Abstract
Purpose
Greater protein intake has been associated with better breast cancer survival in several prospective studies, including among 1,982 women in the Nurses’ Health Study. We proposed to extend this previous finding. We hypothesized that protein, essential amino acid, branched-chain amino acid, and leucine intakes are associated with improved survival and that these associations are stronger in tumors expressing insulin receptor (IR).
Patients and Methods
We included 6,348 women diagnosed with stage I to III breast cancer between 1976 and 2004. There were 1,046 distant recurrences. Relative risks (RRs) and 95% CIs were calculated according to quintiles of updated postdiagnostic diet using adjusted Cox proportional hazards models based on follow-up until 2010.
Results
There was an inverse association between energy-adjusted protein intake and recurrence. Multivariable RRs for increasing quintiles of intake compared with the lowest were 0.95 (95% CI, 0.79 to 1.15), 0.92 (95% CI, 0.76 to 1.11), 0.75 (95% CI, 0.61 to 0.91), and 0.84 (95% CI, 0.69 to 1.03; trend P = .02). For animal protein intake, the RRs were 0.88 (95% CI, 0.73 to 1.06), 0.85 (95% CI, 0.70 to 1.02), 0.75 (95% CI, 0.62 to 0.92), and 0.78 (95% CI, 0.63 to 0.95; trend P = .003). Neither essential amino acids, branched-chain amino acids, nor any individual amino acid stood out as being the source of the association. The association also did not differ by IR status. There was no clear association with any protein-containing foods.
Conclusion
We found a modest survival advantage with higher intake of protein, regardless of IR status. There was no clear mechanism for this association, although it is consistent with prior studies. Our data suggest that there is likely no advantage for women with a history of breast cancer in restricting protein intake or protein-containing foods.
INTRODUCTION
Greater protein intake has been associated with better breast cancer survival in several prospective studies,1-3 including a report of 1,982 women in the Nurses’ Health Study (NHS), the largest to date.4 Reasons for this association are unknown.
There has been recent interest in measures of protein quality and health effects.5 In particular, the role of essential amino acids (lysine, threonine, valine, isoleucine, leucine, methionine, phenylalanine, tryptophan, and histidine) in preservation of lean body mass, cell signaling, glucose metabolism, bone health, and satiety is an area of active research.6 The branched-chain amino acids (leucine, isoleucine, and valine) modulate muscle protein synthesis through the insulin-signaling pathway and are abundant in the food supply, particularly from dairy products.7 Leucine in particular improves weight loss, inhibits skeletal muscle breakdown, and stimulates muscle protein synthesis by enhancing insulin sensitivity,8,9 and glutamine improves insulin sensitivity in vivo.10,11 The beneficial effects of protein on fat-free mass seem to be additive with physical activity.7,12,13
The NHS previously reported a decreased risk of death resulting from any cause with higher protein intake among 1,982 women (relative risk [RR], 0.65).4 With an additional 16 years of follow-up and more than 4,000 additional patient cases, we proposed to study breast cancer recurrence and death resulting from breast cancer as well as the association of survival with intake of total protein and type of protein. Protein types included that from vegetable and animal sources, including fish, dairy, red meat, poultry, and eggs. We also proposed to study essential amino acids, branched-chain amino acids, and individual food sources.
We hypothesized that total protein, essential amino acid, branch-chain amino acid, and leucine intakes are associated with improved survival after breast cancer. We also hypothesized that these associations are strongest in tumors expressing insulin receptor (IR) because of the associations of these amino acids with the insulin pathway.
PATIENTS AND METHODS
Study Population
The NHS is a cohort established in 1976, when 121,700 female nurses in the United States, then age 30 to 55 years, responded to a questionnaire regarding medical and lifestyle factors. Since then, follow-up questionnaires have been sent biannually. Beginning in 1980, participants completed a 61-item food frequency questionnaire (FFQ),14 which was expanded to 116 items beginning in 1984.
The study was approved by the institutional review board of Brigham and Women’s Hospital (Boston, MA), and all participants provided written informed consent. Our analysis included women diagnosed with stage I to III breast cancer between 1976 and 2004 (those with missing stage [n = 726] or stage IV at diagnosis [n = 180] were excluded), with diet data beginning in 1980 (N = 8,004). Women who had died or whose cancer had recurred within 1 year of primary diagnosis (n = 293) or who had missing diet information at least 12 months after diagnosis (n = 1,363) were excluded from analysis. After these exclusions, 6,348 participants were available for this analysis.
Assessment of Dietary Intake
Dietary assessments were administered in 1980, 1984, 1986, 1990, 1994, 1998, 2002, and 2006 using validated semiquantitative FFQs.14 For each food or beverage, participants were asked how often, on average, they had consumed a specified amount over the past year. Mean daily intakes of dietary factors were calculated using US Department of Agriculture food composition sources, supplemented with data from manufacturers and other sources. The baseline diet was the one that most closely followed the diagnosis, with a minimum lag of 12 months. Previous validation studies have reported strong correlations between energy-adjusted nutrients assessed by the FFQ and food records completed over the previous year.15
End Point Ascertainment
Participants self-reported breast cancer diagnosis on the biennial questionnaires. We then obtained permission to view pathology records to confirm diagnosis and obtain information on staging and other relevant information. Participants were observed until death or June 1, 2010, whichever occurred first. Deaths were reported by family members or the postal service or determined through searches in the National Death Index for questionnaire nonresponders.16 Cause of death was ascertained by physician review of the death certificate and medical record. Ascertainment of the cause of death in this cohort is estimated to be 98%.16
Women reported recurrence on supplemental biennial questionnaires. In a validation study, we found that self-reported recurrence had a sensitivity and specificity of 92% compared with medical records. In addition, women who died as a result of breast cancer and did not answer the supplemental questionnaire were considered to have experienced recurrence 2 years before death (ie, median survival time in stage IV breast cancer). We assumed breast cancer had recurred if a woman diagnosed with breast cancer reported a second cancer to a common site of breast cancer recurrence (liver, brain, or bone). Medical records of women who reported a second cancer to the lung were reviewed to distinguish between primary lung cancer and breast cancer metastasis.17 The number of recurrences calculated in this manner was similar to the number expected from recurrence rates found in a large trial of women with early-stage breast cancer (N = 5,569).18 Distant breast cancer recurrence was the primary end point. Death resulting from breast cancer and total mortality were secondary end points.
Assessment of IR and Estrogen Receptor Status
Tissue microarrays were constructed in the Dana-Farber Harvard Cancer Center Tissue Microarray Core Facility (Boston, MA) from 4,308 formalin-fixed, paraffin-embedded incident breast cancers from 1976 to 2008, using three 0.6-mm cores from each breast cancer.19-21 IR expression (cytoplasmic and membranous) was quantified using Definiens image analysis software (http://www.definiens.com). We calculated an IR H score as a weighted sum of the intensity of immunohistochemical cytoplasmic and membranous expression as follows: H score = % of positively stained cells at weak intensity category X 1 + % of positively stained cells at median intensity category X 2 + % of positively stained cells at high intensity category X 3.21 In a subset of 124 patient cases, we observed a sensitivity and specificity of 83% and 69%, respectively, with manual reading as the gold standard. Median H score was chosen as the cutoff for IR positivity. Immunohistochemical staining was performed for estrogen receptor (ER) using previously described methods.19,20
Covariates
Body mass index (BMI; five categories), weight change, menopausal status, hormone therapy use, age at first birth, parity, alcohol consumption, aspirin use, and oral contraceptive use were included, because these factors were previously shown to be associated with breast cancer survival in the NHS cohort.4,22-24 In addition, we adjusted for breast cancer characteristics, including year of diagnosis, disease stage (I, II, or III), self-reported radiation therapy (yes or no), chemotherapy (yes or no), and hormonal treatment (yes or no). Smoking was also included because of its association with total mortality. Physical activity measured in total metabolic equivalent task hours per week was first assessed in 1986 and updated in 1988, 1992, 1996, 1998, 2000, 2004, and 2008. To avoid assessment during active treatment, only measurements taken at least 12 months after diagnosis were considered. Energy intake (five categories) and alcohol consumption (five categories) were first assessed using the questionnaire that most closely followed at least 12 months after breast cancer diagnosis. All other covariates were taken from the questionnaire preceding diagnosis.
