Abstract
The wax ester synthase/acyl coenzyme A (acyl-CoA):diacylglycerol acyltransferase (WS/DGAT) catalyzes the final steps in triacylglycerol (TAG) and wax ester (WE) biosynthesis in the gram-negative bacterium Acinetobacter sp. strain ADP1. It constitutes a novel class of acyltransferases which is fundamentally different from acyltransferases involved in TAG and WE synthesis in eukaryotes. The enzyme was purified by a three-step purification protocol to apparent homogeneity from the soluble fraction of recombinant Escherichia coli Rosetta (DE3)pLysS (pET23a::atfA). Purified WS/DGAT revealed a remarkably low substrate specificity, accepting a broad range of various substances as alternative acceptor molecules. Besides having DGAT and WS activity, the enzyme possesses acyl-CoA:monoacylglycerol acyltransferase (MGAT) activity. The sn-1 and sn-3 positions of acylglycerols are accepted with higher specificity than the sn-2 position. Linear alcohols ranging from ethanol to triacontanol are efficiently acylated by the enzyme, which exhibits highest specificities towards medium-chain-length alcohols. The acylation of cyclic and aromatic alcohols, such as cyclohexanol or phenylethanol, further underlines the unspecific character of this enzyme. The broad range of possible substrates may lead to biotechnological production of interesting wax ester derivatives. Determination of the native molecular weight revealed organization as a homodimer. The large number of WS/DGAT-homologous genes identified in pathogenic mycobacteria and their possible importance for the pathogenesis and latency of these bacteria makes the purified WS/DGAT from Acinetobacter sp. strain ADP1 a valuable model for studying this group of proteins in pathogenic mycobacteria.
In bacteria, the most widespread class of neutral lipids are polyhydroxyalkanoic acids, which serve as intracellular carbon and energy storage compounds (27). Less frequently, triacylglycerols (TAGs)1 and wax esters (WEs; oxoesters of primary long-chain fatty acids and primary long-chain fatty alcohols) serve as lipophilic bacterial storage compounds (1). TAG accumulation seems to be widely distributed among actinomycetes and has been described for members of the genera Streptomyces (23), Mycobacterium (3), and Rhodococcus and Nocardia (2). WE accumulation has been frequently reported for the genus Acinetobacter (22) and less frequently for the genera Moraxella (4) and Micrococcus (24).
In eukaryotes, TAGs are the dominating storage lipids. In animals, TAGs are synthesized and accumulated in specific cells, such as hepatocytes and adipocytes. In plants, TAGs are accumulated mainly in seeds. Three different classes of TAG-synthesizing enzymes that use diacylglycerol (DAG) as a substrate are known (14, 20). Members of the eukaryotic acyl coenzyme A (acyl-CoA):DAG acyltransferase 1 and 2 (DGAT1-DGAT2) protein families and the bacterial WE synthase/acyl-CoA:DAG acyltransferase (WS/DGAT) catalyze the acylation of DAG by using acyl-CoA as the substrate. Mammalian DGAT enzymes have attracted increasing interest recently since they are discussed as a potential target for obesity treatment (8, 28). An acyl-CoA-independent reaction for TAG synthesis is catalyzed by a phospholipid:DAG acyltransferase. This enzyme uses phospholipids as the acyl donor for DAG esterification and has been found in plants and yeasts (10, 26). The third mechanism of TAG synthesis has been described for DAG:DAG transacylases, which where identified in animals and plants utilizing DAG as the acyl donor as well as the acyl acceptor (21).
WEs fulfill a number of biological functions. For example, they occur as epicuticular waxes in plants and serve as protectants against UV light, dehydration, or pathogens. In jojoba seeds (Simmondsia chinensis), the currently most important biological source for WEs, they serve as storage lipids.
WS/DGAT is the key enzyme for biosynthesis of storage lipids in Acinetobacter sp. strain ADP1. It catalyzes the final steps in TAG and WE biosynthesis. It was characterized as an unspecific enzyme catalyzing acyl-CoA-dependent acylation of DAGs and fatty alcohols to TAGs and WEs (14). WS/DGAT represents a new class of acyl-CoA-dependent acyltransferase since it exhibits no sequence homologies to known acyltransferases involved in the biosynthesis of TAGs, WEs, steryl esters, or phospholipids in eukaryotes (14). Previous studies employing total membrane fractions or crude extracts of recombinant Escherichia coli strains (14, 16) revealed that this enzyme has a very broad substrate range, accepting long-chain fatty alcohols and acyl-CoA esters ranging from C12 to C20 as well as monoacylglycerols (MAGs) as substrates. Even alkanethiols are accepted by this enzyme in vivo and in vitro, as was demonstrated for a His6-tagged WS/DGAT (29). Thus, more-detailed investigations concerning the biocatalytic potential of WS/DGAT might lead to the biotechnological production of unusual WE derivatives with new technological, biological, or therapeutic functions.
A large number of genes coding for WS/DGAT-homologous proteins were identified in pathogenic mycobacteria, such as Mycobacterium tuberculosis (14). Only very recently, the genes coding for WS/DGAT-homologous proteins from M. tuberculosis H37Rv have been cloned. DGAT activities, as well as low-level WS activities, were detected when the enzymes were heterologously expressed in E. coli (11). Moreover, transcription of several WS/DGAT-homologous genes was induced under culture conditions simulating the dormancy-like state of M. tuberculosis cells in macrophages, accompanied by intracellular TAG synthesis and accumulation (11). In addition, intracellular lipid accumulation in M. tuberculosis cells was also recently demonstrated in situ with a sputum sample from a patient suffering from clinical tuberculosis syndrome (12). Thus, lipid accumulation might have an important function in pathogenesis and long-term dormancy (11). WS/DGAT-homologous proteins might be potential drug targets against mycobacterial infections (11, 14).
