Abstract
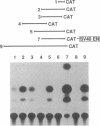
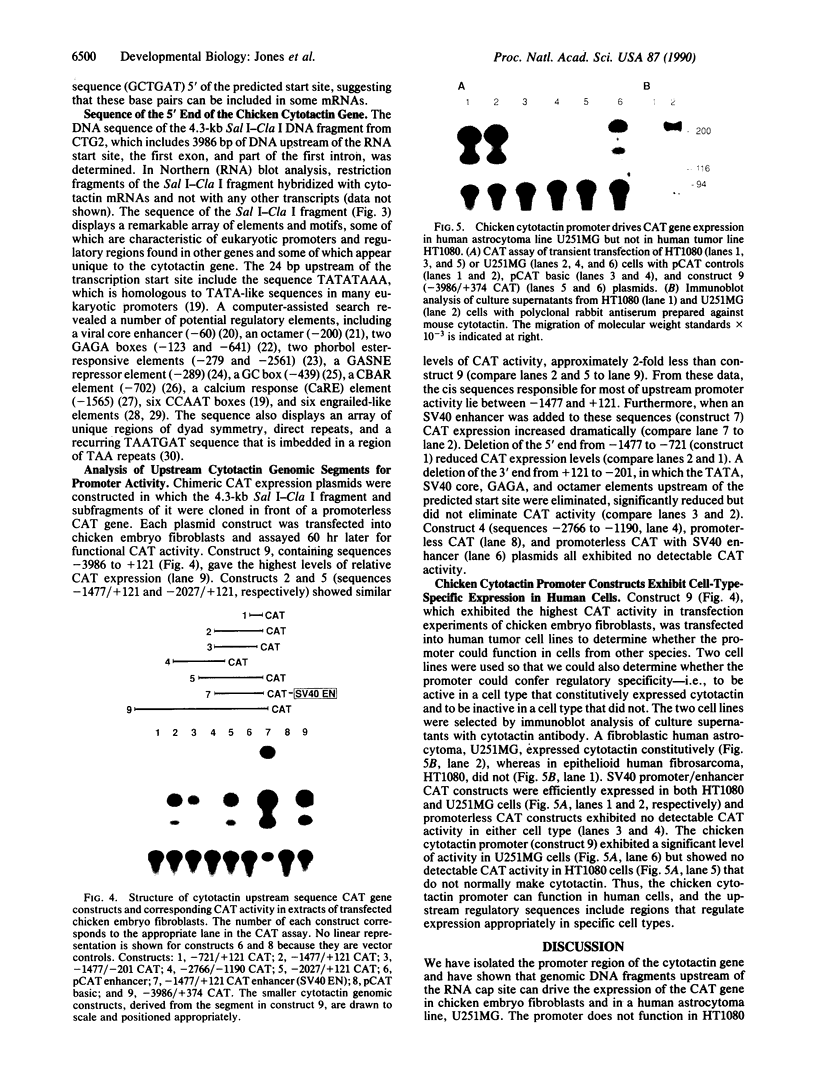
The extracellular glycoprotein cytotactin is expressed in a characteristic and complex spatiotemporal sequence during development of the chicken embryo. To identify the various control elements underlying its expression, the promoter region of the cytotactin gene has been isolated and characterized. Clones were isolated from genomic libraries by using a fragment near the 5' end of the cDNA sequence. The sequence of this cDNA fragment was found to be distributed over two exons separated by a large first intron. The site of transcription initiation was determined by S1 nuclease and primer-extension mapping. Sequencing of a 4.3-kilobase (kb) genomic DNA clone that contains 3986 base pairs (bp) upstream of the RNA start site, the first exon, and part of the first intron revealed a number of sequence motifs implicated in the regulation and expression of eukaryotic genes. These included CCAAT boxes, phorbol ester-responsive elements, enhancer elements, and a consensus TATA sequence located 24 bp upstream of the major RNA cap site. The flanking sequence also contained a number of regions of dyad symmetry and direct repeats unique to cytotactin, as well as an array of A + T-rich sequences that resemble engrailed elements. Constructs containing fragments of the upstream region of the cytotactin gene fused to a promoterless gene for chloramphenicol acetyltransferase were transiently transfected into chicken embryo fibroblasts to define functional promoter sequences. Although sequences from -721 to +121 exhibited minimal promoter activity, the entire region between -3986 to +374 was required to yield maximal expression in chicken embryo fibroblasts. Transfection of the -3986/+374 chloramphenicol acetyltransferase plasmid into the human U251MG astrocytoma cells but not HT1080 fibrosarcoma cells resulted in chloramphenicol acetyltransferase expression, consistent with the observed synthesis of cytotactin protein only by the U251MG cell line. These data indicate that the chicken cytotactin promoter can control expression in a cell type-specific fashion within cells of another species. These studies provide a basis for the dissection of cis elements and trans factors that govern the developmental expression of the cytotactin gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Beachy P. A., Krasnow M. A., Gavis E. R., Hogness D. S. An Ultrabithorax protein binds sequences near its own and the Antennapedia P1 promoters. Cell. 1988 Dec 23;55(6):1069–1081. doi: 10.1016/0092-8674(88)90251-6. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Tjian R. Transcription factors that activate the Ultrabithorax promoter in developmentally staged extracts. Cell. 1988 Jun 3;53(5):699–711. doi: 10.1016/0092-8674(88)90088-8. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Mackie E. J., Pearson C. A., Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell. 1986 Oct 10;47(1):131–139. doi: 10.1016/0092-8674(86)90374-0. [DOI] [PubMed] [Google Scholar]
- Chow K. L., Schwartz R. J. A combination of closely associated positive and negative cis-acting promoter elements regulates transcription of the skeletal alpha-actin gene. Mol Cell Biol. 1990 Feb;10(2):528–538. doi: 10.1128/mcb.10.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong C. M., Crossin K. L., Edelman G. M. Sequential expression and differential function of multiple adhesion molecules during the formation of cerebellar cortical layers. J Cell Biol. 1987 Feb;104(2):331–342. doi: 10.1083/jcb.104.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossin K. L., Hoffman S., Grumet M., Thiery J. P., Edelman G. M. Site-restricted expression of cytotactin during development of the chicken embryo. J Cell Biol. 1986 May;102(5):1917–1930. doi: 10.1083/jcb.102.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossin K. L., Hoffman S., Tan S. S., Edelman G. M. Cytotactin and its proteoglycan ligand mark structural and functional boundaries in somatosensory cortex of the early postnatal mouse. Dev Biol. 1989 Dec;136(2):381–392. doi: 10.1016/0012-1606(89)90264-9. [DOI] [PubMed] [Google Scholar]
- Crossin K. L., Prieto A. L., Hoffman S., Jones F. S., Friedlander D. R. Expression of adhesion molecules and the establishment of boundaries during embryonic and neural development. Exp Neurol. 1990 Jul;109(1):6–18. doi: 10.1016/s0014-4886(05)80004-4. [DOI] [PubMed] [Google Scholar]
