Abstract
Purpose
To perform competing risks analysis and determine short- and long-term cancer- and noncancer-specific mortality and morbidity in patients who had undergone resection for stage I non–small-cell lung cancer (NSCLC).
Patients and Methods
Of 5,371 consecutive patients who had undergone curative-intent resection of primary lung cancer at our institution (2000 to 2011), 2,186 with pathologic stage I NSCLC were included in the analysis. All preoperative clinical variables known to affect outcomes were included in the analysis, specifically, Charlson comorbidity index, predicted postoperative (ppo) diffusing capacity of the lung for carbon monoxide, and ppo forced expiratory volume in 1 second. Cause-specific mortality analysis was performed with competing risks analysis.
Results
Of 2,186 patients, 1,532 (70.1%) were ≥ 65 years of age, including 638 (29.2%) ≥ 75 years of age. In patients < 65, 65 to 74, and ≥ 75 years of age, 5-year lung cancer–specific cumulative incidence of death (CID) was 7.5%, 10.7%, and 13.2%, respectively (overall, 10.4%); noncancer-specific CID was 1.8%, 4.9%, and 9.0%, respectively (overall, 5.3%). In patients ≥ 65 years of age, for up to 2.5 years after resection, noncancer-specific CID was higher than lung cancer–specific CID; the higher noncancer-specific, early-phase mortality was enhanced in patients ≥ 75 years of age than in those 65 to 74 years of age. Multivariable analysis showed that low ppo diffusing capacity of lung for carbon monoxide was an independent predictor of severe morbidity (P < .001), 1-year mortality (P < .001), and noncancer-specific mortality (P < .001), whereas low ppo forced expiratory volume in 1 second was an independent predictor of lung cancer–specific mortality (P = .002).
Conclusion
In patients who undergo curative-intent resection of stage I NSCLC, noncancer-specific mortality is a significant competing event, with an increasing impact as patient age increases.
INTRODUCTION
Among solid tumors, lung cancer carries a relatively high risk of competing cancer and noncancer events because more than two thirds of patients with lung cancer are ≥ 65 years of age at the time of diagnosis,1 and one half of those patients are ≥ 75 years of age with associated high comorbidities.2 As age increases, the risk of competing events increases, such as death from noncancer diseases.3 In this era of personalized cancer therapy, important to the stratification of individualized treatments is the determination of how both cancer and noncancer risk factors—specifically, comorbidities associated with increasing age—contribute to the risk of death.
After the publication of the National Lung Screening Trial results, which demonstrated the efficacy of low-dose computed tomography screening for lung cancer, detection of early-stage lung cancer, for which curative-intent resection is the standard treatment,4 is expected to increase.5-7 The data from the International Association for the Study of Lung Cancer, which were derived from a multinational cohort, are considered the reference standard for overall survival (OS) in patients with non–small-cell lung cancer (NSCLC). According to these data, the estimated 5-year overall mortality from stage IA and IB NSCLC is 17% and 29%, respectively, after curative-intent R0 resection.8 Smoking status, chronic obstructive pulmonary disease (COPD) history, and pulmonary function have been reported to be predictive of OS or lung cancer–related survival after resection of NSCLC.9-20 Noncancer risk factors, such as Charlson comorbidity index (CCI), cardiovascular disease (CVD) history, body mass index, and serum creatinine level, have been reported to influence outcomes.18,21-23 However, a comprehensive analysis of all variables known to affect outcomes has not been published and, to our knowledge, no study has included a cause-specific analysis with an evaluation of competing risks. The goal of this study, therefore, was to perform a competing risks analysis to determine short- and long-term cancer- and noncancer-specific mortality and morbidity in patients who had undergone resection for stage I NSCLC.
By using a large, uniform cohort of patients with stage I NSCLC, we analyzed short- and long-term cause-specific outcomes through competing risks analysis. This comprehensive prognostic analysis included all preoperative variables known to contribute individually to outcomes, including lung-related (forced expiratory volume in 1 second [FEV1]; diffusing capacity of lung for carbon monoxide [DLCO]); age-related (cardiorespiratory comorbidities, renal function, CCI); and cancer-related (tumor size on computed tomography scan) parameters. In light of the ongoing debate about the appropriate type of surgical resection for small NSCLC—lobectomy versus sublobar resection (wedge resection or segmentectomy)—the identification of predictive factors for cancer- and noncancer-specific morbidity and mortality will be of substantial value for stratifying therapy.24,25 The establishment of such factors is even more important for older patients, who comprise the majority of patients with stage I NSCLC.
Our investigation yielded novel and previously unreported observations on outcomes in patients who undergo resection of stage I NSCLC: Noncancer-specific mortality is a prominent competing event, with an increasing impact as age increases; noncancer-specific mortality is higher than lung cancer–specific mortality for up to 1.5 years after resection, particularly in older patients (≥ 65 years of age); and low predicted postoperative (ppo) DLCO is an independent predictor of noncancer-specific mortality, whereas low ppo FEV1 is an independent predictor of lung cancer–specific mortality.
