Abstract
Alkane biosynthesis in the bacterium Vibrio furnissii M1 involves the synthesis of long-chain alkanes via 1-alcohol. Evidence for this novel pathway are the following. (i) Both even- and odd-carbon-number n-alkanes were produced from glucose, while only even-carbon-number fatty acids were produced in V. furnissii M1. This result cannot be explained by the decarbonylation pathway. (ii) Pentadecane and hexadecane were produced from 1-hexadecanoic acid by membrane fractions of V. furnissii M1, and radioisotope precursor-tracer experiments, in which 1-[1-14C]hexadecanoic acid was fed, identified the corresponding alcohol, aldehyde, and alkane derivatives. Since all metabolites maintained the radioisotope label at 1-C, they were produced by a pathway in which the carbon structure was retained, i.e., a reduction pathway. (iii) n-Hexadecane was produced when 1-hexadecanol was fed to membrane preparations.
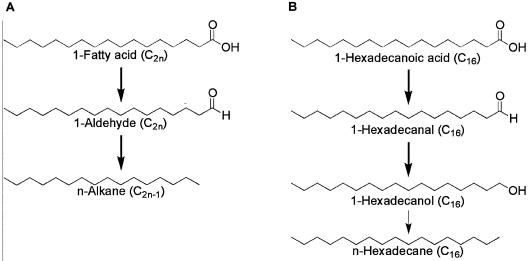
Long-chain alkanes are ubiquitous in living organisms, although only a trace amount of these alkanes are present (9, 16). It has long been believed that the decarbonylation of fatty aldehydes is the only pathway for alkane production (3, 6-8). Figure 1A shows the reactions involved. First, a fatty acid elongase elongates hexadecanoic acid to an even-carbon-number fatty acid (e.g., C2n); then, a fatty acid reductase reduces the fatty acids to aldehydes (C2n); and finally, aldehyde decarbonylase decarbonylates the aldehydes to yield alkanes (C2n−1). It has been reported that a much larger proportion of odd-carbon-number alkanes than even-carbon-number alkanes was present in higher plants (9, 16). Since even-carbon-number fatty acids are produced as the major fatty acid components in most organisms (9), this pathway explains well the predominance of odd-carbon-number n-alkanes.
FIG. 1.
Alkane biosynthesis pathway in eucaryotes (A) and proposed pathway in V. furnissii M1 from the results of this study (B). The thin arrow indicates the pathway elucidated by this study.
In contrast, similar levels of even- and odd-carbon-number alkanes were found in some bacteria (1), while even-carbon-number fatty acids were predominant in these bacteria (1). The decarbonylation pathway does not adequately explain these facts, and this prompted us to consider that an alternative alkane synthesis pathway may exist in the bacteria.
Parker et al. have isolated a Vibrio furnissii M1 strain with a high alkane-producing ability (12) from an environmental sample. The strain produces alkanes of C14 to C22 (Table 1) when using glucose as the sole carbon source (13). No pattern was apparent in the concentration of even- and odd-carbon number alkanes, while even-carbon-number fatty acids were predominant in the culture (Table 1). This result agreed well with the results of the previous report (1).
TABLE 1.
Fatty acids and alkanes produced from glucose by V. furnissii M1
| Compounda | Carbon no. (relative ratio)b |
|---|---|
| Fatty acid | C14:0 (0.08), C15:0 (0.01), C16:0 (0.20), C16:1 (0.30), C18:0 (0.07), C18:1 (0.15), C20:0 (0.01), C24:0 (0.18) |
| n-Alkane | C14 (0.03), C15 (0.13), C16 (0.12), C17 (0.20), C18 (0.27), C19 (0.15), C20 (0.05), C21 (0.02), C22 (0.03) |
Fatty acids were reacted with (N,N)-dimethylformamide dimethyl acetal and detected in the form of methyl ester derivatives in the GC-MS assay. The composition was calculated for a 24-h-old culture.
Mean values for fatty-acids (n = 3, standard deviations < 0.10) and for n-alkanes (n = 3, standard deviations < 0.25).
In the present study, I tested the hypothesis that an alternative pathway for alkane synthesis exists in V. furnissii M1 based on the fact that when 1-[1-14C]hexadecanoic acid was fed, the corresponding alcohol, aldehyde, and alkane derivatives was produced. This pathway involves alkanes being synthesized via 1-alcohol, the reduction of fatty alcohols to alkanes not having previously been reported.
MATERIALS AND METHODS
Culture conditions and preparation of cell extract fraction.