To date little is known about WSs. The only WSs which have been characterized so far are the WS from jojoba (18) and the only very recently discovered mammalian WSs (9). Few examples have been reported from the purification and characterization of DGAT enzymes (7, 17, 19). A greater knowledge about the biochemical properties of WS/DGAT not only will help to elucidate bacterial WE and TAG metabolism but also might give further insights into eukaryotic lipid biosynthesis. Furthermore, if lipid accumulation participates in the pathogenesis of M. tuberculosis, WS/DGAT may serve as a model enzyme for studying this group of enzymes in mycobacteria.
MATERIALS AND METHODS
Materials.
All media and columns used for protein purification as well as molecular mass standard proteins for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and gel filtration were purchased from Amersham Pharmacia Biotech (Uppsala, Sweden). [1-14C]palmitoyl-CoA and [1-14C]hexadecanol were purchased from Hartmann Radiochemicals (Braunschweig, Germany). Unlabeled acyl-CoAs and protease inhibitor cocktail for histidine-tagged proteins were purchased from Sigma-Aldrich (St. Louis, Mo.). All reagents and salts were of the highest purity available.
Expression of WS/DGAT.
The atfA gene (formerly designated wax/dgat [14]), which encodes WS/DGAT, was obtained from the vector pKS::atfA (14) by digestion with SalI and NotI and subcloned into the expression vector pET23a (Novagen, Madison, Wis.). The plasmid pET23a::atfA was transformed into E. coli Rosetta (DE3)pLysS (Novagen).
Cultivation.
Cells of E. coli were grown in Luria-Bertani medium (25) to an optical density at 600 nm of 0.5 at 37°C in Erlenmeyer flasks before IPTG (isopropyl-β-d-thiogalactopyranoside) was added to a final concentration of 1 mM. The induced cultures were incubated at 37°C for 3 h. Cells were then harvested by centrifugation at 4,000 × g for 20 min at 4°C, washed twice with 50 mM sodium phosphate buffer (pH 7.4) containing 1 mM EDTA (buffer A), and resuspended in buffer A. Cell disruption was carried out by a threefold passage through a precooled French pressure cell at 1,000 MPa in the presence of 1% (vol/vol) protease inhibitor cocktail (Sigma-Aldrich) and 1 mM EDTA. The lysate was cleared by ultracentrifugation at 35,000 × g for 1 h at 4°C. All purification steps were carried out at 0 to 4°C.
Concentration of protein solutions.
Protein solutions with a volume of >60 ml were concentrated using ultrafiltration chambers (Amicon, Silver Spring, Md.) equipped with YM 30 membranes (30,000-Da molecular mass cutoff). Smaller volumes were concentrated with Vivaspin 20 centrifugal concentrators (10,000-Da molecular mass cutoff) (Vivascience, Hanover, Germany).
Chromatographic WS/DGAT purification.
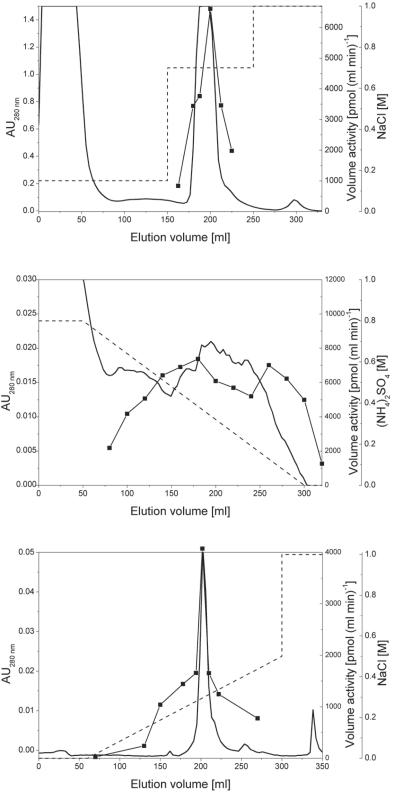
The cleared lysate was applied to a 60-ml sulfopropyl (SP)-Sepharose HP (XK 26/30) column equilibrated with buffer A at a flow rate of 2 ml min−1. After being washed with 150 ml of buffer A containing 150 mM NaCl, proteins were eluted with 100 ml of buffer A containing 700 mM NaCl. Fractions exhibiting WS/DGAT activity were combined (175- to 232-ml elution volume), and 1% (vol/vol) protease inhibitor cocktail was added before purification was continued (SP-Sepharose pool).
The SP-Sepharose pool was adjusted to a final ammonium sulfate concentration of 1 M and subsequently applied to a 20-ml butyl-Sepharose 4 fast flow (YK 26/30) column equilibrated with buffer A containing 1 M ammonium sulfate at a flow rate of 1.5 ml min−1. After the column was washed with buffer A containing 800 mM ammonium sulfate at a flow rate of 2 ml min−1, proteins were eluted by applying a linear gradient of decreasing ammonium sulfate concentrations from 800 to 0 mM. Fractions with WS/DGAT activity were combined (120- to 300-ml elution volume), desalted, and resuspended in 20 mM sodium phosphate buffer (pH 7.7) containing 1 mM EDTA (buffer B). Protease inhibitor cocktail was added at a final concentration of 1% (vol/vol) (butyl-Sepharose pool).
The butyl-Sepharose pool was applied to a 55-ml Q-Sepharose HP (XK 26/30) column equilibrated with 20 mM sodium phosphate buffer (pH 7.7) containing 1 mM EDTA. The sample was applied at a flow rate of 2 ml min−1. After the column was washed with 50 ml of buffer B, proteins were eluted by a linear gradient of increasing NaCl concentration from 0 to 500 mM in a volume of 250 ml. Fractions with WS/DGAT activity were combined (174- to 206-ml elution volume), mixed with 1% (vol/vol) protease inhibitor cocktail, and concentrated to 1.3 mg of protein ml−1.