PATIENTS AND METHODS
Study Cohort
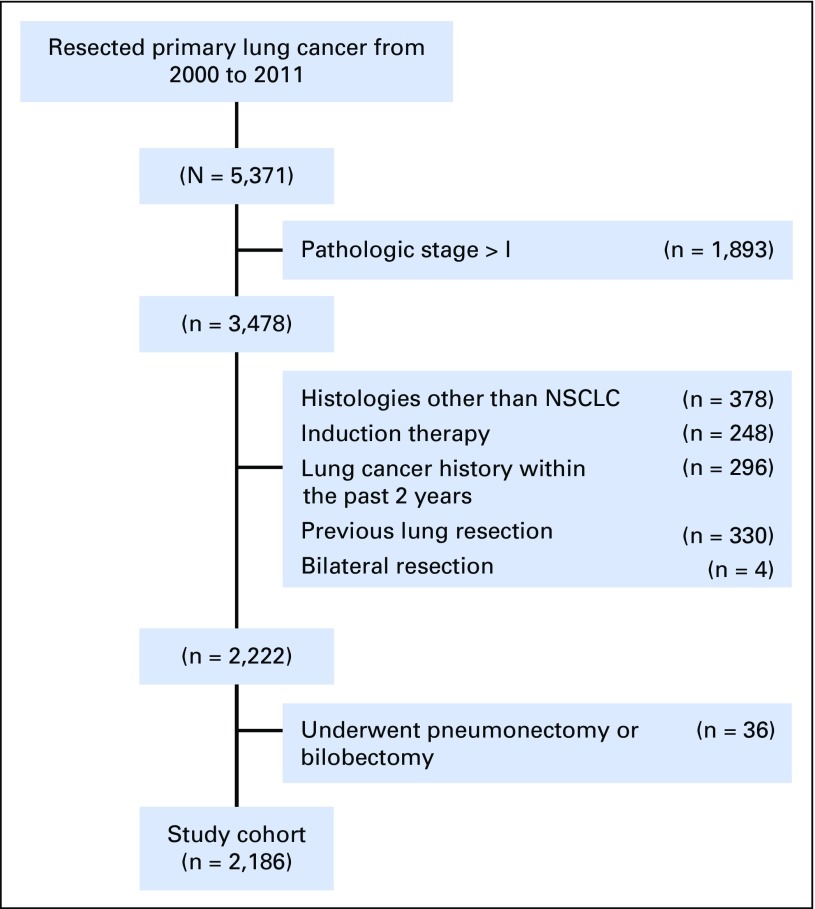
This retrospective study (WA0269-08, WA0219-09, and WA0280-12) was approved by the institutional review board at Memorial Sloan Kettering Cancer Center. Medical records of 5,371 patients with primary lung cancer who had undergone lung resection at our center between 2000 and 2011 were reviewed. Patient exclusion criteria; preoperative, surgical, pathologic data variables; and the surveillance protocol are provided in detail in the Data Supplement. A total of 2,186 patients were included in the study (Fig 1).
Fig 1.
CONSORT diagram. The study cohort included all consecutive patients who underwent R0 resection for pathologic stage I non–small-cell lung cancer (NSCLC). R0, microscopically margin-negative resection.
End Points and Cause of Death
The end points of this study were severe morbidity, 1-year mortality, lung cancer–specific mortality, noncancer-specific mortality, and OS. Severe morbidities were defined as grade 3 and higher, occurring within 30 days after surgery, in accordance with Common Terminology Criteria for Adverse Events (version 4.03).26 The classification of severe morbidities and corresponding incidence rates are shown in the Data Supplement.
The cause of death was classified as lung cancer specific, noncancer specific, other cancer specific, or unknown. Lung cancer–specific mortality was defined as death as a result of recurrent disease associated with resected lung cancer. Patients who had progressive recurrent disease at the last follow-up and death without a documented specific reason were included in the lung cancer–specific group. Noncancer-specific mortality was defined as death as a result of specific causes other than malignant disease, including death without a documented specific reason within 6 months of the last follow-up in the absence of lung cancer recurrence or progressive malignant disease. Death as a result of second primary lung cancer or other malignancies was regarded as other cancer specific.
Statistical Analyses
Patient demographic and clinical characteristics were summarized with descriptive statistics. Associations between variables were analyzed with Fisher’s exact test for categorical variables and Wilcoxon rank sum test for continuous variables by age-group (< 65, 65 to 74, ≥ 75 years) and surgical procedure (lobectomy v sublobar resection).
To evaluate age-related changes in preoperative variables (cardiopulmonary function and CCI), patients were divided into the seven increasing age-groups (≤ 55, 56 to 60, 61 to 65, 66 to 70, 71 to 75, 76 to 80, > 80 years) that were explored graphically. The mean for each variable of the seven groups was summarized and fitted by using cubic B-splines for graphical representation.27 Differences in age-related change between the lobectomy and sublobar resection groups were evaluated by multivariable logistic regression for COPD and CVD history and linear regression for continuous variables. Model covariates included surgical procedure, continuous age, and interaction of both, where applicable. A significant interaction indicates that the pattern of age-related change is significantly different between surgical procedures.
Univariable and multivariable logistic models were constructed to identify factors associated with severe morbidity and 1-year mortality. We elected to use ppo FEV1 and ppo DLCO instead of FEV1 and DLCO in our regression models because risk of postoperative morbidity has been linked to ppo lung function.11,28
The associations between factors and the risk of each cause of death were evaluated with competing risks analysis. Patients were censored if they were alive at the time of the last follow-up. Cumulative incidence functions for each competing event were calculated by competing risks methodology.29 Without loss of generality, analyses of the cumulative incidence of lung cancer–specific mortality considered noncancer-specific and all-other-cause mortality as two separate competing risks, and Fine and Gray’s30 competing risks regressions were used to estimate the subhazard ratio to evaluate the association between preoperative variables and risk of each cause of death. Multivariable regression models included all variables with P < .1 on univariable analysis. Competing risks analyses were conducted with the R project’s Subdistribution Analysis of Competing Risks (cmprsk) package (version 2.13.1) and the Stata 13 Competing Risks Regressions (stcrreg) command (StataCorp, College Station, TX). Estimation of 5-year cumulative incidence of death (CID) by using ppo DLCO, ppo FEV1, and CCI was explored graphically; the estimated 5-year CID by groups (ppo FEV1, ≤ 50%, 51% to 60%, 61% to 70%, 71% to 80%, 81% to 90%, > 90%; ppo DLCO, ≤ 45%, 46% to 55%, 56% to 65%, 66% to 75%, 76% to 85%, > 85%; CCI, 0, 1, 2, 3, ≥ 4) was summarized and fitted by using cubic B-splines for graphical representation. OS was estimated by the Kaplan-Meier method and compared between groups by log-rank test. Hazard ratios were estimated from Cox univariable and multivariable models. As a representation of the performance of multivariable models in terms of discrimination, the concordance index (C-index) was reported for logistic,31 survival,32 and competing risks models.33 Analysis for cumulative incidence of recurrence was performed (Data Supplement). All statistical tests were two sided, and P < .05 was considered significant.