V. furnissii M1 (12) was grown with shaking overnight in 200 ml of Luria-Bertani medium (15) supplemented with 3% (wt/vol) sodium chloride (2) at 37°C. Cells were harvested by centrifugation (6,000 × g; 10 min) and resuspended in 20 ml of 10 mM sucrose-10 mM Tris-HCl (pH 8.0) containing an EDTA-free protease inhibitor (Roche Applied Science) and disrupted by sonication with a Branson Sonifier 250 (Branson) at a power setting of 4 and a 40% duty cycle on ice. The resulting homogenate was centrifuged at 8,000 × g for 10 min at 4°C to remove the cell debris. The resulting supernatant was centrifuged at 105,000 × g for 60 min at 4°C. The pellet formed, which is designated as the membrane fraction, was resuspended in 1 ml of the same buffer containing 0.1% (wt/vol) Triton X-100. The protein concentration was determined according to method of Bradford (4) by a protein detection kit (Bio-Rad).
Enzyme assay.
A 1-mg aliquot of the membrane fraction was suspended in 500 μl of 10 mM Tris-HCl (pH 8.0) containing 25 μM NADH, 25 μM NADPH, 3.3 mM glutathione, and 330 μM MgCl2. 1-Hexadecanoic acid (25 μM; >99% chemical purity; Sigma) was added to the mixture after adding 0.1% (wt/vol) Triton X-100 and was dispersed by sonication (6). 1-Hexadecanoic acid (25 μM) and 740-kBq 1-[1-14C]hexadecanoic acid (99.2% radiochemical purity; Amersham Pharmacia) were used as substrates in the radioisotope tracer experiments. Alternatively, 25 μM 1-hexadecanol (>99% chemical purity; Sigma) and 37-kBq 1-[1-14C]hexadecanol (98% radiochemical purity; Sigma) were used. Each reaction mixture was incubated for 40 min at 37°C, and the neutral lipids were then immediately extracted twice with 1 ml of hexane. After reducing the amount of solvent under nitrogen reflux, the solution was spotted onto a silica gel plate (Merck). Before applying the sample, a mixture of authentic cold hexadecanoic acid, hexadecanal, hexadecanol, and hexadecane was spotted onto the origin point of the plate to prevent any change in Rf value of the alkanes due to downsizing the run in the radioisotope experiments. Developing and visualization were done in the same manner as that described in previous reports (12, 13). In brief, the silica gel plate was developed with a solvent system of 80:20:1 (hexane-diethyl ether-water). The spots were visualized by exposing the plate to iodine vapor.
The alkanes were recovered from the silica gel plate (13), extracted in 1 ml of chloroform, and then analyzed by gas chromatography-mass spectrometry (GC-MS) (a 5890A instrument for GC and a 5989A instrument for MS; Hewlett Packard). Each sample was analyzed twice in the GC-MS assay with electron ionization and positive chemical ionization MS. Electron ionization MS yielded a larger fragment ion peak for n-alkane, while a larger parent ion peak was obtained by positive chemical ionization MS.
The spots corresponding to hexadecanoic acid, hexadecanal, hexadecanol, and hexadecane were extracted from the silica gel plates, and the lipids were dissolved in an AL-1 scintillation cocktail (Dojindo) for radioisotope tracer experiments. The cocktail was directly measured by a liquid scintillation counter as described elsewhere (3). Carbon monoxide was trapped with RhCl[(C6H6)3P]3 and measured as described elsewhere (8). Negative controls were carried out with membrane fractions boiled for 10 min and then quickly cooled on ice for 2 min. Another control without membrane fractions was also run in all experiments. The activity to convert 1-hexadecanoic acid to alkane or to convert 1-hexadecanol to alkane was localized in the membrane fraction.
RESULTS AND DISCUSSION
Synthesis of two alkanes from 1-hexadecanoic acid.
