Abstract
Recently, as a supplement of cement, the utilization of pozzolanic materials in cement and concrete manufacturing has increased significantly. This study investigates the scope to use pozzolanic wastes (slag, palm oil fuel ash and rice husk ash) as an alkali activated binder (AAB) that can be used as an alternative to cement. To activate these materials, sodium hydroxide solution was used at 1.0, 2.5 and 5.0 molar concentration added into the mortar, separately. The required solution was used to maintain the flow of mortar at 110% ± 5%. The consistency and setting time of the AAB-paste were determined. Mortar was tested for its flow, compressive strength, porosity, water absorption and thermal resistance (heating at 700 °C) and investigated by scanning electron microscopy. The experimental results reveal that AAB-mortar exhibits less flow than that of ordinary Portland cement (OPC). Surprisingly, AAB-mortars (with 2.5 molar solution) achieved a compressive strength of 34.3 MPa at 28 days, while OPC shows that of 43.9 MPa under the same conditions. Although water absorption and porosity of the AAB-mortar are slightly high, it shows excellent thermal resistance compared to OPC. Therefore, based on the test results, it can be concluded that in the presence of a chemical activator, the aforementioned pozzolans can be used as an alternative material for cement.
Keywords: slag, palm oil fuel ash, rice husk ash, alkaline activator, alkali activated binder
1. Introduction
As a matter of fact, ordinary Portland cement (OPC) is one of the most consumed materials after water. It is used as the main binding material in construction industries across the globe. However, it is an energy intensive material and is also liable for carbon dioxide (CO2) gas emission. Thus to reduce consumption and dependency on cement, utilization of pozzolanic materials such as ground granulated blast furnace slag, palm oil fuel ash (POFA), and rice husk ash (RHA), fly ash, silica fume, etc. as supplementary cementing materials has become the leading research interest in the area of cement and materials research in recent decades. In addition to CO2 emissions, excess fuel is consumed in cement manufacturing for the burning of Portland cement clinker. Besides, it is one of the most energy intensive materials after aluminum and steel [1]. It has been reported that nearly 850–900 kcal/kg (in the dry process) and 1300–1600 kcal/kg (in the wet process) heat energy is required in cement manufacturing [2]. However, the global average electricity consumption is approximately 111 kWh per ton of cement production [3]. Approximately 4.0 kJ energy [4] and 1.5 tons of raw materials are required to produce one ton of OPC. Thus the cement manufacturing process is not only liable for CO2 emission but also responsible for the gradual depletion of fuel energy and natural stone from our limited stocks in the planet. Therefore, the acquirement and development of sustainable binding materials is one of the prime issues needed to redress the depletion of the world’s most valuable fossil energy and to reduce the negative impacts of cement production on the environment [5]. On the other hand, huge quantities of RHA, POFA, slag, Fly ash (FA), silica fume, etc. are generated regularly worldwide. Table 1 represents a statistics of global production and consumption of various wastes. Table 1 shows that the waste generation rate is high compared to the negligible consumption rate. Only a small portion of slag is consumed as a supplement of cement and an ingredient of concrete [6] and sub-base materials in road construction [7]. Rice husk is consumed for heat generation purposes in rice mills. No promising alternative uses have been developed so far particularly for POFA and RHA and huge costs are incurred for transportation and disposal. Furthermore, enormous environmental pollution is also worth noting.
Table 1.
A statistic of global production and consumption of various wastes.
| Waste | Production Source | Quantity (million ton) | Consumption (million ton) | Reference |
|---|---|---|---|---|
| Slag | Steel industries | 100.00 | 35 | [1] |
| FA | Coal operated power plants | 900.00 | - | [8] |
| POFA | Palm oil mills in Malaysia | 0.06 | - | [9] |
| Rice husk | Rice mills | 110.00 (20% of 550 million tons rice) | - | [10] |
| RHA | Rice mills | 16.50–22.00 | - | [10] |
| Silica fume | Silicon industries | 2.00 | - | [8] |
Nonetheless, it should be noted that thousands of tons of POFA are produced annually in the operation of 200 palm oil mills in Malaysia and are simply disposed of without any salvage value. So the nation’s pollution problem has increased from this sector which includes the annual production of 2.6 million tons of solid waste in the form of oil palm shells [11]. The production of POFA is growing every year, and is disposed of for landfills, and which now also become a noticeable problem. Besides, similar difficulties have also been faced and noticed in association with the generation of slag and RHA. About 650 million tons of rice is produced in the world of which 2.2 million is from Malaysia; after milling, about 20% is converted to rice husk. Finally, 15%–20% ash is produced as RHA after burning [12]. In spite of their technical and ecological benefits, all of these wastes are simply disposed of into ponds, lagoons or rivers. Therefore, reduction of the quantity of waste dumping, environmental sustainability and declining CO2 emission can be ensured by proper consumption or recycling of these materials simultaneously as a supplement of cement or as an ingredient of concrete.
2. Research Significance
An investigation into the development of an alternative binder from pozzolanic waste materials (slag, RHA, POFA) is significant as can be seen by the following aspects. Recently, the utilization of pozzolanic materials in cement and concrete has increased considerably due to their diverse benefits such as less cement use, saving production costs, improvement of the durability properties of the concrete and so on. Pozzolans are fine materials that contain silica and/or alumina. They do not exhibit any cementation properties of their own except in the presence of calcium oxide (CaO) or calcium hydroxide (Ca(OH)2). Silica and alumina in pozzolans also react and form cementitious materials [13]. POFA is one of the pozzolanic materials that contains a moderate percentage of silicon dioxide and has high potential to be used as a cement replacement material. For example, POFA contains silicon dioxide that can react with Ca(OH)2 generated from the hydration process and thus pozzolanic reactions produce more calcium silicate hydrate (C–S–H) gel compound as well as reducing the amount of Ca(OH)2. Thus for concrete production, POFA contributes to making concrete stronger, denser and more durable. POFA can also be used to increase both the compressive strength and the sulfate resistance of mortar, if it is ground properly [14]. POFA contributes to improving the durability and is cost effective due to less use of cement. The ground POFA with high fineness can be used as a cement replacement to produce high-strength concrete with a compressive strength as high as 70 MPa at 90 days when used to replace OPC at 20% by weight of binder [15]. Therefore, it will also be beneficial for the environment with respect to reducing the waste disposal volume of landfills. In addition, POFA, RHA and fly ash can be used as pozzolans to replace part of OPC in making mortar with relatively high strength and good resistance to chloride penetration [16]. RHA has been used in lime pozzolan mixes and could be a suitable partial replacement for Portland cement [17,18,19]. RHA is receiving more attention now since its use generally improves the properties of the blended cement concrete, the cost, and leads to a reduction of negative environmental effects [20]. RHA can be incorporated either as an admixture or as cement replacement material [21]. Slag is commonly used in concrete for the following beneficial reasons: it improves durability and reduces porosity, improves the interfacial zone with the aggregate, requires less quantity of cement, saves energy, and exhibits good performance as well as having better engineering properties [22]. Quaternary-blended cement has been produced from slag, POFA, RHA, and timber ash by 66% OPC replacement to be used in high strength (100–120 MPa), sustainable, and high-performance concrete [23].