Statistical Analyses
Baseline diet was ascertained using the first FFQ at least 12 months after breast cancer diagnosis. This was done to allow completion of active treatment, which may affect diet. To reduce random within-person variation and best represent the long-term effects of dietary intake, cumulative averages of the diet scores from repeated FFQs were computed and updated, as described elsewhere.25 Women were categorized into quintiles of nutrient and food intakes. Two-tailed P values for linear trend tests across quintiles were computed by modeling the median value of each category as a continuous variable.
We used Cox proportional hazards models to assess the association between quintiles of energy-adjusted nutrient and food intakes and outcomes of interest. All Cox models were tested for proportionality of hazards by testing the statistical significance of time-varying covariates, created as interactions between each predictor and the log of the event time.26 Distant breast cancer recurrence was the primary end point. Secondary analyses considered death resulting from breast cancer and total mortality. For each participant, breast cancer diagnosis marked the beginning of follow-up. Person-months were accumulated until the analysis end point or June 2010, whichever occurred first.
Because time since diagnosis is a strong predictor of breast cancer survival, all models were stratified by time since diagnosis. In addition, simple models were adjusted for age. Multivariate models were adjusted for the covariates listed in Covariates and are detailed in the table footnotes. In addition, we conducted analyses stratified by the ER or IR status of the tumor and by BMI at diagnosis (< 30 v ≥ 30 kg/m2). Analyses stratified by IR or ER were performed using a statistical method for disease heterogeneity analysis for categorical subtypes in cohort studies, with a competing risk framework using a Cox proportional hazards model.27 The analysis stratified by BMI was performed using a stratified Cox model, using the likelihood ratio test to determine the statistical significance of the interaction term. We used a nonparametric analysis (SAS PROC LIFETEST; SAS Institute, Cary, NC) to generate Kaplan-Meier survival curves by quintile of protein intake and calculate absolute unadjusted recurrence differences 5 and 10 years after diagnosis. Statistical analyses were performed using SAS software (version 9.3). P values of less than .05 were considered significant.
RESULTS
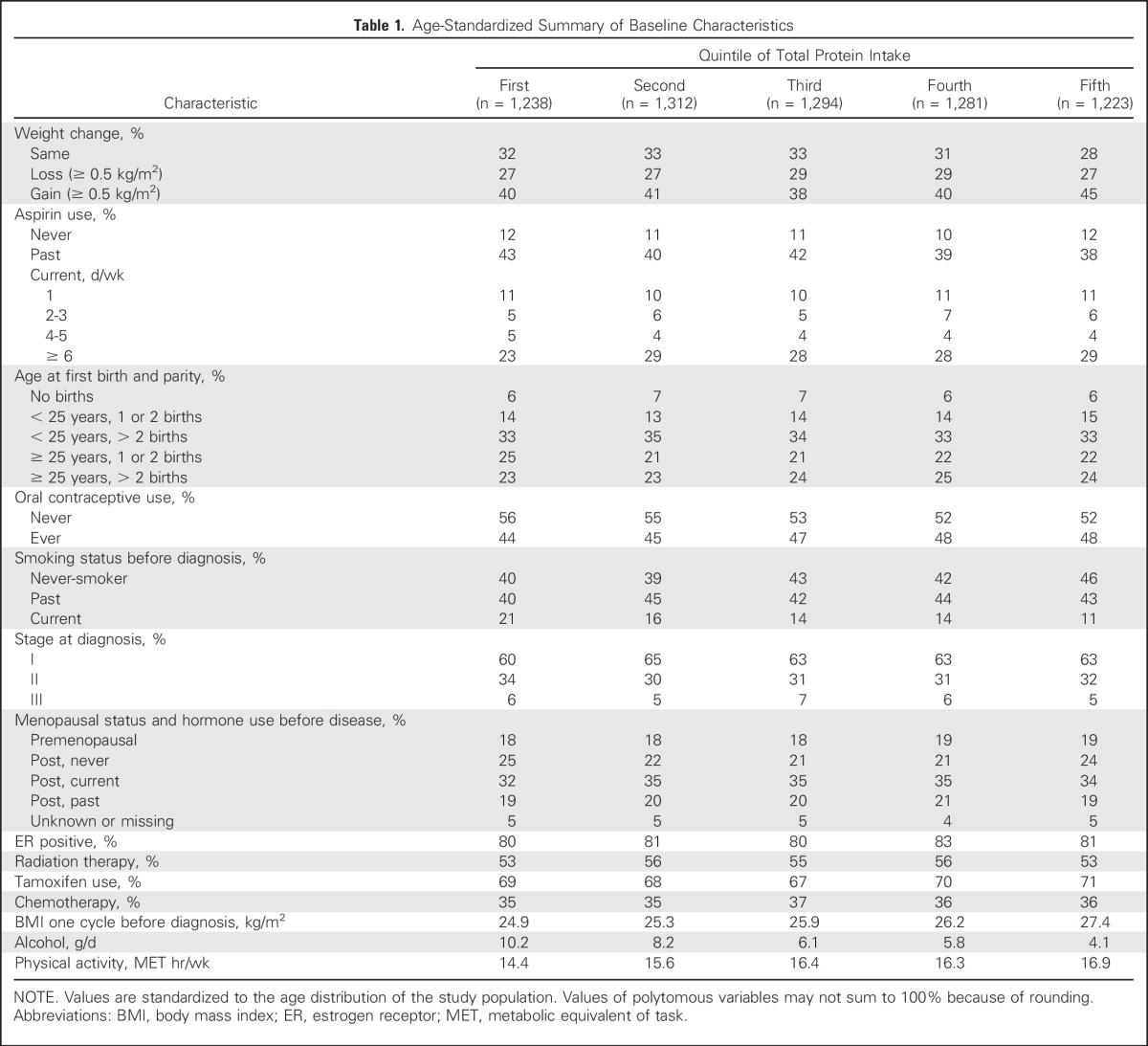
There were 6,348 participants included in the analysis and 1,046 distant recurrences, 919 deaths resulting from breast cancer, and 1,847 total deaths. The age-standardized summary of baseline characteristics according to quintiles of total protein intake is provided in Table 1. Of note, more physically active women seemed to consume more protein.
Table 1.
Age-Standardized Summary of Baseline Characteristics
Main results are listed in Table 2, summarizing the association between intake of protein, amino acids, and protein-containing food groups with distant breast cancer recurrence. The difference between simple (adjusted for age, total energy intake, and time since diagnosis) and multivariable models (also adjusted for stage, treatment, and other covariates) was largely accounted for by adjustment for physical activity. Additional multivariable models adjusted for types of protein and amino acids were constructed to show their independent effect.
Table 2.