Determination of enzyme activities.
WS/DGAT activity was determined in a total volume of 250 μl containing, per ml, 12.5 μg of bovine serum albumin (BSA) (4.26 mg of BSA ml−1 during purification) (Table 1), 4.72 μM [1-14C]palmitoyl-CoA (specific activity, 1.961 Bq pmol−1), 125 mM sodium phosphate buffer (pH 7.4), and different acceptor molecules at a concentration of 3.75 mM. If not stated otherwise below, 1-hexadecanol and 1,2-dipalmitin were used as standard substrates for assaying WS and DGAT activity, respectively. Water-insoluble substrates and BSA were applied as double-concentrated stock solutions emulsified by ultrasonification. The assay mixtures were incubated for 20 min at 35°C, and the reactions were stopped by extraction with 500 μl of chloroform-methanol (1:1, vol/vol). After centrifugation, the chloroform phase was withdrawn and evaporated to dryness, and 40 μg of unlabeled reference substances was added. The reaction products were separated by thin-layer chromatography (TLC) using the solvent system hexane-diethyl ether-acetic acid (90:7.5:1, vol/vol/vol) for the separation of linear, cyclic, and aromatic WEs; hexane-diethyl ether-acetic acid (80:20:1, vol/vol/vol) for the separation of TAGs; and chloroform-methanol-acetic acid (98:2:0.2, vol/vol/vol) for the separation of DAGs. MAGs were separated by applying two consecutive solvent systems; the first development was done in chloroform-methanol-acetic acid-water (70:30:4:2, vol/vol/vol/vol) for the first half of the TLC plate (length, 10 cm), and subsequently the plate was completely developed using diethyl ether-hexane-ethanol-acetic acid (40:50:2:0.2, vol/vol/vol/vol) as the solvent system. After the separation of lipids by TLC and the staining of TLC plates with iodine vapor, spots corresponding to the reaction products were scraped from the plates, and radioactivity was measured by scintillation counting. If reference substances were not available, the radioactive reaction products on the TLC plates were detected by autoradiography. Negative-control experiments were carried out with heat-denatured enzyme.
TABLE 1.
WS/DGAT purification from the soluble fraction of E. coli Rosetta (DE3)pLysS (pET23a::atfA)a
| Enrichment step | Total protein (mg) | Sp act (nmol mg−1 min−1) | Enrichment factor | Yield (%) |
|---|---|---|---|---|
| Crude extract | 1,910.0 | 9.8 | 1 | 100 |
| Soluble fraction | 1,879.0 | 4.1 | 0.4 | 41.2 |
| SP-Sepharose | 345.6 | 17.8 | 1.8 | 32.9 |
| Butyl-Sepharose | 37.4 | 82.1 | 8.4 | 16.4 |
| Q-Sepharose | 19.6 | 183.5 | 18.7 | 19.2 |
The enrichment factor was calculated based on specific activities, with the specific activity of the crude extract set to 1. Yield was calculated based on residual total activity, with the total activity of crude extract set to 100%.
Production and affinity purification of polyclonal antibodies against WS/DGAT from rabbit.
Two hundred fifty micrograms of partially purified WS/DGAT was separated by SDS-PAGE. The band corresponding to WS/DGAT was cut off and used for immunization of a rabbit at Eurogentec (Seraing, Belgium). The immunoglobulin G (IgG) fraction was purified by affinity chromatography with protein A-Sepharose CL-4B (13). Anti-WS/DGAT-specific IgGs were purified by blotting purified WS/DGAT on a nitrocellulose membrane. The blotted protein was incubated in 2.5% (wt/vol) skim milk in phosphate-buffered saline (PBS; 0.5% [wt/vol] NaCl, 10 mM potassium phosphate-buffer [pH 7.0], 0.05% [vol/vol] Tween 20) for 1 h. Subsequently, the blot was washed with PBS, and 400 μl of the IgG solution from protein A chromatography was added. After 3 h, the membrane was washed three times with PBS for 10 min. Anti-WS/DGAT-specific IgGs were eluted with an elution buffer (5 mM glycine [pH 2.3], 0.5 M NaCl, 0.05% [vol/vol] Tween 20) for 2 min. Finally, the solution was neutralized by addition of 1 M potassium phosphate buffer (pH 8.0) and stored at −20°C.
Subcellular fractionation of Acinetobacter sp. strain ADP1.
Cells of Acinetobacter sp. strain ADP1 were cultivated to promote storage lipid accumulation (14). After cell disruption by a threefold passage through a French pressure cell at 1,000 MPa, the crude extract was fractionated by differential centrifugation steps. Centrifugation at 2,700 × g resulted in a pellet which contained lipid inclusion-associated WEs and TAGs. The 2,700 × g supernatant was subsequently centrifuged at 35,000 × g. The resulting pellet contained the total membrane fraction, whereas the supernatant represented the soluble, cytosolic fraction.
Immunogold transmission electron microscopy (TEM).
Acinetobacter sp. strain ADP1 cells cultivated under storage conditions (14) were fixed in 46% paraformylaldehyde, embedded in Lowicryl K4 M (Polyscience, Eppelheim, Germany), and polymerized at −35°C. Ultrathin sections were cut with an ultramicrotome (Ultracut S; Leica Mikroskopie und Systeme GmbH, Wetzlar, Germany). To avoid nonspecific labeling, the sections were kept for 5 min in a solution of 5% (wt/vol) BSA in PBS. Afterwards, the sections were labeled with polyclonal anti-WS/DGAT IgGs. After being washed five times in PBS-BSA solution for 5 min each time and in Tris-BSA solution for 5 min, goat anti-rabbit IgGs coupled to 10-nm-diameter colloidal gold particles (Sigma-Aldrich) were attached to the primary antibodies. Subsequently, the immunogold-labeled sections were washed five times in Tris-BSA solution for 5 min each time and then stained with uranyl acetate and lead citrate. The sections were analyzed in a model H-500 transmission electron microscope (Hitachi Ltd., Tokyo, Japan) operated at 75 kV. Photographs were taken in the bright-field mode with Agfa-Gevaert 23D56 film. Negative-control experiments were carried out with the same cells under the same conditions except that they were not labeled with anti-WS/DGAT IgGs.