Therefore, effective consumption of these waste materials as a replacement for cement will also encourage researchers to investigate a sustainable way of saving material, especially cement as well as reducing the CO2 emission. In addition, the utilization of these waste materials will have the following multiple advantages: enhances the properties of concrete, reduces the production cost of concrete, and minimizes the waste disposal problem. Finally, use of these wastes as a supplement of cement and or as an ingredient of concrete is logical, worthy and attributable to the present situation which demands the achievement of a goal of a sustainable concrete and sustainable binding material. Thus, expectation for the development of an alternative binder from local waste materials is realistic and meaningful. The aim of this research is, therefore, to investigate the possibility of producing an alkali activated binder (AAB) using 100% local industrial slag, RHA and POFA by a mechano-chemical (grinding and chemical activators) activation technique.
3. Materials and Methods
3.1. Materials
Ground granulated blast furnace slag, POFA and RHA were used in this research as components of AAB. OPC (American Society for Testing and Materials (ASTM) type I) was used to compare the different properties including physical, chemical, binding, flow, compressive strength, microstructure and durability of the AAB. Slag and POFA were collected from local industries, Selangor, Malaysia. RHA was produced by a special type of furnace at the concrete lab at the University of Kebangsaan Malaysia (Bangi, Selangor, Malaysia). The details of the furnace were reported by Zain et al. [24]. Local river sand which passed through a 4.75 mm sieve and had a specific gravity of 2.62 was used as fine aggregate. Sodium hydroxide (NaOH) flakes of analytical grade were used as a chemical activator from the manufacturer Merck (Hunterdon, NJ, USA). DarexSuper-20 was used as superplasticizer (SP) from the Grace manufacturing company (Russellville, AR, USA). Though the use of higher amounts of SP has a negative effect on the overall cost and eco efficiency of the materials it was used to increase and maintain a sufficient flow for casting of mortar. SP is considered as grade F according to ASTM C494 specification [25]. Available supplied water was used for mortar preparation and in curing purposes.
3.2. Instruments
The following instruments were utilized to perform different experiments in the research: Malvern Mastersizer 2000 (for grain size analysis, Malvern, UK); X-ray fluorescence (XRF) was done for chemical composition using a Bruker brand (Billerica, MA, USA) XRF machine. A Los Angeles abrasion machine (ELE International Limited, London, UK) was used for grinding of POFA and RHA for increased fineness. X-ray diffraction (XRD) analysis was conducted using a Bruker brand XRD machine. Scanning electron microscope (SEM) analysis was performed by Supra 55 VP, ZEISS (Oberkochen, Germany). A Hobart (Troy, OH, USA) mixing machine was used to mix the paste and mortars. A compression machine (Unit Test scientific, Sendirian Berhad, Selangor, Malaysia) of 3000 kN capacity was used for compressive tests of mortar specimens. An electric furnace was used for thermal testing.
3.3. Preparation of Mortar
Table 2 presents the mixing proportions of the raw materials (slag, RHA and POFA). Paste and mortar were prepared according to ASTM C305-06 specification using a Hobart mixing machine [26]. NaOH solutions of different molar concentrations (1.0, 2.5 and 5.0 M) were prepared before mixing/preparation of mortar. Compaction of the mold was performed using an electrically operated vibration table. After casting, the prism mold of OPC was opened after one day but molds of AAB-mortars for 1.0 M concentration needed two days because of delayed hardening. Finally, samples were immersed into a curing tank at a room temperature of 25 ± 20 °C until the desired testing ages of 3, 7 and 28 days were achieved.
Table 2.
Materials used for preparation of mortar (by weight).
| Binder | Activator | Molar concentration | Solution to binder or water to cement ratio | SP (%) | Sand to binder ratio | ||
|---|---|---|---|---|---|---|---|
| Name | Materials | (%) | |||||
| AAB | Slag | 70 | NaOH | 1.0 M | 0.62 | 4.2 | 2.75 |
| POFA | 20 | 2.5 M | 4.6 | ||||
| RHA | 10 | 5.0 M | 5.0 | ||||
| OPC | - | 100 | - | - | 0.55 | 2.5 | |
3.4. Tests on Paste and Mortar
3.4.1. Normal Consistency of Binder
Consistency (water demand) of AAB and OPC paste was determined according to ASTM C187-04 [27]. In this regard, pastes were prepared and the amount of water required noted to penetrate the Vicat testing (ELE International Limited, London, UK) needle (10 mm diameter) by 10 mm.
3.4.2. Setting Time of Binder
For the observation of the setting time, the ASTM C191-08 testing standard was followed [28]. The Vicat apparatus was also used to determine the setting time of the AAB as well as the OPC paste. The initial setting time was measured for standard paste (prepared using the required water according to the normal consistency) to penetrate the Vicat testing needle (1 mm diameter) by 25 mm. The final setting time was the measured time when there was no penetration of the needle observed by the same testing method.
3.4.3. Flow and Compressive Strength of Mortar
The mortar flow spread test was done using a flow table according to the ASTM C1437 testing standard [29]. The compressive strength and the strength activity index of mortar were determined using a 50 mm cube specimen according to the ASTM C109 testing standard [30].
3.4.4. Water Absorption of Mortar
Water absorption was determined using a mortar prism of 40 mm × 40 mm × 160 mm in size with specimens following the Japanese industrial standard (JIS A6203) as found in a study by Ahmad et al. [31]. In this regard, prism specimens aged for 28 days were dried until constant weight (Wd) was achieved. Then the specimens were immersed into water. Subsequently, the specimens were taken out and their surfaces wiped quickly with a wet cloth. Then they were weighed in air immediately (Wa) after immersion periods of 30 min, 1, 3, 6, 24, 48 and 72 h. Thus, water absorption of the specimen was calculated as 100 × (Wa – Wd)/Wd.