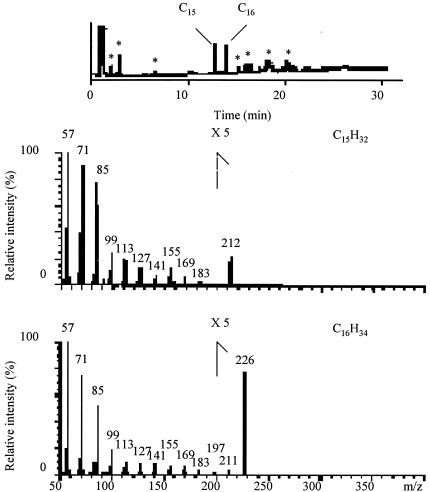
The alkanes produced from 1-hexadecanoic acid in the presence of the membrane fraction of V. furnissii M1 were analyzed by GC-MS, by using simultaneous electron ionization and positive chemical ionization MS. GC chromatograms revealed peaks corresponding to the retention times of authentic n-pentadecane (11.6 min) and n-hexadecane (12.9 min) (Fig. 2). The first GC fraction corresponding to n-pentadecane gave mass spectra at m/z 57 (C4H9+), 71 (C5H11+), 85 (C6H13+), 99 (C7H15+), 113 (C8H17+), 127 (C9H19+), 141 (C10H21+), 155 (C11H23+), 169 (C12H26+), 183 (C13H27+), and 212 (M+: C15H32) in the positive chemical ionization mode (Fig. 2). The same fraction gave mass spectra at m/z 57 (C4H9+), 71 (C5H11+), 85 (C6H13+), 99 (C7H15+), 113 (C8H17+), 127 (C9H19+), 141 (C10H21+), 155 (C11H23+), 169 (C12H26+), 183 (C13H27+), 197 (C14H29+), 211 (M-1), and 212 m/z (M+) in the electron ionization mode, the peak height decreasing with increasing mass. This GC fraction was therefore confirmed as n-pentadecane. The second GC fraction corresponding to n-hexadecane gave mass spectra at m/z 57 (C4H9+), 71 (C5H11+), 85 (C6H13+), 99 (C7H15+), 113 (C8H17+), 127 (C9H19+), 141 (C10H21+), 155 (C11H23+), 169 (C12H26+), 183 (C13H27+), 197 (C14H29+), 211 (C15H31+), and 226 (M+: C16H34) in the positive chemical ionization mode. The same fraction gave mass spectra at m/z 57 (C4H9+), 71 (C5H11+), 85 (C6H13+), 99 (C7H15+), 113 (C8H17+), 127 (C9H19+), 141 (C10H21+), 155 (C11H23+), 169 (C12H26+), 183 (C13H27+), 197 (C14H29+), 211 (C15H31+), 225 (M-1), and 226 (M+) in the electron ionization mode, the peak height decreasing with increasing mass. This GC fraction was therefore confirmed as hexadecane. No alkanes were detected in the negative control runs when using denatured protein or without adding protein (results not shown).
FIG. 2.
GC-MS data for the alkanes produced from 1-hexadecanoic acid in the presence of the membrane fraction from V. furnissii M1. nsrsid87594\delrsid87594 *, background noise, not alkane.
The formation of pentadecane can be explained by decarbonylation of the aldehyde formed by the reduction of 1-hexadecanoic acid which involves the alkane biosynthesis pathway known in higher plants and a microalga, which has not been reported in bacteria yet. On the other hand, the formation of hexadecane from 1-hexadecanoic acid cannot be explained by this decarbonylation route; therefore, the existence of an alternative pathway that does not subtract the carbon of 1-hexadecanoic acid is suggested
Precursors in the alkane synthesis from 1-[1-14C]hexadecanoic acid.
Alcohol as well as aldehyde and alkane with radioisotope labels were produced from 1-[1-14C]hexadecanoic acid in the presence of the membrane fraction of V. furnissii M1 as shown in Table 2. Since only the 1-C site was labeled with the radioisotope in this experiment, these labeled chemicals retaining the 1-C structure respectively corresponded to 1-hexadecanol, 1-hexadecanal, and hexadecane. Carbon monoxide with the radioisotope label was also detected, confirming that decarbonylation had occurred.
TABLE 2.
Radioactivity in bacterial fractions produced from 1-[1-14C]hexadecanoic acid
| Chemical | Radioactivity (cpm)a with:
|
||
|---|---|---|---|
| Membrane fraction | Boiled membrane fraction | No protein | |
| Carbon monoxide | 2,800 ± 900 | 40 ± 5 | 30 ± 3 |
| 1-Hexadecanoic acid | 19,000 ± 590 | 650,000 ± 76,000 | 600,000 ± 100,000 |
| 1-Hexadecanal | 60,000 ± 18,000 | 40 ± 6 | 40 ± 7 |
| 1-Hexadecanol | 14,000 ± 400 | 40 ± 9 | 30 ± 4 |
| n-Hexadecane | 470,000 ± 85,000 | 30 ± 1 | 30 ± 5 |
Each value is presented as the mean ± standard deviation: (n = 3). The reaction was run for 40 min.
Since 1-hexadecanal and 1-hexadecanol were produced from 1-hexadecanoic acid in this experiment, a plausible pathway for alkane synthesis was the successive reduction of the fatty acid. These experimental results support the existence of two pathways for biosynthesis of alkanes.
Alkane synthesis from 1-[1-14C]hexadecanol.
