Abstract
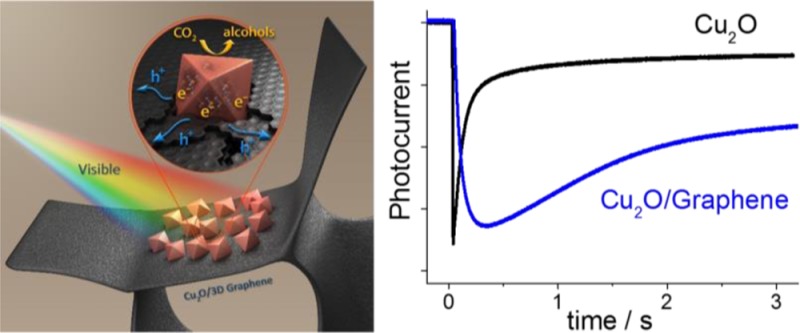
Combination of an oxide semiconductor with a highly conductive nanocarbon framework (such as graphene or carbon nanotubes) is an attractive avenue to assemble efficient photoelectrodes for solar fuel generation. To fully exploit the possible synergies of the hybrid formation, however, precise knowledge of these systems is required to allow rational design and morphological engineering. In this paper, we present the controlled electrochemical deposition of nanocrystalline p-Cu2O on the surface of different graphene substrates. The developed synthetic protocol allowed tuning of the morphological features of the hybrids as deduced from electron microscopy. (Photo)electrochemical measurements (including photovoltammetry, electrochemical impedance spectroscopy, photocurrent transient analysis) demonstrated better performance for the 2D graphene containing photoelectrodes, compared to the bare Cu2O films, the enhanced performance being rooted in suppressed charge carrier recombination. To elucidate the precise role of graphene, comparative studies were performed with carbon nanotube (CNT) films and 3D graphene foams. These studies revealed, after allowing for the effect of increased surface area, that the 3D graphene substrate outperformed the other two nanocarbons. Its interconnected structure facilitated effective charge separation and transport, leading to better harvesting of the generated photoelectrons. These hybrid assemblies are shown to be potentially attractive candidates in photoelectrochemical energy conversion schemes, namely CO2 reduction.
Introduction
The increasing demand for efficient technologies to harvest the energy of the Sun is a major current driver of materials chemistry.1 In addition to batteries and supercapacitors,2 chemical fuels3,4 (such as H2 or methanol) are an attractive option for energy storage, especially given the intermittent nature of renewable sources. Photoelectrochemistry, which relies on chemical reactions initiated at the semiconductor/electrolyte interface5 by photogenerated charge carriers, offers a viable avenue for the direct generation of solar fuels. Photoelectrochemical (PEC) water splitting, on an irradiated TiO2 surface is the pioneering example,6 and the field has grown rapidly during the past decades since the first discovery in the late 1960s.5 On the other hand, CO2 conversion is specifically challenging, because this reaction is multielectron in nature (e.g., 8 e– to CH4) and thus requires long-lived charge carriers7 and good electrocatalysts to obtain the targeted reduction product(s).8,9 While several materials were tested in this vein, ranging from elemental semiconductors (e.g., p-Si)10 to the most frequently studied oxides (mainly Cu-based binary and ternary oxides: e.g., Cu2O,11,12 CuFeO2,13−15 CuBi2O4,16 and CuNb2O617), to other compound semiconductors (e.g., GaP, CdTe, InP, GaAs, GaP, FeS2, ZnTe),5,18−24 none have reached the performance level required for practical utilization.
Considering the very complex requirements that have to be met by a photoelectrode (good light absorption, efficient charge carrier transport, rapid charge transfer kinetics, stability, etc.), it is not too surprising that no single “magic bullet” material has emerged so far.5,25 Consequently, there are ample arguments in favor of assembling and studying intricate architectures with components that have precisely defined functionality and complementarity. There is literature precedence for composite materials outperforming their singular counterparts in PEC applications.26,27 In particular, by providing a highly conductive scaffold, carbon nanotube and graphene-based composite materials are particularly attractive for photocatalytic28,29and PEC30−32 applications in that they facilitate exciton dissociation and charge carrier transport.33 Coherent, interconnected structures have additional benefits, rooted in the lack of carbon/carbon interfaces.34−38
As further, specific examples, enhanced anodic photocurrents were registered in a bioinspired ZnO/graphene assembly, where ZnO nanowires were obtained on honeycomb reduced graphene oxide (rGO).39 Similarly, efficient charge transfer and suppressed electron–hole recombination was reported for a Cu2O/rGO system.40 Liquid phase exfoliated (LPE) graphene nanoplatelets were combined with TiO2 nanoparticles, and improved photocatalytic efficiency was found for both CH3CHO oxidation and CO2 reduction.41 As yet another example, BiVO4/rGO hybrids were employed in PEC water oxidation, and showed 10-times higher anodic photocurrent, compared to BiVO4 alone.42 Similar to the other cases reported, the enhanced activity was rationalized by the instantaneous electron injection to the rGO component from the photoexcited BiVO4. The example of Fe2O3/rGO was used to demonstrate the different time scale of the various processes involved in a PEC reaction.31 Most recently, we demonstrated how ultralong carbon nanotubes could be used as a scaffold for Cu2O nanocrystals to boost the PEC conversion of CO2 to alcohols and formic acid.43 Finally, the combination of carbon nanotubes (CNTs) and graphene can also be employed as conductive platforms, as demonstrated for a hematite (Fe2O3) containing composite, which was successfully employed for PEC water oxidation.44 Notably, the reverse strategy was also demonstrated, where a carbon coating on the semiconductor surface acted both as stabilizer and cocatalyst.45−47
Despite the promising examples above, there is still much to be done to completely understand the effect of the nanocarbon scaffold, and thus to efficiently harness the synergy of the components in the hybrid configurations. Interestingly, there are many examples in the literature for photodriven (sometimes also referred as photocatalytic) reduction of CO2 using suspensions of Cu2O/nanocarbon hybrids,48−50 but only a very few for the corresponding PEC scenario. In our opinion, this trend is rooted in the increased complexity of this latter configuration, where the most important two bottlenecks are (i) the lack of precise control over the composition and morphology in the hybrid architectures, which may deleteriously affect the PEC properties; and (ii) the lack of high quality nanocarbon electrodes with organized, interconnected three-dimensional (3D) structure. Consequently, there are only a few examples in the literature where 3D graphene nanostructures (such as foams) were used as a scaffold for different inorganic oxides. Nanorods of ZnO were hydrothermally deposited on a chemical vapor deposition (CVD)-grown 3D graphene foam and tested as electrochemical sensors.51 Nanosheets of Ni(OH)2 were hydrothermally grown on 3D graphene, and the hybrid was employed for the electrochemical sensing of glucose.52 Finally, a mesoporous Co3O4 nanosheet array was deposited on a graphene foam to obtain high performance charge storage materials.53 Importantly, none of these studies focused on the PEC performance of these nanocomposites.
Electrochemistry is a particularly versatile tool for the controlled synthesis of different semiconductors on nanostructured carbon surfaces. As shown in our recent Minireview article27 it is possible to tailor the composition, particle size and morphology, and the crystallinity of the electrodeposited semiconductor toward specific applications. Similarly, much experience has accumulated on graphene electrochemistry,54,55 which suggests that high quality graphene (or few-layer graphene), obtained by CVD, will be a more versatile electrode materials than their liquid phase exfoliated and partially reduced counterparts (rGO).
In this contribution, we aim to uncover the reasons behind the improved PEC performance of nanocarbon based photoelectrodes, compared to the bare semiconductor counterpart. To this end, various nanostructured carbon electrodes were prepared, including CNT networks, spray-coated graphene films (using LPE few-layer graphene platelets), and 3D graphene foams. Variable amounts of p-Cu2O was loaded on their surface via controlled electrochemical deposition, to explore a broad compositional and morphological space. By comparing and contrasting the PEC behavior of these hybrid photocathodes, we present a comprehensive analysis of the beneficial effects of various nanocarbon scaffolds, with primary focus on graphene. The main outcome of this study is that both the high surface area and the improved charge carrier separation and transport contribute to the enhanced PEC properties. After allowing for the effect of the different surface areas, it was possible to compare the nanocarbons with different morphology. Importantly, the 3D graphene architecture significantly outperformed its random/nonoriented counterparts. This trend was semiquantitatively supported by transient photocurrent measurements, where the magnitude of charge carrier recombination at the surface was estimated. The presented structure–property relationships can be exploited in the rational design of hybrid photoelectrodes in general, to obtain potentially attractive candidates for PEC energy conversion schemes, such as water splitting or CO2 reduction.