3.4.5. Porosity of Mortar
Porosity of mortar was determined with a prism of 40 mm × 40 mm × 160 mm in size of specimens based on the study of Chindaprasirt et al. [32]. After being cured in water until the age of 28 days, prism specimens were taken. The specimens were dried at 100 ± 5 °C until they achieved a constant weight (Wd). Then, the specimens were immersed in clean water for full saturation over three days. After that, the weights of the specimens in water were also recorded (Ww). Subsequently, the specimens were taken out and their surfaces wiped quickly with a wet cloth. Specimens were weighed in air immediately (Wa). Therefore, the porosity of the specimens was obtained in percent as 100 × (Wa − Wd)/(Wa − Ww).
3.4.6. Thermal Resistance of Mortar
For the heat resistance test, using a prism of 40 mm × 40 mm × 160 mm in size, specimens were weighed at the saturated surface in dry condition (Ws). Then, the specimens were put into an electric furnace and heated up to 700 °C for two hours with the temperature increment of the furnace at 9 °C per minute. After completion of the heating period, specimens were cooled to room temperature over approximately the same period of time. Subsequently, the dry weight of the specimens was taken as WD and the strength of the specimens was determined (fcf). Finally, loss in weight was calculated as (Ws – WD)/Ws and the strength loss of the specimens due to the thermal effect was determined as (fc − fcf)/fc; where, fc is the strength of the ordinary sample (without being heated). The same study was successfully performed by Rahel et al. [33].
4. Results and Discussion
4.1. Properties of Materials
4.1.1. Chemical and Physical Properties of Materials
The chemical and physical properties of materials are represented in Table 3. To determine the chemical properties, the raw materials and the new binders were examined by X-ray fluorescence (XRF) test. The Table 3 shows that among all types of raw materials, RHA contains the greater amount of silica (87.75%), which is responsible for the pozzolanic reaction or secondary hydration in mortar or concrete. The total percentage of major oxides (SiO2 + Al2O3 + Fe2O3) is over 70% for RHA which is greater than the minimum (70%) as specified in ASTMC618 [34]. Thus, RHA is categorized as class F pozzolan. While, the sum of these oxides lies between 50% and 70% both for slag and POFA, accordingly, they may be considered as class C pozzolan. Since POFA does not possess any cementing property, it cannot be categorized as class C pozzolan. The sulphur trioxide (SO3) content of these materials is less than 4% which is the maximum allowable content as prescribed by the ASTM C618 standard [34]. POFA contains 11.86% of K2O which is greater when compared to the other materials, because palm oil trees consume a large amount of potassium from the soil during cultivation.
Table 3.
Chemical and physical properties of materials.
| Material | Chemical Properties, Oxide Compositions (%) | |||||||||||
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | P2O5 | TiO2 | MnO | LOI | |
| Slag | 33.05 | 16.36 | 0.53 | 45.00 | 6.41 | 1.21 | 0.13 | 0.42 | - | - | - | 3.05 |
| POFA | 47.22 | 2.24 | 2.65 | 6.48 | 5.86 | 3.34 | 1.22 | 11.86 | 5.37 | 0.17 | 0.10 | 5.42 |
| RHA | 87.75 | 0.38 | 0.19 | 1.04 | 0.69 | 0.56 | 0.05 | 2.83 | 1.31 | 0.02 | 0.07 | 3.04 |
| OPC | 20.99 | 4.60 | 4.44 | 67.17 | 2.53 | 2.98 | 0.03 | 0.16 | - | - | - | 1.30 |
| AAB * | 41.35 | 11.94 | 0.92 | 32.90 | 5.73 | 1.57 | 0.34 | 2.95 | 1.21 | 0.04 | 0.03 | 3.52 |
| Physical Properties | ||||||||||||
| Specific gravity | Average grain size d50 (μm) | Fineness | Color | |||||||||
| Blaine (cm2/g) | Retained on 45 μm sieve (%) | |||||||||||
| Slag | 2.85 | 14.67 | 3,919 | 0.14 | Near white | |||||||
| POFA | 2.16 | 16.08 | 4,582 | 4.23 | Blackish white | |||||||
| RHA | 2.05 | 6.63 | 6,964 | 3.32 | Near white | |||||||
| OPC | 3.14 | 16.17 | 2,850 | 12.52 | Grey | |||||||
| AAB * | 2.63 | 14.15 | 4,356 | 1.28 | Near white | |||||||
* Calculated Value; AAB = 70% Slag + 20% POFA + 10% RHA.
The loss on ignition (LOI) is higher for POFA, but it is less than the prescribed value 10% (ASTM C-618) [34]. The physical properties of the materials are also given in Table 3. The particle size of POFA and RHA decreases after grinding. The average particle sizes of the materials were found to be 14.67 µm for slag, 16.08 µm for POFA, 6.63 µm for RHA and 16.17 µm for OPC. Thus, the raw materials have a lower particle size in comparison to OPC. Specific gravity and fineness of the particles are also shown in Table 3 which shows that pozzolanic materials are lighter than OPC. Similar observations were reported by past researchers [35].
4.1.2. SEM Images of Materials
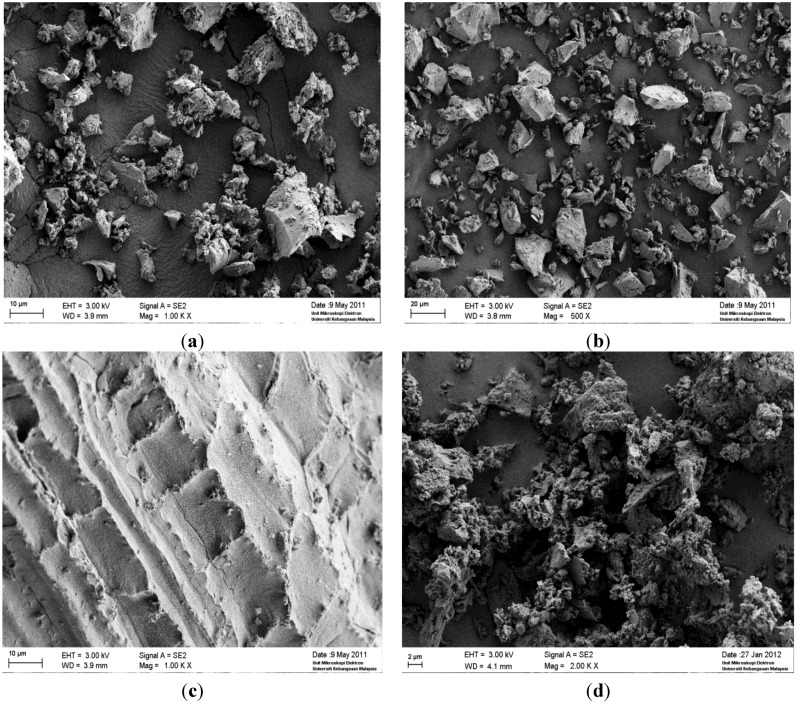
The obtained SEM views of the used raw materials are shown in Figure 1. Slag particles, when seen with a SEM image, appear to be square and diamond in shape and similar to the particles of OPC. The OPC seems to have box and stone-shaped particles observed under SEM. The original RHA shows a spongy and cellular structure. The original POFA (as received) shows that it contains close to spherical particles with a small amount of plerospheres and an irregularly shaped porous cellular structure. After grinding POFA and RHA, the porous structures were crushed and broken down into smaller fractions leading toan increased surface area and improved fineness of the particles.