RESULTS
Patient Characteristics
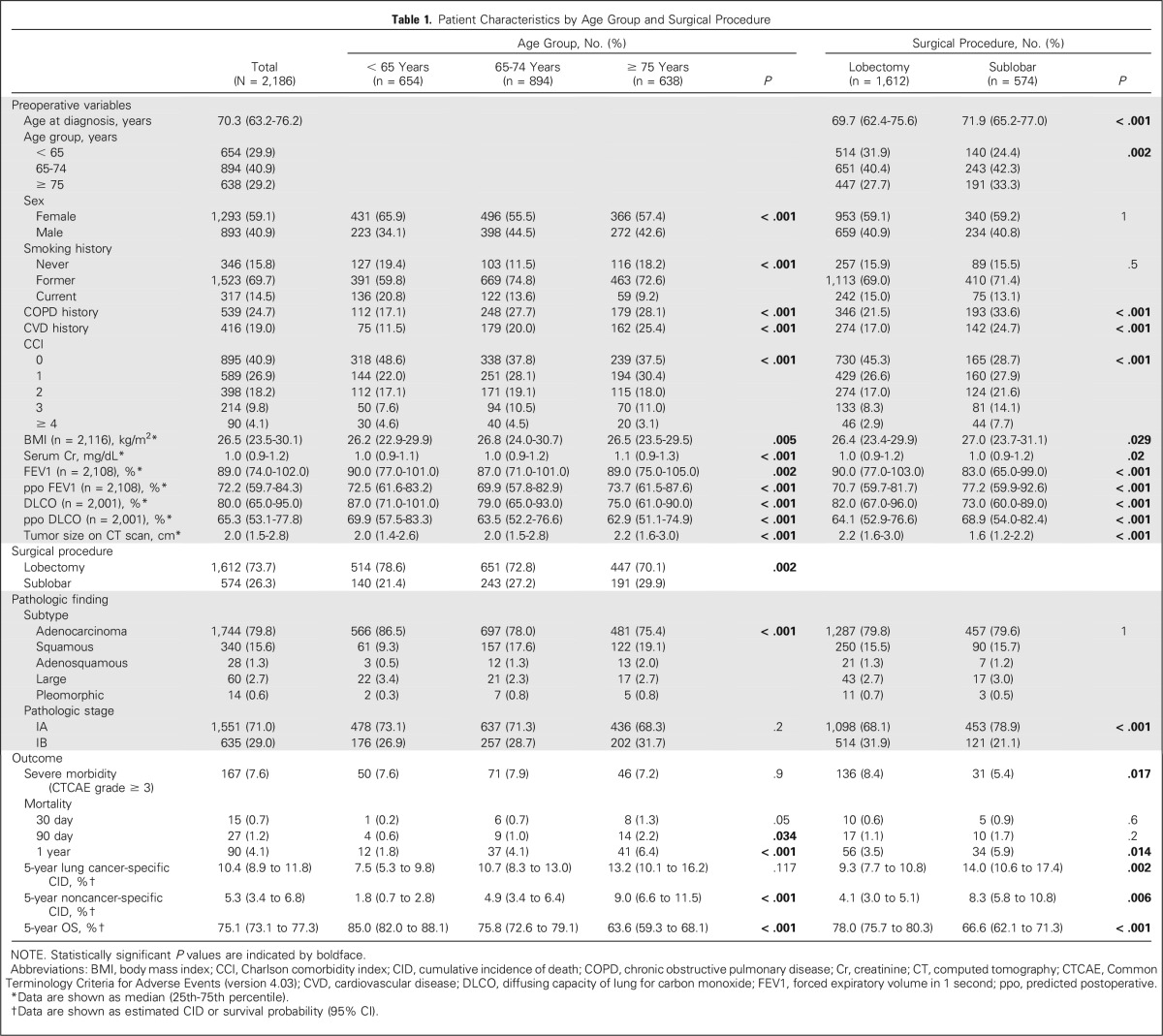
Of the 2,186 patients evaluated, the majority were women (59.1%), were former or current smokers (84.2%), had no history of COPD (75.3%) or CVD (81.0%), had a CCI ≥ 1 (59.1%), were diagnosed with adenocarcinoma (79.8%), and had stage IA disease (71.0%). Patient characteristics are listed in Table 1.
Table 1.
Patient Characteristics by Age Group and Surgical Procedure
Comparison by Age Groups and Surgical Procedures
Among the cohort, 1,532 (70.1%) of patients were ≥ 65 years of age, including 638 (29.2%) ≥ 75 years of age. The majority of patients underwent lobectomy (73.7%). All preoperative variables were statistically different among the three age-groups (< 65, 65 to 74, and ≥ 75 years). All preoperative variables, except sex and smoking status, were statistically different between the lobectomy and sublobar resection groups (Table 1).
The Data Supplement shows preoperative variables stratified by surgical procedure for each age-group. Serum creatinine level, COPD history, CVD history, and CCI increased as age increased; in contrast, DLCO decreased as age increased. For most age-groups, COPD history, CVD history, and CCI were higher in the sublobar resection group than in the lobectomy group. In contrast, DLCO and FEV1 were lower in the sublobar resection group than in the lobectomy group.
Postoperative Severe Morbidities
Of the 2,186 patients evaluated, postoperative severe morbidities developed in 167 (7.6%). Among them, 114 (68.3%) and 31 (18.6%) had respiratory and cardiovascular morbidities, respectively. Overall severe morbidity and respiratory morbidity were significantly more common in the lobectomy group than in the sublobar resection group (P = .017 and .002, respectively) and in patients who underwent right-side lower lobectomy (P = .025 and .029, respectively; Data Supplement).