RESULTS
Expression and purification of WS/DGAT.
By employing the T7 promoter- and polymerase-based pET system, WS/DGAT was heterologously expressed in E. coli. The atfA gene comprises 19 codons which are rarely used in E. coli (AUA [three times], AGA [four times], CUA [six times], CCC [two times], and GGA [four times]); therefore, the expression host E. coli Rosetta (DE3)pLysS (Novagen) was used to complement these rare codons. By using this system, functional expression of WS/DGAT was achieved (Table 1). Forty to 50% of the total WS/DGAT activity was located in the soluble fraction (Table 1). Therefore, the enzyme was purified starting from this fraction. By employing a three-step purification protocol comprising cation-exchange chromatography, hydrophobic-interaction chromatography, and anion-exchange chromatography as described in detail in Materials and Methods (Fig. 1), WS/DGAT was enriched 18.7-fold, with a yield of 19.2% (Table 1). Samples from the different purification steps were analyzed by SDS-PAGE, which revealed that WS/DGAT was purified to near homogeneity, with only trace amounts of contaminating proteins left. The resulting apparent molecular mass was 53 kDa, which is in good agreement with the theoretical molecular mass (51.8 kDa) (Fig. 2). The purified WS/DGAT could be stored at −70°C without significant loss of activity for several months. During the purification procedure the enzyme turned out to be highly sensitive to proteases, especially to metalloproteases. By adding a protease inhibitor cocktail after WS/DGAT-containing fractions were combined and by including EDTA in the elution buffers, proteolysis could be inhibited completely.
FIG. 1.
Purification of WS/DGAT from the soluble fraction of E. coli Rosetta (DE3)pLysS (pET23a::atfA) cells. The values represented are amounts of total protein (solid line, measured as absorption at 280 nm), liquid-phase salt contents [dashed line, NaCl or (NH)2SO4], and enzyme activities (filled squares, measured as WS volume activity). (A) SP-Sepharose HP; (B) butyl-Sepharose; (C) Q-Sepharose HP. SP-Sepharose HP-purified fractions containing WS activity were pooled and further purified with butyl-Sepharose. Similarly, butyl-Sepharose-purified fractions containing WS activity were pooled and further purified with Q-Sepharose HP. AU280 nm, absorption units at 280 nm.
FIG. 2.
SDS-PAGE analysis of WS/DGAT purification steps. Samples from purification steps were subjected to denaturing SDS-PAGE and stained with Coomassie brilliant blue. St, low molecular mass standards; lane 1, crude extract (30 μg of protein); lane 2, soluble fraction (30 μg of protein); lane 3, SP-Sepharose pool (10 μg of protein); lane 4, butyl-Sepharose pool (10 μg of protein); lane 5, Q-Sepharose concentrate (10 μg of protein).
Characteristics of WS/DGAT.
WS/DGAT-mediated WE formation proceeded at a constant rate over a period of 30 min (data not shown). The highest WS activity (403 ± 35 nmol mg−1 min−1) was observed in the presence of 12.5 μg of BSA per ml. Additions of BSA in the range of 1 to 10 mg−1 ml−1 caused a concentration-dependent decrease in activity. However, BSA was required to produce stable emulsions of water-insoluble substrates and was added to all activity assay mixtures at a concentration of 12.5 μg ml−1.
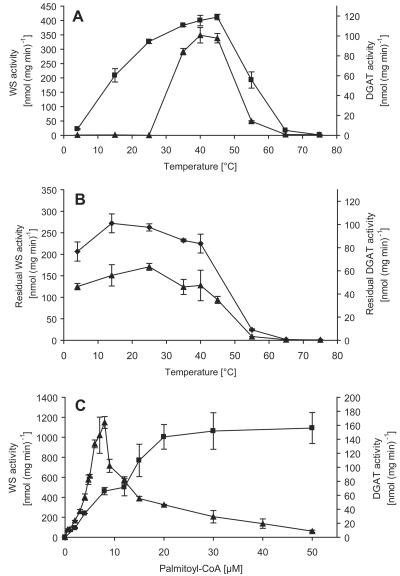
The highest WS and DGAT activities were obtained at 40 to 45°C, whereas higher temperatures led to clearly lower WS and DGAT activities. At 65°C and higher temperatures, almost no WS or DGAT activity was detected (Fig. 3A). Monitoring the temperature stability revealed that WS/DGAT is stable at temperatures ranging from 4 to 40°C. Higher temperatures led to clearly decreased WS and DGAT stability (Fig. 3B).
FIG. 3.
Characteristics of WS/DGAT. The two lines represent measurements of WS activity (filled squares) and DGAT activity (filled triangles) from two independent experiments ± error bars. (A) Temperature dependency of WS/DGAT activity. The reaction mixtures were preincubated for 5 min at the different temperatures before the reaction was started by adding the enzyme. (B) Temperature stability of WS/DGAT. Temperature stability was determined by incubating the WS/DGAT for 20 min at different temperatures before measuring residual activities under standard conditions. (C) Palmitoyl-CoA dependency of purified WS/DGAT. WS and DGAT enzyme assays were performed at various palmitoyl-CoA concentrations.