Figure 1.
SEM image of (a) OPC; (b) Slag. (c) RHA (as produced); (d) RHA (after grinding); (e) POFA (as received); and (f) POFA (after grinding).
4.1.3. Strength Activity Index of Binders
ASTM C 311 defines the strength activity index (SAI) as the ratio between the compressive strength of mortar containing substitute materials of 20% by mass of binder and that of the average compressive strength of the reference cement (OPC) mortar at a designated age [36]; but the material replacement is 50% for the SAI of slag. The SAI of the used materials was determined and the obtained values are presented in Table 4. Experimental results show that the activity index of slag is more than 100% for both 7 and 28 days. Thus, slag is considered as 100 grade based on the ASTM C 989-05 classification [37]. Activity indexes of unground RHA and POFA are below 50% and over 54% at 7 days respectively. They are found to be more than 63% at 28 days. Activity indexes of RHA and POFA are found to be less, due to the use of unground POFA and RHA in preparing testing mortar samples. Usually, the strength activity index of any pozzolans can be improved by grinding or increasing their fineness as can easily be seen from Table 4 as well as found in past studies [38,39]. Thus, the activity index of GPOFA (ground POFA) and GRHA (ground RHA) is much higher than that of the unground sample. Therefore, pozzolans can be further activated by improving their fineness.
Table 4.
Strength activity index (SAI) of materials.
| Binder | SAI (7 days) | ASTM requirement | SAI (28 days) | ASTM requirement |
|---|---|---|---|---|
| Slag | 100.4 | 95 for 120 grade | 103.8 | 95 for 100 grade |
| RHA | 48.6 | - | 63.0 | - |
| GRHA | 86.7 | - | 101.6 | - |
| POFA | 54.2 | - | 65.3 | - |
| GPOFA | 84.9 | - | 99.0 | - |
4.2. Consistency of Binders
Table 5 gives the consistency values of the binders. The Table 5 indicates that the consistency of OPC is 30.0%. In contrast, AAB pastes show high consistency (water demand) compared to OPC (consistency for AAB pastes is 33.5%). This increased consistency occurs due to porous and spongy particles, the fineness and more specific surface areas of the pozzolans, as observed in SEM analysis. Ganesan et al. [40] reported that the consistency of RHA blended pastes gradually increases due to the addition of RHA to the paste. Therefore, it can be understood that a blended paste of pozzolanic materials show a greater consistency than that of OPC pastes. This argument is also supported by Cheerarot et al. [41] who reported that the normal consistency of OPC paste is lower than that of the blended paste of FA.
Table 5.
Consistency, setting time and flow of binder.
| Binder | AAB (1.0 M) | AAB (2.5 M) | AAB (5.0 M) | OPC |
|---|---|---|---|---|
| Consistency (%) | 33.5 | 33.5 | 33.5 | 30.0 |
| Initial setting time (hour:min) | 0:50 | 0:27 | 0:21 | 2:15 |
| Final setting time (hour:min) | 2:10 | 1:45 | 1:05 | 5:25 |
| Flow (%) | 114 | 110 | 106 | 109 |
4.3. Setting Time
The setting time of the binders is presented in Table 5. The setting time of the AAB paste is earlier compared to OPC and it completely depends on the concentration of the NaOH solution, the percentage of raw materials/ingredient of AAB mix and the fineness (unground and ground conditions) of POFA and RHA. For instance, on increasing the molar concentration of NaOH solution, the setting time of the binders gradually decreased because AAB contains 1.0, 2.5 and 5.0 M solutions respectively. The initial setting time of OPC pastes was found to be 2 h and 15 min. The final setting time of AAB paste was observed to be less than that of the value for OPC, as specified in ASTM C150 [42]. However, Tangchirapat et al. [43] reported that the setting time of the paste increases after OPC replacement by POFA. For the present study, the setting time of AAB (100% replacement of OPC) is less which may be due to the chemical reaction of NaOH and pozzolans. NaOH contributes to the earlier setting of the AAB because of its smaller cation (Na+). This is according to Fajan’s rule: (i) the more charged the cation is, the closer and stronger it will pull other molecules to it; (ii) The smaller the cation, the less the levels of electron shielding get in the way, letting other molecules be pulled closer and stronger [44].
4.4. Flow of Binders
The flow of the binders is presented in Table 5. The flow of the OPC mortar is 109% only when using 2.5% super plasticizer. However to obtain the same value of flow, over 4% SP and a greater amount of water solution was used in the case of the AAB-mortar. NaOH in pozzolans contributes to gaining strength quickly and a resultant lesser flow which is a similar argument to that reported by Jae et al. [45] .The lesser flow of mortar containing NaOH may be due to the following reasons:
NaOH has the smallest cation (Na+), which may attract the molecules/constituents of the binder and the mortar quickly and consequently, reduce the flow of mortar.
The lower flow tendency and the higher water demand are due to the porous and spongy nature of pozzolanic materials (particularly for RHA) and the higher fineness or larger surface area; thus a greater amount of water is required. Several researchers reported that a greater amount of water was required to obtain the desired consistency and a lower flow ability is common among pozzolans as reported by Ahmad and Sheikh [46].