Causes of Short- and Long-Term Mortality
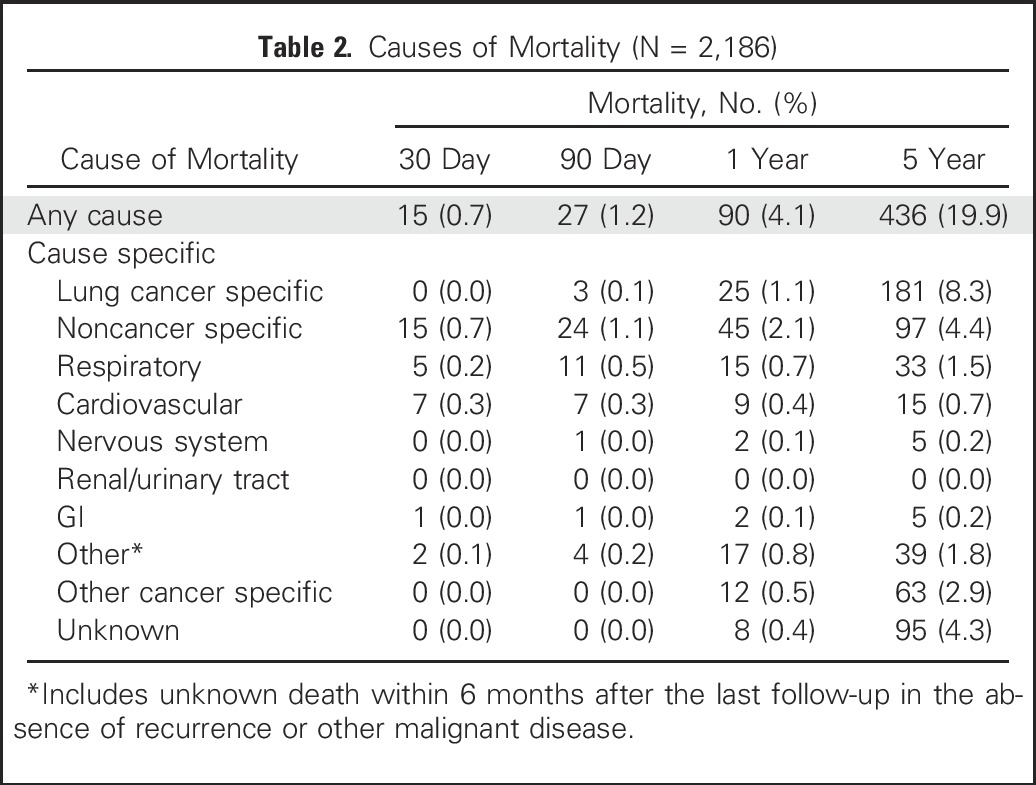
The median follow-up was 4.2 years (range, 0.01 to 14.4 years). The 30-day, 90-day, 1-year, and 5-year mortality rates were 0.7% (n = 15), 1.2% (n = 27), 4.1% (n = 90), and 19.9% (n = 436), respectively. Cardiorespiratory disease was the most frequent specific cause of death at 30 and 90 days. At 1 year, the leading cause of death was noncancer specific (45 of 90 [50.0%]) followed by lung cancer specific (25 of 90 [27.8%]) and other cancer specific (12 of 90 [13.3%]). At 5 years, the leading cause of death was lung cancer specific (181 of 436 [41.5%]) followed by noncancer specific (97 of 436 [22.2%]) and other cancer specific (63 of 436 [14.4%]; Table 2).
Table 2.
Causes of Mortality (N = 2,186)
Univariable and Multivariable Analyses for Short- and Long-Term Outcomes
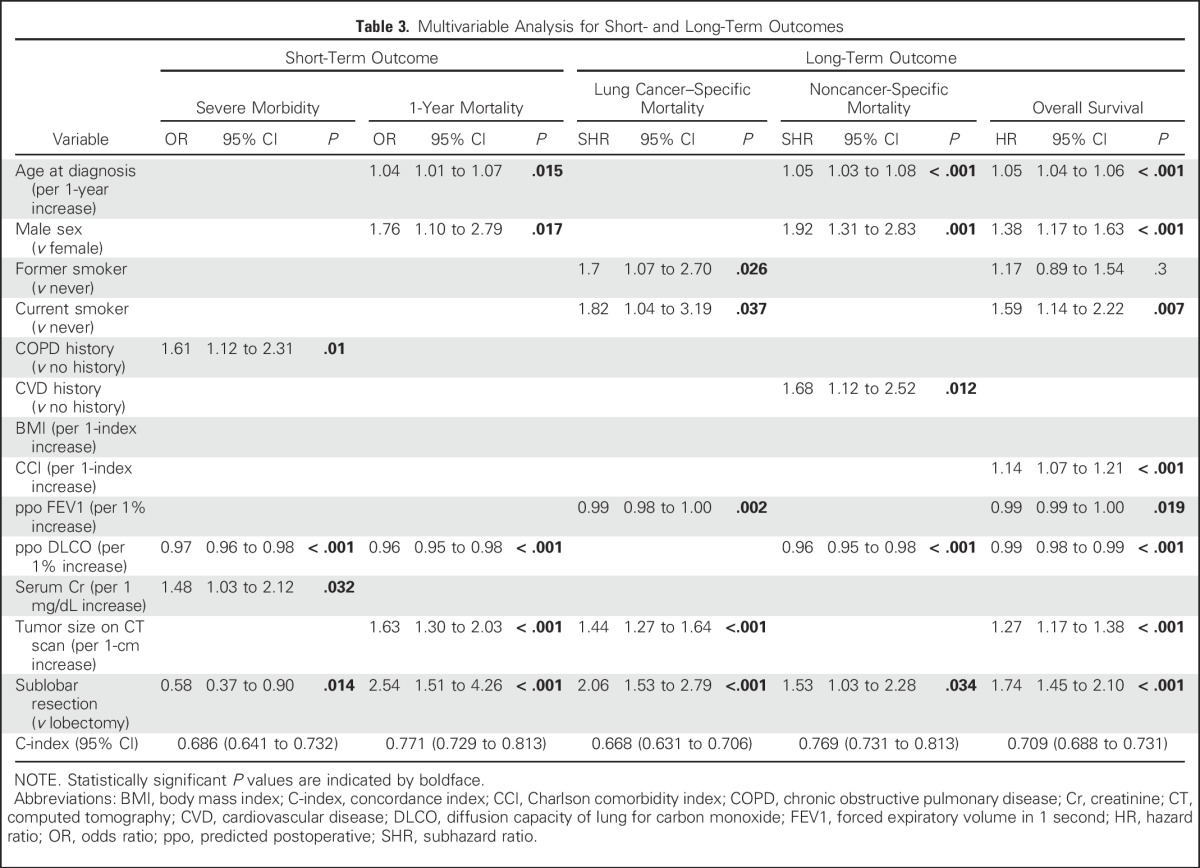
Univariable analyses are outlined in the Data Supplement. Multivariable analyses revealed the following independent predictors of each outcome: COPD history, lower ppo DLCO, higher serum creatinine level, and lobectomy for severe morbidity (C-index, 0.686); older age, male sex, lower ppo DLCO, larger tumor size, and sublobar resection for 1-year mortality (C-index, 0.771); former and current smoker, lower ppo FEV1, larger tumor size, and sublobar resection for lung cancer–specific mortality (C-index, 0.668); older age, male sex, CVD history, lower ppo DLCO, and sublobar resection for noncancer-specific mortality (C-index, 0.769); and older age, male sex, current smoker, higher CCI, lower ppo FEV1, lower ppo DLCO, larger tumor size, and sublobar resection for OS (C-index, 0.709; Table 3).