Experimental Section
3D Graphene Foam Synthesis
3D graphene (3D-GR) foam was prepared via a CVD process using methane as the carbon source. The nickel foam (MTI Corporation, surface density: 350 g m–2) was cleaned by successive sonication for 20 min in ethanol and water. A 1 cm × 3 cm piece of cleaned nickel foam was placed at the center of a fused quartz tube furnace (Lindberg Blue M, inner diameter 22 mm). The furnace tube was evacuated and then heated to 1000 °C with a 40 sccm H2 gas flow under atmospheric pressure. After annealing for 20 min under these conditions, methane was introduced into the reactor. The temperature and gas flows were maintained in the furnace tube for 2 h. After the growth period, the Ar gas flow was stopped and the sample was cooled to room temperature under 40 sccm H2 gas flow. Finally, the samples were immersed in 1 mol dm−3 FeCl3 overnight to dissolve the Ni foam and isolate the free-standing 3D graphene.53
Electrode Preparation
Premium-quality graphene powder (exfoliated graphene platelets from Elicarb) or home-grown arrays of CNTs43 were dispersed in absolute ethanol by ultrasonic treatment. The formed dispersion (graphene: c = 1 mg mL–1, CNT: c ≈ 100 μg mL–1) was spray-coated on ultrasonically cleaned (5–5 min in acetone and ethanol) and preheated indium doped tin oxide-coated glass (ITO) electrodes, using an Alder AD320 type airbrush with a custom-made fully automated spray coater machine (operated with 1 bar compressed air). During the spray-coating process, the electrodes were masked to have an exposed surface area of 1 cm2. To remove ethanol traces and to enhance adhesion of the graphene platelets or the CNTs, the electrodes were kept in an oven (180 °C) for 30 min. Graphene and CNT loading of the electrodes was controlled with the number of spray steps (GR1–6, 30–560 μg cm–2, CNT: 35–690 μg cm–2, see also Table S1), and quantified by quartz crystal microbalance (QCM) measurements using a Stanford Research System QCM-200 type instrument. The 3D graphene samples were fixed on a glass sheet by a small piece of a double-sided tape, and were contacted at the top by graphite adhesive to form the working electrode.
Cu2O Electrodeposition
All chemicals used were of analytical grade and were used as received. CuSO4 × 5H2O and Na2SO4 were purchased from Alfa Aesar, NaOH from VWR International, lactic acid was from Sigma-Aldrich. Ultrapure water (ρ = 18.2 MΩ cm, produced with a Millipore Direct Q3-UV instrument) was used for the preparation of all aqueous solutions. The nanocarbon electrodes were employed as the working electrode, while a Pt sheet and a Ag/AgCl/3 M NaCl were used as counter and reference electrodes, respectively. Cu2O was electrodeposited from an alkaline solution of lactate-stabilized copper sulfate, containing 0.4 mol dm–3 cupric sulfate and 3 mol dm–3 lactic acid in deionized water.43 The pH was adjusted to 9 by the addition of concentrated sodium hydroxide solution. The electrodeposition was performed in a temperature-controlled classical three electrode electrochemical cell. The temperature of the solution was maintained at 60 °C, and the solution was constantly stirred by a magnetic stirrer during the electrodeposition. After recording linear sweep voltammetry data (Figure S2), multiple step potentiostatic electrodeposition protocols were employed (Figure 1).
Figure 1.
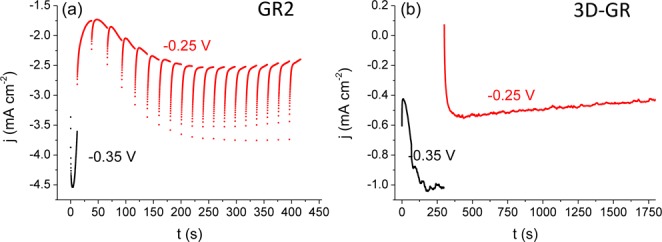
(a) Current response of a GR2/ITO electrode, while applying the multiple-potential step protocol. (b) Current–time curves, registered during the two-step potentiostatic electrodeposition of Cu2O on a 3D-GR electrode. Both syntheses were performed in 0.4 mol dm–3 cupric sulfate and 3 mol dm–3 lactate solution, at pH = 9 and T = 60 °C.
All electrochemical measurements were performed on a Metrohm Autolab PGSTAT302 type potentiostat/galvanostat, equipped with an FRA32 type module for the impedance spectroscopic and the current transient measurements. To establish structure–property relationships, the thickness of the nanocarbon films (CNT: 35–690 μg cm–2, graphene 30–560 μg cm–2), as well as the amount of the electrodeposited Cu2O was systematically varied (50–2000 mC cm–2). Cyclic voltammograms were recorded to estimate the actual surface area of the electrodes (see further discussion in the SI).
Physical Characterization
Raman spectroscopy was performed on a DXR Raman Microscope using a green laser (λ = 532 nm), operating at 10 mW laser power. A FEI Tecnai G2 20 X-Twin type instrument, operating at an acceleration voltage of 200 kV, was used for transmission electron microscopy (TEM). Scanning electron microscopy (SEM) images were recorded on a Hitachi S-4700 field emission scanning electron microscope (coupled with a Röntec EDX detector), operating at an acceleration voltage of 10 kV. X-ray diffraction (XRD) patterns were recorded between 2θ = 20–80° at 1° per minute scan rate on a Rigaku Miniflex II instrument, operating with a Cu Kα,1 radiation source (λ = 0.1541 nm). Diffuse reflectance UV–visible (DR UV–vis) spectra were recorded on an Avantes AVASpec-2048 type instrument, equipped with an AvaSphere-30 type integrating sphere. X-ray photoelectron spectra were recorded with a SPECS instrument equipped with a PHOIBOS 150 MCD 9 hemispherical analyzer. The analyzer was operated in the fixed analyzer transmission (FAT) mode with 20 eV pass energy. The Al Kα radiation (hν = 1486.6 eV) of a dual anode X-ray gun was used as the excitation source. The gun was operated at 210 W power (14 kV, 15 mA). The binding energy scale was corrected by fixing the main C 1s component to 285.0 eV, corresponding to adventitious carbon. For spectrum acquisition and evaluation both manufacturers (SpecsLab2) and commercial (CasaXPS, Origin) software packages were used. Electrochemical impedance spectra (EIS) of the hybrid films were recorded at open-circuit potential in the 10 Hz to 10 kHz frequency range, using a sinusoidal excitation signal (10 mV RMS amplitude). Modulus weighted fitting was performed using the Nova Software of the Autolab Instrument. For Mott–Schottky analysis, the full impedance spectra of the electrodes were recorded at different potentials (E = 0.1–0.35 V vs Ag/AgCl/3 M NaCl).
Photoelectrochemical Measurements
Photovoltammograms were recorded in a sealed electrochemical cell made of quartz. To eliminate the effect of pH, photovoltammograms were recorded in N2-saturated phosphate buffer (pH = 4.0, equal to the pH of the CO2 saturated 0.1 mol dm−3 Na2SO4) solutions as well. The solutions were saturated with N2 or CO2 by bubbling the gases through the cell for 30 min before and during the measurements (by forming a gas pillow above the solutions in the latter case). Linear sweep photovoltammograms were recorded under periodically interrupted light irradiation, using a Newport LCS-100 type solar simulator), operated at full output with a UV-cutoff filter (<400 nm), always placed at a fixed distance of 8 cm from the working electrode. Fast photocurrent transient measurements were performed using the same arrangement. During this measurement, data collection frequency was 20 kHz at five different potentials. Long-term CO2 photoelectrolysis tests were performed in a two-compartment, sealed electrochemical cell (separated by a Nafion-117 membrane). The electrode potential was kept at a given value (−0.05 and +0.05 V vs Ag/AgCl/3 M NaCl), and the electrode was irradiated with the above-described periodically interrupted light source (at 0.033 Hz). Incident photon-to-electron conversion efficiency (IPCE) measurements were performed on a Newport Quantum Efficiency Measurement System (QEPVSI-B) in a single-compartment, three electrode quartz electrochemical cell. The wavelength range was 360–600 nm (Δλ = 10 nm step size). The IPCE profiles were recorded at E = 0.0 V, in CO2 saturated 0.1 mol dm–3 Na2SO4 solution.
Liquid and gas aliquots were taken regularly during photoelectrolysis. The CO2 reduction products in the gas phase were analyzed with a Shimadzu GC-2010 Plus gas-chromatograph equipped with a barrier discharge ionization detector (BID). A HP-PLOT Molisieve column was used for the separation. Samples from the gas phase were taken at 5, 25, 50, and 70 min with a gastight syringe and injected into the GC with split injection. Heating procedure: 40 °C (6 min) to 30 °C/min −200 °C (4 min), injection temperature: 250 °C, linear velocity: 45.6 cm/s, split ratio: 40. The liquid-phase products were analyzed with a Shimadzu GC–MS QP 2010S gas chromatograph–mass spectrometer (column: ZB-FFAP) after removing the electrolyte ions with an Amberlite IRN-150 ion-exchange resin. Liquid samples were taken a few seconds after the gas samples. Heating procedure: 40 °C (3.5 min) to 50 °C/min −70 °C to 10 °C/min 170 °C to 50 °C/min −250 °C (5 min), injection temperature: 200 °C, linear velocity: 40 cm/s, split ratio: 10.
Results and Discussion
Electrodeposition of Cu2O/Graphene Composites
Electrodeposition of the Cu2O nanoparticles on the nanocarbon substrates was realized by a multiple-step potentiostatic method (Figure 1). On the basis of linear sweep voltammetric data (Figure S2), a first nucleation step was conducted at E = −0.35 V to initialize the formation of small seed particles, followed by one or more slow crystal growth steps at a less negative potential (E = −0.25 V). For the spray-coated graphene samples, multiple growth steps were applied to introduce rest periods allowing for continuous supply of copper ions from the bulk solution. We emphasize that although the prenucleation step leads to some copper traces in the formed cuprous oxide, it was found to be essential to reach homogeneous coverage of the nanocarbon supports.