- Dean D. C., Bowlus C. L., Bourgeois S. Cloning and analysis of the promotor region of the human fibronectin gene. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1876–1880. doi: 10.1073/pnas.84.7.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplan C., Theis J., O'Farrell P. H. The sequence specificity of homeodomain-DNA interaction. Cell. 1988 Sep 23;54(7):1081–1090. doi: 10.1016/0092-8674(88)90123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Bourdon M. A. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol. 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Inglesias J. L. A six-armed oligomer isolated from cell surface fibronectin preparations. Nature. 1984 Sep 20;311(5983):267–269. doi: 10.1038/311267a0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M., Hoffman S., Crossin K. L., Edelman G. M. Cytotactin, an extracellular matrix protein of neural and non-neural tissues that mediates glia-neuron interaction. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8075–8079. doi: 10.1073/pnas.82.23.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulcher J. R., Nies D. E., Marton L. S., Stefansson K. An alternatively spliced region of the human hexabrachion contains a repeat of potential N-glycosylation sites. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1588–1592. doi: 10.1073/pnas.86.5.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman S., Crossin K. L., Edelman G. M. Molecular forms, binding functions, and developmental expression patterns of cytotactin and cytotactin-binding proteoglycan, an interactive pair of extracellular matrix molecules. J Cell Biol. 1988 Feb;106(2):519–532. doi: 10.1083/jcb.106.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F. S., Burgoon M. P., Hoffman S., Crossin K. L., Cunningham B. A., Edelman G. M. A cDNA clone for cytotactin contains sequences similar to epidermal growth factor-like repeats and segments of fibronectin and fibrinogen. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2186–2190. doi: 10.1073/pnas.85.7.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F. S., Hoffman S., Cunningham B. A., Edelman G. M. A detailed structural model of cytotactin: protein homologies, alternative RNA splicing, and binding regions. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1905–1909. doi: 10.1073/pnas.86.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen P. D., Burbelo P. D., Martin G. R., Yamada Y. Characterization of the promoter for the alpha 1 (IV) collagen gene. DNA sequences within the first intron enhance transcription. J Biol Chem. 1988 Sep 5;263(25):12310–12314. [PubMed] [Google Scholar]
- Laherty C. D., Gierman T. M., Dixit V. M. Characterization of the promoter region of the human thrombospondin gene. DNA sequences within the first intron increase transcription. J Biol Chem. 1989 Jul 5;264(19):11222–11227. [PubMed] [Google Scholar]
- Okamoto K., Okazawa H., Okuda A., Sakai M., Muramatsu M., Hamada H. A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell. 1990 Feb 9;60(3):461–472. doi: 10.1016/0092-8674(90)90597-8. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Factors involved in specific transcription by human RNA polymerase II: analysis by a rapid and quantitative in vitro assay. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4394–4398. doi: 10.1073/pnas.82.13.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Cromlish J. A., Gerster T., Kawakami K., Balmaceda C. G., Currie R. A., Roeder R. G. A human lymphoid-specific transcription factor that activates immunoglobulin genes is a homoeobox protein. Nature. 1988 Dec 8;336(6199):551–557. doi: 10.1038/336551a0. [DOI] [PubMed] [Google Scholar]
- Sheng M., McFadden G., Greenberg M. E. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990 Apr;4(4):571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- Spring J., Beck K., Chiquet-Ehrismann R. Two contrary functions of tenascin: dissection of the active sites by recombinant tenascin fragments. Cell. 1989 Oct 20;59(2):325–334. doi: 10.1016/0092-8674(89)90294-8. [DOI] [PubMed] [Google Scholar]
- Steindler D. A., Cooper N. G., Faissner A., Schachner M. Boundaries defined by adhesion molecules during development of the cerebral cortex: the J1/tenascin glycoprotein in the mouse somatosensory cortical barrel field. Dev Biol. 1989 Jan;131(1):243–260. doi: 10.1016/s0012-1606(89)80056-9. [DOI] [PubMed] [Google Scholar]
- Tan S. S., Crossin K. L., Hoffman S., Edelman G. M. Asymmetric expression in somites of cytotactin and its proteoglycan ligand is correlated with neural crest cell distribution. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7977–7981. doi: 10.1073/pnas.84.22.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. C., Brand S. J. Islet cell-specific regulatory domain in the gastrin promoter contains adjacent positive and negative DNA elements. J Biol Chem. 1990 May 25;265(15):8908–8914. [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]