Table 3.
Multivariable Analysis for Short- and Long-Term Outcomes
Longitudinal Patterns of Lung Cancer–Specific and Noncancer-Specific CID by Age Group
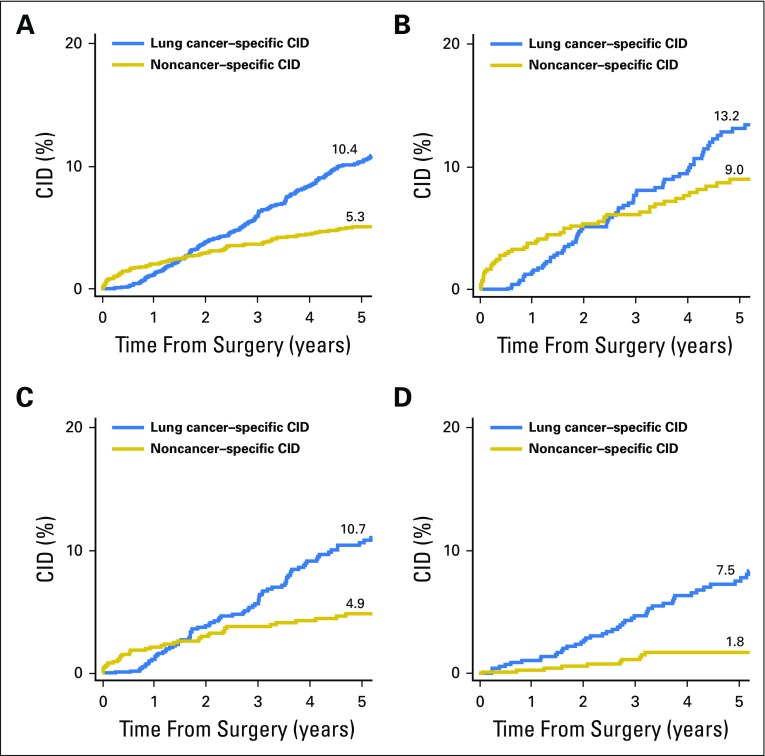
Lung cancer–specific and noncancer-specific CID curves are shown in Fig 2. In patients < 65, 65 to 74, and ≥ 75 years of age, 5-year lung cancer–specific CID was 7.5%, 10.7%, and 13.2%, respectively (overall, 10.4%), and noncancer-specific CID was 1.8%, 4.9%, and 9.0%, respectively (overall, 5.3%). Noncancer-specific CID was higher than lung cancer–specific CID for up to 1.5 years after resection. After 1.5 years, lung cancer–specific CID surpassed noncancer-specific CID. The difference between curves in the early phase after resection was enhanced in the cohort of patients ≥ 75 years of age in which noncancer-specific mortality was higher until approximately 2.5 years postsurgery. In the cohort of patients 65 to 74 years of age, the shapes of both curves were similar to that for the overall cohort. However, in the cohort of patients < 65 years of age, the early-phase increase in noncancer-specific mortality was not observed, and lung cancer–specific mortality was higher than noncancer-specific mortality during most of the postoperative period.
Fig 2.
Lung cancer–specific and noncancer-specific 5-year cumulative incidence of death (CID) by age-group. (A) Up to approximately 1.5 years after surgery, noncancer-specific CID was higher than lung cancer–specific CID. After 1.5 years, lung cancer–specific CID surpassed noncancer-specific CID (N = 2,186). (B) The higher noncancer-specific CID observed in the early postoperative phase increased in patients ≥ 75 years of age, in whom noncancer-specific mortality was higher than lung cancer–specific mortality until approximately 2.5 years postsurgery (n = 638). (C) In patients 65 to 74 years of age, the difference between curves was similar to that for the total cohort (n = 894). (D) In patients < 65 years of age, lung cancer–specific mortality was higher than noncancer-specific mortality during most of the postoperative period (n = 654).
Estimation of 5-Year Lung Cancer–Specific and Noncancer-Specific CID by Using PPO FEV1, PPO DLCO, and CCI
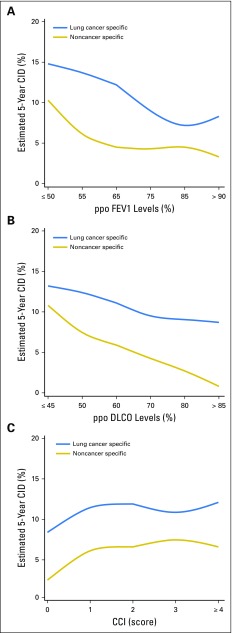
Figure 3A demonstrates that lung cancer–specific CID was the highest (approximately 15%) with the lowest ppo FEV1 and that it decreased linearly as ppo FEV1 increased. Noncancer-specific CID plateaued when ppo FEV1 was ≥ 65% (61% to 70%) but increased as ppo FEV1 decreased below that point. Figure 3B shows that lung cancer–specific CID gradually decreased as ppo DLCO increased, but the gradient was more gradual than it was for ppo FEV1. In contrast, as ppo DLCO increased, noncancer-specific CID decreased linearly, from approximately 11% to 1%. Figure 3C demonstrates an increase of noncancer-specific and lung cancer–specific CID as CCI increased from 0 to 1; however, it plateaued when CCI increased to > 1.