Protein, Amino Acids, and Animal Protein–Containing Food Groups and Distant Breast Cancer Recurrence in NHS
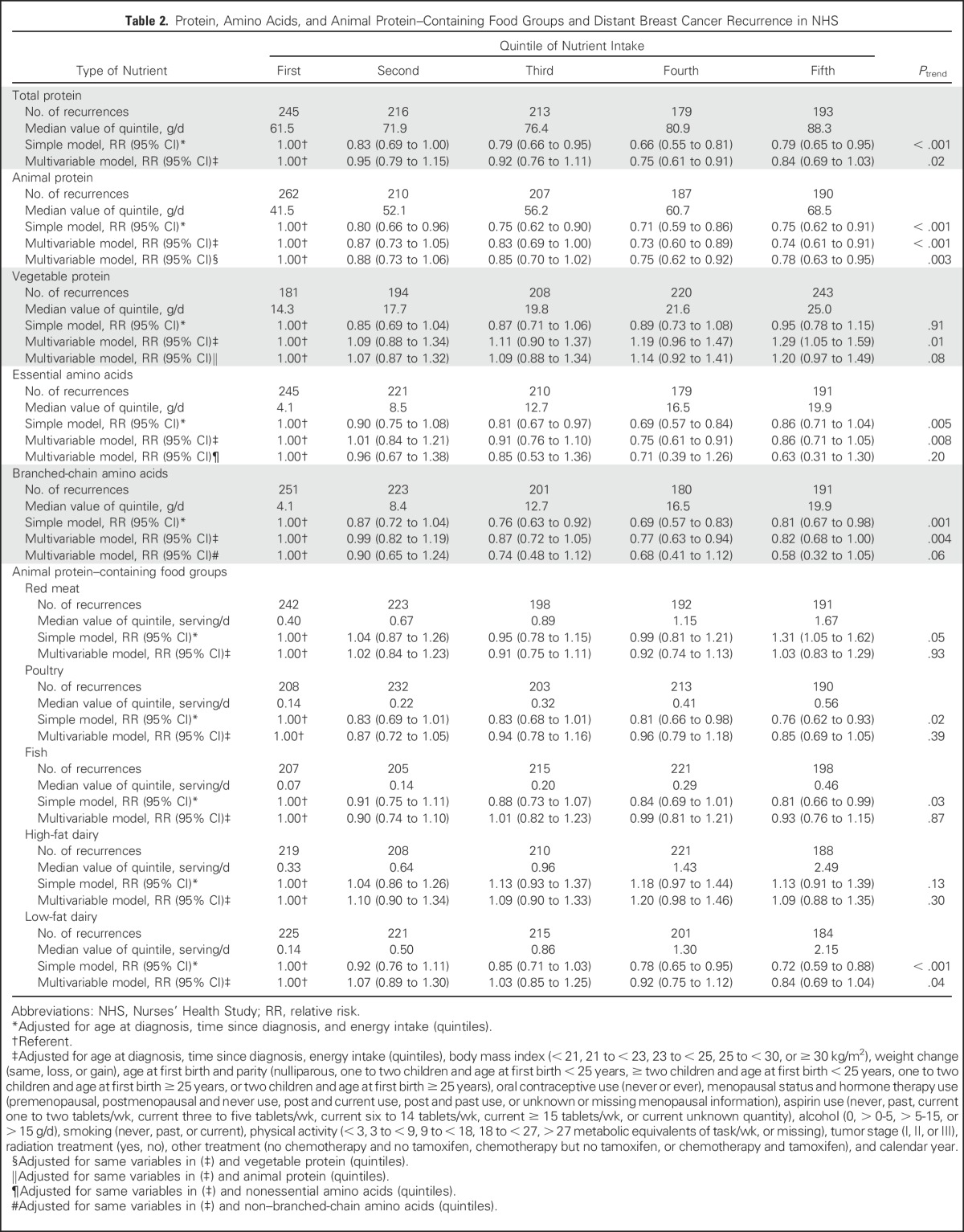
There was an inverse association between total protein intake and distant breast cancer recurrence. Multivariable RRs for increasing quintiles of intake compared with the lowest intake were 0.95 (95% CI, 0.79 to 1.15), 0.92 (95% CI, 0.76 to 1.11), 0.75 (95% CI, 0.61 to 0.91), and 0.84 (95% CI, 0.69 to 1.03; linear trend P = .02). This inverse association was accounted for by animal protein intake; multivariable RRs for quintiles of animal protein intake adjusted for vegetable protein intake were 0.88 (95% CI, 0.73 to 1.06), 0.85 (95% CI, 0.70 to 1.02), 0.75 (95% CI, 0.62 to 0.92), and 0.78 (95% CI, 0.63 to 0.95; P = .003).
The association for higher intake of essential amino acids (adjusted for nonessential amino acids) and branched-chain amino acids (adjusted for nonbranched chain amino acids) tended toward the inverse, although it did not reach statistical significance. Among animal protein–containing food groups, only low-fat dairy showed a borderline inverse association between higher intake and recurrence. Multivariable RRs across quintiles of intake (compared with lowest intake) were 1.07 (95% CI, 0.89 to 1.30), 1.03 (95% CI, 0.85 to 1.25), 0.92 (95% CI, 0.75 to 1.12), and 0.84 (95% CI, 0.69 to 1.05; linear trend P = .04).
Point estimates were similar when participants who contributed to the 1999 NHS report4 were excluded, but because of fewer outcomes, results were less likely to be statistically significant. For example, the multivariable RRs of recurrence for increasing quintiles of intake of total protein compared with the lowest intake were 1.03 (95% CI, 0.76 to 1.39), 0.92 (95% CI, 0.68 to 1.25), 0.78 (95% CI, 0.56 to 1.07), and 0.87 (95% CI, 0.63 to 1.20; linear trend P = .18).
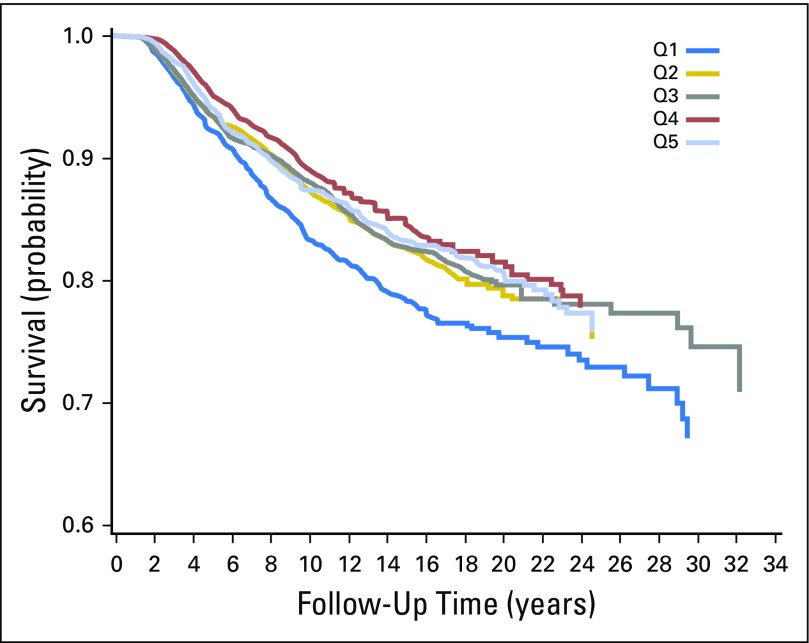
The 5-year recurrence-free survival for women in the highest quintile of protein consumption was 94.0%, and for those in the lowest quintile of protein consumption, it was 92.1% (Appendix Fig A1, online only) The corresponding 10-year recurrence-free survival rates were 87.4% and 83.3%, respectively. The absolute unadjusted risk reduction for recurrence was 1.9% at 5 years and 4.1% at 10 years for women who in the highest quintile of protein consumption compared with the lowest.
Results for death resulting from breast cancer (Table 3) were similar to those for recurrence, although they were less robust because of fewer outcomes. Associations between intakes of protein, amino acids, and protein-containing food groups and breast cancer recurrence did not differ by tumor IR status (Table 4).
Table 3.