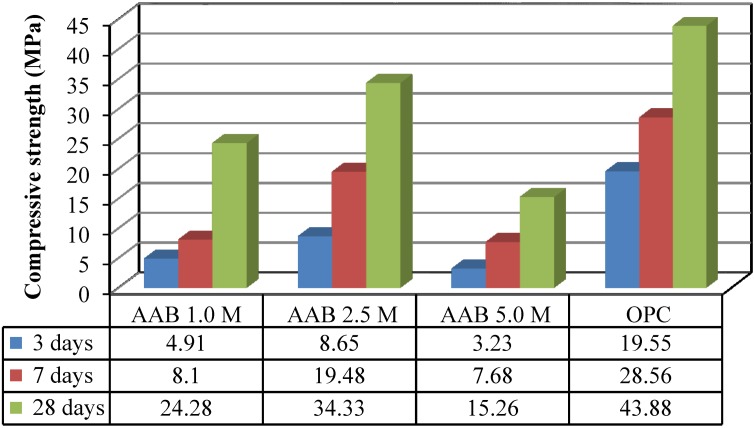
4.5. Compressive Strength of Mortar
In the current study, a compressive strength of 34.3 MPa was found using slag, POFA and RHA with only 2.5 M NaOH solution and an ambient temperature curing method. This better strength development of mortar was also observed by Isaia et al. [47] in binary and/or ternary combination due to the synergic effect of the materials. The compressive strength of AAB-mortars for a different activator concentration is presented in Figure 2. The figure shows that the strength of mortar is influenced significantly by the concentration of activators which is a similar conclusion as reported by other researchers [48,49]. The compressive strength of AAB-mortar has a less value with 1.0 molar (1.0 M) solution at all ages. On the contrary, the strength of all AAB-mortar is less than that of OPC. However, the strength of AAB-mortar is better with 2.5 M solution but worsens for the 5.0 M solution. This reduced strength of mortar obtained may be due to the following reasons:
A lower concentration (1.0 M) may not be sufficient to activate all of the molecules of AAB.
For a higher molarity (5.0 M), excess amounts of activators remain without being bonded or form weak intra-bonds inside the AAB-mortar.
Residual activators or weak bonds of activators may collapse when the mortar is immersed in water for curing. As a result, the strength of the mortar remains the same or decreases.
Figure 2.
Compressive strength of mortar.
The AAB-mortar achieves the highest strength with 2.5 M activators. Therefore, based on experimental results, NaOH contributes towards developing the highest strength of the AAB-mortar with 2.5 M solutions at all ages. It could be noted that the compressive strength of AAB-mortars containing 2.5 M solution is better and so the morphology and durability properties of these mortars were investigated.
4.6. SEM Observation of Mortar
SEM images of AAB exhibit a honeycomb type heterogeneous gel structure with embedded varying morphologies associated with hollow cavities, likened to a geopolymer structure. A similar argument was reported by Rodriguez et al. [50] for an alkali activated binder. From the SEM images (Figure 3), large differences can be observed from the microstructures of OPC and AAB-mortars. In comparison to the shape of the gel formation, the image of OPC shows the existence of ettringite (needle type shape) while that of AAB demonstrates a different view. There is no ettringite observed in the SEM analysis of AAB-mortars, it contains amorphous and a sponge-type gel structure. Therefore, the strength formation of AAB-mortar and that of OPC seems to be completely different.
Figure 3.
SEM views of (a) OPC; (b) AAB-mortar.
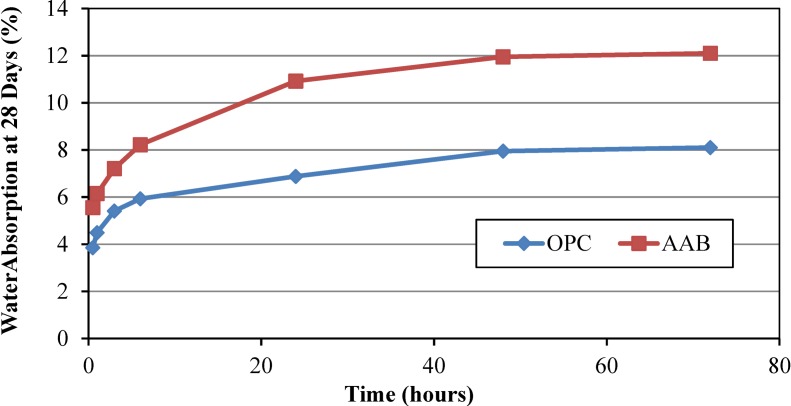
4.7. Water Absorption of Mortars
Figure 4 represents water absorption of OPC and AAB mortar at 28 days of curing age. It was found that water absorption of AAB mortar was over 12%; while, that obtained for OPC mortar was 8.1%, tested after an immersion period of 72 h at 28 days. It is interesting to note that the rate of water absorption is lower at the beginning of the immersion period (i.e., within 30 min to 3 h) at 28 days of testing age. Water absorption of AAB-mortar is higher than that of OPC mortar. The absorption of AAB-mortar was found to be higher due to more voids and pores (Figure 3) present inside the mortar which also lead to a lower compressive strength of the mortar. In addition, AAB has finer particles than those of OPC; consequently, they absorb more water which can be observed from normal consistency data (Table 5).
Figure 4.
Water absorption of AAB and OPC mortar at 28 days of curing age.
In general, for the case of partial replacement of slag, POFA, and RHA results in pore refinement and increased water tightness of the binder matrix. The refinement of the pore structure, however, leads to reduced permeability of the hydrated cement paste and this could lead to a retarded moisture migration through the cement matrix [51]. For the alkali activated binder, Chi and Huang [52] obtained water absorption of 7.5% for an OPC specimen; however, absorption was reduced significantly for the alkali activated binder (1.1%–6.1%); it depends on the ratio of slag-to-fly ash and activator content. This high performance of the water absorption was obtained most probably due to the high compressive strength (20–80 MPa) of mortar.
4.8. Porosity of Mortars
The average porosity of mortar specimens was determined at the age of 28 days. The porosity of OPC and AAB mortar was found as 14.23% and 20.54% respectively. The higher porosity of AAB mortars was found to be due to the small particle size, the siliceous admixture (POFA, RHA and slag) particles have more surface area hence absorb greater amounts of water and have lower compressive strength. A similar observation was reported by Chindaprasirt and Rukzon [32]. In addition, more voids are occupied inside the AAB mortar; the situation can be seen from the SEM image.
However, in the case of partial replacement, incorporation of RHA reduces the porosity [19,51]. Inclusion of FA reduces the average pore size and results in a less permeable paste as reported by Chindaprasirt et al. [53]. It was also found by Chindaprasirt et al. [54] that the permeability of rice husk-bark ash and POFA are lower than that of OPC concrete. Al-Otaib [55] reported that alkali activated slag concrete shows a greater porosity value compared to OPC. He obtained a range of porosity of 13%–10% for 7–360 days of testing time depending on the slag replacement, while OPC concrete showed a porosity of 10.4%–8% for the same age. The main variable to be considered, for obtaining long lasting concrete, is porosity and pore connectivity [56]. Higher porosity and absorption can be reduced by achieving a higher compressive strength of the AAB mortar [32].