Fig 3.
Estimated 5-year lung cancer–specific and noncancer-specific Charlson Comorbidity index (CCI). The mean for each variable was summarized and fitted by using cubic B-splines for graphic representation. Along the x-axis, each group interval was represented as the middle number, such as 55 for 51-60 in panel A. (A) Lung cancer–specific cumulative incidence of death (CID) is highest (approximately 15%) at the lowest predicted postoperative (ppo) forced expiratory volume in 1 second (FEV1) and decreases linearly as ppo FEV1 increases. Noncancer-specific CID plateaus when ppo FEV1 is ≥ 65% (61%-70%) but increases precipitously as ppo FEV1 decreases below that point. (B) Lung cancer–specific CID gradually decreases as ppo diffusing capacity of lung for carbon monoxide (DLCO) increases, but the gradient is more gradual than that for ppo FEV1. By contrast, noncancer-specific CID decreases linearly from approximately 11%-1% as ppo DLCO increases. (C) Noncancer-specific and lung cancer–specific CID increase as CCI increases from 0 to 1 but essentially plateaus when CCI increases to > 1.
DISCUSSION
This study differentiates the influence of cancer-related and noncancer-related risk factors by age-group and further identifies factors that are predictive of short- and long-term cause-specific mortality in patients with stage I NSCLC. We have shown that in patients with stage I NSCLC, the majority of postoperative severe morbidity, 1-year mortality, and 5-year noncancer-specific mortality were attributable to cardiorespiratory diseases. We have also shown that short-term mortality is primarily attributable to noncancer-specific diseases. More importantly, the higher incidence of short-term noncancer-specific mortality was enhanced in the older cohort (≥ 75 years of age) and diminished in the younger cohort (< 65 years of age), which underscores the clinical significance of assessing noncancer-specific mortality as a competing event in older patients (Data Supplement).
Multivariable analyses revealed that ppo DLCO was a strong predictor of severe morbidity, 1-year mortality, and noncancer-specific mortality. Low DLCO has been associated with obstructive,34 restrictive,35 and pulmonary vascular diseases36 as well as with chronic heart failure.37 In previous studies, ppo DLCO represents an independent risk factor for postoperative morbidity and operative mortality.11,13,38 Tumor size, ppo FEV1, and smoking status were found to be independent predictors of lung cancer–specific mortality. In addition, smoking-induced COPD and associated CVD are linked to severe morbidity and CVD to noncancer-specific death; CCI, which includes a history of cancer (other than lung cancer), in addition to noncancer conditions, predicts OS. Because the current study spans a long period, we performed subanalyses of early (2000 to 2005) and later (2006 to 2011) cohorts. Conclusions were similar among all multivariable models of outcomes (Data Supplement).
A review of the prior literature found 11 studies that investigated the prognostic value of preoperative pulmonary function tests or comorbidities in patients with resected NSCLC11,14,15,17-20,39-42; six of these explored national databases14,15,17,39,40,42 (Data Supplement). Only two studies included FEV1, DLCO, and comorbidities as prognostic variables in the multivariable model.14,18 Of the two studies that focused on stage I NSCLC,20,42 only one evaluated both FEV1 and DLCO with OS as an end point.20 None of the studies evaluated cause-specific mortality with competing risks analysis, and only one study investigated both short- and long-term outcomes.40 The strengths of the current study include its comprehensive exploration of short- and long-term cause-specific outcomes, with competing risks analysis, in a large, uniform cohort of patients with stage I NSCLC; a multivariable analysis that included all preoperative variables known to contribute individually to outcomes; and the identification of independent predictors of each outcome, which showed that ppo DLCO and comorbidities are predictors of noncancer-specific mortality and postoperative morbidity and that ppo FEV1 is a predictor of lung cancer–specific mortality.
The study cohort reasonably reflects that of the SEER database, with the representation of patients 65 to 74 years of age approximately 10% higher in our cohort and those > 84 years of age 6% lower (Data Supplement). A possible explanation for these differences is that the SEER database includes patients with more–advanced stage cancer and/or patients treated with nonsurgical intervention. Because one third of patients with stage I NSCLC are ≥ 75 years of age at the time of diagnosis, the significant competing risks outlined in the current study have broad implications for disease management in these patients. These observations can inform conversations with patients and can be used when deciding on aggressive treatments, particularly for high-risk patients. For clinicians who manage patients with stage I NSCLC, the study provides clinically applicable information on the time-related change in mortality risk, including higher noncancer-specific mortality than lung cancer–specific mortality in the early postoperative period; the importance of predicting noncancer-specific mortality in older patients; an estimation of the 5-year CID for each cause-specific mortality; and the importance of cardiorespiratory diseases for postoperative severe morbidity and short-term mortality.
In this study, sublobar resection was associated with a lower incidence of severe morbidity, particularly respiratory events, as well as worse 1-year mortality, lung cancer–specific mortality, noncancer-specific mortality, and OS. Previous studies reported an association between sublobar resection and locoregional recurrence,43-46 which suggests worse lung cancer–specific mortality. Lower pulmonary function test and higher comorbidity status seen in the sublobar resection group indicate selection bias on the basis of cardiorespiratory condition and explains the worse noncancer-specific outcomes (Data Supplement). These observations should be taken into consideration during analysis in ongoing randomized clinical trials (CALGB140503 and JCOG0802) in the assessment of outcomes of sublobar versus lobar resection.
One limitation of this study is the number of patients with an unknown cause of death. Of the 436 deaths within 5 years of surgery, 95 were of unknown causes. Among these patients, 83 experienced no recurrence at the last visit (median follow-up, 0.8 years); the other 12 experienced recurrence but had stable disease at the last visit (median follow-up, 1.6 years). Of these 95 patients, eight died within 1 year. These deaths may have affected our results (Data Supplement). Another limitation is that we did not evaluate patient family and/or social support, which may have affected the analyses, especially that of the long-term, noncancer-specific outcomes in elderly patients.