When this step was omitted, crystal formation occurred exclusively on the edges of graphene sheets (see the inset in Figure 4d). In agreement with our earlier results on Cu2O decorated carbon nanotubes,27,43 we can conclude that homogeneous coverage of carbon nanostructures necessitates the application of a larger driving force (i.e., deposition potential), otherwise only the energetically preferred defect places (e.g., edges, amorphous carbon traces, and grain boundaries) are covered by the electrodeposited Cu2O.
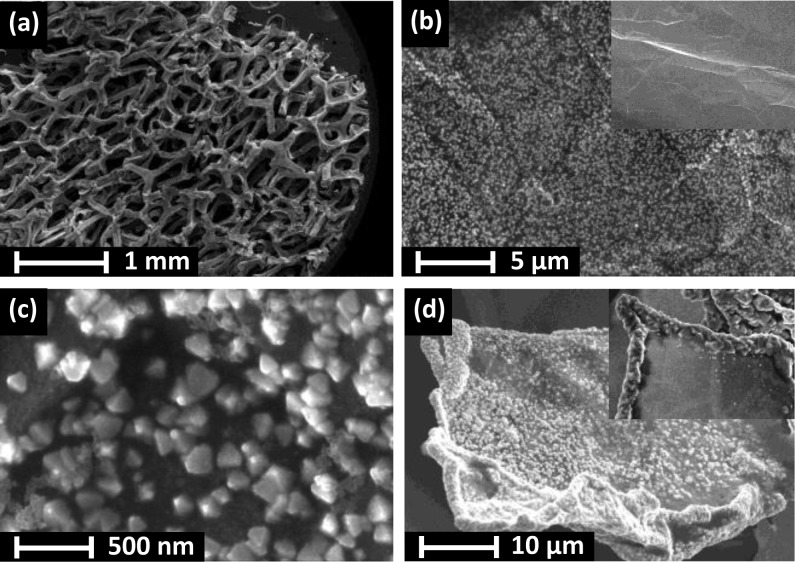
Figure 4.
SEM images of a Cu2O/3D-GR composite (deposited with 1 C cm–2 charge density) at different magnifications. The inset on (b) shows the bare graphene surface while in (d) it shows the structure of the composite formed without the prenucleation step.
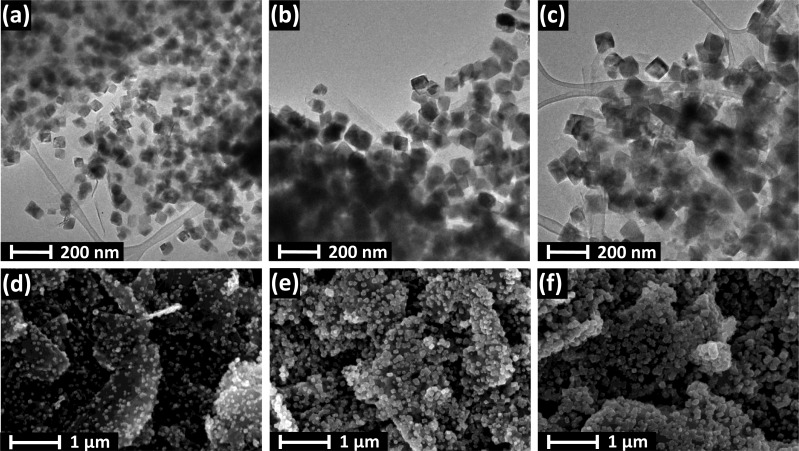
Electron microscopy images were taken for the hybrid Cu2O/GR samples with various compositions to probe their morphological attributes. TEM images confirmed that the Cu2O nanocrystallites are evenly dispersed on the graphene flakes (see Figure 2 and also Figure S3 for more images at both lower and higher magnifications). Additionally, it was also demonstrated that both the size and density of the nanoparticles can be effectively controlled by tuning the deposition protocol (i.e., the transferred charge). As seen in Figure 2 (from left to right), the growing deposition charge (from 200 mC cm–2 to 1 C cm–2) resulted in a gradual increase in both the surface coverage and the particle size. Specifically, the mean particle diameter shifted from 50 to 110 nm as deduced from the histograms in Figure 3. SEM images further confirmed the even dispersion of Cu2O nanoparticles on the graphene platelets (Figure 2d,e). SEM images were also taken for the bare Cu2O film on an ITO electrode (Figure S4). Most importantly, the crystallite sizes were much larger in this case, due to the smaller actual electrode surface area (note that the amount of the deposited Cu2O was identical).
Figure 2.
TEM images of Cu2O/GR4 hybrids obtained with the deposition protocol shown in Figure 1a. The deposition charges were (a) 200 mC cm–2, (b) 600 mC cm–2, and (c) 1000 mC cm–2. SEM images are also shown for the same samples (d–f).
Figure 3.
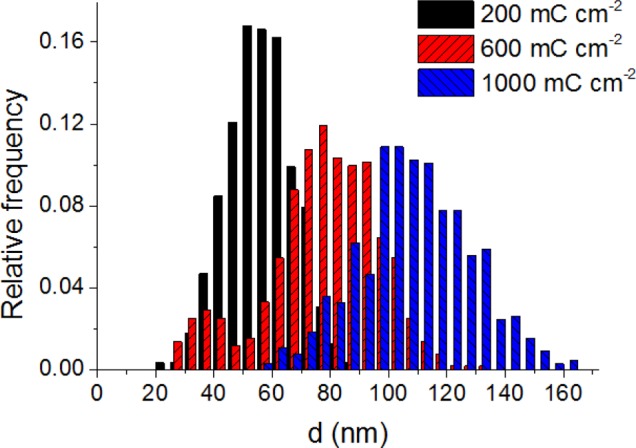
Particle size distribution for the same samples presented in Figure 2. The data were gathered from the TEM images, by measuring the size of 300 particles in each case.
For the 3D graphene-based hybrids, the graphene platelets were homogeneously and almost completely covered with octahedral Cu2O nanoparticles (see also Figure S5 for elemental mapping by EDX), having a size in the range of 100–150 nm (Figure 4). For this system, the initial nucleation step was indeed required to avoid specific deposition at the edge of the graphene platelets (compare with the inset in Figure 4d). Finally, we note that the original structure of the 3D graphene support was preserved in the nanocomposite; it did not suffer any major damage during the electrochemical deposition.
Physical characterization was performed for all the prepared composite systems. The results were very similar for all the samples, confirming that there is no difference in the physicochemical properties of the deposited Cu2O in the different hybrid configurations. For the sake of brevity, we only present the results obtained for the Cu2O/3D-GR composite, and emphasize any differences for the other hybrids. XRD patterns of the Cu2O/graphene composites were recorded to identify the crystal structure and composition of the deposited oxide. The four most intense reflections of the Cu2O can be recognized (Figure 5a).56 Although the presence of some copper traces cannot be completely excluded, the fact that reflections related to copper cannot be identified confirmed that this amount was negligible, if any. We note here that XPS data also confirmed that no Cu was formed during electrodeposition of Cu2O (see the lack of Cu (0) peak around 918.6 eV at the Auger spectrum, Figure S6B).
Figure 5.
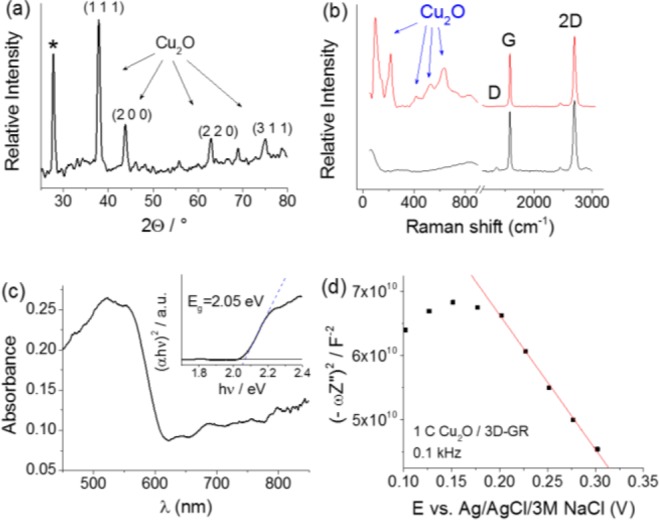
(a) XRD pattern, (b) Raman spectrum (also for the bare 3D-GR sample), (c) Diffuse-reflectance UV–vis spectrum (together with Tauc representation as inset), and (d) Mott–Schottky plot (recorded in 0.1 M NaAc solution), of a Cu2O/3D-GR composite, deposited with 1 C cm–2 charge density. The diffraction marked with asterisk in (a) corresponds to the graphene substrate.
The appearance of the diffraction at 2θ = 27.8° (marked with asterisk in Figure 5a) indicated that we have a multilayer graphene structure instead of a graphene monolayer, as the CVD-grown 3D graphene samples typically consist of both one- and few-layer domains.53 This observation is typical for CVD-grown 3D graphene samples and contributes to the mechanical stability of the 3D electrode.57
The Raman spectrum of the composite showed all the characteristic Raman active vibration modes of Cu2O (Figure 5b). The appearance of the peaks at 145, 220, 416, 532, and 631 cm–1 proves that Cu2O was formed during the deposition.58,59 The lack of the characteristic vibration modes of CuO at 297 and 350 cm–1 proved that the formed oxide is exclusively Cu2O and not CuO (confirming XRD results).60 At 1582.5 cm–1, the G-band of the graphene substrate, associated with the E2g vibration mode of the sp2 framework,61 can be discerned on the Raman spectrum.62 The presence of a very small D-band at 1374 cm–1 indicated that only trace amounts of defects were present in the nanocarbon template. Importantly, these defects were already present before synthesis of the composites, as seen from the spectrum of the bare 3D-GR sample. The intense 2D band centered at 2698 cm–1 confirmed the few-layer character of the 3D-GR sample.