Protein, Amino Acids, and Animal Protein–Containing Food Groups and Death Resulting From Breast Cancer in NHS
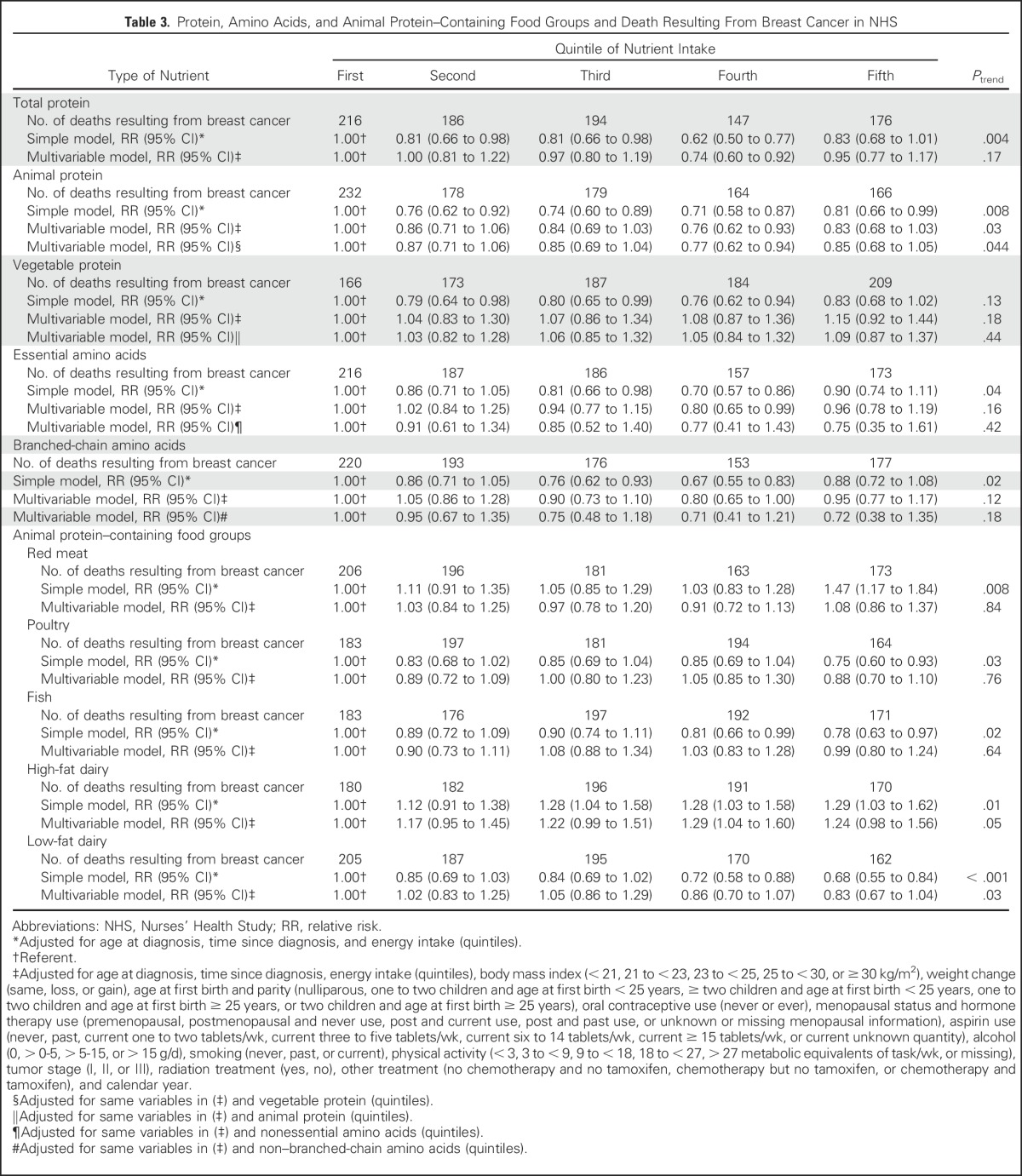
Table 4.
Protein, Amino Acids, and Animal Protein–Containing Food Groups and Distant Breast Cancer Recurrence According to IR Expression in NHS
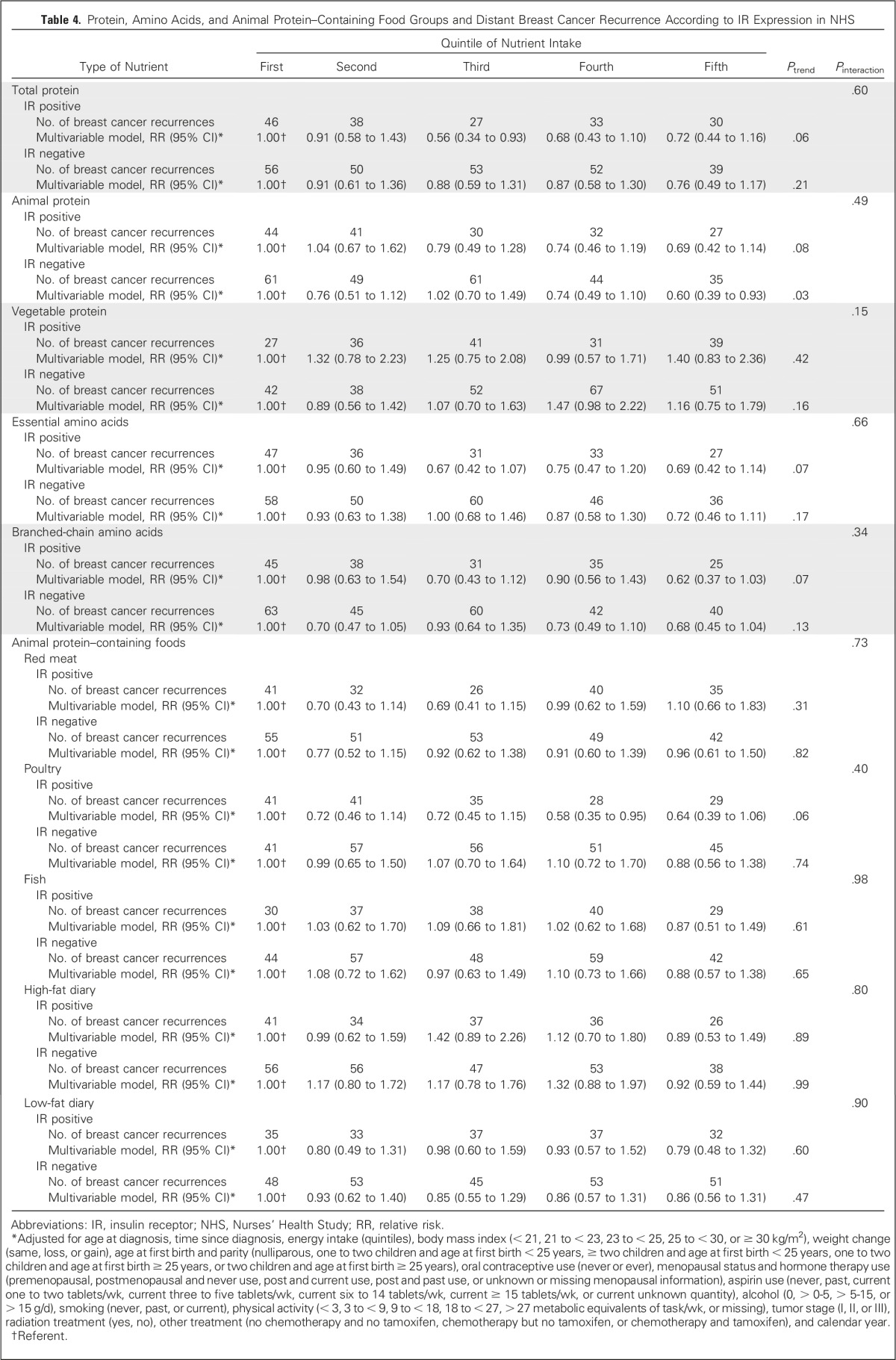
In addition, we examined the association between intakes of individual amino acids and individual animal protein–containing foods and risk of distant breast cancer recurrence (Appendix Table A1, online only). Although all amino acids had an inverse relationship with recurrence, and for many, the P value for the linear trend was statistically significant, no one amino acid stood out from the others. Because we found an inverse association between total death and poultry intake in the 1999 NHS report,4 it is intriguing to examine the results on poultry intake. Among protein-containing foods, intake of poultry with skin was inversely associated with distant recurrence (highest v lowest quintile: RR, 0.72; 95% CI, 0.59 to 0.89; linear trend P = .008), whereas intake of poultry without skin was nearly associated with an increased risk of distant recurrence (highest v lowest quintile: RR, 1.15; 95% CI, 0.94 to 1.41; linear trend P = .08), thus accounting for the lack of association of poultry as a food group with distant recurrence. Results for death resulting from breast cancer (Appendix Table A2, online only) were similar. Results examining total death (Appendix Table A3, online only) showed similar results for poultry with and without skin and no association with protein, type of protein, or individual amino acids. Association between risk of distant recurrence and protein, amino acids, and protein-containing food groups did not differ either by ER status of the tumor (Appendix Table A4, online only) or by BMI at diagnosis (Appendix Table A5, online only).