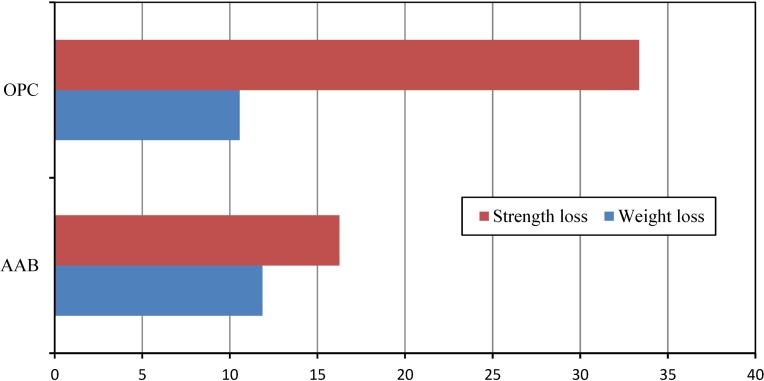
4.9. Thermal Resistance of Mortar
After heating the mortars at 700 °C, the weight loss of OPC samples was found to be 10.56%, while the weight loss of the AAB-specimen obtained was a little over 11.86%. Loss in strength of OPC and AAB-mortar was found as 33.35% and 16.26%, respectively (Figure 5). Although Rahel et al. [33] reported that the strength loss of OPC mortar is 32% after being heated at 700 °C. Alkali activated binders (slag or slag with metakaolin blended) are very good against thermal resistance up to 800–1400 °C as reported by several studies [57,58,59]. In our present study, AAB mortar exhibits a very strong thermal resistance compared to that of OPC up to exposure at 700 °C. This may have occurred due to the strong bond of the NaOH in AAB mortar and the higher boiling point (1390 °C) of NaOH. Thus, the strength of AAB mortar did not reduce significantly due to exposure at 700 °C.
Figure 5.
Losses (%) in weight and strength of mortar after heating at 700 °C.
5. Conclusions
Based on the experimental test results, the following conclusions can be drawn from the present study:
-
1
The physical and chemical test results reveal that the considered pozzolanic waste materials (slag, POFA and RHA) contain a high amount of silica and sufficient amounts of the major oxides. Consequently, they perform as an alternative binder in the presence of a chemical activator, NaOH. It can be predicted that development of a new binder from locally available slag, POFA and RHA might well be possible.
-
2
Experimental results revealed that slag, POFA and RHA could be used as substitutes of cement provided that these wastes are processed properly with maintenance of high fineness and with the use of a chemical activator.
-
3
The alternative binder exhibits reasonable binding, consistency, flow value and setting time compared to OPC. The new binder shows a considerable compressive strength of 34.3 MPa at 28 days.
-
4
The water absorption and porosity of the AAB mortar are slightly higher compared to those of OPC mortar. This occurs due to the porous structure and the less compressive strength of AAB. It can be minimized by improving the strength of AAB.
-
5
An excellent thermal resistance was found with the AAB-mortar when it was exposed to 700 °C for two hours. Only 16.26% of the strength was reduced in the case of AAB-mortar while OPC lost more than 32% of its strength under the same conditions.
-
6
Furthermore, as an alternative material of cement, the consumption of slag, POFA and RHA in the presence of NaOH would be a probable and sustainable solution to reduce the demand of cement which also helps to achieve the goal of sustainable concrete.
Acknowledgments
The authors are greatly indebted to Almighty Allah. Thanks and appreciations are due to the University of Kebangsaan Malaysia, Ministry of Science, Technology and Innovation, Fundamental Research Grant Scheme; and Department of Civil and Structural Engineering for conducting this research.
Author Contributions
For this paper, Muhammad Fauzi Mohd Zain formulated research ideas. Md. Rezaul Karim collected raw materials, carried out the experimental works and performed the literature review. Maslina Jamil and Fook Chuan Lai provided guidence and great assistance during the experimental works. Md. Maruf Hossain and Mohammad Nabi Newaz Khan performed literature reviews and participated in writing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Nehdi M. Ternary and quaternary cements for sustainable development. Concr. Int. 2001;23:35–42. [Google Scholar]
- 2.Climate Policy Assessment for India. University Press; New Delhi, India: 2004. [Google Scholar]
- 3.IEA Tracking Industrial Efficiency and CO2 Emissions, 4a, 2007; Energy Technology Transitions for the Next Industrial Revolution, 2009. [(accessed on 21 June 2011)]. Available online: http//www.ieaetsap.org/web/ETechDS/PDF/I03_cement_June%202010_GS-gct.pdf.
- 4.Bilodeau A., Molhotra V.M. High volume fly ash system: Concrete solution for sustainable development. ACI Mater. J. 2000;97:41–47. [Google Scholar]
- 5.Kartik H.O., Russell L.H., Ross S.M. HVFA concrete—An industry perspective. Concr. Int. 2003;25:29–34. [Google Scholar]
- 6.Miura T., Iwaki I. Strength development of concrete incorporating high level of ground granulated blast-furnace slag at low temperatures. ACI Mater. J. 2000;97:66–70. [Google Scholar]
- 7.Motz H., Geiseler J. Products of steel slags an opportunity to save natural resources. Waste Manag. 2001;21:285–293. doi: 10.1016/S0956-053X(00)00102-1. [DOI] [PubMed] [Google Scholar]
- 8.Malhotra V.M. Reducing CO2 emissions. Concr. Int. 2006;28:42–45. [Google Scholar]
- 9.Yap S.P., Foong K.Y., Alengaram U.J., Zumaat M.Z. Waste materials in Malaysia for the development of sustainable concrete: A review; Proceedings of the 11th International Conference on Concrete Engineering and Technology; Putrajaya, Malaysia. 12–13 June 2012; pp. 113–118. [Google Scholar]
- 10.Torbed Energy technology application description, energy and amorphous silica production from rice husk. [(accessed on 15 April 2011)]. Available online: http://www.torftech.com/pdf/application%20description%20-%20rice%20hulls.pdf.
- 11.Basri H.B., Mannan M.A., Zain M.F.M. Concrete using waste oil palm shells as aggregate. Cem. Concr. Res. 1999;29:619–622. doi: 10.1016/S0008-8846(98)00233-6. [DOI] [Google Scholar]
- 12.FAOSTAT Database. (2008). FAO, Rome. 22 sep 2008 (FAO last access) [(accessed on 20 April 2011)]. Available online: http://beta.irri.org/solutions/index.php?option=com_contentandtask=viewandid=250.