The optical bandgap of the Cu2O/3D-GR composite was determined by diffuse reflectance UV–vis spectroscopy, and the spectrum was analyzed using a Tauc plot (see an example for the 3D-GR support in Figure 5c). The determined Eg = 2.05 eV was in good agreement with values reported for Cu2O.63 Similar data were collected for all Cu2O/nanocarbon samples (not shown here). The most important conclusions were the following: (i) the absorption edge (related to Cu2O, Eg = 2.05 eV, see also Figure 5C) of the samples did not alter with the varying graphene content and (ii) there was a massively increased noncharacteristic absorption related to graphene (especially in the vis-NIR region31,64).
Electrochemical impedance spectroscopic (EIS) measurements were carried out to determine the flatband potential of the composite (Figure 5d). By performing the Mott–Schottky analysis on the measured data, a flatband potential of E = +0.51 V (vs Ag/Ag/3 M NaCl) was obtained for the Cu2O/3D-GR sample, a value slightly higher compared to data reported for electrodeposited Cu2O at neutral pH.65,66 To compare the flatband potential (and thus the apparent Fermi level) of the various photoelectrodes, Table S2 summarizes the onset potential of the photovoltammetry profiles, the open circuit potentials with and without illumination, and the flatband potential obtained from the Mott–Schottky plots. The most important trend observed in these comparisons was the slight positive shift in the flatband potential (apparent Fermi level) in the case of the composite samples (compared to the bare Cu2O). The magnitude of this shift increased with increasing nanocarbon loading, and had a maximum of 100 mV for the spray-coated carbons and 200 mV for the 3D-GR sample. These shifts indicate the intimate contact between the constituents, similar to trends in other studies in the literature on different semiconductor/nanocarbon assemblies.30,31,33,64
Photoelectrochemical Properties
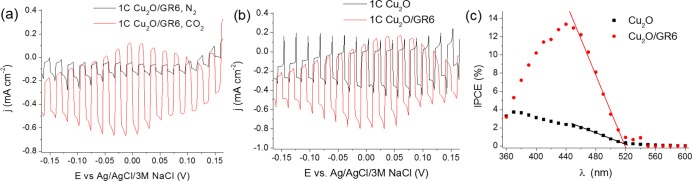
To evaluate the PEC properties of the Cu2O/graphene composites, the photoreduction of CO2 was performed as a model reaction of practical significance (Figure 6a). The most important conclusions to be drawn from these initial measurements are the following: (i) The nanocomposite showed PEC activity toward CO2 reduction, as confirmed by the enhanced photocurrents in the presence of CO2 (note that the pH-effect was deconvoluted by performing control measurements in N2 saturated solution, buffered to pH = 4.0, the pH of the CO2 saturated solution); (ii) There was an increased photocurrent for the GR containing samples as compared to the bare oxide (Figure 6b); and (iii) The overall shape of the photovoltammograms was similar in the presence/absence of the carbon scaffold, except the development of a dark current in the case of the hybrids.
Figure 6.
Linear sweep photovoltammograms recorded for (a) a Cu2O/GR6 hybrid sample (1 C cm–2 Cu2O) in CO2 or N2 saturated 0.1 mol dm–3 Na2SO4 (having the same pH = 4.0). (b) LSV curves for a Cu2O/GR6 hybrid and a Cu2O film (1 C cm–2 Cu2O) in CO2 saturated 0.1 mol dm–3 Na2SO4 solution. The sweep rate was kept at 2 mV s–1, while the light-chopping frequency was 0.2 Hz. A solar simulator was used employing a UV cutoff filter (<400 nm). (c) Photoaction spectra for the two electrodes presented in (b), recorded at E = 0.0 V (vs Ag/AgCl/3 M NaCl) in CO2 saturated 0.1 mol dm–3 Na2SO4 solution.
Additionally, both the onset potential of the CO2 reduction and the maximum photocurrent were very similar to those determined earlier for Cu2O/CNT composites,43 but the shape of the linear sweep voltammetry (LSV) curve was somewhat different. In this case, the photocurrent did not decrease after a certain potential; it was stable in the presented potential regime. Photoaction spectra (incident photon to current efficiency (IPCE) vs wavelength) were recorded, and a massive increase in the IPCE values was observed on the photoaction spectrum (Figure 6c). The identical wavelength threshold value confirmed that the enhanced photocurrents were not related to some new absorption or bandgap shift, but indeed to the better charge carrier extraction in the hybrid (note that the amount of Cu2O was the same).
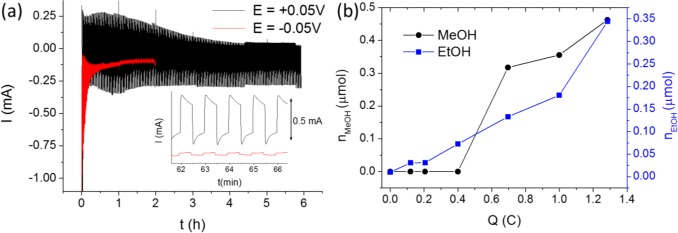
Long-term photoelectrolysis was performed at different potentials to assess the stability of the Cu2O/GR electrode. As shown in Figure 7a, even a 100 mV shift in the external bias potential can cause a massive alteration in the overall shape. Specifically, at E = −0.05 V, the rapid cessation of the photocurrents was seen, as a result of photocorrosion (i.e., formation of metallic Cu).12 On the other hand, at E = +0.05 V, stable photoactivity was reached after an initial decrease. This enhanced PEC behavior is attributed to the dark reoxidation of metallic copper moieties, formed via photocorrosion.12 This hypothesis is supported by the initially observed dark anodic currents at this potential. Notably, a steady-state was reached after 2.5 h, with stable photocurrents and minor dark currents.
Figure 7.
(a) Long-term chronoamperometry data of two Cu2O/GR6 electrodes with an identical Cu2O content (1 C), registered at E = +0.05 V and E = −0.05 V potential (vs Ag/AgCl/3 M NaCl), in CO2 saturated 0.1 M Na2SO4 solution. A solar simulator was used employing a UV cutoff filter (<400 nm). (b) Formation of alcohols during the photoelectrolysis presented in (a).
To verify that the increased photocurrents in the presence of CO2 (see Figure 6a) were related to its conversion to useful products, aliquots were taken periodically both from the liquid and gas phases. Importantly, in the gas phase only minor traces of H2 were detected and no CO formation was confirmed. As for the liquid phase, methanol, ethanol and minor amounts of formic acid were detected; similarly to earlier studies on Cu2O-containing photoelectrodes.43,67 Interestingly, while the ethanol concentration increased continuously, the methanol formation only started after the steady-state PEC behavior was reached (Figure 7b). Quantitatively, the two alcohols accounted for a Faradaic efficiency of 50–60% (with a 2:3 FE ratio for methanol/ethanol) while the rest of the charge was related to H2 and formate production, and the photoreduction of Cu2O to Cu as detailed above. Comparative studies were performed with a bare Cu2O electrode, deposited with identical charge density (Figure S7). The formation of methanol and ethanol was witnessed, although with a different ratio (see additional discussion in the SI).
Degradation of the Cu2O/3D-GR electrode structure during long-term photoelectrolysis is a relevant concern. We found that major degradation of the 3D structure only occurred when the photoelectrolysis was performed at more negative potentials, where water splitting (and thus H2 bubble formation) also occurred. SEM images were taken for samples after photoelectrolysis, and are shown in Figure S9.
Factors Behind the Improved PEC Properties
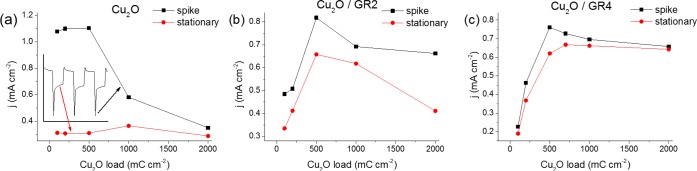
To explore a broad range of the composition and morphology, the effect of the GR thickness and the deposited Cu2O amount were both varied systematically. Figure 8 compares the measured photocurrents (both the initial current spike and the stationary plateau; as shown in the inset of Figure 8a) for the bare Cu2O (on an ITO electrode) and composites (GR supports of three different thicknesses and five different Cu2O loadings for each support). As for the bare Cu2O, there was a huge difference between the initial and the stationary photocurrent values. This observation suggests the insufficient charge carrier extraction in the case of the bare oxide (see below for detailed discussion of this point). In addition, the stationary photocurrents seemed to be independent of the Cu2O loading (film thickness), at least in the studied regime. This constant current suggests that the reacting photoelectrons come from a distinct region of the Cu2O film, independently from the thickness. The overall pattern was totally different for the Cu2O/graphene composites (Figure 8b−c): (i) The transient and the stationary values were much closer to each other, confirming our assumption that the highly conductive graphene support facilitates effective charge separation and charge carrier extraction; (ii) The maximum photocurrent gradually shifts to higher Cu2O loadings with increasing graphene thickness. Similar experiments were carried out with the 3D graphene samples and the composition dependent PEC behavior was also confirmed (Figure S8). These trends suggest that there is an optimal composition (i.e., Cu2O/graphene ratio) in the hybrid configuration.
Figure 8.