DISCUSSION
Our large prospective study of more than 6,000 breast cancer survivors confirmed the modest inverse association with protein intake and breast cancer survival that the NHS first reported in 1999. This association did not vary by IR status, ER status, or BMI at diagnosis. Three other studies (N = 412, 477, and 603) produced similar findings.1-3 A similar association was reported in 1989 for mammary cancer in dogs.28
In the 1999 NHS report, a survival advantage was found for intakes of poultry and dairy products, but not red meat.4 Our study differed from the previous NHS report in both size and ability to examine breast cancer recurrence and death resulting from breast cancer. We again found a lower risk of recurrence and death resulting from breast cancer with higher intake of protein. This was particularly true for protein from animal sources (meat, poultry, fish, dairy, and eggs). However, although the P value for the linear trend for the association with animal protein was .003, it is of note that the values for quintiles two to five (RR, 0.88, 0.85, 0.75, and 0.78, respectively) did not vary much. Thus, the inverse association between animal protein and recurrence seems to be present for any intake above the lowest. In addition, the distributions of intake were different for animal and vegetable proteins. For example, the median of the highest quintile of vegetable protein intake (25.0 g per day) was less than the median of the lowest quintile of animal protein intake (41.5 g per day; Table 2). It may be that intake of vegetable protein was not high enough in our population to detect a benefit.
Although results should be interpreted cautiously because of multiple comparisons, the inverse association with protein intake did not seem to be a result of specific amino acids or groups of amino acids (essential or branched chain). Contrary to our hypothesis, this association was not confined to tumors expressing IR. Therefore, it is difficult to invoke the insulin pathway as a mechanism to explain these findings.
Among animal protein–containing foods, we found only a borderline inverse association with low-fat dairy intake and found no association with total dairy or high-fat dairy intake. In 2013, Kroenke et al29 reported no association between low-fat dairy or total dairy intake and death resulting from breast cancer among 1,893 women in the LACE (Life After Cancer Epidemiology) cohort, but they did find a borderline increased risk of death resulting from breast cancer for high-fat dairy intake; compared with the lowest number of servings per day (zero to < one half serving), the adjusted RR was 1.20 (95% CI, 0.82 to 1.77) for medium intake (half to < one serving) and 1.49 (95% CI, 1.00 to 2.24) for highest intake (≥ one serving; linear trend P = .05). The authors hypothesized that estrogenic hormones found in dairy fat may be detrimental to breast cancer survival. Our findings of an inverse association for poultry with skin and a nonstatistically significant positive association for poultry without skin are likely the results of chance, particularly given the lack of association with poultry intake overall. In summary, no particular animal protein–containing food stood out as beneficial for breast cancer survival.
Any observational study such as this is subject to the limitations of chance, confounding, and bias. Three previous cohort studies reported similar improved survival with higher protein intake, and our study is larger than the other three combined, making chance unlikely. Also, there are no published reports to date of worse breast cancer survival with increased protein intake. We adjusted for the key covariates; the fact that estimates for animal protein did not change much provides some reassurance against confounding to explain the results. Bias is a concern, particularly if women changed their diets as they approached death or developed metastatic disease. However, we used diet assessments updated approximately every 4 years over time. Also, results were similar for death resulting from breast cancer and breast cancer recurrence.
In summary, we found a modest survival advantage with higher protein intake that was not associated with any particular foods. There is no clear mechanism for this surprising finding. Our hypothesis that the effect might be mediated by the IR was not borne out in this study. Given the modest effect and the challenges involved in randomized trials of diet, this association is unlikely to ever be definitively tested in a randomized trial. However, the modest survival advantage with higher protein intake has been found in several studies, and we feel it is important that patients with breast cancer and their clinicians know this. At the least, it may provide reassurance that consuming protein-containing foods is not likely to increase the risk of breast cancer recurrence.
ACKNOWLEDGMENT
We thank the participants and staff of the Nurses’ Health Study for their valuable contributions as well as the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Massachusetts, Minnesota, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming. We also thank Mathew Pazaris for his help with programming. The authors assume full responsibility for analyses and interpretation of these data.
Appendix
Table A1.
Multivariable Analysis of Animal Protein–Containing Foods and Individual Amino Acids and Breast Cancer Recurrence in NHS
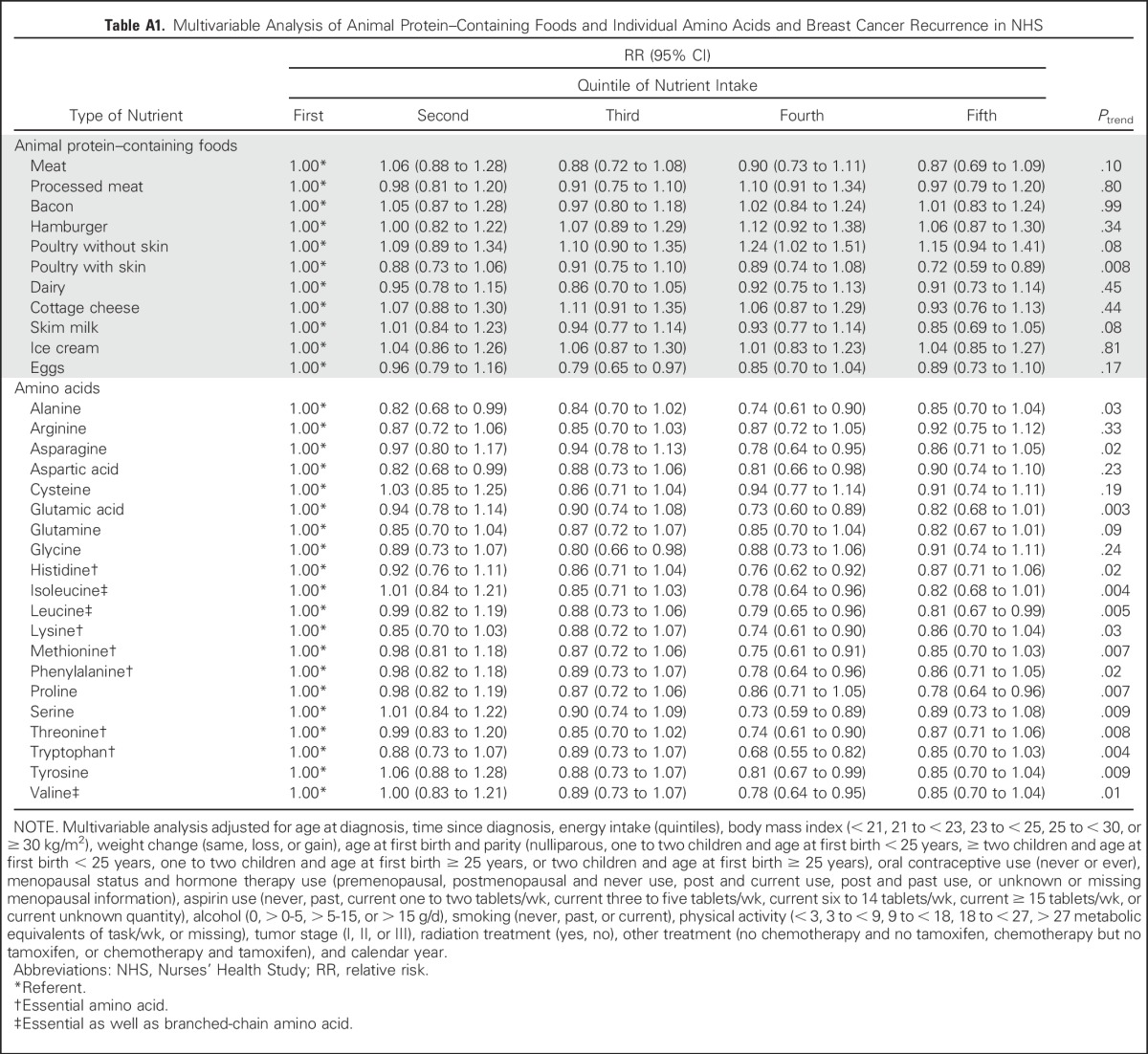
Table A2.
