscience.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willner P, Belzung C. Treatment-resistant depression: are animal models of depression fit for purpose? Psychopharmacology. 2015;232:3473–3495. doi: 10.1007/s00213-015-4034-7. [DOI] [PubMed] [Google Scholar]
- 43.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatmentresistant major depression. Arch. Gen. Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 44.Mion G, Villevieille T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings) CNS Neurosci. Ther. 2013;19:370–380. doi: 10.1111/cns.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moaddel R, Sanghvi M, Ramamoorthy A, Jozwiak K, Singh N, Green C, O’Loughlin K, Torjman M, Wainer IW. Subchronic administration of (R,S)-ketamine induces ketamine ring hydroxylation in Wistar rats. J. Pharm. Biomed. Anal. 2016;127:3–8. doi: 10.1016/j.jpba.2016.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zarate CA, Nancy B, Gonzalo L, Luckenbaugh DA, Vattem Venkata SL, Anuradha R, Ruin M, Wainer IW. Relationship of Ketamine’s Plasma Metabolites with Response, Diagnosis, and Side Effects in Major Depression. Biol. Psychiatry. 2012;72:331–338. doi: 10.1016/j.biopsych.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sigtermans M, Dahan A, Mooren R, Bauer M, Kest B, Sarton E, Olofsen E. S(+)- ketamine effect on experimental pain and cardiac output: a population pharmacokineticpharmacodynamic modeling study in healthy volunteers. Anesthesiology. 2009;111:892–903. doi: 10.1097/ALN.0b013e3181b437b1. [DOI] [PubMed] [Google Scholar]
- 48.Sigtermans M, Marnix S, Ingeborg N, Elise S, Martin B, René M, Erik O, Albert D. An observational study on the effect of S( )-ketamine on chronic pain versus experimental acute pain in Complex Regional Pain Syndrome type 1 patients. Eur. J. Pain. 2010;14:302–307. doi: 10.1016/j.ejpain.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 49.Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol. Pharmacol. 2009;76:215–228. doi: 10.1124/mol.109.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a metaanalysis of neuroimaging studies. Neuropsychologia. 2012;50:1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 51.Becker R, Braun U, Schwarz AJ, Gass N, Schweiger JI, Weber-Fahr W, Schenker E, Spedding M, Clemm von Hohenberg C, Risterucci C, et al. Species-conserved reconfigurations of brain network topology induced by ketamine. Transl. Psychiatry. 2016;6:e786. doi: 10.1038/tp.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]