Comparison of the transient and stationary photocurrents at E = +0.05 V, for five different Cu2O loadings (100–2000 mC cm–2), on (a) a bare ITO and (b,c) two different GR/ITO supports. The inset in (a) shows how the data points were determined. The lines among the data points serve only to guide the eye.
While the effect of GR thickness can be studied by comparing samples with identical Cu2O content, their optical behavior might be different. In fact, this increased light absorption with higher GR thicknesses explains the decrease in the photocurrents at high GR thicknesses, where light absorption of Cu2O is hindered by the presence of GR. This trend is also reflected in Figure 10 below.
Figure 10.
(a) Photocurrent transients of a pristine Cu2O film and four different Cu2O/GR composites deposited with 0.5 C cm–2 charge density, in CO2 saturated 0.1 M Na2SO4 solution at E = 0.05 V potential. A solar simulator was used employing a UV cutoff filter (<400 nm). (b) Dual exponential fit of a transient photocurrent response of the Cu2O/GR2 sample. (c) Time constant values obtained for the samples shown in (a). The lines among the data points serve only to guide visualization.
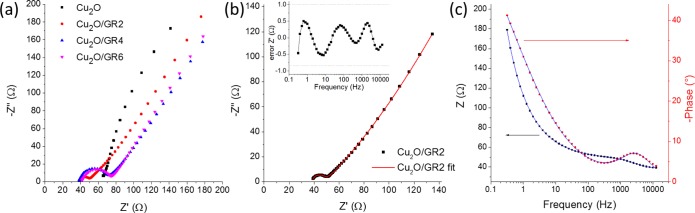
The question, however, still holds: what are the reasons behind the improvement in the PEC properties? EIS measurements were performed to scrutinize the electronic properties of the synthesized photoelectrodes. From qualitative analysis of the Nyquist plots depicted in Figure 9a, one can see the large decrease of semicircle size for the graphene-containing composites. Additionally, there is a higher series resistance for the Cu2O/ITO sample compared to its graphene-containing counterparts (most likely rooted in the resistance of the Cu2O/ITO interface). To quantify these trends an equivalent circuit (see the circuit and its description in the SI, Figure S10) was employed to fit the measured data (Figures 9b and c). The most important conclusions are the following: (i) There was a huge decrease (from 500 Ω to 10 Ω) in the charge transfer resistance (Rct) when Cu2O was electrodeposited on the GR support instead of the bare ITO; (ii) When the GR thickness was increased the Rct value also increased to 30 Ω, and then remained constant at higher GR thicknesses; (iii) The very high surface area of the hybrid electrodes was reflected in the high double-layer capacitance values (C1, 10–30 mF cm–2). Overall, these results confirm that the improved electrical conductivity, facilitating better charge carrier transport, is an important contributor to the superior PEC properties of the hybrid electrodes.
Figure 9.
(a) Nyquist plots recorded for pristine Cu2O and three different Cu2O/GR composites deposited with 1 C cm–2 charge density, in 0.1 M sodium acetate solution at open circuit potential, in the 0.1 Hz to 10 kHz frequency range. (b) Measured (points) and simulated (solid line) Nyquist and (c) Bode-plots of a Cu2O/GR electrode. The inset in (b) shows the error associated with the Bode plot fit (i.e., difference between the measured and calculated value).
To get a better understanding on the charge carrier recombination process, transient photocurrent measurements were performed.68−70 In these measurements the photocurrent was followed after the light was switched on (chopped illumination). The first important observation in all experiments was the slow relaxation of the cathodic photocurrents (on the order of seconds, see Figures 10 and 11). Similarly to previous water oxidation studies,69,70 this slow charge transfer can be attributed to the kinetically hindered, multielectron nature of CO2 reduction (i.e., it involves 6 e– and 6 H+ to form methanol). The competition between electron transfer to CO2 at electrode/electrolyte interface and electron–hole recombination within the space charge layer and at the surface means that only a portion of the photogenerated electrons can react with CO2. Consequently, the achieved solar to chemical conversion efficiencies are much smaller than what could be expected from the optoelectronic properties of Cu2O. Extensive recombination is particularly problematic close to the Fermi level, and while higher external bias potentials can help to suppress it, there is a trade off because of the needed additional energy input. Also note that the stability of the photoelectrode was much lower at higher bias potentials, see also Figure 7.
Figure 11.
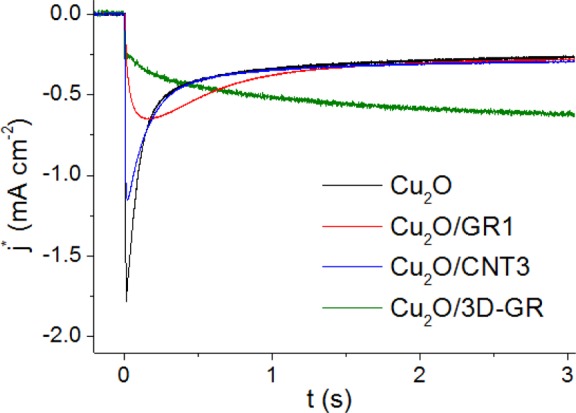
Photocurrent transient analysis of a pristine Cu2O film and three different Cu2O/nanocarbon composites deposited with 0.5 C cm–2 charge density. The measurements were performed in CO2 saturated 0.1 M Na2SO4 solution at E = 0.05 V potential. A solar simulator was used employing a UV cutoff filter (<400 nm). Photocurrent normalization was performed using the relative surface area data in Table S1.
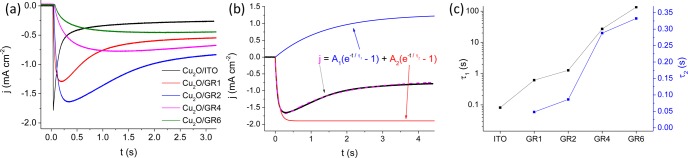
Transient photocurrent curves recorded for the different samples are compared and contrasted in Figure 10a. While for the bare Cu2O there was a sharp transient, this feature gradually disappeared in the series of Cu2O/GR samples with progressively higher GR content. As for Cu2O, the photocurrent developed instantaneously after the light was turned on, followed by an exponential decay to a steady-state cathodic value. These cathodic photocurrent transients can be attributed to accumulation of the photogenerated electrons in the Cu2O space charge layer and recombination of bulk holes with these electrons.68−70 At longer time scales, a steady-state photocurrent was reached, the magnitude of which was dictated by the relative rates of recombination and charge transfer to redox species.68
As for the graphene containing samples, the pattern was more complex. First, the initial spike got gradually smaller and finally disappeared as the graphene thickness increased. Meanwhile, an opposite (i.e., increasing) pattern developed, dictated by charge carrier transport through the relatively thick Cu2O/GR electrode architecture (note that no such behavior was observed for thin nanocarbon supports or bare Cu2O, see also Figures 11 and S11). The relative contribution of these processes is clearly correlated with the graphene content (note that the Cu2O amount was identical in all cases). The ratio of the steady-state and the peak photocurrent increased with the graphene thickness, because of the increasing magnitude of Cu2O/GR junction area (see also Figure 8). This suppressed recombination, however, did not result in constantly growing steady-state currents, mostly because of changing optoelectronic properties in the series of samples (i.e., light absorption of graphene (see above), increased resistance (see Figure 9)).
To facilitate quantitative comparison, the transient photocurrent curves were fitted and time constants were determined for all systems with different composition and morphology. The transient photocurrent curves could be well-fit to a sum of two exponential functions, one related to surface recombination (characterized by τ1) and another one dictated by hole-transport through the electrode assembly (characterized by τ2)71 (see the fitted equation and an example of the fitting in Figure 10b). While A2 gives the absolute value of the maximum achievable photocurrent (i.e., in the absence of recombination), A1 shows the maximal contribution of the recombination process to the decrease in the photocurrent. As seen in Figure 10c, both time constants increased with the growing GR thickness, although at vastly different extents (note the logarithmic scale for τ1). The time constant related to recombination increased rapidly with the higher graphene thicknesses. Note that the Cu2O amount was fixed, therefore this trend is related to the gradually thinner Cu2O coverage on the graphene platelets.
Upon light irradiation the photogenerated electrons (minority carriers) are driven toward the electrode/electrolyte interface by band bending, while the holes are rapidly siphoned off by the graphene phase (note that this process occurs on the nanosecond time scale).72 As a net result, the time constant related to recombination increased by three orders of magnitude for the composite sample with the highest graphene content, compared to the bare Cu2O film. On the other hand, τ2 changed almost proportionally with the GR loading (thickness), which is not too surprising considering that this time constant is related to the transport of holes to the current collector (ITO). We also note here that there are several other processes which contribute to the charge carrier dynamics of the presented photoelectrodes. However, exciton generation, charge carrier separation in Cu2O, and charge transfer from Cu2O to GR occur on a much shorter (fs–ns) time scale, so that their effect cannot be important here.31,73 Pump–probe transient absorption laser spectroscopic studies are planned in the future to study these processes in operando. From these results taken as a whole, we may conclude that both the enhanced specific surface area and improved charge carrier transport result in suppressed recombination, so that a higher fraction of the photogenerated electrons can react with CO2.