In conclusion, we have shown that compared with the common approach of OS or event-free survival, cause-specific outcome analysis better stratifies patients with stage I NSCLC according to their risk of cancer mortality relative to noncancer competing causes of death. Noncancer-specific mortality represents a significant competing event for lung cancer–specific mortality, with an increasing impact as age increases. These findings can provide patients with more accurate information on survivorship on the basis of their individual preoperative status and help determine patients’ optimal treatment options.
ACKNOWLEDGMENT
We thank David Sewell and Alex Torres of the Memorial Sloan Kettering Thoracic Surgery Service for editorial assistance.
Footnotes
Supported by the National Cancer Institute and National Institutes of Health (Grants No. U54 CA137788 and P30 CA008748); Mr William H. Goodwin and Mrs Alice Goodwin; Commonwealth Foundation for Cancer Research; Center for Experimental Therapeutics of Memorial Sloan Kettering; the Joanne and John Dallepezze Foundation; and the Derfner Foundation.
See accompanying Editorial on page 268
AUTHOR CONTRIBUTIONS
Conception and design: Prasad S. Adusumilli
Financial support: Prasad S. Adusumilli
Administrative support: Prasad S. Adusumilli
Provision of study materials: Manjit S. Bains, Robert J. Downey, James Huang, Bernard J. Park, Valerie W. Rusch, Prasad S. Adusumilli
Collection and assembly of data: Takashi Eguchi, Sarina Bains, Ming-Ching Lee, Boris Hristov, Daniel H. Buitrago, Prasad S. Adusumilli
Data analysis and interpretation: Takashi Eguchi, Sarina Bains, Ming-Ching Lee, Kay See Tan, Manjit S. Bains, Robert J. Downey, James Huang, James M. Isbell, Bernard J. Park, Valerie W. Rusch, David R. Jones, Prasad S. Adusumilli
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Impact of Increasing Age on Cause-Specific Mortality and Morbidity in Patients With Stage I Non–Small-Cell Lung Cancer: A Competing Risks Analysis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Takashi Eguchi
No relationship to disclose
Sarina Bains
No relationship to disclose
Ming-Ching Lee
No relationship to disclose
Kay See Tan
No relationship to disclose
Boris Hristov
No relationship to disclose
Daniel H. Buitrago
No relationship to disclose
Manjit S. Bains
No relationship to disclose
Robert J. Downey
Leadership: Cantel Medical (I)
Stock or Other Ownership: Protea Biosciences
James Huang
Research Funding: Bristol-Myers Squibb
James M. Isbell
No relationship to disclose
Bernard J. Park
Honoraria: C. R. Bard, Baxter
Valerie W. Rusch
Research Funding: Genelux (Inst)
David R. Jones
No relationship to disclose
Prasad S. Adusumilli
No relationship to disclose
REFERENCES
- 1.Stat Bite Percentage of New Cases by Age Group For Lung and Bronchus Cancer (2008-2012). JNCI J Natl Cancer Inst 108:djw056, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Janssen-Heijnen ML, Houterman S, Lemmens VE, et al. Prognostic impact of increasing age and co-morbidity in cancer patients: A population-based approach. Crit Rev Oncol Hematol. 2005;55:231–240. doi: 10.1016/j.critrevonc.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Carmona R, Zakeri K, Green G, et al. Improved method to stratify elderly patients with cancer at risk for competing events. J Clin Oncol. 2016;34:1270–1277. doi: 10.1200/JCO.2015.65.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NCCN clinical practice guidelines in oncology (NCCN Guidelines): Non-small cell lung cancer version 2.2016. Ft Washington, PA, National Comprehensive Cancer Network, 2016
- 5.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black WC, Gareen IF, Soneji SS, et al. Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med. 2014;371:1793–1802. doi: 10.1056/NEJMoa1312547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocco G, Allen MS, Altorki NK, et al. Clinical statement on the role of the surgeon and surgical issues relating to computed tomography screening programs for lung cancer. Ann Thorac Surg. 2013;96:357–360. doi: 10.1016/j.athoracsur.2013.05.067. [DOI] [PubMed] [Google Scholar]
- 8.Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Sekine Y, Behnia M, Fujisawa T: Impact of COPD on pulmonary complications and on long-term survival of patients undergoing surgery for NSCLC. Lung Cancer 37:95-101, 2002 [DOI] [PubMed]
- 10.Kawai H, Tada A, Kawahara M, et al. Smoking history before surgery and prognosis in patients with stage IA non-small-cell lung cancer—A multicenter study. Lung Cancer. 2005;49:63–70. doi: 10.1016/j.lungcan.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Brunelli A, Refai MA, Salati M, et al. Carbon monoxide lung diffusion capacity improves risk stratification in patients without airflow limitation: Evidence for systematic measurement before lung resection. Eur J Cardiothorac Surg. 2006;29:567–570. doi: 10.1016/j.ejcts.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Sekine Y, Yamada Y, Chiyo M, et al. Association of chronic obstructive pulmonary disease and tumor recurrence in patients with stage IA lung cancer after complete resection. Ann Thorac Surg. 2007;84:946–950. doi: 10.1016/j.athoracsur.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson MK, Vigneswaran WT: Diffusing capacity predicts morbidity after lung resection in patients without obstructive lung disease. Ann Thorac Surg 85:1158-1164, 2008; discussion 1164-1165 [DOI] [PubMed]
- 14.Ferguson MK, Gaissert HA, Grab JD, et al. Pulmonary complications after lung resection in the absence of chronic obstructive pulmonary disease: The predictive role of diffusing capacity. J Thorac Cardiovasc Surg. 2009;138:1297–1302. doi: 10.1016/j.jtcvs.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 15.Kozower BD, Sheng S, O’Brien SM, et al: STS database risk models: Predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg 90:875-881, 2010; discussion 881-883 [DOI] [PubMed] [Google Scholar]