Free CoA is released as a second product during the WS/DGAT-catalyzed acyltransferase reaction. Thus, the effect of free CoA on WS activity was determined by addition of CoA to the WS assay reaction mixture. Addition of a 50 to 300 μM concentration of free CoA led to a clearly concentration-dependent inhibition of WS activity. In the presence of 300 to 1,000 μM CoA, WS activity was decreased by approximately 70% in comparison with that of the control without CoA addition (data not shown).
The native molecular mass was determined by gel filtration chromatography of purified WS/DGAT by using a Superdex 200 HR 10/30 column. In experiments done in triplicate, the highest level of WS/DGAT activity and the highest protein concentration eluted reproducibly at 11.12 ± 0.11 ml, revealing an apparent molecular mass of 94.1 ± 3.9 kDa, which corresponds to a WS/DGAT homodimer with a theoretical molecular mass of 103.6 kDa. In addition, a minor amount of WS/DGAT activity eluted also at 13.57 ± 0.24 ml under these conditions, corresponding to an apparent molecular mass of 38.1 ± 3.5 kDa, which can be interpreted as the monomeric form of WS/DGAT (data not shown).
Determination of WS/DGAT activity in different buffer systems resulted in a significant decrease of activity in buffers such as bis-Tris, HEPES, MES (morpholineethanesulfonic acid), or Tris compared to that in phosphate buffer at corresponding pH values. Thus, by solely using phosphate buffer, the pH optimum was determined to be pH 8.0 (data not shown).
By monitoring the WS/DGAT activity by employing 1-hexadecanol as a substrate at different palmitoyl-CoA concentrations, a hyperbolic saturation curve was obtained, which indicated that the WS reaction follows a Michaelis-Menten kinetic (Fig. 3C). Assuming substrate saturation for 1-hexadecanol under assay conditions, Lineweaver-Burk plot analysis revealed a Km value for palmitoyl-CoA of 29 μM and a Vmax of 2,000 nmol mg−1 min−1. The DGAT reaction, which was assayed with dipalmitoylglycerol as a substrate at different palmitoyl-CoA concentrations, fit neither Michaelis-Menten nor cooperative enzyme kinetics. At low palmitoyl-CoA concentrations from 0.5 to 8 μM, TAG formation proceeded as sigmoidal but was drastically reduced at palmitoyl-CoA concentrations of >8 μM (Fig. 3C).
Bivalent metal cations decreased WS/DGAT activity by 17% (5 mM MgCl2) and 30% (5 mM CaCl2 and MnCl2), whereas 45 mM ammonium sulfate slightly increased WS/DGAT activity by 10% in comparison with that of the control without the addition of salt (data not shown).
Substrate specificities of WS/DGAT.
WS/DGAT accepted a broad range of various-chain-length linear alcohols. The highest activity was obtained for medium-chain-length alcohols (C14 to C18). Short-chain-length alcohols such as butanol, hexanol, and octanol were accepted with lower specificity, but even ethanol was acylated at a low but significant rate. Long-chain-length alcohols (C20 to C30) were also utilized by this enzyme, with increasing chain length lowering the specific activity (Fig. 4A). Comparing the acylation of 1-, 2-, and 4-decanol revealed the highest specificity towards 1-decanol, indicating that a terminal hydroxy group is more accessible to the enzyme than others (Table 2). Providing different-chain-length acyl-CoAs revealed that palmitoyl-CoA was accepted with highest specificity. However, shorter- and longer-chain-length acyl-CoAs were also accepted but less efficiently (Fig. 4B).
FIG. 4.
Specificity of WS/DGAT towards various-chain-length linear alcohols and acyl-CoAs. (A) Alcohol specificities; (B) acyl-CoA specificities of WS/DGAT. Values are averages of results from experiments done in triplicate ± standard deviations.
TABLE 2.
Acyl acceptor specificites of purified WS/DGAT for different alcohols and aromatic substrates
| Acyl acceptor | Activity (% of that of the hexadecanol control)a |
|---|---|
| Hexadecanol | 100 ± 1.9 |
| 1-Decanol | 48.0 ± 5.6 |
| 2-Decanol | 38.6 ± 2.3 |
| 4-Decanol | 15.6 ± 5.1 |
| Cyclohexanol | 32.0 ± 6.2 |
| Cyclohexandiol | 4.1 ± 0.7 |
| 2-Cyclohexylethanol | 44.8 ± 14.4 |
| Cyclododecanol | 79.7 ± 16.6 |
| Cyclohexanone oxime | 5.2 ± 0.2 |
| Phenol | 4.1 ± 0.4 |
| Phenylethanol | 99.0 ± 32.8 |
| Resorzinol | ND |
Values are specific activities relative to that of hexadecanol as the acyl acceptor. Data are mean values of results of experiments done in triplicate ± standard deviations. ND, not detectable.
Table 3 shows the acyl-acceptor specificities for various acylglycerols. Monitoring the acylation of 1-, 2-, and 3-monopalmitoylglycerol revealed higher specificities for the hydroxy groups in the sn-1 and sn-3 positions, whereas the hydroxy group in the sn-2 position was acylated to a lower extent. Comparing the levels of acylation of 1,2- and 1,3-dipalmitoylglycerols also revealed a clear preference for the sn-3 position. Glycerol could not serve as the substrate for the enzyme and was not acylated.
TABLE 3.
Acyl acceptor specificities of purified WS/DGAT for glycerol and acylglycerolsa
| Acyl acceptor | Amt (nmol mg−1 min−1) formed of:
|
||||
|---|---|---|---|---|---|
| 1- + 3-MPG | 2-MPG | 1,2- + 2,3-DPG | 1,3-DPG | TPG | |
| Glycerol | ND | ND | NT | NT | NT |
| 1-MPG | 58 ± 4 | 373 ± 16 | NT | ||
| 2-MPG | 121 ± 12 | 99 ± 11 | NT | ||
| 3-MPG | 43 ± 2 | 258 ± 9 | NT | ||
| 1,2-DPG | 211 ± 10 | ||||
| 1,3-DPG | 118 ± 22 | ||||
1,2- and 2,3-dipalmitoylglycerol as well as 1- and 3-monopalmitoylglycerol could not be separated under the applied TLC conditions and are therefore summed. Data are averages of results of experiments done in triplicate ± standard deviations. MPG, monopalmitoylglycerol; DPG, dipalmitoylglycerol; TPG, tripalmitoylglycerol; ND, not detectable; NT, not tested.