Comparison of Graphene, 3D Graphene, and CNT as Photoelectrode Support
Finally, to further elucidate the role of the nanocarbon scaffold and possible morphological aspects, the PEC behavior of different nanocarbon containing hybrid samples were compared. To deconvolute the effect of the different surface areas, samples with similar charge capacitance are compared in Figure 11, and the slight differences in the surface area were taken into account by normalizing the photocurrent values (see also Figure S1 and Table S1). The Cu2O content was identical in all cases (0.5 C cm–2, with respect to the geometrical surface area). What is immediately striking is that steady-state currents on the three curves (Cu2O, Cu2O/CNT3, and Cu2O/GR1) almost overlap after normalization, although they have significantly different time constants (slower recombination for CNT and especially the GR-containing composite, compared to the bare Cu2O). These trends suggest that in these instances the improved PEC activity shown above (e.g., Figures 6 and 8) is predominantly rooted in the increased surface area (and the resultant high Cu2O/carbon junction area), provided by the highly conductive nanocarbon scaffold. After factoring out this effect, very similar behavior is seen, at least in terms of the steady-state photocurrents (see also Figure S12a).
On the other hand, much higher normalized steady-state photocurrent flow could be seen for the 3D-GR support, most likely because of the superior charge transport properties rooted in the lack of particle–particle interfaces (also consider that the size of graphene platelets is much higher in this case, compared to the spray-coated samples, see Figure 4 and Figure S3). Note that while this support had relatively small surface area, at the same time, it has a higher thickness. This manifested in the large time constant for the photocurrent development (τ2).
Finally, we recognize that the light penetration depth was limited in this macroscopic architecture. Consequently, design of other 3D architectures, where benefits of the presented 3D graphene structure could be maintained while simultaneously ensuring sufficient light harvesting, would be highly desirable; such studies are in progress in our laboratories.
Conclusions
A broad compositional and morphological space was explored to assemble hybrid photoelectrodes based on a highly conductive nanocarbon support (graphene) and a p-type inorganic semiconductor (Cu2O). A multiple potential-step electrodeposition protocol was employed to control the amount and size (thus the overall coverage) of the Cu2O nanocrystallites on the graphene surface. The PEC activity of the composites was proved in CO2 reduction (to form alcohol products), as a model multielectron transfer reaction of practical significance. It was found that the graphene-containing photoelectrodes outperformed the pure Cu2O, both in terms of the achieved current densities and stability. Composition-dependent PEC studies revealed that there was an optimal loading for both components. Transient photocurrent measurements revealed that the main contribution of the graphene substrate in such structures was the facilitation of effective charge separation and transport, leading to better harvesting of the generated photoelectrons. An added bonus is enhanced stability and durability of the semiconductor components.
A phenomenological model was developed involving: (i) electron transfer from Cu2O to CO2, (ii) recombination of the photoelectrons with holes in the space charge layer, (iii) hole transfer from Cu2O to graphene, and (iv) hole transport through the electrode architecture to the current collector. By fitting the transient photocurrent curve, semiquantitative assessment could be made, for the first time, for the graphene contribution to the enhanced PEC activity of the hybrid photoelectrodes. The presented approach also afforded study of the effect of the nanocarbon morphology: after deconvoluting the effect of the increased surface area, we demonstrated that an interconnected 3D graphene structure (as a new promising member of the self-standing, highly conductive carbon derivative family) has further benefits compared to its nonorganized counterparts (spray-coated graphene and CNT films), because of the lack of carbon–carbon boundaries in the structure. Importantly, the presented design elements for the new family of photoelectrode materials in this study can be utilized for solar fuels generation in general, by assembling hybrid architectures with complementary function and optimized nanomorphology.
Acknowledgments
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 716539). The authors also thank Rendernet Ltd. for assistance in preparing the artwork in the TOC. This research was partially supported by the “Széchenyi 2020” program in the framework of GINOP-2.3.2-15-2016-00013 “Intelligent materials based on functional surfaces—from syntheses to applications” project. Authors from the University of Manchester were supported by EPSRC grants (EP/K007033/1 and EP/K016954/1). The authors thank Dr. Tamás Pajkossy (Research Center for Natural Sciences of the Hungarian Academy of Sciences) for the valuable discussions on the EIS results, Dorottya Hursán for the support with the GC–MS analysis, and Dr. Albert Oszkó (University of Szeged) for recording the XPS spectra during the peer review cycle. Finally, the authors thank the three anonymous reviewers for their constructive and insightful criticisms of an earlier manuscript version.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.7b01820.
Additional synthetic details, morphological studies, EIS data, and photoelectrochemical analysis (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Lewis N. S.; Nocera D. G. Powering the Planet: Chemical Challenges in Solar Energy Utilization. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 15729–15735. 10.1073/pnas.0603395103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Dai H. Strongly Coupled Inorganic–nano-Carbon Hybrid Materials for Energy Storage. Chem. Soc. Rev. 2013, 42, 3088–3113. 10.1039/c2cs35307e. [DOI] [PubMed] [Google Scholar]
- Joshi U. A.; Palasyuk A.; Arney D.; Maggard P. A. Semiconducting Oxides to Facilitate the Conversion of Solar Energy to Chemical Fuels. J. Phys. Chem. Lett. 2010, 1, 2719–2726. 10.1021/jz100961d. [DOI] [Google Scholar]
- Sivula K. Metal Oxide Photoelectrodes for Solar Fuel Production, Surface Traps, and Catalysis. J. Phys. Chem. Lett. 2015, 6, 1624–1633. 10.1021/jz4002983. [DOI] [PubMed] [Google Scholar]
- Rajeshwar K. Solar Energy Conversion and Environmental Remediation Using Inorganic Semiconductor/Liquid Interfaces: The Road Traveled and the Way Forward. J. Phys. Chem. Lett. 2011, 2, 1301–1309. 10.1021/jz200396h. [DOI] [PubMed] [Google Scholar]
- Fujishima A.; Honda K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- Cowan A. J.; Durrant J. R. Long-Lived Charge Separated States in Nanostructured Semiconductor Photoelectrodes for the Production of Solar Fuels W. Chem. Soc. Rev. 2013, 42, 2281–2293. 10.1039/C2CS35305A. [DOI] [PubMed] [Google Scholar]
- Boston D.; Huang K.-L.; de Tacconi N.; Myung N.; MacDonell F.; Rajeshwar K.. Chapter 11. Electro- and Photocatalytic Reduction of CO2: The Homogeneous and Heterogeneous Worlds Collide? In Photoelectrochemical Water Splitting: Materials, Processes and Architectures; Lewerenz H.-J., Peter L., Eds.; The Royal Society of Chemistry, 2013. [Google Scholar]
- Kumar B.; Llorente M.; Froehlich J.; Dang T.; Sathrum A.; Kubiak C. P. Photochemical and Photoelectrochemical Reduction of CO2. Annu. Rev. Phys. Chem. 2012, 63, 541–569. 10.1146/annurev-physchem-032511-143759. [DOI] [PubMed] [Google Scholar]