Since alcohol was found as a potential precursor in alkane synthesis, synthesis of hexadecane from 1-[1-14C]hexadecanol was assayed in the presence of the membrane fraction of V. furnissii M1. High amounts of hexadecane (11,000 ± 2,000 cpm) were detected while no radioactive alkane was detected in the negative control assays.
Our results show that alkane synthesis in V. furnissii M1 can proceed as shown in Fig. 1B. First, 1-hexadecanal is produced from 1-hexadecanoic acid; then, the aldehyde is oxidized to 1-hexadecanol, or alternatively, pentadecane and carbon monoxide are produced from 1-hexadecanal; and then, hexadecane is produced from 1-hexadecanol.
No precedent so far has been detected for the reduction of an alcohol to aldehyde in living organisms for alkane biosynthesis. On the other hand, the reverse pathway, in which an alcohol is produced from an alkane, has been reported for several bacteria (5, 10, 11, 14).
The results of this study offer new insight about the occurrence of even-carbon-number n-alkane biosynthesis in microbes and support the occurrence of even-carbon-number n-alkanes in microorganisms.
Acknowledgments
I am grateful to K. Matsumoto and K. Nanba of Shionogi and Co. Ltd. for technical advice on the GC-MS analysis and to Shigeaki Harayama, Norihide Kurano, Hiroyasu Nagase, Kazumasa Hirata, and Kazuhisa Miyamoto for useful comments.
REFERENCES
- 1.Bagaeva, T. 1997. The ability of sulfate-reducing bacteria of various taxonomic groups to synthesize extracellular hydrocarbons. Microbiology 66:666-668. [Google Scholar]
- 2.Baumann, P., and R. H. W. Schubert. 1984. Family II. Vibrionaceae Véron 1965, 5245AL, p. 516-550. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. Williams & Wilkins, Baltimore, Md. [Google Scholar]
- 3.Belhaddad, F. S., and P. E. Kolattukudy. 2000. Solubilization, partial purification, and characterization of a fatty aldehyde decarbonylase from a higher plant, Pisum sativum. Arch. Biochem. Biophys. 377:341-349. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Cardini, G., and P. Jurtshuk. 1970. The enzymatic hydroxylation of N-octane by Corynebacterium sp. strain 7E1C. J. Biol. Chem. 245:2789-2796. [PubMed] [Google Scholar]
- 6.Cheesbrough, T. M., and P. E. Kolattukudy. 1984. Alkane biosynthesis by decarbonylation of aldehydes catalyzed by a particulate preparation from Pisum sativum. Proc. Natl. Acad. Sci. USA 81:6613-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheesbrough, T. M., and P. E. Kolattukudy. 1988. Microsomal preparation from an animal tissue catalyzes release of carbon monoxide from a fatty aldehyde to generate an alkane. J. Biol. Chem. 263:2738-2743. [PubMed] [Google Scholar]
- 8.Dennis, M., and P. E. Kolattukudy. 1992. A cobalt-porphyrin enzyme converts a fatty aldehyde to a hydrocarbon and CO. Proc. Natl. Acad. Sci. USA 89:5306-5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolattukudy, P. E. 1976. Chemistry and biochemistry of natural waxes. Elsevier, Amsterdam, The Netherlands.
- 10.Marín, M. M., L. Yuste, and F. Rojo. 2003. Differential expression of the components of the two alkane hydroxylases from Pseudomonas aeruginosa. J. Bacteriol. 185:3232-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKenna, E. J., and M. J. Coon. 1970. Enzymatic omega-oxidation. IV. Purification and properties of the omega-hydroxylase of Pseudomonas oleovorans. J. Biol. Chem. 245:3882-3889. [PubMed] [Google Scholar]
- 12.Park, M.-O., M. Tanabe, K. Hirata, and K. Miyamoto. 2001. Isolation and characterization of a bacterium that produces hydrocarbons extracellularly which are equivalent to light oil. Appl. Microbiol. Biotechnol. 56:448-452. [DOI] [PubMed] [Google Scholar]
- 13.Park, M.-O., K. Heguri, K. Hirata, and K. Miyamoto. Production of alternatives to fuel oil from organic waste by the alkane-producing bacterium, Vibrio furnissii M1. J. Appl. Microbiol., in press. [DOI] [PubMed]
- 14.Peterson, J. A., M. Kusunose, E. Kusunose, and M. J. Coon. 1967. Enzymatic omega-oxidation. II. Function of rubredoxin as the electron carrier in omega-hydroxylation. J. Biol. Chem. 242:4334-4340. [PubMed] [Google Scholar]
- 15.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 16.Stumpf, P. K. 1980. The biochemistry of plants, vol. 4. Academic Press, New York, N.Y.