The substrate range of WS/DGAT was further evaluated for cyclic and aromatic alcohols (Table 2). Cyclohexanol, 2-cyclohexylethanol, and cyclododecanol were acylated with high specific activities, whereas the activity with cyclohexandiol was rather low. Aromatic compounds could also serve as the substrate for WS/DGAT. Even phenol was acylated to a minor degree, whereas phenylethanol gave a higher activity. Another unusual substrate, cyclohexanone oxime, was also acylated to a low but significant extent.
Subcellular localization of WS/DGAT.
TLC analysis of lipid extracts of subcellular fractions of the natural WS/DGAT host Acinetobacter sp. strain ADP1 grown under storage conditions (14) revealed that the largest amounts of WEs and TAGs were located in the 2,700 × g pellet, which thus represented the lipid inclusions. High WS/DGAT activities in this fraction indicated that WS/DGAT is associated with these lipophilic inclusions. In the total membrane fraction (35,000 × g sediment), only small amounts of TAGs and WEs were detectable. However, high WS/DGAT activities in this fraction showed that a large amount of the enzyme is also membrane associated. The cytosolic fraction (35,000 × g supernatant) contained nearly no TAGs or WEs but significant WS/DGAT activities, indicating that a minor amount of soluble WS/DGAT is also located in the cytoplasm (Fig. 5). Analogously, the majority of WS/DGAT in recombinant E. coli Rosetta (DE3)pLysS (pET23a::atfA) was membrane associated but to some extent also located in the cytosolic fraction, allowing the purification of the soluble enzyme as described in this study (Table 1).
FIG. 5.
WS/DGAT activity in subcellular fractions of Acinetobacter sp. strain ADP1. (A) TLC analysis of lipid extracts of subcellular fractions. TLC analysis was carried out using lipid extracts obtained from 1.5 mg (dry matter) of subcellular fractions. (B) Specific WS activities (filled bars) and DGAT activities (open bars) in subcellular fractions. Assays employing 0.4 mg of total protein ml−1 were done. Values are averages of duplicate measurements ± error bars. Bars: 1, crude extract; 2, lipid inclusions (2,700 × g pellet); 3, 2,700 × g supernatant; 4, cytosolic fraction (35,000 × g supernatant); 5, total membrane fraction (35,000 × g pellet).
TEM analysis of immunogold-labeled Acinetobacter sp. strain ADP1 cells grown under storage conditions with antibodies raised against WS/DGAT confirmed that this enzyme is predominantly associated with the cytoplasm membrane as well as lipid inclusions, but it is also partially localized in the cytoplasm (Fig. 6). The controls did not show significant gold labeling. Additionally, no significant unspecific background labeling was detectable either in positive or negative controls.
FIG. 6.
Localization of WS/DGAT by immunogold TEM of Acinetobacter sp. strain ADP1. Arrows indicate the gold particles. The scale bars correspond to 500 nm. LI, lipid inclusion; CM, cytoplasm membrane; OM, outer membrane.
DISCUSSION
In this study we have expressed and purified the WS/DGAT from Acinetobacter sp. strain ADP1 to apparent homogeneity from the soluble protein fraction of a recombinant E. coli strain by applying a three-step purification protocol. SDS-PAGE analysis of the purified enzyme revealed a high degree of purity, with only trace amounts of contaminating proteins. This is the first report on the purification of a bacterial enzyme exhibiting WS and/or DGAT activity allowing a detailed biochemical characterization of this novel type of long-chain acyltransferase by employing a homogenous enzyme preparation. Moreover, to date only a few examples of the successful purification of DGAT enzymes have been reported (7, 17, 19), whereas a homogenous WS has not yet been obtained (9, 18). One peculiarity of this WS/DGAT is its partial solubility in the cytosolic fraction, although the enzyme is predominantly associated with the cytoplasm membrane and the lipid inclusions. In contrast, eukaryotic DGAT or WS enzymes that have been described to date are exclusively membrane proteins (5, 6, 17, 18). The amount of WS/DGAT in the cytosolic fraction was dependent on the ionic strength of the buffer. The highest yield in the soluble fraction was obtained with 50 to 125 mM sodium phosphate buffer (pH 7.4). The relatively tight binding of WS/DGAT to a butyl-Sepharose matrix also articulates the anticipated hydrophobic character of this soluble enzyme, since hydrophobic domains are necessary for substrate-protein interaction.
The basic isoelectric point of 9.05 is consistent with those of eukaryotic DGAT enzymes (6) and the WS of jojoba (18). The native molecular mass of purified WS/DGAT was 94.1 kDa, as determined by gel filtration. With the theoretical molecular mass being 51.8 kDa, it can be concluded that the native conformation of the purified soluble enzyme is predominantly a homodimer. The WS/DGAT was sensitive to elevated temperatures and highly accessible to proteases. Use of cation-exchange chromatography as the first purification step reduced proteolytic degradation due to the mainly acidic-to-neutral isoelectric points of E. coli proteases. Protease inhibitors such as AEBSF [4-(2-aminoethyl)-benzenesulfonylfluoride], phosphoramidon, pepstatin A, bestain, E64, and EDTA did not affect the activity of WS/DGAT and may therefore successfully be used to prevent proteolysis during cell disruption and subsequent purification steps.