- Taniguchi I.; Aurian-Blajeni B.; Bockris J. The Reduction of Carbon Dioxide at Illuminated p-Type Semiconductor Electrodes in Nonaqueous Media. Electrochim. Acta 1984, 29, 923–932. 10.1016/0013-4686(84)87137-6. [DOI] [Google Scholar]
- Ghadimkhani G.; de Tacconi N. R.; Chanmanee W.; Janaky C.; Rajeshwar K. Efficient Solar Photoelectrosynthesis of Methanol from Carbon Dioxide Using Hybrid CuO-Cu2O Semiconductor Nanorod Arrays. Chem. Commun. 2013, 49, 1297–1299. 10.1039/c2cc38068d. [DOI] [PubMed] [Google Scholar]
- Janaky C.; Hursán D.; Endrodi B.; Chanmanee W.; Roy D.; Liu D.; Tacconi N. R. De; Dennis B. H.; Rajeshwar K. Electro- and Photoreduction of Carbon Dioxide: The Twain Shall Meet at Copper Oxide/Copper Nanocube Interfaces. ACS Energy Lett. 2016, 1, 332–338. 10.1021/acsenergylett.6b00078. [DOI] [Google Scholar]
- Read C. G.; Park Y.; Choi K. S. Electrochemical Synthesis of p-Type CuFeO2 Electrodes for Use in a Photoelectrochemical Cell. J. Phys. Chem. Lett. 2013, 3, 1872–1876. 10.1021/jz300709t. [DOI] [PubMed] [Google Scholar]
- Gu J.; Wuttig A.; Krizan J. W.; Hu Y.; Detweiler Z. M.; Cava R. J.; Bocarsly A. B. Mg-Doped CuFeO2 Photocathodes for Photoelectrochemical Reduction of Carbon Dioxide. J. Phys. Chem. C 2013, 117, 12415–12422. 10.1021/jp402007z. [DOI] [Google Scholar]
- Prévot M. S.; Guijarro N.; Sivula K. Enhancing the Performance of a Robust Sol-Gel-Processed p-Type Delafossite CuFeO2 Photocathode for Solar Water Reduction. ChemSusChem 2015, 8, 1359–1367. 10.1002/cssc.201403146. [DOI] [PubMed] [Google Scholar]
- Sharma G.; Zhao Z.; Sarker P.; Nail B. A.; Wang J.; Huda M. N.; Osterloh F. E. Electronic Structure, Photovoltage, and Photocatalytic Hydrogen Evolution with p-CuBi2O4 Nanocrystals. J. Mater. Chem. A 2016, 4, 2936–2942. 10.1039/C5TA07040F. [DOI] [Google Scholar]
- Kormányos A.; Thomas A.; Huda M. N.; Sarker P.; Liu J. P.; Poudyal N.; Janáky C.; Rajeshwar K. Solution Combustion Synthesis, Characterization, and Photoelectrochemistry of CuNb2O6 and ZnNb2O6 Nanoparticles. J. Phys. Chem. C 2016, 120, 16024–16034. 10.1021/acs.jpcc.5b12738. [DOI] [Google Scholar]
- Jang J.-W.; Cho S.; Magesh G.; Jang Y. J.; Kim J. Y.; Kim W. Y.; Seo J. K.; Kim S.; Lee K.-H.; Lee J. S. Aqueous-Solution Route to Zinc Telluride Films for Application to CO2 Reduction. Angew. Chem., Int. Ed. 2014, 53, 5852–5857. 10.1002/anie.201310461. [DOI] [PubMed] [Google Scholar]
- Kang U.; Choi S. K.; Ham D. J.; Ji S. M.; Choi W.; Han D. S.; Abdel-Wahab A.; Park H. Photosynthesis of Formate from CO2 and Water at 1% Energy Efficiency via Copper Iron Oxide Catalysis. Energy Environ. Sci. 2015, 8, 2638–2643. 10.1039/C5EE01410G. [DOI] [Google Scholar]
- Halmann M. Photoelectrochemical Reduction of Aqueous Carbon Dioxide on p-Type Gallium Phosphide in Liquid Junction Solar Cells. Nature 1978, 275, 115–116. 10.1038/275115a0. [DOI] [Google Scholar]
- Bockris J.; Wass J. C. On the Photoelectrocatalytic Reduction of Carbon Dioxide. Mater. Chem. Phys. 1989, 22, 249–280. 10.1016/0254-0584(89)90001-1. [DOI] [Google Scholar]
- Barton E.; Rampulla D. M.; Bocarsly A. B. Selective Solar-Driven Reduction of CO2 to Methanol Using a Catalyzed p-GaP Based Photoelectrochemical Cell. J. Am. Chem. Soc. 2008, 130, 6342–6344. 10.1021/ja0776327. [DOI] [PubMed] [Google Scholar]
- Appel A. M.; Bercaw J. E.; Bocarsly A. B.; Dobbek H.; Dubois D. L.; Dupuis M.; Ferry J. G.; Fujita E.; Hille R.; Kenis P. J. A; Kerfeld C. A.; Morris R. H.; Peden C. H. F.; Portis A. R.; Ragsdale S. W.; Rauchfuss T. B.; Reek J. N. H.; Seefeldt L. C.; Thauer R. K.; Waldrop G. L. Frontiers, Opportunities, and Challenges in Biochemical and Chemical Catalysis of CO2 Fixation. Chem. Rev. 2013, 113, 6621–6658. 10.1021/cr300463y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivula K. Toward Economically Feasible Direct Solar-to-Fuel Energy Conversion. J. Phys. Chem. Lett. 2015, 6, 975–976. 10.1021/acs.jpclett.5b00406. [DOI] [PubMed] [Google Scholar]
- Janáky C.; Rajeshwar K. The Role of (Photo)electrochemistry in the Rational Design of Hybrid Conducting Polymer/Semiconductor Assemblies: From Fundamental Concepts to Practical Applications. Prog. Polym. Sci. 2015, 43, 96–135. 10.1016/j.progpolymsci.2014.10.003. [DOI] [Google Scholar]
- Janáky C.; Kecsenovity E.; Rajeshwar K. Electrodeposition of Inorganic Oxide/Nanocarbon Composites: Opportunities and Challenges. ChemElectroChem 2016, 3, 181–192. 10.1002/celc.201500460. [DOI] [Google Scholar]
- Thangavel S.; Krishnamoorthy K.; Krishnaswamy V.; Raju N.; Kim S. J.; Venugopal G. Graphdiyne–ZnO Nanohybrids as an Advanced Photocatalytic Material. J. Phys. Chem. C 2015, 119, 22057–22065. 10.1021/acs.jpcc.5b06138. [DOI] [Google Scholar]
- Thangavel S.; Krishnamoorthy K.; Kim S.; Venugopal G. Designing ZnS Decorated Reduced Graphene-Oxide Nanohybrid via Microwave Route and Their Application in Photocatalysis. J. Alloys Compd. 2016, 683, 456–462. 10.1016/j.jallcom.2016.05.089. [DOI] [Google Scholar]
- Kongkanand A.; Martínez Domínguez R.; Kamat P. V. Single Wall Carbon Nanotube Scaffolds for Photoelectrochemical Solar Cells. Capture and Transport of Photogenerated Electrons. Nano Lett. 2007, 7, 676–680. 10.1021/nl0627238. [DOI] [PubMed] [Google Scholar]
- Meng F.; Li J.; Cushing S. K.; Bright J.; Zhi M.; Rowley J. D.; Hong Z.; Manivannan A.; Bristow A. D.; Wu N. Photocatalytic Water Oxidation by Hematite/Reduced Graphene Oxide Composites. ACS Catal. 2013, 3 (4), 746–751. 10.1021/cs300740e. [DOI] [Google Scholar]
- Meng F.; Cushing S. K.; Li J.; Hao S.; Wu N. Enhancement of Solar Hydrogen Generation by Synergistic Interaction of La2Ti2O7 Photocatalyst with Plasmonic Gold Nanoparticles and Reduced Graphene Oxide Nanosheets. ACS Catal. 2015, 5, 1949–1955. 10.1021/cs5016194. [DOI] [Google Scholar]
- Xiang Q.; Yu J.; Jaroniec M. Graphene-Based Semiconductor Photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. 10.1039/C1CS15172J. [DOI] [PubMed] [Google Scholar]
- Yang M.-Q.; Zhang N.; Pagliaro M.; Xu Y.-J. Artificial Photosynthesis over Graphene–semiconductor Composites. Are We Getting Better?. Chem. Soc. Rev. 2014, 43, 8240–8254. 10.1039/C4CS00213J. [DOI] [PubMed] [Google Scholar]
- Xiang Q.; Cheng B.; Yu J. Graphene-Based Photocatalysts for Solar-Fuel Generation. Angew. Chem., Int. Ed. 2015, 54, 11350–11366. 10.1002/anie.201411096. [DOI] [PubMed] [Google Scholar]
- Lightcap I. V.; Kamat P. V. Graphitic Design: Prospects of Graphene-Based Nanocomposites for Solar Energy Conversion, Storage, and Sensing. Acc. Chem. Res. 2013, 46, 2235–2243. 10.1021/ar300248f. [DOI] [PubMed] [Google Scholar]
- Yokomizo Y.; Krishnamurthy S.; Kamat P. V. Photoinduced Electron Charge and Discharge of Graphene–ZnO Nanoparticle Assembly. Catal. Today 2013, 199, 36–41. 10.1016/j.cattod.2012.04.045. [DOI] [Google Scholar]
- Pathak P.; Gupta S.; Grosulak K.; Imahori H.; Subramanian V. Nature-Inspired Tree-Like TiO2 Architecture: A 3D Platform for the Assembly of CdS and Reduced Graphene Oxide for Photoelectrochemical Processes. J. Phys. Chem. C 2015, 119, 7543–7553. 10.1021/jp512160h. [DOI] [Google Scholar]
- Yin S.; Men X.; Sun H.; She P.; Zhang W.; Wu C.; Qin W.; Chen X. Enhanced Photocurrent Generation of Bio-Inspired graphene/ZnO Composite Films. J. Mater. Chem. A 2015, 3, 12016–12022. 10.1039/C5TA02297E. [DOI] [Google Scholar]
- An X.; Li K.; Tang J. Cu2O/reduced Graphene Oxide Composites for the Photocatalytic Conversion of CO2. ChemSusChem 2014, 7, 1086–1093. 10.1002/cssc.201301194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y. T.; Vijayan B. K.; Gray K. A.; Hersam M. C. Minimizing Graphene Defects Enhances Titania Nanocomposite-Based Photocatalytic Reduction of CO2 for Improved Solar Fuel Production. Nano Lett. 2011, 11, 2865–2870. 10.1021/nl2012906. [DOI] [PubMed] [Google Scholar]