Multivariable Analysis of Animal Protein–Containing Foods and Individual Amino Acids and Death Resulting From Breast Cancer in NHS
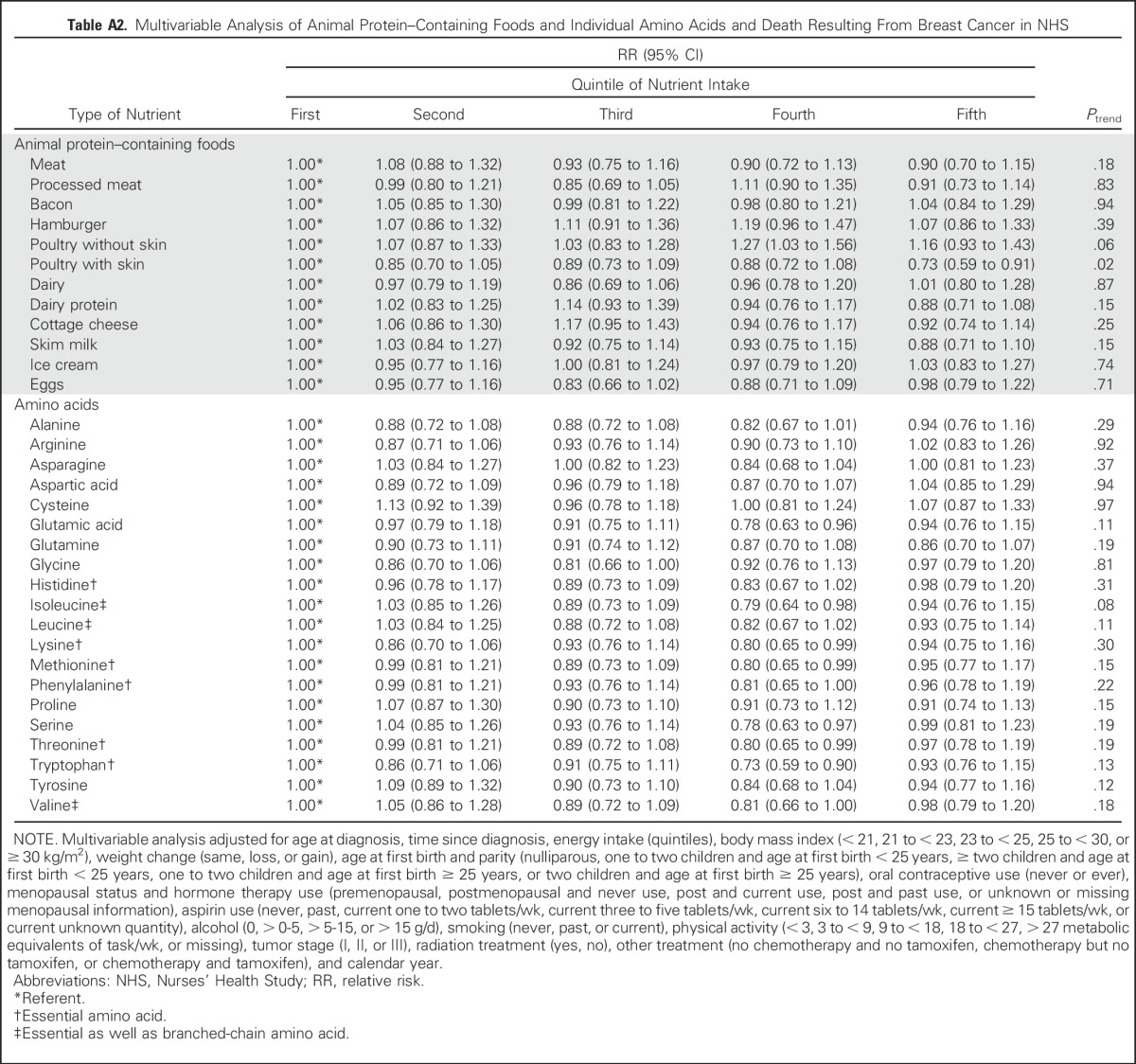
Table A3.
Multivariable Analysis of Animal Protein–Containing Foods and Individual Amino Acids and All Deaths in NHS
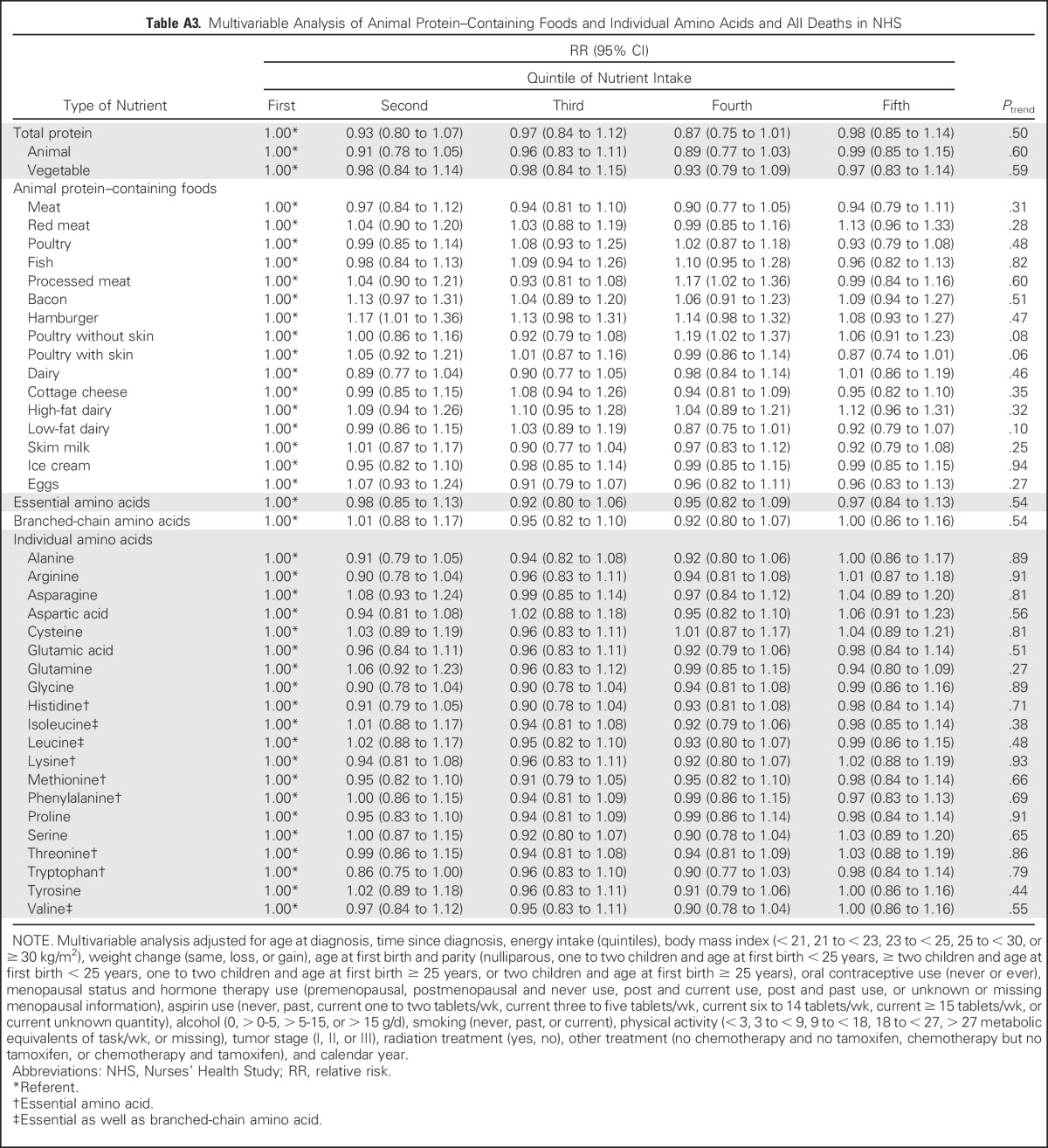
Table A4.