Free CoA is released during the WS/DGAT-mediated acyltransferase reaction with acyl-CoAs as activated substrates. In this study, it was demonstrated that CoA significantly inhibited WS/DGAT-mediated WE formation. At concentrations of ≥300 μM, WS/DGAT activity was decreased by 70%, and even CoA concentrations as low as 50 μM diminished enzyme activity significantly. This product inhibition may constitute a regulatory element for this enzyme in vivo. However, the type of inhibition (competitive or noncompetitive) was not investigated.
The WS/DGAT exhibited DGAT as well as MGAT activity. The exposed hydroxy groups in the sn-1 and sn-3 positions of acylglycerols were clearly preferred, which is most probably due to the better accessibility of these positions. In parallel, primary linear long-chain alcohols were utilized with much higher specific activities than those of secondary alcohols, as was demonstrated for 1-, 2-, and 4-decanol. The higher specific activities for MAGs than for DAGs may be due to better emulsifying properties in aqueous solvents and, thus, a better availability of MAGs for the enzyme. Previous studies of the total membrane fraction of recombinant E. coli XL1-Blue(pKS::atfA) revealed a significantly lower MGAT activity of WS/DGAT, indicating a change in the substrate specificities of the enzyme in soluble and membrane-associated forms (14). The inhibition of DGAT activity at high palmitoyl-CoA concentrations was also reported for the DGAT of Mortierella ramanniana var. angulispora (17). One explanation may be that palmitoyl-CoA has detergent properties due to its amphiphilic character. In fact, the activity of purified WS/DGAT was decreased by addition of different detergents at their critical micellar concentration (data not shown). Interestingly, the WS reaction was not inhibited, so that the reason for this substrate inhibition remains unclear.
The WS/DGAT-mediated acylation of cyclic and aromatic alcohols shown in this study further underlines the remarkably large biocatalytic potential which seems not to be exhausted with the substrates tested so far. The broad range of alternative substrates which can be utilized by WS/DGAT as demonstrated in this study indicates that this enzyme constitutes a highly unspecific, general acyltransferase. The bifunctionality of WS/DGAT in Acinetobacter sp. strain ADP1 reflects the fact that only fatty alcohols and DAGs are provided as substrates by the metabolism of this natural host, resulting in WE and TAG synthesis. In contrast, heterologous expression of WS/DGAT in recombinant organisms providing different substrates can result in the biosynthesis of other fatty acid esters. As an example, the expression of WS/DGAT in Saccharomyces cerevisiae resulted in the accumulation of fatty acid ethyl esters and fatty acid isoamyl esters (15). Thus, exploitation of the low substrate specificity of this acyltransferase might for example be utilized for the biotechnological production of custom-made saturated or unsaturated WEs with physical properties better adapted to their field of application than those available today. The WS/DGAT-mediated acylation of molecules might be used to obtain new applications or altered characteristics, for example, a retarding effect of water soluble pharmaceuticals. Purification of the enzyme is an important prerequisite for the investigation of the substrate range. Further information about the enzyme will increase our knowledge of bacterial TAG and WE biosynthesis. The localization of the enzyme may give further information about the biogenesis of WE or TAG inclusion bodies in bacteria (30).
Intracellular lipid accumulation is supposed to play an important role during the initiation and maintenance of latency when pathogenic mycobacteria like M. tuberculosis enter macrophages and survive intracellularly in a dormancy-like state (11). Since WS/DGAT-homologous proteins are obviously involved in lipid accumulation in M. tuberculosis under conditions simulating the intracellular survival in macrophages in a latent state (11), purified WS/DGAT is a valuable model for studying the possible importance of WS/DGAT-homologous proteins in pathogenic mycobacteria. For example, inhibitor studies may help to develop new strategies for the treatment of diseases like tuberculosis caused by these pathogenic mycobacteria.
Acknowledgments
We thank Torsten Stölting for technical assistance.
REFERENCES
- 1.Alvarez, H. M., and A. Steinbüchel. 2002. Triacylglycerols in prokaryotic microorganisms. Appl. Microbiol. Biotechnol. 60:367-376. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez, H. M., R. Kalscheuer, and A. Steinbüchel. 1997. Accumulation of storage lipids in species of Rhodococcus and Nocardia and effects of inhibitors and polyethylene glycol. Fett-Lipid 99:239-246. [Google Scholar]
- 3.Barksdale, L., and K. S. Kim. 1977. Mycobacterium. Bacteriol. Rev. 41:217-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryn, K., E. Jantzen, and K. Bovre. 1977. Occurrence and patterns of waxes in Neisseriaceae J. Gen. Microbiol. 102:33-43. [DOI] [PubMed] [Google Scholar]
- 5.Cao, J., P. Burn, and Y. Shi. 2003. Properties of the mouse intestinal acyl-CoA:monoacylglycerol acyltransferase, MGAT2. J. Biol. Chem. 278:25657-25663. [DOI] [PubMed] [Google Scholar]
- 6.Cases, S., S. J. Smith, Y. W. Zheng, H. M. Myers, S. R. Lear, E. Sande, S. Novak, C. Collins, C. B. Welch, A. J. Lusis, S. K. Ericson, and R. V. Farese, Jr. 1998. Identification of a gene encoding an acyl-CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 95:13018-13023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cases, S., S. J. Stone, P. Zhou, E. Yen, B. Tow, K. D. Lardizabal, T. Voelker, and R. V. Farese, Jr. 2001. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem. 276:38870-38876. [DOI] [PubMed] [Google Scholar]