- Ng Y. H.; Iwase A.; Kudo A.; Amal R. Reducing Graphene Oxide on a Visible-Light BiVO4 Photocatalyst for an Enhanced Photoelectrochemical Water Splitting. J. Phys. Chem. Lett. 2010, 1, 2607–2612. 10.1021/jz100978u. [DOI] [Google Scholar]
- Kecsenovity E.; Endrődi B.; Pápa Z.; Hernádi K.; Rajeshwar K.; Janáky C. Decoration of Ultra-Long Carbon Nanotubes with Cu2O Nanocrystals: A Hybrid Platform for Enhanced Photoelectrochemical CO2 Reduction. J. Mater. Chem. A 2016, 4, 3139–3147. 10.1039/C5TA10457B. [DOI] [Google Scholar]
- Young Kim J.; Jang J.-W.; Hyun Youn D.; Yul Kim J.; Sun Kim E.; Sung Lee J. Graphene–carbon Nanotube Composite as an Effective Conducting Scaffold to Enhance the Photoelectrochemical Water Oxidation Activity of a Hematite Film. RSC Adv. 2012, 2, 9415. 10.1039/c2ra21169f. [DOI] [Google Scholar]
- Zhang Z.; Dua R.; Zhang L.; Zhu H.; Zhang H.; Wang P. Carbon-Layer-Protected Cuprous Oxide Nanowire Arrays for Efficinet Water Reduction. ACS Nano 2013, 7, 1709–1717. 10.1021/nn3057092. [DOI] [PubMed] [Google Scholar]
- Yu L.; Li G.; Zhang X.; Ba X.; Shi G.; Li Y.; Wong P. K.; Yu J. C.; Yu Y. Enhanced Activity and Stability of Carbon-Decorated Cuprous Oxide Mesoporous Nanorods for CO2 Reduction in Artificial Photosynthesis. ACS Catal. 2016, 6, 6444–6454. 10.1021/acscatal.6b01455. [DOI] [Google Scholar]
- Hou J.; Cheng H.; Takeda O.; Zhu H. Three-Dimensional Bimetal-Graphene-Semiconductor Coaxial Nanowire Arrays to Harness Charge Flow for the Photochemical Reduction of Carbon Dioxide. Angew. Chem., Int. Ed. 2015, 54, 8480–8484. 10.1002/anie.201502319. [DOI] [PubMed] [Google Scholar]
- Yang M.-Q.; Xu Y.-J. Photocatalytic Conversion of CO2 over Graphene-Based Composites: Current Status and Future Perspective. Nanoscale Horiz. 2016, 1, 185–200. 10.1039/C5NH00113G. [DOI] [PubMed] [Google Scholar]
- Low J.; Yu J.; Ho W. Graphene-Based Photocatalysts for CO2 Reduction to Solar Fuel. J. Phys. Chem. Lett. 2015, 6, 4244–4251. 10.1021/acs.jpclett.5b01610. [DOI] [PubMed] [Google Scholar]
- Low J.; Cheng B.; Yu J.; Jaroniec M. Carbon-Based Two-Dimensional Layered Materials for Photocatalytic CO2 Reduction to Solar Fuels. Energy Storage Mater. 2016, 3, 24–35. 10.1016/j.ensm.2015.12.003. [DOI] [Google Scholar]
- Dong X.; Cao Y.; Wang J.; Chan-Park M. B.; Wang L.; Huang W.; Chen P. Hybrid Structure of Zinc Oxide Nanorods and Three Dimensional Graphene Foam for Supercapacitor and Electrochemical Sensor Applications. RSC Adv. 2012, 2, 4364–4369. 10.1039/c2ra01295b. [DOI] [Google Scholar]
- Zhan B. B.; Liu C. B.; Chen H. P.; Shi H. X.; Wang L. H.; Chen P.; Huang W.; Dong X. C. Free-Standing Electrochemical Electrode Based on Ni(OH)2/3D Graphene Foam for Nonenzymatic Glucose Detection. Nanoscale 2014, 6, 7424–7429. 10.1039/c4nr01611d. [DOI] [PubMed] [Google Scholar]
- Zou Y.; Kinloch I. A.; Dryfe R. A. W. Mesoporous Vertical Co3O4 Nanosheet Arrays on Nitrogen-Doped Graphene Foam with Enhanced Charge-Storage Performance. ACS Appl. Mater. Interfaces 2015, 7, 22831–22838. 10.1021/acsami.5b05095. [DOI] [PubMed] [Google Scholar]
- Ambrosi A.; Chua C. K.; Bonanni A.; Pumera M. Electrochemistry of Graphene and Related Materials. Chem. Rev. 2014, 114, 7150–7188. 10.1021/cr500023c. [DOI] [PubMed] [Google Scholar]
- Valota A. T.; Toth P. S.; Kim Y.-J.; Hong B. H.; Kinloch I. A.; Novoselov K. S.; Hill E. W.; Dryfe R. A. W. Electrochemical Investigation of Chemical Vapour Deposition Monolayer and Bilayer Graphene on the Microscale. Electrochim. Acta 2013, 110, 9–15. 10.1016/j.electacta.2013.03.187. [DOI] [Google Scholar]
- Zhang H.; Zhu Q.; Zhang Y.; Wang Y.; Zhao L.; Yu B. One-Pot Synthesis and Hierarchical Assembly of Hollow Cu2O Microspheres with Nanocrystals-Composed Porous Multishell and Their Gas-Sensing Properties. Adv. Funct. Mater. 2007, 17, 2766–2771. 10.1002/adfm.200601146. [DOI] [Google Scholar]
- Cao X.; Yin Z.; Zhang H. Three-Dimensional Graphene Materials: Preparation, Structures and Application in Supercapacitors. Energy Environ. Sci. 2014, 7, 1850–1865. 10.1039/C4EE00050A. [DOI] [Google Scholar]
- Huang M.; Wang T.; Chang W.; Lin J.; Wu C.; Chen I.-C.; Peng K.; Lee S. Temperature Dependence on p-Cu2O Thin Film Electrochemically Deposited onto Copper Substrate. Appl. Surf. Sci. 2014, 301, 369–377. 10.1016/j.apsusc.2014.02.085. [DOI] [Google Scholar]
- Mohemmed Shanid N. A.; Abdul Khadar M.; Sathe V. G. Frohlich Interaction and Associated Resonance Enhancement in Nanostructured Copper Oxide Films. J. Raman Spectrosc. 2011, 42, 1769–1773. 10.1002/jrs.2945. [DOI] [Google Scholar]
- Dar M. A.; Kim Y. S.; Kim W. B.; Sohn J. M.; Shin H. S. Structural and Magnetic Properties of CuO Nanoneedles Synthesized by Hydrothermal Method. Appl. Surf. Sci. 2008, 254, 7477–7481. 10.1016/j.apsusc.2008.06.004. [DOI] [Google Scholar]
- Ferrari A. C.; Meyer J. C.; Scardaci V.; Casiraghi C.; Lazzeri M.; Mauri F.; Piscanec S.; Jiang D.; Novoselov K. S.; Roth S.; Geim A. K. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401–187404. 10.1103/PhysRevLett.97.187401. [DOI] [PubMed] [Google Scholar]
- Malard L. M.; Pimenta M. A.; Dresselhaus G.; Dresselhaus M. S. Raman Spectroscopy in Graphene. Phys. Rep. 2009, 473, 51–87. 10.1016/j.physrep.2009.02.003. [DOI] [Google Scholar]
- Nakano Y.; Saeki S.; Morikawa T. Optical Bandgap Widening of p-Type Cu2O Films by Nitrogen Doping. Appl. Phys. Lett. 2009, 94, 22111. 10.1063/1.3072804. [DOI] [Google Scholar]
- Gao E.; Wang W.; Shang M.; Xu J. Synthesis and Enhanced Photocatalytic Performance of Graphene-Bi2WO6 Composite. Phys. Chem. Chem. Phys. 2011, 13, 2887–2893. 10.1039/C0CP01749C. [DOI] [PubMed] [Google Scholar]
- Paracchino A.; Brauer J. C.; Moser J. E.; Thimsen E.; Graetzel M. Synthesis and Characterization of High-Photoactivity Electrodeposited Cu2O Solar Absorber by Photoelectrochemistry and Ultrafast Spectroscopy. J. Phys. Chem. C 2012, 116, 7341–7350. 10.1021/jp301176y. [DOI] [Google Scholar]
- Wang L. C.; de Tacconi N. R.; Chenthamarakshan C. R.; Rajeshwar K.; Tao M. Electrodeposited Copper Oxide Films: Effect of Bath pH on Grain Orientation and Orientation-Dependent Interfacial Behavior. Thin Solid Films 2007, 515, 3090–3095. 10.1016/j.tsf.2006.08.041. [DOI] [Google Scholar]
- de Brito J. F.; Araujo A. R.; Rajeshwar K.; Zanoni M. V. B. Photoelectrochemical Reduction of CO2 on Cu/Cu2O Films: Product Distribution and pH Effects. Chem. Eng. J. 2015, 264, 302–309. 10.1016/j.cej.2014.11.081. [DOI] [Google Scholar]
- Peter L. M. Dynamic Aspects of Semiconductor Photoelectrochemistry. Chem. Rev. 1990, 90, 753–769. 10.1021/cr00103a005. [DOI] [Google Scholar]
- Ma Y.; Pendlebury S. R.; Reynal A.; Le Formal F.; Durrant J. R. Dynamics of Photogenerated Holes in Undoped BiVO4 Photoanodes for Solar Water Oxidation. Chem. Sci. 2014, 5, 2964. 10.1039/c4sc00469h. [DOI] [Google Scholar]
- Klahr B.; Gimenez S.; Fabregat-Santiago F.; Bisquert J.; Hamann T. W. Electrochemical and Photoelectrochemical Investigation of Water Oxidation with Hematite Electrodes. Energy Environ. Sci. 2012, 5, 7626. 10.1039/c2ee21414h. [DOI] [Google Scholar]
- Noack V.; Weller H.; Eychmuller A. Electron Transport in Particulate ZnO Electrodes: A Simple Approach. J. Phys. Chem. B 2002, 106, 8514–8523. 10.1021/jp0200270. [DOI] [Google Scholar]
- Pu Y. C.; Chou H. Y.; Kuo W. S.; Wei K. H.; Hsu Y. J. Interfacial Charge Carrier Dynamics of Cuprous Oxide-Reduced Graphene Oxide (Cu2O-rGO) Nanoheterostructures and Their Related Visible-Light-Driven Photocatalysis. Appl. Catal., B 2017, 204, 21–32. 10.1016/j.apcatb.2016.11.012. [DOI] [Google Scholar]
- Hansen O.; Seger B.; Vesborg P. C. K.; Chorkendorff I. A Quick Look at How Photoelectrodes Work. Science 2015, 350, 1030–1031. 10.1126/science.aad6060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.