- 16.Kondo R, Yoshida K, Eguchi T, et al. Clinical features of lung cancer in smokers with light and mild chronic obstructive pulmonary disease: A retrospective analysis of Japanese surgical cases. Eur J Cardiothorac Surg. 2011;40:1439–1443. doi: 10.1016/j.ejcts.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Powell HA, Tata LJ, Baldwin DR, et al. Early mortality after surgical resection for lung cancer: An analysis of the English National Lung Cancer Audit. Thorax. 2013;68:826–834. doi: 10.1136/thoraxjnl-2012-203123. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson MK, Watson S, Johnson E, et al. Predicted postoperative lung function is associated with all-cause long-term mortality after major lung resection for cancer. Eur J Cardiothorac Surg. 2014;45:660–664. doi: 10.1093/ejcts/ezt462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhai R, Yu X, Shafer A, et al. The impact of coexisting COPD on survival of patients with early-stage non-small cell lung cancer undergoing surgical resection. Chest. 2014;145:346–353. doi: 10.1378/chest.13-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry MF, Jeffrey Yang CF, Hartwig MG, et al. Impact of pulmonary function measurements on long-term survival after lobectomy for stage I non-small cell lung cancer. Ann Thorac Surg. 2015;100:271–276. doi: 10.1016/j.athoracsur.2015.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivera C, Falcoz PE, Bernard A, et al. Surgical management and outcomes of elderly patients with early stage non-small cell lung cancer: A nested case-control study. Chest. 2011;140:874–880. doi: 10.1378/chest.10-2841. [DOI] [PubMed] [Google Scholar]
- 22.Billmeier SE, Ayanian JZ, He Y, et al. Predictors of nursing home admission, severe functional impairment, or death one year after surgery for non-small cell lung cancer. Ann Surg. 2013;257:555–563. doi: 10.1097/SLA.0b013e31828353af. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Blasberg JD, Pass HI, Donington JS: Sublobar resection: A movement from the Lung Cancer Study Group. J Thorac Oncol 5:1583-1593, 2010 [DOI] [PubMed]
- 25.Sihoe AD, Van Schil P: Non-small cell lung cancer: When to offer sublobar resection. Lung Cancer 86:115-120, 2014 [DOI] [PubMed]
- 26.National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. Washington, DC, US Department of Health and Human Services, 2010
- 27.Hastie TJ: Generalized additive models, in Chambers JM, Hastie TJ (eds): Statistical Models in S. Boca Raton, FL, Chapman and Hall/CRC, 1991, pp 249-287 [Google Scholar]
- 28.Brunelli A, Kim AW, Berger KI, et al: Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143:e166S-1690S, 2013 (suppl 5) [DOI] [PubMed] [Google Scholar]
- 29.Fine JP: Regression modeling of competing crude failure probabilities. Biostatistics 2:85-97, 2001 [DOI] [PubMed]
- 30.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 31.Hanley JA, McNeil BJ: The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29-36, 1982 [DOI] [PubMed]
- 32.Harrell FE Jr: Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY, Springer, 2001 [Google Scholar]
- 33.Wolbers M, Blanche P, Koller MT, et al. Concordance for prognostic models with competing risks. Biostatistics. 2014;15:526–539. doi: 10.1093/biostatistics/kxt059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison NJ, Abboud RT, Ramadan F, et al. Comparison of single breath carbon monoxide diffusing capacity and pressure-volume curves in detecting emphysema. Am Rev Respir Dis. 1989;139:1179–1187. doi: 10.1164/ajrccm/139.5.1179. [DOI] [PubMed] [Google Scholar]
- 35.Watters LC, King TE, Schwarz MI, et al. A clinical, radiographic, and physiologic scoring system for the longitudinal assessment of patients with idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1986;133:97–103. doi: 10.1164/arrd.1986.133.1.97. [DOI] [PubMed] [Google Scholar]
- 36.Steenhuis LH, Groen HJ, Koëter GH, et al. Diffusion capacity and haemodynamics in primary and chronic thromboembolic pulmonary hypertension. Eur Respir J. 2000;16:276–281. doi: 10.1034/j.1399-3003.2000.16b15.x. [DOI] [PubMed] [Google Scholar]
- 37.Agostoni P, Bussotti M, Cattadori G, et al. Gas diffusion and alveolar-capillary unit in chronic heart failure. Eur Heart J. 2006;27:2538–2543. doi: 10.1093/eurheartj/ehl302. [DOI] [PubMed] [Google Scholar]
- 38.Barnett SA, Rusch VW, Zheng J, et al. Contemporary results of surgical resection of non-small cell lung cancer after induction therapy: A review of 549 consecutive cases. J Thorac Oncol. 2011;6:1530–1536. doi: 10.1097/JTO.0b013e318228a0d8. [DOI] [PubMed] [Google Scholar]
- 39.Ferguson MK, Saha-Chaudhuri P, Mitchell JD, et al. Prediction of major cardiovascular events after lung resection using a modified scoring system. Ann Thorac Surg. 2014;97:1135–1140. doi: 10.1016/j.athoracsur.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 40.Lüchtenborg M, Jakobsen E, Krasnik M, et al. The effect of comorbidity on stage-specific survival in resected non-small cell lung cancer patients. Eur J Cancer. 2012;48:3386–3395. doi: 10.1016/j.ejca.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Birim O, Kappetein AP, Waleboer M, et al. Long-term survival after non-small cell lung cancer surgery: Development and validation of a prognostic model with a preoperative and postoperative mode. J Thorac Cardiovasc Surg. 2006;132:491–498. doi: 10.1016/j.jtcvs.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Rueth NM, Parsons HM, Habermann EB, et al. Surgical treatment of lung cancer: Predicting postoperative morbidity in the elderly population. J Thorac Cardiovasc Surg. 2012;143:1314–1323. doi: 10.1016/j.jtcvs.2011.09.072. [DOI] [PubMed] [Google Scholar]
- 43.Ginsberg RJ, Rubinstein LV; Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann Thorac Surg 60:615-622, 1995; discussion 622-623 [DOI] [PubMed]
- 44.Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst. 2013;105:1212–1220. doi: 10.1093/jnci/djt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kadota K, Nitadori JI, Sima CS, et al: Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences following limited resection for small stage I lung adenocarcinomas. J Thorac Oncol, 10:806-814, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Landreneau RJ, Sugarbaker DJ, Mack MJ, et al: Wedge resection versus lobectomy for stage I (T1 N0 M0) non-small-cell lung cancer. J Thorac Cardiovasc Surg 113:691-698, 1997; discussion 698-700 [DOI] [PubMed] [Google Scholar]