- 8.Chen, H. C., and R. V. Farese, Jr. 2000. DGAT and triglyceride synthesis: a new target for obesity treatment? Trends Cardiovasc. Med. 10:188-192. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, J. B., and D. W. Russell. Mammalian wax biosynthesis. II. Expression cloning of wax synthase cDNAs encoding a member of the acyltransferase enzyme family. J. Biol. Chem. 279:37798-37807. [DOI] [PMC free article] [PubMed]
- 10.Dahlqvist, A., U. Ståhl, M. Lenman, A. Banas, M. Lee, L. Sandager, H. Ronne, and S. Stymne. 2000. Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc. Natl. Acad. Sci. USA 97:6487-6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniel, J., C. Deb, V. S. Dubey, T. D. Sirakova, B. Abomoelak, H. R. Morbidoni, and P. E. Kolattukudy. 2004. Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture. J. Bacteriol. 186:5017-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garton, N. J., H. Christensen, D. E. Minnikin, R. A. Adegbola, and M. R. Barer. 2002. Intracellular lipophilic inclusions of mycobacteria in vitro and in sputum. Microbiology 148:2951-2958. [DOI] [PubMed] [Google Scholar]
- 13.Hjelm, H., K. Hjelm, and J. Sjöquist. 1972. Protein A from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Lett. 28:73-76. [DOI] [PubMed] [Google Scholar]
- 14.Kalscheuer, R., and A. Steinbüchel. 2003. A novel bifunctional wax ester synthase/acyl-CoA:diacylglycerol acyltransferase mediates wax ester and triacylglycerol biosynthesis in Acinetobacter calcoaceticus ADP1. J. Biol. Chem. 278:8075-8082. [DOI] [PubMed] [Google Scholar]
- 15.Kalscheuer, R., H. Luftmann, and A. Steinbüchel. 2004. Synthesis of novel lipids in Saccharomyces cerevisiae by heterologous expression of an unspecific bacterial acyltransferase. Appl. Environ. Microbiol. 70:7119-7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalscheuer, R., S. Uthoff, H. Luftmann, and A. Steinbüchel. 2003. In vitro and in vivo biosynthesis of wax diesters by an unspecific bifunctional wax ester synthase/acyl-CoA:diacylglycerol acyltransferase from Acinetobacter calcoaceticus ADP1. Eur. J. Lipid Sci. Technol. 105:578-584. [Google Scholar]
- 17.Kamisaka, Y., S. Mishra, and T. Nakahara. 1997. Purification and characterization of diacylglycerol acyltransferase from the lipid body fraction of an oleaginous fungus. J. Biochem. (Tokyo) 121:1107-1114. [DOI] [PubMed] [Google Scholar]
- 18.Lardizabal, K. D., J. G. Metz, T. Sakamoto, W. C. Hutton, M. R. Pollard, and M. W. Lassner. 2000. Purification of a jojoba embryo wax synthase, cloning of its cDNA, and production of high levels of wax in seeds of transgenic Arabidopsis. Plant Physiol. 122:645-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehner, R., and A. Kuksis. 1995. Triacylglycerol synthesis by purified triacylglycerol synthase of rat intestinal mucosa. Role of acyl-CoA acyltransferase. J. Biol. Chem. 270:13630-13636. [DOI] [PubMed] [Google Scholar]
- 20.Lehner, R., and A. Kuksis. 1993. Purification of an acyl-CoA hydrolase from rat intestinal microsomes. A candidate acyl-enzyme intermediate in glycerolipid acylation. J. Biol. Chem. 268:8781-8786. [PubMed] [Google Scholar]
- 21.Lehner, R., and A. Kuksis. 1996. Biosynthesis of triacylglycerols. Prog. Lipid Res. 35:169-201. [DOI] [PubMed] [Google Scholar]
- 22.Makula, R. A., P. J. Lockwood, and W. R. Finnerty. 1975. Comparative analysis of lipids of Acinetobacter species grown on hexadecane. J. Bacteriol. 121:250-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olukoshi, E. R., and N. M. Packter. 1994. Importance of stored triacylglycerols in Streptomyces: possible carbon source for antibiotics. Microbiology 140:931-943. [DOI] [PubMed] [Google Scholar]
- 24.Russell, N. J., and J. K. Volkman. 1980. The effect of growth temperature on wax ester composition in the psychrophilic bacterium Micrococcus cryophilus ATCC 15174. J. Gen. Microbiol. 118:131-141. [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis (ed.). 1989. Molecular cloning: a laboratory manual, 2nd ed., p. A.1. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 26.Sorger, D., and G. Daum. 2003. Triacylglycerol biosynthesis in yeast. Appl. Microbiol. Biotechnol. 61:289-299. [DOI] [PubMed] [Google Scholar]
- 27.Steinbüchel, A. 1996. PHB and other polyhydroxyalkanoic acids, p. 403-464. In H. J. Rehm, G. Reed, A. Pühler, and P. Stadler (ed.), Biotechnology, 2nd ed., vol. 6. Wiley VCH, Heidelberg, Germany. [Google Scholar]
- 28.Subauste, A., and C. F. Burant. 2003. DGAT: novel therapeutic target for obesity and type 2 diabetes mellitus. Curr. Drug Targets Immune Endocr. Metabol. Disord. 3:263-270. [DOI] [PubMed] [Google Scholar]
- 29.Uthoff, S., T. Stöveken, N. Weber, K. Vosmann, E. Klein, R. Kalscheuer, and A. Steinbüchel. 2004. Thiol wax ester biosynthesis utilizing the unspecific bifunctional wax ester synthase/acyl coenzyme A:diacylglycerol acyltransferase of Acinetobacter sp. strain ADP1. Appl. Environ. Microbiol. 71:•••. [DOI] [PMC free article] [PubMed]
- 30.Wältermann, M., A. Hinz, H. Robenek, D. Troyer, R. Reichelt, U. Malkus, H. J. Galla, R. Kalscheuer, T. Stöveken, P. von Landenberg, and A. Steinbüchel. Mechanism of lipid-body formation in prokaryotes: how bacteria fatten up. Mol. Microbiol., in press (First published 2 December 2004; doi:10.1111/j. 1365-2958.2004.04441.x). [DOI] [PubMed]