- 13.Torgal F.P., Gomes J.C., Jalali S. Alkali-activated binders: A review. Part 1. Historical background, terminology, reaction mechanisms and hydration products. Constr. Build. Mater. 2008;22:1305–1314. doi: 10.1016/j.conbuildmat.2007.10.015. [DOI] [Google Scholar]
- 14.Tangchirapat W., Jaturapitakkul C., Kiattikomol K. Compressive strength and expansion of blended cement mortar containing palm oil fuel ash. J. Mater. Civil. Eng. 2009;21:426–431. doi: 10.1061/(ASCE)0899-1561(2009)21:8(426). [DOI] [Google Scholar]
- 15.Tangchirapat W., Jaturapitakkul C., Chindaprasirt P. Use of palm oil fuel ash as a supplementary cementitious material for producing high-strength concrete. Constr. Build. Mater. 2009;23:2641–2646. doi: 10.1016/j.conbuildmat.2009.01.008. [DOI] [Google Scholar]
- 16.Chindaprasirt P., Rukzon S., Sirivivatnanon V. Resistance to chloride penetration of blended Portland cement mortar containing palm oil fuel ash, rice husk ash and fly ash. Constr. Build. Mater. 2008;22:932–938. [Google Scholar]
- 17.Nicole P.H., Monteiro P.J.M., Carasek H. Effect of silica fume and rice husk ash on alkali silica reaction. ACI Mater. J. 2000;97:486–492. [Google Scholar]
- 18.Sata V., Jaturapitakkul C., Kiattikomol K. Influence of pozzolan from various by-product materials on mechanical properties of high-strength concrete. Constr. Build. Mater. 2007;21:1589–1598. doi: 10.1016/j.conbuildmat.2005.09.011. [DOI] [Google Scholar]
- 19.Zhang M.H., Malhotra V.M. High-performance concrete incorporating rice husk ash as a supplementary cementing material. ACI Mater. J. 1996;93:629–636. [Google Scholar]
- 20.Chindaprasirt P., Jaturapitakkul C., Rattanasak U. Influence of fineness of rice husk ash and additives on the properties of lightweight aggregate. Fuel. 2009;88:158–162. doi: 10.1016/j.fuel.2008.07.024. [DOI] [Google Scholar]
- 21.Mahmud H.B., Majuar E., Zain M.F.M., Hamid N.B.A.A. Mechanical properties and durability of high strength concrete containing rice husk ash. J. Adv. Concr. Tech. 2009;79:21–30. doi: 10.3151/jact.7.21. [DOI] [Google Scholar]
- 22.Oner A., Akyuz S. An experimental study on optimum usage of GGBS for the compressive strength of concrete. Cem. Concr. Compos. 2007;29:505–514. doi: 10.1016/j.cemconcomp.2007.01.001. [DOI] [Google Scholar]
- 23.Lai F.C. High volume quaternary blended cement for sustainable high performance concrete; Proceedings the 34th Conference on Our World in Concrete and Structures; Singapore. 16–18 August 2009; pp. 175–180. [Google Scholar]
- 24.Zain M.F.M., Islam M.N., Mahmud F., Jamil M. Production of rice husk ash for use in concrete as a supplementary cementitious material. Constr. Build. Mater. 2011;25:798–805. doi: 10.1016/j.conbuildmat.2010.07.003. [DOI] [Google Scholar]
- 25.Annual Book of ASTM Standard 2006. Volume 4.2. ASTM International; West Conshohocken, PA, USA: 2006. Standard specification for chemical admixtures for concrete; ASTM C 494/C494M; pp. 222–286. [Google Scholar]
- 26.Annual Book of ASTM Standards 2009 Cement, Lime, Gypsum. Volume 4.1. ASTM International; West Conshohocken, PA, USA: 2009. American society for testing and materials; ASTM C 305–06; pp. 228–230. [Google Scholar]
- 27.Annual Book of ASTM Standard 2009, Cement, Lime, Gypsum. Volume 4.1. ASTM International; West Conshohocken, PA, USA: 2009. Standard test methods for normal consistency of hydraulic cement; ASTM C187–04; pp. 181–183. [Google Scholar]
- 28.Annual Book of ASTM Standard 2009, Cement, Lime, Gypsum. Volume 4.1. ASTM International; West Conshohocken, PA, USA: 2009. Standard test methods for setting time of hydraulic cement by Vicatneedle; ASTM C191–08; pp. 186–191. [Google Scholar]
- 29.Annual Book of ASTM Standards 2009, Cement, Lime, Gypsum. Volume 4.1. ASTM International; West Conshohocken, PA, USA: 2009. American society for testing and materials; ASTM C1437–07; pp. 622–623. [Google Scholar]
- 30.Annual Book of ASTM Standard 2009. Volume 4.1. ASTM International; West Conshohocken, PA, USA: 2009. Standard test methods for compressive strength of hydraulic cement mortar (using 50mm cube); ASTM C109–08; pp. 241–244. [Google Scholar]
- 31.Ahmed S.F.U., Ohama Y., Demura K. Comparison of mechanical properties and durability of mortar modified by silica fume and finely ground blast furnace slag. J. Civil. Eng. 1999;27:143–154. [Google Scholar]
- 32.Chindaprasirt P., Rukzon S. Strength, porosity and corrosion resistance of ternary blend Portland cement, rice husk ash and fly ash mortar. Constr. Build. Mater. 2008;22:1601–1606. doi: 10.1016/j.conbuildmat.2007.06.010. [DOI] [Google Scholar]
- 33.Rahel K.I., Hamid R., Taha M.R. Fire resistance of high-volume fly ash mortars with nanosilica addition. Constr. Build. Mater. 2012;36:779–776. doi: 10.1016/j.conbuildmat.2012.05.028. [DOI] [Google Scholar]
- 34.Annual Book of ASTM Standard 2006. Volume 4.2. ASTM International; West Conshohocken, PA, USA: 2006. Standard specification for coal fly ash and raw or calcined natural pozzolan for use as a mineral admixture in concrete; ASTM C618–05; pp. 326–328. [Google Scholar]
- 35.Awal A.S.M.A., Hussin M.W. The effectiveness of palm oil fuel ash in preventing expansion due to alkali-silica reaction. Cem. Concr. Compos. 1997;19:367–372. doi: 10.1016/S0958-9465(97)00034-6. [DOI] [Google Scholar]