Protein, Amino Acids, and Animal Protein–Containing Foods and Distant Breast Cancer Recurrence According to ER Expression
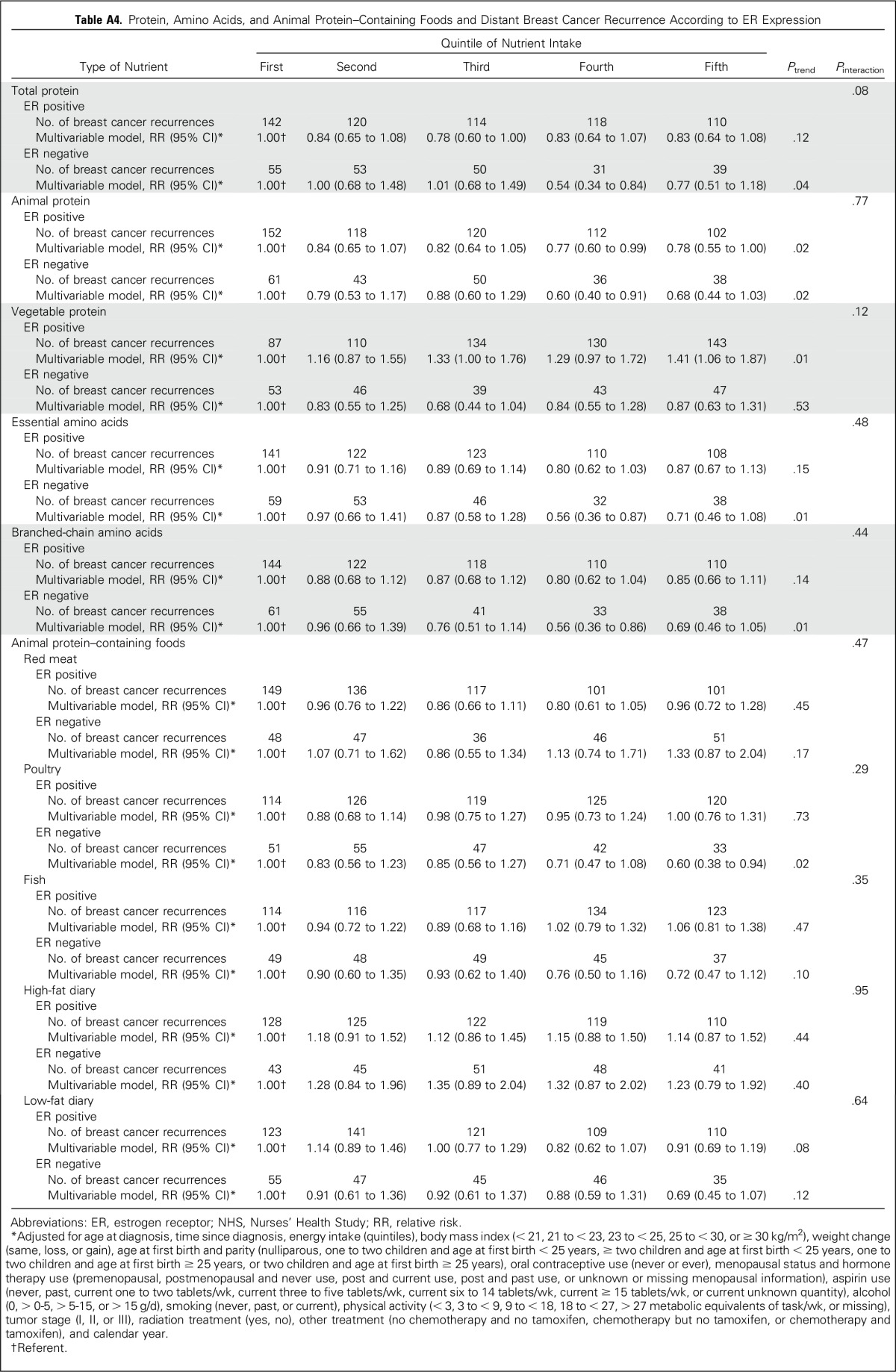
Table A5.
Protein, Amino Acids, and Animal Protein–Containing Foods and Distant Breast Cancer Recurrence According to BMI at Diagnosis
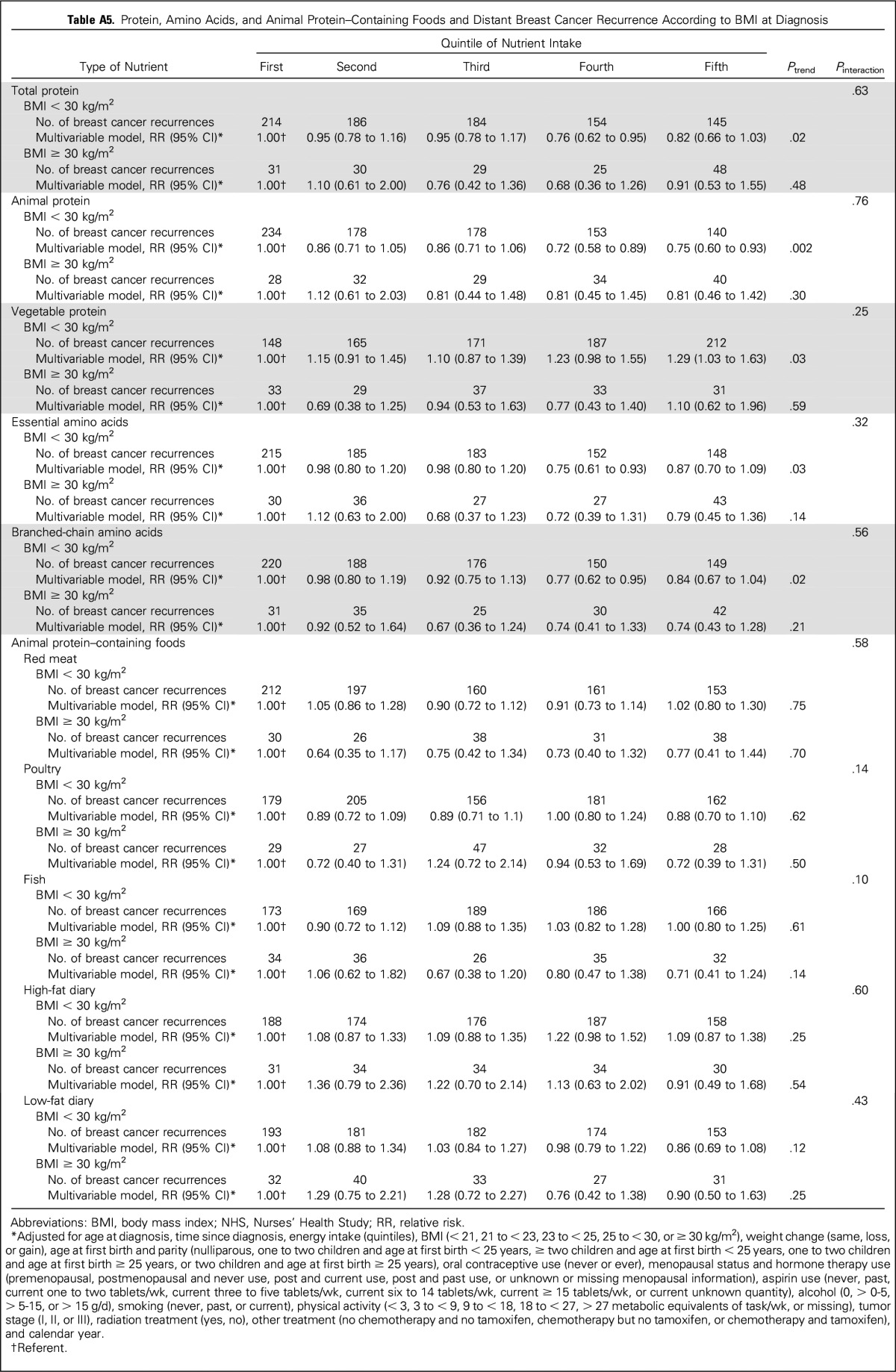
Fig A1.
Kaplan-Meier survival curves of probability of recurrence by quintile (Q) of protein intake.
Footnotes
Supported by National Institutes of Health Grants No. UM1 CA186107 and P01 CA87969.
AUTHOR CONTRIBUTIONS
Conception and design: Michelle D. Holmes, Wendy Y. Chen
Collection and assembly of data: Rulla M. Tamimi
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Protein Intake and Breast Cancer Survival in the Nurses’ Health Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Michelle D. Holmes
Research Funding: Bayer HealthCare Pharmaceuticals (Inst)
Jun Wang
No relationship to disclose
Susan E. Hankinson
No relationship to disclose
Rulla M. Tamimi
No relationship to disclose
Wendy Y. Chen
Research Funding: Bayer HealthCare Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
REFERENCES
- 1.Rohan TE, Hiller JE, McMichael AJ. Dietary factors and survival from breast cancer. Nutr Cancer. 1993;20:167–177. doi: 10.1080/01635589309514283. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin PJ, Ennis M, Pritchard KI, et al. Diet and breast cancer: Evidence that extremes in diet are associated with poor survival. J Clin Oncol. 2003;21:2500–2507. doi: 10.1200/JCO.2003.06.121. [DOI] [PubMed] [Google Scholar]
- 3.Borugian MJ, Sheps SB, Kim-Sing C, et al. Insulin, macronutrient intake, and physical activity: Are potential indicators of insulin resistance associated with mortality from breast cancer? Cancer Epidemiol Biomarkers Prev. 2004;13:1163–1172. [PubMed] [Google Scholar]
- 4. doi: 10.1002/(sici)1097-0142(19990901)86:5<826::aid-cncr19>3.0.co;2-0. Holmes MD, Stampfer MJ, Colditz GA, et al: Dietary factors and the survival of women with breast carcinoma. Cancer 86:826-835, 1999 [Erratum: Cancer 86:2707-2708, 1999] [DOI] [PubMed] [Google Scholar]
- 5.Wolfe RR. Protein Summit: Consensus areas and future research. Am J Clin Nutr. 2008;87:1582S–1583S. doi: 10.1093/ajcn/87.5.1582S. [DOI] [PubMed] [Google Scholar]
- 6.Millward DJ, Layman DK, Tomé D, et al. Protein quality assessment: Impact of expanding understanding of protein and amino acid needs for optimal health. Am J Clin Nutr. 2008;87:1576S–1581S. doi: 10.1093/ajcn/87.5.1576S. [DOI] [PubMed] [Google Scholar]
- 7.Layman DK, Evans E, Baum JI, et al. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J Nutr. 2005;135:1903–1910. doi: 10.1093/jn/135.8.1903. [DOI] [PubMed] [Google Scholar]
- 8.Layman DK. The role of leucine in weight loss diets and glucose homeostasis. J Nutr. 2003;133:261S–267S. doi: 10.1093/jn/133.1.261S. [DOI] [PubMed] [Google Scholar]
- 9.Garlick PJ. The role of leucine in the regulation of protein metabolism. J Nutr. 2005;135:1553S–1556S. doi: 10.1093/jn/135.6.1553S. (suppl) [DOI] [PubMed] [Google Scholar]
- 10.Opara EC, Petro A, Tevrizian A, et al. L-glutamine supplementation of a high fat diet reduces body weight and attenuates hyperglycemia and hyperinsulinemia in C57BL/6J mice. J Nutr. 1996;126:273–279. doi: 10.1093/jn/126.1.273. [DOI] [PubMed] [Google Scholar]
- 11.Borel MJ, Williams PE, Jabbour K, et al. Parenteral glutamine infusion alters insulin-mediated glucose metabolism. JPEN J Parenter Enteral Nutr. 1998;22:280–285. doi: 10.1177/0148607198022005280. [DOI] [PubMed] [Google Scholar]
- 12.Paddon-Jones D, Short KR, Campbell WW, et al. Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr. 2008;87:1562S–1566S. doi: 10.1093/ajcn/87.5.1562S. [DOI] [PubMed] [Google Scholar]
- 13.Houston DK, Nicklas BJ, Ding J, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) study. Am J Clin Nutr. 2008;87:150–155. doi: 10.1093/ajcn/87.1.150. [DOI] [PubMed] [Google Scholar]
- 14.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 15.Willett W. Nutritional Epidemiology. (ed 2) New York, NY: Oxford University Press; 1998. [Google Scholar]
- 16.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119:837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 17.Holmes MD, Murin S, Chen WY, et al. Smoking and survival after breast cancer diagnosis. Int J Cancer. 2007;120:2672–2677. doi: 10.1002/ijc.22575. [DOI] [PubMed] [Google Scholar]
- 18.Bartelink H, Horiot JC, Poortmans P, et al. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med. 2001;345:1378–1387. doi: 10.1056/NEJMoa010874. [DOI] [PubMed] [Google Scholar]
- 19.Tamimi RM, Baer HJ, Marotti J, et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res. 2008;10:R67. doi: 10.1186/bcr2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins LC, Marotti JD, Baer HJ, et al. Comparison of estrogen receptor results from pathology reports with results from central laboratory testing. J Natl Cancer Inst. 2008;100:218–221. doi: 10.1093/jnci/djm270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinsel LB, Szabo E, Greene GL, et al. Immunocytochemical analysis of estrogen receptors as a predictor of prognosis in breast cancer patients: Comparison with quantitative biochemical methods. Cancer Res. 1989;49:1052–1056. [PubMed] [Google Scholar]
- 22.Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 23.Kroenke CH, Chen WY, Rosner B, et al. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:1370–1378. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 24.Holmes MD, Chen WY, Li L, et al. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;28:1467–1472. doi: 10.1200/JCO.2009.22.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: A comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 26.Prentice RL, Kalbfleisch JD. Hazard rate models with covariates. Biometrics. 1979;35:25–39. [PubMed] [Google Scholar]
- 27.Wang M, Spiegelman D, Kuchiba A, et al. Statistical methods for studying disease subtype heterogeneity. Stat Med. 2016;35:782–800. doi: 10.1002/sim.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shofer FS, Sonnenschein EG, Goldschmidt MH, et al. Histopathologic and dietary prognostic factors for canine mammary carcinoma. Breast Cancer Res Treat. 1989;13:49–60. doi: 10.1007/BF01806550. [DOI] [PubMed] [Google Scholar]
- 29.Kroenke CH, Kwan ML, Sweeney C, et al. High- and low-fat dairy intake, recurrence, and mortality after breast cancer diagnosis. J Natl Cancer Inst. 2013;105:616–623. doi: 10.1093/jnci/djt027. [DOI] [PMC free article] [PubMed] [Google Scholar]