- 36.Annual Book of ASTM Standard 2006. Volume 4.2. ASTM International; West Conshohocken, PA, USA: 2006. Standard test methods for sampling and testing of fly ash or natural pozzolan for use in Portland-cement concrete; ASTM C311–05; pp. 207–215. [Google Scholar]
- 37.Annual Book of ASTM Standard 2006. Volume 4.2. ASTM International; West Conshohocken, PA, USA: 2006. Standard specification for ground granulated blast-furnace slag for use in concrete and mortars; ASTM C989–05; pp. 531–534. [Google Scholar]
- 38.Givi N.A., Rashid S.A., Aziz F.N.A., Mohd S.M.A. Assessment of the effects of rice husk ash particle size on strength, water permeability and workability of binary blended concrete. Constr. Build. Mater. 2010;24:2145–2150. doi: 10.1016/j.conbuildmat.2010.04.045. [DOI] [Google Scholar]
- 39.De Sensale G.R. Strength development of concrete with rice-husk ash. Cem. Concr. Compos. 2006;28:158–160. doi: 10.1016/j.cemconcomp.2005.09.005. [DOI] [Google Scholar]
- 40.Ganesan E.K., Rajagopal K., Thangavel K. Rice husk ash blended cement: Assessment of optimal level of replacement for strength and permeability properties of concrete. Constr. Build. Mater. 2008;22:1675–1683. doi: 10.1016/j.conbuildmat.2007.06.011. [DOI] [Google Scholar]
- 41.Cheerarot R., Jaturapitakkul C. A study of disposed fly ash from landfill to replace Portland cement. Waste Manag. 2004;24:701–709. doi: 10.1016/j.wasman.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Annual Book of ASTM Standard 2009. Volume 4.1. ASTM International; West Conshohocken, PA, USA: 2009. Standard specification for Portland cement; ASTM C150–07; pp. 152–157. [Google Scholar]
- 43.Tangchirapat W., Saeting T., Jaturapitakkul C., Kiattikomol K., Siripanichgorn A. Use of waste ash from palm oil industry in concrete. Waste Manag. 2007;27:81–88. doi: 10.1016/j.wasman.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 44.Fajana Fajana’a Rule of Polarization 2012. [(accessed on 10 July 2012)]. Available online: http://www.thebigger.com/chemistry/chemical-bonding/what-is-fajan-rule-ofpolarisation/
- 45.Jae E.O., Monteiro P.M.J., Ssang S.J., Sejin C., Simon M.C. The evaluation of strength and crystalline phases for alkali-activated ground blast furnace slag and fly ash-based geopolymers. Cem. Concr. Res. 2010;40:189–196. doi: 10.1016/j.cemconres.2009.10.010. [DOI] [Google Scholar]
- 46.Ahmad S.Y., Shaikh Z. Portland-pozzolana cement from sugarcane bagasse ash. In: Hill N., editor. Lime and Other Alternative Cements. Intermediate Technology Publications; London, UK: 1992. [Google Scholar]
- 47.Isaia G.C., Gastaldini A.L.G., Moraes R. Physical and pozzolanic action of mineral additions on the mechanical strength of high-performance concrete. Cem. Concr. Compos. 2003;25:69–76. doi: 10.1016/S0958-9465(01)00057-9. [DOI] [Google Scholar]
- 48.Altan E., Erdogan S.T. Alkali activation of a slag at ambient and elevated temperatures. Cem. Concr. Compos. 2012;34:131–139. doi: 10.1016/j.cemconcomp.2011.08.003. [DOI] [Google Scholar]
- 49.Ravikumar D., Peethamparan S., Neithalath N. Structure and strength of NaOH activated concretes containing fly ash or GGBFS as the sole binder. Cem. Concr. Compos. 2010;32:399–410. doi: 10.1016/j.cemconcomp.2010.03.007. [DOI] [Google Scholar]
- 50.Rodriguez E.D., Susan A.B., John L.P., Paya J.L., Jose M.M., Maria V.B. Effect of nanosilica-based activators on the performance of an alkali-activated fly ash binder. Cem. Concr. Compos. 2013;35:1–11. doi: 10.1016/j.cemconcomp.2012.08.025. [DOI] [Google Scholar]
- 51.Saraswathy V., Song H. Corrosion performance of rice husk ash blended concrete. Constr. Build. Mater. 2007;21:1779–1784. doi: 10.1016/j.conbuildmat.2006.05.037. [DOI] [Google Scholar]
- 52.Chi M., Huang R. Binding mechanism and properties of alkali-activated fly ash/slag mortars. Constr. Build. Mater. 2013;40:191–298. doi: 10.1016/j.conbuildmat.2012.11.003. [DOI] [Google Scholar]
- 53.Chindaprasirt P., Jaturapitakkul C., Sinsiri T. Effect of fly ash fineness on compressive strength and pore size of blended cement paste. Cem. Concr. Compos. 2005;27:425–428. doi: 10.1016/j.cemconcomp.2004.07.003. [DOI] [Google Scholar]
- 54.Chindaprasirt P., Homwuttiwong S., Jaturapitakkul C. Strength and water permeability of concrete containing palm oil fuel ash and rice husk-bark ash. Constr. Build. Mater. 2007;21:1492–1499. doi: 10.1016/j.conbuildmat.2006.06.015. [DOI] [Google Scholar]
- 55.Mehta P.K.Y., Monteiro P.J.M. Concrete: Microstructure, Properties, and Materials. 3rd ed. McGraw-Hill; New York, NY, USA: 2006. p. 659. [Google Scholar]
- 56.Al-Otaib S. Durability of concrete incorporating GGBS activated by water-glass. Constr. Build. Mater. 2008;22:2059–2067. doi: 10.1016/j.conbuildmat.2007.07.023. [DOI] [Google Scholar]
- 57.Barbosa V.F.F., MacKenzie K.J.D. Synthesis and thermal behavior of potassium sialategeopolymers. Mater. Lett. 2003;57:1477–1482. doi: 10.1016/S0167-577X(02)01009-1. [DOI] [Google Scholar]
- 58.Bernal S.A., Rodríguez E.D., de Gutiérrez R.M., Provis J.L., Delvasto S. Activation of metakaolin/slag blends using alkaline solutions based on chemically modified silica fume and rice husk ash. Waste Biomass Valoriz. 2012;3:99–108. doi: 10.1007/s12649-011-9093-3. [DOI] [Google Scholar]
- 59.Kong D.L.Y., Sanjayan J.G., Sagoe-Crentsil K. Comparative performance of geopolymers made with metakaolin and fly ash after exposure to elevated temperatures. Cem. Concr. Res. 2007;37:1583–1589. doi: 10.1016/j.cemconres.2007.08.021. [DOI] [Google Scholar]